Introduction
Haemorrhoids are a common condition worldwide and are thought to be a result of prolapsed anal cushions that have undergone pathological changes1. Haemorrhoids can be either external or internal, with the latter graded on a scale of grade I–IV2. A number of non-operative and operative strategies have been implemented to treat symptomatic haemorrhoids, but, for larger grade III–IV haemorrhoids, a surgical haemorrhoidectomy remains the gold standard2–4. However, haemorrhoidectomy is also associated with adverse effects, with postoperative pain being the most common2–4.
Post-haemorrhoidectomy pain is thought to be related to spasm of the internal anal sphincter4–6, but other causes may include local nerve-end damage, creation of an anal fissure, or suture placement below the dentate line4,6–8. Numerous modalities of pain management have been suggested, with multiple studies looking at the use of topical pain relief. The studies have shown promising, albeit mixed, results, examining the use of topical interventions like lignocaine, nitroglycerin, metronidazole, and diltiazem2,6,7,9–13.
Calcium channel blockers (CCBs), available in topical preparations, are thought to decrease internal sphincter spasm due to blockage of myocytes and subsequent relaxation of smooth muscles6,14. One such CCB, diltiazem, has shown success in multiple RCTs in significantly reducing post-haemorrhoidectomy pain and likely reducing overall opioid use amongst patients9–13. Another CCB, nifedipine, has been less documented in the literature with respect to its topical use in relieving post-haemorrhoidectomy pain. One RCT was able to show a significant reduction in pain relief post-haemorrhoidectomy when topical nifedipine was combined with lignocaine versus a control group of lignocaine alone4. Additionally, multiple studies examining chronic anal fissures suggest that nifedipine appears to have more effective healing rates than diltiazem15,16. Thus, given the lack of literature on the use of topical nifedipine in post-haemorrhoidectomy patients, and its reported success in healing anal fissures, the planned study seeks to establish if nifedipine alone, like diltiazem, versus a topical placebo, can reduce postoperative pain, as well as limit opioid use.
Methods
The primary study hypothesis is that topical nifedipine (0.5 per cent) ointment, compared with topical placebo alone, leads to a significant reduction in pain experienced by patients post-haemorrhoidectomy. The secondary study hypothesis is that topical nifedipine ointment leads to less use of rescue oral opioid analgesia by patients, post-haemorrhoidectomy, who are on a standardized postoperative analgesia regimen, with no significant differences in adverse effects between those receiving the topical nifedipine ointment intervention versus those receiving the placebo ointment.
Study design and selection criteria
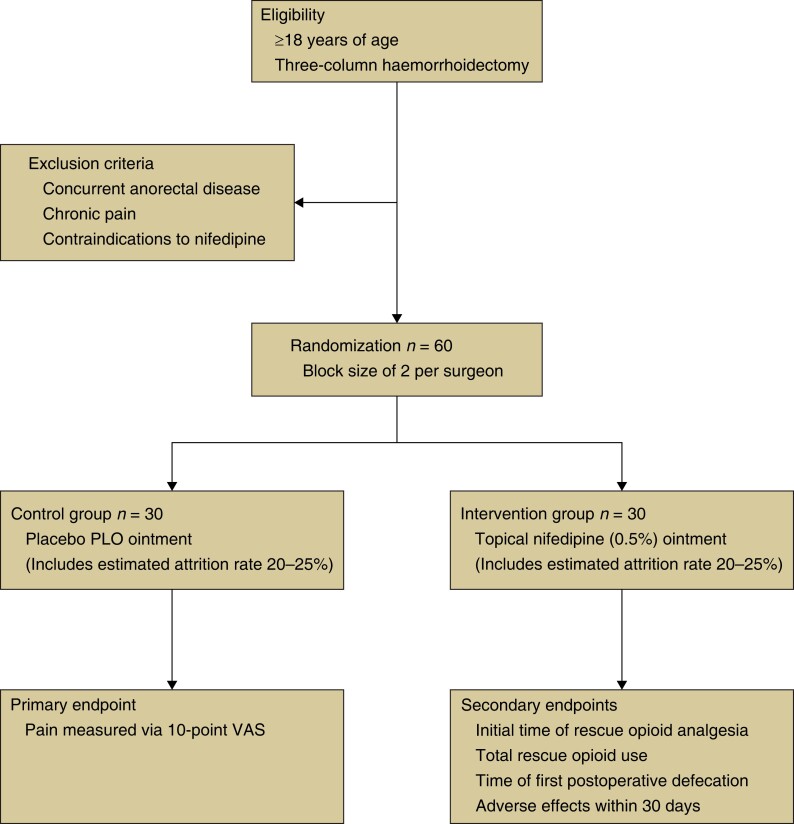
A single-centre, randomized, prospective, double-blind placebo trial will be conducted comparing the efficacy of topical nifedipine ointment versus a topical placebo ointment alone in reducing post-haemorrhoidectomy pain. Secondary outcomes will include rescue oral opioid analgesia use and adverse effects (Fig. 1). The trial began in July 2023 and aims to finish recruitment by July 2024.
Fig. 1.
Trial design
PLO, pluronic lecithin organogel; VAS, visual analogue scale.
Patients over the age of 18 years who are scheduled to undergo three-column haemorrhoidectomy, using a closed technique, will be considered for inclusion and will be recruited by surgeons during either their initial consultation for haemorrhoidectomy surgery or on the day of planned surgery. Exclusion criteria will be patients who: are younger than 18 years; are pregnant or breastfeeding; have concurrent non-haemorrhoidal anorectal disease; have a known allergy to nifedipine; have co-morbidities or take medications that are a contraindication to nifedipine use; have impaired renal function where parecoxib is contraindicated; have a chronic pain condition requiring ongoing regular analgesia; cannot provide informed consent; and cannot have a general anaesthetic.
For each included patient, demographics and clinical data will be recorded as reported in the Supplementary material.
Group allocation
After written informed consent, patients will be randomized to either the intervention group (topical nifedipine ointment) or the control group (placebo ointment) by computer randomization. This will be achieved through the use of a random sequence generated by the study statistician using a package created using MS Excel 2013 software. The patients will be allocated using a randomized block procedure, with a block size of two. Surgeons will be blinded to this allocation.
Standardized analgesia regimen
All patients will receive a general anaesthetic along with intravenous paracetamol (1 g), alfentanil (1 mg), oxycodone (0.05–0.1 mg/kg), and parecoxib (40 mg). After surgery patients will receive recovery analgesia consisting of intravenous oxycodone (2 mg), as required, with a maximum of 10 mg available. Also, local anaesthetic consisting of 20 ml bupivacaine (0.5 per cent) with adrenaline (1 : 200 000) will be used for local wound infiltration.
Upon discharge, patients will be provided with a prescription for regular oral paracetamol (1 g four times per day) and celecoxib (100 mg twice per day) for 5 days (to start 18 h post-parecoxib). They will also receive rescue oxycodone (5 mg every 4 h, with a maximum daily dose of 25 mg), with 20 tablets (100 mg), in total, available.
Intervention and placebo
Patients will receive a supply of anorectal ointment that is de-identified and randomized, that is either the intervention nifedipine ointment or the placebo ointment, thus blinding the patients appropriately. The intervention ointment will consist of a jar containing 30 g of nifedipine (0.5 per cent) in pluronic lecithin organogel (PLO), a common compounding medium for topical medications, with the control group receiving a placebo of PLO only. All patients will be instructed to apply a pea-sized amount of their ointment, using a glove, 1–1.5 cm into the anus circumferentially, as well as to the external anus. This will be done twice a day for 4 weeks.
Outcome measures
As stated, the primary outcome measured will be post-haemorrhoidectomy pain experienced by subjects. This will be measured using a 10-point standardized and validated visual analogue scale (‘VAS’). Scores will range from 0 (no pain at all) to 10 (worst pain imaginable). Pain scores will be recorded at the following time points: before surgery (baseline); at recovery; at 4 h (discharge); daily (week 1); and at 2, 3, and 4 weeks.
Secondary outcomes measured will include rescue analgesic use and adverse effects (Supplementary material).
Statistics and sample size
The statistical plan and intention-to-treat analyses are detailed in the Supplementary material. Compared with similar studies, pre-trial calculations established a required sample size of 48 (24 patients per group), based on α = 0.05 and power = 0.9, based on the primary outcome (pain). Estimating an attrition rate of 20–25 per cent, a sample size of 60 (30 per group) was chosen. The sample size was calculated utilizing STATA 17 (StataCorp, College Station, TX, USA) considering a two-tailed test.
Ethics
An ethics application has been submitted to the local Cabrini Monash University Human Research Ethics Committee. The trial is registered in the Australia New Zealand Clinical Trials Registry (ANZCTR 12623000514606p). Recruitment started in July 2023 and is expected to finish in July 2024.
Discussion
Post-haemorrhoidectomy pain has a pathophysiology that is proposed to be similar to non-healing chronic anal fissures. Given the success of topical nifedipine in healing anal fissures, the authors present a protocol for a single-centre, double-blind, placebo RCT assessing the use of topical nifedipine in haemorrhoidectomy patients, with post-operative pain as the primary outcome.
The literature supports the use of topical CCB diltiazem to relieve post-haemorrhoidectomy pain; however, to the authors’ knowledge, the planned study will be the first to examine the use of nifedipine alone in this cohort. Additionally, the authors aim to better establish guidelines on the administration, dosing, and length of time for topical nifedipine use in this cohort. Furthermore, the planned study will investigate if there is a difference in the use of rescue oral opioid analgesia and any reported adverse effects between the groups.
Supplementary Material
Contributor Information
Christopher J Steen, Department of Surgery, Cabrini Monash University, Melbourne, Victoria, Australia.
Raymond J Yap, Department of Surgery, Cabrini Monash University, Melbourne, Victoria, Australia.
Mohammad Asghari-Jafarabadi, Department of Surgery, Cabrini Monash University, Melbourne, Victoria, Australia.
Adam Sutton, Department of Surgery, Cabrini Monash University, Melbourne, Victoria, Australia.
Martin Chin, Department of Surgery, Cabrini Monash University, Melbourne, Victoria, Australia.
Peter Carne, Department of Surgery, Cabrini Monash University, Melbourne, Victoria, Australia.
Stephen W Bell, Department of Surgery, Cabrini Monash University, Melbourne, Victoria, Australia.
Paul J McMurrick, Department of Surgery, Cabrini Monash University, Melbourne, Victoria, Australia.
Funding
The authors have no funding to declare.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
All relevant data will be published upon completion of the trial.
Author contributions
Christopher J. Steen (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Writing—original draft, Writing—review & editing), Raymond J. Yap (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Writing—original draft, Writing—review & editing), Mohammad Asghari-Jafarabadi (Data curation, Formal analysis, Writing—review & editing), Adam Sutton (Conceptualization, Writing—review & editing), Martin Chin (Conceptualization, Methodology, Writing—review & editing), Peter Carne (Conceptualization, Methodology, Writing—review & editing), Stephen W. Bell (Conceptualization, Methodology, Writing—original draft), and Paul J. McMurrick (Conceptualization, Methodology, Project administration, Resources, Supervision, Writing—original draft, Writing—review & editing).
References
- 1. Nisar PJ, Scholefield JH. Managing haemorrhoids. BMJ 2003;327:847–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davis BR, Lee-Kong SA, Migaly J, Feingold DL, Steele SR. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of hemorrhoids. Dis Colon Rectum 2018;61:284–292 [DOI] [PubMed] [Google Scholar]
- 3. Cengiz TB, Gorgun E. Hemorrhoids: a range of treatments. Cleve Clin J Med 2019;86:612–620 [DOI] [PubMed] [Google Scholar]
- 4. Perrotti P, Dominici P, Grossi E, Cerutti R, Antropoli C. Topical nifedipine with lidocaine ointment versus active control for pain after hemorrhoidectomy: results of a multicentre, prospective, randomized, double-blind study. Can J Surg 2010;53:17–24 [PMC free article] [PubMed] [Google Scholar]
- 5. Roe AM, Bartolo DC, Vellacott KD, Locke-Edmunds J, Mortensen NJ. Submucosal versus ligation excision haemorrhoidectomy: a comparison of anal sensation, anal sphincter manometry and postoperative pain and function. Br J Surg 1987;74:948–951 [DOI] [PubMed] [Google Scholar]
- 6. Rahimi R, Abdollahi M. A systematic review of the topical drugs for post-hemorrhoidectomy pain. Int J Pharmacol 2012;8:628–637 [Google Scholar]
- 7. Wasvary HJ, Hain J, Mosed-Vogel M, Bendick P, Barkel DC, Klein SN. Randomized, prospective, double-blind, placebo-controlled trial of effect of nitroglycerin ointment on pain after hemorrhoidectomy. Dis Colon Rectum 2001;44:1069–1073 [DOI] [PubMed] [Google Scholar]
- 8. Emile SH. Evidence-based review of methods used to reduce pain after excision haemorrhoidectomy. J Coloproctol 2019;39:81–89 [Google Scholar]
- 9. Silverman R, Bendick PJ, Wasvary HJ. A randomized, prospective, double-blind, placebo-controlled trial of the effect of a calcium channel blocker ointment on pain after hemorrhoidectomy. Dis Colon Rectum 2005;48:1913–1916 [DOI] [PubMed] [Google Scholar]
- 10. Hwang DY, Yoon SG, Kim HS, Lee JK, Kim KY. Effect of 0.2 percent glyceryl trinitrate ointment on wound healing after a hemorrhoidectomy: results of a randomized, prospective, double-blind, placebo-controlled trial. Dis Colon Rectum 2003;46:950–954 [DOI] [PubMed] [Google Scholar]
- 11. Amoli HA, Notash AY, Shahandashti FJ, Kenari AY, Ashraf H. A randomized, prospective, double-blind, placebo-controlled trial of the effect of topical diltiazem on posthaemorrhoidectomy pain. Colorectal Dis 2011;13:328–332 [DOI] [PubMed] [Google Scholar]
- 12. Sugimoto T, Tsunoda A, Kano N, Kashiwagura Y, Hirose K, Sasaki T. A randomized, prospective, double-blind, placebo-controlled trial of the effect of diltiazem gel on pain after hemorrhoidectomy. World J Surg 2013;37:2454–2457 [DOI] [PubMed] [Google Scholar]
- 13. Yadav S, Khandelwal RG, Om P, Ravindra K, Choudhary KL. A prospective randomized double-blind study of pain control by topical calcium channel blockers versus placebo after Milligan-Morgan hemorrhoidectomy. Int J Colorectal Dis 2018;33:895–899 [DOI] [PubMed] [Google Scholar]
- 14. Cook TA, Brading AF, Mortensen NJ. Differences in contractile properties of anorectal smooth muscle and the effects of calcium channel blockade. Br J Surg 1999;86:70–75 [DOI] [PubMed] [Google Scholar]
- 15. Perrotti P, Bove A, Antropoli C, Molino D, Antropoli M, Balzano Aet al. Topical nifedipine with lidocaine ointment vs. active control for treatment of chronic anal fissure: results of a prospective, randomized, double-blind study. Dis Colon Rectum 2002;45:1468–1475 [DOI] [PubMed] [Google Scholar]
- 16. Knight JS, Birks M, Farouk R. Topical diltiazem ointment in the treatment of chronic anal fissure. Br J Surg 2001;88:553–556 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data will be published upon completion of the trial.