Abstract
Background
Pulmonary hypertension is common among patients with heart failure (HF). Right ventricular systolic pressure (RVSP) is frequently used to assess its presence and severity. Although RVSP has been associated with adverse outcomes, the importance of serial measurements has not been studied. We evaluated associations between serial RVSP measurements and cardiovascular events in patients with HF.
Methods
Patients with HF and 2 echocardiograms performed ≥ 6 months apart were included. RVSP was categorized, using the second echocardiogram, as follows: normal (< 40 mm Hg); severely elevated (≥ 60 mm Hg); moderately elevated (50-59 mm Hg); or mildly elevated (40-49 mm Hg). Patients also were classified according to change in RVSP categories between echocardiograms. The primary outcome was time to HF hospitalization (HFH) or all-cause mortality (ACM) after the second echocardiogram.
Results
In total, 4319 patients were included (median age: 78 years; 52.1% female). During a median follow-up period of 19.4 months, HFH/ACM occurred in 2714 patients (62.8%). In multivariable analysis, baseline RSVP that was mildly elevated (1069 patients, hazard ratio [HR] 1.31, 95% confidence interval [CI] 1.12-1.54), moderately elevated (797 patients, HR 1.54, 95% CI 1.30-1.82), or severely elevated (837 patients, HR 1.92, 95% CI 1.60-2.31) was independently associated with HFH/ACM. Additionally, improving RVSP was associated with increased HFH/ACM in both categorical (HR 1.16, 95% CI 1.01-1.33) and continuous analyses.
Conclusions
RVSP measurements identify patients at increased risk who may require more-aggressive monitoring and medical therapy. Our study raises the hypothesis that, in addition to the absolute value of RVSP, improving RVSP category may identify higher-risk patients, but further study is needed to elucidate the underlying reasons.
Résumé
Contexte
L’hypertension pulmonaire est fréquente chez les patients atteints d’insuffisance cardiaque. La pression systolique ventriculaire droite (PSVD) est souvent utilisée pour évaluer la présence et la gravité de l’hypertension pulmonaire. Bien que la PSVD ait été associée à des complications, l’importance de mesures répétitives n’a pas été étudiée. Nous avons évalué les liens entre des mesures répétitives de la PSVD et des événements cardiovasculaires chez des patients atteints d’insuffisance cardiaque.
Méthodologie
Ont été inclus des patients atteints d’insuffisance cardiaque pour lesquels on disposait de deux échocardiogrammes réalisés dans un intervalle ≥ 6 mois. La PSVD a été catégorisée comme suit, au moyen du deuxième échocardiogramme : normale (< 40 mmHg); gravement élevée (≥ 60 mmHg); modérément élevée (50 à 59 mmHg) ou légèrement élevée (40 à 49 mmHg). Les patients ont également été classés dans des catégories en fonction de la variation de la PSVD d’un échocardiogramme à l’autre. Le paramètre d’évaluation principal était le temps écoulé avant une hospitalisation pour insuffisance cardiaque ou un décès, toutes causes confondues, après le second échocardiogramme.
Résultats
Au total, 4 319 patients ont été inclus (âge médian de 78 ans; 52,1 % de sexe féminin). Pendant une période de suivi médian de 19,4 mois, une hospitalisation pour insuffisance cardiaque ou un décès, toutes causes confondues, se sont produits chez 2 714 patients (62,8 %). Une analyse multivariée a déterminé qu’une PSVD initiale légèrement élevée (1 069 patients, rapport de risques instantanés [RRI] de 1,31, intervalle de confiance [IC] à 95 % de 1,12 à 1,54), modérément élevée (797 patients, RRI de 1,54, IC à 95 % de 1,30 à 1,82) ou gravement élevée (837 patients, RRI de 1,92, IC à 95 % de 1,60 à 2,31) était indépendamment associée à une hospitalisation pour insuffisance cardiaque ou à un décès, toutes causes confondues. En outre, l’amélioration de la PSVD était associée à une hausse des hospitalisations pour insuffisance cardiaque ou des décès, toutes causes confondues, dans les analyses des catégories (RRI de 1,16, IC à 95 % de 1,01 à 1,33) et continues.
Conclusions
Les mesures de la PSVD ont permis de repérer les patients présentant un risque accru qui pourraient nécessiter une surveillance et un traitement médical plus intenses. Notre étude incite à poser l’hypothèse voulant qu’en plus de la valeur absolue de la PSVD, l’amélioration des catégories de PSVD puisse permettre de repérer les patients présentant un risque accru, mais des études plus approfondies sont nécessaires pour élucider les raisons sous-jacentes.
Pulmonary hypertension is common among patients with chronic heart failure (HF), and it is an important predictor of symptom burden, as well as outcomes,1, 2, 3 for both HF with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF).4 Presently, the gold-standard method of assessing for pulmonary hypertension is to use pulmonary artery pressure measurement through right heart catheterization.5 However, physicians frequently rely on estimates of pulmonary artery pressure from echocardiography, particularly for patients with a high probability of having group 2 pulmonary hypertension.
Echocardiographic evaluation of right ventricular systolic pressure (RVSP) is frequently used to assess for the presence and severity of pulmonary hypertension in patients with chronic HF.6 Previous studies have shown RVSP to be a valuable tool in risk stratification of HF patients in both outpatient and inpatient settings.3,4,7,8 However, no study to date has investigated the importance of serial changes in RVSP as a marker of risk in chronic HF patients. This lack of data is especially important because the right ventricle is exposed to chronic pressure overload, eventually leading to right ventricular dysfunction.9 Given this, the subsequent development of worse outcomes from ongoing pulmonary hypertension may be a dynamic process. Despite this theory having been substantiated in previous hemodynamic studies, in which serial invasive measurements of pulmonary artery pressure were a predictor of HF outcomes in 195 patients,1 it has yet to be demonstrated for echocardiographic assessment.
In the present study, we investigated the prognostic relevance of serial echocardiographic RVSP measurements by examining outcomes in a large cohort of patients with a clinical diagnosis of HF and 2 echocardiograms performed at least 6 months apart.
Methods
Study cohort
The study cohort consisted of all patients living in Alberta, Canada, aged > 18 years, with a physician-assigned diagnosis of HF (at least one hospitalization or emergency department visit with a diagnosis of HF (International Classification of Diseases, 10th revision [ICD-10] code I50.x) between April 1, 2008 and March 31, 2016.10 For the present study, we included the entire spectrum of HF patients, including those with HFrEF, HF with mid-range left ventricular ejection fraction (LVEF), and HFpEF. These categories were established using the index (second) echocardiogram. Moreover, patients with recovered ejection fraction (EF) were defined as those who previously had an EF of ≤ 40% that has improved to > 40%.10 Additionally, patients were only included if they had at least 2 prior echocardiogram studies with a recorded RVSP at least 6 months apart. The 6-month time frame was chosen as a common clinical time frame used to evaluate response to therapy.11 For patients with 3 or more echocardiograms, we included only the data from the first 2 echocardiograms. We excluded patients who underwent cardiac transplantation or left ventricular assist device implantation between the 2 echocardiograms. The present study was approved at the University of Calgary and by the University of Alberta Research Ethics Board, including the waiver of consent. To comply with Alberta’s Health Information Act, the dataset used for this study cannot be made publicly available but is held securely in coded form. Access may be granted to those who meet prespecified criteria for confidential access, available at www.absporu.ca.
Data sources and elements
Standard baseline covariates including comorbidities were identified using Alberta healthcare administrative databases in the 12 months before the initial echocardiogram. Comorbidities were identified with previously validated standard ICD-10-Canada (CA) codes and case-definitions used in Alberta administrative databases.12,13 The duration of HF was determined as the time from the first presentation of HF to the index echocardiogram, which for the purposes of this study was defined as the second echocardiogram. Medication use was defined as at least 2 dispensations in the time between 2 echocardiograms, and this was identified using the pharmaceutical information network, which captures > 90% of prescriptions in Alberta.14 During the study period, angiotensin receptor blocker-neprilysin inhibitor, ivabradine, and sodium glucose cotransporter-2 inhibitors were only available by special authorization and industry support programs; therefore, usage of these medications could not be assessed reliably through the pharmaceutical information network and were not included in the study. Our dataset did not contain information on valvular heart disease, valvular interventions, or percutaneous or surgical revascularization of coronary artery disease.
Echocardiogram information, both outpatient and inpatient, was extracted from databases at the Libin Cardiovascular Institute (Calgary) and the Mazankowski Alberta Heart Institute (Edmonton). RVSP was calculated from peak tricuspid regurgitation velocity using the Bernoulli equation, with right atrial pressure estimated at the time of clinical reporting. Readers typically follow the American Society of Echocardiography recommendations for estimating right atrial pressure.15 Lastly, clinical outcomes were derived from administrative databases from the Discharge Abstract Database, using previously validated ICD-10 codes,12,13 and mortality was extracted from Alberta Vital Statistics. All aforementioned databases are linked to our study cohort through unique study patient identifiers, with automated data-linkage processes. No manual verification of data was performed.
Outcomes
The primary outcome was HF hospitalization or all-cause mortality after the index (second) echocardiogram. The secondary outcome was HF hospitalization and all-cause mortality as separate outcomes.
Statistical analysis
RVSP was defined as follows for the purposes of the present study: severely elevated, for values ≥ 60 mm Hg; moderately elevated, for 50 ≤ RVSP < 60 mm Hg; mildly elevated, for 40 ≤ RVSP < 50; and normal, for values < 40 mm Hg.5,16 Patients were subsequently classified based on RVSP change from first to index echocardiography, with categorization into 4 groups: (i) persistently normal RVSP; (ii) improved RVSP (improvement in at least one category from first echocardiogram); (iii) worsening RVSP (deterioration of at least one category from first echocardiogram); and (iv) persistently elevated RVSP. This classification allowed the analysis to account for the absolute value of RVSP as well as for changes in RVSP between the 2 echocardiograms.
Data are presented as frequencies and percentages for categorical variables, as mean ± standard deviation for normally distributed continuous variables, and median (interquartile range) for continuous variables with a skewed distribution. Categorical variables were compared using the χ2 or Fisher exact test, as appropriate, and continuous variables were compared with 1-way analysis of variance, or the Kruskal-Wallis test, as appropriate.
Kaplan-Meier analysis was used to assess the differences in survival among subjects stratified by changes in RVSP at echocardiographic follow-up evaluation. The log-rank test determined statistical significance among the risk categories for all Kaplan-Meier analyses. Proportional hazards Cox regression models were used to assess associations with the primary and secondary outcomes. RVSP at index echocardiogram and change in RVSP were also modeled as continuous variables with cubic splines to account for nonlinear relationships. Residuals were visually inspected to ensure that no violations of the proportional hazards assumption occurred. The primary analysis was the association of RVSP category and change in RVSP category with the composite outcome of HF hospitalization or all-cause mortality, with follow-up beginning at the time of the second echocardiogram. Time from the second echocardiogram was used to account for immortal time bias related to requiring serial imaging for inclusion in the cohort. Site was included in the model as a shared frailty. Outcomes including death were modelled using Cox proportional hazards models, and outcomes that did not include death (associations with HF hospitalizations) were modelled using Fine-Gray subdistribution hazard models, with death considered to be a competing risk. Follow-up was censored at the first event, March 31, 2017 (last day of follow-up), or at the date of known migration out of the province.
All tests with a 2-sided P-value < 0.05 were considered significant. We assessed the improvement in risk stratification with the use of RVSP measurements by assessing improvement in model fit by likelihood ratio χ2, as well net-reclassification index (NRI).17 We assessed event, non-event, and overall continuous NRI. Statistical analyses were performed using STATA version 13 (StataCorp, College Station, TX) and SPSS version 27.0. (IBM, Armonk, NY).
Results
Baseline characteristics of patient population
Characteristics of the study population are shown in Table 1. In total, 4319 patients were included, with a median time between echocardiograms of 16.7 months (interquartile range [IQR] 10.6- 28.6). The median age was 78 years (IQR 69-85), and the majority of patients were female (52.1%). The median LVEF was 55% (IQR 40%-55%). The median RVSP at the time of first echocardiogram for all patients was mildly elevated, at 42.5 mm Hg (IQR 33.7-53.9), and this remained the case at the time of the second echocardiogram, at which time the median RVSP was 43.8 mm Hg (IQR 34.0-56.0). Compared to patients with normal RVSP, patients with elevated RVSP were older (P < 0.001) and were more likely to be prescribed loop diuretics (P < 0.001).
Table 1.
Baseline characteristics of the study population, stratified by right ventricular systolic pressure (RVSP) on second (index) echocardiogram
| Characteristic | All patients (N = 4319) | Normal (RVSP < 40; n = 1616) | Mildly elevated (RVSP 40–49.9; n = 1069) | Moderately elevated (RVSP 50–59.9; n = 797) | Severely elevated (RVSP > 60; n = 837) | P |
|---|---|---|---|---|---|---|
| Age, y | 78 (67–85) | 75 (63–83) | 79 (70–85) | 79 (70–85) | 79 (70–86) | < 0.001 |
| Male | 2068 (47.9) | 808 (50.0) | 501 (46.9) | 378 (47.4) | 381 (48.7) | < 0.001 |
| Comorbidities | ||||||
| Diabetes | 1468 (34.0) | 484 (30.0) | 374 (35.0) | 299 (37.5) | 311 (37.2) | < 0.001 |
| Hypertension | 2966 (68.7) | 1010 (62.5) | 770 (72.0) | 590 (74.0) | 596 (71.2) | < 0.001 |
| Chronic kidney disease | 1861 (43.1) | 159 (9.8) | 494 (46.2) | 375 (47.1) | 415 (49.6) | < 0.001 |
| Stroke/TIA | 378 (8.8) | 125 (7.7) | 103 (9.6) | 83 (10.4) | 67 (8.0) | 0.001 |
| Atrial fibrillation | 2160 (50.0) | 677 (41.9) | 573 (53.6) | 450 (56.5) | 460 (55.0) | < 0.001 |
| COPD | 1283 (29.7) | 577 (35.7) | 311 (29.1) | 279 (35.0) | 330 (39.4) | < 0.001 |
| Cancer | 428 (9.9) | 159 (9.8) | 94 (8.8) | 87 (10.9) | 88 (10.5) | 0.006 |
| Coronary artery disease | 1797 (41.6) | 636 (39.4) | 473 (44.3) | 337 (42.3) | 351 (41.9) | 0.085 |
| Medications | ||||||
| Diuretics | 2896 (67.1) | 957 (59.2) | 697 (65.2) | 571 (71.6) | 671 (80.2) | < 0.001 |
| Nitrates | 1632 (37.8) | 513 (31.8) | 423 (39.6) | 339 (42.5) | 357 (42.7) | < 0.001 |
| Hydralazine | 245 (5.7) | 86 (5.3) | 54 (5.1) | 53 (6.7) | 52 (6.2) | 0.005 |
| MRA | 997 (23.1) | 361 (22.3) | 236 (22.1) | 175 (22.0) | 225 (26.9) | 0.001 |
| Digoxin | 682 (15.8) | 207 (12.8) | 165 (15.4) | 149 (18.7) | 161 (19.2) | < 0.001 |
| Beta-blockers | 3175 (73.5) | 1186 (73.4) | 803 (75.1) | 587 (73.7) | 598 (71.5) | 0.005 |
| ACEi/ARB | 3342 (77.4) | 1249 (77.3) | 850 (79.5) | 603 (75.7) | 639 (76.3) | 0.003 |
| HF classification | < 0.001 | |||||
| HFpEF | 2,528 (58.5) | 885 (54.8) | 673 (63.0) | 462 (58.0) | 539 (64.4) | |
| HFrEF | 1,101 (25.5) | 522 (32.3) | 281 (26.3) | 204 (25.6) | 195 (23.3) | |
| HFmrEF | 690 (16.0) | 209 (12.9) | 115 (10.8) | 131 (16.4) | 103 (12.3) | |
| Echocardiography | ||||||
| LVEF at second echocardiogram | 55 (40–55) | 55 (37.5–55) | 55 (40–55) | 55 (40–55) | 55 (40–55) | 0.026 |
| RVSP at first echocardiogram | 43 (34–54) | 36 (29–45) | 43 (35–51) | 46 (38–57) | 55 (44–67) | < 0.001 |
| RVSP at second echocardiogram | 44 (34–56) | 31 (27–35) | 44 (42–46) | 54 (51–57) | 70 (64–79) | < 0.001 |
| Change in RVSP | 1 (–9 to 11) | –5 (–14 to 2) | 1 (–8 to 9) | 8 (–2 to 16) | 16 (5 to 29) | < 0.001 |
Values are n (%) or median (interquartile range), unless otherwise indicated. Heart failure (HF) classification was based on ejection fraction (EF) at the second (index) echocardiogram.
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular EF; HFmrEF, HF with midrange EF; HFpEF, HF with preserved EF; HFrEF, HF with reduced EF; MRA, mineralocorticoid receptor antagonist; TIA, transient ischemic attack.
Follow-up characteristics according to changes in RVSP
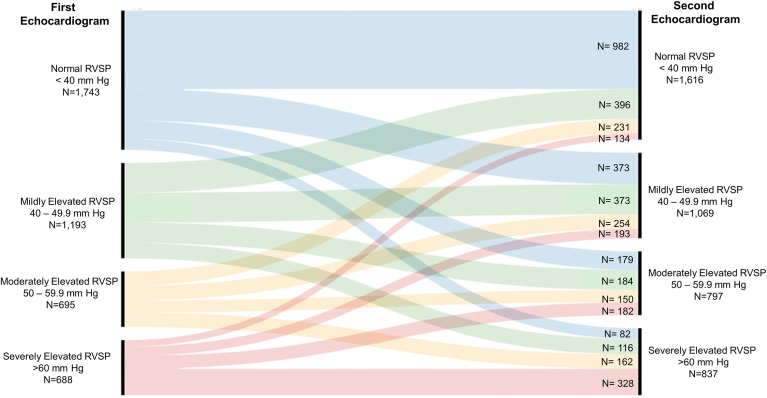
Figure 1 shows RVSP categories at the first and second echocardiogram in individual patients. The schematic tracks patients and assigns their classification based on the RVSP derived from the second echocardiogram, as follows: (i) those who remained in the same RVSP category were considered to have persistently normal RVSP (n = 982) if their first echocardiogram also showed a normal RVSP, or to have persistently elevated RVSP (n = 851) if their RVSP on first echocardiogram was also elevated; (ii) those who moved up at least one category were considered to have worsening RVSP (n = 1390); and (iii) those who moved down at least one category were deemed to have improving RVSP (n = 1096).
Figure 1.
Alluvial diagram outlining patient classification. Changes of right ventricular systolic pressure (RVSP) between the first echocardiogram and the second, performed at least 6 months later (median: 16.7 months).
Table 2 shows baseline clinical and echocardiographic characteristics of patients grouped according to changes in RVSP over time. Compared to patients with persistently normal RVSP, those with worsening or persistently elevated RVSP were older (P < 0.001). Patients with improving or persistently elevated RVSP also had more frequent prescriptions of diuretics (P < 0.001).
Table 2.
Characteristics of the study population according to changes in right ventricular systolic pressure (RVSP) on second (index) echocardiogram
| Characteristic | All patients (N = 4319) | Persistently normal (n = 982) | Improving (n = 1096) | Worsening (n = 1390) | Persistently elevated (n = 851) | P |
|---|---|---|---|---|---|---|
| Age, y | 78 (67–85) | 74 (62–82) | 77 (67–84) | 80 (70–86) | 79 (70–86) | < 0.001 |
| Male | 2068 (47.9) | 500 (50.9) | 500 (45.6) | 649 (46.7) | 419 (49.2) | 0.063 |
| Comorbidities | ||||||
| Diabetes | 1468 (34.0) | 265 (27.0) | 396 (36.1) | 505 (36.3) | 302 (35.5) | < 0.001 |
| Hypertension | 2966 (68.7) | 564 (57.4) | 780 (71.2) | 1,036 (74.5) | 586 (68.9) | < 0.001 |
| Chronic kidney disease | 1861 (43.1) | 309 (31.4) | 489 (44.6) | 648 (46.6) | 415 (48.8) | < 0.001 |
| Stroke/TIA | 378 (8.8) | 77 (7.8) | 80 (7.3) | 153 (11.0) | 68 (8.0) | 0.004 |
| Atrial fibrillation | 2160 (50.0) | 371 (37.8) | 568 (51.8) | 767 (55.2) | 454 (53.4) | < 0.001 |
| COPD | 1283 (29.7) | 183 (18.6) | 328 (29.9) | 469 (33.7) | 303 (35.6) | < 0.001 |
| Cancer | 428 (9.9) | 99 (10.1) | 100 (9.1) | 144 (10.4) | 85 (10.0) | 0.774 |
| Coronary artery disease | 1797 (41.6) | 381 (38.8) | 443 (40.4) | 631 (45.4) | 342 (40.2) | 0.005 |
| Medications | ||||||
| Diuretics | 2896 (67.1) | 533 (54.3) | 768 (70.1) | 966 (69.5) | 629 (73.9) | < 0.001 |
| Nitrates | 1632 (37.8) | 268 (27.3) | 437 (39.9) | 578 (41.6) | 349 (41.0) | < 0.001 |
| Hydralazine | 245 (5.7) | 43 (4.4) | 73 (6.7) | 74 (5.3) | 55 (6.5) | 0.094 |
| MRA | 997 (23.1) | 197 (20.1) | 271 (24.7) | 307 (22.1) | 222 (26.1) | 0.008 |
| Digoxin | 682 (15.8) | 116 (11.8) | 180 (16.4) | 238 (17.1) | 148 (17.4) | 0.001 |
| Beta-blockers | 3175 (73.5) | 709 (72.2) | 824 (75.2) | 1034 (74.4) | 607 (71.3) | 0.167 |
| ACEi/ARB | 3342 (77.4) | 743 (75.7) | 869 (79.3) | 1074 (77.3) | 655 (77.0) | 0.259 |
| HF classification | 0.002 | |||||
| HFpEF | 2528 (58.5) | 557 (56.7) | 627 (57.2) | 791 (56.9) | 553 (65.0) | |
| HFrEF | 1101 (25.5) | 262 (26.7) | 276 (25.2) | 370 (26.6) | 193 (22.7) | |
| HFmrEF | 690 (16.0) | 163 (16.6) | 193 (17.6) | 229 (16.5) | 105 (12.3) | |
| Echocardiography | ||||||
| LVEF at second echocardiogram | 55 (40–55) | 55 (37.5–55) | 55 (40–55) | 55 (37.5–55) | 55 (40–55) | 0.068 |
| RVSP at first echocardiogram | 42.5 (33.7–53.9) | 30.7 (26.6–34.8) | 53.9 (46.5–62.9) | 38.2 (32.3–46.0) | 53.4 (44.9–67.3) | < 0.001 |
| RVSP at second echocardiogram | 43.8 (34.0–56.1) | 30.8 (26.1–34.7) | 37.4 (31.0–45.6) | 54.8 (47.7–64.1) | 53.5 (44.3–69.1) | < 0.001 |
Values are n (%) or median (interquartile range), unless otherwise indicated.
ACEi, ACE inhibitor; ARB, angiotensin II receptor blocker; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; HF, heart failure; HFrEF, HF with reduced EF; HFmrEF, HF with midrange EF; HFpEF, HF with preserved EF; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; TIA, transient ischemic attack.
Outcomes
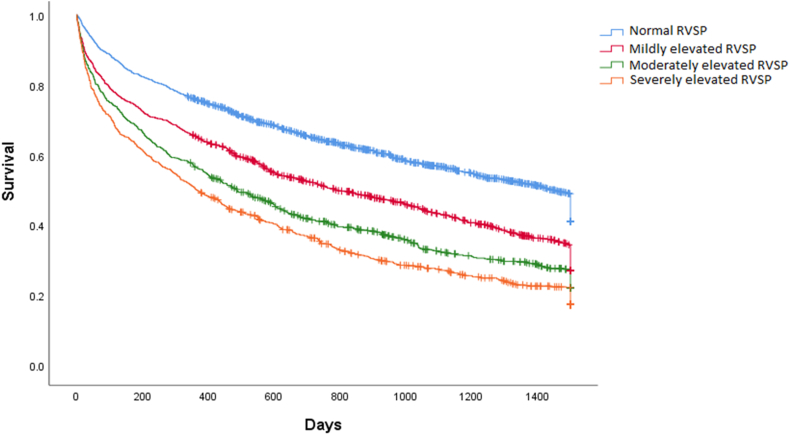
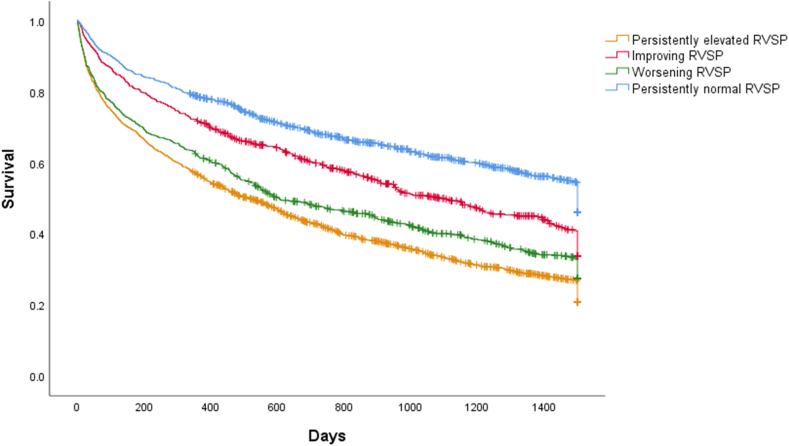
During a median follow-up of 19.4 months, the composite outcome occurred in 2714 patients. The first event included 1183 HF hospitalizations and 1531 deaths, which occurred at median times of 9.6 and 9.0 months. respectively. As shown in Figure 2, patients were at increasing risk of the composite outcome with increasing category of RVSP at index (second) echocardiogram (log-rank P < 0.001). With respect to serial RVSP, patients with persistently normal RVSP were least likely to experience the outcome, followed by those with improving, worsening, and persistently elevated RVSP (log-rank P < 0.001; Fig. 3).
Figure 2.
Kaplan-Meier curve survival estimates according to RVSP categories at the time of the index (second) echocardiogram. Cum, cumulative.
Figure 3.
Kaplan-Meier curve survival estimates according to right ventricular systolic pressure (RVSP) category changes between echocardiograms.
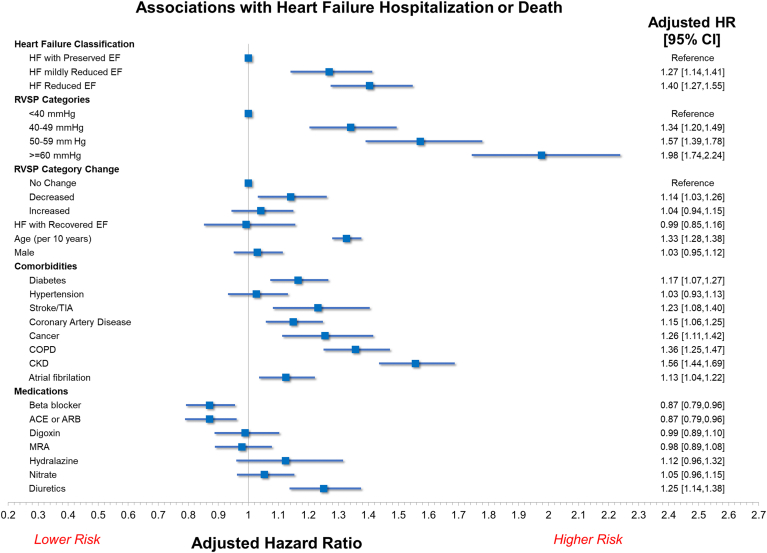
Associations with the composite outcome in the multivariable analysis are shown in Figure 4. Mildly elevated (adjusted hazard ratio [HR] 1.31, 95% confidence interval [CI] 1.12-1.54, P < 0.001), moderately elevated (adjusted HR 1.54, 95% CI 1.30-1.82, P < 0.001), and severely elevated RVSP (adjusted HR 1.92, 95% CI 1.60-2.31, P < 0.001) were independently associated with the outcome. The risk associated with severely elevated RVSP was numerically higher than the risk associated with HFrEF (adjusted HR 1.40, 95% 95% CI 1.27-1.55). Although worsening and persistently abnormal RVSP categories were not associated with increased risk, improving RVSP category was also associated with an increased risk of the composite outcome (adjusted HR 1.16, 95% CI 1.01-1.33, P = 0.040).
Figure 4.
Results of multivariable analysis for the primary outcome of heart failure (HF) hospitalization or all-cause mortality. Increasing right ventricular systolic pressure (RVSP) category at the time of index (second) echocardiogram was associated with increasing risk of events. Improving RVSP category was also associated with an increased risk of events. ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; HR, hazard ratio; MRA, mineralocorticoid receptor antagonist; TIA, transient ischemic attack.
Inclusion of change in RVSP category improved model fit, with an increase in likelihood ratio χ2 of 12.5 (P < 0.01). Improvement occurred in overall net-reclassification (NRI 0.111, 95% CI 0.027-0.259), and classification of patients without events (nonevent NRI 0.245, 95% CI 0.011-0.386), but not classification of patients with events (event NRI -0.133, 95% CI -0.271-0.221).
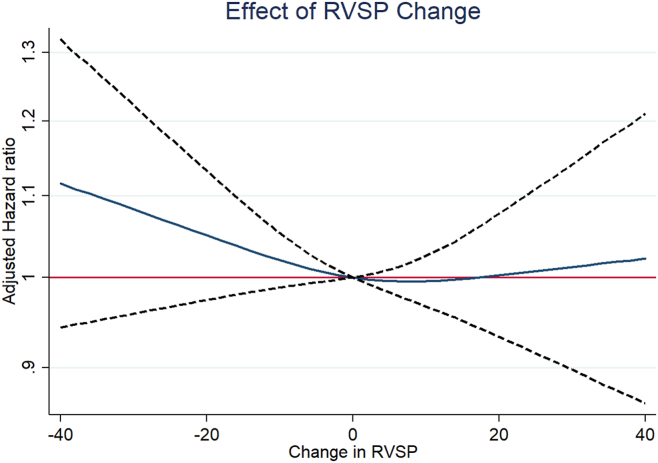
Continuous analysis of change in RVSP suggested that the risk is related to larger degrees of change in RVSP (Fig. 5). Associations with each component of the composite outcome were consistent with the overall results (Supplemental Table S1). In multivariable analysis, both improving and worsening RVSP category were associated with increased risk in patients with HFrEF, whereas no significant associations occurred in patients without HFrEF (Supplemental Table S2).
Figure 5.
Continuous analysis of change in right ventricular systolic pressure (RVSP). The blue line represents the point estimate; dotted black lines represent the 95% confidence intervals.
Discussion
The present study showed in a large cohort of chronic HF patients that pulmonary hypertension is common, as evidenced by 60.4% of patients having an elevated RVSP on echocardiography, and this was associated with poor outcomes. Changes from one RVSP category to another were frequent, and included both improving and worsening of RVSP category. Higher RVSP category was associated with worse outcomes. However, we also found that improving RVSP between echocardiograms was associated with a small increase in risk of HF hospitalization or death. Our results suggest that serial RVSP measurements may be helpful in identifying higher-risk patients, but absolute values of RVSP seem to be more important than the relative change between studies.
The pathophysiologic changes that occur to the right ventricle in response to pulmonary hypertension are best viewed as a continuum. Over time, pressure overload leads to maladaptive ventricular remodeling, including fibrosis, progressive dilatation, decreased capillary density, and in some cases, even right ventricular ischemia.9,18,19 As an expected consequence of these adaptations, we found that patients with elevated RVSP at the second echocardiogram were at higher risk of death or HF hospitalization, compared to patients with normal RVSP. However, we also noted that patients with a decrease in RVSP between echocardiograms were at higher risk of these outcomes than would be expected from absolute RVSP value alone. For example, among patients with RVSP of 40-49 mm Hg at the second echocardiogram, those patients with RVSP > 50 mm Hg on the first echocardiogram were at higher risk. Although the reasons for this finding in our study are not entirely clear, one possibility is that declining RVSP is a marker of worsening right ventricular (RV) function. Unfortunately, measures of RV function are not available in our cohort. However, this hypothesis is supported by the finding that risk is highest in patients with large drops in RVSP (Fig. 5), which would be explained most readily by a decline in RV function. Alternatively, decreasing RVSP could be a consequence of systemic hypotension,20 which also cannot be elucidated with our existing data.
Our study adds to the growing body of literature highlighting the potential clinical utility of serial cardiac imaging in patients with HF. In a previous study, we showed that patients with HF who exhibited a recovery in their EF on serial echocardiograms had significantly better prognosis, compared to those with persistently reduced EF.10 Similarly, change in left ventricular mass is a predictor of adverse outcomes.21 Dini et al. evaluated serial tricuspid annular plane systolic excursion (TAPSE) measurements in 706 patients with HFrEF.22 They found that worsening or persistently reduced TAPSE was associated with an increased risk of all-cause mortality, and improving TAPSE was associated with improved survival. In conjunction with our findings, this finding suggests that the presence of elevated RVSP in patients who also have abnormal or worsening TAPSE could be used to identify the highest-risk patients. In addition, the presence of normal RVSP and improving TAPSE can be used to identify the lowest-risk patients.
Our study should be viewed in the context of a few important limitations. The first is that our data are derived from estimates of pulmonary artery pressure from echocardiography rather than right heart catheterization. RVSP values were calculated from tricuspid regurgitation peak velocity and right atrial pressure estimates at the time of clinical reporting. Although readers typically estimate right atrial pressure based on American Society of Echocardiography recommendations,15 we do not know how it was estimated in situations in which right atrial pressure estimates might need to be adjusted, such as in the presence of positive pressure ventilation. One possibility is that some patients had discrepant measurements on right heart catheterization, but it was not feasible to perform serial measurements without use of an implantable pulmonary artery pressure sensor.23 Second, we are unable to elicit the class of pulmonary hypertension that was present in those patients with elevated pulmonary arterial systolic pressure. Third, given the number of missing values in our dataset, we do not have comprehensive data for TAPSE, left ventricular end-systolic volume index, or left ventricular end-diastolic volume index, RV size, or RV function in the present study, which are variables that may have been significantly associated with the outcome. Decreasing RVSP could be a marker of worsening RV function, a possibility that could be investigated in future studies. Moreover, we did not have data regarding severity of valvular disease or serum biomarkers, such as creatinine and B-type natriuretic peptide, in our analyses. Fourth, given that our goal was to assess the value of serial RVSP measurements, we included patients with at least 2 echocardiograms at least 6 months apart. However, by definition, this approach excludes patients who died within 6 months of the initial echocardiogram, leading to some selection bias. Fifth, we do not have longitudinal prescription information, or insight into patient adherence or other medical therapy that patients may have received over the course of the study, which may be further confounding factors. Last, the retrospective design of the study creates biases in the patients enrolled in the present study. Given that HF was diagnosed based on initial hospitalization or emergency department visit, those with more severe disease were included in the study. Moreover, those that were followed up with a repeat echocardiogram likely had a greater severity of disease, as those who were clinically stable may not have needed a repeat echocardiogram.
Conclusions
Our study showed, in a cohort of 4319 chronic HF patients, that RVSP is an independent predictor of adverse outcomes. Additionally, serial measurements of RVSP through echocardiography may provide some further risk stratification, but larger studies with details regarding relevant confounding features are needed to evaluate these hypotheses.
Acknowledgments
Ethics Statement
The present study was approved at the University of Calgary and by the University of Alberta Research Ethics Board, including the waiver of consent.
Patient Consent
The authors confirm that patient consent is not applicable to this article. This is a retrospective case report using de-identified data and a waiver of consent was granted by the research ethics boards.
Funding Sources
The authors have no funding sources to declare.
Disclosures
R.J.H.M. has received consulting and research support from Pfizer. The other authors have no conflicts of interest to disclose.
Footnotes
See page 678 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2023.05.011.
Supplementary Material
References
- 1.Grigioni F., Potena L., Galiè N., et al. Prognostic implications of serial assessments of pulmonary hypertension in severe chronic heart failure. J Heart Lung Transplant. 2006;25:1241–1246. doi: 10.1016/j.healun.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Damy T., Goode K.M., Kallvikbacka-Bennett A., et al. Determinants and prognostic value of pulmonary arterial pressure in patients with chronic heart failure. Eur Heart J. 2010;31:2280–2290. doi: 10.1093/eurheartj/ehq245. [DOI] [PubMed] [Google Scholar]
- 3.Bursi F., McNallan S.M., Redfield M.M., et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59:222–231. doi: 10.1016/j.jacc.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghio S., Temporelli P.L., Klersy C., et al. Prognostic relevance of a non-invasive evaluation of right ventricular function and pulmonary artery pressure in patients with chronic heart failure. Eur J Heart Fail. 2013;15:408–414. doi: 10.1093/eurjhf/hfs208. [DOI] [PubMed] [Google Scholar]
- 5.Galiè N., Hoeper M.M., Humbert M., et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 6.Hellenkamp K., Unsöld B., Mushemi-Blake S., et al. Echocardiographic estimation of mean pulmonary artery pressure: a comparison of different approaches to assign the likelihood of pulmonary hypertension. J Am Soc Echocardiogr. 2018;31:89–98. doi: 10.1016/j.echo.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Aronson D., Darawsha W., Atamna A., et al. Pulmonary hypertension, right ventricular function, and clinical outcome in acute decompensated heart failure. J Card Fail. 2013;19:665–671. doi: 10.1016/j.cardfail.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Lam C.S., Roger V.L., Rodeheffer R.J., et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin K.M., Kim N.H., Rubin L.J. The right ventricle in pulmonary hypertension. Coron Artery Dis. 2005;16:13–18. doi: 10.1097/00019501-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Ghimire A., Fine N., Ezekowitz J.A., et al. Frequency, predictors, and prognosis of ejection fraction improvement in heart failure: an echocardiogram-based registry study. Eur Heart J. 2019;40:2110–2117. doi: 10.1093/eurheartj/ehz233. [DOI] [PubMed] [Google Scholar]
- 11.Ezekowitz J.A., O'Meara E., McDonald M.A., et al. 2017 comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol. 2017;33:1342–1433. doi: 10.1016/j.cjca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Quan H., Sundararajan V., Halfon P., et al. Coding algorithms for defining comorbidities in ICD-9-cm and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 13.Tonelli M., Wiebe N., Fortin M., et al. Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2015;15:31. doi: 10.1186/s12911-015-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye M., Vena J.E., Johnson J.A., Xu J.Y., Eurich D.T. Validation of drug prescription records for senior patients in Alberta's tomorrow project: assessing agreement between two population-level administrative pharmaceutical databases in Alberta, Canada. Pharmacoepidemiol Drug Saf. 2019;28:1417–1421. doi: 10.1002/pds.4861. [DOI] [PubMed] [Google Scholar]
- 15.Rudski L.G., Lai W.W., Afilalo J., et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. ; quiz 786-8. [DOI] [PubMed] [Google Scholar]
- 16.Strange G., Stewart S., Celermajer D.S., et al. Threshold of pulmonary hypertension associated with increased mortality. J Am Coll Cardiol. 2019;73:2660–2672. doi: 10.1016/j.jacc.2019.03.482. [DOI] [PubMed] [Google Scholar]
- 17.Kerr K.F., Wang Z., Janes H., et al. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology. 2014;25:114–121. doi: 10.1097/EDE.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haddad F., Doyle R., Murphy D.J., Hunt S.A. Right ventricular function in cardiovascular disease, part ii: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 19.Zelt J.G.E., Chaudhary K.R., Cadete V.J., Mielniczuk L.M., Stewart D.J. Medical therapy for heart failure associated with pulmonary hypertension. Circ Res. 2019;124:1551–1567. doi: 10.1161/CIRCRESAHA.118.313650. [DOI] [PubMed] [Google Scholar]
- 20.Itelman E., Segel M.J., Kuperstein R., et al. Pulmonary hypertension is associated with systemic arterial hypertension among patients with normal left ventricular diastolic function. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.023603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L., Pagano J., Chow K., et al. Cardiac remodelling predicts outcome in patients with chronic heart failure. ESC Heart Fail. 2021;8:5352–5362. doi: 10.1002/ehf2.13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dini F.L., Carluccio E., Simioniuc A., et al. Right ventricular recovery during follow-up is associated with improved survival in patients with chronic heart failure with reduced ejection fraction. Eur J Heart Fail. 2016;18:1462–1471. doi: 10.1002/ejhf.639. [DOI] [PubMed] [Google Scholar]
- 23.Gibson J., McGrath K., Miller R.J.H., Sumner G., Clarke B. Ambulatory pulmonary artery pressure monitoring reduces costs and improves outcomes in symptomatic heart failure: a single-centre Canadian experience. CJC Open. 2023;5:237–249. doi: 10.1016/j.cjco.2022.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.