Abstract
Thyroid cancer (TC) is caused by genetic factors and or their cross talk with lifestyle and environment. An important role of miRNA involvement has been identified in different human diseases alongside the cancer. The growing cloud of miRNA discoveries narrates miRNA-221 and miRNA-222 as key elements of ready arsenal in the cancer micro-niches. The aim of present study was to identify the variations of miRNA-221 and miRNA-222 expression in TC tissues and their likely association with TC. miRNA-221 and miRNA-222 were investigated for their expressional alterations in TC tissue samples and healthy thyroid tissue. Expression of miRNA-221 and -222 was analyzed through real time PCR. The relative gene expression of both the miRNA was quantified and statistically evaluated. miRNA-221 and miRNA-222 were found to be highly over expressed when compared with samples of multinodular goiter (MNG) and normal controls. Interestingly, it was also noted that miRNA-221 and miRNA-222 expression is working in a cluster in thyroid cancer patients. So, it can be concluded that the expressional alterations of miRNA-221 and -222 are playing their potential role in the development of thyroid cancer.
Subject terms: Biochemistry, Cancer, Cell biology, Genetics, Molecular biology, Biomarkers, Diseases, Health care, Medical research, Molecular medicine, Oncology, Risk factors
Introduction
The endocrine related malignancy of thyroid which is recently found to be increasing rapidly from last few decades is considered to be thyroid cancer as its prevalence in many populations is increased. Differentiated thyroid carcinoma which constitute about 90% of thyroid cancers includes papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC)1. Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer (85–90%)2. microRNAs (miRNAs) found endogenously and are single-stranded, noncoding 18- to 25-nucleotide RNAs which are playing their role in various biological and pathological processes of cell proliferation, differentiation and apoptosis3. miRNAs are contributing in the development of cancer in two ways. First, there may be the up regulation of some miRNAs which resulted in the silencing of tumor suppressor genes. Secondly there may be the down regulation of miRNAs which could resulted in more expression of oncogenes. So, the deregulations of miRNAs may lead to the over expression of some oncogenes or decreased expression of tumor suppressor genes leading to cancer4. Recent studies have also observed several miRNAs that are transcriptionally deregulated in papillary thyroid cancer (PTC) tissues in comparison with benign thyroid nodules and normal thyroid tissues5. miRNA-221 and miRNA-222 are important microRNAs that are abnormally expressed in thyroid cancer tissues compared with healthy controls6. Blocking miRNA-221 function by antisense methodology led to reduced cell growth of a human PTC cell line, while its overexpression led to increased colony formation, indicating a critical role of miRNA-221 in thyroid carcinogenesis7. Studies demonstrated that miRNA-221 and miRNA-222 are transcriptionally up-regulated in PTC tumors in comparison with normal thyroid tissue8. A functional study showed that miRNA-221 and miRNA-222 are endogenous regulators of P27Kip1 protein expression, which represents a very important regulator of the cell cycle9. With the up-regulation of these miRNAs, there is a dramatic loss of KIT transcript and Kit protein, both of which are involved in the pathogenesis of thyroid cancer.
In Hashimoto’s Thyroiditis small extracellular vesicle (sEV) mediated miR-142-3p is a communication mode between thyrocyte cells and T-lymphocytes favoring the progression of the disease10.
It is confirmed that the down-regulation of HOXB5 by endogenous or exogenous miRNA-221 involves the progression of PTC11. It is investigated that expression of miRNA-221 and miRNA-222 had 5- to a 35-fold differential in FNAB samples of PTCs compared with other thyroid nodules7. It was also observed that the overexpression of miRNA-221, and miRNA-222 were significantly associated with extrathyroidal invasion in PTCs12. In the current study, aberrant expression of these miRNAs in thyroid cancer tissue samples playing their potential role in the development and progression of PTC was investigated.
Overexpressed miRNA-221 and miRNA-222 in PTC are closely correlated with clinical and pathological characteristics13. Overexpression of miRNA-221 in PTC samples was considered a potential biomarker for PTC recurrence and excessively secreted miRNA-221 levels have been observed in PTC, follicular and anaplastic thyroid cancers9. Previous studies also indicated that overexpression of miRNA-221 played an important role in the proliferation of thyroid cancer cells14. All these findings strongly suggest a close correlation between miRNA-221and miRNA-222 and the development of PTC. By keeping this in view the present study was conducted to check the expression of miRNA-221 and miRNA-222 in thyroid cancer patients and multinodular goiter subjects along with their adjacent normal tissue samples. This study aimed to check whether these miRNAs are overexpressed in the studied population and have a risk associated with the development of thyroid cancer in the Pakistani population like other populations.
Material and methods
Sampling and data collection
Fresh tissue samples were taken in falcon tubes having RNA later and immediately stored at 4 °C. Trizol method of RNA extraction was used to extract the RNA from tissue samples. RNA quantification was done by Nano drop to check the concentration of RNA. After extraction of RNA, cDNA was synthesized by using a commercially available kit according to manufacturer protocol. Synthesized cDNA was used and RT-PCR was carried out for the expressional analysis of miRNA-221 and miRNA-222.
Thyroid stimulating hormone (TSH), Thyroglobulin (TG), Antithyroglobulin (ATG) antibodies, and tumor grades were recorded on a questionnaire specially designed for the study. The study protocol was approved by the ethical review committee of PMAS Arid Agriculture University Rawalpindi and from the hospitals of Rawalpindi and Islamabad Pakistan.
Expressional analysis
The subjects were studied for expressional analysis of miRNA-221 and miRNA-222. Briefly, cDNA was prepared and real time PCR was performed for the cDNA samples using specific primers for specified miRNAs. The primers, F-5′-GCATGAACCTGGCATACA-3′-F and reverse R-5′-AGCAGACAATGTAGCTGTTG-3′-R were selected for miRNA-221. Similarly, the primers F-5′-AAGGTGTAGGTACCCTCAAT-3′-F and reverse R-5′-CCAGATGTAGCTGCTGATTAC-3′-R were selected for miRNA-222 for their expressional analysis. RT-PCR was carried out in a total volume of 25 µl in each PCR tube. It includes 0.5 µl of 100 pmol/µl of each of forward and reverse primers, 13 µl of SYBR Green PCR Master Mix, 2 µl cDNA template RT+/RT−/water/patient sample/control and 9 µl of PCR water.
Statistical analysis
The results were statistically calculated and analyzed using SPSS (version 20) and GraphPad Prism version 9.5 for Mac, GraphPad Software, http://www.graphpad.com. The statistical tests; T-test, Chi-square test for p-value calculation for the comparison of gene expression among thyroid cancer patients, MNG and controls were conducted.
Use of human participants
The study protocol for the induction of human participants in the study was approved by the ethical review committee of PMAS Arid Agriculture University Rawalpindi Pakistan on October 03, 2018 and confirmed that all research will be performed in accordance with relevant guidelines/regulations, and it is confirmed that informed consent was obtained from all participants and/or their legal guardians. In addition, the research involving human participants will be performed in accordance with the Declaration of Helsinki.
Results
Demographic and clinical data
The subjects included 30 thyroid cancer patients along with 30 adjacent normal tissue samples of the same patient and 30 multinodular goiter patients along with their normal thyroid tissue. Patients were categorized into three different age groups i.e. later age (more than 60), middle-age (40–60), and early age (below 40). 34% patients in early age, 51.1% in middle and 14.9% patients in the later age group were present. It was seen that all the patients were having papillary thyroid cancer with its different subtypes. Age, gender and metastatic status of the patients was also recorded (Table 1).
Table 1.
Clinical hallmarks of thyroid cancer patients.
| Variables | Patients | Frequency | Comparative data |
|---|---|---|---|
| Age | < 50 (years) | 34.78 | ± 45.6(years) [27] |
| 50–60 (years) | 51.97 | ||
| > 60 (years) | 15.59 | ||
| Gender | Females | 86.4 | |
| Males | 14.6 | ||
| Metastasis | Metastatic | 6.41 | 0.7% [28] |
| Non-metastatic | 93.53 |
TSH, TG, ATG, and tumor grades were also recorded for the thyroid cancer patient group as well as for MNG patient group (Figs. 1 and 2).
Figure 1.
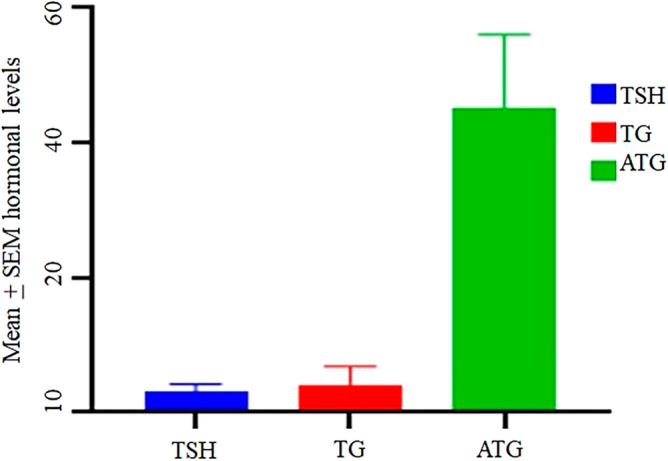
Hormonal levels TSH, TG and ATG in thyroid cancer patients. The mean values of the levels were calculated with standard error of the mean (± SEM). SEM error bars are shown on the top of each bar on the chart.
Figure 2.
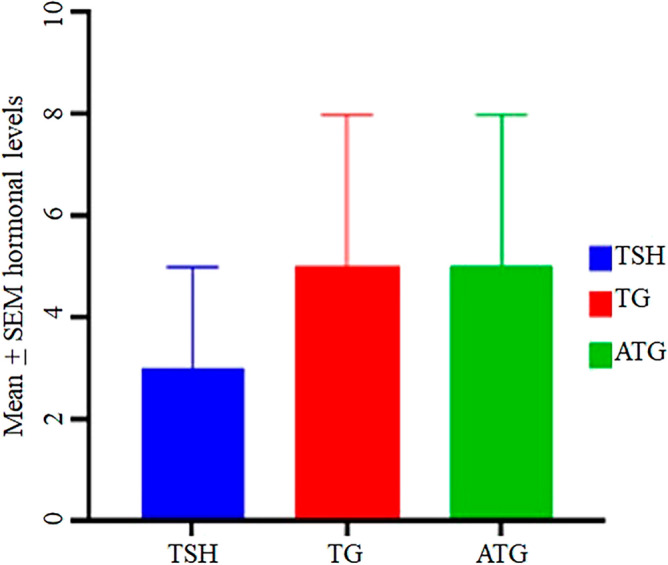
Hormonal levels TSH, TG and ATG in MNG patients. The mean values of the levels were calculated with standard error of the mean (± SEM). SEM error bars are shown on the top of each bar on the chart.
Analysis of expression of miRNA-221 and miRNA-222
Expression of miRNA-221 and thyroid cancer
In the present study 30 thyroid cancer tissue samples were checked for their expression (Fig. 3) and then the expression was compared with 30 MNG tissue samples along with their adjacent normal control samples in the Pakistani population. The expression of miRNA-221 was found to be significantly higher (p value 0.00012) in thyroid cancer samples when its expression was compared through One way ANOVA with the patients of MNG and normal controls (Fig. 4). The ANOVA was followed by Post Hoc Tukey’s test showing multiple comparison among controls, MNGs and thyroid cancer patients (Table 2).
Figure 3.
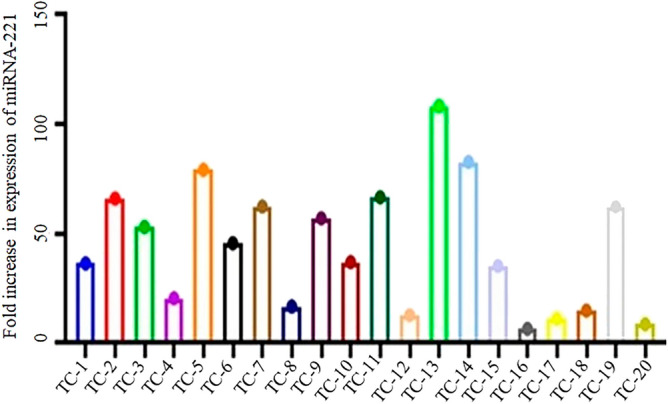
Fold increase in expression of miRNA-221 in thyroid cancer patients (TC-1 to TC-20). The fold increase in the expression was determined by relative gene expression through real time PCR.
Figure 4.
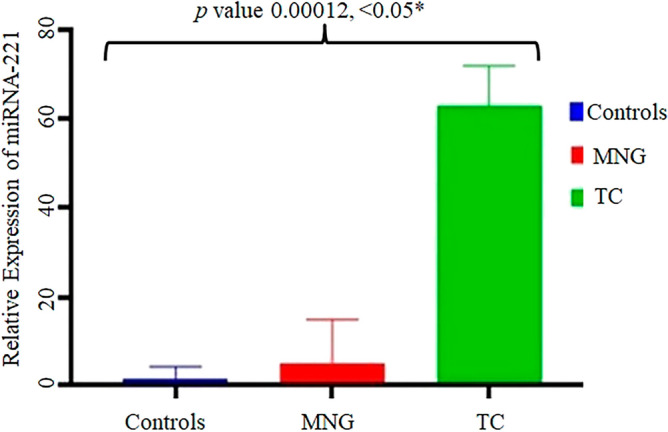
Relative expression of miRNA-221 in thyroid cancer patients, MNG and normal controls. ANOVA test was applied to thyroid cancer, MNG and controls. *p value determined by comparison of the relative gene expression observed in the three groups i.e. Controls, MNG and TC in ANOVA test.
Table 2.
miRNA-221 expression analysis by (a) one way ANOVA followed by (b) Post hoc test multiple comparisons for Tukey’s HSD.
| (a) | ||||||
|---|---|---|---|---|---|---|
| Groups | Sum of squares | df | Mean square | F | Significance | |
| Between groups | 22,821.606 | 2 | 11,410.803 | 40.984 | 0.00012* | |
| Within groups | 15,870.012 | 57 | 278.421 | |||
| Total | 38,691.618 | 59 | ||||
| (b) | ||||||
|---|---|---|---|---|---|---|
| Group A | Group B | Mean difference (A-B) | Std. error | Significance | 95% confidence interval | |
| Lower bound | Upper bound | |||||
| Control | MNG | − 3.64700 | 5.27656 | 0.770 | − 16.3446 | 9.0506 |
| Thyroid Cancer Patients | − 43.07450** | 5.27656 | 0.000 | − 55.7721 | − 30.3769 | |
| MNG | Control | 3.64700 | 5.27656 | 0.770 | − 9.0506 | 16.3446 |
| Thyroid Cancer Patients | − 39.42750** | 5.27656 | 0.000 | − 52.1251 | − 26.7299 | |
| Thyroid Cancer Patients | Control | 43.07450** | 5.27656 | 0.000 | 30.3769 | 55.7721 |
| MNG | 39.42750** | 5.27656 | 0.000 | 26.7299 | 52.1251 | |
*Significance at the 0.05 level.
**The mean difference is significant at the 0.05 level.
Expression of miRNA-222 and thyroid cancer
It was observed in this study that the expression of miRNA-222 was significantly high (p value 0.00001) as revealed by One way ANOVA in thyroid cancer samples while the expression was quite low in MNG and normal control samples (Figs. 5 and 6). The ANOVA test for the multiple comparisons among controls, MNGs and thyroid cancer patients was followed by Post Hoc Tukey’s test (Table 3).
Figure 5.
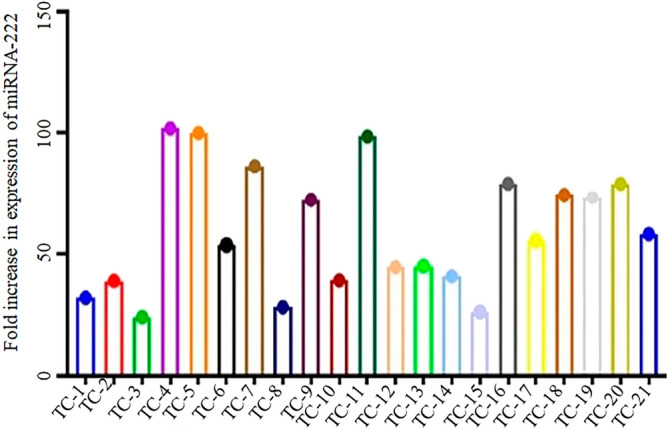
Fold increase in expression of miRNA-222 in thyroid cancer patients (TC-1 to TC-21). The fold increase in the expression was determined by relative gene expression through real time PCR.
Figure 6.
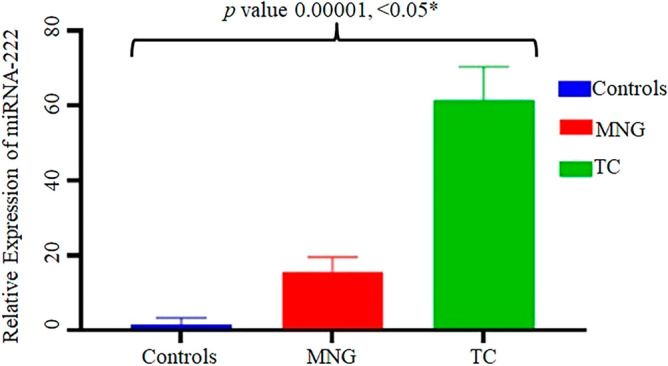
Relative expression of miRNA-222 in thyroid patients compared to MNG and normal controls. ANOVA test was applied to thyroid cancer, MNG and controls. *p value determined by comparison of the relative gene expression observed in the three groups i.e. Controls, MNG and TC in ANOVA test.
Table 3.
miRNA-222 expression analysis by (a) one way ANOVA followed by (b) post hoc test multiple comparisons for Tukey’s HSD.
| (a) | ||||||
|---|---|---|---|---|---|---|
| Groups | Sum of squares | df | Mean square | F | Significance | |
| Between groups | 40,662.521 | 2 | 20,331.260 | 89.587 | 0.00001* | |
| Within groups | 12,935.862 | 57 | 226.945 | |||
| Total | 53,598.382 | 59 | ||||
| (b) | ||||||
|---|---|---|---|---|---|---|
| Group A | Group B | Mean difference (A-B) | Std. error | Significance | 95% confidence interval | |
| Lower bound | Upper bound | |||||
| Control | MNG | − 7.49700 | 4.76387 | 0.265 | − 18.9609 | 3.9669 |
| Thyroid cancer patients | − 58.5895** | 4.76387 | 0.000 | − 70.0534 | − 47.1256 | |
| MNG | Control | 7.49700 | 4.76387 | 0.265 | − 3.9669 | 18.9609 |
| Thyroid cancer patients | − 51.09250** | 4.76387 | 0.000 | − 62.5564 | − 39.6286 | |
| Thyroid cancer patients | Control | 58.58950** | 4.76387 | 0.000 | 47.1256 | 70.0534 |
| MNG | 51.09250** | 4.76387 | 0.000 | 39.6286 | 62.5564 | |
*Significance at the 0.05 level.
**The mean difference is significant at the 0.05 level.
miRNA-221 and miRNA-222 working in a cluster in thyroid cancer
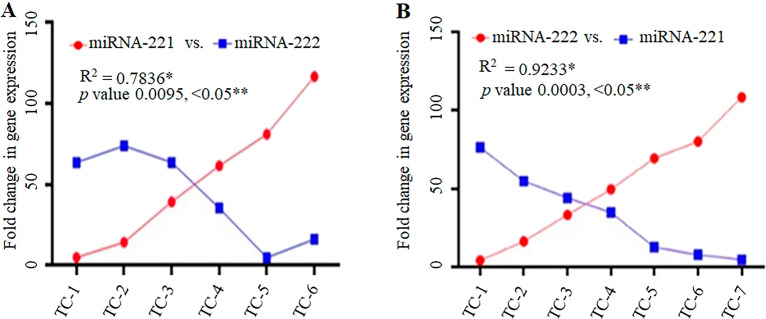
In the present study, it was demonstrated that both miRNA-221 and miRNA-222 showed their expression in a cluster, and also, they are compensating each other’s expression in cancer progression. It was seen that in the same patient if the expression of miRNA-221 was low the expression of miRNA-222 was very high to continue the cell proliferation of cancerous cells (Fig. 7A). In the same way when the expression of miRNA-222 was low in some patients the expression of miRNA-221 was very high to compensate for the miRNA-222 to continue the cell growth and proliferation to increase the progression of cancerous cells (Fig. 7B).
Figure 7.
Clustered fold change in the expression of miRNA-221 and miRNA-222 in thyroid cancer patients (A) TC-1 to TC-6, (B) TC-1 to TC-7. The clustered or combined fold change in the gene expression was calculated by correlation of the data test. *R-squared (R2) and **p values are represented from the correlation of the data test.
Discussion
miRNAs are potential biomarkers for the detection of cancer, and numerous studies have been performed to determine the relationship between miRNAs and thyroid cancer. The relative gene expression of miRNA-221 and miRNA-222 was investigated in TC, MNG and normal controls. The target genes of miR-222 and miR-221 are p27 and p57 and they may interfere essentially to onset thyroid oncogenesis9. Additionally, miRNA-221 and miRNA-222 were found at the highest levels in BRAF- and RAS-positive PTCs15. Moreover, the up regulation of miRNA-221 and miRNA-222 may not only be involved in the development of PTC but also in invasion and metastasis of PTC11.
The results of present study showed significantly high expression of the miRNAs in thyroid cancer patients than MNG and normal samples. Strong evidence of up-regulated expression of miRNA genes i.e. miRNA-221 and 222 in PTC and an up to 70-fold boosted expression of miRNA-221in PTC compared to negligible expression in the healthy thyroid tissues has been reported6,16. Additionally, miRNA-222 and miR-146b have been shown tenfolds up-regulated in classic variants of PTC in contrast to normal thyroid tissue17.
Additionally miRNA-221 over expression has been identified in clear cell renal cell carcinoma18. The up regulation of miRNA-221 and miRNA-222 has been reported in breast neoplasm, glioblastomas, and lung and hepatocellular carcinoma19 and significant over expression associated with extra-thyroidal invasion in PTCS11. Moreover miRNA-221 was the most reported as the most favorable miRNA in differentiating benign from malignant thyroid pathology20. These gene have been shown to modulate cell proliferation, telomere activity, apoptosis, tumor suppressors, angiogenesis, and autophagy21.
The present study remarkably showed clustered working of the miRNA-221 and miRNA-222 in TC. This can be explained as if expression of one of the miRNAs was lower in a any of the patient, the expression of the other miRNA was found up-regulated in the same patient. This clustered mechanism in miRNAs expression indicates their certainly crucial role in the TC. The amalgam of scientific literature comprehensively suggests combined involvement of miRNA221- and miRNA-222 together. But surprisingly findings of present study represented an unidentified expressional regulation of these miRNAs in thyroid cancer in alternate manner i.e. one of the miRNA if down-regulated the other one’s expression is boosted. This leads to the concept that there is a dire augmented back-to-back support and or involvement of this miRNA221 and miRNA-222 combo.
Conclusion
The current study revealed that expression of both miRNA-221 and miRNA-222 is working in an alternate clustered fashion in TC tumor. Since this effect is first of its kind at the expression level in the tumor microenvironment the study regards and proposes it as “Khan’s microRNAs clustering in thyroid cancer tumor”. This specific miRNA signature composed of miR-221, and miR-222 could serve as potential clinical biomarker as therapeutic target in TC patients, particularly in PTC. These findings showing up regulation of miRNA-221 and miRNA-222 might be helpful and facilitate the surgical management of thyroid cancer patients and can be integrated into the prognostication system of thyroid cancer in near future.
Acknowledgements
This study was supported by the Higher Education Commission of Pakistan (Grant number: 10168/Punjab/NRPU/R&D/HEC). The authors would like to acknowledge patients and normal individuals who contributed to this research work. We also acknowledge Nuclear Medicine Oncology and Radiotherapy Institute Islamabad Pakistan, Department of Zoology, PMAS-Arid Agriculture University, Rawalpindi, Pakistan, and Institute of Biomedical and Genetic Engineering (IBGE), Islamabad, Pakistan for experimental help.
Author contributions
R.K.: responsibility of conducting experiments, management of patients, organizing and reporting data, responsibility for presentation and logical explanation of results. A.R.: lab space and experimental work. S.A., T.S.: sampling and data compilation. Q.M.: provided equipment, space and personnel vital to the project and critical reviewer of the manuscript. R.M.B.: the idea for research or article/hypothesis generation, supervision and responsibility for the organization and financial support.
Data availability
The data related to this study is included in the manuscript. The first author RK can be contacted at the email address rashidakhan3919@gmail.com for the availability of any data related to this study.
Competing interests
All of the authors have declared that they participated in the design, execution, and analysis of the study and that they have approved the final version. Additionally, there are no conflicts of interest in connection with this paper.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ruqia Mehmood Baig, Email: dr.ruqia@uaar.edu.pk.
Qaisar Mansoor, Email: qmibge@gmail.com.
References
- 1.Heidari Z, Mohammadpour-Gharehbagh A, Eskandari M, Harati-Sadegh M, Salimi S. Genetic polymorphisms of miRNA let7a-2 and pri-mir-34b/c are associated with an increased risk of papillary thyroid carcinoma and clinical/pathological features. J. Cell. Biochem. 2019;120(5):8640–8647. doi: 10.1002/jcb.28152. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol. Prev. Biomarkers. 2016;25(1):16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Abdel-Mageed AB, Mondal D, Kandil E. MicroRNA expression profiles in differentiated thyroid cancer, a review. Int. J. Clin. Exp. Med. 2013;6(1):74. [PMC free article] [PubMed] [Google Scholar]
- 4.Suresh R, Sethi S, Ali S, Giorgadze T, Sarkar FH. Differential expression of MicroRNAs in papillary thyroid carcinoma and their role in racial disparity. J. Cancer Sci. Ther. 2015;7(5):145. doi: 10.4172/1948-5956.1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikiforova MN, Tseng GC, Steward D, Diorio D, Nikiforov YE. MicroRNA expression profiling of thyroid tumors: Biological significance and diagnostic utility. J. Clin. Endocrinol. Metab. 2008;93(5):1600–1608. doi: 10.1210/jc.2007-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YS, Lim YS, Lee J-C, Wang S-G, Park H-Y, Kim SY, et al. Differential expression levels of plasma-derived miR-146b and miR-155 in papillary thyroid cancer. Oral Oncol. 2015;51(1):77–83. doi: 10.1016/j.oraloncology.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Pallante P, Battista S, Pierantoni GM, Fusco A. Deregulation of microRNA expression in thyroid neoplasias. Nat. Rev. Endocrinol. 2014;10(2):88–101. doi: 10.1038/nrendo.2013.223. [DOI] [PubMed] [Google Scholar]
- 8.He L, Hannon G. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004;5(7):522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 9.Visone R, Russo L, Pallante P, De Martino I, Ferraro A, Leone V, et al. MicroRNAs (miR)-221 and miR-222, both overexpressed in human thyroid papillary carcinomas, regulate p27Kip1 protein levels and cell cycle. Endocr. Relat. Cancer. 2007;14(3):791–798. doi: 10.1677/ERC-07-0129. [DOI] [PubMed] [Google Scholar]
- 10.Li G, He L, Huang J, Liu J, Chen W, Zhong J, Wei T, Li Z, Zhu J, Lei J. miR-142-3p encapsulated in T lymphocyte-derived tissue small extracellular vesicles induces Treg function defect and thyrocyte destruction in Hashimoto's thyroiditis. BMC Med. 2023;21(1):206. doi: 10.1186/s12916-023-02914-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HJ, Kim YH, Lee DS, Chung J-K, Kim S. In vivo imaging of functional targeting of miR-221 in papillary thyroid carcinoma. J. Nucl. Med. 2008;49(10):1686–1693. doi: 10.2967/jnumed.108.052894. [DOI] [PubMed] [Google Scholar]
- 12.Chou C-K, Chen R-F, Chou F-F, Chang H-W, Chen Y-J, Lee Y-F, et al. miR-146b is highly expressed in adult papillary thyroid carcinomas with high risk features including extrathyroidal invasion and the BRAFV600E mutation. Thyroid. 2010;20(5):489–494. doi: 10.1089/thy.2009.0027. [DOI] [PubMed] [Google Scholar]
- 13.Chou C-K, Yang KD, Chou F-F, Huang C-C, Lan Y-W, Lee Y-F, et al. Prognostic implications of miR-146b expression and its functional role in papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2013;98(2):E196–E205. doi: 10.1210/jc.2012-2666. [DOI] [PubMed] [Google Scholar]
- 14.Pallante P, Visone R, Ferracin M, Ferraro A, Berlingieri M, Troncone G, et al. MicroRNA deregulation in human thyroid papillary carcinomas. Endocr. Relat. Cancer. 2006;13(2):497–508. doi: 10.1677/erc.1.01209. [DOI] [PubMed] [Google Scholar]
- 15.Fornari F, Gramantieri L, Ferracin M, Veronese A, Sabbioni S, Calin G, et al. MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene. 2008;27(43):5651–5661. doi: 10.1038/onc.2008.178. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira AL, Dias F, Gomes M, Fernandes M, Medeiros R. Circulating biomarkers in renal cell carcinoma: The link between microRNAs and extracellular vesicles, where are we now? J. Kidney Cancer VHL. 2014;1(8):84. doi: 10.15586/jkcvhl.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keutgen XM, Filicori F, Crowley MJ, Wang Y, Scognamiglio T, Hoda R, et al. A panel of four miRNAs accurately differentiates malignant from benign indeterminate thyroid lesions on fine needle aspiration. Clin. Cancer Res. 2012;18(7):2032–2038. doi: 10.1158/1078-0432.CCR-11-2487. [DOI] [PubMed] [Google Scholar]
- 18.Felli N, Fontana L, Pelosi E, Botta R, Bonci D, Facchiano F, et al. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc. Natl. Acad. Sci. 2005;102(50):18081–18086. doi: 10.1073/pnas.0506216102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pallante P, Visone R, Croce CM, Fusco A. Deregulation of microRNA expression in follicular cell-derived human thyroid carcinomas. Endocr. Relat. Cancer. 2010;17(1):F91–F104. doi: 10.1677/ERC-09-0217. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Yan G-Y. Novel human lncRNA–disease association inference based on lncRNA expression profiles. Bioinformatics. 2013;29(20):2617–2624. doi: 10.1093/bioinformatics/btt426. [DOI] [PubMed] [Google Scholar]
- 21.Yoon SO, Chun S-M, Han EH, Choi J, Jang SJ, Koh SA, et al. Deregulated expression of microRNA-221 with the potential for prognostic biomarkers in surgically resected hepatocellular carcinoma. Hum. Pathol. 2011;42(10):1391–1400. doi: 10.1016/j.humpath.2010.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data related to this study is included in the manuscript. The first author RK can be contacted at the email address rashidakhan3919@gmail.com for the availability of any data related to this study.