Abstract
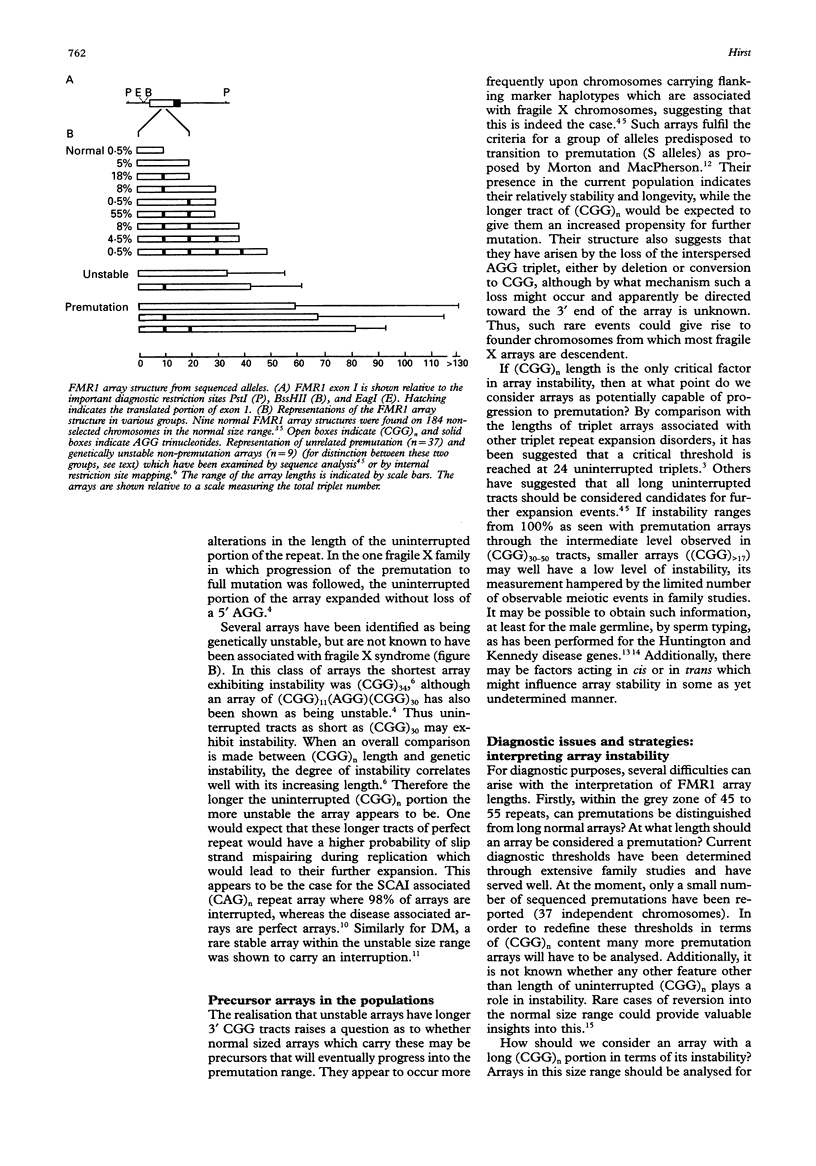
Our understanding of FMRI trinucleotide instability has increased dramatically with knowledge of its detailed structures. While most arrays seem to be protected by interspersions, for a few the price of perfection is instability. Although there remain many unanswered questions, diagnosis in the “grey zone” can be greatly improved by studying array content. For the future, as we strive to delineate normal from premutation, we should increasingly be able to estimate rates of instability for future generations and predict the risk of conversion to the full mutation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arinami T., Asano M., Kobayashi K., Yanagi H., Hamaguchi H. Data on the CGG repeat at the fragile X site in the non-retarded Japanese population and family suggest the presence of a subgroup of normal alleles predisposing to mutate. Hum Genet. 1993 Nov;92(5):431–436. doi: 10.1007/BF00216445. [DOI] [PubMed] [Google Scholar]
- Chung M. Y., Ranum L. P., Duvick L. A., Servadio A., Zoghbi H. Y., Orr H. T. Evidence for a mechanism predisposing to intergenerational CAG repeat instability in spinocerebellar ataxia type I. Nat Genet. 1993 Nov;5(3):254–258. doi: 10.1038/ng1193-254. [DOI] [PubMed] [Google Scholar]
- Eichler E. E., Holden J. J., Popovich B. W., Reiss A. L., Snow K., Thibodeau S. N., Richards C. S., Ward P. A., Nelson D. L. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat Genet. 1994 Sep;8(1):88–94. doi: 10.1038/ng0994-88. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Kuhl D. P., Pizzuti A., Pieretti M., Sutcliffe J. S., Richards S., Verkerk A. J., Holden J. J., Fenwick R. G., Jr, Warren S. T. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991 Dec 20;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Hirst M. C., Grewal P. K., Davies K. E. Precursor arrays for triplet repeat expansion at the fragile X locus. Hum Mol Genet. 1994 Sep;3(9):1553–1560. doi: 10.1093/hmg/3.9.1553. [DOI] [PubMed] [Google Scholar]
- Kunst C. B., Warren S. T. Cryptic and polar variation of the fragile X repeat could result in predisposing normal alleles. Cell. 1994 Jun 17;77(6):853–861. doi: 10.1016/0092-8674(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Leeflang E. P., Arnheim N. A novel repeat structure at the myotonic dystrophy locus in a 37 repeat allele with unexpectedly high stability. Hum Mol Genet. 1995 Jan;4(1):135–136. doi: 10.1093/hmg/4.1.135. [DOI] [PubMed] [Google Scholar]
- Macpherson J. N., Curtis G., Crolla J. A., Dennis N., Migeon B., Grewal P. K., Hirst M. C., Davies K. E., Jacobs P. A. Unusual (CGG)n expansion and recombination in a family with fragile X and DiGeorge syndrome. J Med Genet. 1995 Mar;32(3):236–239. doi: 10.1136/jmg.32.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton N. E., Macpherson J. N. Population genetics of the fragile-X syndrome: multiallelic model for the FMR1 locus. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4215–4217. doi: 10.1073/pnas.89.9.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss A. L., Kazazian H. H., Jr, Krebs C. M., McAughan A., Boehm C. D., Abrams M. T., Nelson D. L. Frequency and stability of the fragile X premutation. Hum Mol Genet. 1994 Mar;3(3):393–398. doi: 10.1093/hmg/3.3.393. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Kondo I., Holman K., Yamauchi M., Seki N., Kishi K., Staples A., Sutherland G. R., Hori T. Haplotype analysis at the FRAXA locus in the Japanese population. Am J Med Genet. 1994 Jul 15;51(4):412–416. doi: 10.1002/ajmg.1320510422. [DOI] [PubMed] [Google Scholar]
- Snow K., Tester D. J., Kruckeberg K. E., Schaid D. J., Thibodeau S. N. Sequence analysis of the fragile X trinucleotide repeat: implications for the origin of the fragile X mutation. Hum Mol Genet. 1994 Sep;3(9):1543–1551. doi: 10.1093/hmg/3.9.1543. [DOI] [PubMed] [Google Scholar]
- Telenius H., Almqvist E., Kremer B., Spence N., Squitieri F., Nichol K., Grandell U., Starr E., Benjamin C., Castaldo I. Somatic mosaicism in sperm is associated with intergenerational (CAG)n changes in Huntington disease. Hum Mol Genet. 1995 Feb;4(2):189–195. doi: 10.1093/hmg/4.2.189. [DOI] [PubMed] [Google Scholar]
- Vits L., De Boulle K., Reyniers E., Handig I., Darby J. K., Oostra B., Willems P. J. Apparent regression of the CGG repeat in FMR1 to an allele of normal size. Hum Genet. 1994 Nov;94(5):523–526. doi: 10.1007/BF00211019. [DOI] [PubMed] [Google Scholar]
- Zhang L., Fischbeck K. H., Arnheim N. CAG repeat length variation in sperm from a patient with Kennedy's disease. Hum Mol Genet. 1995 Feb;4(2):303–305. doi: 10.1093/hmg/4.2.303. [DOI] [PubMed] [Google Scholar]
- Zhong N., Liu X., Gou S., Houck G. E., Jr, Li S., Dobkin C., Brown W. T. Distribution of FMR-1 and associated microsatellite alleles in a normal Chinese population. Am J Med Genet. 1994 Jul 15;51(4):417–422. doi: 10.1002/ajmg.1320510423. [DOI] [PubMed] [Google Scholar]


