Abstract
The performance of the Etest for fluconazole susceptibility testing of 402 yeast isolates was assessed against the National Committee for Clinical Laboratory Standards (NCCLS) microdilution broth method. The NCCLS method employed RPMI 1640 broth medium, and MICs were read after incubation for 48 h at 35°C. Etest MICs were determined with RPMI agar containing 2% glucose (RPG), Casitone agar (CAS), and Mueller-Hinton agar (MHA) and were read after incubation for 48 h at 35°C. The yeast isolates included Candida albicans (n = 161), Candida glabrata (n = 41), Candida tropicalis (n = 35), Candida parapsilosis (n = 29), Candida krusei (n = 32), Candida lusitaniae (n = 31), Candida species (n = 19), Cryptococcus neoformans (n = 40), and miscellaneous yeast species (n = 14). The Etest results correlated well with reference MICs. Overall agreement was 94% with RPG, 97% with CAS, and 53% with MHA. When RPG was used, agreement ranged from 89% for Candida spp. to 100% for C. krusei. When CAS was utilized, agreement ranged from 93% for Cryptococcus neoformans to 100% for C. tropicalis, C. parapsilosis, C. lusitaniae, Candida spp., and miscellaneous yeast species. With MHA, agreement ranged from 17% for C. parapsilosis to 90% for C. krusei. Both RPG and CAS supported growth of all yeast species, whereas growth on MHA was comparatively weaker. Etest results were somewhat easier to read on CAS. The Etest method using either RPG or CAS, but not MHA, appears to be a viable alternative to the NCCLS reference method for determining fluconazole susceptibilities of yeasts.
The assessment of alternative antifungal susceptibility testing methods such as the colorimetric MIC microdilution procedure (8–10, 18) and agar-based methods like the disk diffusion method (1) and Etest stable gradient method (2–4, 6, 12, 16, 17, 19) has become possible with the standardization of broth dilution methods for in vitro susceptibility testing of yeasts (7, 13, 15). Studies by Sewell et al. (16) and Colombo et al. (3, 4) demonstrated that the Etest was potentially useful for in vitro testing of susceptibility to fluconazole and other azoles. Additional evaluations with the Etest have suggested that the use of RPMI 1640 agar supplemented with 2% glucose (RPG) or Casitone agar may optimize the growth of certain yeasts and facilitate the examination of MIC endpoints for azoles (6, 12, 19).
It is well known that a concentration-dependent partial inhibition of fungal growth by fluconazole and other azoles results in the so-called trailing endpoints and causes problems with reliable interpretation of MIC results (5, 14). Partial inhibition in the Etest corresponds to the presence of microcolonies around or inside the whole area of a discernable inhibition ellipse (2–4, 6). This trailing is minimized by adding 2% glucose to RPMI agar and is less frequently seen on Casitone agar (6). In the present study, we provided an expanded evaluation of the Etest, using RPMI, Casitone, and Mueller-Hinton agars, in comparison to the National Committee for Clinical Laboratory Standards (NCCLS) reference microdilution method by testing the susceptibilities of 402 clinical yeast isolates to fluconazole.
MATERIALS AND METHODS
Test organisms.
Four hundred and two clinical yeast isolates were selected for testing. The collection included 161 Candida albicans, 41 Candida glabrata, 35 Candida tropicalis, 29 Candida parapsilosis, 32 Candida krusei, 31 Candida lusitaniae, 19 Candida spp. (five Candida quilliermondii, four Candida lipolytica, five Candida rugosa, two Candida kefyr, and three Candida zeylanoides), 40 Cryptococcus neoformans, and 14 miscellaneous yeast species (two Cryptococcus laurentii, one Cryptococcus albidus, four Trichosporon beigelii, four Rhodotorula spp., and three Saccharomyces cerevisiae) isolates. The members of this collection were all recent clinical isolates from geographically diverse medical centers in the United States. The majority were isolated from blood or normally sterile body fluids. The isolates were identified by standard methods (20) and were stored as suspensions in water at ambient temperature until used in the study. Prior to testing, each isolate was subcultured at least twice on Sabouraud dextrose agar (Remel, Lenexa, Kans.) to ensure optimal growth characteristics.
Antifungal agents.
Etest strips containing fluconazole (0.016 to 256 μg/ml) were supplied by AB BIODISK (Solna, Sweden). Fluconazole was obtained as a reagent-grade powder from Pfizer Pharmaceuticals, Roerig Division (New York, N.Y.). Stock solutions prepared in water were further diluted in RPMI 1640 medium buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) buffer (Sigma, St. Louis, Mo.) and dispensed into 96-well microdilution trays. Trays containing a 0.1-ml aliquot of appropriate drug solution (two times the final concentration) in each well were subjected to quality control (QC) and then sealed and stored at −70°C until they were used. The final concentrations of fluconazole were 0.12 to 128 μg/ml.
Media.
Agar formulations used for the Etest were RPMI 1640 medium (American Biorganics, Buffalo, N.Y.) supplemented with 1.5% agar and 2% glucose (RPG) and buffered with MOPS, Casitone agar (Difco), and Mueller-Hinton agar (BBL). The RPMI 1640 broth medium used for the microdilution testing was buffered with MOPS in accordance with the NCCLS M27-A method (7).
Antifungal susceptibility test methods.
Broth microdilution tests were performed according to NCCLS document M27-A (7). An inoculum concentration of 0.5 × 103 to 2.5 × 103 cells per ml was standardized spectrophotometrically. Microdilution trays were incubated at 35°C and read after 48 h of incubation. With trailing endpoints, the MIC was determined visually according to NCCLS recommendations of an 80% reduction in turbidity.
For the Etest, 90-mm-diameter plates containing agar at a depth of 4.0 mm were used. The agar surface was inoculated by using a nontoxic swab dipped in a cell suspension adjusted spectrophotometrically to the turbidity of a 0.5 McFarland standard. After excess moisture was absorbed into the agar and the surface was completely dry, an Etest strip was applied to each plate. The plates were incubated at 35°C and read at 24 and 48 h. The MIC was read at the lowest concentration at which the border of the elliptical inhibition zone intercepted the scale on the strip. Any growth, such as microcolonies, throughout a discernable inhibition ellipse was ignored.
QC.
QC, in accordance with NCCLS document M27-A (7), was performed by testing C. krusei ATCC 6258 and C. parapsilosis ATCC 22019. QC determinations made on each day of testing were within the control limits for fluconazole (7, 11).
Analysis of results.
Etest MICs read at 24 and 48 h on the three media were compared to reference microdilution MICs read at 48 h. The reference microdilution and Etest MIC determinations were performed in two physically separate laboratories and were read independently; i.e., the testing was blinded. Since the Etest scale has a continuous gradient of concentrations, the MIC values in between twofold dilutions were raised to the next twofold level of the reference method for comparison (3, 4). Off-scale MICs at the upper limit were converted to the next higher concentration, and off-scale results at the lower limit were left unchanged. Discrepancies between MIC values of no more than two dilutions were used to calculate the percent agreement.
RESULTS AND DISCUSSION
Table 1 summarizes the in vitro susceptibilities of 402 yeast isolates to fluconazole as determined by the reference broth microdilution panel. A broad range of MICs was observed for each organism group. In general, the fluconazole MICs obtained were typical for individual yeast species (8, 9, 13, 16, 17). Both fluconazole-susceptible and -resistant strains were represented in the collection (15).
TABLE 1.
In vitro activity of fluconazole against 402 clinical yeast isolates as determined by the reference broth microdilution methoda
| Organism | No. of isolates tested | MIC (μg/ml)b
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| C. albicans | 161 | 0.25–64 | 1.0 | 4.0 |
| C. glabrata | 41 | 1.0–256 | 32 | 128 |
| C. tropicalis | 35 | 0.5–8.0 | 2.0 | 4.0 |
| C. parapsilosis | 29 | 0.25–64 | 4.0 | 32 |
| C. krusei | 32 | 2.0–64 | 64 | 64 |
| C. lusitaniae | 31 | 0.25–256 | 2.0 | 8.0 |
| Candida speciesc | 19 | 1.0–128 | 4.0 | 64 |
| Cryptococcus neoformans | 40 | 2.0–32 | 8.0 | 16 |
| Miscellaneous speciesd | 14 | 1.0–>256 | 4.0 | >256 |
| All | 402 | 0.25–>256 | 4.0 | 64 |
Performed according to NCCLS M27-A (7).
50% and 90%, MICs at which 50 and 90% of isolates tested, respectively, are inhibited.
Includes C. guilliermondii (n = 5), C. lipolytica (n = 4), C. rugosa (n = 5), C. kefyr (n = 2), and C. zeylanoides (n = 3).
Includes Cryptococcus laurentii (n = 2), C. albidus (n = 1), T. beigelii (n = 4), Rhodotorula spp. (n = 4), and S. cerevisiae (n = 3).
Table 2 summarizes the percentage of 48-h fluconazole MICs obtained by the Etest on the three agar media that were within two dilutions of the reference method result. Overall, the percent agreement was 94% with RPMI and 97% with Casitone. The agreement between the Etest and microdilution MICs was ≥90% for all species with Casitone agar and for all species with the exception of Candida spp. (89%) with RPMI agar. The agreement between Etest and microdilution MICs was poor when Mueller-Hinton agar was used (53%), with ≥80% agreement achieved with only C. krusei (90%), Cryptococcus neoformans (87%), and miscellaneous species (85%).
TABLE 2.
Agreement between Etest and reference fluconazole MICs for 402 clinical yeast isolates
| Organism | No. of isolates tested | % Agreement when tested ona:
|
||
|---|---|---|---|---|
| RPG | Casitone agar | Mueller-Hinton agar | ||
| C. albicans | 161 | 90 | 95 | 50 |
| C. glabrata | 41 | 100 | 95 | 28 |
| C. tropicalis | 35 | 91 | 100 | 47 |
| C. parapsilosis | 29 | 100 | 100 | 17 |
| C. krusei | 32 | 100 | 97 | 90 |
| C. lusitaniae | 31 | 97 | 100 | 45 |
| Candida species | 19 | 89 | 100 | 53 |
| Cryptococcus neoformans | 40 | 97 | 93 | 87 |
| Miscellaneous species | 14 | 100 | 100 | 85 |
| All | 402 | 94 | 97 | 53 |
Percentage of Etest MICs (read at 48 h), determined with three different agar media, that are within ±2 log2 dilutions of the reference microdilution MICs (RPMI broth, 48 h).
An acceptable level of agreement was also achieved when Etest MICs were read after 24 h of incubation (Table 3). This agreement was independent of the species of yeast tested. The overall level of agreement was 90% or greater with both RPMI (95%) and Casitone (97%) agar. The Mueller-Hinton agar results were slightly better (62% agreement) at 24 h but still inferior to those obtained with either RPMI or Casitone agar.
TABLE 3.
Effect of Etest incubation time on agreement of resultant data with NCCLS reference MICs of fluconazole against 402 yeast isolates
| Etest agar test medium | % Agreement ata:
|
|
|---|---|---|
| 24 h | 48 h | |
| RPG | 95 | 94 |
| Casitone | 97 | 97 |
| Mueller-Hinton | 62 | 53 |
Percentage agreement, within two doubling dilutions, of MICs obtained by the Etest, following 24 and 48 h incubation, with MICs obtained by the broth microdilution reference method following 48 h of incubation.
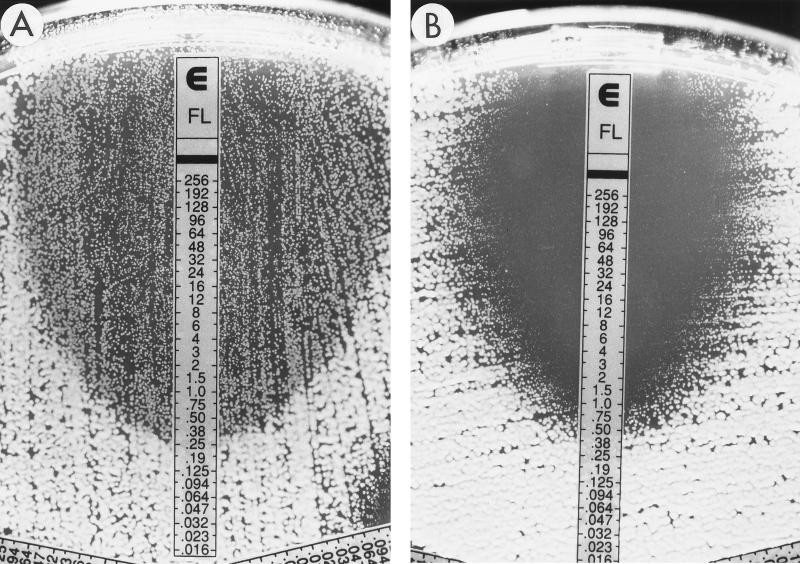
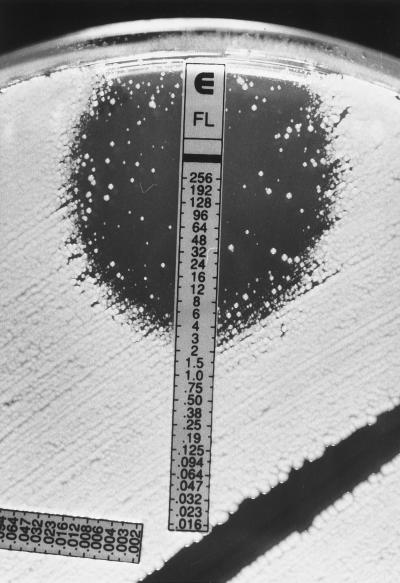
The results of this study provide further documentation of the applicability of the Etest stable agar gradient method for determining the in vitro susceptibilities of yeast isolates to fluconazole. Previous studies in our laboratory using RPMI agar without additional glucose (0.2% final concentration) found poor agreement between Etest and broth dilution MICs for fluconazole tested against C. tropicalis and C. glabrata (16). The supplementation of RPMI agar with additional glucose (2% final concentration) provided optimal growth of these species as well as Cryptococcus neoformans and led to excellent agreement with the MICs obtained by the microdilution reference method (Tables 2 and 3). Similarly, the problem of trailing endpoints due to partial inhibition of growth by azoles was minimized with the use of either RPG or Casitone agar and specific criteria for reading Etest MICs as described in the Etest package insert and technical guide for yeasts (AB BIODISK). In this study, the inhibition ellipses were quite clear with most isolates tested on either RPG or Casitone agar. Good agreement with broth dilution MICs was obtained when discernable growth within the ellipse was ignored. Thus, if an ellipse was visualized, the MIC was read at the point at which the ellipse intersected the strip, irrespective of the presence of inner colonies or a lawn of weaker or less-pigmented growth within the ellipse (Fig. 1). For those isolates in which such inner growth was observed, retesting the organisms from the inner growth produced the same result and did not indicate that the inner colonies exhibited a greater degree of resistance. However, it is important to distinguish the microcolonies from isolated macrocolonies that can be seen especially with C. glabrata and C. tropicalis on RPMI and Casitone agars (Fig. 2). These often represent subpopulations which on retesting can give higher MICs. Thus, the presence of macrocolonies, as shown in Fig. 2, should be considered indicative of a resistant subpopulation. It is notable that the presence of growth within the inhibition ellipse was almost completely eliminated with Casitone agar for a significant number of isolates (Fig. 1).
FIG. 1.
Fluconazole (FL) Etest reading patterns for C. albicans. (A) Growth of microcolonies inside the entire inhibition zone (ellipse); MIC, 0.38 μg/ml. (B) Clear ellipse on Casitone agar; MIC, 0.5 μg/ml. The numbers on the scale correspond to the fluconazole concentrations on the strip (in micrograms per milliliter).
FIG. 2.
Fluconazole (FL) Etest reading patterns for C. glabrata. A resistant subpopulation appears as macrocolonies within the ellipse on Casitone agar. MIC, >256 μg/ml. The numbers on the scale correspond to fluconazole concentrations on the strip (in micrograms per milliliter).
The use of RPG or Casitone agar also allows for earlier (24 h) reading of fluconazole Etest MICs. As noted by others (3, 4, 16), occasional isolates of Candida spp. and all Cryptococcus neoformans isolates require 48 h of incubation for optimal growth. We encountered seven isolates of Candida spp. that required 48 h of incubation on RPG or Casitone agar before MICs could be read. The ability to determine MIC results within 24 h is potentially advantageous for an early clinical application of antifungal susceptibility testing results.
We included Mueller-Hinton agar in this comparison in an effort to evaluate the applicability of an agar medium that is widely available and used in clinical laboratories for agar-based susceptibility testing of bacterial isolates. Unfortunately, the performance of this medium for fluconazole susceptibility testing by Etest was suboptimal. In general, MICs obtained by Etest on Mueller-Hinton agar were considerably lower than those obtained by either the reference broth dilution or the Etest with RPG or Casitone agar. The likely explanation for the poor agreement with MICs obtained with Mueller-Hinton agar is the relatively poor growth of most isolates on this medium.
In summary, we have provided further documentation of the ability of the Etest to generate fluconazole MIC data that are comparable to those obtained by the NCCLS broth microdilution method. The use of more-enriched media, such as Casitone agar and RPG, provides optimal growth for a broad range of yeast species and improves the ease of fluconazole MIC endpoint determination by the Etest. Both RPG and Casitone agar performed equally well in this study, with Casitone agar providing clearer MIC endpoints. We recommend the use of either of these media, but not Mueller-Hinton agar, for performing fluconazole susceptibility testing by the Etest.
ACKNOWLEDGMENTS
The excellent secretarial support of K. Meyer is greatly appreciated.
This study was supported in part by Pfizer Pharmaceuticals, Roerig Division, and by AB BIODISK.
REFERENCES
- 1.Barry A L, Brown S D. Fluconazole disk diffusion procedure for determining susceptibility of Candida species. J Clin Microbiol. 1996;34:2154–2157. doi: 10.1128/jcm.34.9.2154-2157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S C A, O’Donnell M L, Gordon S, Gilbert G L. Antifungal susceptibility testing using the Etest: comparison with the broth macrodilution technique. J Antimicrob Chemother. 1996;37:265–273. doi: 10.1093/jac/37.2.265. [DOI] [PubMed] [Google Scholar]
- 3.Colombo A L, Barchiesi F, McGough D A, Rinaldi M G. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for azole antifungal susceptibility testing. J Clin Microbiol. 1995;33:535–540. doi: 10.1128/jcm.33.3.535-540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo A L, Barchiesi F, McGough D A, Fothergill A W, Rinaldi M G. Evaluation of the Etest system versus a microtitre broth method for antifungal susceptibility testing of yeasts against fluconazole and itraconazole. J Antimicrob Chemother. 1995;36:93–100. doi: 10.1093/jac/36.1.93. [DOI] [PubMed] [Google Scholar]
- 5.Cormican M G, Pfaller M A. Standardization of antifungal susceptibility testing. J Antimicrob Chemother. 1996;38:561–578. doi: 10.1093/jac/38.4.561. [DOI] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff A, Pfaller M, Erwin M E, Jones R N. Interlaboratory evaluation of Etest method for testing antifungal susceptibilities of pathogenic yeasts to five antifungal agents by using Casitone agar and solidified RPMI 1640 medium with 2% glucose. J Clin Microbiol. 1996;34:848–852. doi: 10.1128/jcm.34.4.848-852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 8.Pfaller M A, Barry A L. Evaluation of a novel colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1994;32:1992–1996. doi: 10.1128/jcm.32.8.1992-1996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaller M A, Grant C, Morthland V, Rhine-Chalberg J. Comparative evaluation of alternative methods for broth dilution susceptibility testing of fluconazole against Candida albicans. J Clin Microbiol. 1994;32:506–509. doi: 10.1128/jcm.32.2.506-509.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaller M A, Vu Q, Lancaster M, Espinel-Ingroff A, Fothergill A, Grant C, McGinnis M R, Pasarell L, Rinaldi M G, Steele-Moore L. Multisite reproducibility of colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1994;32:1625–1628. doi: 10.1128/jcm.32.7.1625-1628.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaller M A, Bale M, Buschelman B, Lancaster M, Espinel-Ingroff A, Rex J H, Rinaldi M G, Cooper C R, McGinnis M R. Quality control guidelines for National Committee for Clinical Laboratory Standards recommended broth macrodilution testing of amphotericin B, fluconazole, and flucytosine. J Clin Microbiol. 1995;33:1104–1107. doi: 10.1128/jcm.33.5.1104-1107.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller M A, Messer S A, Bolmström A, Odds F C, Rex J H. Multisite reproducibility of the Etest MIC method for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1996;34:1691–1693. doi: 10.1128/jcm.34.7.1691-1693.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller M A, Rex J H, Rinaldi M G. Antifungal susceptibility testing: technical advances and potential clinical applications. Clin Infect Dis. 1997;24:776–784. doi: 10.1093/clinids/24.5.776. [DOI] [PubMed] [Google Scholar]
- 14.Rex J H, Pfaller M A, Rinaldi M G, Polak A, Galgiani J N. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 16.Sewell D L, Pfaller M A, Barry A L. Comparison of broth macrodilution, broth microdilution, and E Test antifungal susceptibility tests for fluconazole. J Clin Microbiol. 1994;32:2099–2102. doi: 10.1128/jcm.32.9.2099-2102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simor A E, Goswell G, Louie L, Lee M, Louie M. Antifungal susceptibility testing of yeast isolates from blood cultures by microbroth dilution and the Etest. Eur J Clin Microbiol Infect Dis. 1997;16:693–697. doi: 10.1007/BF01708563. [DOI] [PubMed] [Google Scholar]
- 18.Tiballi R N, He X, Zarins L T, Revankar S G, Kauffman C A. Use of a colorimetric system for yeast susceptibility testing. J Clin Microbiol. 1995;33:915–917. doi: 10.1128/jcm.33.4.915-917.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wanger A, Mills K, Nelson P W, Rex J H. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob Agents Chemother. 1995;39:2520–2522. doi: 10.1128/aac.39.11.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warren N G, Hazen K C. Candida, Cryptococcus, and other yeasts of medical importance. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 723–737. [Google Scholar]