Abstract
Aims
Identifying patients with cardiac sarcoidosis (CS) who are at an increased risk of sudden cardiac death (SCD) poses a clinical challenge. We sought to identify the optimal cutoff for left ventricular ejection fraction (LVEF) in predicting ventricular arrhythmia (VA) and all-cause mortality and to identify clinical and imaging risk factors in patients with known CS.
Methods and results
This retrospective cohort included 273 patients with well-established CS. The primary endpoint was a composite of VA and all-cause mortality. A modified receiver operating curve analysis was utilized to identify the optimal cutoff for LVEF in predicting the primary composite endpoint. Cox proportional hazard regression analysis was used to identify independent risk factors of the outcomes. At median follow-up of 7.9 years, the rate of the primary endpoint was 38% (83 VAs and 32 all-cause deaths). The 5-year overall survival rate was 97%. The optimal cutoff LVEF for the primary composite endpoint was 42% in the entire cohort and in subjects without a history of VA. Younger age, history of VA, lower LVEF, and any presence of scar by cardiac magnetic resonance (CMR) imaging and/or positron emission tomography (PET) were found to be independent risk factors for the primary endpoint and for VA, whereas lower LVEF, baseline NT-proBNP, and any presence of scar were independent risk factor of all-cause mortality.
Conclusion
Among patients with CS, a mild reduction in LVEF of 42% was identified as the optimal cutoff for predicting VA and all-cause mortality. Prior VA and scar by CMR or PET are strong risk factors for future VA and all-cause mortality.
Keywords: Cardiac sarcoidosis, ventricular arrhythmia, ICD shock, multimodality imaging, outcomes
Graphical Abstract
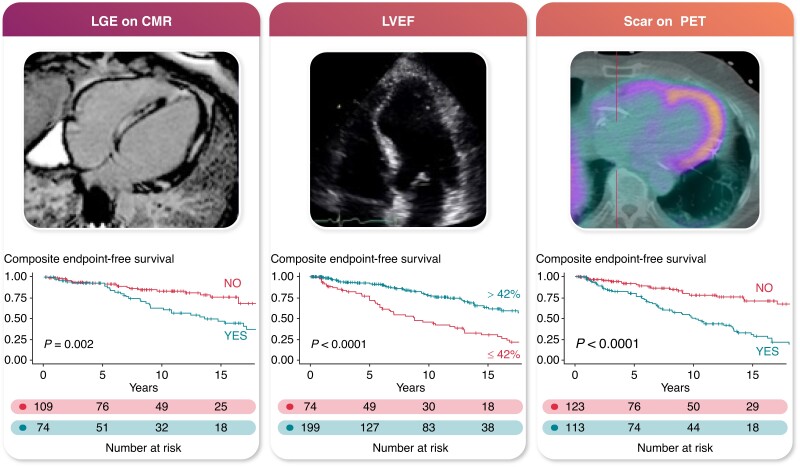
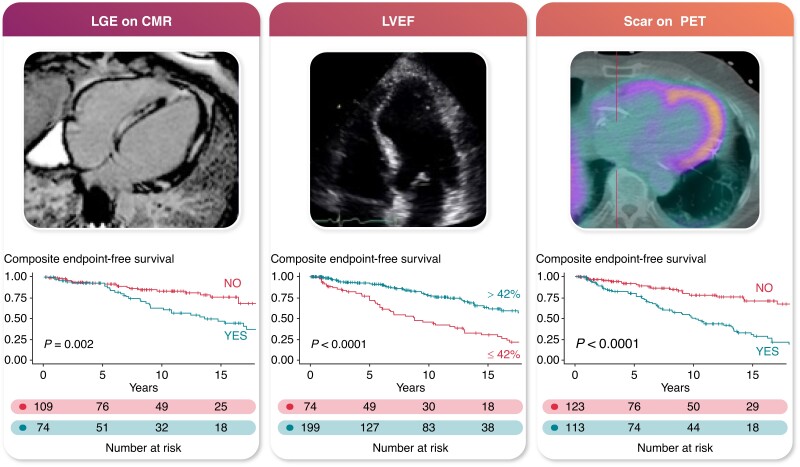
Graphical abstract.
Multimodality imaging predictors of VA and all-cause mortality in cardiac sarcoidosis.
What’s new?
In a large cohort of patients with cardiac sarcoidosis (CS), the optimal cutoff for left ventricular ejection fraction (LVEF) in predicting a composite endpoint of ventricular arrhythmia (VA) and all-cause mortality was identified to be ≤42%, even in patients without prior VA.
Younger age, history of VA, lower LVEF by echocardiogram, and any presence of scar by cardiac magnetic resonance (CMR) and/or positron emission tomography (PET) were shown to be independent risk factors for the primary endpoint and the secondary endpoint of VA.
Ventricular arrhythmia events in this cohort of patients with known CS were common, with a 5-year event rate of 11%. The appropriate implantable cardioverter–defibrillator (ICD) shock event rate at 5 years was 7.9%, highlighting the importance of ICD therapy in this patient population.
Introduction
Sarcoidosis is a rare multisystem granulomatous disease, characterized by the accumulation of T-lymphocytes and mononuclear cells resulting in the formation of noncaseating granulomas.1,2 Clinically manifesting cardiac sarcoidosis (CS) has been described in <10% of patients with sarcoidosis3,4 but autopsies of patients with sarcoidosis, histopathology of explanted hearts, and multimodality cardiac imaging studies have reported a higher prevalence, close to 30%.1,5–8 Recently, the prevalence of CS has been noted to be increasing, likely owing to the increased awareness of diagnosis, advances in cardiac imaging, and possibly changing environmental factors.5
Cardiac sarcoidosis can present with left ventricular dysfunction with or without clinical heart failure, ventricular arrhythmia (VA), conduction abnormalities, and sudden cardiac death (SCD) representing the second leading cause of sarcoidosis-related mortality, following pulmonary sarcoidosis.9 Despite the increased awareness for this disease, the diagnosis of CS and identifying patients who are at increased risk of SCD pose a clinical challenge.8 Recently, expert guidelines have incorporated recommendations for placement of implantable cardioverter–defibrillator (ICD) in patients with CS.10,11 However, these recommendations have been based on small observational studies of known or suspected CS and the left ventricular ejection fraction (LVEF) cutoff of 35% has been extrapolated from ischemic and mixed ischemic and nonischemic population data.12–16
The aim of this study was to (i) characterize a cohort of patients with known CS, (ii) identify the optimal cutoff for LVEF in predicting composite VA and all-cause mortality, and (iii) identify risk factors of VA and all-cause mortality.
Methods
Study population
Patients with CS seen at our tertiary care center between April 2001 and February 2021 were included in a retrospectively collected registry. Subjects were identified through the cardiac magnetic resonance imaging (CMR) and positron emission tomography (PET) databases. Electronic medical records were used to retrieve clinical, laboratory, and imaging data and treatment strategies and outcomes. This study was approved by the institutional review board and ethics committee at our center (IRB number: 19-1136). Patient consent was waived due to minimal risk criteria.
Included patients were adults, ≥18 years of age, with an established diagnosis of CS by the Heart Rhythm Society (HRS) criteria10 who had histology-proven tissue (cardiac or extracardiac) compatible with a diagnosis of sarcoidosis. Patients with imaging and clinical criteria suggestive of sarcoidosis but without histological evidence of the disease were excluded.
Imaging
Transthoracic echocardiogram was performed using Vivid7 or Vivid9 (GE Medical, Milwaukee), or EPIQ (Philips Medical Systems, N.A., Bothell, WA) ultrasound systems. Conventional quantitative parameters for left atrial volumes, LVEF, right ventricular systolic pressure, and diastolic function were measured according to valid guidelines at the time of each study. The preferred method for LVEF determination was the biplane disk summation method. The initial (first) echocardiogram was used for analysis when more than one study was present.
Cardiac magnetic resonance (CMR) imaging was performed using Phillips Achieva 1.5 Tesla or Phillips Ingenia 3.0 Tesla (Philips Medical Systems, Best, The Netherlands) scanners. CVI-42 software (Circle Cardiovascular Imaging, Calgary, Alberta, Canada) was used for CMR analysis of studies performed within our institution and after the year 2015. For studies performed before 2015, we used Phillips proprietary software. The presence or absence of late gadolinium enhancement (LGE) of the myocardium was recorded. When multiple CMR studies were done in one subject, all were reviewed, and if any study documented LGE, this was collected as present.
Cardiac and whole-body PET with computed tomography (CT) was acquired using resting myocardial perfusion with Rb-82 and metabolic imaging with F18-fluorodeoxyglucose (FDG) following our standard sarcoidosis protocol.17,18 PET imaging was performed on Siemens Biograph mCT PET/CT scanners (Siemens Healthcare, Erlanger, Germany). Images were interpreted after attenuation correction using 4DM-SPECT software (4DM, INVIA, Medical Imaging Solutions, Ann Arbor, Michigan) and Syngo Via software (Siemens Healthineers, Erlanger, Germany). Prior to PET imaging, a dietary protocol consisting of 24 h high fat, no carbohydrate, and no sugar followed by >12 h fast, was implemented. When multiple PET studies were done in one subject, all were reviewed and the study with the higher degree of FDG uptake was used to collect data on the presence of abnormal FDG metabolism, number of abnormal segments, presence of resting perfusion defect, and presence of a mismatch pattern (defined as area of abnormal perfusion with F18-FDG uptake). Perfusion defect not explained by artifact was taken to represent myocardial scar. The Corridor4DM automatic algorithm19 with manual reorientation of F18-FDG PET and Rb-82 PET tomograms was used to collect standardized uptake values (SUV) by two physicians to quantify the percentage of FDG uptake and scar (perfusion defect) in all studies performed after 2018. Interobserver and intraobserver variability was evaluated by remeasuring 10 random studies twice and calculating the intraclass correlation coefficient.
Outcomes
The prespecified primary endpoint of interest was a composite of VA (sustained) and all-cause mortality. All-cause mortality rather than cardiovascular mortality was used to maintain consistency with prior ICD outcome studies. Secondary endpoints were individual rates of VA and all-cause mortality. VA events were collected from electronic medical records by reviewing ICD interrogation reports and clinician notes. Sustained VA was defined as ventricular tachycardia or fibrillation lasting more than 30 s or appropriate ICD therapy. All-cause mortality and death dates were obtained from electronic medical records. Heart transplantation was censored as a mortality event.
Follow-up was defined from the time of CS diagnosis to the time of event or last follow-up.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation or median and interquartile ranges for skewed distributions. Categorical variables are expressed as absolute number and frequency. A modified receiver operating curve analysis was utilized to identify the optimal cutoff for LVEF by echocardiogram in predicting the primary composite endpoint. A sensitivity analysis for the cutoff determination using a cohort of subjects without history of VA (primary prevention cohort) was also performed. The method for cutoff determination was evaluated using bootstrapping with 1000 iterations. Univariate Cox proportional hazards regression models were constructed to evaluate potential risk factors for the primary and secondary endpoints. Following this, we constructed multivariate Cox proportional hazards models for the primary and secondary endpoints, using those variables with a P-value of <0.05 in the univariate analysis. Variance inflation factors were calculated for each model to evaluate for multicollinearity. For the primary endpoint, we first created a multivariate model for each imaging modality that included all significant variables and then created a final multimodality imaging model using significant variables but removing repeated variables (i.e. LVEF by PET, echocardiogram, or CMR) to avoid multicollinearity. In the case of repeated multimodality imaging variables, we used the one with the least amount of missing data in the final model. In addition, we created a competitive risk model using the Fine-Gray method for our final model to measure the association of the predictors with VA. Survival curves were plotted using the Kaplan–Meier method.
A two-sided P < 0.05 was considered statistically significant. We assumed missing data completely at random and thus performed a complete case analysis. Statistical analyses were performed using R studio, version 4.3.1. (The R foundation, https://www.r-project.org/).
Results
A total of 2358 patients with systemic sarcoidosis or suspected CS who underwent CMR or PET with a sarcoidosis protocol between April 2001 and February 2021 were identified. Of these, 1914 were excluded due to nondefinitive imaging evidence of cardiac involvement. A total of 444 subjects with abnormal imaging findings were reviewed to identify those with histology-proven sarcoidosis. Of these, 273 patients had histology-proven sarcoidosis and were included in the final cohort.
Clinical and imaging characteristics
Baseline clinical characteristics are shown in Table 1. The mean age was 59 ± 11 years, and 40% were female. Isolated CS (ICS), defined as biopsy-proven CS without extracardiac involvement, was present in 13 subjects (5%). The prevalence of atrial fibrillation (AF) was 41%, and the mean CHADS2VASc was 2.3.
Table 1.
Baseline characteristics of study population
| Characteristics | Overall population (N = 273) |
|---|---|
| Age (years) | 59 ± 11 |
| Female gender | 108 (40%) |
| Race | |
| White | 200 (73%) |
| Black | 64 (23%) |
| Other | 9 (3%) |
| History of smoking | 86 (32%) |
| Body mass index (kg/m2) | 31 ± 7 |
| Hypertension | 134 (49%) |
| Diabetes mellitus | 53 (19%) |
| Nonobstructive coronary artery disease | 53 (19%) |
| Congestive heart failure | 159 (58%) |
| Cerebrovascular disease | 23 (8%) |
| Peripheral artery disease | 38 (14%) |
| Pulmonary sarcoidosis | 226 (82%) |
| Isolated cardiac sarcoidosis | 13 (5%) |
| Atrial fibrillation (AF) | 113 (41%) |
| Paroxysmal AF | 92 (81%) |
| Permanent AF | 21 (19%) |
| CHADS2Vasc | 2.3 ± 1.6 |
| Syncope | 74 (27%) |
| History of ventricular arrhythmia | 36 (14%) |
| Presence of ICD | 157 (58%) |
| Primary prevention ICD | 119 (76%) |
| Secondary prevention ICD | 38 (24%) |
| Presence of permanent pacemaker | 22 (8%) |
| Medications | |
| Anticoagulation | 87 (32%) |
| Antiplatelet | 41 (15%) |
| Antiarrhythmic | 69 (26%) |
| Rate control | 95 (35%) |
| Prednisone | 232 (85%) |
| Methotrexate | 176 (64%) |
| Leflunomide | 84 (31%) |
ICD, implantable cardioverter–defibrillator.
Sarcoidosis histology was obtained from mediastinal lymph node in 204 (75%) cases, followed by endomyocardial biopsy in 34 (12%), skin in 15 (6%), peripheral lymph nodes in 5 (2%), and lungs in 4 (1%). Other less common sites of tissue biopsy included the bone marrow (N = 3), orbit (N = 2), liver (N = 2), and spleen (N = 1).
Treatment strategy during the entire study period included use of prednisone in 85%, methotrexate in 64%, and leflunomide in 31%. Antiarrhythmic and anticoagulant medical therapy was utilized in 26% and 32%, respectively. A total of 157 subjects (58%) had an implantable cardiac defibrillator for primary (77%) or secondary (23%) prevention of SCD. Permanent pacemaker was present in 22 subjects (8%) for treatment of atrioventricular conduction block, and 94 subjects (34%) did not have a device.
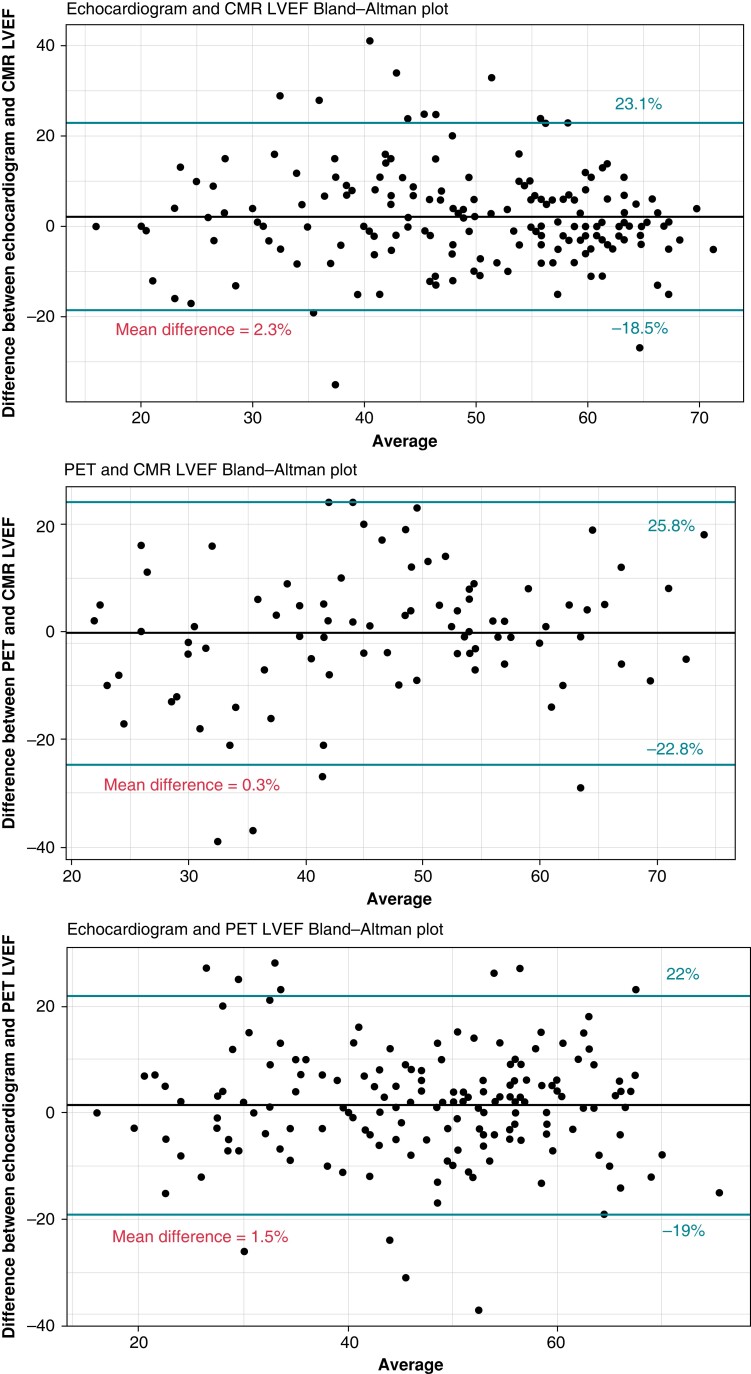
All 273 subjects underwent echocardiography evaluation at a median time from diagnosis of 26 months. PET was performed in 235 (86%) subjects at a median time from diagnosis of 40 months (IQR 2–49), and CMR was done in 185 (68%) subjects at a median time from diagnosis of 3 months (IQR 0–29). All three imaging modalities were done in 148 (54%). Median time between PET and CMR was 3 months (IQR 0–26). Imaging and laboratory findings are summarized in Table 2. The agreement for LVEF between imaging modalities was modest as shown in Figure 1. Myocardial scar was documented in 43% of subjects who underwent PET and 40% of those who underwent CMR, for a total of 55% (N = 149) subjects with any scar documentation. Abnormal metabolism by PET was present in 63% of subjects and the mean percentage of total FDG uptake was 12.4%. Agreement on percentage of FDG uptake was adequate with an intraclass correlation coefficient of 0.959 (95% CI 0.88–0.98).
Table 2.
Laboratory and imaging characteristics of study population
| Laboratory | |
| Initial creatinine (mg/dL) | 1 ± 0.7 |
| Initial glomerular filtration rate (mL/min/1.73 m2) | 71 ± 21 |
| Initial NT-proBNP (pg/mL) | 384 (IQR 137–1134) |
| Electrocardiography | |
| QRS duration by electrocardiogram (ms) | 125 (IQR 92–156) |
| Left bundle branch block | 83 (31%) |
| Right bundle branch block | 104 (38%) |
| High-degree atrioventricular block | 94 (34%) |
| Echocardiography | |
| Left ventricular ejection fraction (%) | 49.7 ± 13.4 |
| Left atrial volume index (mL/m2) | 35.5 ± 17.7 |
| Right ventricular systolic pressure (mmHg) | 31.6 ± 12.5 |
| E/e′ lateral mitral annulus | 9.1 ± 4.4 |
| E/e′ septal mitral annulus | 11.6 ± 4.8 |
| E/A wave ratio | 1.2 ± 0.7 |
| Deceleration time (ms) | 217 ± 69 |
| Interventricular wall thickness (cm) | 1.0 ± 0.2 |
| Grade of diastolic dysfunction | |
| None | 94 (34%) |
| Grade I | 85 (31%) |
| Grade II | 28 (10%) |
| Grade III | 17 (6%) |
| Indeterminate | 49 (18%) |
| Positron emission tomography (N = 235) | |
| Abnormal metabolism | 147 (63%) |
| FDG uptake extent | |
| Small (<3 segments) | 21 (25%) |
| Medium (3–5 segments) | 42 (51%) |
| Large (>5 segments) | 20 (24%) |
| Total FDG uptake by SUV (%) | 12.36 ± 24 |
| Right ventricular FDG uptake | 9 (3%) |
| Presence of resting perfusion defect | 113 (43%) |
| Total perfusion defect by SUV (%) | 4 ± 9 |
| Mismatch pattern | 72 (31%) |
| LVEF by PET (%) | 48 ± 18 |
| Cardiac magnetic resonance (N = 185) | |
| Delayed gadolinium enhancement | 74 (40%) |
| LVEF by CMR (%) | 49 ± 14 |
Values are reported as mean ± standard deviation, median and interquartile range (IQR), or absolute and percentage value.
Figure 1.
Bland–Altman plots showing agreement between imaging modalities for LVEF.
Outcomes
Median follow-up was 7.9 years (IQR 0.1–25 years). At median follow-up, the rate of the composite primary endpoint was 38% (N = 103). The rates of the secondary endpoints of VA and all-cause mortality were 30% (N = 83) and 12% (N = 32), respectively. A total of seven patients (2.6%) who underwent heart transplantation were censored. The 5-year overall survival probability was 97%. The 1-, 3-, 5-, and 10-year event rates for VA were 2%, 8%, 11%, and 26%, respectively.
From a total of 157 subjects who had an ICD, 119 (76%) were implanted for primary prevention of SCD. The indications for primary prevention ICD implantation were low LVEF in 41% (N = 49), high–degree AV block in 30% (N = 36), nonsustained VA in 23% (N = 27), unexplained syncope without inducible VA in 4% (N = 5), and high LGE burden in 2% (N = 2). Among the 157 subjects who had an ICD, 65 (41%) documented ICD shocks, of which 59 (91%) were appropriate. Antitachycardia pacing (ATP) therapy was documented in 48% (N = 76) of patients with an ICD. Among these, 13 patients (17%) had successful ATP therapy without experiencing ICD shocks, 33 patients (48%) had unsuccessful ATP therapy resulting in subsequent appropriate ICD shocks, and 30 patients (39%) had successful ATP but recurrent VA that resulted in one or more ICD shocks. All devices were transvenous and thus had ATP capabilities, and all were programed to deliver ATP prior to ICD shocks. The rates of appropriate ICD shock at 1, 3, and 5 years were 1.3%, 8.3%, and 10.7%, respectively. The rates of appropriate ICD shock in the primary prevention cohort at 1, 3, and 5 years were 0.9%, 7.4%, and 9.5%, respectively. The rates of appropriate ICD shock in the secondary prevention cohort at 1, 3, and 5 years were 2.6%, 11.1%, and 14%, respectively.
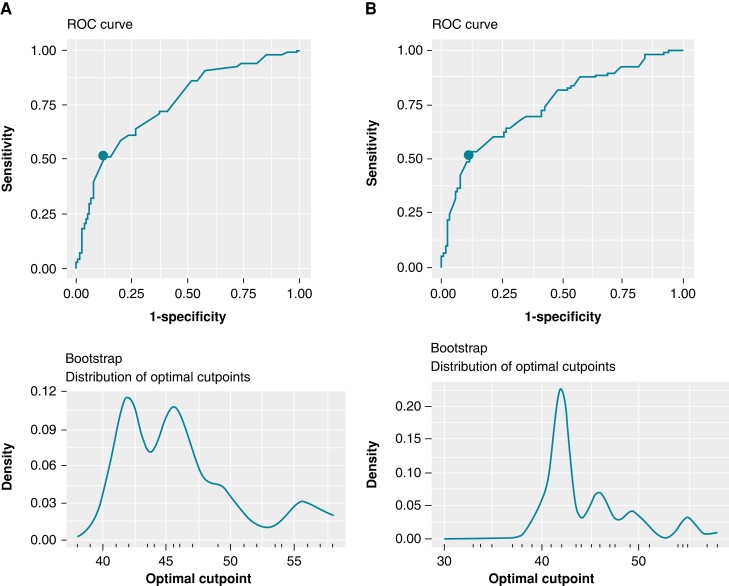
The optimal cutoff for LVEF by echocardiography in predicting the primary composite endpoint for the entire study population was identified to be ≤42% (IQR 42–60), which corresponds to an area under the receiver operating curve of 76%, sensitivity of 51%, and specificity of 88%. This reflects a 5-year survival probability of 0.78 for patients with LVEF ≤ 42% compared to 0.93 for patients with LVEF > 42%, for an absolute risk of 15% for the primary endpoint. Among subjects with LVEF ≤ 42% (N = 74), a total of 59% (N = 26) had LGE on CMR compared to 35% (N = 48) among subjects with LVEF > 42% (N = 199), (P = 0.007).
In the cohort of subjects without a history of VA (N = 236), the optimal cutoff for LVEF by echocardiography in predicting the primary composite endpoint was again identified to be ≤42% (IQR 25–60), with an area under the receiver operating curve of 75%, sensitivity of 53%, and specificity of 88% (Figure 2).
Figure 2.
Receiver operating curve analysis for the entire cohort (panel A) and selected cohort without history of VA (panel B).
We evaluated the effect of age, sex, body mass index, coronary artery disease, atrial fibrillation, history of VA, QRS interval duration by ECG, high-degree AV block, NT-proBNP at baseline, and LVEF by echocardiogram, CMR, and PET; LGE on CMR; scar, abnormal metabolism, and mismatch on PET; and any presence of scar as potential risk factors for the primary and secondary endpoints. The univariate Cox proportional hazard models for each of the outcomes are summarized in Table 3. Table 4 summarizes the multivariate Cox proportional hazard models for the primary and secondary endpoints. Younger age, history of VA, lower LVEF by echocardiogram, and any presence of scar by CMR and/or PET were shown to be independent risk factors for the primary endpoint and the secondary endpoint of VA. For the secondary endpoint of all-cause mortality, we found that lower LVEF by echocardiogram, baseline NT-proBNP, and any presence of scar were independent risk factors of all-cause mortality. The proportionality test for all models had a P > 0.05, indicating that the model’s assumptions were met. Hazard ratios for VA using competitive risk regression did not differ significantly with our final model (see Supplementary material online, Table S1).
Table 3.
Univariate cox regression analysis for the primary and secondary endpoints of interest
| Predictor | Univariate analysis | |
|---|---|---|
| HR (95% CI) | P-value | |
| Primary composite endpoint | ||
| Age | 0.97 (0.96–0.99) | 0.003 |
| Male gender | 1.6 (1–2.4) | 0.03 |
| Body mass index | 0.98 (0.95–1) | 0.2 |
| Coronary artery disease | 0.7 (0.43–1.1) | 0.2 |
| Atrial fibrillation | 1.1 (0.75–1.6) | 0.6 |
| History of VA | 3.3 (2.2–5) | <0.001 |
| QRS duration | 1 (1–1) | 0.6 |
| High-degree AV block | 1.1 (0.8–1.6) | 0.7 |
| Baseline NT-proBNP | 1 (1–1) | 0.5 |
| LVEF by echocardiogram | 0.97 (0.95–0.98) | <0.001 |
| LGE on CMR | 2.2 (1.3–3.6) | 0.003 |
| LVEF by CMR | 0.98 (0.97–1) | 0.08 |
| Abnormal metabolism by PET | 1 (0.68–1.6) | 0.9 |
| Total FDG uptake (%) | 1 (0.99–1) | 0.7 |
| Scar in PET | 3 (1.9–4.6) | <0.001 |
| Total scar (%) | 1 (0.97–1) | 0.9 |
| Mismatch in PET | 1.7 (1.1–2.6) | 0.01 |
| LVEF by PET | 0.98 (0.97–1) | 0.05 |
| Any scar | 2.4 (1.6–3.7) | <0.001 |
| Secondary endpoint of VA | ||
| Age | 0.97 (0.49–0.96) | 0.003 |
| Male gender | 1.5 (0.94–2.4) | 0.09 |
| Body mass index | 0.98 (0.95–1) | 0.3 |
| Coronary artery disease | 0.72 (0.72–1.2) | 0.2 |
| Atrial fibrillation | 1.2 (0.78–1.9) | 0.4 |
| History of VA | 4.6 (3–7.2) | <0.001 |
| QRS duration | 1 (1–1) | 0.2 |
| High-degree AV block | 1.3 (0.82–2) | 0.3 |
| Baseline NT-proBNP | 1 (1–1) | 0.86 |
| LVEF by echocardiogram | 0.96 (0.95–0.98) | <0.001 |
| LGE on CMR | 2.4 (1.3–4.3) | 0.004 |
| LVEF by CMR | 0.99 (0.97–1) | 0.2 |
| Abnormal metabolism by PET | 1 (0.65–1.6) | 0.9 |
| Total FDG uptake (%) | 1 (0.99–1) | 0.7 |
| Scar in PET | 3.6 (2.2–5.9) | <0.001 |
| Total scar (%) | 1 (0.97–1) | 0.9 |
| LVEF by PET | 0.99 (0.97–1) | 0.08 |
| Mismatch in PET | 1.8 (1.2–2.8) | 0.008 |
| Any scar | 2.9 (1.8–4.8) | <0.001 |
| Secondary endpoint of all-cause mortality | ||
| Age | 0.9 (0.96–1) | 0.8 |
| Male gender | 1.9 (0.86–4.1) | 0.1 |
| Body mass index | 0.94 (0.89–1) | 0.05 |
| Coronary artery disease | 1 (0.46–2.2) | 1 |
| Atrial fibrillation | 1.2 (0.62–2.5) | 0.5 |
| History of VA | 1.1 (0.43–2.9) | 0.8 |
| QRS duration | 1 (1–1) | 0.1 |
| High-degree AV block | 0.5 (0.21–1.2) | 0.1 |
| Baseline NT-proBNP | 1 (1–1) | 0.009 |
| LVEF by echocardiogram | 0.96 (0.93–0.98) | <0.001 |
| LGE on CMR | 3.3 (1.4–7.6) | 0.006 |
| LVEF by CMR | 0.96 (0.95–1) | 0.06 |
| Abnormal metabolism by PET | 1.1 (0.49–2.5) | 0.8 |
| Total FDG uptake (%) | 1 (0.99–1) | 0.8 |
| Scar in PET | 2.8 (1.2–6.6) | 0.01 |
| Total scar (%) | 0.97 (0.88–1.1) | 0.5 |
| Mismatch in PET | 1.8 (0.82–4) | 0.2 |
| LVEF by PET | 0.95 (0.92–0.99) | 0.01 |
| Any scar | 2.7 (1.2–6) | 0.02 |
CMR, cardiac magnetic resonance; LGE, delayed gadolinium enhancement; LVEF, left ventricular ejection fraction; PET, positron emission tomography.
Table 4.
Results of multivariate cox regression models for the primary and secondary endpoints
| Model | Independent variable | HR (95% CI) | P-value |
|---|---|---|---|
| Echocardiogram model (N = 273, events = 103) | Age | 0.97 (0.95–0.99) | 0.007 |
| Male gender | 1.3 (0.8–2.0) | 0.3 | |
| History of VA | 2.9 (1.9–4.4) | <0.001 | |
| LVEF by echocardiogram | 0.97 (0.95–0.98) | <0.001 | |
| CMR model (N = 183, events = 63) | Age | 0.96 (0.94–0.98) | 0.002 |
| Male gender | 1.5 (0.8–2.6) | 0.2 | |
| History of VA | 3.2 (1.9–5.6) | <0.001 | |
| LVEF by CMR | 1 (0.97–1.0) | 0.5 | |
| LGE on CMR | 2.3 (1.4–3.9) | 0.001 | |
| PET model (N = 234, events = 95) | Age | 0.97 (0.96–0.99) | 0.005 |
| Male gender | 1.2 (0.7–1.9) | 0.5 | |
| History of VA | 2.6 (1.7–4) | <0.001 | |
| Scar in PET | 3.2 (1.8–5.8) | <0.001 | |
| Mismatch in PET | 0.6 (0.4–1) | 0.06 | |
| Final model primary endpoint (N = 234, events = 95) | Age | 0.98 (0.96–99) | 0.03 |
| Male gender | 1.3 (0.8–2.1) | 0.3 | |
| History of VA | 2.6 (1.7–4.1) | <0.001 | |
| LVEF by echocardiogram | 0.97 (0.95–0.98) | <0.001 | |
| Any scar | 2.0 (1.1–3.4) | 0.02 | |
| Mismatch in PET | 0.8 (0.5–1.3) | 0.3 | |
| Final model VA (N = 234, events = 82) | Age | 0.97 (0.95–0.99) | 0.004 |
| History of VA | 3.3 (2.0–5.2) | <0.001 | |
| LVEF by echocardiogram | 0.97 (0.96–0.98) | <0.001 | |
| Any scar | 2.1 (1.1–3.8) | 0.02 | |
| Mismatch in PET | 0.8 (0.4–1.3) | 0.3 | |
| Final model all-cause mortality (N = 176, events = 26) | Baseline NT-proBNP | 1 (1–1) | 0.008 |
| LVEF by echocardiogram | 0.95 (0.92–0.97) | 0.03 | |
| Any scar | 2.3 (0.88–5.81) | 0.03 |
The Kaplan–Meier curves for the composite endpoint-free survival stratified by imaging modality risk factors are shown in Figure 3.
Figure 3.
Multimodality imaging predictors of VA and all-cause mortality in cardiac sarcoidosis.
Discussion
To our knowledge, this is the first study to determine the optimal LVEF cutoff for risk stratification in patients with definite diagnosis of CS. We found that (i) the optimal LVEF by echocardiography for predicting a composite of VA and all-cause mortality was 42% in the entire cohort and in patients without history of VA (primary prevention cohort); (ii) at median follow-up of 7.9 years, the rate of the composite endpoint of VA and all-cause mortality was 38%; and (iii) lower age, history of VA, lower LVEF by echocardiogram, and any presence of scar by CMR and/or PET are independent risk factors for the composite endpoint and for VA.
The strength of this study is the high certainty that patients in the cohort had a diagnosis of CS given the exclusion of probable or possible disease. Thus, it provides characterization of a patient population with a rare disease for which there are many unanswered questions due to the lack of large-sized studies.
Our LVEF cutoff determination suggests that moderate to severe left ventricular systolic dysfunction is not required for adverse outcomes to occur in CS. Current guidelines utilize an LVEF cutoff of 35% as a class I indication for ICD implantation in primary prevention of SCD in patients with CS and recommend ICD for primary prevention with LVEF above 35% in the presence of other risk factors.10,11 Our study findings are concordant with current guideline recommendations in that prevention of clinically relevant events should focus on identification of risk enhancers such as complete heart block,20 presence of LGE on CMR,21 and abnormal perfusion and metabolism on PET22 but suggest reconsideration of the LVEF cutoff to 42%. In fact, the distribution of optimal LVEF cut points shown in Figure 2A is bimodal peaking at 42% and at 46% in the entire cohort but unimodal at 42% in subjects without prior VA. This difference may be explained by risk enhancers such as LGE in patients with prior VA. A recent study by Nordenswan et al.23 reported a large cohort of patients with clinically manifesting CS and found that fatal and nonfatal arrhythmic events occurred at a comparable rate between patients with class IIa indication for an ICD and those without an indication for an ICD. This group has also suggested a lower threshold for ICD implantation in subjects with clinically manifesting CS and call for a revision of current societal guidelines. Moreover, the ILLUMINATE-CS registry24 which included 512 patients with CS by HRS or Japanese Circulation Society criteria found that patients with LVEF of 41%–49% had a high incidence of fatal VA events to a similar degree compared to patients with LVEF ≤ 40%. Others have described that the incidence rate of VA in CS is high, up to 15% per year, and thus, this patient population needs better risk stratifying tools to prevent SCD.25 Furthermore, mortality following ICD implantation has been shown to be similar compared to propensity-matched NICM patients highlighting the importance of ICD therapy in the prevention of SCD in this population.26 Recently, the European Society of Cardiology published 10 novel key aspects for the management of VA and prevention of SCD underlining the importance of individualized SCD risk stratification with the use of risk calculators, emphasizing the value of CMR and the identification of risk enhancers beyond LVEF.27 Our final multimodality model confirms the strong predictive capacity of LGE on CMR that has been shown in previous data28,29 and also demonstrates that the presence of any myocardial scar, by either perfusion defect on PET or LGE on CMR, and lower LVEF by echocardiogram are independent predictors of VA and all-cause mortality. While we identified an optimal LVEF cutoff by echocardiogram in predicting composite VA and all-cause mortality, we acknowledge that LVEF alone should not guide ICD implantation but prompt further investigation with advanced cardiac imaging and monitoring to identify risk enhancers and facilitate shared decision making for ICD implantation. In this regard, our model may serve as a preliminary guide to calculate individual risk of VA and mortality.
We found that the mismatch pattern on PET was associated with adverse events in univariate analysis but this did not remain true after adjusting for the history of VA and presence of myocardial scar. The landmark study evaluating the prognostic role of PET in CS included 118 subjects with suspected CS (as initial diagnostic tool) and found that a mismatch pattern and RV uptake of FDG were independent predictors of death or VA on multivariable modeling.22 More recent cohorts have shown discrepant findings regarding the prognostic ability of FDG uptake in PET or Gallium scintigraphy alone when LGE on CMR is accounted for.24 Our findings may be explained by a larger proportion of PET studies used as a tool to tailor therapy (in established CS) rather than as a diagnostic tool. Hence, therapeutic adjustments of pharmacotherapy prompted by abnormal metabolism or mismatch pattern on PET may have positively impacted outcomes and explain the lack of negative predictability we observed. Our findings call for larger prospective studies to evaluate the utility of PET using modern dietary protocol and uniform interpretation with quantification of the degree and extent of FDG uptake in the prognosis and follow-up of patients with CS.
Lastly, while long-term mortality in our study was favorable, with a 5-year overall survival of 97%, VA events were common with a 5-year event rate of 11%. Thus, the high survival rate could be explained by a high percentage of patients with an ICD and a high rate of appropriate ICD therapy.
Limitations
Our study is limited by the single-center and the retrospective nature of its design. Due to our strict inclusion criteria of CS with tissue evidence of sarcoidosis, our results are not generalizable to patients with possible or probable CS. Given the retrospective nature of the study and the fact that clinical events may be distant to abnormal imaging findings, it is possible that unmeasured confounders are explaining some of our results.
A major limitation of our study is that PET and CMR were not done in the entire cohort, and in subjects that had both imaging modalities, these were done at different mean times from diagnosis. This likely explains why our CMR and PET models had lower event rates and may have been underpowered to show significant prognostic value (i.e. lack of prognostic value of LVEF by CMR and PET). In addition, patients that underwent CMR likely differ from those that did not based on the presence or absence of an ICD; thus, it is possible that selection bias may explain some of our findings. Because our study comprises patients treated over 20 years, there could be disparities in imaging quality and interpretation in earlier studies compared to more contemporary studies. Furthermore, because we did not perform core lab analysis of imaging data (aside for SUV measurement on PET) and rather collected imaging reports, interpretation and interobserver variability may introduce bias to our findings. Core lab LGE quantification was not possible due to unavailable images from earlier studies and outside institutions. However, we believe this reflects real-world practice and highlights the variability in management of patients with CS.
While a higher percentage of patients with LVEF ≤ 42% had LGE on CMR, our multimodality model demonstrates that LVEF by echocardiography and LGE on CMR are independent predictors of poor outcomes and LGE on CMR does not entirely explain our cutoff determination value. Our final model confirms what prior cohorts have shown with regard to the prognostic role of age, prior VA, LGE, and LVEF in patients with CS and adds that perfusion defect by PET has independent prognostic value. Finally, external validation of our model for its use as a risk calculator is necessary for generalized use. We hope our study stimulates the development of randomized controlled trials and prospective studies to validate our findings.
Conclusion
In this large cohort of subjects with CS who were managed longitudinally at a tertiary care center, we found that a mild reduction in LVEF was associated with VA and all-cause mortality, with an optimal LVEF cutoff of 42%. History of VA and presence of scar by either CMR or PET were associated with the strongest risk for future VA and all-cause mortality.
Supplementary Material
Contributor Information
Erika Hutt, Section of Cardiovascular Imaging, Heart, Vascular and Thoracic Institute, Cleveland Clinic, OH 44195, USA.
Maria Vega Brizneda, Department of Internal Medicine, Medicine Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
Ghazaleh Goldar, Department of Internal Medicine, Medicine Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
Jose Aguilera, Section of Cardiac Electrophysiology and Pacing, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
Tom Kai Ming Wang, Section of Cardiovascular Imaging, Heart, Vascular and Thoracic Institute, Cleveland Clinic, OH 44195, USA.
Ziad Taimeh, Section of Heart Failure and Transplantation Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
Daniel Culver, Sarcoidosis Center, Respiratory Institute Cleveland Clinic, Cleveland, OH 44195, USA.
Thomas Callahan, Section of Cardiac Electrophysiology and Pacing, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
W H Wilson Tang, Section of Heart Failure and Transplantation Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
Paul C Cremer, Section of Cardiovascular Imaging, Heart, Vascular and Thoracic Institute, Cleveland Clinic, OH 44195, USA.
Wael A Jaber, Section of Cardiovascular Imaging, Heart, Vascular and Thoracic Institute, Cleveland Clinic, OH 44195, USA.
Manuel L Ribeiro Neto, Sarcoidosis Center, Respiratory Institute Cleveland Clinic, Cleveland, OH 44195, USA.
Christine L Jellis, Section of Cardiovascular Imaging, Heart, Vascular and Thoracic Institute, Cleveland Clinic, OH 44195, USA.
Supplementary material
Supplementary material is available at Europace online.
Funding
No extramural funding was used to support this work.
Data availability
The dataset analyzed to support the findings of this study is available upon reasonable request from the corresponding author (E.H.).
References
- 1. Ribeiro Neto ML, Jellis CL, Joyce E, Callahan TD, Hachamovitch R, Culver DA. Update in ardiac sarcoidosis. Ann Am Thorac Soc 2019;16:1341–50. [DOI] [PubMed] [Google Scholar]
- 2. Houston BA, Mukherjee M. Cardiac sarcoidosis: clinical manifestations, imaging characteristics, and therapeutic approach. Clin Med Insights Cardiol 2014;8:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lyle MA, Cooper LT Jr. Cardiovascular outcomes in sarcoidosis. J Am Coll Cardiol 2020;76:778–80. [DOI] [PubMed] [Google Scholar]
- 4. Hulten E, Aslam S, Osborne M, Abbasi S, Bittencourt MS, Blankstein R. Cardiac sarcoidosis-state of the art review. Cardiovasc Diagn Ther 2016;6:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo Ket al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation 2015;131:624–32. [DOI] [PubMed] [Google Scholar]
- 6. Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978;58:1204–11. [DOI] [PubMed] [Google Scholar]
- 7. Fussner LA, Karlstedt E, Hodge DO, Fine NM, Kalra S, Carmona EMet al. Management and outcomes of cardiac sarcoidosis: a 20-year experience in two tertiary care centres. Eur J Heart Fail 2018;20:1713–20. [DOI] [PubMed] [Google Scholar]
- 8. Gilotra NA, Griffin JM, Pavlovic N, Houston BA, Chasler J, Goetz Cet al. Sarcoidosis-related cardiomyopathy: current knowledge, challenges, and future perspectives state-of-the-art review. J Cardiac Fail 2022;28:113–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kazmirczak F, Chen K-HA, Adabag S, von Wald L, Roukoz H, Benditt DGet al. Assessment of the 2017 AHA/ACC/HRS guideline recommendations for implantable cardioverter-defibrillator implantation in cardiac sarcoidosis. Circ Arrhythmia Electrophysiol 2019;12:e007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CSet al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014;11:1305–23. [DOI] [PubMed] [Google Scholar]
- 11. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis ABet al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation 2018;138:e272–391. [DOI] [PubMed] [Google Scholar]
- 12. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DSet al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Eng J Med 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 13. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau Ret al. Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. N Eng J Med 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 14. Poole JE, Olshansky B, Mark DB, Anderson J, Johnson G, Hellkamp ASet al. Long-term outcomes of implantable cardioverter-defibrillator therapy in the SCD-HeFT. J Am Coll Cardiol 2020;76:405–15. [DOI] [PubMed] [Google Scholar]
- 15. Kadish A, Dyer A, Daubert JP, Quigg R, Estes NAM, Anderson KPet al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Eng J Med 2004;350:2151–8. [DOI] [PubMed] [Google Scholar]
- 16. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup Eet al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Eng J Med 2016;375:1221–30. [DOI] [PubMed] [Google Scholar]
- 17. Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala Set al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol 2016;23:1187–226. [DOI] [PubMed] [Google Scholar]
- 18. Sperry BW, Tamarappoo BK, Oldan JD, Javed O, Culver DA, Brunken Ret al. Prognostic impact of extent, severity, and heterogeneity of abnormalities on 18F-FDG PET scans for suspected cardiac sarcoidosis. JACC Cardiovasc Imaging 2018;11:336–45. [DOI] [PubMed] [Google Scholar]
- 19. Ficaro EP, Lee BC, Kritzman JN, Corbett JR. Corridor4DM: the Michigan method for quantitative nuclear cardiology. J Nucl Cardiol 2007;14:455–65. [DOI] [PubMed] [Google Scholar]
- 20. Azoulay LD, Waintraub X, Haroche J, Amoura Z, Cohen Aubart F. Factors associated with implantable cardioverter defibrillators appropriate therapy in cardiac sarcoidosis: a meta-analysis. Sarcoidosis Vasc Diffuse Lung Dis 2020;37:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coleman GC, Shaw PW, Balfour PC Jr, Gonzalez JA, Kramer CM, Patel ARet al. Prognostic value of myocardial scarring on CMR in patients with cardiac sarcoidosis. JACC Cardiovasc Imaging 2017; 10: 411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VLet al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014;63:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nordenswan H-K, Pöyhönen P, Lehtonen J, Ekström K, Uusitalo V, Niemelä Met al. Incidence of sudden cardiac death and life-threatening arrhythmias in clinically manifest cardiac sarcoidosis with and without current indications for an implantable cardioverter defibrillator. Circulation 2022;146:964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nabeta T, Kitai T, Naruse Y, Taniguchi T, Yoshioka K, Tanaka Het al. Risk stratification of patients with cardiac sarcoidosis: the ILLUMINATE-CS registry. Eur Heart J 2022;43:3450–9. [DOI] [PubMed] [Google Scholar]
- 25. Betensky BP, Tschabrunn CM, Zado ES, Goldberg LR, Marchlinski FE, Garcia FCet al. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart Rhythm 2012;9:884–91. [DOI] [PubMed] [Google Scholar]
- 26. Higgins AY, Annapureddy AR, Wang Y, Minges KE, Bellumkonda L, Lampert Ret al. Risk and predictors of mortality after implantable cardioverter-defibrillator implantation in patients with sarcoid cardiomyopathy. Am Heart J 2022;246:21–31. [DOI] [PubMed] [Google Scholar]
- 27. Könemann H, Dagres N, Merino JL, Sticherling C, Zeppenfeld K, Tfelt-Hansen Jet al. Spotlight on the 2022 ESC guideline management of ventricular arrhythmias and prevention of sudden cardiac death: 10 novel key aspects. Europace 2023;25:euad091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kouranos V, Tzelepis GE, Rapti A, Mavrogeni S, Aggeli K, Douskou Met al. Complementary role of CMR to conventional screening in the diagnosis and prognosis of cardiac sarcoidosis. JACC Cardiovasc Imaging 2017;10:1437–47. [DOI] [PubMed] [Google Scholar]
- 29. Greulich S, Deluigi CC, Gloekler S, Wahl A, Zürn C, Kramer Uet al. CMR Imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging 2013;6:501–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analyzed to support the findings of this study is available upon reasonable request from the corresponding author (E.H.).