Abstract
Introduction
The incidence of post vitrectomy endophthalmitis (PVE) is reported to be between 0.02 and 0.84%. Resterilization of single use instruments is a common practice amidst developing countries to make it more affordable to the patients by reducing the cost of the surgery and also reduce the environmental hazard. The aim of our study is to evaluate the incidence of PVE amidst existing sterilization practices of reused instruments in multiple vitreoretinal centres in India.
Methodology
Centres with an endophthalmitis tracking system were invited to participate in a survey. Twenty-five centres were sent a questionnaire via email. The questionnaire included details about the institution, number of vitrectomies performed in a year, sterilization practices followed pre-operatively, intraoperatively and postoperatively, incidence of endophthalmitis and instrument reuse policies.
Results
A total of 29 cases of endophthalmitis were reported out of the 47,612 vitrectomies performed across various centres. The mean incidence of endophthalmitis was 0.06%. There was no difference in the rates of endophthalmitis based on various pre-operative, intraoperative or postoperative prophylactic measures. Nearly 80% of the centres change most of the instruments after every case, while the rest reused. The mean number of times a cutter was being reused until discarded was 4.7. Nearly 76% followed a performance-based protocol, and the remaining 24% had a fixed protocol for the number of times an instrument can be reused before discarding it.
Conclusion
PVE rates are not significantly different in India despite the multiuse of single use instruments. The purpose of this paper is not to suggest an alternate protocol but to creating one in the future with these results in mind, to rationalise the use of single use instruments, make VR surgery more affordable and also have a positive impact on the carbon footprint of consumables in surgery.
Subject terms: Retinal diseases, Outcomes research
Introduction
Post-operative endophthalmitis is an uncommon and devastating ocular event that can occur after any intraocular surgery [1]. Although post cataract surgery endophthalmitis is well known, there is very limited information about endophthalmitis after pars plana vitrectomy (PPV). The incidence of post vitrectomy endophthalmitis (PVE) can range between 0.02% [2] to 0.05% [3] in India which is comparable to 0.05% in China [4], 0.11% in Malaysia [5] and 0.09% in USA [6]. Reported endophthalmitis rates post PPV surgery in India are comparable to that of the rest of the world despite differing sterilization practices and reuse of instruments typically considered single use.
The cost of PPV surgery is significantly different between developing countries and the rest of the world. The average base cost of a pars plana vitrectomy varies from $500 to 1000 in India to $220–322 in Indonesia [7] to $3037 in Germany [8] and $2500–13,000 in USA [9]. Despite the vastly different cost of surgery, the cost of consumables used in the surgery are not very different in India compared to the rest of the world.
Resterilization of single use instruments is a prevalent practice amidst developing economies, with comparable results [10]. To ameliorate the cost of the vitreoretinal (VR) surgery, the wide reuse of expensive consumables deemed necessary.
The aim of our study is to describe the incidence of PVE amidst existing sterilization practices in multiple vitreoretinal centres in India. The study compares rates of PVE with various existing sterilization practices and instrument reuse policies followed by various centres across the country.
Methods
The study was approved by the Ethics committee board of respective participating centres and it adhered to the tenets of the Declaration of Helsinki. The study was conducted by the Vitreoretinal Society of India (VRSI) Study group. This was a retrospective, multicentric, observational study of all the patients who underwent PPV between 1st January 2019 to 31st December 2019, in 25 tertiary eye care centres across various states and union territories in India [Andhra Pradesh (1), Chandigarh (1), New Delhi (3), Gujarat (1), Karnataka (4), Kerala (2), Madhya Pradesh (1), Maharashtra (1), Punjab (1), Pondicherry (1), Tamil Nadu (6), Telangana (1) and West Bengal (2)]. This period was selected to minimize the effect of COVID on number of patients operated.
All the centres were sent a questionnaire (Supplementary Information) via email. The questionnaire included details about the institution, number of vitrectomy surgeries performed in a year, sterilization practices followed, incidence of endophthalmitis and instrument reuse policies which were as follows:
Fixed protocol- Instrument usage was assessed based on number of times to be used and then discarded
Performance based protocol- used until the surgeon felt that it was functioning suboptimally
Details about sterilization practices of all the instruments used in VR surgery and reuse of single use instruments were sought and obtained. Also instruments were segregated based on single or multiple reuse, fibreoptic instruments like light pipe and laser probes, instruments with lumen like cutters, flute, backflush etc.
The centres were anonymized to mask the identity and given a centre code instead. Endophthalmitis incidence was assessed based on the type of centre (non-governmental organization (NGO) vs. private vs. government funded etc.,), practice patterns based on preoperative, intraoperative and postoperative measures, number of new cutter packs used, instrument reuse policies and sterilization practices of individual instruments. Possible practice patterns amidst centres reporting the highest incidence of endophthalmitis were also evaluated.
The prerequisite for including a centre in the study was the presence of an endophthalmitis tracking system in that particular centre. All participating centres were high volume centres that had an infection monitoring committee to which every case of endophthalmitis was reported to; root cause analysis assessing the cause of endophthalmitis was performed; treatment and its outcome were tracked for each patient.
Cases with endophthalmitis due to other pre-disposing factors like trauma were excluded.
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics for Windows, Version 20.0 (Armonk, NY: IBM Corp; 2011). The mean, median, frequency and standard deviation were calculated. A multivariate analysis was performed amongst various factors to predict the correlation between rate of endophthalmitis and various practice patterns. Fisher’s exact test, Wilcoxon Mann Whitney U test and Kruskal Wallis test were used for non-parametric data. Spearman Correlation test was used to explore the correlation between variables, as the data was not normally distributed.
Results
A total of 25 centres across India participated in the study, of which 52% were private centres, 44% were non-governmental organizations and 4% were government centres. A total of 47 612 vitrectomies were performed in one year across various centres.
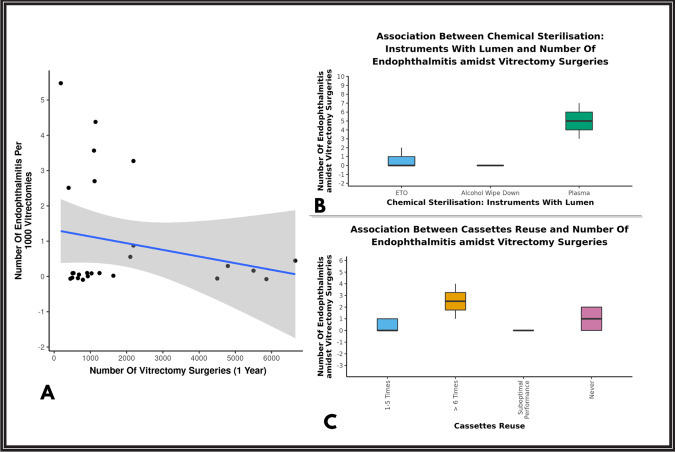
Of the total 25 centres who participated in the survey, the mean overall percentage of endophthalmitis incidence was 0.06%. A total of 29 cases of endophthalmitis were reported out of the 47,612 vitrectomies performed across various centres. The incidence of endophthalmitis ranged from 0.02 to 0.54 (Table 1). There was no statistically significant correlation between number of vitrectomies performed per centre and the number of endophthalmitis cases (p = 0.08) [Fig. 1A]. Also, there was no statistically significant difference between the type of practice/centre and endophthalmitis rates. (p = 0.171).
Table 1.
Number of vitrectomies performed in a year and incidence of endophthalmitis centre-wise.
| Centre code | Number of vitrectomies/year | Number of endophthalmitis/year | Number of endophthalmitis/1000 vitrectomies/year | Percentage of endophthalmitis/year |
|---|---|---|---|---|
| 01 | 1100 | 4 | 3.6 | 0.36 |
| 02 | 500 | 0 | 0 | 0 |
| 03 | 2188 | 7 | 3.1 | 0.31 |
| 04 | 400 | 1 | 2.5 | 0.25 |
| 05 | 912 | 0 | 0 | 0 |
| 06 | 5856 | 0 | 0 | 0 |
| 07 | 509 | 0 | 0 | 0 |
| 08 | 794 | 0 | 0 | 0 |
| 09 | 535 | 0 | 0 | 0 |
| 10 | 1634 | 0 | 0 | 0 |
| 11 | 1032 | 0 | 0 | 0 |
| 12 | 6652 | 3 | 0.4 | 0.04 |
| 13 | 4792 | 1 | 0.2 | 0.02 |
| 14 | 1143 | 5 | 4.3 | 0.43 |
| 15 | 5500 | 1 | 0.2 | 0.02 |
| 16 | 686 | 0 | 0 | 0 |
| 17 | 929 | 0 | 0 | 0 |
| 18 | 1116 | 3 | 2.6 | 0.26 |
| 19 | 2102 | 1 | 0.5 | 0.05 |
| 20 | 2188 | 2 | 0.9 | 0.09 |
| 21 | 185 | 1 | 5.4 | 0.54 |
| 22 | 450 | 0 | 0 | 0 |
| 23 | 1252 | 0 | 0 | 0 |
| 24 | 4500 | 0 | 0 | 0 |
| 25 | 657 | 0 | 0 | 0 |
| Total | 47,612 | 29 | 0.6 | 0.06% |
Fig. 1. Graphs depicting rate of endophthalmitis.
A The scatterplot above depicts the correlation between number of vitrectomies performed in a year and number of endophthalmitis per 1000 vitrectomies. The blue line represents the correlation trend and grey area shows 95% confidence interval. B The Box-and-Whisker plot above depicts the distribution of endophthalmitis cases amidst vitrectomy surgeries in the 3 groups of chemical sterilisation of instruments with lumen. C The Box-and-Whisker plot above depicts the distribution of endophthalmitis cases amidst vitrectomy surgeries amidst cassette reuse.
The most common vitrectomy machine used was Alcon Constellation® i.e. 96% (24 centres). The remaining 4% (1 centre) used Geuder.® In addition to Constellation, 12% (3 centres) also used DORC Eva® and 4% (1 centre) also used Reticare.®
Preoperative protocols
Antibiotic prophylaxis
There was no significant difference in the endophthalmitis rates irrespective of the type of preoperative (p = 0.345) antibiotic prophylaxis.
Topical povidone alone was used by 44% (11 centres), povidone with topical antibiotics was used by 28% (7), povidone with parenteral antibiotics by 12% (3), povidone with topical and parenteral antibiotics was used by 4% (1) and topical antibiotic prophylaxis alone was used by 12% (3).
Scrub before each surgery
In nearly half of the centres surveyed, i.e., (52%), the surgical team scrubbed for each subsequent case while the other half did not. There was however no difference in endophthalmitis rates between the two groups. (p = 0.763)
Glove change before each surgery
Majority of the centres (96%), change of gloves was practiced after each surgery except for one centre(4%), where gloves were changed after a set of 2–5 cases. There was no incidence of endophthalmitis reported from this centre.
Instrument related protocols
Number of times of reuse
Nearly three-quarters of the centres, i.e., 76% (19) followed a performance based protocol, and the remaining one-quarter 24% (6) had a fixed protocol for number of times an instrument can be reused. There was no statistically significant difference in the rate of endophthalmitis between the centres following different protocols. (p = 1.0). (Table 2).
Table 2.
Centre-wise practise pattern on reuse of vitreoretinal instruments and incidence of endophthalmitis.
| Instrument reuse | Reuse between 1 and 5 times (Fixed Protocol) | Reuse until suboptimal performance (Performance based protocol) | Endophthalmitis incidence (p-value) |
|---|---|---|---|
| Cutter | |||
| Average endophthalmitis rate | 0.79 ± 1.48 (0.09%) | 1.64 ± 2.25 (0.1%) | 0.276 |
| Number of centres | 56% (14 centres) | 44% (11 centres) | |
| Fiberoptic instruments | |||
| Average endophthalmitis rate | 1.67 ± 1.97 (0.17%) | 1.12 ± 1.93 (0.08%) | 0.296 |
| Number of centres | 24% (6 centres) | 76% (19 centres) | |
| Instruments with lumen | |||
| Average endophthalmitis rate | 1.00 ± 2.00 (0.16%) | 1.22 ± 1.93 (0.08%) | 0.757 |
| Number of centres | 24% (6 centres) | 76% (19 centres) | |
| Forceps/Scissors | |||
| Average endophthalmitis rate | 1.33 ± 2.80 (0.14%) | 1.11 ± 1.56 (0.08%) | 0.673 |
| Number of centres | 24% (6 centres) | 76% (19 centres) | |
| Trocar/Cannula | |||
| Average endophthalmitis rate | 0.54 ± 0.88 (0.08%) | 1.83 ± 2.41 (0.11%) | 0.241 |
| Number of centres | 52% (13 centres) | 48% (12 centres) | |
The average number of times, a cutter was being re-used before being discarded was 4.7. Centres reusing cutters for more numbers of cases/until suboptimal performance did not show a significant difference compared to those reusing for less than 5 times in terms of incidence of endophthalmitis rates(p = 0.276). (Fig. 1B)
Instrument reuse policy and cleaning methods
Nearly 80% of the centres changed instruments after every case, while of the 20% who cleaned and reused, 8% performed alcohol wipe down and 12% cleaned using irrigating solution in between the cases. For tubings, the cleaning methods followed at the end of each case included, aspirating copious amount of distilled water/balanced salt solution (BSS) through the tubing using the vitrectomy console, followed by flushing with air or using positive air pressure alone; some added antibiotic to distilled water/BSS during rinsing. There was no difference in the incidence of endophthalmitis among various cleaning methods followed, (p = 0.724) i.e., whether the instruments were changed at the end of each case or reused after each case by alcohol wipe down or cleaning with irrigating solutions. (Table 3)
Table 3.
Centre-wise practise pattern on cleaning of vitreoretinal instruments and incidence of endophthalmitis.
| Instrument cleaning between cases | Average endophthalmitis rate ± SD | P-value |
|---|---|---|
| Vitreous cutter | 0.652 | |
| Change Instrument After Every Case | 0.85 ± 1.23 | |
| Reuse Same Instrument | 2.40 ± 3.36 | |
| Fiberoptic instruments | 0.682 | |
| Change Instrument After Every Case | 1.14 ± 1.80 | |
| Reuse Same Instrument | 1.25 ± 2.50 | |
| Instruments with lumen | 0.139 | |
| Change Instrument After Every Case | 1.32 ± 1.94 | |
| Reuse Same Instrument | 0.00 ± 0.00 | |
| Instruments like forceps/scissors | 0.430 | |
| Change Instrument After Every Case | 1.20 ± 1.82 | |
| Reuse Same Instrument | 1.00 ± 2.24 | |
| Trocar/Cannula | 0.139 | |
| Change Instrument After Every Case | 1.32 ± 1.94 | |
| Reuse Same Instrument | 0.00 ± 0.00 |
Instrument sterilization methods
The most common mode of sterilization used was ethylene oxide (ETO), used by 88%, followed by 12% using plasma chambers.
There was a slightly higher incidence of endophthalmitis rate between chemical sterilization using plasma (0.29%) when compared to ETO in instruments with lumen like cutters and flute/backflush (p = 0.045), although the number of centres using plasma sterilization was only 3. Also, this difference was noted for cassette sterilization, with plasma sterilization showing slightly higher incidence of endophthalmitis (0.22%) when compared to ETO (p = 0.014) while the number of centres using plasma sterilization for vitrectomy cassette was only 2. (Fig. 1B, C).
Viewing systems were either placed on a sterile drape between cases or cleaned and reused or resterilised between cases. Various practices followed however did not show any difference in the rates of endophthalmitis incidence (Table – Supplementary Information).
Impact of postoperative measures on endophthalmitis
There was no significant difference in the endophthalmitis rates irrespective of the type of prophylaxis used at the end of surgery (p = 0.136).
Topical antibiotics alone at the end of surgery were used by 28% (7 centres), topical povidone alone at the end of surgery by 28% (7 centres), topical povidone and antibiotics by 28% (7 centres), subconjunctival antibiotics alone by 8% (2 centres) and topical along with subconjunctival antibiotics by another 8% (2 centres).
Intraoperative and post operative antibiotic prophylaxis protocols are shown in Table 4.
Table 4.
Antibiotic prophylaxis protocols.
| YES | NO | |
|---|---|---|
| Topical antibiotics at the end of surgery | 14 centres (56%) | 11 centres (44%) |
| Subconjunctival antibiotics | 3 centres (12%) | 22 centres (88%) |
| Antibiotics added to infusion intraoperatively | 3 centres (12%) | 22 centres (88%) |
| Parenteral antibiotics before surgery | 4 centres (16%) | 21 centres (84%) |
| Oral antibiotics before surgery | 4 centres (16%) | 21 centres (84%) |
| Topical antibiotics postoperatively | 24 centres (96%) | 1 centres (4%) |
| Oral antibiotics after surgery | 14 centres (56%) | 11 centres (44%) |
Discussion
The overall incidence of PVE reported from previous studies ranges from 0.01 to 0.84% and the same is comparable with the Indian studies [2–6]. In our study the rate of PVE was 0.06%, which is comparable to the published studies.
The main objective of this study is to show that despite the reuse policy which is practised all across the country our endophthalmitis incidence rate, is comparable to the published studies from the western world where single use instruments are not reused. The study is not intended to propose recommendations out of the results but to study the various reuse strategies across various centres providing vitreoretinal treatment services in India. The major advantage of reusing the instrument is to reduce the cost burden on the patient (since most of the patients pay out of their pocket due to poor insurance coverage) and also to reduce the environmental hazard.
Surgeries involving maximal utilization of single-use disposable instruments, generate large quantities of material waste and environmental emissions contributing to global warming and climate change [10]. This is particularly important in vitreoretinal surgery wherein a large number of single use consumables are used. Today the healthcare sector is responsible for 5% of the United Kingdom’s and 10% of United States’ greenhouse gases respectively [11, 12]. Thus optimizing the use of reusable instruments and supplies, maximizing single-use device reprocessing, promoting minimum waste and recycling practices, using energy-efficient appliances and air-handling systems, and investing in low-carbon energy sources can help in reducing the health care related environmental hazard to a great extent. The major advantages of the reuse policy are reducing the carbon footprint and being more environment friendly, while also to reduce the cost burden on the patient and the hospital.
In the U.S.A, vitrectomy costs anywhere between 2500 and 13,000 U.S dollars while, it is around 500 to 1000 US$ in India. Also, the per-capita income of U.S.A is 66,080 $ in contrast to 6920 $ of India. A recent study by Berkowitz et al. from USA, showed that actual vitrectomy costs versus reimbursement from insurance companies, does not allow to recover even break-even costs unless reimbursement is increased by 40.15%. In addition, they also showed that 68% of cases are completely unprofitable, with increasing losses directly proportional to the length of the case [13]. If this is the situation in the USA, wherein the cost of vitrectomy is nearly 7–10 times that of it in India, recovering costs with multi-use of instruments is an uphill task in a developing country like India. In addition, a large number of centres perform free vitreoretinal surgeries as a social obligation to serve the underprivileged section of the society, making it particularly important to rationalize the cost of care. Our study shows that despite rationalizing the cost of care, patient safety is not compromised and endophthalmitis rates are comparable to that of the developed world.
Thus, to reduce the cost-burden, most of the eye hospitals in our country reuse light probes, endolaser probes, trocars and cutters multiple times after subjecting to enzymatic sterilisation followed by using ethylene oxide after drying.
A study by Zacharias et al. on safety and cost-effectiveness of reusing single use endolaser probes also concluded that reprocessing was safe with no associated increase in endophthalmitis rate and the sterility tests were negative for any microbial growth after reprocessing [14].
In another study by Silpa-Archa et al. from Thailand, incidence of PVE was reported 0.10% over 13 years of reuse with single use instruments [5]. The authors have also reused vitrectomy cassettes, trocar cannulas, vitreous cutters, endoilluminators, intraocular forceps, laser probes, and diathermy probes. Our results are also comparable to their study.
Our study is the pioneer study to evaluate the incidence of endophthalmitis post vitreous surgery amidst existing sterilisation practices across various tertiary eye care centres in India. It also gives a detailed insight about the possible association of any factors, including type of the centre, type of preoperative and intraoperative antibiotic prophylaxis, sterilization techniques followed for each case, reuse of various equipment required for the procedures, methods of sterilisation followed for the reuse. There was no significant statistical association found in any of the variables when compared to the incidence of PVE.
There was a slightly higher incidence of endophthalmitis rates between chemical sterilisation using plasma when compared to ETO in instruments with lumen like cutters and flute/backflush (p = 0.045). The incidence of endophthalmitis was also slightly higher when the cassette was reused for more than five times(0.31%) although not statistically significant and when the cassette sterilisation was performed using plasma compared to ETO (p = 0.014). As only 3 centres employed plasma sterilisation, (total 8804 vitrectomies performed in these centres, i.e., 18.5%), it may be imprudent to conclude that plasma sterilization may be a factor in causing endophthalmitis.
Limitations of our study are
Limited centres were recruited based on survey invite basis. We did not differentiate endophthalmitis rates amongst 23 G/25 G/27 G vitrectomies as a cause of endophthalmitis were not considered. Another limitation is that post-operative inflammation has not been tracked. There is a risk of increased post-op inflammation with reuse of instruments due to endotoxins and possible chemical residues from the sterilization procedures. Though unlikely, we also do not know if the reuse of instruments results in compromised anatomical and visual outcomes. These are questions that are best answered by a multicentric prospective studies.
To the best of our knowledge, this is the first study evaluating incidence of endophthalmitis rates over large number of vitrectomy surgeries performed (47,612) amongst various practice patterns and sterilization protocols highlighting the reuse of single use VR instruments. The above mentioned practices are not our recommendations. We urge to change instruments after every case, follow sterile precautions as much as possible and rationalise the system by judicious reuse of instruments.
Conclusion
PVE rates are not significantly different in India despite the multiuse of single use instruments. The purpose of this paper is not to suggest an alternate protocol but to creating one in the future with these results in mind, to rationalise the use of single use instruments, make VR surgery more affordable and also have a positive impact on the carbon footprint of consumables in surgery.
Summary
What was known before
Vitreoretinal surgery incurs a huge cost due to the consumables required, causing a cost burden and an environmental hazard.
What this study adds
Reusing consumables in vitreoretinal surgery is a common practice in India with similar rates of endophthalmitis with a lower cost and reduced environmental hazard.
Supplementary information
Author contributions
PNS: Review of study design, conducting review of literature, data analysis, drafting the manuscript, editing and revising, and submission process. DKM: Study design, writing the study protocol, data collection, submission to institutional review board, manuscript writing, and editing. MPS: Proposed the study idea and design, overall supervision of the study, revising it critically for important intellectual content, final approval of the study. MA: Study design, review of literature, data acquisition, and final approval of the version to be published. PS: data acquisition and final approval of the version to be published. ACS: data acquisition and final approval of the version to be published. RR: data acquisition and final approval of the version to be published. VD: data acquisition and final approval of the version to be published. VS: data acquisition and final approval of the version to be published. NK: data acquisition and final approval of the version to be published. TS: data acquisition and final approval of the version to be published. MDS: data acquisition and final approval of the version to be published. SRS: data acquisition and final approval of the version to be published. MKR: data acquisition and final approval of the version to be published. AGA: data acquisition and final approval of the version to be published. AM: data acquisition and final approval of the version to be published. NKY: data acquisition and final approval of the version to be published. DB: data acquisition and final approval of the version to be published. EN: data acquisition and final approval of the version to be published. RN: data acquisition and final approval of the version to be published. SMK: data acquisition and final approval of the version to be published. NA: data acquisition and final approval of the version to be published. SM: data acquisition and final approval of the version to be published. HM: data acquisition and final approval of the version to be published. PSM: data acquisition and final approval of the version to be published. GSP: data acquisition and final approval of the version to be published. MN: data acquisition and final approval of the version to be published. JW: data acquisition and final approval of the version to be published. VG: data acquisition and final approval of the version to be published. AK: Study design, data acquisition, and final approval of the version to be published.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-023-02430-1.
References
- 1.Lemley CA, Han DP. Endophthalmitis: A review of current evaluation and management. Retina. 2007;27:662–80. doi: 10.1097/IAE.0b013e3180323f96. [DOI] [PubMed] [Google Scholar]
- 2.Bhende M, Raman R, Jain M, Shah PK, Sharma T, Gopal L, et al. Incidence, microbiology, and outcomes of endophthalmitis after 111,876 pars plana vitrectomies at a single, tertiary eye care hospital. PLoS ONE. 2018;13:e0191173. doi: 10.1371/journal.pone.0191173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dave VP, Pathengay A, Basu S, Gupta N, Basu S, Raval V, et al. Endophthalmitis after pars plana vitrectomy: clinical features, risk factors, and management outcomes. Asia Pac J Ophthalmol. 2016;5:192–5. doi: 10.1097/APO.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 4.Shi XY, Zhao HS, Wei WB. Analysis of post-operative endophthalmitis after pars plana vitrectomy: A 10-year experience at a single center. Chin Med J (Engl) 2013;126:2890–3. [PubMed] [Google Scholar]
- 5.Silpa-Archa S, Kumsiang K, Preble JM. Endophthalmitis after pars plana vitrectomy with reused single-use devices: A 13-year retrospective study. Int J Retin Vitreous. 2021;7:2. doi: 10.1186/s40942-020-00274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunimoto DY, Kaiser RS, Wills Eye Retina Service. Incidence of endophthalmitis after 20- and 25-gauge vitrectomy. Ophthalmology. 2007;114:2133–7. doi: 10.1016/j.ophtha.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Simanjuntak GW, Djatikusumo A, Adisasmita A, Nadjib M, Mailangkay H, Hussain N. Cost analysis of vitrectomy under local versus general anesthesia in a developing country. Clin Ophthalmol. 2018;12:1987–91. doi: 10.2147/OPTH.S179369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Framme C, Franz D, Mrosek S, Helbig H. Kosteneffizienz von netzhaut- und glaskörperchirurgischen Eingriffen mittels ppV unter DRG-Bedingungen [Cost recovery for the treatment of retinal and vitreal diseases by pars plana vitrectomy under the German DRG system] Ophthalmologe. 2007;104:866–74. doi: 10.1007/s00347-007-1619-5. [DOI] [PubMed] [Google Scholar]
- 9.Nicod E, Jackson TL, Grimaccia F, Angelis A, Costen M, Haynes R, et al. Direct cost of pars plana vitrectomy for the treatment of macular hole, epiretinal membrane and vitreomacular traction: A bottom-up approach. Eur J Health Econ. 2016;17:991–9. doi: 10.1007/s10198-015-0741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiel CL, Schehlein E, Ravilla T, Ravindran RD, Robin AL, Saeedi OJ, et al. Cataract surgery and environmental sustainability: Waste and lifecycle assessment of phacoemulsification at a private healthcare facility. J Cataract Refract Surg. 2017;43:1391–8. doi: 10.1016/j.jcrs.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbon Footprint Update for NHS in England 2012. Cambridge, UK: Sustainable Development Unit; 2013. [Accessed September 25, 2017].
- 12.Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. PLoS One. 2016;11:e0157014. doi: 10.1371/journal.pone.0157014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkowitz ST, Sternberg P, Jr, Patel S. Cost analysis of routine vitrectomy surgery. Ophthalmol Retin. 2021;5:496–502. doi: 10.1016/j.oret.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Zacharias LC, da Silva Conci L, Megnis BP, Falabretti JG, Dos Santos Rodrigues Neto T, da Silva Neto ED, et al. Safety and cost-effectiveness of single-use endolaser probe reprocessing in vitreoretinal surgery. Int J Retin Vitr. 2021;7:22.. doi: 10.1186/s40942-021-00292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.