3.1. Summary
Companies are increasingly marketing a wide range of synthetic nicotine products—products that contain, or are promoted as containing, nicotine that is chemically synthesized rather than found in tobacco plants. These products have not been shown to have fewer risks than products containing tobacco-derived nicotine, though their marketing sometimes claims or implies that they do. In many countries, synthetic nicotine products are not clearly subject to current tobacco control regulations (though the products may be subject to other kinds of laws, such as consumer protection laws, in some of those countries). In some countries, however, tobacco control laws have been updated to cover these products in various ways. Countries should consider the ways in which legal adjustments might be made to close regulatory gaps for synthetic nicotine products, including considering adjustments that cover both the broad range of products currently on the market and those products that might emerge in the future.
3.2. Background
The recent appearance of products promoted as containing synthetic nicotine or “tobacco-free” nicotine in many markets, including products sold worldwide over the internet, has brought to the attention of many World Health Organization (WHO) Member States the need for regulators to share information with each other about this development. Many WHO Member States have also requested technical assistance from WHO to address this issue and synthesize available evidence to provide authoritative advice to countries on how to deal with products which are claimed to contain synthetic nicotine. This report, commissioned by WHO, was prepared to help address these issues.
This report covers synthetic nicotine products marketed for recreational uses (rather than for medical uses, such as smoking cessation). The report provides an overview of the kinds of synthetic nicotine products being sold, the claims with which they are marketed, and the science of synthetic nicotine production, toxicology, pharmacology, and detection. It then provides information about the global legal landscape for synthetic nicotine products, focused on tobacco control laws. More specifically, the paper provides the results of coding 210 countries’ laws and the EU’s tobacco control directives to identify laws that cover synthetic nicotine products, and it describes the variation in the ways that those laws cover synthetic nicotine products. Finally, it proposes some recommendations for consideration by policy makers.
3.3. Introduction
The rise of novel and emerging tobacco products, electronic nicotine and non-nicotine delivery systems, imitation tobacco products, and nicotine pouches has led to new forms of nicotine use, including by young people. The success of tobacco control measures and the social stigma associated with consuming conventional tobacco products (including cigarettes, cigars, waterpipe tobacco and smokeless tobacco products) contributed to industry motivation to develop e-cigarettes and other novel products distinct from conventional products. Lately, companies have begun to sell versions of these novel or unconventional products that claim to contain synthetic, rather than tobacco-derived, nicotine (1). These products are sometimes sold with youth-appealing flavours. Additionally, although there currently is no evidence that products containing synthetic nicotine have different health effects or are less addictive than products containing tobacco-derived nicotine, synthetic nicotine products are being sold with marketing claims that may suggest they are safer than tobacco-derived nicotine products (2). This section briefly surveys the kinds of synthetic nicotine products being sold, and the marketing and promotion of those products.
3.3.1. Types of Synthetic Nicotine Products
News reports suggest that the United States (US) is currently the largest market for synthetic nicotine products (though this may change as a result of an amendment to US law in March 2022 that brings synthetic nicotine products within the US Food & Drug Administration’s [FDA’s] tobacco products authorities), followed by South Korea (3). Currently, most products marketed as containing synthetic nicotine are either e-cigarettes, e-liquids, or nicotine pouches. But these are not the only kinds of synthetic nicotine products being sold (2). For example, several companies sell gum products described as containing synthetic or “tobacco-free” nicotine (4, 5), at least two companies are similarly marketing synthetic nicotine toothpicks (6, 7), a Canadian company, PODA, announced in 2021 plans to launch a “heat-not-burn product” that uses “pelletized tea leaves infused with synthetic nicotine,” (8). This company was purchased by Philip Morris, and it is unclear whether its products will be marketed. At least one company, Outlaw Dip, is offering “100% tobacco free” moist snuff “that does NOT come from tobacco” (9), and at least one company, Ronin, is selling a combustible cannabidiol cigarette infused with “non-tobacco nicotine.” (10). There is, thus, wide variety in the kinds of products sold as containing synthetic, rather than tobacco-derived, nicotine, and new types of products may continue to emerge.
Additionally, many of these synthetic or “tobacco-free” nicotine products are designed with flavours that are likely to appeal to youth. For instance, some toothpicks are sold in flavors like “butterscotch cake” and “strawberry cheesecake.” (7). Similarly, certain disposable e-cigarettes are sold in flavour concepts like “banana ice” and “blue razz.” (11).
3.3.2. Marketing and Promotion of Synthetic Nicotine Products
Many companies marketing synthetic nicotine products make claims that may suggest—implicitly or explicitly—that their products are “safer” than products using tobacco-derived nicotine in various ways. These include claims suggesting that synthetic nicotine contains fewer impurities than tobacco-derived nicotine and that synthetic nicotine is equivalent to pharmaceutical grade nicotine. Companies also make claims that suggest that synthetic nicotine products provide other advantages over products with tobacco-derived nicotine, including claims that they provide more satisfaction and a better taste experience, and that they are more environmentally friendly. Additionally, some synthetic nicotine products are marketed as effective smoking cessation aids or equivalent to approved nicotine replacement therapies (sometimes alongside disclaimers that products are not smoking cessation products). A few specific examples are provided below.
3.4. The Science of Synthetic Nicotine
The rapid and poorly regulated introduction of synthetic nicotine products (electronic cigarettes, oral pouches, and other product categories) in the United States and other countries raises questions about product safety and potential differences in the addictive and reinforcing properties of synthetic nicotine. In this section, we review the chemical synthesis strategies, the different forms of synthetic nicotine in products, manufacturers and patent landscape, and the toxicological, pharmacological, and metabolic properties of synthetic nicotine.
3.4.1. Methodology
Research databases, including PubMed and Web of Science, were searched with the terms such as “synthetic nicotine”, “R-nicotine”, “(+)-nicotine”, “L-nicotine”, “D-nicotine”, “racemic nicotine”, and “nicotine synthesis” for journal articles on synthetic nicotine and studies comparing the effects of nicotine enantiomers (defined below). Patents were searched on patents.google.com with the combinations of the terms such as “nicotine” “synthesis” and / or “stereoselective”, “enantioselective”. The tobacco legacy database at www.industrydocuments.ucsf.edu/tobacco/ was searched with terms such as “synthetic nicotine”, “nicotine synthesis”, “synthesis of nicotine”, and “R-nicotine”.
3.4.2. Results and discussion
3.4.2.1. Synthetic nicotine: What is it, and how does it differ from tobacco-derived nicotine?
Nicotine exists in two chemical forms that are structural mirror images of each other. These two forms, also termed enantiomers, are called S- and R-nicotine (Figure 1A). Nicotine in the tobacco plant consists of >99% S-nicotine and only minimal amounts of R-nicotine (17). Chemists first synthesized nicotine in 1904 (18). The synthesis resulted in a mixture containing both S- and R-nicotine at a 50/50 ratio (18, 19). Such a 50/50 mixture is called a racemic mixture. This mixture differs from tobacco-derived nicotine in that it has a much higher R-nicotine content and a lower S-nicotine content.
Figure 1. Structure and chemistry of synthetic nicotine.
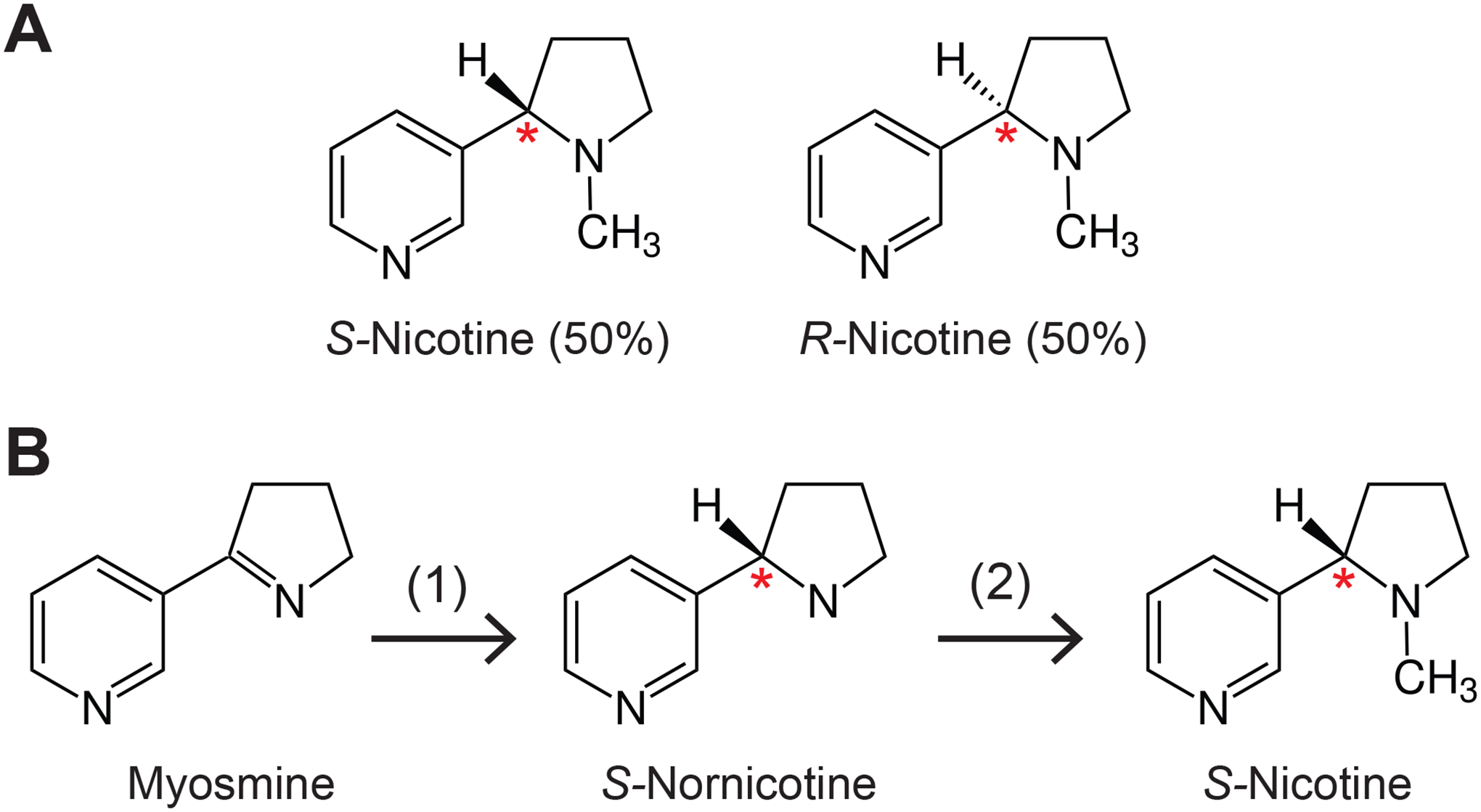
(A). Structures of S- and R-nicotine. The compounds differ in their configuration at the carbon atom labeled with a red asterisk, also called a chiral center. In tobacco leaf >99% of nicotine is present as S-nicotine. Synthetic “Tobacco-Free Nicotine” (TFN), marketed by Next Generation Labs, is racemic, containing 50% S-nicotine and 50% R-nicotine. Pure synthetic S-nicotine is chemically indistinguishable from S-nicotine purified from tobacco.
(B). Synthesis of S-nicotine as described in a patent assigned to Zanoprima involving a biotechnological step. The starting material is myosmine, first converted to S-nornicotine using a recombinant enzyme (1), a NADH/NADPH dependent imine reductase. Such a reaction is also called “stereoselective”. S-nornicotine is then converted to S-nicotine through methylation (2).
A search in the Truth Tobacco Industry Documents, the database of tobacco industry internal corporate documents produced during litigation in the United States, with the term “synthetic nicotine” revealed that the industry had already considered the use of synthetic nicotine in the 1960s. Employees of British American Tobacco (BAT) proposed the addition of synthetic nicotine as a method to increase the nicotine / tar ratio in combustible cigarettes (20). However, the proposal was not further pursued due to concerns that synthetic nicotine was only available as a racemic mixture with unknown health effects. Also, the price of synthetic nicotine was much higher than the price of tobacco-derived nicotine (20). Employees of RJ Reynolds and Liggett & Myers also considered using synthetic nicotine to adjust nicotine levels in cigarettes, however, this idea was abandoned for the same reasons (21, 22). Beyond 1978, the Truth Tobacco Industry Documents database provides no further evidence for deliberations on the use of synthetic nicotine use by the major US tobacco companies. In the following years, chemists developed new strategies for the synthesis of nicotine, including strategies to produce pure S-nicotine, the form of nicotine prevalent in tobacco leaf (19, 23).
3.4.2.2. The synthetic nicotine marketplace: Manufacturers, patents, and pricing
In 2015, the company Next Generation Labs (NGL) began marketing synthetic nicotine in the United States under the trademarked brand names TFN® (Tobacco Free Nicotine) for consumer products and PHARMANIC® for pharmaceutical products. In the same year, NGL applied for US and world-wide patents with the title “Process for the Preparation of (RS)-Nicotine” (24). The US patent was assigned to NGL in 2017. This patent describes a synthetic pathway using ethyl nicotinate as starting material. Ethyl nicotinate is derived from nicotinic acid (niacin), a synthetic chemical produced from petrochemical sources. Ethyl nicotinate is reacted with N-vinyl-2-pyrrolidinone to form myosmine, a tobacco alkaloid. Myosmine is then converted to nornicotine. Subsequent methylation of nornicotine then results in a racemic (50/50) nicotine mixture of S- and R-nicotine (Figure 1A) (24). NGL also filed a patent for the use of their synthetic nicotine in smoking cessation products (25). In 2016, Hellinghausen et al. analyzed the nicotine content of US-marketed electronic cigarette liquids containing TFN-branded synthetic nicotine manufactured by NGL, confirming that the product is racemic nicotine (26). While vaping products containing synthetic nicotine were marketed in the United States since 2015, they only attracted public attention in 2021, when popular vaping brand Puffbar announced a switch to synthetic nicotine in their products (27). Analytical studies revealed that these products contained racemic nicotine (28). The source of the synthetic racemic nicotine in Puffbar products has not been revealed.
At the same time, advances in chemistry enabled the optimization of strategies for the manufacture of pure S-nicotine. Several companies have filed patent applications for the synthesis of S-nicotine. Contraf-Nicotex-Tobacco (CNT GmbH, Germany), the world’s largest supplier of pharmaceutical grade nicotine, developed a manufacturing process first synthesizing racemic nicotine from ethyl nicotinate and n-vinylpyrrolidone nicotinic acid. A subsequent selective purification is used to enrich for pure S-nicotine (29, 30). Vaping products containing CNT’s synthetic S-nicotine have been marketed in the United States since 2020 (31, 32). Zanoprima Life Sciences Ltd. (London, UK) is another manufacturer of synthetic S-nicotine (33). Zanoprima was granted a US patent for a process involving a biotechnological step for the synthesis of S-nicotine in 2021. (34). The start material is myosmine, first converted to S-nornicotine using a commercially available recombinant enzyme, a NADH/NADPH dependent imine reductase. S-nornicotine is then converted to S-nicotine through methylation (Figure 1B). This product is currently marketed under the brand name “SyNic” (33). Hangsen International Group, a major manufacturer of vaping devices and e-liquids, applied for a Chinese and World Patent for a similar process, marketing synthetic S-nicotine under the brand name “Motivo” (35, 36). NJOY, a major E-cigarette manufacturer (soon to be owned by cigarette-maker Altria (37)), was also awarded a patent for nicotine synthesis and purification (38). Some patents describe the resulting nicotine as >99.9% pure, with chiral purity of >99.6% S-nicotine or higher. Wholesale products list purities of 99.9% S-nicotine (39).
In 2019, a representative of Next Generation Lab stated that the company’s synthetic racemic nicotine product, the racemic mix of R- and S-nicotine, “is only three to four times the current cost of tobacco-derived nicotine” (40). As of March 21, 2023, the wholesale price of 1 liter of Next Generation Labs TFN racemic synthetic nicotine was quoted as USD 1,800, while the same wholesaler offered 1 liter of tobacco-derived nicotine for USD 229.99 – 429.99, depending on brand, equivalent to a 4–8 – fold higher price for the synthetic version (41–43). 1 liter of Zanoprima’s SyNic synthetic S-nicotine was marketed at a price of USD 999.99 while the same seller quoted a price of USD 229.99 for tobacco-derived nicotine, a ~4-fold difference (39, 44). In summary, the pricing of synthetic nicotine remains substantially higher than for tobacco-derived nicotine. Nevertheless, synthetic nicotine products continue to be marketed, including electronic cigarette products and oral nicotine pouches (ONPs), also known as “white snus”. These products are often advertised using claims of purity and healthfulness when compared to products containing tobacco-derived nicotine.
Manufacturers of synthetic nicotine (Table 2) have begun to leverage their intellectual property, leading to legal conflicts and market consolidation. NGL’s intellectual property was recently affirmed by Chinese authorities, enabling the company to enforce its patents in the country where the large majority of E-cigarette products are manufactured (45). Zanoprima sued a major E-cigarette and liquid manufacturer, Hangsen, for infringement of its patent in a United States District Court (46). Hangsen-manufactured nicotine was added to US-marketed “Geekbar” products in 2021, however, Hangsen ceased marketing its “Motivo”-branded synthetic S-nicotine in the US after the lawsuit was filed, while continuing sales outside the US (35, 47).
Table 2.
Major manufacturers of synthetic nicotine and their synthesis strategies
| Manufacturer Name | Starting material | Resulting product | Stereoselective step |
|---|---|---|---|
| Next Generation Labs LLC (NGL) | ethyl nicotinate | racemic (50/50) R-/S-nicotine | n/a |
| Contraf-Nicotex-Tobacco GmbH (CNT) | ethyl nicotinate | S-nicotine | stereoselective recrystallization |
| Zanoprima Lifesciences Ltd | Myosmine | S-nicotine | enzymatic stereoselective step |
| Hangsen International Group | Myosmine | S-nicotine | enzymatic stereoselective step |
| NJOY LLC | racemic (50/50) R-/S-nicotine | S-nicotine | stereoselective recrystallization |
3.4.2.3. Health claims by synthetic nicotine manufacturers
Similar to the sellers of end products, the companies manufacturing synthetic nicotine promote their products with health-related statements. Next Generation Labs claims that “TFN is devoid of many of the residual impurities that tobacco derived nicotine contain … TFN is virtually tasteless and odorless … there is no need to mask the off-flavor and aroma of tobacco-based nicotine.” (48). Next Generation Labs also claims that “specific ratios of the ‘R’ to the ‘S’ isomers could potentially offer nicotine use at satisfying but non-addictive or less addictive levels.”. CNT opposes this notion, claiming that its synthetic S-nicotine is superior to the racemic version, stating “If you look at the European and the U.S. Pharmacopeias, the percentage of S-isomers in nicotine must be higher than 99 percent” (40, 49). Zanoprima claims that its synthetic S-nicotine “is free of related tobacco alkaloids, TSNAs (tobacco-specific nitrosamines), odour, and harsh taste” (33). These statements may represent claims of superior drug-like properties for synthetic nicotine. Companies also claim they use a sustainable “green chemistry” approach for production that is environmentally more friendly than agricultural tobacco production requiring pesticides, fertilizer, extensive land use and hazardous production methods.
3.4.2.4. Toxicological, pharmacological, and metabolic properties of synthetic nicotine
As described above, there are currently two forms of synthetic nicotine in marketed products, S-nicotine and racemic nicotine, the latter consisting of 50% S-nicotine and 50% R-nicotine. Since synthetic S-nicotine is chemically identical to tobacco-derived S-nicotine, its toxicological, metabolic, and pharmacological properties should also be identical, especially if added at the purity the major manufacturers are selling the synthetic product (>99.9%). Nevertheless, even at this high degree of purity, trace amounts of other chemicals remaining from the chemical process might be present, deserving further attention.
If a consumer uses a product containing synthetic racemic nicotine, 50% of the nicotine intake is R-nicotine. Compared to S-nicotine, the knowledge about R-nicotine’s toxicological, metabolic, and pharmacological effects remains limited. A study using mice established the dose required to produce a lethal effect in 50% of the animals (LD50) 60 minutes after intravenous injection. The LD50 for S-nicotine was 0.33 mg/kg, while the LD50 for R-nicotine was 6.15 mg/kg, >18 fold higher, suggesting that R-nicotine has less acute toxicity than S-nicotine under the chosen conditions (50). This study also found that a higher dose of R-nicotine was required to induce convulsions compared to S-nicotine.
Pharmacological studies revealed that R-nicotine is a significantly less potent (~10-fold) agonist of nicotine receptors than S-nicotine (51). A study examining nicotine binding in the brain found that S-nicotine is more potent than R-nicotine, by 10-fold or greater (52). Chronic administration of either form of nicotine was shown to increase the number of nicotine binding sites in the rat brain (53).
In an operant behavioral study evaluating the capability of rats to discriminate injected R- or S-nicotine from saline, S-nicotine was 9 times more potent than R-nicotine (54). A study characterizing the locomotor stimulant action of nicotine in rats observed that S-nicotine was at least 10 times more potent in stimulating motor activity (55). S-nicotine was 4–5 times more potent than R-nicotine in conditioned taste aversion assays in rats (56). In contrast to S-nicotine, R-nicotine did not induce weight loss in rats and did not trigger epinephrine release (51, 53). Altogether, pharmacological studies comparing the enantiomers in the standard experimental paradigms for nicotinic pharmacology revealed that S-nicotine, the prevalent nicotine enantiomer in tobacco (>99%), is between 4–28 times more potent than R-nicotine that is present at high levels (50%) in synthetic racemic nicotine (51, 52, 54, 55, 57–60).
S-nicotine and R-nicotine also differ in their metabolism. Studies in guinea pigs revealed that S-nicotine formed only oxidative metabolites, whereas R-nicotine formed both oxidative and N-methylated metabolites (61). The degradation kinetics of the resulting S- and R-cotinine also differed. Studies comparing metabolism in different laboratory animal species observed strong differences in the degradation and excretion of S-nicotine vs. R-nicotine, and also sex differences in R-nicotine metabolism (60–62). Species differences were also observed when the N-methylation of S- and R-nicotine were compared in human, rat and guinea pig liver cytosol extracts (63). While human extract catalyzed N-methylation of both forms of nicotine, rat extract did not form any N-methylation products, and guinea pig extract only transformed R-nicotine, but not S-nicotine (63). It is unknown whether these N-methylation products are bioactive, and whether S- and R-nicotine methylation products act differently. These findings indicate that the human metabolism of R-nicotine and its behavioral effects need to be investigated further, and that predictions about toxicological outcomes of R-nicotine consumption should not rely on animal model studies alone. At this time, lack of such key data and the observed species differences preclude a toxicological risk assessment for R-nicotine in humans.
In addition to the differences in nicotinic receptor-mediated pharmacological effects, R- and S-nicotine have differential effects on other pharmacological targets. For example, a tobacco industry-sponsored study on acetylcholinesterase, the enzyme that degrades the neurotransmitter acetylcholine in the synaptic cleft to terminate neurotransmission, revealed that R-nicotine is a more potent inhibitor of the enzyme than S-nicotine, binding to a different site on the enzyme protein (64). The experiments were performed using acetylcholine esterase isolated from electric eels, and at nicotine concentrations much higher than in smokers. Whether such effects occur in humans, and how they impact acetylcholine levels and neurotransmission, needs to be further studied. Both forms of nicotine were shown to interfere with the production of certain lipid mediators involved in regulation of inflammation with similar potency, demonstrating that some biological processes are equally affected by both forms of nicotine (65).
3.4.3. Human psychophysical studies
Human psychophysical studies examined whether R- and S-nicotine elicited different odor or irritant sensations. Humans perceive nicotine vapor as aversive when exposed in the nose. At higher concentrations, nicotine vapor causes nasal irritation, including stinging and burning sensations, mediated by the trigeminal nerve that transmits pain signals to the brain. Test subjects reported lower detection thresholds for S-nicotine than for R-nicotine, higher burning and stinging intensity, while olfactory perceptions were elicited at similar levels (66). In electrical recordings of the mucosal potential, S-nicotine elicited stronger responses than R-nicotine. Smokers perceived S-nicotine as more hedonic than non-smokers, likely due to prior experience (66). These are, so far, the only systematic human studies comparing responses to S- and R-nicotine within the very limited timeline of the experiments, with individual vapor stimuli applied for only 250 milliseconds.
3.4.4. Analytical detection of synthetic nicotine
In order to validate vaping products labeled to contain synthetic racemic nicotine, Hellinghausen et al. developed a methodology, using a chiral stationary phase for separation of R- and S-nicotine by High-Pressure Liquid Chromatography, HPLC, followed by circular dichroism detection and electrospray ionization mass spectrometry (26). The authors reported that one product contained twice as much total nicotine (sum of R- and S-nicotine) as stated on the product label, effectively listing only the S-nicotine strength. In contrast, the nicotine amount listed on other labels was equivalent to the measured total nicotine amount, with effectively half present as S-nicotine (26). These observations suggest that uniform product labeling practices should be imposed by regulators. Otherwise, unknowing users may be exposed to high levels of R-nicotine, or to lower S-nicotine levels than they are used to. Inappropriate labeling of nicotine levels may motivate consumers to purchase products with higher total nicotine content, potentially leading to significantly higher S-nicotine intake. The authors also detected impurities that require further characterization (26). A more recent study examined Puffbar vaping products for the presence of S- and R-nicotine, using 1H NMR spectroscopy, polarimetry, and Gas Chromatography-Mass Spectrometry (GCMS) (28). The authors confirmed that these products indeed contained both nicotine forms, albeit with a slightly higher content of S-nicotine than R-nicotine. The authors speculate that the manufacturer may have added tobacco-derived nicotine, however, further analysis would be needed to confirm this.
Some methods have been proposed to differentiate nicotine derived from tobacco from synthetic nicotine. With synthetic S-nicotine available now at high purity, differentiation of synthetic S-nicotine from tobacco-derived S-nicotine is challenging. Since the compounds are chemically identical, they cannot be differentiated by standard analytical techniques. Carbon isotope analysis may offer a solution. Carbon exists as three isotopes 12C, 13C and 14C. 14C has a half-life of 5,700 years, a property used in radiocarbon dating of biological materials. 14C is constantly replenished in the atmosphere by the sun’s radiation and is then integrated into living plant matter, including tobacco plants and their natural products such as nicotine. Synthetic nicotine is produced from petrochemical precursors that were formed in the earth millions of years ago, having a much lower 14C content. For example, a 14C analytical method has been developed to differentiate between naturally sourced and fossil chemical-derived vanillin, a popular flavor chemical (67). Depending on the metabolic pathways involved, natural products may also contain a higher ratio of 13C. High-temperature liquid chromatography coupled to isotope ratio mass spectrometry (HT RPLC/IRMS) has become a standard approach to identify foods adulterated with synthetic additives, capable of differentiating between natural and synthetic caffeine, ethanol and sugars, among other chemicals (67, 68). A method developed by Cheetham et al. used a 14C isotope based approach to compare samples of tobacco-derived and synthetic nicotine, finding that the tobacco-derived samples contained 100% “modern” biocarbon, meaning that their carbon isotope distribution is identical to the current distribution in earth’s atmosphere (69). The synthetic nicotine samples contained only ~35% biocarbon, meaning that some naturally sourced precursors were likely used in the synthetic process. The commercial synthetic nicotine preparations tested in this study were found to be of high purity (>99.9% nicotine content) (69). They contained only minor amounts of nicotine derivatives and degradants, fulfilling the United States Pharmacopeia (USP) criteria for pharmaceutical grade nicotine (70). The commercial purified tobacco-derived nicotine samples were of similar high purity, also fulfilling USP criteria for pharmaceutical grade nicotine (69). The authors also devised methodologies to identify products containing mixtures of both synthetic and tobacco-derived nicotine, and they developed a method for purification of nicotine from electronic cigarette liquids, an essential first step for the analysis of marketed products (69). Purification of nicotine is required due to the presence of carbon-based solvents (propylene glycol, glycerol), flavor chemicals and other additives in marketed products.
Methodologies involving the analysis of hydrogen isotopes (hydrogen and deuterium), and nitrogen isotopes (15N) in nicotine, either qualitative or quantitative, also revealed substantial differences between tobacco-derived nicotine sourced from different growth locations, and from synthetic nicotine (71, 72). Taken together, while significant advances have been made in the analytical methodologies to discriminate synthetic from tobacco-derived nicotine, no standard methodology is available at this time and the instrumentation and skills required to apply such methodology are costly to acquire, with few countries having such capabilities.
3.4.5. Summary and discussion
Manufacturers have developed several synthetic strategies that enabled more efficient and economical production of synthetic nicotine. At this time, two forms of synthetic nicotine are added to marketed products, racemic nicotine, consisting of 50% S-nicotine and 50% R-nicotine, and pure S-nicotine. The pricing of synthetic nicotine remains significantly higher than for tobacco-derived nicotine. Consumers using products containing racemic nicotine take in much higher amounts of R-nicotine than users of tobacco-derived nicotine or pure S-nicotine, raising questions about the long-term safety of such products. While R-nicotine is significantly less potent than S-nicotine in standard nicotinic pharmacological assays and behavioral tests, toxicological studies examining the effects of R-nicotine are limited to acute studies. There is also evidence that R-nicotine differentially affects other pharmacological and toxicological targets, raising concerns about unexpected toxicological effects. None of the published pharmacological studies exposed animals for longer than 1–2 weeks, and none examined pathological effects thereafter. None of the published studies examined the effects of racemic nicotine, with both R- and S-nicotine present, and none compared their effects when inhaled or ingested, the routes through which consumer products dispense nicotine. Most studies comparing the effects of R- and S-nicotine were published between the 1970s and 90s. Toxicological methods have advanced significantly since then and should be applied to examine long-term effects of R-nicotine intake. Chemical analysis strategies are capable of differentiating between synthetic and tobacco-derived nicotine, however, they have not been standardized and require substantial investments in advanced equipment and training. Analytical studies raise concerns about inaccurate labeling of marketed products and undisclosed addition of tobacco-derived nicotine, likely added to increase addictiveness and raise profits while maintaining health claims associated with synthetic nicotine. Tested commercial preparations of both purified tobacco-derived nicotine and synthetic nicotine fulfill USP purity criteria for pharmaceutical grade nicotine, however, not all currently marketed preparations have been compared. Due to the very similar purity of the synthetic and tobacco-derived preparations, specific health claims attributed to synthetic nicotine over purified tobacco-derived nicotine will require strong scientific evidence.
If regulators restrict the use of synthetic nicotine in marketed products, the chemical synthesis strategies developed by the manufacturers can be rapidly modified to generate nicotine analogs (19). The tobacco industry has a long history of studying the addictive and reinforcing effects of nicotine-related tobacco alkaloids, including anabasine, nornicotine, anatabine, cotinine and myosmine (19, 59, 73–76). Regulators need to be aware that these analogs may be used to replace nicotine in marketed products.
3.5. Legal Landscape
Allowing an unregulated market of synthetic nicotine products risks undermining public health progress in mitigating the harms of tobacco use (2, 77, 78). US lawmakers, for instance, wrote a letter to the FDA in November 2021 expressing concern that the unregulated sale of synthetic nicotine products was “undermin[ing] efforts to reduce the continued popularity of youth vaping.” (79) Additionally, current marketing claims for certain synthetic products may be misleading people who use those products by, for example, suggesting that they are safer than tobacco-derived nicotine products even though existing evidence does not support such a claim.
Where synthetic nicotine products are left unregulated, companies are likely to make business choices to sell products containing synthetic, rather than tobacco-derived, nicotine (or at least claim to be doing so), undermining efforts to comprehensively regulate novel tobacco and nicotine products (77). Companies are aware that synthetic nicotine products may not be covered by some countries’ tobacco control laws. Two of the major global suppliers of synthetic nicotine, Hangsen and NGL, have both touted “[f]ewer restrictions on entering new markets” as one of synthetic nicotine’s key benefits (80). Going further, before recent changes to US law, an investment analyst in the US referred to synthetic nicotine as a potential “golden ticket,” because use of synthetic nicotine in place of tobacco-derived nicotine might mean “no FDA regulation, no tobacco taxes, no flavor restrictions, and no restrictions on direct to consumer e-commerce.” (81). As a final example, Puff Bar, which produces disposable e-cigarettes popular among youth, took advantage of the former regulatory gap in the US, when, following an FDA enforcement action, it relaunched its products in early 2021 claiming that it was using synthetic nicotine that exempted it from regulation as a tobacco product (82).
A key question for policy makers, accordingly, is whether or not synthetic nicotine (or other non-tobacco-derived nicotine alternative) products fall within existing regulatory frameworks for tobacco products. This depends on the definitions of the key terms used in relevant laws and whether or not those definitions are specific to (and limited to) tobacco-derived products. The WHO Framework Convention on Tobacco Control (WHO FCTC) defines “tobacco products” as “products entirely or partly made of the leaf tobacco as raw material which are manufactured to be used for smoking, sucking, chewing or snuffing,” which would appear to exclude non-tobacco synthetic nicotine products (83). However, the WHO FCTC language does not limit the ability of Member States to include synthetic nicotine products within the definition of “tobacco products” in their national laws, or to otherwise incorporate products containing synthetic nicotine into national (or sub-national) tobacco control laws. Notably, the Conference of the Parties has requested the Secretariat of the WHO FCTC “to advise, as appropriate, on the adequate classification of novel and emerging tobacco products such as heated tobacco products to support regulatory efforts and the need to define new product categories.” (83).
To better understand the global legal landscape for synthetic nicotine products and inform how Member States may want to revise existing regulatory definitions, including to comply with international obligations (83), we surveyed the tobacco control laws of 210 countries and the EU to determine whether and how those laws applied to synthetic nicotine products.
3.5.1. Methodology
Our review covered tobacco control regulations pertaining to market entry requirements (e.g., requirements for registration before marketing), sales restrictions (e.g., age restrictions for sales, or restrictions on where retailers place tobacco products), packaging and labeling requirements (e.g., requirements for certain warning statements or images), and advertising regulations (e.g., restrictions on television advertisements). We excluded other kinds of tobacco-related laws, such as tax laws, smoke-free laws, and the regulation of flavours.
Laws generally were collected from the Tobacco Control Laws website (84), which provides laws for 210 countries and the EU. The United States’ March 2022 amendment to its “tobacco products” definition was not yet available on this website, and was accessed elsewhere (85–87). Accordingly, laws from a total of 211 jurisdictions were included in the analysis.
Of the 211 jurisdictions reviewed, 21 countries either did not have any laws available or did not have laws in English available. For the remaining 190 jurisdictions (189 countries and the EU) in our sample, three English-speaking individuals with US legal training (two of the authors, MLB and PJZ, and Annamarie Beckmeyer, JD) reviewed the relevant laws. For the EU, its directives are not binding law but are instead “legislative act[s] that set [] out a goal that all EU countries must achieve [with…] the individual countries [left to] to devise their own laws on how to reach these goals.” However, because of the importance of the Tobacco Products Directive (TPD) to tobacco policymaking in Europe, we coded the EU as a separate jurisdiction (88).
Using MonQcle, a legal research software (89), laws were first coded for whether they applied to any synthetic nicotine products. If the laws did apply to synthetic nicotine products, they were then coded for whether they applied to any synthetic nicotine products in addition to e-cigarettes, as well as whether the covered synthetic nicotine products are subject to market entry requirements, sales restrictions, packaging and labeling requirements, and advertising restrictions.
3.5.2. Results and Discussion
Some countries have laws with broad enough phrasing to cover some synthetic nicotine products, and other countries have begun to amend their tobacco control laws such that they apply to products using nicotine that is not tobacco-derived, but many countries’ tobacco control laws do not clearly apply to such products.
3.5.3. Laws that Do Not Cover Synthetic Nicotine Products
Of the 190 jurisdictions’ laws coded, 92 did not apply to any type of synthetic nicotine product. The jurisdictions within this category often had laws defining covered products by reference to tobacco content. For example, before March 2022, U.S. law defined “tobacco products” (for purposes of federal regulation) as product that were “made or derived from tobacco.” (86, 87, 90).
Some laws in this category did not expressly define the relevant terms in the laws, but the terms themselves suggested synthetic nicotine products likely do not fall within the scope of the laws. For example, some laws used the term “tobacco product” without defining it. Countries in the WHO Africa Region were most likely to have laws that did not apply to any type of synthetic nicotine product.
3.5.4. Laws that Clearly Cover Only Certain Synthetic Nicotine Products
52 jurisdictions’ laws include definitions broad enough to cover certain synthetic nicotine—typically e-cigarettes—but not other currently marketed synthetic nicotine products like pouches, toothpicks, and gums. In many of these cases, the laws define “tobacco products” with reference to tobacco content (as in the category above), but then separately define “electronic cigarettes” or similar terms without limiting the nicotine source to tobacco or without requiring any nicotine content.
Other laws in this category do not expressly define relevant terms but use terms that can encompass e-cigarettes that contain synthetic nicotine. For example, some laws use terms like “electronic cigarettes” or “electronic nicotine delivery systems” without defining the limits of the terms. These terms are likely broad enough to cover e-cigarettes that contain synthetic, rather than tobacco-derived, nicotine.
Some countries’ laws have limited application beyond e-cigarettes. For instance, some countries’ laws also cover herbal smoking products that contain no tobacco, leaving room to cover combustible products infused with synthetic nicotine.
Of the entire group of laws covering only certain synthetic nicotine products, a few were complete bans on the sale and distribution of e-cigarettes. There is no clear geographic trend in which jurisdictions have implemented laws covering only certain synthetic nicotine products. Instead, there appears to be a temporal trend; most laws in this category were enacted in 2017 or later.
3.5.5. Laws that Cover Synthetic Nicotine Products More Broadly
29 jurisdictions’ laws are drafted broadly enough to cover all or most of the synthetic nicotine products that are currently marketed, as well as synthetic nicotine products that may emerge in the future. Of these laws, only a few amounted to complete bans on a broader range of synthetic nicotine products.
The US provides a recent example of a country in this category that now regulates, but does not explicitly ban, synthetic nicotine products (91). In March 2022, the US Federal Food, Drug, and Cosmetic Act was amended to bring synthetic nicotine products within FDA’s tobacco product authorities. The US definition of a “tobacco product” now covers “any product made or derived from tobacco or containing nicotine from any source that is intended for human consumption, including any component, part, or accessory of a tobacco product” (emphasis added). This definition, accordingly, would cover all or most of the currently marketed synthetic nicotine products, as well as products that may emerge in the future. Under the U.S. law, synthetic nicotine products now require premarket authorization from the FDA before they can be legally sold. No synthetic nicotine products have yet received such authorization, but the FDA reports that > 1 million marketing applications from more than 200 companies were received (91). FDA refused to accept 925,000 of these for further review. 8,600 applications were accepted for further review.
As another example, Moldova defines “related products” separately from “tobacco products” to include “products made of plants for smoking and products that contain nicotine, including electronic cigarettes,” which again seems to provide broad coverage of existing synthetic nicotine products, as well as those that may emerge. Singapore provides an additional example of a country where the law contains a definition providing broad coverage over synthetic nicotine products. Its law defines “tobacco product” to include “a tobacco substitute,” which in turn means “any article, object or thing that contains nicotine” without requiring that nicotine be tobacco derived. (And the law expressly excludes from the “tobacco substitute” definition “(a) a cigarette or cigar, or any other form of tobacco; (b) a tobacco derivative; (c) a mixture containing any form of tobacco or a tobacco derivative; (d) a therapeutic product registered under the Health Products Act”).
Countries in the WHO Europe Region are most likely to have laws that reach a broader range of synthetic nicotine products. A majority of these laws were created since 2016.
3.5.6. Laws that are Unclear Regarding Synthetic Nicotine Product Coverage
17 jurisdictions’ laws were unclear as to whether their definitions cover synthetic nicotine products. For example, some of these laws referred to tobacco when defining nicotine products but did not clarify whether the laws’ application was limited to nicotine derived from tobacco. For some countries, the Tobacco Control Laws website did not have sufficient information available in English to determine whether its tobacco-related laws applied to products containing synthetic nicotine.
3.5.7. Discussion
There are varied options for legal adjustments that can bring synthetic nicotine products within the scope of tobacco control regulations. Some of the approaches that countries have adopted cover only e-cigarette or e-liquid synthetic nicotine products. These approaches leave potentially unregulated other kinds of synthetic nicotine products that are currently marketed or that may emerge in the future, which may in turn undermine efforts to comprehensively regulate novel tobacco and nicotine products. However, as approaches in countries like Moldova, Singapore, and, most recently, the US demonstrated, there are also legal adjustments that can bring the full range of currently marketed synthetic nicotine products, as well as products that may emerge in the future, within tobacco control regulations.
Though our analysis is limited to synthetic nicotine, it provides an example of how the tobacco industry may seek to exploit gaps or uncertainty in legal rules in order to market new products or evade tobacco-related regulations. Further work on the legal landscape for nicotine analogs may be useful to help countries develop appropriate regulatory approaches (73).
3.5.8. Limitations
There are several limitations to the above description of the global legal landscape for synthetic nicotine products. Only laws available in English, and only tobacco control laws regarding market entry, sales restrictions, packaging and labeling, and advertising, were coded. There may be additional variation in the ways that countries address synthetic nicotine products in laws not available in English or in other kinds of tobacco laws, like tax laws. Similarly, the coded laws generally did not include sub-national jurisdictions within the same country, which may also have laws that define key terms differently. There also may be laws missing from the Tobacco Control Laws website, or court decisions that could impact the interpretation or enforceability of these laws. For example, the most recently adopted laws for some countries may not yet be reflected on the Tobacco Control Laws website. Likewise, there may be translation errors in the English versions of laws originally enacted in another language.
Importantly, the coded laws also did not include laws that regulate products other than tobacco products. Even if synthetic nicotine products do not fall within a country’s regulatory scheme for tobacco products, they may be subject to regulation as drugs (or drug-device combination products), or subject to other consumer protection laws. Such laws may give countries opportunities to regulate synthetic nicotine products without any changes to tobacco control laws (77). For example, before US law was amended in March 2022 to bring synthetic nicotine products within FDA’s tobacco product authorities, public health groups urged FDA to regulate synthetic nicotine products as drugs (95). Synthetic nicotine manufacturers like Hangsen and NGL boast that their products “provide the same satisfaction smokers are seeking from their nicotine,” which is an implicit acknowledgement that the products being sold and used for its effects as an addictive drug (48). Most product websites also include warnings or disclaimers acknowledging that the nicotine in their products is addictive and may be hazardous. As another example, the Australian Therapeutic Goods Administration (TGA) requires a prescription for the sale of e-cigarettes containing nicotine. Although this requirement appears to cover synthetic nicotine products, it is not imposed through Australia’s tobacco control laws and thus is outside the scope of this analysis (96).
Finally, whether requirements are imposed through tobacco control laws or other kinds of laws, the coding analysis cannot assess whether any given law is being enforced. Levels of enforcement may also vary within and between countries.
3.6. Recommendations for consideration by policymakers
If a regulatory gap for synthetic nicotine products (as compared to products containing nicotine derived from tobacco) exists, countries may consider amending tobacco control laws to ensure that synthetic nicotine products fall within their scope.
Countries that choose to amend tobacco control laws to cover synthetic nicotine products may consider legal adjustments that extend the scope of the laws to cover the full range of synthetic nicotine products that are currently marketed as well as products that may emerge in the future. These may include products containing synthetic nicotine analogs or any other chemical with similar properties, or chemical systems that generate nicotine or analogs in situ.
Countries are advised to enforce purity standards for synthetic nicotine in products, preferably at U.S. Pharmacopeia/ European Pharmacopeia (USP / Ph. Eur.) standards. Regulators should consider the implementation of product standards that ban the mixing of tobacco-derived nicotine with synthetic nicotine in marketed products.
Policymakers are advised to enforce uniform labelling rules for products containing nicotine, either natural or synthetic, declaring the content of S-nicotine and, separately, the content of R-nicotine and any other nicotine analog, or any other chemical with similar properties.
Countries should consider implementing bans of synthetic nicotine products containing levels of R-nicotine, or any nicotine analog besides S-nicotine, that exceed the compounds’ levels in tobacco-sourced products, until the safety of the consumption of these chemicals from such products is established.
Regulators should consider restricting marketing practices promoting synthetic nicotine as generally “tasteless and odorless”, “more pure” or “healthier” than purified tobacco-derived nicotine, unless scientific evidence supporting these claims is provided.
3.7. Conclusions
Companies are increasingly marketing a wide range of synthetic nicotine products, which, if left unregulated, may undermine efforts to reduce tobacco and nicotine use and addiction, as well as WHO Member States’ efforts to comprehensively regulate tobacco and nicotine products. Additionally, there are still many knowledge gaps relating to the human health effects of using synthetically derived nicotine in different types of consumer products. Although synthetic nicotine products are not clearly subject to current tobacco control regulations in many countries, some countries’ tobacco control laws have been updated to cover these products. The information presented in this paper shows that there are various options for countries to make legal adjustments to close regulatory gaps for synthetic nicotine products, including ways to make adjustments that cover both the broad range of products currently on the market and those products that might emerge in the future.
Table 1.
Example Promotional Claims about Synthetic Nicotine Products
| Product | Owner/Manufacturer | Claim |
|---|---|---|
| Juice Head pouches | Juice Head (USA) |
“[ ] may offer higher nicotine satisfaction with potentially less risk than tobacco-derived nicotine. In addition, while tobacco nicotine often features a strong pungent odor and taste, synthetic nicotine is virtually tasteless and odorless.” “[ ] it is important to note that tobacco cultivation (which is commonly very subsidized) can be very damaging to the environment and is often a process that is highly labor-intensive, cumbersome, and wasteful.” “It should be noted that tobacco-derived nicotine may come along with more risks of side effects than pouches made without tobacco.” (12) |
| Pacha Mama vape pen | Charlie’s Holdings, Inc. (USA) | “increased purity and consistency over traditionally harvested nicotine” (13) |
| Outlaw Dip | Outlaw Dip Company (USA) | “pharmaceutical grade” (9) |
| Bidi Pouch | Kaival Brands Innovations Group, Inc. (USA) | “aims to help adult smokers take their first steps in going smokeless” (14) |
| ZIA gum | Next Generation Labs LLC (USA) | “the Only Nicotine Gum Developed with Synthetic Nicotine” (4) “offers the same nicotine satisfaction as any tobacco-derived product containing nicotine,” (4) and “ZIA™ gum is not intended to assist in quitting efforts” (15) |
| VaporX e-juice and disposable e-cigarettes | Vaporex Co., Ltd. (South Korea) | “We are committed to protecting the health of smokers by providing them with a valuable and appropriate vaping experience” (16) |
Table 3:
Overview of Tobacco Control Laws’ Applicability to Synthetic Nicotine (SN) Products
| Category | Number of Jurisdictions | Characteristics |
|---|---|---|
| Laws That Do Not Cover SN Products | 92 | “Tobacco products” defined as products containing elements made from tobacco plant. |
| Laws that Clearly Cover Only Certain SN Products | 52 | E-cigarettes (or other specific product types) defined to include nicotine derived from any source, but “tobacco product” definition otherwise limited to products made from tobacco plant. |
| Laws that Cover SN Products More Broadly | 29 | “Tobacco products” defined to explicitly include synthetic nicotine or to encompass nicotine derived from any source. |
| Laws for which coverage of SN Products was unclear | 17 | Product definitions reference tobacco plant or smoke without expressly requiring that the products be made or derived from tobacco. |
| Laws that Were Unavailable | 21 | |
| Total in Sample | 211 |
Table 4:
Examples of Countries with Product Definitions Covering All Synthetic Nicotine Products
| Country | Date | Comments/Definitions |
|---|---|---|
| Singapore | 2010 | Singapore’s law has included the regulation of “tobacco substitutes” since 2010. Though the definition has been amended over time, it has consistently been used as catch-all term to regulate products that contain nicotine (regardless of source) but are not otherwise included in the other defined categories in the Tobacco Act (92). |
| Moldova | 2015 | Moldova adopted comprehensive revisions to its Tobacco Control Act to comply with its obligations under the WHO FCTC and to align its policies with the EU pursuant to the Moldova-EU Association Agreement. In addition to regulating “tobacco products,” the revised law also regulates “related products,” defined to include “products made of plants for smoking and products that contain nicotine, including electronic cigarettes” (emphasis added) (93). |
| United States | 2022 | In response to the introduction of synthetic nicotine products that claimed to be outside the reach of the U.S. Tobacco Control Act, the U.S. amended to definition of “tobacco product” in the Act to include “any product…containing nicotine from any source, that is intended for human consumption[.]”(94) |
Key Findings:
Synthetic nicotine products—including nicotine pouches, e-liquids, disposable e-cigarettes, gums, toothpicks, and infused combustible products—are being marketed and sold throughout the world.
Synthetic nicotine products are being sold with marketing claims (e.g., “tobacco-free”) that may suggest they are safer than products using tobacco-derived nicotine and some products are being sold with flavour concepts (e.g., “chocolate dream,” “pink lemonade”) that are likely to appeal to youth.
Synthetic nicotine is added to marketed products in two forms—S-nicotine and R-nicotine. S-nicotine is the primary form of nicotine found in tobacco plants, but the pharmacological, metabolic, and toxicological effects of R-nicotine, and of mixtures and R- and S-nicotine, remain poorly understood.
No standard methodologies for the chemical analysis of synthetic nicotine exist, and product adulteration with tobacco-derived nicotine is of concern.
Whether synthetic nicotine products fall within current tobacco control regulations depends on how the laws of each country define products covered under the regulations. Laws that only apply to “tobacco products” or “tobacco-derived” products may not be broad enough to cover synthetic nicotine products, because synthetic nicotine is not derived from tobacco plants.
Companies are aware that some tobacco control laws do not cover synthetic nicotine products and have sought to take advantage of such regulatory gaps.
Some countries have amended their tobacco control laws such that they apply to products using nicotine that is not made or derived from tobacco, such as synthetic nicotine. But many countries’ tobacco control laws do not clearly apply to such products or do not apply to the full range of currently marketed products.
For this analysis, we reviewed 210 countries’ laws, as well as the European Union’s (EU) Tobacco Products Directive, by examining the laws available on the Tobacco Control Laws website (www.tobacccocontrollaws.org). Of those 211 jurisdictions’ laws, 21 did not have laws available or did not have laws available in English. Of the remaining 190 jurisdictions, 52 included definitions broad enough to clearly cover at least certain synthetic nicotine products (e.g., e-cigarettes but not other synthetic nicotine products), and 29 included definitions covering a broader range of synthetic nicotine products. 92 included definitions that did not apply to any type of synthetic nicotine product, while, for 17 jurisdictions, it was unclear as to whether laws cover synthetic nicotine.
References
- 1.Initiative T. What you need to know about new synthetic nicotine products 2021. [updated 04/06/2021; cited 2023 03/24/2023]. Available from: https://truthinitiative.org/research-resources/harmful-effects-tobacco/what-you-need-know-about-new-synthetic-nicotine-products; https://web.archive.org/web/20230307030020/https://truthinitiative.org/research-resources/harmful-effects-tobacco/what-you-need-know-about-new-synthetic-nicotine-products.
- 2.Ramamurthi D, Chau C, Lu Z, Rughoobur I, Sanaie K, Krishna P, Jackler RK. Marketing of “Tobacco-Free” and “Synthetic Nicotine” Products (White Paper) Stanford University 2022. [updated 03/08/2022; cited 2023 03/24/2023]. Available from: https://tobacco-img.stanford.edu/wp-content/uploads/2022/03/13161808/Synthetic-Nicotine-White-Paper-3-8-2022F.pdf; https://web.archive.org/web/20220727130135/https://tobacco-img.stanford.edu/wp-content/uploads/2022/03/13161808/Synthetic-Nicotine-White-Paper-3-8-2022F.pdf.
- 3.Schmid T A Real Up and Comer: Synthetic Nicotine 2021. [updated 02/14/2021; cited 2023 03/24/2023]. Available from: https://www.tobaccoasia.com/features/a-real-up-and-comer-synthetic-nicotine/; https://web.archive.org/web/20221202133322/https://www.tobaccoasia.com/features/a-real-up-and-comer-synthetic-nicotine/.
- 4.Ray K, Schuman V. Next Generation Labs CEO Vincent Schuman Announces ZIA™ Gum with TFN® Synthetic Nicotine 2017. [updated 10/31/2017; cited 2023 03/24/2023]. Available from: https://www.prweb.com/releases/2017/11/prweb14861999.htm: https://web.archive.org/web/20230324200234/https://www.prweb.com/releases/2017/11/prweb14861999.htm.
- 5.Lucy TM Gum 2023. [cited 2023 03/24/2023]. Available from: https://lucy.co/products/chewpark; https://web.archive.org/web/20230324200955/https://lucy.co/products/gum.
- 6.Nicotine Picks 2023. [Available from: https://nicotinepicks.com/; https://web.archive.org/web/20230324202011/https://nicotinepicks.com/.
- 7.Crave Nicotine Picks 2023. [cited 2023 03/24/2023]. Available from: https://cravepicks.com/; https://web.archive.org/web/20230313174220/https://cravepicks.com/.
- 8.Ltd PLW. Poda and our flagship Beyond Burn™ Poda Pods are set to revolutionize the heat-not-burn industry 2021. [cited 2023 03/24/2023]. Available from: https://web.archive.org/web/20210609132039/https://podalifestyle.com/.
- 9.Outlaw Dip Co. 2023. [cited 2023 03/25/2023]. Available from: https://outlawdip.com/faq/; https://web.archive.org/web/20230325040748/https://outlawdip.com/faq/.
- 10.Ronin Smokes 2023. [cited 2023 03/25/2023]. Available from: https://www.roninsmokes.com/; https://web.archive.org/web/20230325042020/https://www.roninsmokes.com/.
- 11.The Puff Bar - New & improved 2021. [updated 07/26/2021; cited 2023 03/25/2023]. Available from: https://web.archive.org/web/20210726184612/https://puffbar.com/collections/puff-bar.
- 12.Juice Head Pouches 2023. [cited 2023 03/29/2023]. Available from: https://juicehead.co/collections/juice-head-pouches; https://perma.cc/AF7J-BSHT.
- 13.Pachamama SYN Vape Disposable 2023. [cited 2023 03/25/2023]. Available from: https://www.vaporfi.com/pachamama-synthetic-disposable-vape-pen/; https://web.archive.org/web/20230325044756/https://www.vaporfi.com/pachamama-synthetic-disposable-vape-pen/.
- 14.BidiTM Vapor Launches BidiTM Pouch, a Nicotine Delivery Product in a Tin Pack 2020. [updated 10/29/2020; cited 2023 03/25/2023]. Available from: https://www.newswire.com/news/biditm-vapor-launches-biditm-pouch-a-nicotine-delivery-product-in-a-21245979; https://web.archive.org/web/20210419195703/https://www.newswire.com/news/biditm-vapor-launches-biditm-pouch-a-nicotine-delivery-product-in-a-21245979.
- 15.ZIA (TM). Can’t Light Up? 2018. [cited 2022 06/07/2022]. Available from: https://www.nextgenerationlabs.com/wp-content/uploads/2017/07/ZIA-gum-presentation-final.pptx; https://web.archive.org/web/20180107215049/https://www.nextgenerationlabs.com/wp-content/uploads/2017/07/ZIA-gum-presentation-final.pptx.
- 16.VAPORX 2022. [cited 2023 03/25/2023]. Available from: http://vaporx.co.kr/22; https://web.archive.org/web/20221130133455/http://vaporx.co.kr/22.
- 17.Zhang H, Pang Y, Luo Y, Li X, Chen H, Han S, et al. Enantiomeric composition of nicotine in tobacco leaf, cigarette, smokeless tobacco, and e-liquid by normal phase high-performance liquid chromatography. Chirality. 2018;30(7):923–31. [DOI] [PubMed] [Google Scholar]
- 18.Pictet A, Rotschy A. Synthese des Nicotins. Berichte der deutschen chemischen Gesellschaft. 1904;37(2):1225–35. [Google Scholar]
- 19.Wagner FF, Comins DL. Recent advances in the synthesis of nicotine and its derivatives. Tetrahedron. 2007;63(34):8065–82. [Google Scholar]
- 20.Anderson H. Manufacture of Nicotine. In: Tobacco BA, editor. British American Tobacco Records 1964. p. 100048807–8. [Google Scholar]
- 21.Moates RF. Feasibility of synthetic nicotine production. In: Reynolds R, editor. RJ Reynolds Records; Master Settlement Agreement 1967. p. 500613486–9. [Google Scholar]
- 22.Southwick E. Synthesis of Nicotine. In: Myers L, editor. Liggett & Myers Records 1978. p. lg0292754-lg5. [Google Scholar]
- 23.Ye X, Zhang Y, Song X, Liu Q. Research Progress in the Pharmacological Effects and Synthesis of Nicotine. ChemistrySelect. 2022;7(12):e202104425. [Google Scholar]
- 24.Arnold M, inventor; Next Generation Labs, LLC, assignee. Process for the preparation of (R,S)-nicotine. USA patent 9,556,142. 2017.
- 25.Arnold M, inventor; Next Generation Labs LLC, Kaival Labs LLC, assignee. Nicotine replacement therapy products comprising synthetic nicotine USA patent 10,610,526. 2020.
- 26.Hellinghausen G, Lee JT, Weatherly CA, Lopez DA, Armstrong DW. Evaluation of nicotine in tobacco-free-nicotine commercial products. Drug Test Anal. 2017;9(6):944–8. [DOI] [PubMed] [Google Scholar]
- 27.Puffbar. Puffbar about - Tobacco Free: Cool Clouds Distribution Inc.,; 2021. [cited 2021 02/28/2021]. Available from: https://puffbar.com/pages/about-puff-bar; https://perma.cc/5Q2W-93NU.
- 28.Duell AK, Kerber PJ, Luo W, Peyton DH. Determination of (R)-(+)- and (S)-(-)-Nicotine Chirality in Puff Bar E-Liquids by (1)H NMR Spectroscopy, Polarimetry, and Gas Chromatography-Mass Spectrometry. Chem Res Toxicol. 2021;34(7):1718–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber B, Pan B, inventors; Siegfried AG, Contraf-Nicotex-Tobacco GmbH, assignee. Enantiomeric separation of racemic nicotine by addition of an o,o’-disubstituted tartaric acid enantiomer patent US20200331883A1. 2019. 06/27/2019.
- 30.Weber BT, Lothschütz C, Pan B, inventors; Siegfried AG Contraf-Nicotex-Tobacco GmbH assignee. Preparation of racemic nicotine by reaction of ethyl nicotinate with n-vinylpyrrolidone in the presence of an alcoholate base and subsequent process steps USA2020.
- 31.Pawns F. S-isomer, the purest form of tobacco free nicotine 2020. [updated 08/08/2020; cited 2023 03/16/2023]. Available from: https://web.archive.org/web/20200811035816/https://fivepawns.com/blogs/five-pawns-news-events/s-isomer-tobacco-free-nicotine.
- 32.E-liquid TT. Synthetic Nicotine 2021. [cited 2021 06/05/2021]. Available from: https://web.archive.org/web/20211028035558/https://teatimeliquid.com/pages/synthetic-nicotine.
- 33.Zanoprima. Zanoprima: Patented synthetic nicotine for a clean nicotine future 2023. [cited 2023 03/16/2023]. Available from: https://www.zanoprima.com/; https://perma.cc/CW8M-YT7Z.
- 34.McCague R, Narasimhan AS, inventors; Zanoprima Lifesciences Limited (London, GB), assignee. Process of making (S)-nicotine. USA patent 10,913,962. 2021. 02/09/2021.
- 35.Tech H. What is MOTiVO TM ? 2023. [cited 2023 03/15/2023]. Available from: https://www.hkhangsen.com/show/index/cid/7/id/6.html; https://perma.cc/BE6K-P3US.
- 36.李家全, 魏庚辉, 孟宪强. Method for preparing nicotine of high optical purity 2020. [updated 05/27/2022; cited 2023 03/27/2023]. Available from: https://patents.google.com/patent/WO2022105482A1/en.
- 37.Altria Announces Definitive Agreement to Acquire NJOY Holdings, Inc. 2023. [Available from: https://investor.altria.com/press-releases/news-details/2023/Altria-Announces-Definitive-Agreement-to-Acquire-NJOY-Holdings-Inc/default.aspx; https://perma.cc/H2JH-X8QB.
- 38.Willis B, Ahmed MM, Freund W, Sawyer D, inventors; NJOY, LLC, assignee. Synthesis and resolution of nicotine. USA patent US10759776B2. 2020. 09/01/2020.
- 39.Nicotineriver - SyNic Pure Nicotine 1000mg/mL 2023. [cited 2023 03/20/2023]. Available from: https://nicotineriver.com/collections/synic%E2%84%A2-nicotine/products/synic-pure-nicotine-1000mg-ml; https://perma.cc/BF6D-B2GT?type=image.
- 40.Rossel S. Synthetic Nicotine is Gaining Acceptance Tobacco Reporter; 2019. [updated 12/01/2019; cited 2021 03/01/2021]. Available from: https://tobaccoreporter.com/2019/12/01/mirror-image/.
- 41.Liquid Nicotine Wholesalers - Synthetic Nicotine TFN® Pure Nicotine 2023. [cited 2023 03/20/2023]. Available from: https://liquidnicotinewholesalers.com/tfn-pure-liquid-nicotine.html; https://web.archive.org/web/20230321011109/https://liquidnicotinewholesalers.com/tfn-pure-liquid-nicotine.html.
- 42.Liquid Nicotine Wholesalers - CNT 2023. [cited 2023 03/20/2023].
- 43.Liquid Nicotine Wholesalers - Cultra Pure Liquid Nicotine 2023. [cited 2023 03/20/2023]. Available from: https://liquidnicotinewholesalers.com/cultra-pure-liquid-nicotine.html; https://web.archive.org/web/20230321022645/https://liquidnicotinewholesalers.com/cultra-pure-liquid-nicotine.html.
- 44.Nicotineriver - PurNic Pure Nicotine 1000mg/mL 2023. [cited 2023 03/20/2023]. Available from: https://nicotineriver.com/collections/purnic%E2%84%A2-nicotine/products/purnic-pure-nicotine-1000mg-ml; https://perma.cc/8276-UT6Q?type=image.
- 45.Next Generation Labs LLC has been granted a Notice of Allowance from China for its Process for The Preparation of (R-S) Synthetic Nicotine – Patent #201580069647.2: Cision PRWeb; 2021. [cited 2021 06/18/2021]. Available from: https://www.prweb.com/releases/next_generation_labs_llc_has_been_granted_a_notice_of_allowance_from_china_for_its_process_for_the_preparation_of_r_s_synthetic_nicotine_patent_201580069647_2/prweb17809747.htm.
- 46.Zanoprima Lifesciences Ltd. v. Hangsen International Group Ltd (6:22-cv-00268) District Court, W.D. Texas; 2022. [Google Scholar]
- 47.Hangsen Releases Synthetic Nicotine 2020. [cited 2023 03/19/2023]. Available from: https://tobaccoreporter.com/2020/09/11/hangsen-and-geek-vape-release-synthetic-nicotine-product/; https://web.archive.org/web/20210306015552/https://tobaccoreporter.com/2020/09/11/hangsen-and-geek-vape-release-synthetic-nicotine-product/.
- 48.NextGenerationLabs. NextGenerationLabs - What is TFN® 2021. [updated 02/28/2021; cited 2021 02/28/2021]. Available from: http://www.nextgenerationlabs.com/; https://perma.cc/D3K3-KXAV.
- 49.Specific Nicotine Isomers Ratios Could Potentially Offer Nicotine use at Satisfying but Non-Addictive Levels as Revealed by Next Generation Labs CEO Vincent Schuman: CISION PRWeb; 2017. [cited 2021 06/17/2021]. Available from: https://www.prweb.com/releases/2017/11/prweb14911138.htm.
- 50.Shimada A, Iizuka H, Kawaguchi T, Yanagita T. [Pharmacodynamic effects of d-nicotine--Comparison with l-nicotine]. Nihon Yakurigaku Zasshi. 1984;84(1):1–10. [PubMed] [Google Scholar]
- 51.Ikushima S, Muramatsu I, Sakakibara Y, Yokotani K, Fujiwara M. The effects of d-nicotine and l-isomer on nicotinic receptors. J Pharmacol Exp Ther. 1982;222(2):463–70. [PubMed] [Google Scholar]
- 52.Martin BR, Aceto MD. Nicotine binding sites and their localization in the central nervous system. Neurosci Biobehav Rev. 1981;5(4):473–8. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Gong ZH, Nordberg A. Effects of chronic treatment with (+)- and (-)-nicotine on nicotinic acetylcholine receptors and N-methyl-D-aspartate receptors in rat brain. Brain Res. 1994;644(1):32–9. [DOI] [PubMed] [Google Scholar]
- 54.Meltzer LT, Rosecrans JA, Aceto MD, Harris LS. Discriminative stimulus properties of the optical isomers of nicotine. Psychopharmacology (Berl). 1980;68(3):283–6. [DOI] [PubMed] [Google Scholar]
- 55.Clarke PB, Kumar R. Characterization of the locomotor stimulant action of nicotine in tolerant rats. Br J Pharmacol. 1983;80(3):587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar R, Pratt JA, Stolerman IP. Characteristics of conditioned taste aversion produced by nicotine in rats. Br J Pharmacol. 1983;79(1):245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romano C, Goldstein A, Jewell NP. Characterization of the receptor mediating the nicotine discriminative stimulus. Psychopharmacology (Berl). 1981;74(4):310–5. [DOI] [PubMed] [Google Scholar]
- 58.Rosecrans JA, Meltzer LT. Central sites and mechanisms of action of nicotine. Neurosci Biobehav Rev. 1981;5(4):497–501. [DOI] [PubMed] [Google Scholar]
- 59.Goldberg SR, Risner ME, Stolerman IP, Reavill C, Garcha HS. Nicotine and some related compounds: effects on schedule-controlled behaviour and discriminative properties in rats. Psychopharmacology (Berl). 1989;97(3):295–302. [DOI] [PubMed] [Google Scholar]
- 60.Jacob P 3rd, Benowitz NL, Copeland JR, Risner ME, Cone EJ. Disposition kinetics of nicotine and cotinine enantiomers in rabbits and beagle dogs. J Pharm Sci. 1988;77(5):396–400. [DOI] [PubMed] [Google Scholar]
- 61.Nwosu CG, Godin CS, Houdi AA, Damani LA, Crooks PA. Enantioselective metabolism during continuous administration of S-(-)- and R-(+)-nicotine isomers to guinea-pigs. J Pharm Pharmacol. 1988;40(12):862–9. [DOI] [PubMed] [Google Scholar]
- 62.Nwosu CG, Crooks PA. Species variation and stereoselectivity in the metabolism of nicotine enantiomers. Xenobiotica. 1988;18(12):1361–72. [DOI] [PubMed] [Google Scholar]
- 63.Crooks PA, Godin CS. N-methylation of nicotine enantiomers by human liver cytosol. J Pharm Pharmacol. 1988;40(2):153–4. [DOI] [PubMed] [Google Scholar]
- 64.Yang J, Chen YK, Liu ZH, Yang L, Tang JG, Miao MM, et al. Differences between the binding modes of enantiomers S/R-nicotine to acetylcholinesterase. Rsc Adv. 2019;9(3):1428–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saareks V, Mucha I, Sievi E, Vapaatalo H, Riutta A. Nicotine stereoisomers and cotinine stimulate prostaglandin E2 but inhibit thromboxane B2 and leukotriene E4 synthesis in whole blood. Eur J Pharmacol. 1998;353(1):87–92. [DOI] [PubMed] [Google Scholar]
- 66.Thuerauf N, Kaegler M, Dietz R, Barocka A, Kobal G. Dose-dependent stereoselective activation of the trigeminal sensory system by nicotine in man. Psychopharmacology (Berl). 1999;142(3):236–43. [DOI] [PubMed] [Google Scholar]
- 67.Mao H, Wang H, Hu X, Zhang P, Xiao Z, Liu J. One-Pot Efficient Catalytic Oxidation for Bio-Vanillin Preparation and Carbon Isotope Analysis. ACS Omega. 2020;5(15):8794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang L, Kujawinski DM, Federherr E, Schmidt TC, Jochmann MA. Caffeine in your drink: natural or synthetic? Anal Chem. 2012;84(6):2805–10. [DOI] [PubMed] [Google Scholar]
- 69.Cheetham AG, Plunkett S, Campbell P, Hilldrup J, Coffa BG, Gilliland S 3rd, Eckard S. Analysis and differentiation of tobacco-derived and synthetic nicotine products: Addressing an urgent regulatory issue. PLoS One. 2022;17(4):e0267049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.United States Pharmacopeial Convention. United States Pharmacopeia and National Formulary (USP 29-NF 24): Nicotine 2020. [cited 2023 04/23/2023]. Available from: https://online.uspnf.com/uspnf/document/1_GUID-3D851985-2C16-408D-99E1-F241A9767168_4_en-US; http://uspbpep.com/usp32/pub/data/v32270/usp32nf27s0_m56620.html.
- 71.Liu B, Chen Y, Ma X, Hu K. Site-specific peak intensity ratio (SPIR) from 1D (2)H/(1)H NMR spectra for rapid distinction between natural and synthetic nicotine and detection of possible adulteration. Anal Bioanal Chem. 2019;411(24):6427–34. [DOI] [PubMed] [Google Scholar]
- 72.Han S, Cui L, Chen H, Fu Y, Hou H, Hu Q, Yuan Y. Stable isotope characterization of tobacco products: A determination of synthetic or natural nicotine authenticity. Rapid Commun Mass Spectrom. 2023;37(3):e9441. [DOI] [PubMed] [Google Scholar]
- 73.Vagg R, Chapman S. Nicotine analogues: a review of tobacco industry research interests. Addiction. 2005;100(5):701–12. [DOI] [PubMed] [Google Scholar]
- 74.Clemens KJ, Caillé S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int J Neuropsychopharmacol. 2009;12(10):1355–66. [DOI] [PubMed] [Google Scholar]
- 75.Hall BJ, Wells C, Allenby C, Lin MY, Hao I, Marshall L, et al. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacol Biochem Behav. 2014;120:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harris AC, Tally L, Muelken P, Banal A, Schmidt CE, Cao Q, LeSage MG. Effects of nicotine and minor tobacco alkaloids on intracranial-self-stimulation in rats. Drug Alcohol Depend. 2015;153:330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zettler PJ, Hemmerich N, Berman ML. Closing the Regulatory Gap for Synthetic Nicotine Products. Boston Coll Law Rev. 2018;59(6):1933–82. [PMC free article] [PubMed] [Google Scholar]
- 78.Jordt SE. Synthetic nicotine has arrived. Tob Control. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merkley JA, Kaine T, Warren E, Brown S, Markey EJ, Baldwin T, et al. Concern over the public health challenge presented by synthetic nicotine products 2021. [updated 11/16/2021; cited 2023 03/25/2023]. Available from: https://www.merkley.senate.gov/imo/media/doc/21.11.16%20Signed%20Synthetic%20Nicotine%20Letter%20to%20FDA.pdf; https://web.archive.org/web/20211117213625/https://www.merkley.senate.gov/imo/media/doc/21.11.16%20Signed%20Synthetic%20Nicotine%20Letter%20to%20FDA.pdf.
- 80.Hangsen Tech 2023. [cited 2023 03/16/2023]. Available from: https://web.archive.org/web/20210509192635/http:/hkhangsen.com/show/index/cid/7/id/6.html
- 81.Lavery MS. Tobacco Synthetic Nicotine Bursts on to the Scene 2021. [
- 82.Maloney J. Puff Bar Defies FDA Crackdown on Fruity E-Cigarettes by Ditching the Tobacco 2021. [updated 03/02/2021; cited 2023 03/25/2023]. Available from: https://www.wsj.com/articles/puff-bar-defies-fda-crackdown-on-fruity-e-cigarettes-by-ditching-the-tobacco-11614681003; https://web.archive.org/web/20210303091223/https://www.wsj.com/articles/puff-bar-defies-fda-crackdown-on-fruity-e-cigarettes-by-ditching-the-tobacco-11614681003.
- 83.WHO. FCTC/COP8(22) Novel and emerging tobacco products 2018. [cited 2023 03/25/2023]. Available from: https://fctc.who.int/who-fctc/governance/conference-of-the-parties/eight-session-of-the-conference-of-the-parties/decisions/fctc-cop8(22)-novel-and-emerging-tobacco-products;.
- 84.Tobacco Control Laws 2023. [cited 2023 03/25/2023]. Available from: https://www.tobaccocontrollaws.org/; https://web.archive.org/web/20230316014939/https://www.tobaccocontrollaws.org/.
- 85.H.R.2471 - Consolidated Appropriations Act, 2022 2022. [updated 03/15/2022; cited 2023 03/25/2023]. Available from: https://www.congress.gov/bill/117th-congress/house-bill/2471/text.
- 86.21 USC 321: Definitions; generally - From Title 21-FOOD AND DRUGS - CHAPTER 9-FEDERAL FOOD, DRUG, AND COSMETIC ACT SUBCHAPTER II-DEFINITIONS 2022. [updated 12/29/2022; cited 2023 03/25/2023]. Available from: https://uscode.house.gov/view.xhtml?req=granuleid:USC-prelim-title21-section321&num=0&edition=prelim.
- 87.Section 101 of the Tobacco Control Act - Amendment of Federal Food, Drug, and Cosmetic Act (FDCA) 2023. [updated 03/17/2023; cited 2023 03/25/2023]. Available from: https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/section-101-tobacco-control-act-amendment-federal-food-drug-and-cosmetic-act-fdca; https://web.archive.org/web/20230326033207/https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/section-101-tobacco-control-act-amendment-federal-food-drug-and-cosmetic-act-fdca.
- 88.European Union: Types of legislation [cited 2023. 04/24/2023]. Available from: https://european-union.europa.eu/institutions-law-budget/law/types-legislation_en.
- 89.The Policy Surveillance Program - About LawAtlas.org 2023. [Available from: https://lawatlas.org/page/lawatlas-about; https://web.archive.org/web/20230124172410/https://lawatlas.org/page/lawatlas-about.
- 90.Family Smoking Prevention and Tobacco Control Act 2009. [updated 06/22/2009; cited 2023 03/25/2023]. Available from: https://www.govinfo.gov/content/pkg/PLAW-111publ31/pdf/PLAW-111publ31.pdf; https://web.archive.org/web/20161231192826/https://www.govinfo.gov/content/pkg/PLAW-111publ31/pdf/PLAW-111publ31.pdf.
- 91.Regulation and Enforcement of Non-Tobacco Nicotine (NTN) Products 2023. [updated 01/31/2023; cited 2023 03/25/2023]. Available from: https://www.fda.gov/tobacco-products/products-ingredients-components/regulation-and-enforcement-non-tobacco-nicotine-ntn-products; https://web.archive.org/web/20230326034710/https://www.fda.gov/tobacco-products/products-ingredients-components/regulation-and-enforcement-non-tobacco-nicotine-ntn-products.
- 92.TOBACCO (CONTROL OF ADVERTISEMENTS AND SALE) ACT 1993 2021. [updated 03/26/2023; cited 2023 03/25/2023]. Available from: https://sso.agc.gov.sg/Act/TCASA1993; https://web.archive.org/web/20230326142639/https://sso.agc.gov.sg/Act/TCASA1993.
- 93.Republica Moldova PARLAMENTUL LEGE Nr. 278 din 14–12-2007 2023. [updated 04/22/2022. Available from: https://www.legis.md/cautare/getResults?doc_id=128322&lang=ro.
- 94.Lipstein A, Zeller M. E-cigarette companies found a loophole in synthetic nicotine — it won’t stop the FDA 2022. [updated 04/07/2022; cited 2023 03/26/2023]. Available from: https://thehill.com/opinion/healthcare/3260879-e-cigarette-companies-found-a-loophole-in-synthetic-nicotine-it-wont-stop-the-fda/; https://web.archive.org/web/20220407181249/https://thehill.com/opinion/healthcare/3260879-e-cigarette-companies-found-a-loophole-in-synthetic-nicotine-it-wont-stop-the-fda/
- 95.Pediatrics AAo, Network ACSCA, Association AH, Association AL, Kids CfTF, E-cigarettes PAV, Initiative T. Letter to Dr. Janet Woodcock, Acting FDA Commissioner RE: Synthetic nicotine and Puff Bar 2021. [updated 03/18/2021; cited 2023 03/26/2023]. Available from: https://www.tobaccofreekids.org/assets/content/what_we_do/federal_issues/fda/regulatory/2021_03_18_puff-bar-synthetic-nicotine.pdf; https://web.archive.org/web/20210603101623/https://www.tobaccofreekids.org/assets/content/what_we_do/federal_issues/fda/regulatory/2021_03_18_puff-bar-synthetic-nicotine.pdf
- 96.(Australia) TGA. TGA confirms nicotine e-cigarette access is by prescription only 2020. [updated 12/21/2020; cited 2023 03/26/2023]. Available from: https://www.tga.gov.au/news/media-releases/tga-confirms-nicotine-e-cigarette-access-prescription-only; https://web.archive.org/web/20230321060557/https://www.tga.gov.au/news/media-releases/tga-confirms-nicotine-e-cigarette-access-prescription-only.


