Abstract
Many orphan G protein-coupled receptors (GPCRs) remain understudied because their endogenous ligands are unknown. Here, we show that a group of class A/rhodopsin-like orphan GPCRs including GPR61, GPR161 and GPR174 increase the cAMP level similarly to fully activated D1 dopamine receptor (D1R). We report cryo-electron microscopy structures of the GPR61‒Gs, GPR161‒Gs and GPR174‒Gs complexes without any exogenous ligands. The GPR174 structure reveals that endogenous lysophosphatidylserine (lysoPS) is copurified. While GPR174 fails to respond to exogenous lysoPS, likely owing to its maximal activation by the endogenous ligand, GPR174 mutants with lower ligand binding affinities can be specifically activated by lysoPS but not other lipids, in a dose-dependent manner. Moreover, GPR174 adopts a non-canonical Gs coupling mode. The structures of GPR161 and GPR61 reveal that the second extracellular loop (ECL2) penetrates into the orthosteric pocket, possibly contributing to constitutive activity. Our work definitively confirms lysoPS as an endogenous GPR174 ligand and suggests that high constitutive activity of some orphan GPCRs could be accounted for by their having naturally abundant ligands.
Subject terms: Cryoelectron microscopy, Cryoelectron microscopy, G protein-coupled receptors
A number of orphan GPCRs show high constitutive activity. Here, the authors show that the high basal activity of some receptors can be explained by their sensitivity to naturally abundant lipids or by penetration of ECL2 in the orthosteric binding pocket.
Introduction
Given their wide distribution in the human body, versatile ligand recognition, and diverse downstream pathways, GPCRs contribute to virtually every aspect of physiology and pathology. As such, GPCRs are the most important class of targets for drug discovery campaigns. GPCRs are known to recognize a wide range of stimuli including light, odors, neurotransmitters, hormones, and peptides. However, there are over 100 GPCRs with no characterized endogenous ligands and clear biological functions, which are called orphan GPCRs (oGPCRs)1,2. Defining the endogenous ligands for oGPCRs is very important for understanding their physiological functions, and opens new possibilities for drug discovery against diseases with unmet medical needs. Notably, a large number of GPCRs including some oGPCRs show constitutive activity3,4, isomerizing from inactive state to active state in the absence of exogenous agonists. The discovery of engineered adrenoceptors mutants and disease-causing GPCR mutants with increased constitutive activity suggested that the constitutive activity of GPCRs is partly attributed to their intrinsic molecular properties3,5. For instance, an ‘ionic lock’ between the conserved D/ERY motif on TM3 is required to maintain the inactive state. Mutations that disrupt the ionic lock lead to receptor activation and increase constitutive activity6,7. Moreover, recent structural studies have shown that some oGPCRs that exhibit high constitutive activity may be self-activated by the second extracellular loop (ECL2), which penetrates into the orthosteric binding pocket (OBP)8–11.
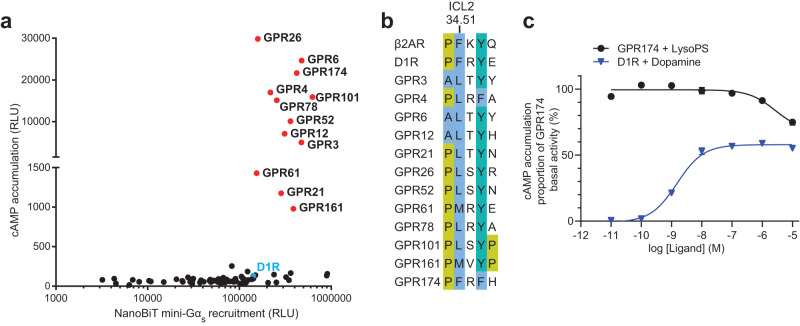
Building upon previous studies that evaluated the constitutive activity of 40 class A oGPCRs using a cAMP-dependent response element (CRE) based reporter assay12, we comprehensively analyzed the constitutive activity of 81 class A oGPCRs (annotated in GPCRdb13) expressed in Expi293F cells using the GloSensor cAMP accumulation assay. We found that 12 oGPCRs remarkably increased the intracellular cAMP level, compared with other oGPCRs and D1 dopamine receptors (D1R) without ligand treatment (Fig. 1a and Supplementary Table 1). Most of these receptors are expressed at lower levels compared to D1R (Supplementary Fig. 1a). Further efforts to increase the expression level of D1R have little effect on its basal activity, which is still remarkably lower than that of GPR174 even when expressed at a low level (Supplementary Fig. 1b), suggesting that the higher constitutive activities of these receptors are not attributed to their expression levels. These constitutively active oGPCRs are abbreviated as caoGPCRs hereafter. Interestingly, most of the caoGPCRs are closely related to GPCRs known to be activated by lipids (Supplementary Fig. 1c). Based on their sequence and structural similarity, they are further classified into three major groups: I (GPR26, GPR78, GPR101, GPR161), II (GPR3, GPR6, GPR12), III (GPR21, GPR52). The receptors in group I are closely related to prostanoid receptors. Structural predictions by Alphafold reveal that ECL2 in group I form an antiparallel β-sheet, covering the ligand-binding pocket, which is observed in prostanoid receptors (Supplementary Fig. 1d). A phytoestrogen, genistein has been reported to increase the cAMP level in cells expressing GPR26 and GPR78 (US patents: US7872101B1). GPR3, GPR6, and GPR12 in group II share about 60% sequence identity, and are phylogenetically related to sphingosine-1-phosphate receptors (S1PR) and cannabinoid receptors (Supplementary Fig. 1c). S1P, sphingosylphosphorylcholine (SPC) and cannabidiol have been identified as putative modulators of GPR3, GPR6, and GPR1214. GPR21 and GPR52 with 71% sequence identity in group III share about 30% sequence similarity with GPR119 that can be activated by lysophosphatidylcholine (lysoPC)15,16. While the endogenous ligands of GPR21 and GPR52 have not been identified, several surrogate agonists for GPR52 show high lipophilicity and poor water solubility, suggesting the hydrophobicity of the ligand binding pocket. Recent structural studies suggested that GPR21 and GPR52 can be self-activated by ECL2, which contributes to their high constitutive activity. GPR174 has been shown to be activated by lysophophatidylserine (lysoPS) via Gα12/13 and Gαs4,17,18. LysoPS-mediated GPR174 activation restrains T regulatory cell development and function and conventional T cell proliferation and modulates B cell gene expression via Gαs19–23. Given the possible relationship between lipids and caoGPCRs, we reasoned that the high constitutive activity of caoGPCRs may be attributed to the stimulation by endogenous lipids released from transfected cells where they are expressed. Therefore, we sought to determine the cryo-electron microscopy (cryo-EM) structures of caoGPCR‒Gs signaling complexes purified from mammalian cells to unravel molecular mechanisms underlying constitutive activity.
Fig. 1. A group of class A oGPCRs dramatically increase cAMP levels via the Gs pathway.
a GloSensor cAMP accumulation assay and NanoBiT mini-Gs recruitment assay performed in Expi293F cells transiently expressing any of 81 oGPCRs or D1R with relative luminescence unit (RLU) value shown. Receptors that show high cAMP levels compared with the other oGPCRs are colored in red. b Sequence alignment of ICL2 in caoGPCRs, D1R and β2AR. Residues are highlighted based on the Clustal color scheme. c Concentration–response curves in the cAMP accumulation assay at Expi293F cells transiently transfected with GPR174 or D1R treated with lysoPS or dopamine. Each data point represents mean ± SEM from three independent experiments. Source data are provided as a Source Data file.
In this work, we show that the high constitutive activity of GPR174 can be attributed to endogenous lysoPS, which occupies the OBP of GPR174, whereas penetration of ECL2 in the OBP of GPR61 and GPR161 possibly contributes to the high constitutive activity. Some Gs-coupled receptors including GPR174 adopt a non-canonical Gs coupling mode, where TM6 shows less pronounced movement compared with that in Gi/o-coupled receptors. We found that the non-canonical Gs coupling mode is due to the presence of a larger hydrophobic residue (L or C) at position 5.65 of receptors, while a small hydrophobic residue (A or V) at this position leads to the canonical Gs coupling mode.
Results
Structural approach to identify endogenous ligands of oGPCRs
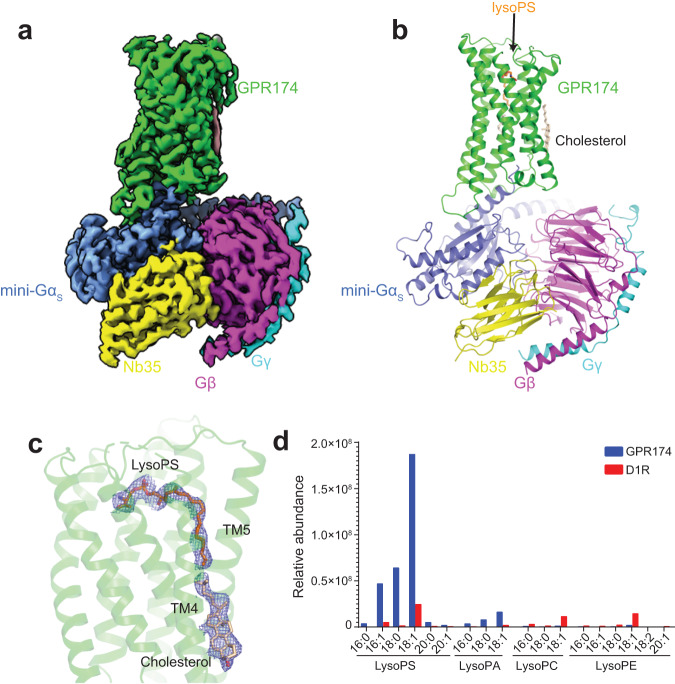
In addition to increasing cAMP levels, caoGPCRs recruit Gαs more efficiently than most other oGPCRs (Fig. 1a and Supplementary Table 1), as shown by NanoBiT mini-Gαs recruitment assay24. Moreover, they all contain a large hydrophobic residue (L, M, or F) at position 34.51 of the second intracellular loop (ICL2) (Fig. 1b), which is essential for Gs coupling25. These results indicate that caoGPCRs can increase the cAMP level through Gs. Notably, the cAMP levels induced by expression of GPR174 alone are almost two-fold of those induced by fully activated D1R treated with dopamine (Fig. 1c). Although previous studies have shown that lysoPS can activate GPR174 in a dose-dependent manner using a transforming growth factor-α (TGFα) shedding assay17, our GloSensor cAMP assay results revealed that lysoPS failed to further increase cAMP levels in cells expressing GPR174 (Fig. 1c). Owing to its amphipathic characteristics, very high concentration of lysoPS disrupts membrane structure and causes cell lysis, thereby leading to a reduced cAMP level. We speculated the endogenous ligands might have occupied the receptor, leading to its maximal activation in the cAMP assay. To test this hypothesis, we attempted to identify the endogenous ligands of caoGPCRs using structural approaches complemented by mass spectrometry (MS). Since G protein binding with GPCRs can enhance agonist binding26, we purified GPR174‒mini-Gαs fusion protein from Expi293F cells (the same cell type used for our signaling assays) through affinity purification and assembled GPR174‒mini-Gαs fusion protein with purified Gβ1γ2 and Nb35 using size-exclusion chromatography in the absence of exogenous ligands (Supplementary Fig. 2a). The structure of the GPR174‒Gs complex was determined using cryo-EM to a nominal resolution of 2.9 Å (Supplementary Fig. 2b–f and Supplementary Table 2). An unassigned density was observed in the orthosteric pocket of GPR174 after building all protein structure models (Fig. 2a–c). LysoPS with 15 carbons in the acyl chain can be precisely modeled due to the high quality of EM density. To further confirm the identity of lysoPS, we performed lipidomics analysis of GPR174‒Gs, using D1R‒Gs as a control, by MS (Fig. 2d and Supplementary 3a). LysoPS isoforms with acyl chain lengths ranging from 16:0 to 20:1 were remarkably more abundantly present in GPR174‒Gs, compared with D1R‒Gs. As 18:1 lysoPS is the most dominant lipid present in GPR174, we further verified its binding pose using molecular dynamics (MD) simulations. LysoPS stably associates with GPR174 during the course of 100-ns MD (Supplementary Fig. 3b). The acyl chain particularly the first two carbons displayed more flexibility than the polar group, explaining the absence of the terminal carbons in the structure. Noteworthily, lysophospholipids (LPs) present in protein samples are mainly synthesized by cells as they are barely detected in the fresh culture medium. Although cells release a larger quantity of lysoPE and lysoPC than lysoPS27, lysoPS is specifically copurified with GPR174 after a harsh purification procedure, indicating their high affinity and specific binding. Since lysoPS can be produced by many and possibly all mammalian cells, it is not surprising that GPR174 also exhibits high basal activity when expressed in other cell lines such as Chinese Hamster Ovary (CHO) cells and HeLa cells (Supplementary Fig. 3c, d).
Fig. 2. The endogenous lysoPS is copurified with GPR174.
a Cryo-EM map of the GPR174 and mini-Gs complex. b Ribbon representation of the GPR174 and mini-Gs complex. LysoPS and cholesterol are shown as sticks. c Cryo-EM density map of lysoPS and cholesterol in the GPR174‒Gs complex at a contour level of 3.5σ. d Lipidomics analysis of the GPR174‒Gs and D1R‒Gs complexes by MS. The relative abundance of representative LPs with the acyl chain length from 16:0 to 20:1 determined by MS is shown. Source data are provided as a Source Data file.
LysoPS recognition by GPR174
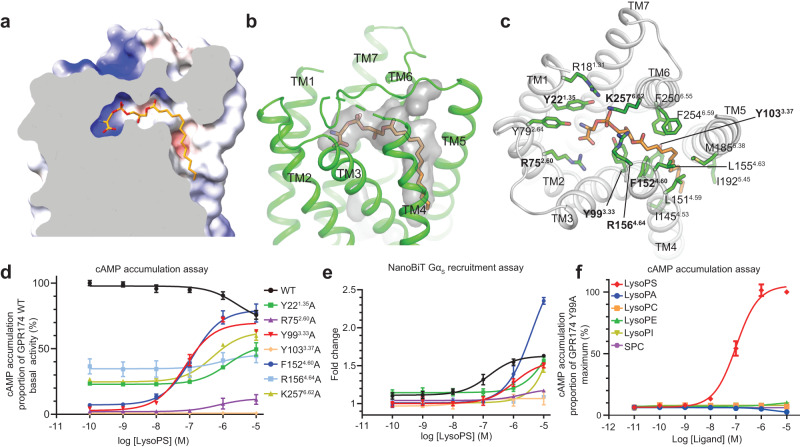
When bound to GPR174, lysoPS forms an L-shape configuration. Its polar head group lies in the orthosteric pocket, parallel to the membrane plane, whereas the fatty acyl chain is nearly perpendicular to the membrane and extends into the membrane portal between TM4 and TM5 (Fig. 3a, b). LysoPS shows perfect charge and shape complementarity with the binding pocket of the receptor and buries a surface area of 614 Å2, accounting for its high-affinity binding with GPR174. Notably, underneath lysoPS is a cholesterol molecule that binds on the surface of TM3, TM4, and TM5. Because of their close contact, cholesterol may potentially further enhance lysoPS binding to the receptor, suggesting a positive allosteric effect of the endogenous cholesterol (Fig. 2c). Cholesterol is also observed at a similar position in D1R, and interacts with a positive allosteric modulator LY315420728. Specifically, the terminal amine group of serine in lysoPS engages in a hydrogen bond and a cation–π interaction with Y792.64 and Y221.35, respectively, while it’s carboxyl acid group forms salt bridge interactions with R752.60 (Fig. 3c and Supplementary Fig. 2f). The phosphate group is covered by three spatially separated basic residues, namely R181.31, R1564.64, and K2576.62 (Fig. 3c). Y993.33 underneath lysoPS forms hydrogen bonds with hydroxyl groups at the sn-2 and sn-3 positions of the glycerol moiety. The acyl chain is encircled by a number of hydrophobic residues including Y1033.37, L1514.59, F1524.60, L1554.63, M1855.38, F2506.55, and F2546.59. Consistent with our structural observations, mutation of R752.60, Y993.33, Y1033.37, and F1524.60 almost completely suppressed the basal activity; mutation of Y221.35, R1564.64, or K2576.62 reduced the basal activity by more than 50% (Supplementary Fig. 3e). Interestingly, in contrast to GPR174 wild-type (WT), most mutants can be activated by lysoPS in a dose-dependent manner with distinct potency (EC50) and maximal effect (Emax) (Fig. 3d). LysoPS can activate the Y993.33A and F1524.60A mutants with Emax nearly approaching the basal activity of WT and potencies of 71.6 and 119.7 nM, respectively (Supplementary Table 3). Given the lack of potency of lysoPS for GPR174 WT in the cAMP assay, it is unclear to what extent these mutations alter the potency of lysoPS. Therefore, we sought to measure the potency of lysoPS for WT using the NanoBiT Gs recruitment assay which has a lower amplification than the cAMP assay (Fig. 3e). For D1R, the potencies of dopamine obtained from the NanoBiT assay and cAMP assay are 2.1 μM and 3.6 nM (about 1000-fold difference), respectively (Supplementary Fig. 3f, g). As expected, lysoPS can activate GPR174 WT in a dose-dependent manner with a potency of 155.7 nM using the NanoBiT Gs recruitment assay (Supplementary Table 3), and all mutations of GPR174 tested above remarkably reduce the potency of lysoPS, which is consistent with results using the cAMP assay (Fig. 3d and e and Supplementary Fig. 3e). For the Y993.33A mutant, the potency is reduced by about 7-fold compared to GPR174 WT in the NanoBiT assay (Supplementary Table 3). Therefore, we speculate that the potency of lysoPS for WT using the cAMP assay could be in a single-digit nanomolar range. The concentration of lysoPS generated by cells is likely to be higher than that required to produce the maximal effect of WT, explaining how GPR174 exhibits extremely high basal activity and that the exogenous lysoPS cannot further increase the cAMP level. The potency of the Y993.33A mutant using the cAMP assay is comparable to that of WT using the TGFα shedding assay, which exceeds the concentration of lysoPS released by Expi293F or HEK293 cells explaining that they show dose–response curves when treated with exogenous lysoPS. Although GPR119 can be activated by lysoPC and is more abundantly synthesized than lysoPS in cells15,16, its basal activity is similar to D1R without ligand treatment because of the low micromolar potency of lysoPC for GPR119 (Supplementary Table 1). Moreover, we tested activity of other LPs for the Y993.33A mutant, and none of them could activate this mutant, supporting the specific recognition of lysoPS by GPR174 (Fig. 3f). From a structural perspective, lysoPE, lysoPC and lysophosphatidic acid (lysoPA) that lack the terminal carboxylic acid group involved in binding R752.60 is analogous to R752.60 mutation which almost abolished the activity of lysoPS (Fig. 3d and e and Supplementary Fig. 3a). The inositol group in lysophosphatidylinositol (lysoPI) that is larger than serine in lysoPS cannot be accommodated in the binding pocket of GPR174 (Fig. 3b).
Fig. 3. Specific recognition of lysoPS by GPR174.
a Electrostatic potential of the lysoPS binding surface colored from red to blue for negatively and positively charged regions. b GPR174 shows perfect shape complementarity with lysoPS. The surface of interior pocket of GPR174 is shown in gray. c Detailed interactions between GPR174 and lysoPS. d and e Concentration–response curves of GPR174 mutants treated with increasing concentrations of lysoPS in the cAMP accumulation assay (d) and the NanoBiT Gαs recruitment assay (e). All measurements were performed in three independent times, shown as mean ± SEM. Source data are provided as a Source Data file. f Concentration–response curves of GPR174/Y993.33A mutants treated with different LPs in the cAMP accumulation assay. Each data point represents mean ± SEM from three independent experiments. Source data are provided as a Source Data file.
Occupation of ECL2 in the orthosteric pocket of GPR161 and GPR61
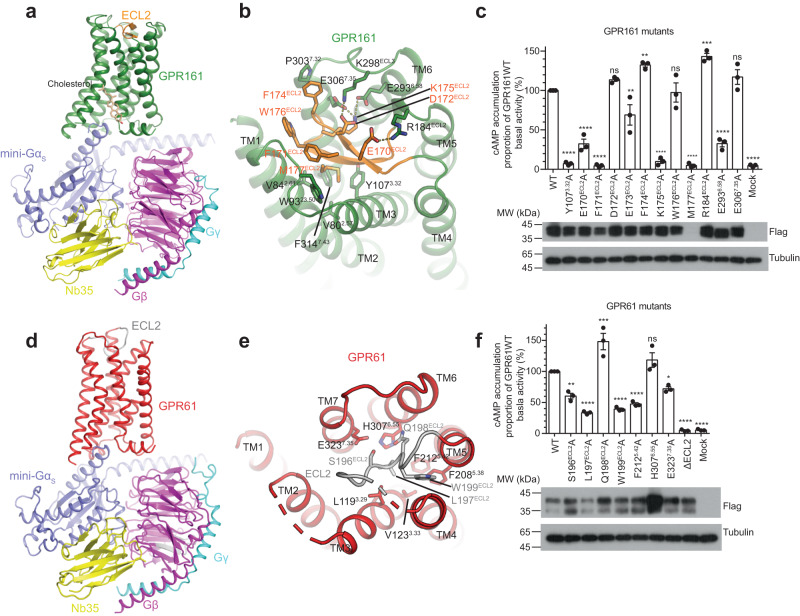
Recently published structures of GPR1211 in subgroup II and GPR219,10 and GPR528 in subgroup III in complex with Gs revealed no ligands bound to the receptors, but instead showed that ECL2 of these receptors penetrate into the orthosteric pocket, leading to a self-activation model. Since no structures of receptors in group I have been reported, we further determined the cryo-EM structures of GPR161‒Gs and GPR61‒Gs complexes using the same strategy as GPR174 at nominal resolutions of 3.1 and 3.2 Å, respectively (Supplementary Figs. 4, 5 and Supplementary Table 2). The constitutively active GPR161 localizes to cilia and inhibits Sonic hedgehog signaling (Shh) by proteolysis of full-length Gli3 into its repressor form via the Gs-cAMP-protein kinase A pathway29–31, which is required for normal embryonic development. GPR161 has been shown to be associated with medulloblastoma and breast cancer32–34. The GPR161 structure reveals that ECL2 forms an anti-parallel β sheet and is buried inside the receptor (Fig. 4a). The β-sheet structure of ECL2 universally exists in GPCRs that recognize prostanoids and peptides35. In the absence of ligands, ECL2 in peptide GPCRs is flexible and solvent-accessible and adopts distinct conformations when bound to different ligands. In contrast, reminiscent of prostanoid receptors, one β strand of ECL2 in GPR161 penetrates into the receptor pocket, and is completely occluded from the solvent, whereas the other β strand faces the solvent (Fig. 4b and Supplementary Fig. 6a). In the solvent-accessible strand, E170ECL2, and D172ECL2 potentially form salt bridge interactions with R184ECL2 and K298ECL3 respectively (Fig. 4b and Supplementary Fig. 4f); F171ECL2 makes van der Waals contact with the backbone and W9323.50 in ECL1. K175ECL2 in the β-turn potentially engages two salt bridges with E2936.58 and E3067.35. M177ECL2 in the other strand is inserted into a hydrophobic pocket formed by V802.57, V842.61, Y1073.32, and F3147.43. Consistently, mutation of Y1073.32, F171ECL2, or K175ECL2 completely suppressed the basal activity of GPR161; and mutation of E170ECL2 or E2936.58 significantly reduced its basal activity (Fig. 4c). However, it is unclear how mutation of F174ECL2 or R184ECL2 can enhance the basal activity. All these mutants except M177ECL2A are expressed at comparable levels with WT. Instead of forming β hairpin structure, ECL2 in the structure of GPR61 and previously reported structures of GPR21, GPR52, and GPR12 are organized into a short loop structure (Fig. 4d). Nevertheless, ECL2 in GPR61 shows shallower penetration in the orthosteric pocket compared to that in GPR21 and GPR52 (Supplementary Fig. 6b). Major contacts between ECL2 and the other parts of GPR61 are limited to the residues, namely S196, L197, and W199 (Fig. 4e and Supplementary Fig. 5f). S196ECL2 make a hydrogen bond with E3237.35; L197ECL2 penetrates into a hydrophobic pocket formed by V1233.33, L1193.29, F2125.42, and H3076.55; and W199ECL2 makes van der Waals contact with the backbone of TM4. Mutation of S196ECL2, L197ECL2, or W199ECL2 dramatically reduced the basal activity of GPR61 (Fig. 4f), and combined mutations of residues in ECL2 (S196ECL2G/L197ECL2G/Q198ECL2G/W199ECL2G, ΔECL2) completely impaired the basal activity of GPR61. In contrast, mutation of Q198ECL2 alone increased the basal activity. We speculate that the interaction between Q198ECL2 and H3076.55 limits the outward movement of TM6. Therefore, abolishing their interactions may lower the energy barrier of the active state. These mutations had minimal or no effect on receptor expression level compared with WT (Fig. 4f). These results suggest that penetration of ECL2 in the orthosteric pocket of receptors contributes to the constitutive activity.
Fig. 4. ECL2 is penetrated into the orthosteric binding pocket of GPR161 and GPR61.
a and d Ribbon representation of the GPR161‒Gs (a) and GPR61‒Gs (d) complexes with ECL2 colored in orange and gray respectively. b and e Detailed interactions between ECL2 and other regions of GPR161 (b) or GPR61 (e). c and f The basal activity of GPR161 (c) or GPR61 (f) mutants determined by the cAMP assay. Bar graphs represent mean ± SEM from three independent experiments. Expression levels of mutants were determined by western blot using an anti-Flag antibody. Statistical analysis was performed using one-way ANOVA method (ns, not significant; *P < 0.1; **P < 0.01; ***P < 0.001; ****P < 0.0001). Source data are provided as a Source Data file. P values for GPR161 mutants versus WT are <0.0001, <0.0001, <0.0001, 0.5954, 0.0098, 0.0067, <0.0001, 0.9996, <0.0001, 0.0003, <0.0001, 0.3316, <0.0001 (from left to right). P values for GPR61 mutants versus WT are 0.001, <0.0001, 0.0001, <0.0001, <0.0001, 0.2203, 0.0230, <0.0001, <0.0001 (from left to right).
Non-canonical Gs coupling mode
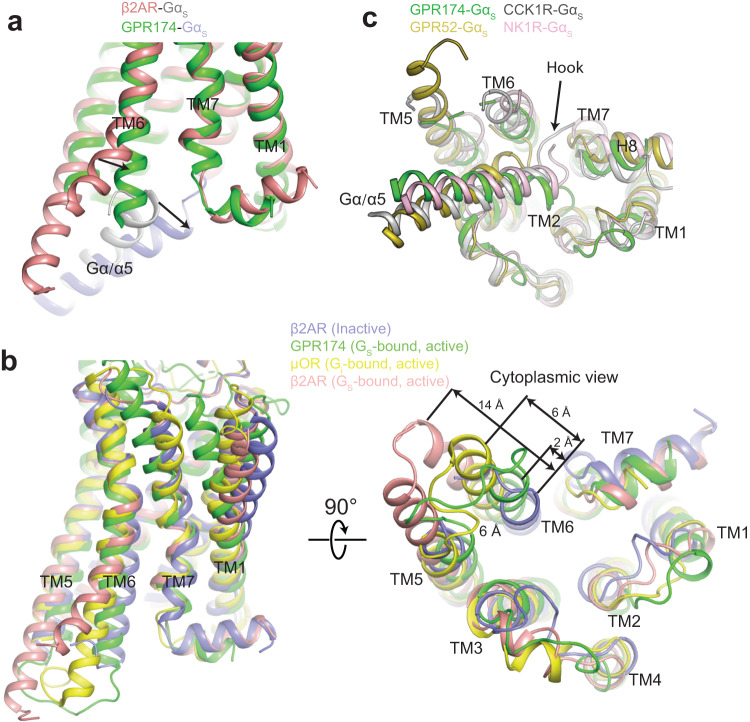
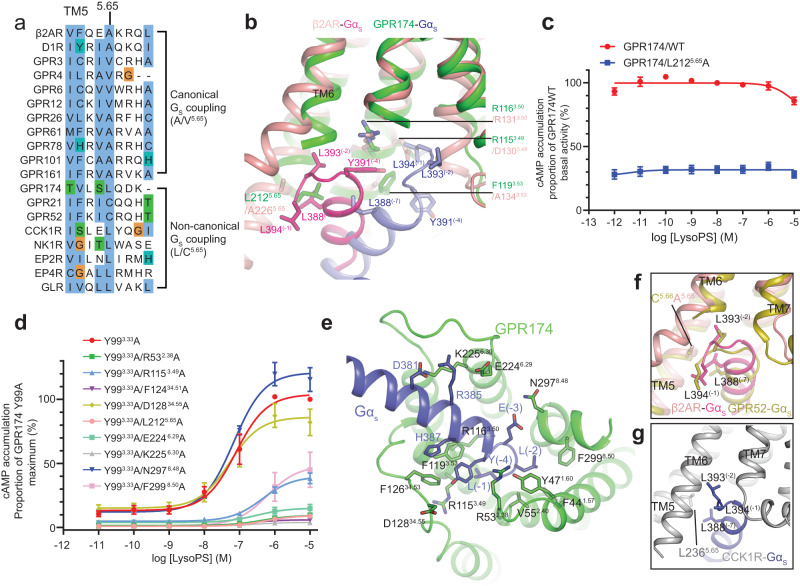
Both GPR61 and GPR161 adopt a canonical Gs coupling mode, which is observed in most class A Gs-coupled receptors such as β2 adrenergic receptors (β2AR)36 and D1R37 (Supplementary Fig. 6c). When bound to the receptors, the α5 helix of Gαs undergoes rotational and translational movement, with the extreme C-terminus of α5 assuming a “hook” conformation. The “hook” is inserted into the intracellular cavity of the receptor and is in direct contact with TM6 and TM5, leading to an outward movement of TM6 and TM5. Owing to the bulkier size of the “hook” in Gαs, the outward movement of TM6 in Gs-coupled GPCRs is more pronounced than in GPCRs that couple to the other G protein subtypes. Strikingly, when bound to GPR174 instead of contacting TM6, the “hook” is distorted and protruded into a groove formed by TM1, TM2, and TM7. Compared with inactive β2AR, TM6 in GPR174 moves outward by only 2 Å, which is smaller than the outward displacement of TM6 in the active β2AR (14 Å) and the active μ opioid receptor that is bound to Gαi (6 Å)38 (Fig. 5b). The less pronounced movement of TM6 in active Gs-coupled receptors can be considered as a non-canonical Gs coupling mode, which has been observed in GPR21/528, neurokinin 1 receptor (NK1R)39,40 and cholecystokinin 1 receptor (CCK1R)41,42 (Fig. 5c). Although they share a similar extent of the outward movement of TM6, they induce various conformational changes of the “hook”. Comparison of structures of GPR174‒Gs and β2AR‒Gs complexes reveals unique sequence features in GPR174 that determine the non-canonical coupling. Our previous studies have identified an A/V5.65Ф5.69Ф5.72 (Ф represents hydrophobic residues) motif at TM5 that is prevalent in the majority of Gs-coupled receptors including most caoGPCRs (Fig. 6a)37. In receptors such as β2AR and D1R that adopt canonical Gs coupling mode, A5.65 is projected into a hydrophobic pocket formed by L394(−1), L393(−2), and L388(−7) (−1 indicates the last residue) at the extreme C-terminus of Gαs, which we called a tri-leucine pocket (Fig. 6a and b). Mutation of A5.65 to the large hydrophobic residue leucine causes a steric clash with the tri-leucine pocket, and thereby dramatically reduces the Gs coupling efficiency of D1R, while its mutation to the relatively small hydrophobic residue valine has little effect37. Therefore, as a result of the substitution of A5.65 with L5.65 in GPR174, the tri-leucine pocket observed in the canonical Gs coupling mode collapses due to steric clash with L5.65, leading to a distorted “hook”. Instead, L2125.65 in GPR174 forms strong hydrophobic interactions with L388(−7) (Fig. 6b). In contrast to D1R that prefers a small hydrophobic residue (A or V) at position 5.65, mutation of L2125.65 in alanine remarkably reduces the Gs coupling efficiency either in the context of GPR174WT or GPR174/Y99A mutant (Fig. 6c and d). Meanwhile, GPR174 acquires unique sequence features to accommodate tri-leucine residues. D/E3.49 in the D/E3.49R3.50Y3.51 motif that exists in most class A GPCRs is replaced by R in GPR174. L394(−1) is positioned in the hydrophobic pocket formed by the aliphatic part of R1153.49, R1163.50 and F1193.53, while L393(−2) is projected into a hydrophobic groove formed by F441.57, Y471.60, V552.40, and F2998.50 (Fig. 6e). Instead of contacting with R3.50, Y391(−4) engages hydrophobic interactions with R1153.49, F1193.53, and F12634.53 in GPR174. Moreover, D381 and R385 at α5 make additional salt bridge interactions with K2256.30 and E2246.29, respectively; R532.38 forms a hydrogen bond with the backbone of α5 (Fig. 6e). In agreement with structure observations, mutation of R532.38 or K2256.30 dramatically reduced the basal activity of GPR174 (Supplementary Fig. 6g). Furthermore, we analyzed the effect of mutations on the potency and Emax of lysoPS in the context of the GPR174/Y99A mutant which can be activated in a dose-dependent manner by lysoPS (Fig. 6d). Consistently, mutation of R532.38, E2246.29 or K2256.30 almost completely abolished Gs coupling, and mutations of R1153.49 or F2998.50 reduced the potency by almost ten-fold and Emax by about 50% compared with the Y99A mutant (Fig. 6d and Supplementary Table 3). Noteworthily, Gs-coupled receptors such as GPR21, GPR52, CCK1R, NK1R, prostaglandin receptor EP4R43 and EP2R44 that adopt the non-canonical Gs coupling mode all possess a larger hydrophobic residue (L or C) at the position 5.65 than A/V (Fig. 6a, f, and g and Supplementary Fig. 7a–c). In the GPR52‒Gs complex, the tri-leucine pocket in Gs moves downward to avoid steric clash with C5.66 (equivalent to the position 5.65 in other GPCRs due to an extra residue in TM5 of GPR52), making the cytoplasmic end of TM6 in GPR52 flexible (Fig. 6f). L394(−1) in CCK1R‒Gs and NK1R-Gs are oriented outside the receptor core to avoid a steric clash with L2365.65 (Fig. 6g and Supplementary 7a). Similar to GPR174‒Gs, the “hook” in the EP2R‒Gs and EP4R‒Gs protrudes into a hydrophobic groove formed by TM1, TM2, and TM7 (Supplementary Fig. 7b, c). In addition, most class B Gs-coupled receptors including glucagon receptor (GLR) and corticotropin-releasing factor receptor also have a larger hydrophobic residue L at 5.65, which is accommodated by a more pronounced movement of TM5 and TM6, triggered by a sharp kink at the conserved PXXG motif in the middle of TM645–47 (Supplementary Fig. 7d). The absence of the PXXG motif in class A Gs-coupled receptors limits the further outward movement of TM6, thereby leading to a distorted hook when bound to class A Gs-coupled receptors that possess a larger hydrophobic residue at 5.65. Notably, most receptors including GPR174, GPR21, GPR52, CCK1R, and NK1R that adopt non-canonical Gs coupling mode have been reported to promiscuously couple to other G protein subtypes9,39–42. Our previous studies have shown that a larger hydrophobic residue (L) at 5.65 is dominant in Gq- and Gi/o-coupled receptors37,48. Mutation of L2145.65 in galanin receptor 2 to alanine almost abolished Gq recruitment, indicating its critical role for Gq coupling48. Despite differences in conformational changes of TM6 and the hook in Gα between canonical and non-canonical Gs coupling mode, a common feature of GPCRs and Gs coupling is the insertion of a large hydrophobic residue at the position 34.51 of ICL2 into a hydrophobic groove in the Ras domain of Gα (Fig. 1b and Supplementary Fig. 6d–f). Mutation of F12434.51 in GPR174 completely abolished Gs coupling (Fig. 6d and Supplementary Fig. 6g). Taken together, these data suggest that the non-canonical Gs coupling mode is determined by a larger hydrophobic residue (L or C) at position 5.65 of receptors, which may be critical for coupling to other G protein subtypes, whereas receptors that adopt canonical Gs coupling mode prefer a small hydrophobic residue (A or V) at this position.
Fig. 5. In contrast to GPR61, GPR161, and β2AR, GPR174 adopts a non-canonical Gs coupling mode.
a Structural overlay of β2AR‒Gs and GPR174‒Gs complexes from the side view. b Comparison of structures of inactive β2AR (PDB: 2RH1), active β2AR (PDB: 3SN6), active μOR (PDB: 6DDE) and active GPR174 in two orthogonal views. c Structural overlay of GPR174‒Gs, CCK1R‒Gs (PDB: 7MBX), GPR52‒Gs (PDB: 6LI3), and NK1R‒Gs (PBD: 7RMI) complexes from the cytoplasmic view. All these complexes adopt a non-canonical Gs coupling mode where the hook of Gαs is distorted and the outward movement of TM6 is less pronounced compared to β2AR. α5 of Gαs is shown with other regions hidden.
Fig. 6. The non-canonical Gs coupling mode is determined by a larger hydrophobic residue at position 5.65 of TM5.
a Receptors that adopt canonical Gs coupling mode have a small hydrophobic residue A/V at position 5.65, while receptors that adopt non-canonical Gs coupling prefer a large hydrophobic residue (L/C5.65). b Comparison of the binding interface between β2AR–Gαs and GPR174–Gαs. The hook of Gαs is distorted when bound to GPR174 due to a potential steric clash between L2125.65 and the tri-leucine pocket formed by L394(-1), L393(−2), and L388(−7). c Mutation of L2125.65 in GPR174 remarkably reduces its basal activity. Each data point represents mean ± SEM from three independent experiments. Source data are provided as a Source Data file. d The effects of mutations in the GPR174‒Gs interface on the potency and maximum effect of lysoPS in the context of the Y993.33A mutant evaluated by the cAMP assay. Each data point represents mean ± SEM from three independent experiments. Source data are provided as a Source Data file. e Detailed interactions between GPR174 and Gαs. f and g The presence of a larger hydrophobic residue (L or C) at 5.65 in GPR52 (f) and CCK1R (g) leads to a non-canonical Gs coupling mode.
Discussion
Here, we found a group of class A Gs-coupled oGPCRs named caoGPCRs show extremely high basal activity, compared with the other oGPCRs. The cryo-EM structure of GPR174, one of the caoGPCRs in complex with Gs without exogenous ligands revealed that endogenous lysoPS had occupied the receptor, leading to maximal activation and making it not respond to exogenous lysoPS in the cAMP assay. The perfect shape and charge complementarity between lysoPS and GPR174 account for their specific recognition and high-affinity binding. Occupancy of the receptor by endogenous lysoPS is consistent with functional studies showing that lysoPS needs to be added in micromolar concentrations to promote GPR174-mediated suppression of T cell proliferation20–23 and with the finding that exogenous lysoPS fails to increase the GPR174-Gs-PKA dependent up-regulation of CD86 in cultured B cells19. LPs including lysoPC, lysoPE, and lysoPS are synthesized via cleavage of one acyl group from membrane lipids by phospholipases. LysoPC is the most abundant LP in plasma, with a concentration reaching hundreds of micromolars, while the concentration of lysoPS is in the hundreds of nanomolar range27. This amount of lysoPS would be anticipated to induce full activation of GPR174 in terms of the Gs pathway. However, LPs can be rapidly metabolized in tissues and interstitial concentrations are often considerably lower than in plasma21,27,49. In contrast to in vitro culture systems, exogenous lysoPS can induce GPR174-dependent CD86 expression in vivo19, suggesting that lysoPS may not fully occupy GPR174 in some microenvironments. Thus, the baseline amount of GPR174 signaling in cells is likely to reflect the local balance of lysoPS synthetic and degradative activity. Increases in lysoPS generation under inflammatory conditions are likely to increase GPR174 signaling and function21,23,50.
Liang et al. published the cryo-EM structure of GPR174‒Gs purified from insect cells with exogenous lysoPS51 after we submitted this work. Our structure shows an almost identical binding pose of lysoPS in GPR174 and a similar Gs coupling mode with theirs, further supporting the identity of lysoPS in our structure, and that the GPCR-Gs fusion protein strategy for structural characterization does not influence the binding mode of Gα. The weak NanoBiT Gs dissociation response mentioned in Liang’s work is also probably due to the maximal activation of GPR174 by the endogenous lysoPS.
While ECL2 is penetrated into the orthosteric pocket of GPR61 and GPR161 as well as GPR12, GPR21, and GPR52, and possibly contributes to their constitutive activity, we cannot rule out the possibility that the existence of endogenous ligands that may dissociate after harsh purification procedure lead to extremely high basal activity. Residues on ECL2 that show reduced basal activity when mutated may be involved in ligand binding or be required for proper folding of the receptors. As G protein coupling to GPCRs stabilizes a “closed” receptor conformation which impedes agonist binding as well as prevents the dissociation of bound ligands from the receptor26, the penetration of ECL2 in the orthosteric binding pocket observed in these GPCRs‒Gs complexes could be attributed to G protein coupling to the receptors in the absence of agonists. As mentioned above, all caoGPCRs share relatively high sequence similarity with GPCRs known to be activated by lipids. ECL2 in EP2R occupies a similar position as that in GPR161, with the endogenous ligand PGE2 bound in the side pocket44 (Supplementary Fig. 6a), suggesting that unknown endogenous lipids may bind GPR161 in a similar manner. Based on their sequence homology with S1PR, GPR3/6/12 accommodate a conserved hydrophobic pocket for binding with the acyl chain of lipids (Supplementary Fig. 7e). Previous studies have shown that S1P or SPC can induce intracellular Ca2+ mobilization in cells expressing GPR3, GPR6, or GPR1214. However, controversial pharmacological data were obtained using different functional assays such as the cAMP assay or β-arrestin recruitment assay with different cell lines. In the case of GPR174, owing to the higher potency of lysoPS in the Gs pathway than G12/13, the exogenous lysoPS cannot further increase the cAMP level in our cell line but can activate GPR174 in a dose-dependent manner using the TGFα shedding assay. GPR174 mutants with a reduced potency of lysoPS can respond to exogenous lysoPS in the cAMP assay. Therefore, different potencies among distinct signaling assays and different concentrations of ligands released by different cell lines or present in different cell media can cause functional discrepancies. However, it is very challenging to identify the endogenous ligands of caoGPCRs, since most of them may have been fully activated by the endogenous ligands in the Gs pathway and no longer respond to the exogenous ones. Structural approaches in complement with functional assays will be important to identify and confirm what the endogenous ligands for caoGPCRs are.
Unlike conventional GPCRs in which their activity is spatially and temporally controlled by the release of ligands such as neurotransmitters under certain stimulation, the caoGPCRs are partially or fully activated once expressed in vivo. The question is how the activity of caoGPCRs is precisely modulated in vivo. In adipose tissue, GPR3 is expressed at a very low level, and cold exposure triggers transcription of Gpr3 that drives thermogenesis via Gs52. GPR161 is trafficked to primary cilia through the Tulp3/IFT-A complex29, which is essential for inhibiting Shh signaling and normal embryonic development. The transcriptional induction of GPR3 and trafficking of GPR161 to cilia are analogous to ligand-regulated activation of conventional GPCRs. The activity of caoGPCRs can be terminated via receptor internalization and degradation. Moreover, the existence of endogenous antagonists (agouti family) for melanocortin receptors53 raises the possibility that the endogenous antagonists may exist in vivo and modulate the activity of caoGPCRs. To investigate how the activity of caoGPCRs is modulated will be an exciting research area in the future.
Methods
Cloning
All GPCR sequences used in this study were subcloned from the PRESTO-Tango GPCR Kit54 into the pcDNA3.1 vector, with an N-terminal hemagglutinin (HA) signaling peptide (MKTIIALSYIFCLVFA) following by a FLAG tag (DYKDDDDA). For the NanoBiT mini-Gαs recruitment assay, the smBiT55 tag (VTGYRLFEEIL) was added to the C-terminus of GPCRs with a flexible linker (GGGSGGGGSGGSSSGG) in between, and the lgBiT tag was fused to the N-terminus of mini-Gαs56. For GPR61‒, GPR161‒, or GPR174‒mini-Gαs fusion proteins, the C-terminus of GPCRs was fused with the N-terminus of mini-Gαs399 with a 34 amino acid linker. All point mutations were introduced by quick change following standard procedure.
Expression and purification of Gβ1γ2 and Nb35
The human His6-tagged Gβ1 and Gγ2 with a C68S mutation were cloned into pFastBac™ Dual vector and expressed in Sf9 incest cell using Bac-to-Bac Baculovirus expression system (ThermoFisher Scientific). For protein expression, the Sf9 cell was infected at 3 × 106 cell/ml density. After 48 h, cells were harvested by centrifugation, and the pellet was resuspended in wash buffer I (20 mM HEPES, pH 7.4, 150 mM NaCl, and 20 mM imidazole) supplemented with 5 mM CaCl2 and 0.5 mM NiSO4, and were by Dounce homogenizer and sonication. The homogenate was centrifuged at 35,000×g at 4 °C for 30 min. The supernatant was filtered through a 0.45 μm membrane filter, and loaded onto Ni-NTA resin by gravity. Ni beads were washed by wash buffer, and Gβ1γ2(C68S) protein was eluted by elution buffer (20 mM HEPES, pH 7.4, 150 mM NaCl, 250 mM imidazole), and further purified by HiTrap Q HP anion exchange chromatography column.
For Nb35 protein purification36, a plasmid encoding Nb35 with a periplasmic signal peptide was transformed into Escherichia coli strain BL21 (DE3). 2 L bacteria were cultured in LB media until OD600 reached 0.6–0.8. Bacteria were further shaken at 37 °C for 12 h after the addition of 500 mM isopropyl 1-thio-β-d-glucopyranosided (IPTG). Bacteria were collected by centrifugation, resuspended in 100 ml SET buffer (0.5 M Sucrose, 0.5 mM EDTA, 0.2 M Tris, pH 8.0), and stirred at room temperature (RT) for 45 min. The lysate was added with 200 ml H2O to induce osmotic shock and stirred for 45 min before adding a final concentration of 150 mM NaCl, 2 mM MgCl2, and 20 mM Imidazole. The lysate was clarified by centrifugation and loaded onto Ni-NTA resin followed by the same purification procedure as above. After elution from nickel resin, Nb35 was further purified by a Superdex 75 increase 10/300 GL column. Both Gβ1γ2(C68S) and Nb35 were concentrated to 1 mg/ml, flash frozen in liquid nitrogen, and stored at −80 °C.
Expression and purification of GPR61/GPR161/GPR174‒mini-Gαs fusion protein
All GPCRs were transiently expressed in Expi293F cells using a serum-free SMM 293-TII expression medium (SinoBiological). 500 ml cells were transfected with 500 μg plasmids using polyethyleneimine “MAX” (Polysciences, Inc) at 2.3 × 106 cells per ml density. Cells were harvested by centrifugation at 48 h post-transfection, and lysed in in hypotonic buffer containing 25 mM HEPES (pH 7.4) and 50 mM NaCl by glass dounce. Membranes were pelleted by centrifugation at 38,000×g and solubilized in solubilization buffer (20 mM HEPES pH 7.4, 150 mM NaCl, 2 mM CaCl2, 0.5% LMNG, and 0.05% CHS). Homogenate was stirred at 4 °C for 2 h before centrifugation at 35,000×g for 30 min at 4 °C to remove debris. Supernatant was filtered through a 0.45 μm membrane filter, and loaded onto M1-Flag agarose beads. The beads were washed with wash buffer II (20 mM HEPES pH 7.4, 150 mM NaCl, 2 mM CaCl2, 0.01% LMNG, 0.001% CHS). The bound receptors were eluted with elution buffer (20 mM HEPES pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.1 mg/ml Flag peptide, 0.01% LMNG, and 0.001% CHS). The eluted proteins were concentrated and loaded onto a Superose 6 increase 10/300 GL column to further remove aggregation.
Complex assembly
For complex assembly, the purified GPCRs‒mini-Gαs fusion protein, Gβ1γ2, and Nb35 were mixed at a molar ratio of 1:1.2:1.2 supplemented with 2 mM MgCl2, 1 μl apyrase, and 1 μl 10% LMNG stock solution and incubated on ice overnight. The complexes were purified by a Superose 6 increase 10/300 GL column in SEC buffer (20 mM HEPES pH 7.4, 150 mM NaCl, 0.01% LMNG, and 0.001% CHS). The peak fractions corresponding to the complex were pooled and concentrated to 7–8 mg/ml for cryo-EM analysis.
Cryo-EM sample preparation and data collection
3.0 μl of protein complex was loaded onto a glow-charged Quantifoil Au R1.2/1.3 300 mesh grids and incubated for 8 s in a chamber maintained at 8 °C and 100% humidity, blotted for 3.0–5.0 s and plunge frozen into liquid ethane using a FEI Vitrobot MarkIV (Thermo Fisher Scientific). Cryo-EM movies for all complexes were collected on a Titan Krios (Thermo Fisher Scientific) at a nominal magnification of ×64,000 equipped with a BioQuantum GIF/K3 (Gatan) direct electron detector in a superresolution mode. Data were collected using EPU at a dose rate of 22 e− pixel−1 s−1 for a total dose of 50 e− Å−2 over 32 frames. Cryo-EM data collection parameters are summarized in Supplementary Table 2.
Cryo-EM data processing
A total of 923 movies were collected for the GPR61‒miniGαs–Gβ1γ2–Nb35 complex, 1058 movies for the GPR161‒miniGαs–Gβ1γ2–Nb35 complex, and 2043 movies for the GPR174‒miniGαs–Gβ1γ2–Nb35 complex. Movies were motion-corrected and 2× binned to a pixel size of 1.08 Å using MotionCor257. Contrast transfer function (CTF) was estimated using patch-based CTF estimation in cryoSPARC58. Then the particles were picked by a Blob picker with a 180-pixel box size and subjected to a round of 2D classification in cryoSPARC. 2D class averages with clear secondary structural features were combined and used as a template for another round of particle picking by a Template picker. The selected particles were subjected to several rounds of 2D classification, a round of Ab initio reconstruction, and a round of heterogenous refinement. Finally, non-uniform (NU) refinement and local refinement were performed in cryoSPARC to obtain density maps with global resolution of 3.16 Å for the GPR61‒mini-Gαs‒Gβ1γ2‒Nb35 complex, 3.10 Å for the GPR161‒miniGαs‒Gβ1γ2‒Nb35 complex and 2.83 Å for the GPR174‒mini-Gαs‒Gβ1γ2‒Nb35 complex.
Model building
Structural models of GPR61, GPR161, and GPR174 were generated by AlphaFold59. The structures of the receptor and the mini-Gαs‒Gβ1γ2‒Nb35 complex (PDB: 7F0T) are fitted into the density map using UCSF Chimera60,61 to generate the atomic model for the full complex. All three models were manually adjusted in COOT 0.9-pre and refined in Phenix62 using the secondary structure restraints. Structure and density map figures were prepared by Pymol and UCSF Chimera X60.
NanoBiT mini-Gαs recruitment assay
NanoBiT55 assay is a NanoLuc-based enzyme complementation system, in which the large fragment (LgBiT) element is fused to the N-terminus of mini-Gαs proteins, and the small fragment (SmBiT) is fused to the C-terminus of GPCRs. Expi293F cells cultured in the serum-free SMM 293-TII medium were seeded into a six-well plate at a density of 2 × 106 cells per ml and transfected with 1 μg of GPCR-smBit plasmid and 1 μg of mini-Gαs-lgBit plasmid using PEI. After 24 h, cells were centrifuged, washed once with Hank’s balanced salt solution (HBSS), and resuspended into the NanoBiT assay buffer (HBSS supplemented with 0.01% bovine serum albumin (BSA, Sigma), 10 mM HEPES (pH 7.3, Beyotime) and 10 μM coelenterazine-h (Yeasen)). Cells were seeded into a black-bottom 96-well plate and equilibrated at room temperature (RT) for 1 h. Relative bioluminescence units (RLUs) were measured by Spark multimode microplate reader (Tecan).
cAMP accumulation assay
In the cAMP accumulation assay, Expi293F cells cultured in the serum-free SMM 293-TII medium were seeded into a six-well plate at a density of 2 × 106 cells per ml and transfected with 1 μg of GPCR plasmid and 1 μg of pGloSensor-22F cAMP plasmid (Promega). At 24 h post-transfection, cells were washed once with HBSS and resuspended into the cAMP assay buffer (HBSS supplemented with 0.01% bovine serum albumin (BSA, Sigma), 10 mM HEPES (pH 7.3, Beyotime) and 500 μg/ml d-luciferin (Beyotime)), and then seeded into black-bottom 96-well plate with 99 μl cells. After incubation at RT for 30 min, cells were stimulated using 1 μl of ligands with titrated concentrations, and RLUs were measured by Spark microplate reader in 3–5 min. Data were fitted to a three-parameter sigmoidal concentration–response in GraphPad Prism 7.0.
NanoBit Gs recruitment assay
2 ml Expi293F cell was seeded into a six-well plate at a density of 2 × 106 cells/ml and transfected with five plasmids (200 ng GPCR-SmBit, 200 ng WT-Gαs-LgBit, 500 ng Gβ1, 500 ng Gγ2 and 100 ng RIC8A) using PEI. LgBit is inserted between L113 and V114 of Gαs, with a 15 aa linker in between. CMV promoter in pcDNA3.1 driving GPCR expression was changed to a weaker expression promoter spleen focus-forming virus (SFFV). 24 h after transfection, the cell was centrifuged at 100×g for 3 min and washed by HBSS 2 times. Then, the cell pellet was resuspended into 4 ml NanoBiT assay buffer and seeded into a white bottom 96-well plate with 95 μl cells. After incubation for 30–60 min, 5 μl of ligand was added into each well, and RLUs were measured by Spark microplate reader in 3–5 min.
Flow cytometry
2 ml of Expi293F cells were transfected by Flag-tagged GPCR plasmids by PEI at 2.3 × 106 cells per ml density and cultured in a six-well plate. After 24 h, 100 μl of cells were centrifuged at 250×g for 3 min to remove the culture medium and washed twice with the FACS buffer (20 mM HEPES, 150 mM NaCl, 2 mM CaCl2, 0.01% BSA, pH 7.4). Cells were resuspended in FASC buffer supplemented with 1 μl of Alexa Fluor 647-conjugated M1-Flag antibody for 15 min and washed once by the FACS buffer to remove free labeled antibody before analysis using flow cytometry (BD Accuri™ C6 Plus). APC-derived fluorescent signal was recorded in an FL4 channel of the flow cytometry and analyzed by FlowJo 10 software.
MS analysis
240 µl of methanol was added to 60 µl of 29 μM GPR174‒mini-Gαs‒Gβ1γ2‒Nb35 or D1R‒mini-Gαs‒Gβ1γ2‒Nb35, mixed by vortex and centrifuged at 14,000×g at 4 °C for 15 min. The supernatant was transferred to liquid chromatography (LC) vials with a micro-insert and 3 µl was injected into LC–MS. LC–MS analysis was performed on a Thermo Vanquish UHPLC equipped with a Thermo Q Exactive HF-X hybrid quadrupole-Orbitrap mass spectrometer in negative ESI mode. A Waters Acquity CSH C18 column (2.1 × 100 mm, 1.7 μm) was used for separation. The mobile phase contains 60:40 acetonitrile:water (A) and 90:10 isopropanol:acetonitrile (B), both with 10 mM ammonium acetate. The following gradient was applied: 0–2 min,15–30% B; 2–2.5 min, 30–48% B; 2.5–11 min, 48–82% B; 11–11.5 min, 82–99% B; 11.5–12 min, 99% B; 12–12.1 min, 99–15% B; 12.1–15 min, 15% B. Column temperature was maintained at 65 °C and the flow rate was 0.6 ml/min. Full-scan mass spectra were acquired in the range of m/z 120–1800 with the following ESI source settings: spray voltage: 2.5 kV, auxiliary gas heater temperature: 380 °C, capillary temperature: 320 °C, sheath gas flow rate: 30 units, auxiliary gas flow: 10 units. MS1 scan parameters included resolution 60000, AGC target 3e6, and maximum injection time of 200 ms. MS/MS data was collected with a normalized collision energy (NCE) of 20. Data processing was performed with MS-DIAL63 software (v. 4.16).
MD simulations
Six simulation systems were prepared using the Membrane Builder module in CHARMM-GUI with cryo-EM structure as an initial model. GPR174 was inserted in an explicit bilayer of 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (POPC) lipids. A 22.5 Å water layer was added to the top and bottom of the lipid bilayer. 150 mM of Na+ and Cl− ions were added to the bulk water to achieve a charge-neutral condition in the restrained equilibration. In all the simulations, the systems were parameterized using the CHARMM36 force field64 for protein, lipids, and water; and CGenFF65 for the small molecules. All simulations were performed using the GROMACS (2021.4)66. The systems were minimized for 5000 steps with the steepest descent algorithm. Six turns of 500 ps restrained equilibration were carried out to relax the system following the CHARMM-GUI protocol67. After equilibration, a 100 ns NPT production run was performed for each system. All simulations were performed under the following protocol. A 2-fs time-step with the LINCS algorithm was used. The van der Waals interactions were smoothly switched off at 10–12 Å by a force-switching function, and long-range electrostatic interactions were calculated using the particle-mesh Ewald method. Temperature and pressure were held at 298.15 K and 1 bar, respectively. Temperature and pressure controls were achieved with a Nose–Hoover thermostat and Parrinello–Rahman barostat for dynamics.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
We thank the staff at Shuimu BioSciences for their help with cryo-EM data collection. All EM images were collected at Shuimu BioSciences. This work has been supported by the Beijing Municipal Science & Technology Commission (Z201100005320012, N.H.)
Author contributions
Y.N. and Z.Q purified the protein complexes, collected cryo-EM data and performed cryo-EM data processing and model building, and performed functional assays with the supervision of S.Z. S.C. performed NanoBiT Gs recruitment assay. X.S. and Y.M. performed MS experiments. Z.C. and N.H. performed MD simulations. S.Z. wrote the manuscripts with helpful inputs by J.G.C.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The atomic structures of GPR174‒Gs, GPR161‒Gs, and GPR61‒Gs have been deposited at the Protein Data Bank (PDB) under the accession codes 8KH5, 8KH4, and 8KGK. The EM maps have been deposited at the Electron Microscopy Data Bank (EMDB) under the accession numbers EMD-37237, EMD-37236, and EMD-37224. Source data are provided with this paper. Uncropped scans of all blots and gels used in Supplementary Information are shown in Supplementary Fig. 8. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yingying Nie, Zeming Qiu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-41654-3.
References
- 1.Tang XL, Wang Y, Li DL, Luo J, Liu MY. Orphan G protein-coupled receptors (GPCRs): biological functions and potential drug targets. Acta Pharmacol. Sin. 2012;33:363–371. doi: 10.1038/aps.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laschet C, Dupuis N, Hanson J. The G protein-coupled receptors deorphanization landscape. Biochem. Pharmacol. 2018;153:62–74. doi: 10.1016/j.bcp.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- 4.Lu S, Jang W, Inoue A, Lambert NA. Constitutive G protein coupling profiles of understudied orphan GPCRs. PLoS ONE. 2021;16:e0247743. doi: 10.1371/journal.pone.0247743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefkowitz RJ, Cotecchia S, Samama P, Costa T. Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol. Sci. 1993;14:303–307. doi: 10.1016/0165-6147(93)90048-O. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen SG, et al. Mutation of a highly conserved aspartic acid in the beta2 adrenergic receptor: constitutive activation, structural instability, and conformational rearrangement of transmembrane segment 6. Mol. Pharmacol. 1999;56:175–184. doi: 10.1124/mol.56.1.175. [DOI] [PubMed] [Google Scholar]
- 7.Scheer A, Fanelli F, Costa T, De Benedetti PG, Cotecchia S. Constitutively active mutants of the alpha 1B-adrenergic receptor: role of highly conserved polar amino acids in receptor activation. EMBO J. 1996;15:3566–3578. [PMC free article] [PubMed] [Google Scholar]
- 8.Lin X, et al. Structural basis of ligand recognition and self-activation of orphan GPR52. Nature. 2020;579:152–157. doi: 10.1038/s41586-020-2019-0. [DOI] [PubMed] [Google Scholar]
- 9.Lin X, et al. Cryo-EM structures of orphan GPR21 signaling complexes. Nat. Commun. 2023;14:216. doi: 10.1038/s41467-023-35882-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong TS, et al. Cryo-EM structure of orphan G protein-coupled receptor GPR21. MedComm. 2023;4:e205. doi: 10.1002/mco2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, et al. Structural insight into the constitutive activity of human orphan receptor GPR12. Sci. Bull. 2023;68:95–104. doi: 10.1016/j.scib.2022.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Martin AL, Steurer MA, Aronstam RS. Constitutive activity among orphan Class-A G protein coupled receptors. PLoS ONE. 2015;10:e0138463. doi: 10.1371/journal.pone.0138463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kooistra AJ, et al. GPCRdb in 2021: integrating GPCR sequence, structure and function. Nucleic Acids Res. 2021;49:D335–D343. doi: 10.1093/nar/gkaa1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morales P, Isawi I, Reggio PH. Towards a better understanding of the cannabinoid-related orphan receptors GPR3, GPR6, and GPR12. Drug Metab. Rev. 2018;50:74–93. doi: 10.1080/03602532.2018.1428616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu P, et al. Structural identification of lysophosphatidylcholines as activating ligands for orphan receptor GPR119. Nat. Struct. Mol. Biol. 2022;29:863–870. doi: 10.1038/s41594-022-00816-5. [DOI] [PubMed] [Google Scholar]
- 16.Soga T, et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 2005;326:744–751. doi: 10.1016/j.bbrc.2004.11.120. [DOI] [PubMed] [Google Scholar]
- 17.Inoue A, et al. TGFalpha shedding assay: an accurate and versatile method for detecting GPCR activation. Nat. Methods. 2012;9:1021–1029. doi: 10.1038/nmeth.2172. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura H, et al. GPR34 is a receptor for lysophosphatidylserine with a fatty acid at the sn-2 position. J. Biochem. 2012;151:511–518. doi: 10.1093/jb/mvs011. [DOI] [PubMed] [Google Scholar]
- 19.Wolf EW, et al. GPR174 signals via Galphas to control a CD86-containing gene expression program in B cells. Proc. Natl Acad. Sci. USA. 2022;119:e2201794119. doi: 10.1073/pnas.2201794119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes MJ, Cyster JG. Lysophosphatidylserine suppression of T-cell activation via GPR174 requires Galphas proteins. Immunol. Cell Biol. 2018;96:439–445. doi: 10.1111/imcb.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes MJ, et al. The lysophosphatidylserine receptor GPR174 constrains regulatory T cell development and function. J. Exp. Med. 2015;212:1011–1020. doi: 10.1084/jem.20141827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinjo Y, et al. Lysophosphatidylserine suppresses IL-2 production in CD4 T cells through LPS(3)/GPR174. Biochem. Biophys. Res. Commun. 2017;494:332–338. doi: 10.1016/j.bbrc.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, et al. GPR174 knockdown enhances blood flow recovery in hindlimb ischemia mice model by upregulating AREG expression. Nat. Commun. 2022;13:7519. doi: 10.1038/s41467-022-35159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan Q, et al. Mini G protein probes for active G protein-coupled receptors (GPCRs) in live cells. J. Biol. Chem. 2018;293:7466–7473. doi: 10.1074/jbc.RA118.001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moro O, Lameh J, Hogger P, Sadee W. Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J. Biol. Chem. 1993;268:22273–22276. [PubMed] [Google Scholar]
- 26.DeVree BT, et al. Allosteric coupling from G protein to the agonist-binding pocket in GPCRs. Nature. 2016;535:182–186. doi: 10.1038/nature18324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan ST, Ramesh T, Toh XR, Nguyen LN. Emerging roles of lysophospholipids in health and disease. Prog. Lipid Res. 2020;80:101068. doi: 10.1016/j.plipres.2020.101068. [DOI] [PubMed] [Google Scholar]
- 28.Teng X, et al. Ligand recognition and biased agonism of the D1 dopamine receptor. Nat. Commun. 2022;13:3186. doi: 10.1038/s41467-022-30929-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukhopadhyay S, et al. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell. 2013;152:210–223. doi: 10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Bachmann VA, et al. Gpr161 anchoring of PKA consolidates GPCR and cAMP signaling. Proc. Natl Acad. Sci. USA. 2016;113:7786–7791. doi: 10.1073/pnas.1608061113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tschaikner PM, et al. Feedback control of the Gpr161–G(alphas)–PKA axis contributes to basal Hedgehog repression in zebrafish. Development. 2021;148:dev192443. doi: 10.1242/dev.192443. [DOI] [PubMed] [Google Scholar]
- 32.Begemann M, et al. Germline GPR161 mutations predispose to pediatric medulloblastoma. J. Clin. Oncol. 2020;38:43–50. doi: 10.1200/JCO.19.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feigin ME, Xue B, Hammell MC, Muthuswamy SK. G-protein-coupled receptor GPR161 is overexpressed in breast cancer and is a promoter of cell proliferation and invasion. Proc. Natl Acad. Sci. USA. 2014;111:4191–4196. doi: 10.1073/pnas.1320239111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimada IS, et al. Basal suppression of the sonic hedgehog pathway by the G-protein-coupled receptor Gpr161 restricts medulloblastoma pathogenesis. Cell Rep. 2018;22:1169–1184. doi: 10.1016/j.celrep.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu F, Song G, de Graaf C, Stevens RC. Structure and function of peptide-binding G protein-coupled receptors. J. Mol. Biol. 2017;429:2726–2745. doi: 10.1016/j.jmb.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen SG, et al. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teng X, et al. Structural insights into G protein activation by D1 dopamine receptor. Sci. Adv. 2022;8:eabo4158. doi: 10.1126/sciadv.abo4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koehl A, et al. Structure of the micro-opioid receptor-Gi protein complex. Nature. 2018;558:547–552. doi: 10.1038/s41586-018-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thom C, et al. Structures of neurokinin 1 receptor in complex with G(q) and G(s) proteins reveal substance P binding mode and unique activation features. Sci. Adv. 2021;7:eabk2872. doi: 10.1126/sciadv.abk2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris JA, et al. Selective G protein signaling driven by substance P-neurokinin receptor dynamics. Nat. Chem. Biol. 2022;18:109–115. doi: 10.1038/s41589-021-00890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mobbs JI, et al. Structures of the human cholecystokinin 1 (CCK1) receptor bound to Gs and Gq mimetic proteins provide insight into mechanisms of G protein selectivity. PLoS Biol. 2021;19:e3001295. doi: 10.1371/journal.pbio.3001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Q, et al. Ligand recognition and G-protein coupling selectivity of cholecystokinin A receptor. Nat. Chem. Biol. 2021;17:1238–1244. doi: 10.1038/s41589-021-00841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nojima S, et al. Cryo-EM structure of the prostaglandin E receptor EP4 coupled to G protein. Structure. 2021;29:252–260.e256. doi: 10.1016/j.str.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Qu C, et al. Ligand recognition, unconventional activation, and G protein coupling of the prostaglandin E(2) receptor EP2 subtype. Sci. Adv. 2021;7:eabf1268. doi: 10.1126/sciadv.abf1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiao A, et al. Structural basis of G(s) and G(i) recognition by the human glucagon receptor. Science. 2020;367:1346–1352. doi: 10.1126/science.aaz5346. [DOI] [PubMed] [Google Scholar]
- 46.Hilger D, et al. Structural insights into differences in G protein activation by family A and family B GPCRs. Science. 2020;369:eaba3373. doi: 10.1126/science.aba3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang YL, et al. Toward a structural understanding of class B GPCR peptide binding and activation. Mol. Cell. 2020;77:656–668.e655. doi: 10.1016/j.molcel.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 48.Jiang W, Zheng S. Structural insights into galanin receptor signaling. Proc. Natl Acad. Sci. USA. 2022;119:e2121465119. doi: 10.1073/pnas.2121465119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogasawara D, et al. Selective blockade of the lyso-PS lipase ABHD12 stimulates immune responses in vivo. Nat. Chem. Biol. 2018;14:1099–1108. doi: 10.1038/s41589-018-0155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamat SS, et al. Immunomodulatory lysophosphatidylserines are regulated by ABHD16A and ABHD12 interplay. Nat. Chem. Biol. 2015;11:164–171. doi: 10.1038/nchembio.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang J, et al. Structural basis of lysophosphatidylserine receptor GPR174 ligand recognition and activation. Nat. Commun. 2023;14:1012. doi: 10.1038/s41467-023-36575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sveidahl Johansen O, et al. Lipolysis drives expression of the constitutively active receptor GPR3 to induce adipose thermogenesis. Cell. 2021;184:3502–3518.e3533. doi: 10.1016/j.cell.2021.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu D, et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 54.Kroeze WK, et al. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat. Struct. Mol. Biol. 2015;22:362–369. doi: 10.1038/nsmb.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dixon AS, et al. NanoLuc complementation reporter optimized for accurate measurement of protein interactions in cells. ACS Chem. Biol. 2016;11:400–408. doi: 10.1021/acschembio.5b00753. [DOI] [PubMed] [Google Scholar]
- 56.Nehmé R, et al. Mini-G proteins: novel tools for studying GPCRs in their active conformation. PLoS ONE. 2017;12:e0175642. doi: 10.1371/journal.pone.0175642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng SQ, et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Punjani A, Rubinstein JL, Fleet DJ, Brubaker MA. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods. 2017;14:290–296. doi: 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 59.Jumper J, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pettersen EF, et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 2021;30:70–82. doi: 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 62.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsugawa H, et al. MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods. 2015;12:523–526. doi: 10.1038/nmeth.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacKerell AD, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 65.Vanommeslaeghe K, et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010;31:671–690. doi: 10.1002/jcc.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Der Spoel D, et al. GROMACS: fast, flexible, and free. J. Comput. Chem. 2005;26:1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- 67.Jo S, Lim JB, Klauda JB, Im W. CHARMM-GUI Membrane Builder for mixed bilayers and its application to yeast membranes. Biophys. J. 2009;97:50–58. doi: 10.1016/j.bpj.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The atomic structures of GPR174‒Gs, GPR161‒Gs, and GPR61‒Gs have been deposited at the Protein Data Bank (PDB) under the accession codes 8KH5, 8KH4, and 8KGK. The EM maps have been deposited at the Electron Microscopy Data Bank (EMDB) under the accession numbers EMD-37237, EMD-37236, and EMD-37224. Source data are provided with this paper. Uncropped scans of all blots and gels used in Supplementary Information are shown in Supplementary Fig. 8. Source data are provided with this paper.