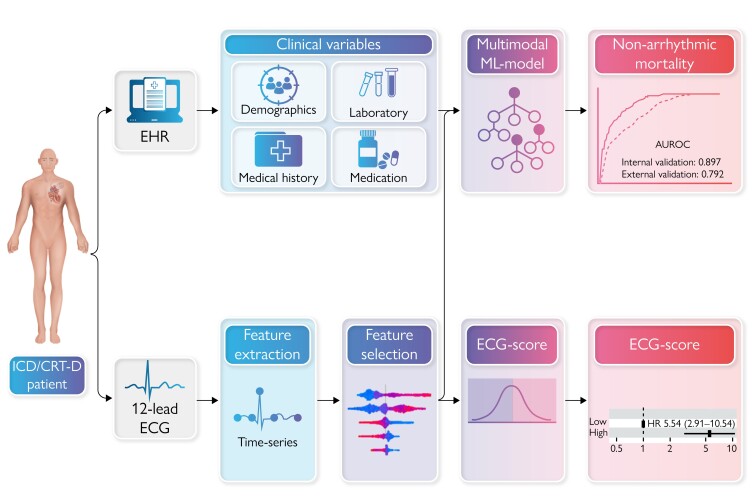
Abstract
Aims
Left ventricular ejection fraction (LVEF) is suboptimal as a sole marker for predicting sudden cardiac death (SCD). Machine learning (ML) provides new opportunities for personalized predictions using complex, multimodal data. This study aimed to determine if risk stratification for implantable cardioverter-defibrillator (ICD) implantation can be improved by ML models that combine clinical variables with 12-lead electrocardiograms (ECG) time-series features.
Methods and results
A multicentre study of 1010 patients (64.9 ± 10.8 years, 26.8% female) with ischaemic, dilated, or non-ischaemic cardiomyopathy, and LVEF ≤ 35% implanted with an ICD between 2007 and 2021 for primary prevention of SCD in two academic hospitals was performed. For each patient, a raw 12-lead, 10-s ECG was obtained within 90 days before ICD implantation, and clinical details were collected. Supervised ML models were trained and validated on a development cohort (n = 550) from Hospital A to predict ICD non-arrhythmic mortality at three-year follow-up (i.e. mortality without prior appropriate ICD-therapy). Model performance was evaluated on an external patient cohort from Hospital B (n = 460). At three-year follow-up, 16.0% of patients had died, with 72.8% meeting criteria for non-arrhythmic mortality. Extreme gradient boosting models identified patients with non-arrhythmic mortality with an area under the receiver operating characteristic curve (AUROC) of 0.90 [95% confidence intervals (CI) 0.80–1.00] during internal validation. In the external cohort, the AUROC was 0.79 (95% CI 0.75–0.84).
Conclusions
ML models combining ECG time-series features and clinical variables were able to predict non-arrhythmic mortality within three years after device implantation in a primary prevention population, with robust performance in an independent cohort.
Keywords: Machine learning, Ventricular arrhythmia, Implantable cardioverter-defibrillator, Artificial intelligence
Graphical Abstract
Graphical Abstract.
What’s new?
Patients with a low left ventricular ejection fraction (LVEF ≤35%) despite 90 days of optimal guideline-directed medical therapy may benefit from prophylactic implantable cardioverter-defibrillator (ICD) implantation for prevention of sudden cardiac death (SCD). However, LVEF alone is inadequate for predicting the likelihood of benefit from prophylactic ICD treatment, especially considering the competing risk of nonarrhythmic mortality.
We developed ML models that incorporate both clinical variables and 12-lead electrocardiogram time-series features to predict the risk of non-arrhythmic mortality in a primary prevention ICD population.
Accurate prediction of ICD non-benefit was achieved by ML models that combined ECG time-series features and clinical variables, demonstrating robust performance on an external patient cohort.
Future studies are warranted to prospectively validate the identified ECG features and their electrophysiological substrate for arrhythmic risk prediction.
Introduction
Patients with a low left ventricular ejection fraction (LVEF ≤35%) despite 90 days of optimal guideline-directed medical therapy benefit from prophylactic implantable cardioverter-defibrillator (ICD) implantation for prevention of sudden cardiac death (SCD).1–3 However, LVEF alone is inadequate for predicting the likelihood of benefit from prophylactic ICD treatment, especially considering the competing risk of non-arrhythmic mortality.4 This results in approximately two-thirds of patients with an ICD for primary prevention who never receive appropriate ICD-therapy.5 Moreover, advancements in medical therapy for heart failure have led to a decrease in the risk of ventricular arrhythmia and prolonged life-expectancy, affecting the proportion of patients who benefit from ICD treatment.6,7 Conversely, these patients remain at risk for device complications, inappropriate ICD shock and reduced health-related quality of life.8,9 Various risk scores have been developed to better estimate the proportional risks of SCD and non-SCD for heart failure patients who would qualify for primary prevention ICD population using clinical variables, such as the Seattle Proportional Risk Model (SPRM) and the MADIT-ICD score.10–14 The MADIT-ICD score incorporating clinical variables recently demonstrated a C-index of 0.67 in predicting mortality without prior ventricular tachycardia/fibrillation (VT/VF) on an external cohort.10 Machine and deep learning models are able to detect intricate and non-linear interactions and reveal patterns within datasets that may not be apparent through traditional statistical methods, providing opportunities for personalized predictions of patient outcomes.15 In particular, the complex interplay between clinical risk factors, structural cardiac abnormalities and arrhythmic substrates captured within a machine learning (ML) framework, could potentially improve the accuracy of prediction models.15,16 We hypothesized that ML may enable personalized prediction of non-arrhythmic mortality risk within 3 years after device implantation, which could be used to refine current broad guidelines and aid the decision-making process for ICD implantation, using a combination of baseline clinical variables and time-series features derived from 12-lead electrocardiograms (ECG).
Methods
Study design
A multicenter, retrospective, observational study using patient data obtained from two academic hospitals in Amsterdam (Amsterdam Medical Center and the VU University Medical Center), The Netherlands, was performed. Patients with an LVEF ≤35% implanted with an ICD with or without resynchronization therapy (CRT) between 2007 and 2021 for primary prevention of SCD were included. All patients were diagnosed with an ischaemic, dilated, or non-ischaemic cardiomyopathy before implantation of the ICD. Implanted devices included single-chamber ICDs (VR), dual-chamber ICDs (DR), subcutaneous ICDs (S-ICD), and cardiac resynchronization therapy-defibrillators (CRT-D). ICDs were manufactured by Biotronik (Germany), Medtronic (USA), St. Jude Medical/Abbott (USA), or Boston Scientific (USA). Patients were followed from device implantation onwards. Clinical data at baseline was obtained from the electronic health records (EHR). All patients <18 years old at device implantation were excluded. This study adheres to the reporting guidelines for Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) where applicable.17
Outcome
The outcome of interest was non-arrhythmic mortality within 3 years of ICD implantation, defined as all-cause mortality without any appropriate ICD-therapy before death. Appropriate ICD-therapy was defined as any shock and/or anti-tachycardia pacing (ATP) in response to VT or VF according to the interpretation of the clinician as reported in the EHR. Secondary outcomes were appropriate ICD-therapy and all-cause mortality.
ECG time-series features
Raw format, standard 12-lead 10-s resting ECGs were collected retrospectively on both sites and considered eligible if recorded within 90 days prior to ICD implantation. ECGs were visually inspected to assess the quality (e.g. presence of baseline wander, high-frequency noise, lack of signal in one or more leads). ECGs of low quality and/or with pacing artefacts were excluded. If more than one eligible ECG was available per subject, the ECG closest to ICD implantation was selected. Detailed description of the ECG pre-processing and feature extraction are provided in the Supplementary material online, Methods. The pre-processed ECGs were characterized by their time-series features, extracted using a systematic feature engineering technique (tsfresh version 0.12.0 in Python 3.6).18 This algorithm extracts 65 unique features, calculated using different parameter settings, which results in a total of 783 features per 10-s ECG lead. Subsequently, a two-step feature selection approach that combined the Benjamini-Yekutieli procedure and a recursive feature elimination algorithm that removed the lowest importance features was used, which resulted in a total of 50 selected features.19 ECG pre-processing steps were identical in the development and external testing cohorts. Feature selection was performed on the development cohort alone.
Clinical variables
Clinical baseline information from the moment of device implantation was extracted from the EHR. Clinical variables included medical history, medication usage (i.e. anti-arrhythmic, anti-coagulant, anti-hypertensive, lipid-lowering), demographics (age, sex), body mass index (BMI), and laboratory values (i.e. creatinine, potassium, sodium). Missing data was imputed using Random Forests for non-parametric imputation, in two separate procedures for the development and testing cohort [missForest package (v. 1.5) in Python].20 Variables with ≥30% missing values were excluded (see Supplementary material online, Table S1 shows missing values per variable). Out-of-bag errors were calculated for each imputed variable. Categorical variables were one-hot encoded, continuous variables were standardized using z-score.
Model development and evaluation
We compared different supervised ML models: support vector machines (SVM), extreme gradient boosting algorithms (XGBoost), and random forest (RF) classifiers.21 Hyperparameter tuning was performed for each model, and various configurations of model parameters were assessed to find the optimal set of hyperparameters. ML models were developed and tested using the scikit-learn library (version 1.1.1.) and the XGBoost library (version 1.6.2.).21,22 All modelling was performed using Python (version 3.6.7). Internal model evaluation was performed by repeated stratified k-fold (k = 10, 5 repeats) cross-validation (CV), to ensure similar proportions of the target classes in the train and validation splits. Models were trained using a combination of clinical and ECG time-series features (multimodal ML model), and with ECG features only (ECG ML model). The performance of the final model was evaluated on the external testing cohort from Hospital B which was not used during model development. The interpretation of individual predictions was explained using the SHAP (SHapley Additive exPlanations) method.23 The SHAP summary plot presents a combination of feature importance and feature effect, represented by each point as a Shapley value for a specific feature and instance. To further clarify predictions, mean waveforms for predicted high-risk and predicted low risk of non-arrhythmic mortality in the external patient cohort were displayed.
Statistical analysis
Continuous variables were presented by their median, mean, interquartile range (IQR), and standard deviation. Categorical sociodemographic and clinical variables were presented as frequencies (percentages) and compared using Fisher’s exact test when appropriate, otherwise using Chi-square test. Model performances were assessed by using the following metrics: area under the receiver operating characteristics curve (AUROC), area under the precision recall curve (AUPRC), F1-score, sensitivity, and specificity. Youden’s J index was used to find the optimal threshold on the receiver operating characteristic (ROC) curve for classification. Model calibration was visualized by plotting the calibration curve and assessed by its slope and intercept. Confidence intervals (CI) and standard errors (SE) around the performance measures were obtained using 2000 bootstrap samples. To assess the robustness of the model, the performance was assessed in the following sub-populations: with or without CRT-D, old vs. young (age 65 years), male vs. female, ischaemic vs. non-ischaemic cardiomyopathy, implantation before 2013 vs. after 2013 and guideline-directed medical therapy vs. non-guideline directed medical therapy (GDMT). Optimal GDMT was defined as β-blocker, angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARB) inhibitors, and a mineralocorticoid receptor antagonists (MRA). On top of evaluating model performance, we analysed the potential clinical value of the model through decision curve analysis. The decision curve depicts the net benefit of the model, defined as the weighted combination of true positive and false positive predictions (i.e. incorrect withholding of an ICD), for a wide range of risk thresholds.24 Survival analysis was performed using the Kaplan–Meier method, and survival curves for low risk, intermediate risk and high-risk predicted probability quantiles were compared using the log-rank test. The predicted probability for the ECG ML model was dichotomized to low and high ECG-scores relative to Youden’s J index. Multivariate Cox proportional hazard models with the ECG ML predicted probability and clinical covariates age, sex, non-sustained VT, BMI, ventricular rate, myocardial infarction, cerebral vascular accident, diabetes mellitus, atrial arrhythmia, and CRT-D as independent variables. Schoenfeld residuals were used to check the proportional hazards assumption. A two-sided P-value <0.05 was considered significant. Statistical analyses were performed using R statistical software (version 3.6.2, R Core Team).25
Ethics
The requirement for written informed consent for this retrospective study was waived by the Institutional Review Board, as the medical research involving Human Subjects Act did not apply.
Role of the funding source
The funding source had no role in the study design, data collection, data analyses, interpretation, or writing of report.
Results
Cohort description
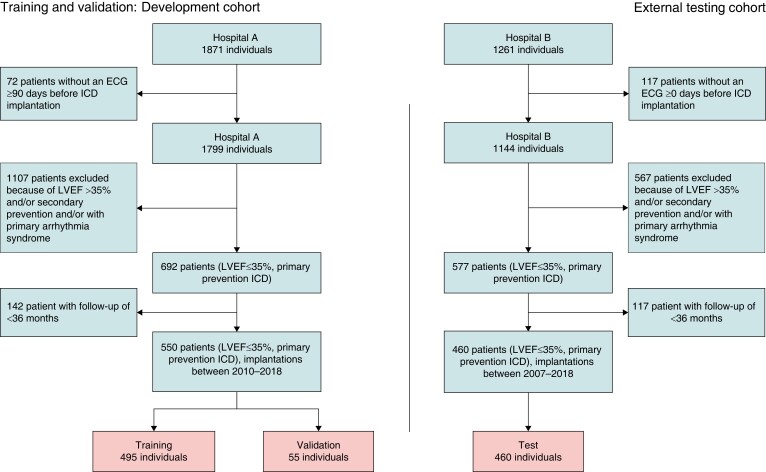
Figure 1 displays the patient selection flow diagram. The development cohort (Hospital A) consisted of 1871 ICD recipients; the external testing cohort (Hospital B) consisted of 1261 patients. Selection of patients with an ECG within 90 days before ICD implantation resulted in 1799 patients in the development cohort and 1144 patients in the external testing cohort. Exclusion of patients with an ICD indication for secondary prevention of SCD, patients with LVEF >35%, and patients with a primary arrhythmia syndrome resulted in a dataset of in total 1010 patients. The development cohort consisted of 550 patients, the external cohort consisted of 460 individuals. Patient characteristics of both patient cohorts are displayed in Table 1. Significant differences were observed between the development cohort and external testing cohort in age hypertension, hypercholesterolaemia, atrial arrhythmia, and congenital heart disease. The distribution in aetiology of cardiomyopathy, prior ventricular arrhythmias before implantation, and implanted device were significantly different between the two cohorts. A total of 382 (37.8%) patients were implanted with a CRT-D, 246 (24.4%) patients were using guideline-directed medical therapy (GDMT) at baseline.
Figure 1.
Flowchart of patient selection and assignment to the training cohort and the testing cohort.
Table 1.
Baseline characteristics of the development cohort and the external testing cohort
| Development cohort (n = 550) | External testing cohort (n = 460) | P-value | |
|---|---|---|---|
| Age, mean (SD) | 64.4 (11.4) | 67.0 (9.4) | <0.001 |
| Sex, male (%) | 407 (74.0) | 350 (76.1) | 0.491 |
| BMI, mean (SD) | 27.3 (3.7) | 27.1 (4.4) | 0.336 |
| OHCA, n (%) | 22 (4.0) | 11 (2.4) | 0.210 |
| Cardiomyopathy, n (%) | |||
| Ischaemic | 296 (53.8) | 262 (57.0) | <0.001 |
| Dilated | 185 (33.6) | 195 (42.4) | |
| Non-ischaemic | 69 (12.5) | 3 (0.7) | |
| Prior ventricular arrhythmia, n (%) | |||
| VF | 14 (2.5) | 18 (3.9) | 0.024 |
| Sustained VT | 12 (2.2) | 2 (0.4) | |
| Non-sustained VT | 72 (13.1) | 43 (9.3) | |
| Medical history, n (%) | |||
| Myocardial infarction | 292 (53.1) | 228 (49.6) | 0.292 |
| Percutaneous coronary intervention | 202 (36.7) | 165 (35.9) | 0.829 |
| Coronary artery bypass grafting | 97 (17.6) | 97 (21.1) | 0.192 |
| Cerebral vascular accident | 81 (14.7) | 51 (11.1) | 0.106 |
| Peripheral artery disease | 29 (5.3) | 35 (7.6) | 0.165 |
| Atrial arrhythmia | 191 (34.7) | 121 (26.3) | 0.005 |
| Congenital heart disease | 7 (1.3) | 0 (0) | 0.018 |
| COPD | 51 (9.3) | 49 (10.7) | 0.532 |
| Diabetes mellitus | 144 (26.2) | 126 (27.4) | 0.718 |
| Hypertension | 228 (41.5) | 251 (54.6) | <0.001 |
| Hypercholesterolaemia | 88 (16.0) | 242 (52.6) | <0.001 |
| Laboratory, mean (SD) | |||
| Sodium, mmol/L | 139.3 (2.8) | 139.5 (2.7) | 0.146 |
| Potassium, mmol/L | 4.3 (0.4) | 4.4 (0.5) | 0.004 |
| Creatinine, µmol/L | 109.8 (63.5) | 106.8 (61.4) | 0.454 |
| Medication, n (%) | |||
| Vitamin K antagonist | 175 (31.8) | 209 (45.4) | <0.001 |
| Diuretics | 377 (68.5) | 315 (68.5) | 1.000 |
| ARB/ACEi | 34 (24.4) | 76 (16.5) | 0.003 |
| Sotalol | 24 (4.4) | 6 (1.3) | 0.008 |
| Digoxin | 49 (8.9) | 36 (7.8) | 0.615 |
| Amiodarone | 43 (7.8) | 23 (5.0) | 0.094 |
| β-blocker | 430 (78.2) | 384 (83.5) | 0.041 |
| NOAC | 55 (10.0) | 9 (2.0) | <0.001 |
| Mineralocorticoid receptor antagonist | 177 (32.2) | 179 (38.9) | 0.030 |
| Device type, n (%) | |||
| Single-chamber | 205 (37.3) | 95 (20.7) | <0.001 |
| Dual-chamber | 65 (11.8) | 179 (38.9) | |
| CRT-D | 203 (36.9) | 179 (38.9) | |
| S-ICD | 77 (14.0) | 7 (1.5) | |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers inhibitors; BMI, body mass index; COPD, Chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; NOAC, novel oral anti-coagulant; OHCA, out-of-hospital cardiac arrest; S-ICD, subcutaneous ICD; SD, standard deviation; VF, ventricular fibrillation; VT, ventricular tachycardia.
At 3-year follow-up, 162 (16.0%) patients had died of which 118 (72.8%) were classified as having non-arrhythmic mortality. In total 196 (19.4%) patients received appropriate ICD-therapy, 104 patients (10.3%) received appropriate shock, 44 received ATP followed by shock (4.4%), and 48 received ATP only (4.8%). There were significant differences between the development cohort and external testing cohort in all-cause mortality (13.5% vs. 19.1%, P = 0.018) and non-arrhythmic mortality (9.5% vs. 14.3%, P = 0.021). An overview of patient outcomes is shown in Table 2, survival curves are shown in Supplementary material online, Figure S1. Patients with a CRT-D had a higher risk of non-arrhythmic mortality (P = 0.028) and all-cause mortality (P = 0.031), and a lower risk of appropriate ICD-therapy (P = 0.031) compared to ICD patients (see Supplementary material online, Figure S1B).
Table 2.
Incidence of outcomes in the development cohort and external testing cohort
| Development cohort (n = 550) | External testing cohort (n = 460) | P-value | |
|---|---|---|---|
| All-cause mortality, yes (%) | 74 (13.5%) | 88 (19.1%) | 0.018 |
| Appropriate ICD-therapy, yes (%) | 113 (20.5%) | 83 (18.0%) | 0.357 |
| ATP | 25 (4.5%) | 23 (5.0%) | |
| ATP and shock | 24 (4.4%) | 20 (4.3%) | |
| Shock | 64 (11.6%) | 40 (8.7%) | |
| Non-arrhythmic mortality, yes (%) | 52 (9.5%) | 66 (14.3%) | 0.021 |
Abbreviations: ATP, anti-tachycardia pacing; ICD, implantable cardioverter-defibrillator.
Multimodal ML model
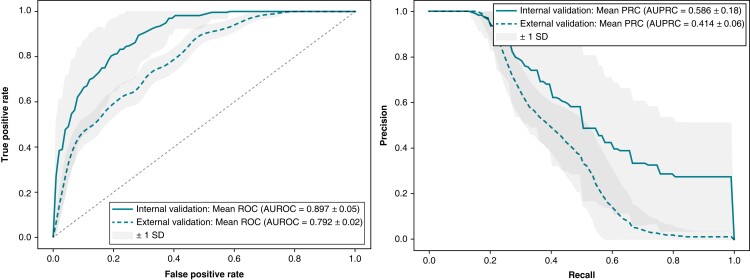
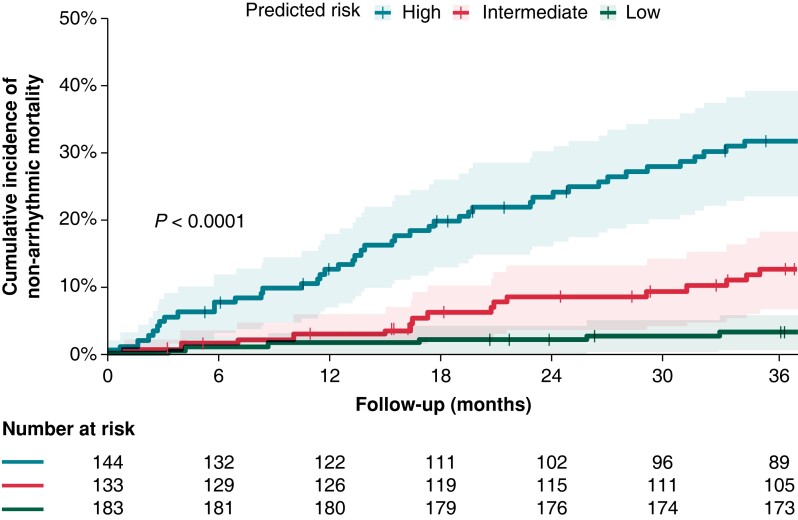
The predictive performance during model development and internal validation was highest for the XGBoost model. On internal validation, the model predicted non-arrhythmic mortality at 3 years with an AUROC of 0.897 ± 0.05 and an AUPRC of 0.546 ± 0.18. External validation of the model in an external patient cohort showed an AUROC of 0.792 (95% CI: 0.746–0.841) and an AUPRC of 0.414 (95% CI: 0.284–0.500), Figure 2 displays the ROC and Precision-Recall curves. The model sensitivity, specificity, F1-score, calibration intercept, and calibration slope for the external testing cohort are presented in Table 3. The calibration plot shown in Supplementary material online, Figure S2 indicates that the model underestimates at low probabilities, high predicted probabilities were rare as shown in the grey histogram of the prediction distributions. Kaplan–Meier survival curves were plotted to visualize non-arrhythmic mortality in the external testing cohort during 3-year follow-up stratified by predicted probability, as depicted in Figure 3. The probability of non-arrhythmic mortality at 3-year follow-up for the low, intermediate and high predicted risk groups were respectively 3.3%, 12.7%, and 31.7% (P < 0.001). The performance of the multimodal ML model on the outcomes appropriate ICD-therapy and all-cause mortality are displayed in Supplementary material online, Table S2. No differences in AUROC were observed for subgroups, the AUPRC was higher in a CRT-D population and in patients older than 65 year (see Supplementary material online, Figure S5). The MADIT-ICD score had an AUROC of 0.67 in the external cohort.
Figure 2.
Receiver operating characteristic curve (Panel A) and precision-recall curve (Panel B) of the multimodal ML model at internal validation and external testing.
Table 3.
Comparison of model performances in the external patient cohort
| Multimodal model | ECG model | |
|---|---|---|
| AUROC | 0.792 (0.746–0.841) | 0.742 (0.689–0.797) |
| AUPRC | 0.414 (0.284–0.500) | 0.326 (0.216–0.410) |
| Calibration slope | 1.048 (0.727–1.430) | 0.382 (0.669–0.968) |
| Calibration intercept | 0.072 (0.046–0.100) | 0.098 (0.068–0.127) |
| F1-score | 0.746 (0.714–0.778) | 0.658 (0.621–0.693) |
| Sensitivity | 0.703 (0.664–0.741) | 0.600 (0.558–0.641) |
| Specificity | 0.696 (0.654–0.738) | 0.564 (0.520–0.609) |
Abbreviations: AUROC, area under the receiver operator curve; AUPRC, area under the precision recall curve.
Figure 3.
Kaplan–Meier survival curves for non-arrhythmic mortality during 3-year follow-up stratified to low, intermediate, and high predicted risk.
ECG ML model
The ECG ML model reached an AUROC of 0.742 (95% CI: 0.689–0.797) and an AUPRC of 0.326 (95% CI: 0.216–0.410) on the external testing cohort. The sensitivity, specificity, F1-score, calibration intercept, and calibration slope for the external testing cohort are shown in Table 3 (calibration plots Supplementary material online, Figures S3andS4). There was a significant association between low vs. high ECG-score and non-arrhythmic mortality [hazard ratio (HR) 5.54, 95% CI: 2.91–10.54, P < 0.001] adjusted for clinical covariates (see Supplementary material online, Figure S6). Other significant associations were found for age (HR 2.66 for ≤75 vs. > 75 years, 95% CI: 1.59–4.45, P < 0.001) and CRT-D implanted (HR 2.07, 95% CI: 1.23–3.49, P = 0.006).
Model explainability
Supplementary material online, Figures S7–SS9 show SHAP summary plots with the 50 features with the highest feature importance in descending order for the multimodal ML model for non-arrhythmic mortality, appropriate ICD-therapy, and all-cause mortality. Age and serum creatinine had the highest feature importance followed by some of the ECG time-series features for prediction of non-arrhythmic mortality. For both prediction of non-arrhythmic mortality and appropriate ICD-therapy, features representing the decomposed ECG into its frequency components were consistently of high importance. The Fourier coefficients corresponding lower frequencies were particularly important for prediction of appropriate ICD-therapy, as compared to higher frequencies for non-arrhythmic mortality (see Supplementary material online, Figure S12).
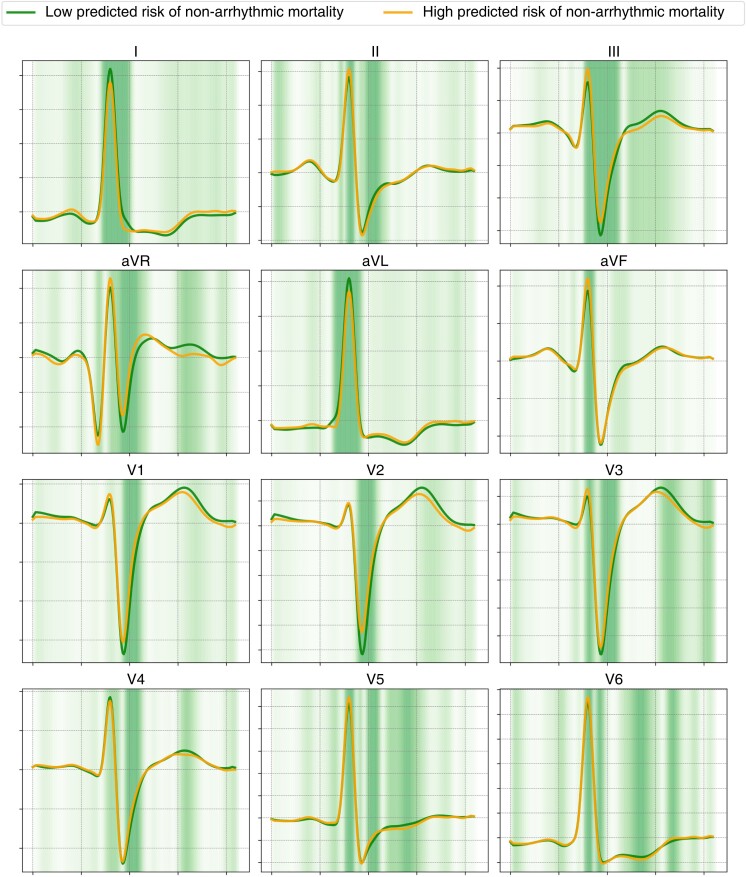
Mean waveforms for high predicted risk (orange) vs. low predicted risk (green) of non-arrhythmic mortality are displayed in Figure 4, Supplementary material online, Figure S10 shows the mean waveforms for prediction of appropriate ICD-therapy. Mean waveforms for individuals with predicted non-arrhythmic mortality showed smaller T-wave and larger R-wave amplitudes in the precordial leads and a shorter QRS-duration. In a CRT-D only population the anterior leads showed deeper Q-waves for the high predicted probability of non-arrhythmic mortality (see Supplementary material online, Figure S11). Mean waveforms for high predicted risk of appropriate ICD-therapy showed ST-T-wave abnormalities in the precordial leads, characterized by lower T-wave amplitudes, flattened ST-segments and shallow S-waves in the anterior leads.
Figure 4.
Mean ECG waveforms for multimodal ML model predictions of non-arrhythmic mortality (orange: high predicted risk of non-arrhythmic mortality, green: low predicted risk of non-arrhythmic mortality). The heatmap indicates the magnitude of the (normalized) differences between the two waveforms.
Net benefit curve
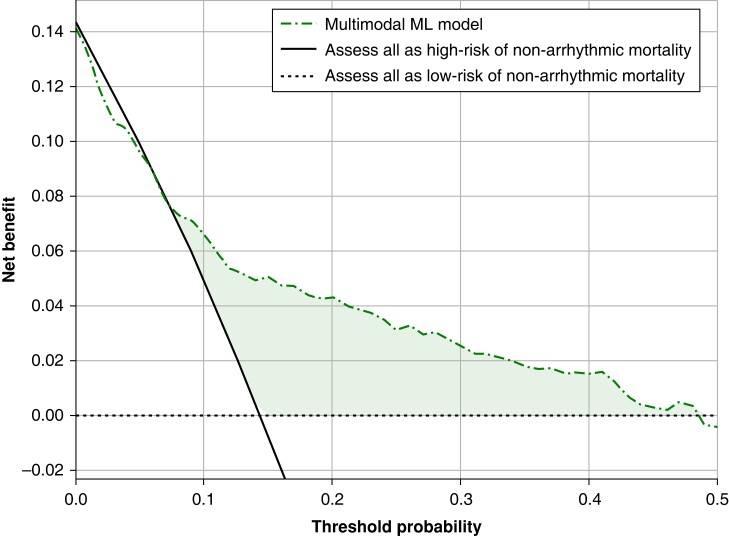
Figure 5 displays the net benefit curve for the multimodal ML model. Not using a model would assume that all subjects have the same risk and is illustrated by the two alternatives of either assuming all are at low risk or that all are at high-risk of non-arrhythmic mortality 3 years following ICD implantation. According to Figure 5, the most significant benefits would be gained when probabilities between 10% and 40% would be used as threshold. For example, at a decision threshold of 20% for the 3-year risk of non-arrhythmic mortality, the multimodal ML model would achieve a net benefit of 0.042 over assessing all patients as low risk of non-arrhythmic mortality. This would translate to identifying 42 additional true cases of non-arrhythmic mortality per 1000 subjects, without increasing the number of false positive predictions, compared to a situation where all individuals were considered at low risk of non-arrhythmic mortality.
Figure 5.
Net benefit decision curve analysis of the multimodal ML model to guide ICD implantation through non-arrhythmic mortality prediction.
Discussion
We present the development and external validation of a multimodal ML model for risk prediction of non-arrhythmic mortality 3 years following device implantation. Accurate risk-stratification tools for guiding primary prevention ICD implantation are essential for balancing an individual's risk of non-arrhythmic mortality and malignant ventricular tachyarrhythmias.4 By leveraging ML techniques to extract time-series features from real-world 12-lead ECG signals, we were able to achieve accurate personalized predictions on an independent external testing cohort.5,10,11,26 Second, we further observed that the probabilities obtained using an ECG ML model could provide additional predictive value on top of standard clinical variables. On balance, our findings suggest that time-series characteristics derived from 12-lead ECG have predictive value and could improve risk-stratification tools to guide ICD implantation.
Patient selection for ICD implantation
In line with prior studies, we observed that the non-arrhythmic mortality accounted for 72.8% of the total mortality at 3-year follow-up.5 Conversely, these patients have been exposed to potential side-effects of device implantation, inappropriate ICD-therapy, and reduced health-related quality of life.8,9 The presented multimodal ML model was developed with the aim to inform clinicians on the patient’s risk of having no benefit from an ICD due to the competing risk of (early) non-arrhythmic mortality. This information could aid physicians to defer ICD implantation in patients with high probability of non-arrhythmic mortality, and provide tools for better informed shared decision making. Various prediction models have been developed to improve patient selection for primary prevention of ICD implantation, using a combination of clinical variables, electrophysiological signals, and cardiac imaging.10–14 The Dutch outcome in ICD-therapy (DO-IT) study developed and validated a model for all-cause mortality and ICD shock using medical history, medication, and laboratory values.27 At external validation, these models had a C-statistic of 0.74 (95% CI: 0.70–0.78) and 0.60 (95% CI: 0.53–0.67), respectively. In terms of non-arrhythmic mortality, validation of three predictions risk-scores [Functional class, Age, Diabetes mellitus, Ejection fraction and Smoking, Multicenter Automatic Defibrillator Implantation Trial (MADIT), and Seattle Heart Failure Model for Defibrillator] on an external cohort showed AUROCs of 0.66, 0.69, and 0.75.5 We aimed to identify ECG features that reflect arrhythmic risk and could complement these clinical predictors, which led to an AUROC of 0.897 during internal validation and 0.792 on external validation. Additionally, the net benefit curve, a weighted ratio between true positive and false positive predictions at different risk thresholds, was used to explore the theoretical clinical utility of the multimodal ML model. Currently, in hypertrophic cardiomyopathy patients, the risk threshold to consider an individual at high-risk of SCD requiring ICD treatment is between 4% and 6%, however, such a threshold has not been defined for non-arrhythmic mortality.28 Based on decision curve analyses for the multimodal ML model, a risk threshold for non-arrhythmic mortality above 10% but below 40%, to guide ICD implantation would have conferred benefit over a situation where all individuals were considered to be at low risk of non-arrhythmic mortality. Selecting probability thresholds for non-arrhythmic mortality to guide ICD implantation remains challenging due to the difficulty in quantifying the consequences of false predictions, such as incorrect withholding of ICD implantation in patients who experience sustained ventricular arrhythmias or complications from the ICD in patients without ventricular arrhythmias. Nonetheless, for these findings to be translated to a clinical setting and aid the decision-making process for ICD implantation, a clinically defined threshold probability is crucial.24
Similar to the previously reported accuracy of the MADIT-ICD score in an external patient cohort from the RAID randomied trial, the MADIT-ICD score had an AUROC for non-arrhythmic mortality of 0.67 in our external patient cohort.10 Despite the substantially higher predictive accuracy of the multimodal ML model in the external cohort, it should be noted that the model was developed and validated on retrospective data that could make it susceptible to unfair predictions within underrepresented subgroups. One of such is patients with CRT, where there is a challenge of balancing the risks of malignant ventricular arrhythmias and non-arrhythmic mortality as CRT affects non-arrhythmic mortality and pro-arrhythmic risks.29–31 Kaplan–Meier time-to-event analyses demonstrated higher non-arrhythmic mortality in CRT-D patients compared to patients with an ICD, potentially attributed to the anti-arrhythmic effect exerted by CRT. Both the MADIT-ICD score and the multimodal ML model, which was trained on a real-world ICD population (37.8% had CRT), showed differences in AUROCs between CRT-D only (0.81, 95% CI: 0.73–0.88) and ICD-only populations (0.72, 95% CI: 0.60–0.81), which emphasizes the relevance of validating model performance in subgroups.32 In line with prior observational studies, the retrospective nature of this study, and inherent lack of information on the patient selection for CRT, may have introduced selection bias, as patients who receive CRT may differ in important ways from those who do not.33,34 Future studies may provide additional insights into the differences in arrhythmic substrate and triggering mechanisms among different clinical phenotypes, including patients undergoing CRT, and their impact on time-series ECG data. In addition, guideline-recommended medical therapy for heart failure with reduced ejection fraction has changed over the past years to include β-blockers, ACE-inhibitors/ARB, angiotensin receptor neprilysin inhibitors (ARNI), sodium-glucose cotransporter 2 inhibitors (SGLT2) inhibitor, and MRA.35 These have reduced mortality risks in primary prevention ICD patients, affecting the balance between non-arrhythmic and arrhythmic mortality.36 The multimodal ML model demonstrated similar AUROC and AUPRC between patients using GDMT (comprising 24.4% of patients) and non-GDMT, nevertheless the effect of more novel heart failure medication such as SGLT2 inhibitors and ARNI on the model performance is unclear. Prospective validation to assess the robustness of the model in a contemporary, real-world ICD population is pivotal for clinical adoption.
Machine learning for outcome prediction
Features derived from non-invasive electrophysiological signals have been demonstrated to reflect arrhythmic risk, such as T-wave alternans, heart rate variability, and fragmented QRS.37,38 In particular, there has been a growing interest in using non-linear dynamic analyses of ECGs to identify predictors for ventricular arrhythmias.15,39–42 Periodic repolarisation dynamics (PRD) have been proposed as a method to quantify sympathetic modulation of ventricular repolarisation by measuring low frequency (<0.1 Hz) oscillations in the T-wave vector.40 In addition, fibrosis and scar lead to structural changes that may impact repolarisation and induce phasic increases in dispersion of repolarisation (at least partly) reflected by PRD.43 From the SHAP summary plots, we observed that the Fourier coefficients that correspond to low frequencies were particularly important for prediction of appropriate ICD-therapy (see Supplementary material online, Figure S12). This observation aligns with previous reports of PRD’s predictive value for the onset of ventricular arrhythmias, and merits further examination to better understand the pro-arrhythmic mechanisms and the potential use as a predictor of arrhythmic risk.
Moreover, artificial intelligence (AI) facilitates automated extraction of features from signals that have predictive capacity for mortality and ventricular tachyarrhythmia.39,44–46 The value of time-series features extracted from electrophysiological signals as input for ML models was previously demonstrated by Rogers et al. who applied this to the ventricular monophasic action potential (MAP) of 42 patients with ischaemic cardiomyopathy.46 This ML model trained on time-series features alone reached an AUROC of 0.90 for sustained VT/VF (95% CI: 0.76–1.00) on 10-fold CV. Aside from electrocardiographic features, the presence of ventricular fibrosis assessed by late gadolinium is a promising risk marker for ventricular arrhythmia risk.47,48 The recent Survival Study of Cardiac Arrhythmia Risk prediction model that was developed to predict the 10-year risk of SCD using a combination of clinical features and raw cardiac magnetic resonance images (CMR) confirmed the high potential of CMR data as input to a deep learning model.49 External validation of this model on a cohort of patients with mild to moderate LVEF reduction who did not qualify for an ICD yielded an AUROC of 0.72 and AUPRC of 0.73.49 Furthermore, the ongoing PROFID project funded by the European Union aimed to develop a personalized prediction of SCD after myocardial infarction using CMR and clinical data, however, no model based upon baseline characteristics and imaging substantially improved the predictive performance of LVEF.50 Considering the potential prognostic information in both CMR, ECG, and clinical parameters, we ultimately envision a multimodal model that exploits features from each modality for personalized prediction of SCD.
Explainability of model predictions
Machine learning is often criticized for a lack of transparency on the basis for model predictions, nevertheless explainability and actionability of ML predictions are necessary for the adoption of an AI-based tool in clinical practice. Our models were developed using times-series features extracted from the ECGs which are all to a certain extent human-interpretable, however, the relationship between cardiac pathology, specific physiological events, and these time-series features is largely unknown. We aimed to clarify model predictions by calculating SHAP values and visualizing the mean ECG waveforms for model predictions. Although mean waveform analysis is constrained as the time-series features extracted may capture variations between beats rather than solely the QRS-T morphology, we observed substantial differences between high vs. low predicted probabilities of non-arrhythmic mortality and appropriate ICD-therapy. Mean waveform analysis demonstrated distinct differences in the morphology of the ST-segments and T-wave reflecting ventricular repolarisation, especially in precordial leads. Over the past decade, multiple parameters that reflect T-wave morphology, or changes in T-wave morphology over time, have been associated with ventricular arrhythmia risk.51,52 Moreover, a recent subanalysis of the prospective EU-CERT-ICDs study of primary prevention ICD patient (no CRT) evaluated several electrocardiographic variables (e.g. fragmented QRS, early repolarisation, T-wave inversion), reporting that the presence of pathological Q-waves was found to be the only electrocardiographic predictor for ICD benefit.53 In our waveform analysis, the prominence of the Q-waves was not different between high vs. low predicted probabilities of non-arrhythmic mortality or appropriate ICD-therapy, however, in a CRT-D only population we found Q-waves in the anterior leads to be more prominent for high-risk of non-arrhythmic mortality. Future investigations are necessary to clarify the prognostic relevance of Q-waves in a CRT-D population, and the correlations between these novel time-series ECG features and established ECG variables reflecting T-wave morphology.
Limitations
One of the major limitations of this study is the discrepancy between appropriate ICD-therapy and actual SCD. Previous research has shown that appropriate ICD-therapy does not necessarily equate to SCD, which may lead to an overestimation of the proportion of patients who truly benefit from the ICD.54 In addition, variations in device programming at baseline and during follow-up may have affected the incidence of appropriate ICD-therapy. Due to the retrospective nature of this study, programming details could not be included in analyses. Secondly, our dataset did not include clinical variables that are known to have predictive capacity such as imaging data.10 Thirdly, the current model requires manual selection of ECGs of sufficient quality and excludes ECGs where cardiac pacing is present, which may affect the generalisability of the model. Moreover, to utilize the multimodal model for clinical purposes a set of ECG features needs to be extracted that go beyond standard ECG parameters, either manually or through a systematic feature engineering technique, which may require additional resources. Finally, changes in the guidelines for optimal medical therapy and CRT have changed considerably of the past decade, and have affected the prevalence of non-arrhythmic mortality and ventricular arrhythmias. By training the multimodal ML model on a real-world ICD population including CRT, and assessing model performance on subgroups, we aimed to assess the robustness of the model and potential data drift. For clinical uptake of the multimodal ML model, we aim to perform external, prospective validation on other cohorts to assess the stability in model performance.
Conclusion
Multimodal ML models can leverage ECG time-series features and clinical data to achieve accurate and personalized predictions of non-arrhythmic mortality risk in a primary prevention ICD population. A digital tool that provides clinicians with a personalized risk-score for early non-arrhythmic mortality could aid the decision-making process for ICD-implantation. The presented multimodal ML model outperformed established clinical prediction scores in an external patient cohort, however, future studies are warranted to prospectively validate the identified ECG features and their electrophysiological substrate for arrhythmic risk prediction.
Supplementary Material
Acknowledgements
We thank Dr Selder MD, Dr Götte MD, PhD, and Mr Geijtenbeek MSc, for their contributions to this research.
Contributor Information
Maarten Z H Kolk, Department of Cardiology, Heart Center, Amsterdam UMC location University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Amsterdam Cardiovascular Sciences, Heart Failure and Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Samuel Ruipérez-Campillo, Department of Medicine and Cardiovascular Institute, Stanford University, 780 Welch Road, MC 5773, Stanford, CA 94305, USA; Department of Information Technology and Electrical Engineering, Swiss Federal Institute of Technology Zurich (ETHz), Zurich, Switzerland.
Brototo Deb, Department of Medicine and Cardiovascular Institute, Stanford University, 780 Welch Road, MC 5773, Stanford, CA 94305, USA.
Erik J Bekkers, Faculty of Science, University of Amsterdam, Science Park 904, 1098 XH Amsterdam, the Netherlands.
Cornelis P Allaart, Department of Cardiology, Amsterdam UMC location Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
Albert J Rogers, Department of Medicine and Cardiovascular Institute, Stanford University, 780 Welch Road, MC 5773, Stanford, CA 94305, USA.
Anne-Lotte C J Van Der Lingen, Department of Cardiology, Amsterdam UMC location Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
Laura Alvarez Florez, Department of Cardiology, Heart Center, Amsterdam UMC location University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Amsterdam Cardiovascular Sciences, Heart Failure and Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Department of Biomedical Engineering and Physics, Amsterdam UMC location University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Ivana Isgum, Faculty of Science, University of Amsterdam, Science Park 904, 1098 XH Amsterdam, the Netherlands; Department of Biomedical Engineering and Physics, Amsterdam UMC location University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Department of Radiology and Nuclear Medicine, Amsterdam UMC location University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Bob D De Vos, Department of Biomedical Engineering and Physics, Amsterdam UMC location University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Paul Clopton, Department of Medicine and Cardiovascular Institute, Stanford University, 780 Welch Road, MC 5773, Stanford, CA 94305, USA.
Arthur A M Wilde, Department of Cardiology, Heart Center, Amsterdam UMC location University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Amsterdam Cardiovascular Sciences, Heart Failure and Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Reinoud E Knops, Department of Cardiology, Heart Center, Amsterdam UMC location University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Amsterdam Cardiovascular Sciences, Heart Failure and Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Sanjiv M Narayan, Department of Medicine and Cardiovascular Institute, Stanford University, 780 Welch Road, MC 5773, Stanford, CA 94305, USA.
Fleur V Y Tjong, Department of Cardiology, Heart Center, Amsterdam UMC location University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Amsterdam Cardiovascular Sciences, Heart Failure and Arrhythmias, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Department of Medicine and Cardiovascular Institute, Stanford University, 780 Welch Road, MC 5773, Stanford, CA 94305, USA.
Supplementary material
Supplementary material is available at Europace online.
Funding
This publication is part of the project DEEP RISK ICD (with project number 452019308) of the research programme Rubicon which is (partly) financed by the Dutch Research Council (NWO). This research is partly funded by the Amsterdam Cardiovascular Sciences.
Conflict of interest
S.M.N. reports grants or contracts from National Institutes of Health (NIH), consulting fees from Abbott Inc., and royalties and licences from Up-to-date. R.E.K. reports consultancy fees and research grants from Boston Scientific, Medtronic and Abbott Inc. and has the stock options from AtaCor Medical Inc. F.V.Y.T. has grants or contracts from the Dutch Research Council (NWO) and Amsterdam Cardiovascular Sciences, and received honoraria fees from Boston Scientific and Abbott Inc. (no personal financial gain). A.A.W. has consultancy fees from ARMGO and Thyrv Therapeutics, and participates on a Data Safety Monitoring Board or Advisory Board for the LEAP trial. C.P.A. reports grants or contracts from Biotronik. I.I. reports research grants or contracts from participation of Philips, Quantib, Pie Medical Imaging and Esaote, has patents planned, issued or pending, has stock option from RadNet and has leadership or fiduciary for Chair Medical Imaging with Deep Learning (MIDL) board, MIDL Foundation and SPIE Medical Imaging, Image processing. M.Z.H.K., S.R.C., B.D., L.A.F., E.J.B., P.C., and A.C.J.L have nothing to declare.
Data availability
Data sharing requests will be considered upon a reasonable request. Programming code for ECG pre-processing, time-series feature extraction, and model development is available through: https://github.com/DeepRiskAUMC/Optimizing-Patient-Selection-for-Primary-Prevention-ICD-Implantation.
References
- 1. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis ABet al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation 2018;138:e210–71. [DOI] [PubMed] [Google Scholar]
- 2. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NAet al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. [DOI] [PubMed] [Google Scholar]
- 3. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IMet al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. EP Europace 2022;24:71–164. [DOI] [PubMed] [Google Scholar]
- 4. Wellens HJ, Schwartz PJ, Lindemans FW, Buxton AE, Goldberger JJ, Hohnloser SHet al. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J 2014;35:1642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Heijden AC, van Rees JB, Levy WC, van der Bom JG, Cannegieter SC, de Bie MKet al. Application and comparison of the FADES, MADIT, and SHFM-D risk models for risk stratification of prophylactic implantable cardioverter-defibrillator treatment. EP Europace 2017;19:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Disertori M, Mase M, Rigoni M, Nollo G, Ravelli F. Declining clinical benefit of ICD in heart failure patients: temporal trend of mortality outcomes from randomized controlled trials. J Cardiol 2020;75:148–54. [DOI] [PubMed] [Google Scholar]
- 7. Dagres N, Hindricks G. Devices for management of sudden cardiac death: successes, challenges and perspectives. Int J Cardiol 2017;237:34–7. [DOI] [PubMed] [Google Scholar]
- 8. Ezzat VA, Lee V, Ahsan S, Chow AW, Segal O, Rowland Eet al. A systematic review of ICD complications in randomised controlled trials versus registries: is our ‘real-world’ data an underestimation? Open Heart 2015;2:e000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Magnusson P, Mattsson G, Wallhagen M, Karlsson J. Health-related quality of life in patients with implantable cardioverter defibrillators in Sweden: a cross-sectional observational trial. BMJ Open 2021;11:e047053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Younis A, Goldberger JJ, Kutyifa V, Zareba W, Polonsky B, Klein Het al. Predicted benefit of an implantable cardioverter-defibrillator: the MADIT-ICD benefit score. Eur Heart J 2021;42:1676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bilchick KC, Wang Y, Cheng A, Curtis JP, Dharmarajan K, Stukenborg GJet al. Seattle Heart failure and proportional risk models predict benefit from implantable cardioverter-defibrillators. J Am Coll Cardiol 2017;69:2606–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He Het al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol 2008;51:288–96. [DOI] [PubMed] [Google Scholar]
- 13. van Rees JB, Borleffs CJ, van Welsenes GH, van der Velde ET, Bax JJ, van Erven Let al. Clinical prediction model for death prior to appropriate therapy in primary prevention implantable cardioverter defibrillator patients with ischaemic heart disease: the FADES risk score. Heart 2012;98:872–7. [DOI] [PubMed] [Google Scholar]
- 14. Shadman R, Poole JE, Dardas TF, Mozaffarian D, Cleland JG, Swedberg Ket al. A novel method to predict the proportional risk of sudden cardiac death in heart failure: derivation of the Seattle proportional risk model. Heart Rhythm 2015;12:2069–77. [DOI] [PubMed] [Google Scholar]
- 15. Kolk MZH, Deb B, Ruiperez-Campillo S, Bhatia NK, Clopton P, Wilde AAMet al. Machine learning of electrophysiological signals for the prediction of ventricular arrhythmias: systematic review and examination of heterogeneity between studies. eBioMedicine 2023;89:104462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barker J, Li X, Khavandi S, Koeckerling D, Mavilakandy A, Pepper Cet al. Machine learning in sudden cardiac death risk prediction: a systematic review. EP Europace 2022;24:1777–87. [DOI] [PubMed] [Google Scholar]
- 17. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Br J Surg 2015;102:148–58. [DOI] [PubMed] [Google Scholar]
- 18. Christ M, Kempa-Liehr AW, Feindta M. Distributed and parallel time series feature extraction for industrial big data applications. arXiv, 10.48550/arXiv.1610.07717 (May 19, 2017, preprint: not peer reviewed). 2016. [DOI]
- 19. Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat 2001;29:1165–88. [Google Scholar]
- 20. Stekhoven DJ, Buhlmann P. Missforest—non-parametric missing value imputation for mixed-type data. Bioinformatics 2012;28:112–8. [DOI] [PubMed] [Google Scholar]
- 21. Chen T, Guestrin C. XGBoost: A scalable tree boosting system. arXiv, 10.48550/arXiv.1603.02754(March 9, 2016, preprint: not peer reviewed). 2016. [DOI]
- 22. Pedregosa FVG, Gramfort A, Michel V, Thirion B, Grisel O, Blondel Met al. Scikit-learn: machine learning in python. J Mach Learn Res 2011;12:2825–30. [Google Scholar]
- 23. Lundberg S, Lee S-I. A unified approach to interpreting model predictions. arXiv, 10.48550/arXiv.1705.07874(May 22, 2017, preprint: not peer reviewed). 2017. [DOI]
- 24. Vickers AJ, van Calster B, Steyerberg EW. A simple, step-by-step guide to interpreting decision curve analysis. Diagn Progn Res 2019;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. R Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 26. Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DTet al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation 2009;120:835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verstraelen TE, van Barreveld M, van Dessel P, Boersma LVA, Delnoy P, Tuinenburg AEet al. Development and external validation of prediction models to predict implantable cardioverter-defibrillator efficacy in primary prevention of sudden cardiac death. EP Europace 2021;23:887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron Pet al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–79. [DOI] [PubMed] [Google Scholar]
- 29. Yuyun MF, Erqou SA, Peralta AO, Hoffmeister PS, Yarmohammadi H, Echouffo Tcheugui JBet al. Risk of ventricular arrhythmia in cardiac resynchronization therapy responders and super-responders: a systematic review and meta-analysis. EP Europace 2021;23:1262–74. [DOI] [PubMed] [Google Scholar]
- 30. Gras M, Bisson A, Bodin A, Herbert J, Babuty D, Pierre Bet al. Mortality and cardiac resynchronization therapy with or without defibrillation in primary prevention. EP Europace 2020;22:1224–33. [DOI] [PubMed] [Google Scholar]
- 31. Schrage B, Lund LH, Melin M, Benson L, Uijl A, Dahlstrom Uet al. Cardiac resynchronization therapy with or without defibrillator in patients with heart failure. EP Europace 2022;24:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dauw J, Martens P, Nijst P, Meekers E, Deferm S, Gruwez Het al. The MADIT-ICD benefit score helps to select implantable cardioverter-defibrillator candidates in cardiac resynchronization therapy. EP Europace 2022;24:1276–83. [DOI] [PubMed] [Google Scholar]
- 33. Mullens W, Auricchio A, Martens P, Witte K, Cowie MR, Delgado Vet al. Optimized implementation of cardiac resynchronization therapy: a call for action for referral and optimization of care. EP Europace 2021;23:1324–42. [DOI] [PubMed] [Google Scholar]
- 34. Hadwiger M, Dagres N, Haug J, Wolf M, Marschall U, Tijssen Jet al. Survival of patients undergoing cardiac resynchronization therapy with or without defibrillator: the RESET-CRT project. Eur Heart J 2022;43:2591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm Met al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. [DOI] [PubMed] [Google Scholar]
- 36. Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGFet al. Declining risk of sudden death in heart failure. N Engl J Med 2017;377:41–51. [DOI] [PubMed] [Google Scholar]
- 37. Narayan SM. T-wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol 2006;47:269–81. [DOI] [PubMed] [Google Scholar]
- 38. Nolan J, Andrews R, Lindsay SJ, Brooksby P, Mullen M, Baig Wet al. Prospective study of heart rate variability and mortality in chronic heart failure results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart). Circulation 1998;98:1510–6. [DOI] [PubMed] [Google Scholar]
- 39. Bauer A, Klemm M, Rizas KD, Hamm W, von Stulpnagel L, Dommasch Met al. Prediction of mortality benefit based on periodic repolarisation dynamics in patients undergoing prophylactic implantation of a defibrillator: a prospective, controlled, multicentre cohort study. Lancet 2019;394:1344–51. [DOI] [PubMed] [Google Scholar]
- 40. Rizas KD, McNitt S, Hamm W, Massberg S, Kaab S, Zareba Wet al. Prediction of sudden and non-sudden cardiac death in post-infarction patients with reduced left ventricular ejection fraction by periodic repolarization dynamics: MADIT-II substudy. Eur Heart J 2017;38:2110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boas R, Sappler N, von Stulpnagel L, Klemm M, Dixen U, Thune JJet al. Periodic repolarization dynamics identifies ICD responders in nonischemic cardiomyopathy: A DANISH substudy. Circulation 2022;145:754–64. [DOI] [PubMed] [Google Scholar]
- 42. Palacios S, Cygankiewicz I, Bayes de Luna A, Pueyo E, Martinez JP. Periodic repolarization dynamics as predictor of risk for sudden cardiac death in chronic heart failure patients. Sci Rep 2021;11:20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taggart P, Pueyo E, Duijvenboden SV, Porter B, Bishop M, Sampedro-Puente DAet al. Emerging evidence for a mechanistic link between low-frequency oscillation of ventricular repolarization measured from the electrocardiogram T-wave vector and arrhythmia. EP Europace 2021;23:1350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kwon JM, Kim KH, Jeon KH, Lee SY, Park J, Oh BH. Artificial intelligence algorithm for predicting cardiac arrest using electrocardiography. Scand J Trauma Resusc Emerg Med 2020;28:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sammani A, van de Leur RR, Henkens M, Meine M, Loh P, Hassink RJet al. Life-threatening ventricular arrhythmia prediction in patients with dilated cardiomyopathy using explainable electrocardiogram-based deep neural networks. EP Europace 2022;24:1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rogers AJ, Selvalingam A, Alhusseini MI, Krummen DE, Corrado C, Abuzaid Fet al. Machine learned cellular phenotypes in cardiomyopathy predict sudden death. Circ Res 2021;128:172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klem I, Klein M, Khan M, Yang EY, Nabi F, Ivanov Aet al. Relationship of LVEF and myocardial scar to long-term mortality risk and mode of death in patients with nonischemic cardiomyopathy. Circulation 2021;143:1343–58. [DOI] [PubMed] [Google Scholar]
- 48. Dawson DK, Hawlisch K, Prescott G, Roussin I, Di Pietro E, Deac Met al. Prognostic role of CMR in patients presenting with ventricular arrhythmias. JACC: Cardiovasc Imaging 2013;6:335–44. [DOI] [PubMed] [Google Scholar]
- 49. Popescu DM, Shade JK, Lai C, Aronis KN, Ouyang D, Moorthy MVet al. Arrhythmic sudden death survival prediction using deep learning analysis of scarring in the heart. Nat Cardiovasc. Res 2022;1:334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dagres N, Peek N, Leclercq C, Hindricks G. The PROFID project. Eur Heart J 2020;41:3781–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ramirez J, Kiviniemi A, van Duijvenboden S, Tinker A, Lambiase PD, Junttila Jet al. ECG T-Wave morphologic variations predict ventricular arrhythmic risk in low- and moderate-risk populations. J Am Heart Assoc 2022;11:e025897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Seegers J, Hnatkova K, Friede T, Malik M, Zabel M. T-wave loop area from a pre-implant 12-lead ECG is associated with appropriate ICD shocks. PLoS One 2017;12:e0173868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pelli A, Junttila MJ, Kentta TV, Schlogl S, Zabel M, Malik Met al. Q waves are the strongest electrocardiographic variable associated with primary prophylactic implantable cardioverter-defibrillator benefit: a prospective multicentre study. EP Europace 2022;24:774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tung R, Zimetbaum P, Josephson ME. A critical appraisal of implantable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol 2008;52:1111–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing requests will be considered upon a reasonable request. Programming code for ECG pre-processing, time-series feature extraction, and model development is available through: https://github.com/DeepRiskAUMC/Optimizing-Patient-Selection-for-Primary-Prevention-ICD-Implantation.