Abstract
The chemistry and mineralogy of slabs subducted into lower mantle control slab rheology and impact the deep volatile cycle. It is known that the metamorphism of little-altered oceanic crust results in eclogite rocks with subequal proportions of garnet and clinopyroxene. With increasing pressure, these minerals react to stabilize pyrope-rich tetragonal majoritic garnet. However, some eclogites contain higher proportions of omphacitic clinopyroxene, caused by Na- and Si-rich metasomatism on the ocean floor or during subduction. The mineralogy of such eclogites is expected to evolve differently. Here, we discuss the results of the crystallization products of omphacitic glass at ~ 18 and ~ 25 GPa and 1000 °C to simulate P–T regimes of cold subduction. The full characterization of the recovered samples indicates evidence of crystallization of Na-, Si-rich cubic instead of tetragonal majorite. This cubic majorite can incorporate large amounts of ferric iron, promoting redox reactions with surrounding volatile-bearing fluids and, ultimately, diamond formation. In addition, the occurrence of cubic majorite in the slab would affect the local density, favoring the continued buoyancy of the slab as previously proposed by seismic observations. Attention must be paid to omphacitic inclusions in sublithospheric diamonds as these might have experienced back-transformation from the HP isochemical cubic phase.
Subject terms: Mineralogy, Petrology
Introduction
The fate of the oceanic lithosphere’s chemical budget during subduction is linked to the complex motion and morphology of the subducting slab, linked to trench migration rate and slab-dip angle that determine the conditions for slab penetration at depth1. Density and viscosity of the subducting slab are key variables that control positive buoyancy vs. sinking processes at depth2–6. Knowledge of these variables allows a better understanding of the origin of deep-focus earthquakes, generally defined as seismic events with hypocenters located at depths greater than 350 km7. To date, such events have been interpreted as due to shear instabilities associated with polymorphic transformations of olivine (Mg,Fe)2SiO4, the most abundant mineral in the upper mantle (UM), to wadsleyite/ringwoodite with increasing depth, accompanied by positive density changes of 6% and 2%, respectively8. In subduction zones, this transformation of olivine can be promoted at shallower depths under lower temperature regimes, and this may affect the dynamics of the subducting slab and the origin of deep-focus earthquakes9–11. According to the common mantle adiabat, where temperatures are expected to be ~ 1500 °C in the transition zone (TZ)12, olivine transforms to wadsleyite at a depth of 410 km, to ringwoodite at a depth of 520 km, and finally to (Mg,Fe)SiO3 bridgmanite + (Mg,Fe)O (magnesio-wüstite) at a depth of about 660 km13. Subducting oceanic crust is also exposed to important phase transitions and reactions, such as clinopyroxene (Cpx) plus garnet (Grt) contained in the eclogitic portions of the slab initially roughly at 1:1 volume ratio14 forming majoritic garnet (Maj). In turn, the decomposition of Maj under anhydrous conditions leads to the formation of both calcic (i.e., davemaoite) and ferromagnesian (i.e., bridgmanite) silicate perovskites plus additional minor phases like stishovite, NAL (new aluminous) and CF (calcium-ferrite) phases. The presence of such phases is either substantiated by experimental studies15,16 or corroborated directly by the analysis of mineral inclusions present in sub lithospheric diamonds17,18. Among the several mineral phase transformations occurring during subduction, the continuous reaction of eclogitic Cpx (i.e. omphacite) with Grt to form Maj is of particular interest because of its use as a geobarometer for sub lithospheric diamonds19, and the associated increase in slab density (~ 6%; ref.20) at about 350–450 km. The transformations Cpx + Grt → Cpx + Maj → Maj should occur over a broader depth interval, by about an order of magnitude, than those involving olivine21–25. The effect on buoyancy forces by the majorite reactions occurs over a large P-interval relative to the olivine-waddsleyite-ringwoodite transitions. During subduction, omphacitic Cpx dissolves progressively into Grt within a pressure range of 7–17 GPa, to form Maj16,26. The occurrence of majoritic Grt included in sub-lithospheric diamonds along with the frequent report of E-type inclusions in diamonds suggest the potential role of the involved Fe-bearing minerals (i.e., Cpx, Grt and Maj) to buffer the local redox conditions and promote diamond precipitation through redox reactions at the expense of oxidized CO2-bearing fluids27–29. Eclogite xenoliths exhumed from the mantle are commonly interpreted as having subducted oceanic crust precursors30. These rocks are characterized by chemical and mineralogical variability resulting from variations in the nature of the protolith (various portions of oceanic crust) as well as interaction with metasomatic fluids (on the ocean floor, during subduction and in the mantle)31. Experimental studies, observations in nature and thermodynamic modelling suggest that typical oceanic crust metamorphoses to eclogite with subequal modal abundances of Grt and omphacitic Cpx30–32. However, some eclogitic xenoliths have been reported to contain much more Cpx than Grt, up to almost 80%33,34, and jadeitites and omphacitites are minor lithologies that are often reported from high-pressure-low-temperature metamorphic terranes35. As a consequence, subduction of very Cpx-rich lithologies may result in incomplete dissolution in garnet to form majorite, resulting in retention of excess omphacitic cpx at very high pressures.
To date, experimental studies on the stability of omphacitic Cpx at high pressure and temperature are sparse in the literature and arrive at two main contrasting results: (1) decomposition to an assemblage of tetragonal Na-bearing Maj + Ca-perovskite + stishovite, or (2) formation of a post-Cpx phase36,37. In detail, Bobrov et al.38–40 proved that most of the Na present in majoritic Grt is accommodated via the pressure dependent exchange reaction Na+ + Si4+ = Mg2+ + Al3+ (Na for Mg in the X site and Si for Al in the Y site). Such a mechanism of sodium incorporation in tetragonal majoritic Grt supported the idea of the presence of a Na-Maj end member in Grt solid solution41,42. The Na2MgSi5O12 end-member, indeed, was later synthesized in the model system Mg3Al2Si3O12–Na2MgSi5O12 at 17.5 GPa and 1700°C43 and structurally characterized by Bindi et al.44. These authors confirmed the ability of majoritic Grt to incorporate significant concentrations of Na and Si as previously reported by authors45,46 who experimentally observed the immiscibility between Al-rich Maj and Na, Si-rich Maj (along with other minerals) between 13 and 15 GPa at 1550–1700 °C in the Na2O-CaO-FeO-MgO-Al2O3-SiO2 (NCFMAS) system. The finding of majorite with both peridotitic and eclogitic affinity as inclusion in diamonds have raised important considerations on the role that this mineral might have in diamond formation processes at expenses of C-saturated fluids27–29. Therefore, mineralogical and petrological evidence of the existence of Na, Si-rich Maj would imply an important role as host for Na and diamond formation from an alkali-silica rich growth medium in the lower part of the upper mantle (UM) and transition zone (TZ).
We here report the synthesis, using the multi anvil press technique, of Na-, Si-rich Fe-bearing Maj from a starting glass with Na-, Si-rich omphacitic composition (see Table S1) with a cubic structure rather than the common tetragonal symmetry after quench from HP experiments, and at lower T than previously reported. We describe the results of its characterization by FE-SEM, powder and single X-ray diffraction along with Raman spectroscopy, Nano-IR microscopy and Mössbauer spectroscopy techniques. Results are discussed in terms of effects on the density of the subducting slab and on redox interactions between slab and surrounding mantle components, with implications for the behavior of the slab at transition zone (TZ) and lower mantle (LM) depths and the origin of sub-lithospheric diamonds.
Results
Texture and composition
A summary of the experimental conditions and recovered run products is reported in the Supplementary Information (Table S2) along with a sketch of the cell assembly used to reach the target pressure and temperature (Fig. S1). The chemical composition of the recovered samples is shown in Table 1. Results from these experiments are summarized in Fig. 1. These experiments were held for 30 min at 1000 °C and resulted in the presence of variably well-shaped grains of Maj, with sizes up to 100 µm also constituted by an aggregate of smaller crystals (run M81). No additional minerals were observed. The oxygen fugacity in these runs was buffered by the coexistence of both Re and ReO2 to allow comparison of the Fe3+/∑Fe ratio with that of previous studies (see “Discussion” section).
Table 1.
Chemical composition in wt% of the recovered samples.
| Run | N meas | Na2O | MgO | Al2O3 | SiO2 | CaO | Fe2O3 | Total |
|---|---|---|---|---|---|---|---|---|
| M81 | 10 | 5.84 (72) | 9.64 (30) | 11.32 (37) | 55.17 (27) | 14.72 (69) | 4.03 (38) | 100.72 (1.02) |
| M82-rim | 12 | 5.88 (45) | 9.44 (42) | 11.17 (29) | 55.31 (81) | 15.06 (62) | 4.31 (41) | 101.17 (1.09) |
| M82-core | 7 | 5.87 (17) | 9.44 (39) | 10.97 (47) | 54.86 (85) | 15.13 (59) | 4.27 (44) | 100.54 (1.42) |
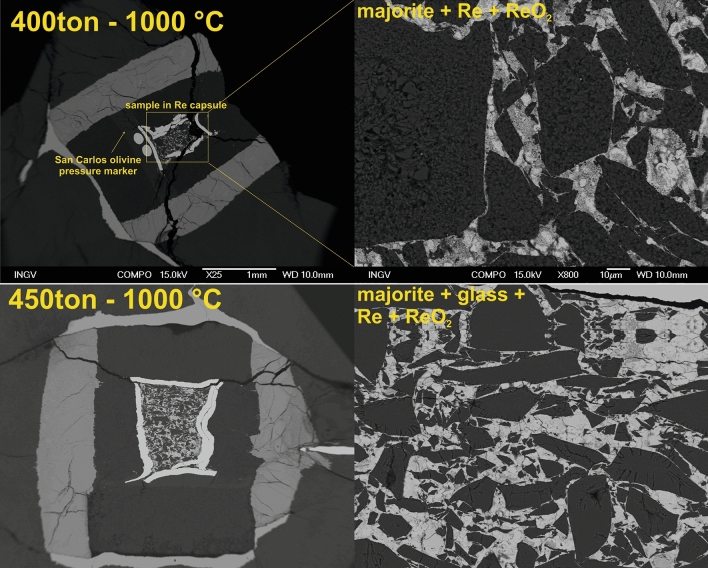
Figure 1.
Back-scattered electron (BSE) images of the runs M81 (top—400ton–1000 °C) and M82 (bottom—450ton–1000 °C). Capsules and mineral phases crystallized in the charges are depicted in the images reported on the left and on the right, respectively.
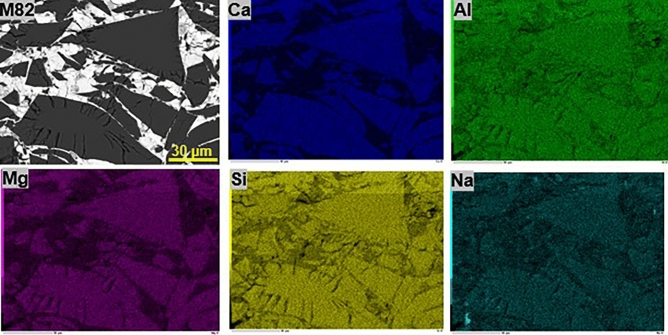
In run M81, the mineral phases observed in the capsule with the SEM are Maj + Re0 + ReO2. In run M82, Maj rims the residual starting glass (Figs. 1 and 2). The recovered products were found to retain the same chemical composition of the initial Cpx glass with ~ 5.8 wt% Na2O content and 55 wt% SiO2 corresponding to ~ 20% Na-Maj (Na2MgSi5O12) and ~ 58% Maj (Mg4Si4O12) end-members. This can be seen in Fig. 2 where a map of chemical elements in the quenched products is shown.
Figure 2.
Chemical maps collected on sample M82 showing no differences of the chemical composition between the rim and core of the recovered phases.
X-ray diffraction measurements and Mössbauer spectroscopy
The results from single-crystal XRD revealed that the phases in Fig. 1 consist of multiple crystallites. Only 4 single-crystal reflections (i.e., d = 1.54, 2.46, 2.58 and 2.89 Ả) belonging to the cubic Grt structure were unambiguously indexed giving a ~ 11.5 Å. On the other hand, the powder diffraction pattern collected on the same fragment showed the presence of several diffraction peaks (Fig. S2 of Supplementary Information), again all belonging to the cubic Grt structure. The least squares refinement of the diffraction peaks led to a = 11.4137(3) Å. Such a value is smaller than that observed for Na-bearing synthetic (Mg2.802Na0.198)(Al1.790Si0.150Mg0.060)Si3O12 Grt [a = 11.456(2) Å] studied by38, but it is larger than that measured for the Na-Maj (Na2MgSi5O12) end member44. Although synthetic Na2MgSi5O12 exhibits a tetragonal distortion, which is not observed in the present sample (no evidence of splitting of high-θ reflections), we can re-calculate an average cubic unit-cell parameter (a = 11.377 Å), which well reflects the decrease of the unit-cell volume with the increase of the amount of octahedral silicon. The ideal chemical formula of the crystal studied here, [CaNaMg][FeSiAl]Si3O12, verifies the pressure dependent exchange reaction NaX + SiY = MgX + AlY 19,40 assumed in Na-bearing Grt on the basis of the obtained experimental data38–44. The average cubic unit-cell parameter of the quenched Maj is also smaller than the cell parameter determined for (Na0.92Mg2.08)(Mg0.02Al1.06Si0.92)Si3O12 (~ 49% En-5% pyrope-46% Jd), as synthesized within 1 h by ref. 47 at 22 GPa and 2000 °C pointing out, therefore, the effect of the chemical composition on the Maj crystal structure, while the P–T dependence on the lattice parameter remains to be investigated.
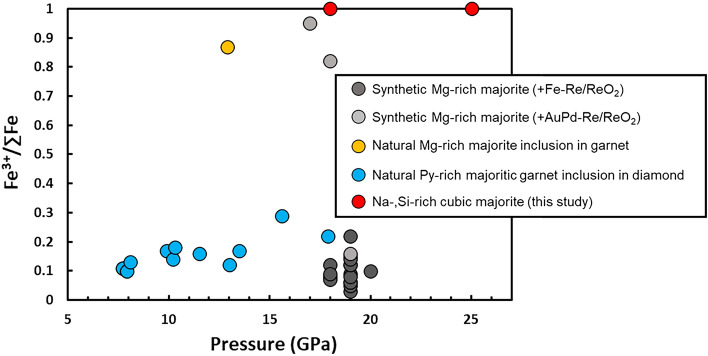
Mössbauer spectra were collected on the quenched cubic Maj in runs M81 and M82 to investigate the Fe oxidation state. The quenched crystalline phases were found to contain entirely Fe3+, with a detection limit for Fe2+ estimated as 2% for M81 and 3% for M82. M81 and M82 spectra were fitted to one doublet for Fe3+ (Fig. S3). The hyperfine parameters for the Fe3+ doublet are consistent with those reported in the literature for both natural29,48 and synthetic49,50 tetragonal Maj (Fig. S4). A high Fe3+ content was previously observed in a natural Maj included in an eclogitic Grt48 and in synthetic hydrous Maj50 (Fig. 3). Conversely, some natural29 and synthetic49 majorites of peridotitic composition only show Fe3+ contents up to ~ 30%.
Figure 3.
Variation of Fe3+/∑Fe with pressure for Na-rich Maj (this study) compared with literature data for majoritic Grt included in sub-lithospheric diamonds29, a natural eclogitic Maj included in Grt48, and synthetic peridotitic Maj under redox buffered conditions49,50.
Raman spectroscopy and nano-infrared microscopy
Strong evidence of the direct synthesis of cubic Maj from omphacitic glass is shown by the Raman spectra in Fig. 4 collected on M81, M82 core and M82 rim. The spectrum of Maj from run M81 appears similar to that from M82 rim, particularly regarding the positions and relative intensities of peaks at low to intermediate frequencies. By comparison with Raman frequencies reported by51, these Raman modes are assigned to dodecahedral translations (Tdod, ~ 200 cm−1), A-type vibration due to rotation/libration (R, ~ 360 cm−1), symmetric (ν2, ~ 576 cm−1) and asymmetric bending motions (ν4, ~ 650 cm−1 and ~ 800–850 cm−1) of SiO4 tetrahedra. The high-frequency region is dominated by broad peaks due to symmetric (ν1, ~ 930 cm−1) and asymmetric (ν1, ~ 968 cm−1 in M81) SiO4 stretching modes, while the peaks at 1065 cm−1 in M81 and 1057 cm−1 in M82 from the rim are associated with Si–O stretching between linked SiO4 and SiO6 polyhedra, in agreement also with that found in the case of natural Maj48. Peak widths are very broad compared to naturally occurring Maj, which most likely reflects strong cation disorder51,52 typically observed in samples produced on a laboratory timescale. However, the extreme broadening observed in the M82 core spectrum (Fig. 4, gray line) is more of a testament to the amorphous, glassy nature of the starting material.
Figure 4.
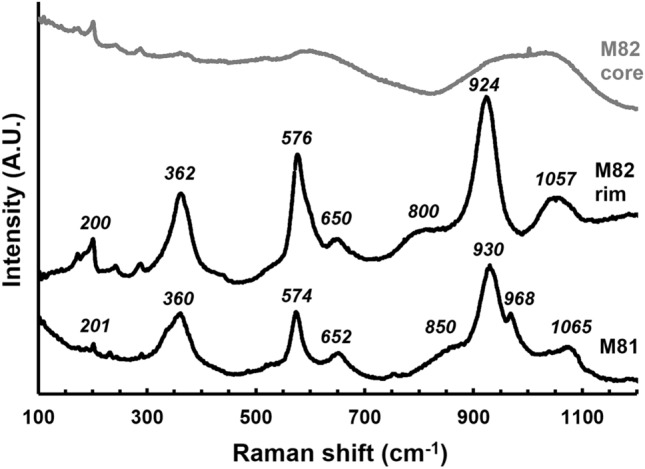
Raman spectra collected on samples M81, M82 core and M82 rim (in grey). The numbers reported above each peak indicate the Raman shift (in cm−1).
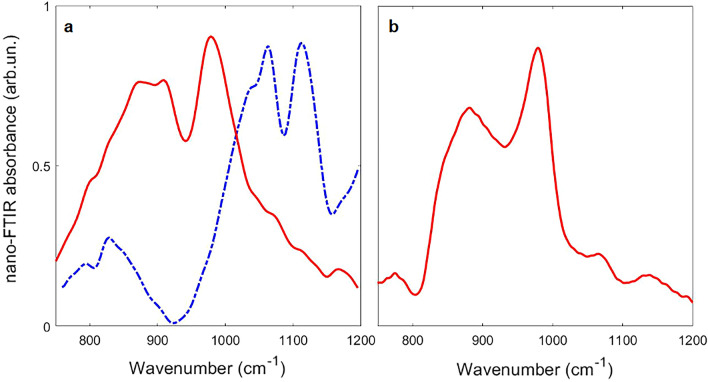
In view of the small grain size of the recovered quenched product and the absence of minor phases (e.g., exsolved minerals or amorphous unreacted portions), the homogeneous crystalline Maj product was further investigated by Nano-Infrared microscopy. Figure 5a,b shows spectra collected on M81 and M82 runs on an average of seven acquisitions on different spots. The absorption spectra of M81 and M82 in the spectral region between 750 and 1200 cm−1 are reported (with red continuous lines) respectively in Fig. 5a,b. An intense band between 850 and 1000 cm−1 is observed for both samples. In particular, three overlapping peaks are clearly detected for M81 at 870, 910 and 980 cm−1, while a peak at 880 cm−1 and two shoulders at 910 and 978 cm−1 are observed for M82. The discussed features are reproducible and match well with what found by author51 for Maj80. Remarkably, a previously unreported band is detected as a shoulder around 795 cm−1 in M81 spectrum and clearly observed as an isolated band for M82 at 774 cm−1. We suggest this to be characteristic of the Na-, Si- rich cubic Maj produced in the present study. These latter peaks must not be confused with that at ~ 785 cm−1 along with the peaks visible above 1000 cm−1 measured on the pockets filled by the Re-ReO2 buffer (blue dashed line in Fig. 5a).
Figure 5.
(a) Nano-Infrared spectra collected on two areas of samples M81 corresponding to the polycrystalline matrix (red continuous line) and the Re-ReO2 buffer (blue dotted line) for comparison. (b) Nano-Infrared spectrum collected on M81 polycrystalline matrix.
Discussion
Some considerations on the crystal-chemistry of quenched cubic majorite
Our results show textural and chemical evidence of the formation of a cubic Na, Fe3+ and Si-rich Maj single phase crystallized from a reduced omphacitic glass resembling the chemical composition of eclogitic Na-rich Cpx within 15–30 min at estimated pressures of about 18 (M81) and 25 GPa (M82) at 1000 °C, thus extending our knowledge of the pressure-dependent exchange reaction NaX + SiY = MgX + AlY. In light of previous studies45–47, we hypothesize that the quenched synthetic Na, Si-rich phase is the product of isochemical phase transformation of metastable Cpx, which in nature occurs over the first ~ 200 km during subduction of cold slabs. The synthesized cubic Na, Si-rich Maj phase appears distinct from that reported in literature being Si4+ ~ 4 a.p.f.u., which is higher than 3.1–3.55 a.p.f.u. of Maj trapped in sub-lithospheric diamonds29,53–57, while the sum of 8-coordinated cations is 2.393 a.p.f.u. in M81 and 2.409 a.p.f.u. in M82, therefore lower than 2.7–3.4 a.p.f.u. of natural inclusions. Before the quenched phase can be equaled to the Si-rich Grt reported by authors45–47, several points must be considered. First of all, Gasparik and co-authors45,46 reported the occurrence of Si-rich Maj from a more ultramafic composition used as starting material that brought to the formation of additional minerals like Al-rich majorite coexisting with pyroxene and olivine (wadsleyite). In contrast, our runs resulted in the formation of Na,Si-rich Maj as single phase using an omphacitic glass as starting material. Secondly, Gasparik and co-authors45,46 reported much lower P (13.5, 14.5 and 15 GPa), higher T (1550–1650 °C) and longer runs (4 h) of equilibration for the Na,Si-rich Maj. In contrast, our experiments were conducted at higher P and much lower T and duration resulting in a well crystallized and chemically homogeneous Maj phase with cubic symmetry, taking into account that the aim of this study was neither to delineate the phase stability P–T field nor to constrain the kinetics of the Cpx—to—Maj transformation for which additional experiments are certainly needed. Finally, a cubic Na, Si-rich Maj was synthesized by ref.47 at 22 GPa and 2000 °C in 1 h, but with no Ca and Fe so that the composition cannot be considered representative of eclogitic Cpx. We conclude, therefore, that although a Na-, Si-rich cubic Maj has been shown to quench in this and a previous study within 15 min to 4 h, we cannot exclude the possibility that this mineral could have turned into either tetragonal Na-, Si-rich Maj or decomposed to a mineral assemblage in the case of much longer runs. Obviously, such an assessment would require time series experiments to demonstrate the long-time metastability of this polymorph. Here, we propose the origin of dominantly Na-rich Maj in a scenario where omphacite-rich lithologies persist during subduction and transform to a cubic Na, Si-rich Maj layer due to the absence or paucity of Grt as a reactant.
Potential implications for the rheology of the subducted slab
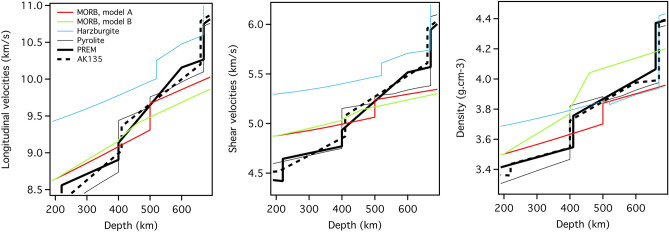
To understand the potential effects of the omphacitic Cpx → cubic Na,Si-rich Maj transformation on the rheology of the subducted slab, we used our results to model the density, longitudinal (VP) and shear (VS) wave velocities of mid-ocean ridge basalt (MORB) at the pressures of the mantle transition zone (MTZ), using elastic parameters of major minerals available in the literature (Table 2). The elastic properties of the [CaNaMg][SiFeAl]Si3O12 cubic Grt, as well as MORB-like58 and pyrolite-like59 Maj, were estimated by a weighted summation of the individual elastic properties of Mg3Al2Si3O12 pyrope60, MgSiO3 majorite61, Ca3Al2Si3O12 grossular58, Ca3Fe2Si3O12 andradite62, Fe3Al2Si3O12 almandine63 and Na2MgSi3O12 majorite64. In the absence of direct measurements, the pressure and temperature dependences of the adiabatic bulk (KS) and shear (G) moduli of Na2MgSi3O12 Maj were derived from the elasticity dataset given by41. Comparison of our models for MORB and pyrolite Maj with experimental data allows to test the effect of non-ideal mixing on the elasticity of Maj Grt solid solutions. Our results show that this effect may be negligible at P higher than ~ 7 GPa (Fig. S5 in extended data) and therefore we assumed [CaNaMg][SiAl]Si3O12 cubic Grt is also behaving as an ideal solid solution at high pressure. Our model predicts that the longitudinal and shear velocities of [CaNaMg][SiAl]Si3O12 Maj are slightly higher than those of pyrolitic Maj59 while they are substantially lower than those of MORB Maj58 at the P conditions of the mantle transition zone (Fig. S5a,b).
Table 2.
Elastic parameters of MORB-derived silicate minerals and end members.
| Mineral | KS0 (GPa) | KS’ | dKS/dT (GPa K−1) | G0 (GPa) | G’ | dG/dT (GPa K−1) |
|---|---|---|---|---|---|---|
| CaMgSi2O6 diopside a | 116.4 | 4.9 | − 0.012 | 73 | 1.6 | − 0.011 |
| Pyrope b | 170 | 4.5 | − 0.017 | 93.2 | 1.51 | − 0.011 |
| Grossular c | 171 | 4.4 | − 0.013 | 108 | 1.3 | − 0.011 |
| Andradite d | 154 | 4.7 | − 0.013* | 90 | 1.2 | − 0.011* |
| Almandine e | 174 | 4.6 | − 0.027 | 95 | 1.1 | − 0.013 |
| MgSiO3 Maj f | 164 | 4.1 | − 0.022 | 86 | 1.1 | − 0.009 |
| Na-Maj g | 173 | 4.4* | − 0.013** | 115 | 1.5 | − 0.010** |
| MORB Maj h | 155.8 | 4.5 | − 0.013** | 89.7 | 1.5 | − 0.010** |
| Pyrolite Maj i | 164.4 | 4.2 | − 0.013 | 94.9 | 1.1 | − 0.010 |
| SiO2 + 2wt% Al2O3 Stishovite j | 286 | 5.2 | − 0.045 | 199 | 2.5 | − 0.017 |
a. Ref. 90, b. Ref. 60, c. Ref. 91, d. Ref. 62, e. Ref. 63, f. derived from Ref. 61, g. Ref. 42,64, h. Ref. 58, i. Ref. 59, j. Ref. 92. Value fixed to those of *grossular Grt and ** pyrolitic Maj, respectively. (+) KS and G were taken from ref. 64, which has the closest composition to our cubic majorite. Ks’ was derived from Kt’ of 42 and G’ from assuming a constant Poisson ratio. Thermal parameters were assumed = pyrolitic Maj.
The elasticity of MORB aggregates was derived through a Voigt-Reuss-Hill (VRH) average of the individual mineral elastic properties, assuming relative proportions of each phase from phase equilibrium data proposed by65. In this calculation, we used a cold slab geotherm with an adiabatic temperature gradient as proposed by Thompson66, and which has a root temperature T = 280 K at 0 km depth (e.g., T = 1140 K at ~ 500 km depth). The calculated longitudinal velocities, shear velocities and density of MORB aggregates along a cold slab geotherm as a function of depth are shown in Fig. 6a–c. In the UM, we hypothesize a MORB-derived eclogitic slab that consists of 75 vol% Cpx, 20 vol% cubic Grt (e.g., almandine-pyrope-grossular solid solution) and ~ 5 vol% stishovite. Although the modal composition is strictly controlled by P, T and composition14,32, a representative seismic model can be drawn with the UM Grt being calculated following weighted average methods assuming a solid solution (in mol%) of 43% pyrope, 27% grossular and 30% almandine as suggested by the phase relation of MORB at 5 GPa and 1200 °C67. When equilibrated, the MORB assemblage transforms to majoritic Grt (95%) and stishovite (5%) upon the gradual dissolution of pyroxenes into the Grt at P between 7 and 15 GPa (Model B of Fig. 6). In contrast, at conditions where metastable minerals might be present such as those in the subducted cold slab, our results raise the question whether the assemblage of Cpx, Grt and stishovite remains stable up to ~ 18 GPa where omphacitic Cpx is here shown to transform directly to cubic [CaNaMg][SiAl]Si3O12 majoritic Grt, thus forming an assemblage of 75% majoritic Grt, 20% cubic Grt and 5% stishovite in the MTZ (Model A, Fig. 6). If this is the case, the transition of omphacitic Cpx to [CaNaMg][SiAl]Si3O12 cubic majoritic Grt at ~ 520 km depth would be accompanied by a ~ 0.2 g cm−3 density increase while VP and VS would increase by 0.5 and 0.2 km s−1, respectively (Fig. 6). These results inferred from our experiments show that unequilibrated MORB mineral assemblages would have density similar to that of the surrounding mantle and harzburgite (blue line in Fig. 6), hence, forcing the slab to float between 500 and 660 km depth. These results are strengthened by harzburgite, which constitutes the main body of the subducted slab, and whose density is even further below those of pyrolite while yielding higher velocities59,68,69. The presence of harzburgite and unequilibrated MORB mineral assemblages (with the hypothesized proportions) might have favored regimes of slab floating at middle-to-lowermost MTZ (see Fig. 6c) for millions of years such as those observed by seismic tomography beneath Europe or North America70. Obviously, an experimental study aimed to investigate the kinetics of the cubic-to-tetragonal majorite transformation is needed to strengthen such a conclusion. In contrast, equilibrated MORB mineral assemblage (green line in Fig. 6c) would cause the slab to sink into the LM as the density is far higher than those of pyrolite and harzburgite in the MTZ71. Importantly, after transformation of omphacitic Cpx to Grt, only VP contrast appears significant while VS are almost identical. Finally, as dense majorite MORB forms upon equilibration of the slab (e.g., model A transforms to model B), this sinks down into the LM carrying, therefore, incompatible and refractory elements such as Na and Al, respectively.
Figure 6.
(a) Longitudinal, (b) Shear velocities and (c) density of MORB aggregates along a cold slab geotherm [ref.66] as a function of depth. Red line represents the disequilibrium MORB where the transition of Cpx to [CaNaMg][SiAl]Si3O12 majoritic Grt was fixed to 520 km depth. Green line represents equilibrium MORB where Cpx dissolved gradually in Grt, increasing in turn proportions of MORB majoritic Grt. Thin black line represents velocities and density of pyrolite for comparison, as well as seismological 1D models, PREM (thin black line) and AK135 (broken black line).
Majorite as diamond growth-medium
The calculated proportions of Na-majorite component, Na2MgSi5O12 [(Na)/2], and majorite component, Mg3MgSiSi3O12 [(Si-2Na-Maj)-3] can be used as a geobarometer19. From our experiments, we calculated proportions of 0.20 and 0.57, respectively, for the two components giving a pressure estimate of ~ 20 GPa. The Mg# [(Mg/Mg + Fe2+)] of 0.81–0.83 and Ca# [Ca/(Ca + Mg + Fe2+)] of 0.47–0.48 (Fe2+ recalculated from the measured Fe3+) are both intermediate with respect to Mg# and Ca# measured in natural majoritic inclusions with peridotitic and eclogitic affinity (see Fig. 7a,b in Ref.19). Conversely, the unique chemical composition of our synthesized Maj is very similar to that reported for two mineral inclusions (BZ237A and BZ259B) found in a diamond from Juina (Brazil)72 and referred to a III-type mineral association representative of the TZ and LM. Indeed, the chemical composition of these inclusions match well with our quenched cubic Maj much more than that of another diamond inclusion from the kimberlite pipe in Liaoning Province (China)73. In this latter case, the mineral was proposed to contain 16 mol% Na-Maj and 84 mol% Mg-Maj, corresponding to 2.3 wt% Na2O and 33 wt% MgO. Experiments at HP-T suggested the possible origin of this Na-rich Maj as unmixing of Al-rich Maj and as reaction product of MgSiO3 bridgmanite with an alkali-rich carbonatitic melt at depths the TZ and LM74,75. However, the temperature of these experiments is unfeasibly high (1900–2000 °C) within the context of any plausible subduction scenario. We emphasize that the new synthesized cubic Maj contains about 5.8 wt% Na2O, more than 9 wt% MgO, along with CaO (~ 14–15 wt%), Al2O3 (~ 11 wt%) and Fe2O3 (~ 3.7 wt%), and it was quenched at P representative of the TZ and LM depths. The lack of evidence for cubic Na-, Si- rich majorite inclusions in diamonds with chemical compositions similar to that reported in this study can be explained as a consequence of the long equilibration times required to precipitate diamonds compared to the those of synthesis reported here. Noteworthy, the high Fe3+ content of cubic Maj implies a major role in redox reactions at the expense of oxidized C-bearing melts to precipitate diamonds via the chemical equilibrium,
| 1 |
On the kinetics of cubic-to-tetragonal majorite
It is known that cubic Maj with composition Wo1En78Fs21 was first found within minerals in shock impact in meteorites76. An accurate literature search reveals that majoritic Grt from shocked chondrites76–81 are characterized by Si ranging from 3.55 to 3.99 a.p.f.u., with the highest contribution to the majoritic end member given by the low-Al Grt that transformed directly from enstatite, whereas the more Al-rich compositions refers to Grt that crystallized from a shock-induced melt. Regardless, all of these majorite garnets are characterized by a cubic symmetry explained as a consequence of either the rapid quench on the atomic ordering (requiring longer time than a metastable phase transition) or their relatively high Fe/(Fe + Mg) ratios of 0.20–0.27 responsible for the stabilization of the cubic structure82. The incorporation of elements such as Fe3+ and Na, as well as the T effect were indicated as likely to further stabilize the cubic structure of Maj in the TZ82–84. Our current results would confirm these expectations.
Materials and methods
Synthesis
The starting material used in this study is a synthetic omphacitic glass with composition as reported for natural Bavarian (Weissenstein) eclogite85 (COMP2, ref.27). The synthetic omphacitic glass was prepared by melting a mixture of oxides and carbonates at 1650 °C in an iron-saturated platinum crucible followed by rapid quenching in water and ice. The glass was analyzed using the electron microprobe (details in ref.27) and then ground and reduced in a gas mixing furnace at 800 °C using a H2-CO2 gas mixture at a fo2 of ~ 2 log units above the iron-wüstite buffer. The glass was, then, powdered and mixed with 25% of a Re and ReO2 (1:1 mol ratio) mixture to act as an oxygen buffer. Two experiments were performed at pressures of ~ 18, and ~ 25 GPa at 1000 °C using the 840-ton Walker-type press available at the High-Pressure High Temperature laboratory of the National Institute of Geophysics and Volcanology (INGV, Italy). Tungsten carbide anvils (F grade) with 3 mm truncation edge lengths (TEL) were used with cobalt-doped MgO octahedra as pressure media. Rhenium capsules were employed in our experiments to prevent loss of Fe from the starting material during the experiments. After loading with the starting powder, the capsule was placed in the central portion of a cylindrical straight LaCrO3 furnace separated by MgO sleeve. MgO disks as spacers were placed at the bottom and top of the capsule to serve as pressure medium. The temperature during the experiments was monitored with a W-5%Re/W-26%Re (C-type) thermocouple inserted through a hole drilled in the pressure medium and the heater above which the capsule was located. The gaps between the thermocouple and the capsule were filled with olivine (San Carlos) powder used as pressure marker based on the olivine-ringwoodite and ringwoodite-periclase + bridgmanite phase transitions86. The sample was compressed to the target pressure at a rate of ~ 0.5 GPa/h, then heated and kept at a constant temperature within ± 10 °C for a period of 30 min. The run was quenched by turning off the power to the furnace and then, decompressed to ambient pressure within 15 h.
Field emission scanning electron microscopy
Textural observations and quantitative chemical compositions of the run products were performed using a JEOL JSM-6500F field emission scanning electron microscope at the Microanalysis Lab of National Institute of Geophysics and Volcanology (INGV, Rome). The FE-SEM apparatus is equipped with back-scattered electron detector and energy dispersion system (JEOL 133 eV resolution), and the operative acquiring conditions established for accurate analyses are an accelerating voltage of 15 kV and a probe current of 0.8 nA. Samples were C-coated before being analyzed.
X-ray diffraction
X-ray diffraction investigations were performed at the CRIST, Centro di Studi per la Cristallografia Strutturale, Università di Firenze, Italy. A small fragment (size about 17 × 22 × 34 μm3) was extracted from the polished section of one of the recovered samples (M82) under a reflected light microscope and mounted on a 5 µm diameter carbon fiber, which was, in turn, attached to a glass rod. The single-crystal X-ray study was done with an Oxford Diffraction Xcalibur3 CCD single-crystal diffractometer using MoKα radiation (λ = 0.71073 Å), working conditions 60 kV × 50 nA and with 300 s exposure time per frame; the detector-to-sample distance was 6 cm. Then, to get a powder diffraction pattern, the same grain was studied with an Oxford Diffraction Xcalibur PX Ultra diffractometer equipped with a 165-mm diagonal Onyx CCD detector at 2.5:1 demagnification operating with CuKα radiation (λ = 1.5406 Å). The working conditions were 50 kV × 50 nA with 7 h of exposure; the detector-to-sample distance was 7 cm. Data were processed using the CrysAlis software package version 1.171.36.28 running on the Xcalibur PX control PC.
Mössbauer spectroscopy
Fe3+/ΣFe ratios were estimated using Mössbauer spectroscopy. Octahedra from high-pressure runs were mounted in epoxy resin and cut into slices to expose the sample on both sides. Slices were double polished to a thickness of 600 μm, which is close to the optimum thickness based on sample composition87 and corresponds to a dimensionless effective thickness of 2 (5 mg Fe/cm2). A region of recovered products containing majorite with dimensions of 800 μm × 600 μm was exposed on each side of sample slices, and the outside area was covered with 25 μm thick Ta foil, which absorbs 99% of 14.4 keV gamma rays. Mössbauer spectra were recorded at room temperature in transmission mode on a constant acceleration spectrometer using a nominal 370 MBq 57Co point source at Bayerisches Geoinstitut, Bayreuth. The collecting time for each spectrum was 2 weeks. The velocity scale, set at ~ 5 mm/s, was calibrated relative to an α-Fe foil reference standard. Once folded, spectra were fitted to Lorentzian line-shapes using the fitting program MossA88.
Raman spectroscopy and nano-infrared microscopy
The crystallinity of the recovered products was checked by Raman spectroscopy using a Horiba Jobin Yvon LABRAM HR800 spectrometer at the Experimental Volcanology and Petrology Laboratory (EVP Lab) (University of Roma Tre, Rome). The spectrometer is equipped with two gratings (1800 and 600 grooves/mm), a CCD detector (operating at − 70 °C), an Olympus optical microscope (objectives 10X, 20X, 50X and 100X) and a solid-state Nd-YAG laser as source (wavelength 532 nm, power 60 Mw).
Nano-Infrared Microscopy measurements were carried out at the SISSI beamline of Elettra/CNR-IOM at Elettra Sincrotrone (Basovizza, Trieste, Italy) by using a NEASPEC s-SNOM instrument. Measurements were performed in tapping mode at a tapping frequency of 260 kHz. Tapping amplitude was set to 80 nm (with approach at 80% of free amplitude). Spectra were acquired at a resolution of 6 cm−1. A N2-cooled MCT (Mercury–Cadmium–Tellurium) detector was used to detect infrared signal. The absorption was calculated directly from Neaspec acquisition software following the procedure described in ref.89.
Supplementary Information
Acknowledgements
V.S. gratefully acknowledges financial support from PRIUS program (GRC, Ehime University) and “Fondi di Ateneo 2021” by Sapienza University (Rome). LB was supported by the “Progetto di Ateneo 2015” issued by the Università di Firenze, Italy. SA was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project number AU356/12. Nano-Infrared Microscopy measurements were performed at Elettra Synchrotron of Trieste.
Author contributions
The study was initially conceived by V.S. and P.S.. V.S. and B.B. performed the synthesis experiment. L.B. performed diffraction measurements. B.B., V.S., B.P. and C.R. collected the Raman spectra from the recovered samples. B.B., VS and M.N. performed chemical analyses of the recovered run products. S.G. prepared the cell assemblies for the experiments and with T.I. worked on the seismic model. G.M. and C.A.M collected and fitted the Mössbauer spectra. F.P. and S.L. performed nano-FTIR measurements and elaborated the acquired spectra. V.S., L.B., S.A. and S.G. wrote the paper. All the authors discussed the results, commented on the manuscript and contributed to its final version.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-43037-6.
References
- 1.Christensen UR. The influence of trench migration on slab penetration into the lower mantle. Earth Planet. Sci. Lett. 1996;140(1):27–39. doi: 10.1016/0012-821X(96)00023-4. [DOI] [Google Scholar]
- 2.Torii Y, Yoshioka S. Physical conditions producing slab stagnation: Constraints of the Clapeyron slope, mantle viscosity, trench retreat, and dip angles. Tectonophysics. 2007;445(3):200–209. doi: 10.1016/j.tecto.2007.08.003. [DOI] [Google Scholar]
- 3.Christensen UR, Yuen DA. The interaction of a subducting lithospheric slab with a chemical or phase boundary. J. Geophys. Res. 1984;89(B6):4389–4402. doi: 10.1029/JB089iB06p04389. [DOI] [Google Scholar]
- 4.Gurnis M, Hager BH. Controls of the structure of subducted slabs. Nature. 1988;335(6188):317–321. doi: 10.1038/335317a0. [DOI] [Google Scholar]
- 5.Yoshida M. The role of harzburgite layers in the morphology of subducting plates and the behavior of oceanic crustal layers. Geophys. Res. Lett. 2013;40:5387–5392. doi: 10.1002/2013GL057578. [DOI] [Google Scholar]
- 6.Garel F, Goes S, Davies DR, Davies JH, Kramer SC, Wilson CR. Interaction of subducted slabs with the mantle transition-zone: A regime diagram from 2-D thermo-mechanical models with a mobile trench and an overriding plate. Geochem. Geophys. Geosyst. 2014;15:1739–1765. doi: 10.1002/2014GC005257. [DOI] [Google Scholar]
- 7.Carminati E, Negredo AM, Valera JL, Doglioni C. Subduction-related intermediate depth and deep seismicity in Italy: Insights from thermal and rheological modeling. Phys. Earth Planet. Int. 2005;149:65–79. [Google Scholar]
- 8.Akaogi M, Ito E, Navrotsky A. Olivine-modified spinel-spinel transitions in the system Mg2SiO4-Fe2SiO4: Calorimetric measurements, thermochemical calculation, and geophysical application. J. Geophys. Res. 1989;94(B11):15671–15685. [Google Scholar]
- 9.Rubie DC, Ross CR., II Kinetics of the olivine-spinel transformation in subducting lithosphere: Experimental constraints and implications for deep slab processes. Phys. Earth Planet. Int. 1994;86(1–3):223–243. [Google Scholar]
- 10.Green HW, Houston H. The mechanics of deep earthquakes. Annu. Rev. Earth Planet. Sci. 1995;23(1):169–213. [Google Scholar]
- 11.Kirby SH, Stein S, Okal EA, Rubie DC. Metastable mantle phase transformations and deep earthquakes in subducting oceanic lithosphere. Rev. Geophys. 1996;34(2):261–306. [Google Scholar]
- 12.Ito E, Katsura T. A temperature profile of the mantle transition zone. Geophys. Res. Lett. 1989;16(5):425–428. [Google Scholar]
- 13.Solomatov VS, Stevenson DJ. Can sharp seismic discontinuities be caused by non-equilibrium phase transformations? Earth Planet. Sci. Lett. 1994;125(1–4):267–279. [Google Scholar]
- 14.Knapp N, Woodland AB, Klimm K. Experimental constraints on coesite abundances in eclogite and implications for the X seismic discontinuity. J. Geophys. Res. Solid Earth. 2015;120:4917–4930. doi: 10.1002/2015JB011933. [DOI] [Google Scholar]
- 15.Hirose K, Fei Y. Subsolidus and melting phase relations of basaltic composition in the uppermost lower mantle. Geochim. Cosmochim. Acta. 2002;66(12):2099–2108. [Google Scholar]
- 16.Irifune T, Ringwood AE. Phase transformations in primitive MORB and pyrolite compositions to 25 GPa and some geophysical implications. In: Manghnani M, Syono Y, editors. High Pressure Research in Mineral Physics. American Geophysical Union; 1987. pp. 231–242. [Google Scholar]
- 17.Harte B, Harris JW. Lower mantle mineral associations preserved in diamonds. Mineral. Mag. A. 1994;58:384–385. [Google Scholar]
- 18.Thomson AR, Kohn SC, Bulanova GP, Smith CB, Araujo D, Walter MJ. Origin of sub-lithospheric diamonds from the Juina-5 kimberlite (Brazil): Constraints from carbon isotopes and inclusion compositions. Contrib. Mineral. Petrol. 2014;168(6):1081. [Google Scholar]
- 19.Beyer C, Frost DJ. The depth of sub-lithospheric diamond formation and the redistribution of carbon in the deep mantle. Earth Planet. Sci. Lett. 2017;461:30–39. [Google Scholar]
- 20.Christensen U. Effect of phase transitions on mantle convection. Annu. Rev. Earth Planet. Sci. 1995;23:65–87. [Google Scholar]
- 21.Bina CR, Wood BJ. The eclogite to garnetite transition: Experimental and thermodynamic constraints. Geophys. Res. Lett. 1984;11(10):955–958. [Google Scholar]
- 22.Akaogi, M., Navrotsky, A., Yagi, T. & Akimoto, S. I. Pyroxene‐Garnet Transformation: Thermochemistry and Elasticity of Garnet Solid Solutions, and Application to a Pyrolite Mantle. High-Pressure Research in Mineral Physics: A Volume in Honor of Syun-iti Akimoto 251–260 (1987).
- 23.Bina CR, Liu M. A note on the sensitivity of mantle convection models to composition-dependent phase relations. Geophys. Res. Lett. 1995;22(19):2565–2568. [Google Scholar]
- 24.Wood BJ, Rubie DC. The effect of alumina on phase transformations at the 660-kilometer discontinuity from Fe-Mg partitioning experiments. Science. 1996;273(5281):1522–1524. [Google Scholar]
- 25.Vacher P, Mocquet A, Sotin C. Computation of seismic profiles from mineral physics: The importance of the non-olivine components for explaining the 660 km depth discontinuity. Phys. Earth Planet. Inter. 1998;106(3–4):275–298. [Google Scholar]
- 26.Akaogi M, Akimoto SI. Pyroxene-garnet solid-solution equilibria in the systems Mg4Si4O12-Mg3Al2Si3O12 and Fe4Si4O12-Fe3Al2Si3O12 at high pressures and temperatures. Phys. Earth Planet. Inter. 1977;15(1):90–106. doi: 10.1016/0031-9201(77)90013-9. [DOI] [Google Scholar]
- 27.Stagno V, Frost DJ, McCammon CA, Mohseni H, Fei Y. The oxygen fugacity at which graphite or diamond forms from carbonate-bearing melts in eclogitic rocks. Contrib. Miner. Petrol. 2015;169:16. [Google Scholar]
- 28.Stagno V. Carbon, carbides, carbonates and carbonatitic melts in the Earth’s interior. J. Geol. Soc. 2019 doi: 10.1144/jgs2018-095. [DOI] [Google Scholar]
- 29.Kiseeva ES, Vasiukov DM, Wood BJ, et al. Oxidized iron in garnets from the mantle transition zone. Nat. Geosci. 2018;11:144–147. doi: 10.1038/s41561-017-0055-7. [DOI] [Google Scholar]
- 30.Aulbach S, Jacob DE. Major- and trace-elements in cratonic mantle eclogites and pyroxenites reveal heterogeneous sources and metamorphic processing of low-pressure protoliths. Lithos. 2016;262:586–605. [Google Scholar]
- 31.Aulbach S, Massuyeau M, Garber JM, Gerdes A, Heaman LM, Viljoen KS. Ultramafic carbonated melt- and auto-metasomatism in mantle eclogites: Compositional effects and geophysical consequences. Geochem. Geophys. Geosyst. 2020;5:e2019GC008774. [Google Scholar]
- 32.Garber JM, Maurya S, Hernandez J-A, Duncan MS, Zeng L, Zhang HL, et al. Multidisciplinary constraints on the abundance of diamond and eclogite in the cratonic lithosphere. Geochem. Geophys. Geosyst. 2018 doi: 10.1029/2018GC007534. [DOI] [Google Scholar]
- 33.Agashev AM, Pokhilenko LN, Pokhilenko NP, Shchukina EV. Geochemistry of eclogite xenoliths from the Udachnaya Kimberlite Pipe: Section of ancient oceanic crust sampled. Lithos. 2018;314–315:187–200. [Google Scholar]
- 34.Usui T, Nakamura E, Helmstaedt H. Petrology and geochemistry of eclogite xenoliths from the Colorado plateau: Implications for the evolution of subducted oceanic crust. J. Petrol. 2006;47(5):929–964. doi: 10.1093/petrology/egi101. [DOI] [Google Scholar]
- 35.Harlow GE, Tsujimori T, Sorensen SS. Jadeitites and plate tectonics. Annu. Rev. Earth Planet. Sci. 2015;43(1):105–138. [Google Scholar]
- 36.Irifune T, Hibberson WO, Ringwood AE, et al. Eclogite-garnetite transformation at high pressure and its bearing on the occurrence of garnet inclusions in diamond. In: Ross J, et al., editors. Kimberlites and Related Rocks 2. Blackwell; 1989. pp. 877–882. [Google Scholar]
- 37.Nishi M, Kubo T, Ohfuji H, Kato T, Nishihara Y, Irifune T. Slow Si-Al interdiffusion in garnet and stagnation of subducting slabs. Earth Planet. Sci. Lett. 2013;361:44–49. doi: 10.1016/j.epsl.2012.11.022. [DOI] [Google Scholar]
- 38.Bobrov AV, Litvin YuA, Bindi L, Dymshits AM. Phase relations and formation of sodium-rich majoritic garnet in the system Mg3Al2Si3O12–Na2MgSi5O12 at 7.0 and 8.5 GPa. Contrib. Mineral. Petrol. 2008;156:243–257. [Google Scholar]
- 39.Bobrov AV, Dymshits AM, Litvin YuA, Bindi L. The Mg3Al2Si3O12–Na2MgSi5O12 system at pressures of 7.0 and 8.5 GPa and a temperature of 1300–1800 °C: Phase relationships and crystallization of Na-bearing majoritic garnet. Moscow Univ. Geol. Bull. 2012;67:289–297. [Google Scholar]
- 40.Bobrov AV, Litvin YA, Kuzyura AV, Dymshits AM, Jeffries T, Bindi L. Partitioning of trace elements between Na-bearing majoritic garnet and melt at 8.5 GPa and 1500–1900 °C. Lithos. 2014;189:159–166. [Google Scholar]
- 41.Dymshits AM, Bobrov AV, Bindi L, Litvin YA, Litasov KD, Shatskiy AF, Ohtani E. Na-bearing majoritic garnet in the system Na2MgSi5O12–Mg3Al2Si3O12 at 11–20 GPa: Phase relations, structural peculiarities and solid solutions. Geochim. Cosmochim. Acta. 2013;105:1–13. [Google Scholar]
- 42.Dymshits AM, Litasov KD, Shatskiy A, Sharygin IS, Ohtani E, Suzuki A, Pokhilenko NP, Funakoshi K. P-V–T equation of state of Na-majorite to 21 GPa and 1673 K. Phys. Earth Planet. Inter. 2014;227:68–75. [Google Scholar]
- 43.Dymshits AM, Bobrov AV, Litasov KD, Shatskiy AF, Ohtani E, Litvin YA. Experimental study of the pyroxene-garnet phase transition in the Na2MgSi5O12 system at pressures of 13–20 GPa: First synthesis of sodium majorite. Dokl. Earth Sci. 2010;434:1263–1266. [Google Scholar]
- 44.Bindi L, Dymshits AM, Bobrov AV, Litasov KD, Shatskiy AF, Ohtani E, Litvin YA. Crystal chemistry of sodium in the Earth’s interior: The structure of Na2MgSi5O12 synthesized at 17.5 GPa and 1700 °C. Am. Mineral. 2011;96:447–450. [Google Scholar]
- 45.Gasparik T. Evidence for immiscibility in majorite garnet from experiments at 13–15 GPa. Geochim. Cosmochim. Acta. 2000;64:1641–1650. [Google Scholar]
- 46.Gasparik T. Experimental investigation of the origin of majoritic inclusions in diamonds. Phys. Chem. Minerals. 2002;29:170–180. [Google Scholar]
- 47.Reichmann HJ, Sinogeikin SV, Bass JD, Gasparik T. Elastic moduli of Jadeite-Enstatite Majorite. Geophys. Res. Lett. 1936;29(19):2002. doi: 10.1029/2002GL015106. [DOI] [Google Scholar]
- 48.Xu C, Kynický J, Tao R, Liu X, Zhang L, Pohanka M, Wenlei S, Fei Y. Recovery of an oxidized majorite inclusion from Earth’s deep asthenosphere. Sci. Adv. 2017;3(4):e1601589. doi: 10.1126/sciadv.1601589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCammon CA, Ross NL. Crystal chemistry of ferric iron in (Mg, Fe)(Si, Al)O3 majorite with implications for the transition zone. Phys. Chem. Minerals. 2003;30(4):206–216. doi: 10.1007/s00269-003-0309-3. [DOI] [Google Scholar]
- 50.McCammon CA, Frost DJ, Smyth JR, Laustsen HMS, Kawamoto T, Ross NL, Van Aken PA. Oxidation state of iron in hydrous mantle phases: Implications for subduction and mantle oxygen fugacity. Phys. Earth Planet. Inter. 2004;143:157–169. doi: 10.1016/j.pepi.2003.08.009. [DOI] [Google Scholar]
- 51.Hofmeister AM, Giesting PA, Wopenka B, Gwanmesia GD, Jolliff BL. Vibrational spectroscopy of pyrope-majorite garnets: Structural implications. Am. Mineral. 2004;89:132–146. [Google Scholar]
- 52.McMillan P, Akaogi M, Ohtani E, Williams Q, Nieman R, Sato R. Cation disorder in garnets along the Mg3Al2Si3O12-Mg4Si4O12 join: An infrared, Raman and NMR study. Phys. Chem. Minerals. 1989;16:428–435. [Google Scholar]
- 53.Thomson AR, Walter MJ, Kohn SC, Brooker RA. Slab melting as a barrier to deep carbon subduction. Nature. 2016;529:76–79. doi: 10.1038/nature16174. [DOI] [PubMed] [Google Scholar]
- 54.Collerson KD, Williams Q, Kamber BS, Omori S, Arai H, Ohtani E. Majoritic garnet: A new approach to pressure estimation of shock events in meteorites and the encapsulation of sub-lithospheric inclusions in diamond. Geochim. Cosmochim. Acta. 2010;74:5939–5957. [Google Scholar]
- 55.Grütter HS, Gurney JJ, Menzies AH, Winter F. An updated classification scheme for mantle-derived garnet, for use by diamond explorers. Lithos. 2004;77:841–857. [Google Scholar]
- 56.Kiseeva ES, Yaxley GM, Stepanov AS, Tkalčic H, Litasov KD, Kamenetsky VS. Metapyroxenite in the mantle transition zone revealed from majorite inclusions in diamonds. Geology. 2013;41:883–886. [Google Scholar]
- 57.Stachel T, Brey GP, Harris JW. Kankan diamonds (Guinea)I: From the litho-sphere down to the transition zone. Contrib. Mineral. Petrol. 2000;140:1–15. [Google Scholar]
- 58.Kono Y, Higo Y, Ohfuji H, Inoue T, Irifune T. Elastic wave velocities of garnetite with a MORB composition up to 14 GPa. Geophys. Res. Lett. 2007;34:L14308. [Google Scholar]
- 59.Irifune T, Higo Y, Inoue T, Kono Y, Ohfuji H, Funakoshi K. Sound velocities of majorite garnet and the composition of the mantle transition region. Nature. 2008;451(7180):814–817. doi: 10.1038/nature06551. [DOI] [PubMed] [Google Scholar]
- 60.Zou Y, Irifune T, Gréaux S, Whitaker ML, Shinmei T, Ohfuji H, Negishi R, Higo Y. Elasticity and sound velocities of polycrystalline Mg3Al2(SiO4)3 garnet up to 20 GPa and 1700 K. J. Appl. Phys. 2012;112:014910. doi: 10.1063/1.4736407. [DOI] [Google Scholar]
- 61.Zhou C, Gréaux S, Liu Z, Higo Y, Arimoto T, Irifune T. Sound velocity of MgSiO3 majorite garnet up to 18 GPa and 2000 K. Geophys. Res. Lett. 2021;48:e2021GL093499. doi: 10.1029/2021GL093499. [DOI] [Google Scholar]
- 62.Jiang F, Speziale S, Shieh SR, Duffy TS. Single-crystal elasticity of andradite garnet to 11 GPa. J. Phys. 2004;16:S1041–S1052. [Google Scholar]
- 63.Arimoto T, Gréaux S, Irifune T, Zhou C, Higo Y. Sound velocities of Fe3Al2Si3O12 almandine up to 19 GPa and 1700 K. Phys. Earth Planet. Int. 2015;246:1–8. doi: 10.1016/j.pepi.2015.06.004. [DOI] [Google Scholar]
- 64.Pacalo REG, Weidner DJ, Gasparik T. Elastic properties of sodium rich majorite garnet. Geophys. Res. Lett. 1992;19(18):1895–1898. [Google Scholar]
- 65.Nishi M, Kubo T, Kato T. Metastable transformation of eclogite to garnetite in subducting oceanic crust. J. Mineral. Petrol. Sci. 2009;104:192–198. [Google Scholar]
- 66.Thompson AB. Water in the Earth's upper mantle. Nature. 1992;358(6384):295–302. [Google Scholar]
- 67.Aoki I, Takahashi E. Density of MORB eclogite in the upper mantle. Phys. Earth Planet. Inter. 2004;143–144:129–143. [Google Scholar]
- 68.Irifune T, Ringwood AE. Phase transformations in a harzburgite composition to 26 GPa: Implications for dynamical behaviour of the subducting slab. Earth Planet. Sci. Lett. 1987;86(2–4):365–376. [Google Scholar]
- 69.Irifune T, Ringwood AE. Phase transformations in subducted oceanic crust and buoyancy relationships at depths of 600–800 km in the mantle. Earth Planet. Sci. Lett. 1993;117(1–2):101–110. [Google Scholar]
- 70.Ballmer MD, Schmerr NC, Nakagawa T, Ritsema J. Compositional mantle layering revealed by slab stagnation at ~1000-km depth. Sci. Adv. 2015;1(11):e1500815. doi: 10.1126/sciadv.1500815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirose K, Fei Y, Ma Y, Mao H-K. The fate of subducted basaltic crust in the Earth’s lower mantle. Nature. 1999;397:53–56. [Google Scholar]
- 72.Hutchison MT, Hursthouse MB, Light ME. Mineral inclusions in diamonds: Associations and chemical distinctions around the 670-km discontinuity. Contrib. Mineral. Petrol. 2001;142:119–126. [Google Scholar]
- 73.Wang W, Sueno S. Discovery of a NaPx-En inclusion in diamond: Possible transition zone origin. Mineral. J. 1996;18(1):9–16. [Google Scholar]
- 74.Wang W, Gasparik T. Evidence for a deep-mantle origin of a NaPx-En inclusion in diamond. Int. Geol. Rev. 2000;42(11):1000–1006. doi: 10.1080/00206810009465122. [DOI] [Google Scholar]
- 75.Gasparik T, Hutchison MT. Experimental evidence for the origin of two kinds of inclusions in diamonds from the deep mantle. Earth Planet. Sci. Lett. 2000;181:103–114. [Google Scholar]
- 76.Coleman LC. Ringwoodite and majorite in the Catherwood meteorite. Can. Mineral. 1977;15:97–101. [Google Scholar]
- 77.Smith JV, Mason B. Pyroxene-garnet transformation in Coorara meteorite. Science. 1970;168:832–833. doi: 10.1126/science.168.3933.832. [DOI] [PubMed] [Google Scholar]
- 78.Price GD, Putnis A, Agrell SO. Electron petrography of shock-produced veins in the Tenham chondrite. Contrib. Mineral. Petrol. 1979;71:211–218. [Google Scholar]
- 79.Langenhorst F, Joreau P, Doukhan JC. Thermal and shock metamorphism of the Tenham meteorite: A TEM examination. Geochim. Cosmochim. Acta. 1995;59:1835–1845. [Google Scholar]
- 80.Chen M, Sharp TG, El Goresy A, Wopenka B, Xie X. The majorite-pyrope1magnesiowüstite assemblage: Constraints on the history of shock veins in chondrites. Science. 1996;271:1570–1573. [Google Scholar]
- 81.Ghosh S, Tiwari K, Miyahara M, Rohrbach A, Vollmer C, Stagno V, Ohtani E, Ray D. Natural Fe-bearing aluminous bridgmanite in the katol meteorite. Proc. Natl. Acad. Sci. USA. 2021;118:40. doi: 10.1073/pnas.2108736118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heinemann S, Sharp T, Seifert F, Rubie D. The cubic-tetragonal phase transition in the system majorite (Mg4Si4O12)–pyrope (Mg3Al2Si3O12), and garnet symmetry in the Earth's transition zone. Phys. Chem. Miner. 1997;24:206–221. doi: 10.1007/s002690050034. [DOI] [Google Scholar]
- 83.Hatch DM, Ghose S. Symmetry analysis of the phase transition and twinning in MgSiO3 garnet: Implications to mantle mineralogy. Am. Mineral. 1989;74:1221–1224. [Google Scholar]
- 84.Angel RJ, Finger LW, Hazen RM, Kanzaki M, Weidner DJ, Liebermann RC, Veblen DR. Structure and twinning of single crystal MgSiO3 garnet synthesized at 17 GPa and 1800 °C. Am. Mineral. 1989;74:509–512. [Google Scholar]
- 85.Stosch HG, Lugmair GW. Geochemistry and evolution of MORB-type eclogites from the Münchberg Massif, southern Germany. Earth Planet. Sci. Lett. 1990;99(3):230–249. [Google Scholar]
- 86.Keppler H, Frost DJ. Introduction to minerals under extreme conditions. In: Miletich R, editor. EMU Notes in Mineralogy, 7. Eötvös University Press; 2005. pp. 1–30. [Google Scholar]
- 87.Long GJ, Cranshaw TE, Longworth G. The ideal Mössbauer effect absorber thickness. Mössbauer Eff. Ref. Data J. 1983;6(2):42–49. [Google Scholar]
- 88.Prescher C, McCammon C, Dubrovinsky L. MossA: A program for analyzing energy-domain Mössbauer spectra from conventional and synchrotron sources. J. Appl. Crystallogr. 2012;45(2):329–331. doi: 10.1107/S0021889812004979. [DOI] [Google Scholar]
- 89.Piccirilli F, Tardani F, D’Arco A, Birarda G, Vaccari L, Sennato S, Casciardi S, Lupi S. Infrared nanospectroscopy reveals DNA structural modifications upon immobilization onto clay nanotubes. Nanomaterials. 2021;11:1103. doi: 10.3390/nano11051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li B, Neuville DR. Elasticity of diopside to 8 GPa and 1073 K and implications for the upper mantle. Phys. Earth Planet. Inter. 2010;183(3–4):398–403. doi: 10.1016/j.pepi.2010.08.009. [DOI] [Google Scholar]
- 91.Kono Y, Greaux S, Higo Y, Ohfuji H, Irifune T. Pressure and temperature dependences of elastic properties of grossular garnet up to 17 GPa and 1 650 K. J. Earth Sci. 2010;21(5):782–791. [Google Scholar]
- 92.Gréaux S, et al. Sound velocities of aluminum-bearing stishovite in the mantle transition zone. Geophys. Res. Lett. 2016;43:4239–4246. doi: 10.1002/2016GL068377. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.