Abstract
Background/Objectives
Rheumatoid arthritis (RA) is a multisystem autoimmune disorder characterized by articular and extra-articular manifestations. Neuropathy is a poorly studied manifestation of RA. The aim of this study was to utilize the rapid non-invasive ophthalmic imaging technique of corneal confocal microscopy to identify whether there is evidence of small nerve fibre injury and immune cell activation in patients with RA.
Subjects/Methods
Fifty consecutive patients with RA and 35 healthy control participants were enrolled in this single-centre, cross-sectional study conducted at a university hospital. Disease activity was assessed with the 28-Joint Disease Activity Score and erythrocyte sedimentation rate (DAS28-ESR). Central corneal sensitivity was measured with a Cochet-Bonnet contact corneal esthesiometer. A laser scanning in vivo corneal confocal microscope was used to quantify corneal nerve fibre density (CNFD), nerve branch density (CNBD), nerve fibre length (CNFL), and Langerhans cell (LC) density.
Results
Corneal sensitivity (P = 0.01), CNFD (P = 0.02), CNBD (P < 0.001), and CNFL (P < 0.001) were lower, and mature (P = 0.001) and immature LC densities (P = 0.011) were higher in patients with RA compared to control subjects. CNFD (P = 0.016) and CNFL (P = 0.028) were significantly lower in patients with moderate to high (DAS28-ESR > 3.2) compared to mild (DAS28-ESR ≤ 3.2) disease activity. Furthermore, the DAS28-ESR score correlated with CNFD (r = −0.425; P = 0.002), CNBD (ρ = −0.362; P = 0.010), CNFL (r = −0.464; P = 0.001), total LC density (ρ = 0.362; P = 0.010) and immature LC density (ρ = 0.343; P = 0.015).
Conclusions
This study demonstrates reduced corneal sensitivity, corneal nerve fibre loss and increased LCs which were associated with the severity of disease activity in patients with RA.
Subject terms: Autoimmune diseases, Inflammatory diseases, Neurological disorders, Medical imaging
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease characterized by symmetrical erosive polyarthritis. It is also associated with multiple extra-articular manifestations involving the skin, eyes, respiratory, cardiovascular and nervous systems, which are associated with increased morbidity and mortality [1]. Peripheral nerve involvement may occur in up to 75% of patients with RA and manifests as a distal symmetric sensory neuropathy, mononeuritis multiplex, multifocal sensorimotor neuropathy, and entrapment neuropathy [2–5]. Peripheral neuropathy is mostly asymptomatic, but may manifest with pain, paraesthesia, numbness and muscle weakness, which may overlap with symptoms of arthritis, resulting in underdiagnosis of neuropathy. The assessment of neuropathic symptoms and quantitative sensory testing, psychophysical tests of sensory function included in the German Research Network on Neuropathic Pain (DFNS) [6] may allow evaluation of neuropathy; but are subjective and primarily identify established neuropathy. Abnormal nerve conduction studies have been found in patients with RA [4, 5, 7, 8] but they cannot detect small nerve fibre damage. Indeed, in patients with RA, skin biopsy studies have shown reduced intraepidermal nerve fibre density [9, 10] and a sural nerve biopsy study showed loss of myelinated fibres and necrotizing vasculitis with perivascular lymphomononuclear cell infiltration [4]. Whilst, skin and nerve biopsy are objective and provide insights into the underlying pathophysiology, they are invasive and require expert laboratory assessment. Impaired sudomotor function has also been reported in RA indicating autonomic nerve fibre damage [11].
Corneal confocal microscopy (CCM) is an objective, repeatable and reproducible ophthalmic imaging technique for the quantification of small nerve fibre degeneration [12–14]. Multiple studies have demonstrated corneal nerve fibre loss in a range of peripheral neuropathies including diabetic neuropathy [15–18], fibromyalgia [19], idiopathic small fibre neuropathy [20], Fabry disease [21], chemotherapy-induced peripheral neuropathy [22], and chronic inflammatory demyelinating polyneuropathy (CIDP) [23]. In addition, CCM has been used to identify increased Langerhans cells (LCs) in immune mediated and inflammatory neuropathies such as multiple sclerosis, CIDP and Behçet’s disease [23–25]. We have recently utilized CCM to demonstrate corneal nerve fibre loss and increased LCs in patients with systemic lupus erythematosus [26].
We hypothesized that CCM could serve as an objective method for assessing neurodegeneration and immune cell activation in relation to disease severity and progression in RA. We have quantified corneal sensitivity and corneal subepithelial nerve plexus morphology and LC density in relation to disease activity in patients with RA.
Materials and methods
This cross-sectional comparative study undertaken at a tertiary referral university hospital included 50 patients with RA and 35 healthy control participants. The study design adhered to the tenets of the Declaration of Helsinki and was approved by the Research Ethics Committee of the Necmettin Erbakan University. Written informed consent was obtained from each participant after a detailed explanation of the study protocol.
Patients with RA were consecutively recruited from the Rheumatology department. The diagnosis was established according to the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria [27]. Patients were excluded if they had diabetes mellitus or any coexisting systemic disease that might cause neuropathy, secondary Sjögren’s syndrome, a previous history of ocular surgery or other corneal pathology. Patients with a non-anesthetized Schirmer’s test score of ≤5 mm in 5 min were also excluded. Control participants were recruited from visitors to the hospital who had no corneal pathology and systemic inflammatory or neurological disease or history of contact lens use.
The severity of disease activity was determined by an experienced rheumatology specialist using the 28-Joint Disease Activity Score and erythrocyte sedimentation rate (DAS28-ESR), which consists of 4 components: ESR, tender joint count, swollen joint count, and visual analogue scale for general health [28]. The scores for DAS28 range from 0 to 9.4 and disease activity is interpreted as follows: low activity (DAS28 ≤ 3.2), moderate activity (DAS28 > 3.2 and ≤ 5.1), and high activity (DAS28 > 5.1) [29]. Laboratory data including complete blood count, ESR, C-reactive protein (CRP), rheumatoid factor (RF), anti-cyclic citrullinated peptide (anti-CCP), anti-nuclear antibody (ANA) and anti-extractable nuclear antigen (ENA) panel were recorded.
After a complete ophthalmologic evaluation, a contact corneal esthesiometer (Cochet-Bonnet; Luneau, France) was used to determine the central corneal sensitivity threshold. A pressure was applied perpendicular to the central cornea with a 0.12-mm-diameter nylon monofilament extended to the maximal length of 6.0 cm, corresponding to the lowest possible pressure, and was gradually reduced in 5-mm steps until the first response was elicited. The longest filament length (cm) that resulted in a positive response was verified twice and recorded as the central corneal sensitivity threshold.
All participants underwent laser scanning CCM using the Rostock Corneal Module of the Heidelberg Retina Tomograph lll (Heidelberg Engineering, Germany). The section mode was used to scan the full thickness of the central cornea, and two-dimensional digital images were obtained with a lateral digital resolution of 1 μm/pixel, a depth resolution of 2 μm/pixel, and an image size of 400 × 400 μm. The total duration of examination was approximately 2 min/eye. A standardized image selection protocol was used; three high-quality subepithelial nerve plexus images from the central cornea of each participant containing the highest, intermediate, and least number of nerve fibres were selected and the average of these results was considered [30]. A validated, purpose-designed manual image segmentation algorithm (CCMetrics, University of Manchester, Manchester, UK) was used to analyze the CCM images. Three corneal nerve plexus parameters were quantified: corneal nerve fibre density (CNFD) (no./mm2); corneal nerve branch density (CNBD) (no./mm2); and corneal nerve fibre length (CNFL) (mm/mm2) [31]. The total number of hyper-reflective cells was quantified in the same image frames used to assess the nerve plexus, and LC density (no./mm2) was established. As per previously described criteria, large cells with dendriform structures were considered as mature LCs and smaller cells without clearly visible dendritic structures were considered as immature LCs [32]. An experienced observer (GB) quantified nerve parameters and LC densities and was unmasked as to whether the images belonged to a patient with RA or a control subject but was masked in relation to disease severity. To evaluate the intra- and inter-observer agreements of LC quantification, a randomly selected subset of 50 CCM images were re-analyzed by the same clinician (GB) and by an independent masked clinician (MCE). For all subjects, only the data obtained from the right eye were included in the analyses.
Statistical analysis was performed using IBM SPSS Statistics v21.0 software. Basic descriptive statistics were calculated and reported as the mean ± SD or median (interquartile range [IQR]), as appropriate. A pre-study sample size calculation was not performed, however a sample size similar to that of prior CCM studies on RA patients [33, 34] was obtained. A post-hoc power analysis based on CNFL revealed a power of 99.7%. All categorical variables were compared using the Pearson χ2-test. Normal distribution of continuous variables was confirmed with the Shapiro–Wilk test. Independent samples t-test for normally distributed data and Mann-Whitney U-test for non-normally distributed data were used to compare the parameters between patients with RA and healthy control participants. The associations among variables were analyzed using the Pearson correlation coefficient for normally distributed data and the Spearman correlation coefficient for non-normally distributed data. Intra- and inter-observer agreements were calculated using the intraclass correlation coefficients (ICC) and their 95% confidence intervals (CI). Values of ICC were interpreted as poor reliability (<0.50), moderate reliability (0.50–0.75), good reliability (0.75–0.90), and excellent reliability (>0.90) [35]. For all evaluations, a two-sided P value of less than 0.05 was considered statistically significant.
Results
Clinical measures
Table 1 summarizes clinical characteristics of the patients with RA and control participants. No significant differences were observed between patients and controls for age (P = 0.983) and gender (P = 0.793). The median (IQR) time from the initial diagnosis of RA was 9.5 (4.0–20.0) years. The mean DAS28-ESR was 3.13 ± 1.09, and 25 (50%) patients had DAS28-ESR greater than 3.2, indicating moderate to high disease activity. Thirty (60%) patients were receiving low dose corticosteroids, 25 (50%) methotrexate, 20 (40%) anti-TNF agents, 15 (30%) hydroxychloroquine, 14 (28%) leflunomide, 13 (26%) sulfasalazine, 6 (12%) tocilizumab, and 1 (2%) was receiving baricitinib as monotherapy or in combination.
Table 1.
Baseline characteristics of the study participants.
| Control subjects (n = 35) | Patients with RA (n = 50) | |
|---|---|---|
| Age (years), mean ± SD | 52.0 ± 13.1 | 52.1 ± 9.7 |
| Gender (F/M), n | 25/10 | 37/13 |
| Duration of disease (years), median (IQR) | – | 9.5 (4.0–20.0) |
| DAS28-ESR, mean ± SD | – | 3.13 ± 1.09 |
| ESR (mm/h), median (IQR) | – | 25.5 (13.3–43.5) |
| C-reactive protein (mg/L), median (IQR) | – | 5.9 (3.3–13.5) |
| Rheumatoid factor (IU/mL), median (IQR) | – | 57.0 (13.7–133.3) |
| Anti-CCP (IU/mL), median (IQR) | – | 40.0 (3.5–177.5) |
| ANA positivity, n (%) | – | 17 (34%) |
| Anti-ENA positivity, n (%) | – | 0 (0%) |
| Ongoing therapies | ||
| Corticosteroids, n (%) | – | 30 (60%) |
| Methotrexate, n (%) | – | 25 (50%) |
| Anti-TNF agents, n (%) | – | 20 (40%) |
| Hydroxychloroquine, n (%) | – | 15 (30%) |
| Leflunomide, n (%) | – | 14 (28%) |
| Sulfasalazine, n (%) | – | 13 (26%) |
| Tocilizumab, n (%) | – | 6 (12%) |
| Baricitinib, n (%) | – | 1 (2%) |
ANA anti-nuclear antibody, Anti-CCP anti-cyclic citrullinated peptide, Anti-ENA anti-extractable nuclear antigen, DAS28-ESR 28-Joint Disease Activity Score based on the erythrocyte sedimentation rate.
Corneal sensitivity and corneal confocal microscopy
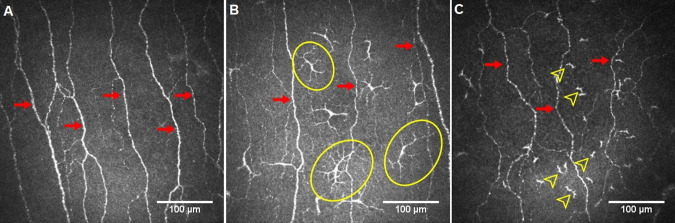
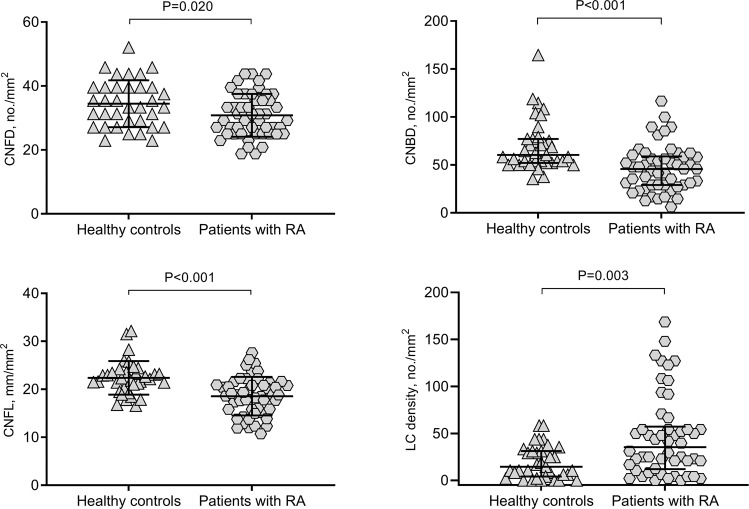
Figure 1 demonstrates CCM images of the central corneal subepithelial nerve plexus in a control participant and two patients with RA. Central corneal sensitivity (P = 0.01), CNFD (P = 0.02), CNBD (P < 0.001), and CNFL (P < 0.001) were lower, and total (P = 0.003), mature (P = 0.001) and immature LC densities (P = 0.011) were higher in patients with RA compared to control subjects (Table 2, and Fig. 2). CNFD (P = 0.016) and CNFL (P = 0.028) were lower, with no difference in corneal sensitivity (P = 0.491), CNBD (P = 0.103), and total, mature and immature LC densities (P = 0.079, P = 0.403, and P = 0.069, respectively) in patients with moderate to high (DAS28-ESR > 3.2, n = 25 [50%]) compared to mild (DAS28-ESR ≤ 3.2) disease activity (Table 3). CNBD was lower (P = 0.024) and total LC density was higher (P = 0.017) in ANA-positive (34%) compared to ANA-negative patients with RA (SupplementaryTable S1). The duration of disease was significantly longer (P = 0.01) and CNFD (P = 0.036) was lower with no difference in disease severity, CNBD, CNFL or LC density between patients with RA on first- or second-line therapy (Supplementary Table S2). Intra-observer agreements for total LC density (ICC = 0.986 [95% CI: 0.975–0.992]), mature LC density (ICC = 0.990 [95% CI: 0.982–0.994]), and immature LC density (ICC = 0.982 [95% CI: 0.969–0.990]) were excellent. Inter-observer agreements were also found to be excellent for total LC density (ICC = 0.902 [95% CI: 0.833–0.943]), mature LC density (ICC = 0.918 [95% CI: 0.861–0.953]), and immature LC density (ICC = 0.910 [95% CI: 0.846–0.948]).
Fig. 1. Representative corneal confocal microscopy images.
Subepithelial nerve plexus in a control participant (A) and two patients with rheumatoid arthritis (B and C), showing reduced nerve fibres (arrows) and increased mature (circles) and immature (arrowheads) Langerhans cells.
Table 2.
Corneal sensitivity thresholds and corneal nerve and LC parameters in patients with rheumatoid arthritis (RA) and healthy control participants.
| Control subjects (n = 35) |
Patients with RA (n = 50) |
P value | |
|---|---|---|---|
| Central corneal sensitivity (cm), median (IQR) | 6.0 (6.0–6.0) | 6.0 (5.5–6.0) | 0.010a |
| CNFD (no./mm2), mean ± SD | 34.5 ± 7.3 | 30.8 ± 6.7 | 0.020b |
| CNBD (no./mm2), median (IQR) | 60.4 (52.1–77.1) | 45.8 (29.2–58.8) | <0.001a |
| CNFL (mm/mm2), mean ± SD | 22.4 ± 3.5 | 18.5 ± 4.0 | <0.001b |
| LC density (no./mm2), median (IQR) | 14.6 (4.2–31.3) | 35.4 (12.0–57.3) | 0.003a |
| Mature LC density (no./mm2), median (IQR) | 0 (0–4.2) | 5.2 (0–15.3) | 0.001a |
| Immature LC density (no./mm2), median (IQR) | 10.4 (2.1–29.1) | 21.9 (7.8–45.8) | 0.011a |
CNFD corneal nerve fibre density, CNBD corneal nerve branch density, CNFL corneal nerve fibre length, LC Langerhans cell.
aMann–Whitney U-test.
bIndependent samples t-test.
Fig. 2. Corneal nerve fibre parameters and Langerhans cell (LC) density in patients with rheumatoid arthritis (RA) and healthy control participants, showing significantly lower corneal nerve fibre density (CNFD), corneal nerve branch density (CNBD) and corneal nerve fibre length (CNFL), and higher LC density in patients with RA.
Error bars indicate mean (SD) for CNFD and CNFL, and median (IQR) for CNBD and LC density.
Table 3.
Corneal sensitivity thresholds and corneal nerve and LC parameters according to disease activity in patients with rheumatoid arthritis.
| DAS28-ESR ≤ 3.2 (n = 25) |
DAS28-ESR > 3.2 (n = 25) |
P value | |
|---|---|---|---|
| Central corneal sensitivity (cm), median (IQR) | 6.0 (5.5–6.0) | 5.5 (5.5–6.0) | 0.491a |
| CNFD (no./mm2), mean ± SD | 33.1 ± 6.8 | 28.6 ± 5.9 | 0.016b |
| CNBD (no./mm2), median (IQR) | 50.0 (32.3–62.5) | 35.4 (25.0–56.3) | 0.103a |
| CNFL (mm/mm2), mean ± SD | 19.8 ± 3.5 | 17.3 ± 4.2 | 0.028b |
| LC density (no./mm2), median (IQR) | 22.9 (4.2–54.2) | 47.9 (22.9–79.2) | 0.079a |
| Mature LC density (no./mm2), median (IQR) | 4.2 (0–13.5) | 8.3 (0–16.7) | 0.403a |
| Immature LC density (no./mm2), median (IQR) | 20.8 (4.2–38.5) | 35.5 (16.5–65.6) | 0.069a |
CNFD corneal nerve fibre density, CNBD corneal nerve branch density, CNFL corneal nerve fibre length, LC Langerhans cell.
aMann–Whitney U-test.
bIndependent samples t-test.
Correlations
The DAS28-ESR score correlated inversely with CNFD (r = −0.425; P = 0.002), CNBD (ρ = −0.362; P = 0.010), CNFL (r = −0.464; P = 0.001) and directly with total LC density (ρ = 0.362; P = 0.010) and immature LC density (ρ = 0.343; P = 0.015). There were significant correlations between central corneal sensitivity and CNFD (ρ = 0.328; P = 0.020), CNBD (ρ = 0.306; P = 0.030), and CNFL (ρ = 0.496; P < 0.001). CNBD correlated with total (ρ = −0.403; P = 0.004), mature (ρ = −0.421; P = 0.002), and immature LC densities (ρ = −0.292; P = 0.040), and CNFL correlated with total (ρ = −0.341; P = 0.015) and immature LC densities (ρ = −0.303; P = 0.032). RF levels correlated with CNFD (ρ = −0.283; P = 0.046), and CNFL (ρ = −0.291; P = 0.041). There was no correlation between duration of disease and DAS28-ESR, corneal sensitivity, or CCM measures.
Discussion
We have shown reduced corneal sensitivity, loss of corneal nerve fibres and an increase in corneal immune cells associated with disease activity in patients with RA. The prevalence of neuropathy in patients with RA ranges from 17% to 75%, depending on the criteria used to identify nerve damage [2, 5, 7]. In a study of 108 patients with RA, 21% had neuropathic symptoms, but 57% had electrophysiological abnormalities [4]. The aetiology of peripheral neuropathy in RA is not well-understood and various mechanisms have been proposed, including vasculitis, autoimmunity, drug toxicity, nerve entrapment and amyloidosis [4, 36, 37]. In a sural nerve biopsy study of patients with RA and symptomatic peripheral neuropathy, there was evidence of axonal degeneration and epineurial and endoneurial arteritis [38]. In another histopathological study of 32 RA patients with peripheral neuropathy there was evidence of necrotizing vasculitis with demyelination and axonal degeneration [39]. In sural nerve biopsies from 23 patients with RA, Agarwal et al. [4]. demonstrated a loss of myelinated nerve fibres and perivascular lymphomononuclear cell infiltration. We now demonstrate corneal nerve fibre loss and increased LCs using CCM. This is a rapid non-invasive ophthalmic imaging technique which is comparable to skin biopsy in relation to demonstrating a loss of small nerve fibres in diabetic neuropathy, fibromyalgia, and amyloid neuropathy [19, 40, 41].
Villani et al. [33]. have reported a reduction in corneal nerve fibres, epithelial cell and stromal keratocyte density in patients with RA in relation to the severity of dry eye. Corneal nerve fibre loss and increased dendritic cells have also been demonstrated in patients with dry eye [42, 43]. Sjögren’s syndrome is associated with small fibre neuropathy and dry eye as well as corneal nerve fibre damage [43–45], especially in patients with primary Sjögren’s syndrome [46], but not in those with secondary Sjögren’s syndrome [33]. Nevertheless, to avoid the potential confounding effects of Sjögren’s syndrome and dry eye, we excluded patients with secondary Sjögren’s syndrome and those with a Schirmer’s score equal to or less than 5 mm. Previously Villani et al. [33]. showed a reduction in corneal nerve fibre number but no relation to RA disease severity evaluated by the Lansbury index. In the present study we have shown more extensive alterations in corneal nerve morphology and increased LCs, which were associated with the severity of disease activity in RA. Given that corneal nerve degeneration and inflammation play key roles in the pathophysiology of dry eye [47], longitudinal analyses are needed to investigate the causal relation between loss of corneal nerves and increased LCs, with dry eye in patients with RA.
In relation to underlying mechanisms of nerve damage, we have shown that an increase in corneal LC density was associated with the severity of disease activity in patients with RA. Different methods have been used to evaluate LC morphology, either using continuous variables measured by performing cell morphometric analysis with image analysis software, or categorical variables by classifying LCs as mature/immature cells or assigning a morphology score ranging from 1 to 3, with 1 representing LCs without dendrites, 2, LCs with small dendritic processes and 3 representing LCs with long processes [34, 48, 49]. In this study we have categorized LC morphology according to the cell size and presence or absence of dendritic structures, according to an established protocol [32]. Previous studies have demonstrated increased mature and immature dendritic cells in synovial fluid and synovial tissue and a reduction of dendritic cells in the peripheral blood of patients with RA; suggesting that inflammatory cells migrate and accumulate in the synovium [50, 51]. A similar process may also occur in the cornea. Indeed, Marsovszky et al. [34]. showed an increase in corneal LC density and mature LCs in RA patients and Villani et al. [52]. showed that systemic treatment for RA was associated with a reduction in disease activity and corneal dendritic cells in patients with RA and secondary Sjögren’s syndrome. Leppin et al. [53]. showed that dendritic cells may mediate corneal nerve fibre damage in a murine model of diabetic neuropathy. Increased corneal dendritic cells and a change in their morphology has been correlated with a reduction in corneal nerve fibres in ocular surface diseases [54]. More recently, in patients with Sjögren’s syndrome and RA, corneal nerve fibre length correlated negatively with systemic disease activity and circulating IL21+ CD8+ T cells and positively with total memory, unswitched memory and CD24Hi CD27+ B cells; indicating that corneal nerve damage may be associated with circulating inflammatory cell dynamics [55]. We now demonstrate inverse correlations between corneal nerve parameters and LC densities, suggesting an association between corneal immune cells and nerve fibre degeneration in RA.
Anti-nuclear antibody positivity is associated with an increased prevalence of extra-articular manifestations in patients with RA [56, 57]. Indeed, Caspi et al. [57]. reported a higher risk of vasculitis and more severe pain in ANA-positive RA patients. In the current study, we show a greater reduction in CNBD and increase in LC density in ANA-positive patients with RA, but no difference in disease activity.
Limitations of this study include a relatively small sample size, the absence of long-term evaluation, and a lack of additional measures of peripheral neuropathy such as quantitative sensory testing and intraepidermal nerve fibre density at baseline. Another limitation of this study is the selection of the CCM images by an unmasked observer, which may increase the risk of bias. However, we used a standardized image selection protocol which has excellent intra- and inter-observer repeatability [30].
In conclusion, we have demonstrated significant corneal nerve fibre loss and increased LCs which were associated with the severity of disease activity in patients with RA. Corneal confocal microscopy is a rapid ophthalmic imaging modality which could be utilized in ophthalmic or optometry practices to help evaluate the severity and progression of RA.
Summary
What was known before
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease associated with multiple extra-articular manifestations.
Peripheral nervous system involvement is an underdiagnosed manifestation of RA.
Corneal confocal microscopy enables rapid, non-invasive and objective assessment of nerve damage and immune activation.
What this study adds
Corneal confocal microscopic analysis showed evidence of subepithelial nerve fibre loss and increased immune cells in patients with RA.
Corneal nerve loss and immune cell activation are associated with disease activity in RA.
Supplementary information
Author contributions
GB, AK, and RAM contributed to the conception and design of the study; GB, AK, MCE, GS, and RAM contributed to acquisition and analysis of data; GB, MCE and GS drafted the text and prepared the figures; AK and RAM reviewed the draft, provided suggestions and improvements.
Funding
This work was supported by The Scientific Research Coordination Center of Necmettin Erbakan University (Project ID: 201218001). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
All anonymized data that support the findings of this study are available to any qualified researcher upon reasonable request to the corresponding author (ORCID: 0000-0002-0509-5649).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-023-02447-6.
References
- 1.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62–67. [PubMed] [Google Scholar]
- 2.Lanzillo B, Pappone N, Crisci C, di Girolamo C, Massini R, Caruso G. Subclinical peripheral nerve involvement in patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:1196–202. doi: 10.1002/1529-0131(199807)41:7<1196::AID-ART8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.DeQuattro K, Imboden JB. Neurologic manifestations of rheumatoid arthritis. Rheum Dis Clin N Am. 2017;43:561–71. doi: 10.1016/j.rdc.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal V, Singh R, Wiclaf, Chauhan S, Tahlan A, Ahuja CK, et al. A clinical, electrophysiological, and pathological study of neuropathy in rheumatoid arthritis. Clin Rheumatol. 2008;27:841–4. doi: 10.1007/s10067-007-0804-x. [DOI] [PubMed] [Google Scholar]
- 5.Kaeley N, Ahmad S, Pathania M, Kakkar R. Prevalence and patterns of peripheral neuropathy in patients of rheumatoid arthritis. J Fam Med Prim Care. 2019;8:22–26. doi: 10.4103/jfmpc.jfmpc_260_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rolke R, Baron R, Maier C, Tölle TR, Treede -DR, Beyer A, et al. Quantitative sensory testing in the German Research Network on Neuropathic. Pain. 2006;123:231–43. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Bayrak AO, Durmus D, Durmaz Y, Demir I, Canturk F, Onar MK. Electrophysiological assessment of polyneuropathic involvement in rheumatoid arthritis: relationships among demographic, clinical and laboratory findings. Neurol Res. 2010;32:711–4. doi: 10.1179/016164109X12581096870195. [DOI] [PubMed] [Google Scholar]
- 8.Sim MK, Kim DY, Yoon J, Park DH, Kim YG. Assessment of peripheral neuropathy in patients with rheumatoid arthritis who complain of neurologic symptoms. Ann Rehabil Med. 2014;38:249–55. doi: 10.5535/arm.2014.38.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gemignani F, Giovanelli M, Vitetta F, Santilli D, Bellanova MF, Brindani F, et al. Non-length dependent small fibre neuropathy. a prospective case series. J Peripher Nerv Syst. 2010;15:57–62. doi: 10.1111/j.1529-8027.2010.00252.x. [DOI] [PubMed] [Google Scholar]
- 10.Birnbaum J. Small fibre neuropathy presenting during the antecedent period of undifferentiated arthritis prior to rheumatoid arthritis. Neurol Clin Pract. 2017;7:e47–e50. doi: 10.1212/CPJ.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Syngle V, Syngle A, Garg N, Krishan P, Verma I. Predictors of autonomic neuropathy in rheumatoid arthritis. Auton Neurosci. 2016;201:54–9. doi: 10.1016/j.autneu.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Efron N, Edwards K, Roper N, Pritchard N, Sampson GP, Shahidi AM, et al. Repeatability of measuring corneal subbasal nerve fibre length in individuals with type 2 diabetes. Eye Contact Lens. 2010;36:245–8. doi: 10.1097/ICL.0b013e3181eea915. [DOI] [PubMed] [Google Scholar]
- 13.Pacaud D, Romanchuk KG, Tavakoli M, Gougeon C, Virtanen H, Ferdousi M, et al. The reliability and reproducibility of corneal confocal microscopy in children. Invest Ophthalmol Vis Sci. 2015;56:5636–40. doi: 10.1167/iovs.15-16995. [DOI] [PubMed] [Google Scholar]
- 14.Wang EF, Misra SL, Patel DV. In vivo confocal microscopy of the human cornea in the assessment of peripheral neuropathy and systemic diseases. Biomed Res Int. 2015;2015:951081. doi: 10.1155/2015/951081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik RA, Kallinikos P, Abbott CA, van Schie CH, Morgan P, Efron N, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46:683–8. doi: 10.1007/s00125-003-1086-8. [DOI] [PubMed] [Google Scholar]
- 16.Messmer EM, Schmid-Tannwald C, Zapp D, Kampik A. In vivo confocal microscopy of corneal small fiber damage in diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 2010;248:1307–12. doi: 10.1007/s00417-010-1396-8. [DOI] [PubMed] [Google Scholar]
- 17.Hertz P, Bril V, Orszag A, Ahmed A, Ng E, Nwe P, et al. Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabet Med. 2011;28:1253–60. doi: 10.1111/j.1464-5491.2011.03299.x. [DOI] [PubMed] [Google Scholar]
- 18.Misra SL, Craig JP, Patel DV, McGhee CN, Pradhan M, Ellyett K, et al. In vivo confocal microscopy of corneal nerves: an ocular biomarker for peripheral and cardiac autonomic neuropathy in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2015;56:5060–5. doi: 10.1167/iovs.15-16711. [DOI] [PubMed] [Google Scholar]
- 19.Evdokimov D, Frank J, Klitsch A, Unterecker S, Warrings B, Serra J, et al. Reduction of skin innervation is associated with a severe fibromyalgia phenotype. Ann Neurol. 2019;86:504–16. doi: 10.1002/ana.25565. [DOI] [PubMed] [Google Scholar]
- 20.Tavakoli M, Marshall A, Pitceathly R, Fadavi H, Gow D, Roberts ME, et al. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol. 2010;223:245–50. doi: 10.1016/j.expneurol.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bitirgen G, Turkmen K, Malik RA, Ozkagnici A, Zengin N. Corneal confocal microscopy detects corneal nerve damage and increased dendritic cells in Fabry disease. Sci Rep. 2018;8:12244. doi: 10.1038/s41598-018-30688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang JCB, Goldstein D, Park SB, Krishnan AV, Markoulli M. Corneal nerve changes following treatment with neurotoxic anticancer drugs. Ocul Surf. 2021;21:221–37. doi: 10.1016/j.jtos.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Stettner M, Hinrichs L, Guthoff R, Bairov S, Petropoulos IN, Warnke C, et al. Corneal confocal microscopy in chronic inflammatory demyelinating polyneuropathy. Ann Clin Transl Neurol. 2015;3:88–100. doi: 10.1002/acn3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bitirgen G, Akpinar Z, Malik RA, Ozkagnici A. Use of corneal confocal microscopy to detect corneal nerve loss and increased dendritic cells in patients with multiple sclerosis. JAMA Ophthalmol. 2017;135:777–82. doi: 10.1001/jamaophthalmol.2017.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bitirgen G, Tinkir Kayitmazbatir E, Satirtav G, Malik RA, Ozkagnici A. In vivo confocal microscopic evaluation of corneal nerve fibres and dendritic cells in patients with Behçet’s disease. Front Neurol. 2018;9:204. doi: 10.3389/fneur.2018.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bitirgen G, Kucuk A, Ergun MC, Baloglu R, Gharib MH, Al Emadi S, et al. Subclinical corneal nerve fibre damage and immune cell activation in systemic lupus erythematosus: a corneal confocal microscopy study. Transl Vis Sci Technol. 2021;10:10. doi: 10.1167/tvst.10.14.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 28.Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 29.van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998;41:1845–50. doi: 10.1002/1529-0131(199810)41:10<1845::AID-ART17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 30.Kalteniece A, Ferdousi M, Adam S, Schofield J, Azmi S, Petropoulos I, et al. Corneal confocal microscopy is a rapid reproducible ophthalmic technique for quantifying corneal nerve abnormalities. PLoS ONE. 2017;12:e0183040. doi: 10.1371/journal.pone.0183040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dabbah MA, Graham J, Petropoulos IN, Tavakoli M, Malik RA. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med Image Anal. 2011;15:738–47. doi: 10.1016/j.media.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Khan A, Li Y, Ponirakis G, Akhtar N, Gad H, George P, et al. Corneal immune cells are increased in patients with multiple sclerosis. Transl Vis Sci Technol. 2021;10:19. doi: 10.1167/tvst.10.4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villani E, Galimberti D, Viola F, Mapelli C, Del Papa N, Ratiglia R. Corneal involvement in rheumatoid arthritis: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2008;49:560–4. doi: 10.1167/iovs.07-0893. [DOI] [PubMed] [Google Scholar]
- 34.Marsovszky L, Resch MD, Németh J, Toldi G, Medgyesi E, Kovács L, et al. In vivo confocal microscopic evaluation of corneal Langerhans cell density, and distribution and evaluation of dry eye in rheumatoid arthritis. Innate Immun. 2013;19:348–54. doi: 10.1177/1753425912461677. [DOI] [PubMed] [Google Scholar]
- 35.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–63. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pouget J. Neuropathies des vascularites [Vascular neuropathies] Rev Prat. 2000;50:749–52. [PubMed] [Google Scholar]
- 37.Salih AM, Nixon NB, Gagan RM, Heath P, Hawkins CP, Dawes PT, et al. Anti-ganglioside antibodies in patients with rheumatoid arthritis complicated by peripheral neuropathy. Br J Rheumatol. 1996;35:725–31. doi: 10.1093/rheumatology/35.8.725. [DOI] [PubMed] [Google Scholar]
- 38.Conn DL, McDuffie FC, Dyck PJ. Immunopathologic study of sural nerves in rheumatoid arthritis. Arthritis Rheum. 1972;15:135–43. doi: 10.1002/art.1780150202. [DOI] [PubMed] [Google Scholar]
- 39.Puéchal X, Said G, Hilliquin P, Coste J, Job-Deslandre C, Lacroix C, et al. Peripheral neuropathy with necrotizing vasculitis in rheumatoid arthritis. A clinicopathologic and prognostic study of thirty-two patients. Arthritis Rheum. 1995;38:1618–29. doi: 10.1002/art.1780381114. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Graham J, Dabbah MA, Petropoulos IN, Ponirakis G, Asghar O, et al. Small nerve fibre quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fibre density. Diabetes Care. 2015;38:1138–44. doi: 10.2337/dc14-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rousseau A, Cauquil C, Dupas B, Labbé A, Baudouin C, Barreau E, et al. Potential role of in vivo confocal microscopy for imaging corneal nerves in transthyretin familial amyloid polyneuropathy. JAMA Ophthalmol. 2016;134:983–9. doi: 10.1001/jamaophthalmol.2016.1889. [DOI] [PubMed] [Google Scholar]
- 42.Xu J, Chen P, Yu C, Liu Y, Hu S, Di G. In vivo confocal microscopic evaluation of corneal dendritic cell density and subbasal nerve parameters in dry eye patients: a systematic review and meta-analysis. Front Med. 2021;8:578233. doi: 10.3389/fmed.2021.578233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tepelus TC, Chiu GB, Huang J, Huang P, Sadda SR, Irvine J, et al. Correlation between corneal innervation and inflammation evaluated with confocal microscopy and symptomatology in patients with dry eye syndromes: a preliminary study. Graefes Arch Clin Exp Ophthalmol. 2017;255:1771–8. doi: 10.1007/s00417-017-3680-3. [DOI] [PubMed] [Google Scholar]
- 44.Birnbaum J, Lalji A, Saed A, Baer AN. Biopsy-proven small-fibre neuropathy in primary Sjögren’s syndrome: Neuropathic pain characteristics, autoantibody findings, and histopathologic features. Arthritis Care Res. 2019;71:936–48. doi: 10.1002/acr.23762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerrero-Moreno A, Baudouin C, Melik Parsadaniantz S, Réaux-Le Goazigo A. Morphological and functional changes of corneal nerves and their contribution to peripheral and central sensory abnormalities. Front Cell Neurosci. 2020;14:610342. doi: 10.3389/fncel.2020.610342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li F, Zhang Q, Ying X, He J, Jin Y, Xu H, et al. Corneal nerve structure in patients with primary Sjögren’s syndrome in China. BMC Ophthalmol. 2021;21:211. doi: 10.1186/s12886-021-01967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438–510. doi: 10.1016/j.jtos.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 48.Dehghani C, Frost S, Jayasena R, Fowler C, Masters CL, Kanagasingam Y, et al. Morphometric changes to corneal dendritic cells in individuals with mild cognitive impairment. Front Neurosci. 2020;14:556137. doi: 10.3389/fnins.2020.556137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chinnery HR, Zhang XY, Wu CY, Downie LE. Corneal immune cell morphometry as an indicator of local and systemic pathology: a review. Clin Exp Ophthalmol. 2021;49:729–40. doi: 10.1111/ceo.13972. [DOI] [PubMed] [Google Scholar]
- 50.Page G, Lebecque S, Miossec P. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J Immunol. 2002;168:5333–41. doi: 10.4049/jimmunol.168.10.5333. [DOI] [PubMed] [Google Scholar]
- 51.Canavan M, Marzaioli V, Bhargava V, Nagpal S, Gallagher P, Hurson C, et al. Functionally mature CD1c+ dendritic cells preferentially accumulate in the inflammatory arthritis synovium. Front Immunol. 2021;12:745226. doi: 10.3389/fimmu.2021.745226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villani E, Galimberti D, Del Papa N, Nucci P, Ratiglia R. Inflammation in dry eye associated with rheumatoid arthritis: cytokine and in vivo confocal microscopy study. Innate Immun. 2013;19:420–7. doi: 10.1177/1753425912471692. [DOI] [PubMed] [Google Scholar]
- 53.Leppin K, Behrendt AK, Reichard M, Stachs O, Guthoff RF, Baltrusch S, et al. Diabetes mellitus leads to accumulation of dendritic cells and nerve fibre damage of the subbasal nerve plexus in the cornea. Invest Ophthalmol Vis Sci. 2014;55:3603–15. doi: 10.1167/iovs.14-14307. [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi T, Hamrah P, Shimazaki J. Bilateral alterations in corneal nerves, dendritic cells, and tear cytokine levels in ocular surface disease. Cornea. 2016;35(Suppl 1):S65–S70. doi: 10.1097/ICO.0000000000000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barcelos F, Hipólito-Fernandes D, Martins C, Ângelo-Dias M, Cardigos J, Monteiro R, et al. Corneal sub-basal nerve plexus assessment and its association with phenotypic features and lymphocyte subsets in Sjögren’s Syndrome. Acta Ophthalmol. 2021;99:e1315–e1325. doi: 10.1111/aos.14811. [DOI] [PubMed] [Google Scholar]
- 56.Cimmino MA, Salvarani C, Macchioni P, Montecucco C, Fossaluzza V, Mascia MT, et al. Extra-articular manifestations in 587 Italian patients with rheumatoid arthritis. Rheumatol Int. 2000;19:213–7. doi: 10.1007/PL00006853. [DOI] [PubMed] [Google Scholar]
- 57.Caspi D, Elkayam O, Eisinger M, Vardinon N, Yaron M, Burke M. Clinical significance of low titer anti-nuclear antibodies in early rheumatoid arthritis: implications on the presentation and long-term course of the disease. Rheumatol Int. 2001;20:43–7. doi: 10.1007/s002960000073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All anonymized data that support the findings of this study are available to any qualified researcher upon reasonable request to the corresponding author (ORCID: 0000-0002-0509-5649).