Abstract
Background
To examine the association between optical coherence tomography angiography (OCTA) retinal measurements and Parkinson’s disease (PD).
Methods
We searched MEDLINE and EMBASE from inception up to November 5th, 2021 for studies examining the differences between OCTA retinal measurements in PD patients and healthy controls. We used the Hartung–Knapp–Sidik–Jonkman random-effects method to combine study-specific standardized mean differences (SMD) in pooled effect estimates and a meta-analytic extension of the E-value metric to quantify the confounding bias capable of nullifying the pooled estimates.
Results
Nine eligible studies for our systematic review were identified through our search strategy. The pooled SMD between the retinal vessel density of PD patients and healthy participants in the whole superficial vascular plexus (SVP), foveal SVP, parafoveal SVP and foveal avascular zone (FAZ) was −0.68 (95% CI: −1.18 to −0.17, p value = 0.02, n = 7 studies), −0.14 (95% CI: −0.88 to 0.59, p value = 0.62, n = 5 studies), −0.59 (95% CI: −1.41 to 0.23, p value = 0.12, n = 5 studies) and −0.20 (95% CI: −0.79 to 0.38, p value = 0.39, n = 5 studies), respectively. An unmeasured confounder would need to be associated with a 3.01-fold, 1.54-fold, 2.81-fold and 1.70-fold increase in the risk of PD and OCTA retinal measurements, in order for the pooled SMD estimate of vessel density in whole SVP, parafoveal SVP and FAZ, respectively, to be nullified.
Conclusions
Our results provide evidence on an inverse association between whole SVP vessel density and PD.
Subject terms: Predictive markers, Diseases of the nervous system
Abstract
背景: 探讨视网膜影像检查相干光断层扫描血流成像技术 (OCTA) 与帕金森病 (PD) 之间的相关性。
方法: 我们在MEDLINE和EMBASE上搜索了从开始时的相关文献到2021年11月5日之间的、研究PD患者和健康对照组的OCTA视网膜参数差异性的文献。我们使用Hartung-Knapp-Sidik-Jonkman随机效应法, 将研究特定的标准化平均差异 (SMD) 结合到集合效应估计中, 在合并效应估计和E值指标的元分析扩展中, 以量化能够合并估计无效的混杂偏差。
结果: 通过搜索, 我们确定了9项符合系统评价的研究。PD患者和健康参与者在整个浅层血管丛 (SVP) 、中心凹 (SVP) 、旁中心凹SVP和中心凹无血管区 (FAZ) 的视网膜血管密度集合的SMD为-0.68 (95% CI: −1.18 to −0.17, p = 0.02, n = 7)、−0.14 (95% CI: −0.88–0.59, p = 0.62, n = 5)、−0.59 (95% CI: −1.41–0.23, p = 0.12, n = 5) 和 -0.20 (95% CI: −0.79 至 0.38, p = 0.39, n = 5)。一个未测量的混杂因素需要与PD和OCTA视网膜测量的风险增加3.01倍、1.54倍、2.81倍和1.70倍相关, 以便使整个SVP、旁中心凹 SVP和FAZ的血管密度集合的SMD估计值失效。
结论: 我们的结果为整个SVP血管密度和PD之间呈负相关提供了证据。
Introduction
Neurological disorders are the leading causes of disability worldwide, with Parkinson’s disease (PD), the second most common neurodegenerative disease after Alzheimer’s disease, having the fastest increase not only in disability, but also in prevalence and mortality [1]. PD is a progressive disorder which affects predominantly dopaminergic neurons and subsequently leads to a variety of symptoms ranging from motor impairment to non-motor neurological symptoms [2], including visual impairment and cognitive deficits, which may have a detrimental impact on the quality of life of patients with PD [3].
The histopathological hallmark of PD is the intracellular accumulation of misfolded alpha-synuclein mainly in the dopaminergic neurons of the substantia nigra, with subsequent dopaminergic neurodegeneration [4]. Since, the central nervous system shares anatomical and histological similarities with the retina due to their common embryological origin, neurodegenerative changes in the brain disorders like Alzheimer’s disease and multiple sclerosis, have been associated with structural alterations of retinal tissue [5, 6]. Similarly, structural and functional retinal changes have been linked to PD in observational [7], as well as postmortem studies [8], and visual symptoms sometimes precede motor symptoms in PD patients.
In recent years, apart from neurodegeneration, brain microvasculature changes have also been deemed a contributing factor to the incidence and progression of neurodegenerative disorders [9]. The advent of optical coherence tomography angiography (OCTA) has given us the opportunity to assess noninvasively whether microvascular retinal changes can serve as potential surrogate biomarkers for neurodegenerative diseases, including PD. Additionally, since noninvasive objective tests for early diagnosis of PD remain an unmet need, several studies in the last years have examined the association of OCTA metrics, such as retinal vascular density and foveal avascular zone (FAZ), with PD occurrence [10–12].
Therefore, our aim in this study was to perform a systematic review and meta-analysis of the literature on the differences of OCTA retinal measurements between PD patients and healthy controls, as well as to assess the robustness of these meta-analytic associations to unobserved confounding by bias analysis.
Materials
Eligibility criteria
To conduct the respective systematic review and meta-analysis, we adhered to the guideline of “Meta-analysis of Observational Studies in Epidemiology” (Supplementary Table 1) [13]. We attempted to discern if there was truly a connection between OCTA measurements and PD, and thus, our search algorithm was structured accordingly. The inclusion criteria that studies needed to fulfill in order to be considered eligible for inclusion in our systematic review and meta-analysis were: 1) cross-sectional, case–control, or prospective design; 2) data of OCTA measurements as mean or mean difference and standard deviation between PD patients and healthy controls was reported; 3) PD diagnosis in participants was based on established diagnostic systems (e.g. UK Brain Bank Criteria, International Parkinson and Movement Disorder Society clinical criteria (MDS-PD) diagnostic criteria); 4) PD patients were recruited in addition to controls; 5) sample size of the study was >10. Moreover, studies in which the data provided could be used to calculate the association estimates indirectly were considered eligible for inclusion in our meta-analysis. We excluded case-report studies, letters to the editor, non-English studies, non-human studies, and low-quality studies using the Newcastle-Ottawa Scale (NOS) [14].
Literature search
A literature search was performed independently by two authors (AK, IP), utilizing the databases MEDLINE and EMBASE (OvidSP) from database inception up to November 5th, 2021. The search strategy employed was tailored to the research question and the respective inclusion and exclusion criteria considered in our review. Keywords from the hierarchically organized terminology for indexing and cataloging were used and synonyms of these terms. We also used free-text words in order to retrieve “In Process” and “publisher-supplied citations” as they are not indexed with structured terminology. Finally, in order not to omit relevant articles, a manual search was performed of the reference lists of all the eligible studies (“snowball” procedure). The exact combination of search terms that were put in the search query of the OvidSP databases is provided in Supplementary Table 2. Additionally, other sources of gray literature were searched, such as Google Scholar and suggested citations.
Study selection and study quality assessment
Upon discarding duplicate articles, eligible studies were identified using a selection process involving two steps. Initially, two authors (AK, IP) independently screened the titles and abstracts of the studies yielded from the computerized literature search. Secondly, the authors fully assessed the texts of the remaining studies in order to identify relevant articles. Studies that did not meet the aforementioned eligibility criteria were not considered and any discrepancies were resolved by consensus.
The methodologic quality of the studies included was assessed by the same two investigators through a modified version of NOS for cross-sectional studies, which has been previously described [15]. They independently reviewed and graded the eligible articles obtained from the literature search to assess their quality. The main domains assessed with the modified NOS are representativeness of the sample, whether the sample size is justified and satisfactory, description of respondents and non-respondents, characteristics and response rate, ascertainment of the exposure, comparability of the subjects in different outcome groups, assessment of the outcome, and adequacy of statistical analysis. Only studies including subjects diagnosed with PD according to established diagnostic systems were considered representative of the average exposed cohort in the target population and were allotted a star in the “Selection” section of the NOS. In the “Comparability” section, age was set as the most important factor for controlling confounding and can be awarded a maximum of 2 stars. Similarly, the “Ascertainment of the exposure” and “Assessment of the outcome” sections can be awarded a maximum of 2 stars respectively, while the remaining sections can be awarded a maximum of 1 star. A study can be given a maximum score of 10. Studies with scores of less than 6 were deemed low quality and were excluded from this meta-analysis, while studies with scores of 6 or higher were considered of moderate to high quality.
Data extraction
The data of the studies deemed eligible were independently checked by two authors (AK, IP) and were entered in a customized extraction form. The information which was extracted from each selected study included: first author’s name, publication year, the country in which the study was conducted, sample size, number of male and female participants, mean age, mean disease severity score, mean PD duration, mean Unified Parkinson’s Disease Rating Scale (UPDRS) score, OCTA machine type used, the OCTA parameters that each study assessed on each outcome group and control covariates. All reported data were extracted from published articles. Furthermore, the authors were contacted for additional information.
Statistical analysis
We used means and standard deviations from each outcome group to calculate standardized mean differences (SMDs) of each OCTA measurement between different outcome groups, with corresponding 95% confidence intervals (95% CI). In case the OCTA measurements weren’t directly available, the values where indirectly calculated by combining means and standard deviations. The Hartung–Knapp/Sidik–Jonkman random-effects method was employed in order to combine study-specific SMDs in pooled effect estimates with respective 95% confidence intervals and to estimate variance between studies (τ2). The Hartung–Knapp/Sidik–Jonkman method boasts several advantages which were mentioned previously [16, 17]. This becomes evident especially in the case of high heterogeneity among studies and when the number of studies in the meta-analysis is small. Furthermore, the percentage of total variation due to heterogeneity was calculated (I2) and the Cochran Q was used to test for heterogeneity between the studies. We conducted a meta-analysis on the association between OCTA retinal measurements and PD only in the case where 5 or more studies were eligible for a particular OCTA parameter.
At the same time sensitivity analyses were performed to assess unmeasured confounding since random-effect meta-analyses can incur biased estimates when the included studies are subject to unmeasured confounding [18]. More specifically, we calculated the minimum magnitude of unmeasured confounding on the risk ratio scale required to nullify the SDM between the outcome groups. This approach is a meta-analytic extension of the E value metric [19] that estimates the confounding bias capable of bringing the effect estimate, of single studies, to a specific threshold. Due to the relatively low number of eligible studies, no tests for the assessment of publication bias nor meta-regression to identify sources of heterogeneity were conducted [20]. P values less than 0.05 were considered statistically significant, and all statistical tests were two-sided. All analyses were performed using the statistical software R (version 3.5.1, Foundation for Statistical Computing, Vienna, Austria; package) [21].
Results
Systematic review
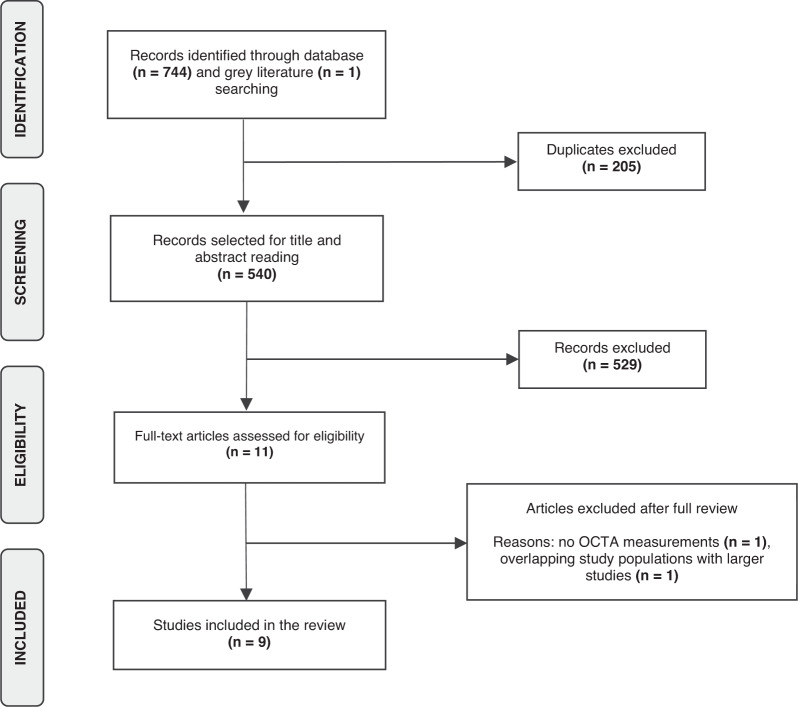
Through our literature search, we identified 745 articles in total and upon removing duplicates, 540 articles were selected for the title and abstract screening and 529 of them were excluded (Fig. 1). The remaining 11 articles were considered for full-text review. One article was excluded since it included retinal microvasculature measurements derived from fluorescein angiography [22] and another one was due to overlapping study populations with a larger study [23]. Ultimately, nine articles [10, 11, 24–30] were included in our systematic review and were eligible for meta-analysis. All eligible studies were of moderate or high quality with the NOS score ranging from 7/10 to 9/10 with a median score of 9/10 (Supplementary Table 3).
Fig. 1. Flowchart of the selection strategy of eligible studies.
A two-step screening process was adopted for the identification of eligible articles for our systematic review and meta-analysis.
The main characteristics of the eligible studies are summarized in Tables 1 and 2. Among the nine included articles, five were conducted in Asia [25, 27–30], two in Europe [10, 26], and two in the U.S.A. [11, 24]. All studies were cross-sectional and the total number of participants’ eyes was 1280 (499 eyes from PD patients and 781 eyes from healthy participants), ranging from 47 to 372 participants in individual studies. Moreover, the mean age of participants ranged from 55.92 to 71.7 years and from 54.68 to 70.9 years in PD patients and healthy participants, respectively. The mean disease severity score of PD patients was reported from seven out of nine eligible studies [10, 11, 26–30]. More specifically, the UK Brain Bank Criteria and the International Parkinson and MDS-PD clinical criteria were utilized for PD diagnosis in six [25–30] and two studies [10, 11], respectively. In order to assess the retinal microvasculature the AngioVue software of Optovue spectral domain-OCT [31], was utilized in four studies [10, 24, 25, 27], three studies [11, 29, 30] used the AngioPlex software of Carl Zeiss spectral domain-OCT, one study [28] utilized the SVision commercial SSOCT system and one study [26] used the Spectralis spectral-domain OCT system.
Table 1.
Main characteristics of eligible studiesa.
| Author, country, year | Study design | No. of participants/no. of eyes | Males/females | Mean age ± SD | Mean disease severity score ± SD | Mean PD duration | Mean UPDRS score ± SD | Adjustments | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Kwapong et al., China, 2018 | Cross- sectional |
38/49 PD 28/34 controls |
NR NR |
62.95 ± 7.97 61.18 ± 5.74 |
NR NR |
3.84 ± 2.80 NA |
NR NR |
Age and IOP | 9/10 |
| Berkowitz et al., USA, 2020 | Cross- sectional |
7/14 PD NR/135 controls |
NR NR |
NR NR |
NR NR |
NR NR |
NR NR |
Age matching | 8/10 |
| Rascunà et al., Italy, 2020 | Cross- sectional |
21/41 PD 17/33 controls |
12/9 9/8 |
61.5 ± 6.5 65.1 ± 10.7 |
HY: 1.9 ± 0.4 NA |
2.28 ± 1.19 NA |
25.0 ± 6.9 3.2 ± 2.7 |
None | 7/10 |
| Shi et al., China, 2020 | Cross- sectional |
25/50 PD 25/50 controls |
13/12 13/12 |
61.9 ± 7.6 59.0 ± 5.8 |
HY: 2.2 ± 1.0 NA |
3.7 ± 2.4 NA |
NR NR |
Age and sex matching | 9/10 |
|
Zou et al., China, 2020 |
Cross- sectional |
35/35 PD 35/35 controls |
16/19 20/15 |
61.86 ± 5.46 60.20 ± 6.75 |
HY: 1.7 ± 0.7 NA |
3.2 ± 2.0 NA |
NR NR |
Age matching | 8/10 |
| Robbins et al., USA, 2020 | Cross- sectional |
69/124 PD 137/248 controls |
15/14 10/16 |
71.7 ± 7.0 70.9 ± 6.7 |
MMSE: 28.4 ± 2.4 MMSE: 29.0 ± 2.8 |
NR NA |
30.9 ± 6.7 NA |
Age and sex matching | 9/10 |
| Zhang et al., China, 2021 | Cross- sectional |
42/75 PD 75/150 controls |
NR 32/43 |
55.92 ± 7.53 54.68 ± 6.66 |
HY: 1.42 ± 0.55 NA |
3.2 ± 2.0 NA |
16.92 ± 7.6 NA |
Age, sex, IOP, inter-eye correlation | 9/10 |
| Murueta-Goyena et al., Spain, 2021 | Cross- sectional |
49/87 PD 40/73 controls |
33/16 13/27 |
64.6 ± 7.9 62.1 ± 8.0 |
MoCA: 24.4 ± 4.1 MoCA: 25.7 ± 2.5 |
7.1 ± 4.1 NA |
16.92 ± 7.6 NA |
Age, sex, and hypertension | 9/10 |
| Zhou et al., China, 2021 | Cross- sectional |
24/24 PD 23/23 controls |
18/6 11/12 |
65.88 ± 6.50 63.43 ± 7.11 |
HY: 2.0 ± 0.3 MMSE: 28.5 ± 1.6 NA |
5.3 ± 4.2 NA |
26.5 ± 12.3 NA |
Age, sex, and hypertension | 9/10 |
aPD Parkinson’s disease, HY Hoehn and Yahr, MMSE Mini-Mental State Exam, MoCA Montreal Cognitive Assessment, NOS Newcastle-Ottawa Scale, NR not reported, NA not applicable, SD standard deviation.
Table 2.
OCTA machine and parameters of included studiesa.
| Author, Country, Year | OCTA machine | OCTA parameters assessed within macula | Macular scan diameter (mm) |
|---|---|---|---|
| Kwapong et al., China, 2018 | Optovue | Vessel density (%) of SVP, DVP and whole retinal capillary plexus (Ø 0.6–2.5 mm) and FAZ (mm2) | 2.5 |
| Berkowitz et al., USA, 2020 | Optovue | FAZ (mm2) | 6 |
| Rascunà et al., Italy, 2020 | Optovue | Vessel density (%) of SVP, DVP, outer retinal layer (whole [3 mm diameter circle], fovea [1 mm diameter circle] and parafovea [Ø 1-3 mm]) | 3 and 4.5 |
| Shi et al., China, 2020 | Optovue | Vessel density (%) and skeleton density (%) of SVP and DVP (Ø 0.6–2.5 mm) | 2.5 |
| Zou et al., China, 2020 | Zeiss | Perfusion density (%) and vessel length density (mm) of SVP (1 mm, 3 mm and 6 mm diameter circles, Ø 1-3 mm and Ø 3-6 mm) and FAZ (mm2) | 6 |
| Robbins et al., USA, 2020 | Zeiss | Perfusion density (%) and vessel density (/mm) of SVP (whole [6 mm diameter circle], parafovea [Ø 1-3 mm] and perifovea [Ø 3-6 mm]) and FAZ (mm2) | 6 |
| Zhang et al., China, 2021 | SVision | Flow density (mm2) and flow ratio (%) of SVP and DVP (whole [3 mm and 6 mm diameter circles] and fovea [1 mm diameter circle]) | 6 |
| Murueta-Goyena et al., Spain, 2021 | Heidelberg Engineering | Perfusion density (%), skeleton density (1/mm) and fractal dimension (Dbox) of SVP and DVP (whole [2.5 mm diameter circle], fovea [1 mm circle diameter] and parafovea [Ø 1-2.5 mm]) and FAZ (mm2) | 2.5 |
| Zhou et al., China, 2021 | Zeiss | Vessel density (/mm) of SVP (whole [6 mm diameter circle], fovea [1 mm circle diameter], parafovea [Ø 1-3 mm] and perifovea [Ø 3-6 mm]) and FAZ (mm2) | 6 and 3 |
Meta-analyzed metrics of each study appear in bold.
aDVP deep vascular plexus, FAZ Foveal Avascular Zone (mm2), SVP superficial vascular plexus, Ø ring around fovea.
The methodology of participants’ eye selection varied among studies; in seven studies [10, 11, 24–28] values from both eyes of every participant were used, unless only one suitable image was available, in one study [29] the eye with the highest signal quality score was selected for each participant and in one study [30] one eye of each participant was included in the analysis without reporting the reasoning behind the selection. Finally, two studies [25, 27] recruited controls from the working staff at the hospitals where the studies were conducted, one study [11] included community-dwelling volunteers as healthy subjects, in one study [29] controls were recruited from the patients’ non-consanguineous families or friends and five studies [10, 24, 26, 28, 30] did not report the way that their controls were recruited.
Meta-analysis
Considering the fact that several OCTA parameters exist, which assess the vessel density of retinal microvasculature and may differ among different OCTA machines (Table 2), use of the SMD was made as a summary statistic in our meta-analyses. This method is particularly useful when studies assess the same outcome but measure it in various ways [32]. OCTA data on the vessel density of the whole superficial vascular plexus (SVP), foveal SVP, parafoveal SVP, and foveal avascular zone (FAZ), were obtained in more than five studies and thus, these were the OCTA metrics that were meta-analyzed. Regarding the whole SVP vessel density, foveal SVP vessel density, and FAZ, estimates were obtained from seven studies [10, 11, 25, 27–30], five studies [10, 26, 28–30] and five studies [11, 24, 26, 29, 30], respectively. Since Murueta-Goyena et al. [26] provided two estimates for FAZ (FAZ of SVP and DVP) we included the average of them in our meta-analysis. More specifically, we meta-analyzed seven estimates of the whole SVP vessel density measured over the 3 × 3 mm circle centered on the fovea. [10, 11, 25, 27–30]. Of these seven estimates, three of them [10, 25, 27] were directly obtained from the data of each study, while the rest four were indirectly calculated [11, 28–30]. We were not able to meta-analyze the estimates of the whole SVP vessel density of 6 × 6 mm scans since only four of them could be obtained [11, 28–30]. Regarding the meta-analysis of parafoveal SVP, in four studies [11, 24, 26, 30] the effect estimates were obtained directly from the data provided by each study, while in the remaining study [29] the estimate was indirectly calculated. When more than one type of vessel density metrics was provided by a study for a specific vascular plexus, we selected the one that was used by most of the remaining studies for the meta-analyses.
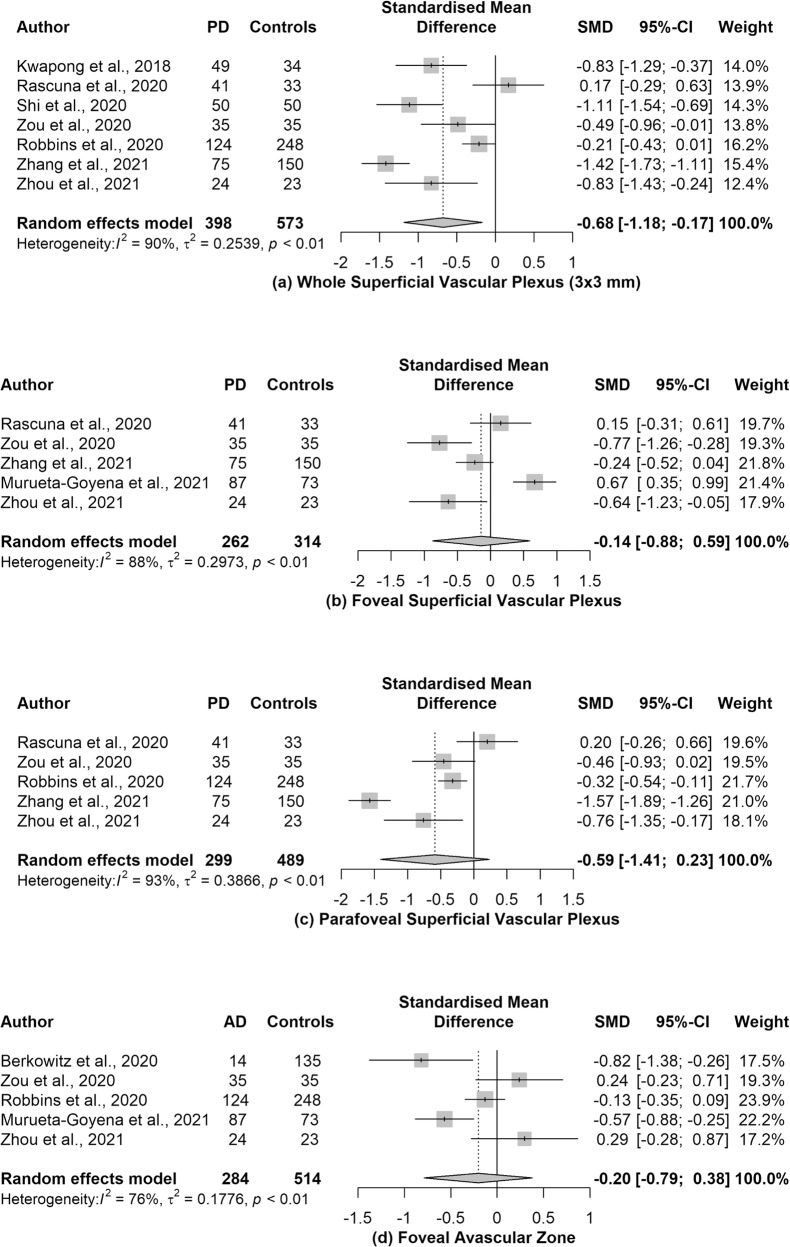
The pooled SMD between the retinal vessel density of PD patients and healthy participants in the whole SVP, foveal SVP, parafoveal SVP and FAZ was -0.68 (95% CI: −1.18 to −0.17, p value = 0.02, I2 = 90%, n = 7 studies), −0.14 (95% CI: −0.88 to 0.59, p value = 0.62, I2 = 88%, n = 5 studies), −0.59 (95% CI: −1.41 to 0.23, p value = 0.12, I2 = 93%, n = 5 studies) and −0.20 (95% CI: −0.79 to 0.38, p value = 0.39, I2 = 76%, n = 5 studies), respectively (Fig. 2). High and statistically significant heterogeneity was observed among the studies examining all the meta-analyzed associations, which justifies our use of the Hartung–Knapp/Sidik–Jonkman method. In order for the pooled SMD estimate of vessel density in whole SVP, foveal SVP parafoveal SVP, and FAZ to be nullified, an unmeasured confounder would have to be associated with a risk ratio of 3.01, 1.54, 2.81, and 1.70, respectively, with the risk of PD and the corresponding OCTA metrics.
Fig. 2. Forest plots of the pooled standardized mean differences (SMDs) on patients with Parkinson’s disease (PD) and healthy participants.
Association estimates between PD and a the whole superficial vascular plexus, b the foveal superficial vascular plexus, c the parafoveal superficial vascular plexus and d the foveal avascular zone.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to assess the associations between OCTA retinal measurements and PD. In our study, a statistically significant inverse association was found, of whole SVP vessel density with PD.
Changes in the brain microvasculature in PD as well as in its variants, like multiple system atrophy and progressive supranuclear palsy, have been identified by several studies [33, 34]. Additionally, dopamine is a crucial retinal neuromodulator, which regulates several aspects of visual function, including circadian nature of light-adapted vision and contrast acuity [35]. Even though the exact pathophysiological mechanism of reduced vessel density in PD patients remains unclear, it has been suggested that degeneration of dopamine neurons could lead to vessel fragmentation and loss of capillary connections due to the interactions of endothelial cells, neurons and glial cells, which together form a functional and structural unit [33]. Moreover, several postmortem studies have found structural and physiological changes, like abnormal capillaries and string vessel formation with no functional blood flow, in retinal vessels of PD patients [33, 36]. Finally, in a recent systematic review assessing the use of OCTA in Parkinson’s disease [37], the authors have also shown that alterations of the macular capillary plexus may comprise useful biomarkers for PD diagnosis. Since it is difficult to identify the exact mechanism of retinal microvascular changes in PD patients through observational studies, further experimental and histopathology studies are required.
As it has also been highlighted in a previous meta-analysis conducted by us [36], which examined the associations between OCTA metrics and Alzheimer’s disease, the variation in the way that vessel density is assessed among different OCTA machines is a significant concern. A specific OCTA machine can utilize various OCTA metrics to quantify retinal vessel density. We addressed this concern by using SMD as the summary statistic in our meta-analyses, giving us the possibility to summarize OCTA metrics that assess the same outcome (vessel density) on a different scale. In most of our included studies, vessel density was defined either as the percentage of perfused retinal area (unit of measurement was %) or as the ratio of total retinal vessels’ length per unit area in the region of measurement (unit of measurement was /mm). Apart from these two common OCTA metrics, several OCTA parameters of retinal vessel density were additionally included in some studies, like flow density (mm2) [28], fractal dimension (Dbox) [26], flow ratio (%) [28], skeleton density (%) [28] and vessel length density (mm) [30].
Our findings have important clinical implications as far as PD screening and diagnosis is concerned. Current diagnostic methods for PD are based on clinical assessment using the UPDRS (scale), which despite having been identified as a reliable, and valid tool for PD diagnosis, it has several drawbacks, including ambiguities in the written text, metric flaws, and a tangible lack of screening questions for several non-motor symptoms of PD [38]. Moreover, by the time that a PD diagnosis has been made, a substantial amount of dopaminergic neuronal loss has already taken place [39]. Consequently, faster, more patient-friendly, reliable, and objective diagnostic techniques, which could detect PD before a considerable neuronal loss has occurred, constitute a large unmet need for efficient screening of those at risk. In this context, quantitative OCTA metrics may represent promising biomarkers for monitoring the progression of pathological neural degeneration associated with PD, considering also that OCT metrics of retinal structure have already been identified as potential surrogate biomarkers of PD [7]. However, our results need also to be interpreted with caution, since the differences in vessel density between patients with PD and controls for several vascular plexuses are small and below the repeatability levels of vessel density assessed by OCTA. In particular, Pappelis et al. [40] found the coefficient of repeatability (standard deviation) of the parafoveal perfused capillary density to be 2.7% (1.8%), corresponding to an SMD of 1.5, larger than all of our meta-analyzed SMDs. Thus, it is unlikely that the reported differences could become clinically relevant, at least not until inherent OCTA limitations are overcome.
Although considering exclusively moderate to high-quality studies in accordance to the NOS score in our meta-analyses, several potential limitations should be simultaneously taken into consideration. First of all, several factors may have influenced the pooled estimates of our meta-analyses such as the methodological heterogeneity among studies with regard to the OCTA machine and the metrics utilized, the reasoning behind the selection of the eyes of participants and the covariates included in analysis. Second, although most studies made use of official disease severity scales which reflect the severity of disease with accuracy, there was heterogeneity in the types of disease severity scales utilized, as well as, in the disease severity scores. This variability in disease severity in individual studies could have resulted in over- or underestimation of the true effect sizes. Third, despite the majority of the studies having been controlled in the analysis for the age of the participants (a strong potential confounder on the association estimates), other potential confounding variables such as socioeconomic status, were not considered. The small sample size of most studies could be the culprit for this, which at the same time restricts the number of covariates in the analysis and constitutes an additional limitation of our meta-analysis. Fourth, several of the considered studies which evaluated both eyes of the participants, failed to adjust for the inter-eye correlation in their analyses, consequently increasing the likelihood of overestimating the correlation between retinal microvascular measurements and PD. Lastly, considering that all meta-analyzed studies are cross-sectional, no temporal ordering of the OCTA metrics and PD can be established.
In conclusion, this systematic review and meta-analysis provides evidence of an inverse association of whole SVP vessel density with PD. Due to several limitations, causal associations cannot be established and thus, future longitudinal studies, with more robust design and analysis are warranted to support our results.
Summary
What is known about this topic
It has been shown that an association exists between OCTA retinal measurements and PD.
What this study adds
This study underlines that OCTA metrics may constitute promising biomarkers for PD.
Supplementary information
Supplementary Table 1: Meta-analyses Of Observational Studies in Epidemiology Checklist
Supplementary Table 2: MEDLINE and EMBASE were searched using the Ovid interface on 05/11/2021 from inception until 05/11/2021
Supplementary Table 3: Modified Newcastle-Ottawa Quality Scale scores of cross-sectional studies
Author contributions
AK conceived and designed the presented study and performed the analysis. All authors wrote and critically reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Andreas Katsimpris, Iason Papadopoulos.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-023-02438-7.
References
- 1.Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–80. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeMaagd G, Philip A. Parkinson’s disease and its management: part 1: disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. J Formul Manag. 2015;40:504–32. [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson’s disease: the relative importance of the symptoms. Mov Disord. 2008;23:1428–34. doi: 10.1002/mds.21667. [DOI] [PubMed] [Google Scholar]
- 4.Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27:27–42. doi: 10.1111/ene.14108. [DOI] [PubMed] [Google Scholar]
- 5.Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, Green A, et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2010;9:921–32. doi: 10.1016/S1474-4422(10)70168-X. [DOI] [PubMed] [Google Scholar]
- 6.Chan VTT, Sun Z, Tang S, Chen LJ, Wong A, Tham CC, et al. Spectral-domain OCT measurements in Alzheimer’s disease: a systematic review and meta-analysis. Ophthalmology. 2019;126:497–510. doi: 10.1016/j.ophtha.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chrysou A, Jansonius NM, van Laar T. Retinal layers in Parkinson’s disease: A meta-analysis of spectral-domain optical coherence tomography studies. Parkinsonism Relat Disord. 2019;64:40–9. doi: 10.1016/j.parkreldis.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Ortuño-Lizarán I, Beach TG, Serrano GE, Walker DG, Adler CH, Cuenca N. Phosphorylated α-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Movem Disord. 2018;33:1315–24. doi: 10.1002/mds.27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21:1318–31. doi: 10.1038/s41593-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rascuna C, Russo A, Terravecchia C, Castellino N, Avitabile T, Bonfiglio V, et al. Retinal thickness and microvascular pattern in early Parkinson’s disease. Front Neurol. 2020;11:533375. doi: 10.3389/fneur.2020.533375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robbins CB, Thompson AC, Bhullar PK, Koo HY, Agrawal R, Soundararajan S, et al. Characterization of retinal microvascular and choroidal structural changes in Parkinson disease. JAMA Ophthalmol. 2021;139:182–8. doi: 10.1001/jamaophthalmol.2020.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauermann JL, Sochurek JAM, Plottner P, Alten F, Kasten M, Prasuhn J, et al. Applicability of optical coherence tomography angiography (OCTA) imaging in Parkinson’s disease. Sci Rep. 2021;11:5520. doi: 10.1038/s41598-021-84862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.Wells G, Shea B, O’Connell D, Robertson J, Peterson J, Welch V et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. www.ohri.ca/programs/clinical_epidemiology/. Accessed 10 Nov 2019.
- 15.Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One. 2016;11:e0147601. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IntHout J, Ioannidis JPA, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7:55–79. doi: 10.1002/jrsm.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M, Schneider M, Davey Smith G. Spurious precision? Meta-analysis of observational studies. BMJ. 1998;316:140–4. doi: 10.1136/bmj.316.7125.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathur MB, VanderWeele TJ. Sensitivity analysis for unmeasured confounding in meta-analyses. J Am Stat Assoc. 2020;115:163–72. doi: 10.1080/01621459.2018.1529598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane, 2019. www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
- 21.R Core Team (2018). R: a language and environment for statistical computing. R Foundation for Statistical Computing V, Austria. https://www.R-project.org/.
- 22.Miri S, Shrier EM, Glazman S, Ding Y, Selesnick I, Kozlowski PB, et al. The avascular zone and neuronal remodeling of the fovea in Parkinson’s disease. Ann Clin Transl Neurol. 2015;2:196–201. doi: 10.1002/acn3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhullar PK, Pead E, Thompson AC, Yoon SP, Grewal DS, Polascik B, et al. Evaluation of potential biomarkers in multimodal retinal images for diagnosis of Parkinson’s disease: a pilot study. In Proc. Investigative Ophthalmology and Visual Science Conference. 2019;60.
- 24.Berkowitz S, Patel S. Pilot study for neurological and retinal imaging as biomarkers for Parkinson’s disease using optical coherence tomography-angiography. In: Proc. Investigative Ophthalmology and Visual Science Conference. 2020;61.
- 25.Kwapong WR, Ye H, Peng C, Zhuang X, Wang J, Shen M, et al. Retinal microvascular impairment in the early stages of Parkinson’s disease. Invest Ophthalmol Vis Sci. 2018;59:4115–22. doi: 10.1167/iovs.17-23230. [DOI] [PubMed] [Google Scholar]
- 26.Murueta-Goyena A, Del Pino R, Galdós M, Arana B, Acera M, Carmona-Abellán M, et al. Retinal thickness predicts the risk of cognitive decline in Parkinson’s disease. Ann Neurol. 2021;89:165–76. doi: 10.1002/ana.25944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi C, Chen Y, Kwapong WR, Tong Q, Wu S, Zhou Y, et al. Characterization by fractal dimension analysis of the retinal capillary network in Parkinson’s disease. Retina. 2020;40:1483–91. doi: 10.1097/IAE.0000000000002641. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Zhang D, Gao Y, Yang L, Tao Y, Xu H, et al. Retinal flow density changes in early-stage Parkinson’s disease investigated by swept-source optical coherence tomography angiography. Curr Eye Res. 2021;46:1886–91. doi: 10.1080/02713683.2021.1933054. [DOI] [PubMed] [Google Scholar]
- 29.Zhou M, Wu L, Hu Q, Wang C, Ye J, Chen T, et al. Visual impairments are associated with retinal microvascular density in patients with Parkinson’s disease. Front Neurosci. 2021;15:718820. doi: 10.3389/fnins.2021.718820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou J, Liu K, Li F, Xu Y, Shen L, Xu H. Combination of optical coherence tomography (OCT) and OCT angiography increases diagnostic efficacy of Parkinson’s disease. Quant Imaging Med Surg. 2020;10:1930–9. doi: 10.21037/qims-20-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang D, Jia Y, Gao SS, Lumbroso B, Rispoli M. Optical coherence tomography angiography using the optovue device. Dev Ophthalmol. 2016;56:6–12. doi: 10.1159/000442770. [DOI] [PubMed] [Google Scholar]
- 32.HJ Schünemann, GE Vist, JPT Higgins, N Santesso, JJ Deeks, P Glasziou, et al. Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook.
- 33.Guan J, Pavlovic D, Dalkie N, Waldvogel HJ, O’Carroll SJ, Green CR, et al. Vascular degeneration in Parkinson’s disease. Brain Pathol. 2013;23:154–64. doi: 10.1111/j.1750-3639.2012.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Holst HM, van Uden IWM, Tuladhar AM, de Laat KF, van Norden AGW, Norris DG, et al. Cerebral small vessel disease and incident parkinsonism: the RUN DMC study. Neurology. 2015;85:1569–77. doi: 10.1212/WNL.0000000000002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson CR, Ruan GX, Aseem F, Abey J, Gamble K, Stanwood G, et al. Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci. 2012;32:9359–68. doi: 10.1523/JNEUROSCI.0711-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang P, Pavlovic D, Waldvogel H, Dragunow M, Synek B, Turner C, et al. String Vessel Formation is Increased in the Brain of Parkinson Disease. J Parkinsons Dis. 2015;5:821–36. doi: 10.3233/JPD-140454. [DOI] [PubMed] [Google Scholar]
- 37.Christou EE, Asproudis I, Asproudis C, Giannakis A, Stefaniotou M, Konitsiotis S. Macular microcirculation characteristics in Parkinson’s disease evaluated by OCT-Angiography: a literature review. Semin Ophthalmol. 2022;37:399–407. doi: 10.1080/08820538.2021.1987482. [DOI] [PubMed] [Google Scholar]
- 38.Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18:738–50. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 39.Rees RN, Acharya AP, Schrag A, Noyce AJ. An early diagnosis is not the same as a timely diagnosis of Parkinson’s disease. F1000Research. 2018;7:F1000 Faculty Rev-106. doi: 10.12688/f1000research.14528.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pappelis K, Jansonius NM. Quantification and repeatability of vessel density and flux as assessed by optical coherence tomography angiography. Transl Vis Sci Technol. 2019;8:3. doi: 10.1167/tvst.8.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Meta-analyses Of Observational Studies in Epidemiology Checklist
Supplementary Table 2: MEDLINE and EMBASE were searched using the Ovid interface on 05/11/2021 from inception until 05/11/2021
Supplementary Table 3: Modified Newcastle-Ottawa Quality Scale scores of cross-sectional studies