Abstract
Anopheles arabiensis and Anopheles funestus sensu stricto mosquitoes are major East African malaria vectors. Understanding their dispersal and population structure is critical for developing effective malaria control tools. Three mark-release-recapture (MRR) experiments were conducted for 51 nights to assess daily survival and flight range of An. arabiensis and An. funestus mosquitoes in south-eastern, Tanzania. Mosquitoes were marked with a fluorescent dye as they emerged from breeding sites via a self-marking device. Mosquitoes were collected indoors and outdoors using human landing catches (HLC) and Centers for Disease Control and Prevention light traps (CDC-LT). In total, 4210 An. arabiensis and An. funestus were collected with 316 (7.5%) marked and recaptured (MR). Daily mean MR was 6.8, standard deviation (SD ± 7.6) for An. arabiensis and 8.9 (SD ± 8.3) for An. funestus. Probability of daily survival was 0.76 for An. arabiensis and 0.86 for An. funestus translating into average life expectancy of 3.6 days for An. arabiensis and 6.5 days for An. funestus. Dispersal distance was 654 m for An. arabiensis and 510 m for An. funestus. An. funestus life expectancy was substantially longer than that of An. arabiensis. The MRR method described here could be routinely utilized when evaluating the impact of new vector control tools on mosquito survival.
Subject terms: Ecology, Ecology
Introduction
Mainland Tanzania has been classified by World Health Organization (WHO) as a country with high malaria burden that requires targeted application of malaria control tools1. Both malaria cases and malaria deaths have increased in recent years due to population growth2, insufficient coverage of vector control tools3 and to some extent the increase of malaria vector resistance to insecticides used in vector control4. Malaria transmission is highly heterogeneous5 due to geographical differences including altitude, urbanization and vegetation6. For these reasons, the country has stratified malaria control deploying tools based on the intensity of malaria transmission7 as a response to the WHO high burden to high impact (HBHI) strategy8. Much of the finer scale (district) differences in malaria transmission intensity is attributable to the vector species composition in that area. To maximize resources, the deployment of malaria vector control needs to be targeted against those vectors that transmit most of the disease.
In south-eastern Tanzania, malaria transmission is mediated by Anopheles funestus sensu stricto and Anopheles arabiensis that differ markedly in their vectoral capacity9. An. funestus feeds on humans primarily indoors and late at night10 while An. arabiensis feeds on humans and cattle indoors or outdoors11. These differences in ecology result in different man-vector contact that to some extent explains their differing vectoral capacity12.
Adult survival is a critical component of vectoral capacity because adult females must survive the extrinsic incubation period of Plasmodium before they can transmit pathogens13,14. Vector control tools reduce mosquito infectivity rate15, density and daily survival, with the later having the greatest impact on malaria transmission16,17. In addition, the use of insecticides may also disproportionally increase mortality among older mosquitoes and can partially reduce the impact of insecticide resistance18. Other than old age, several other factors such as disease, predation or environmental factors contribute to mosquito mortality19. Therefore, it can be argued that vector population age structure is a more valid metric20 than mosquito density as an outcome in entomological trials of vector control tools, as has been elegantly demonstrated in early trials of Insecticide Treated Nets (ITNs)21.
Dispersal of Anopheles mosquito range between few meters to several kilometres depending on resource availability i.e. larval habitats, sugar and blood hosts22 and species-specific environmental adaptability23. The range has been demonstrated to be bigger in rural areas, with relatively higher mobility compared to urban areas24. Laboratory-reared Anopheles have been found to disperse more than wild mosquitoes, possibly due to the presence of a memorised home range25. Anopheles mosquitoes actively disperse only or primarily during part of their gonotrophic cycle26. This is epidemiologically important as it influences the extent of malaria parasite acquisition and distribution that leads to transmission by female mosquitoes.
Mosquito population parameters can be estimated through a number of morphological, biochemical, genetic and spectroscopic methods, each of which has limitations in reliability, specificity, validation, and requires high cost or the need for an expert’s technical ability27. MRR is a technique where mosquitoes collected in the wild or reared in the laboratory, are marked, released then recaptured at a given distance and time interval from the releasing point28–30. MRR is an effective and low cost means of investigating adult mosquito dispersal and survivorship that can be utilized in most settings and has been evaluated against multiple species31. MRR experiments include a single mark and release of mosquitoes, followed by one or repeated recaptures32.
This study investigated the survival and dispersal capabilities of An. arabiensis and An. funestus in Ikungua village by marking mosquitoes as they emerged from their breeding sites33. Using mosquitoes as they emerge means that the technique is more reflective of natural dispersal and ethically less challenging as additional mosquitoes are not introduced and wild mosquitoes from the environment are recaptured. This data is needed to inform mathematical models used to optimize the selection of malaria control tools7. Furthermore, knowledge of survival and dispersal of malaria vectors is critical for planning, evaluating and implementing new tools which are intended to interrupt the pathogen’s transmission34.
Results
Mosquito release
A total of 4210 mosquitoes (both An. arabiensis and An. funestus) were marked and released into the wild mosquito population (with correction factor of 86% marking efficiency33), over three separate releases and 17 days follow up for each release.
Mosquito recapture
A total of 13,359 (An. arabiensis and An. funestus) marked and unmarked mosquitoes were collected and morphologically identified as An. gambiae s.l. (7260) and An. funestus s.l. (6099). Polymerase chain reaction (PCR) showed all the An. gambiae s.l. tested to be An. arabiensis (50/50 succesful amplifications). Furthermore, for An. funestus group, 92% (46/50 successful amplifications) showed to be An. funestus s.s whilst 8% (4/50) of the samples did not amplify. Recapture rate was 3% (n = 138) and 4% (n = 178) with a daily average of 6.8 (SD ± 7.6) for An. arabiensis and 9 (SD ± 8) for An. Funestus, respectively. Daily recapture declined over time from 16 (5.3%) on the first day to 1 (0.3%) on the 15th day after each release. There were few recaptured male mosquitoes, 12 An. gambiae s.l. and 4 An. funestus s.l. because we used recapture methods that targeted host seeking female mosquitoes. Males were excluded from the analysis.
Daily survival probability
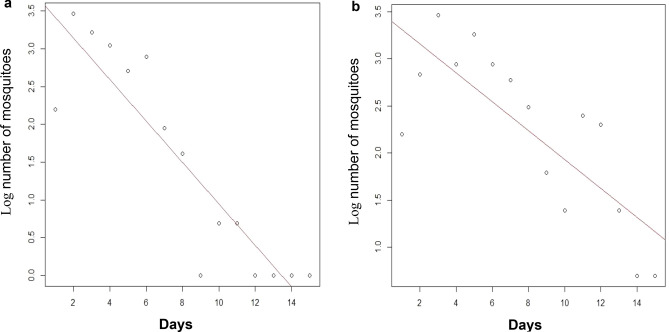
The daily survival probability was 0.76 for An. arabiensis and 0.86 for An. funestus which equates to a life expectancy of 3.64 and 6.51 days, respectively (Fig. 1).
Figure 1.
Probability of survival trends for An. arabiensis (a) and An. funestus (b) over a period of 2 weeks. Overall, for both species’ survival reduced over time but, An. funestus seemed to live longer than An. arabiensis since recapture of marked and released mosquitoes extended beyond the 15th day.
Average dispersal distance
The average dispersal distance of marked mosquitoes was 654 m (95% CI 543–763) for An. arabiensis and 510 m (95% CI 450–570) metres for An. funestus. The probability of capturing a marked mosquito declined over distance (Table 1). The majority (95.6%) of An. arabiensis and a lower proportion (75.8%) of An. funestus were recaptured within 500 m of the releasing point. Fewer mosquitoes were recaptured in the third annulus compared to the fourth annulus; 0.7% versus 3.6% for An. arabiensis and 2.2% versus 21.9% for An. funestus. There was a similarity between maximum recapture for An. arabiensis (20.5%) and An. funestus (20.0%) at 6 days after mosquito release, while the rate dropped dramatically from day 7 to 15 with 1.6% An. arabiensis and 6.4% An. funestus recaptured (Fig. 2).
Table 1.
Number of An. arabiensis and An. funestus recaptured by distance from the release point in Ikungua village.
| Annulus | Distance from release (m) | An. Arabiensis n (%) | An. Funestus n (%) |
|---|---|---|---|
| 1st | 215 | 78 (56.5) | 71 (39.9) |
| 2nd | 430 | 54 (39.1) | 64 (35.9) |
| 3rd | 645 | 1 (0.7) | 4 (0.02) |
| 4th | 860 | 5 (3.6) | 39 (21.9) |
| Total | 138 (100) | 178 (100) |
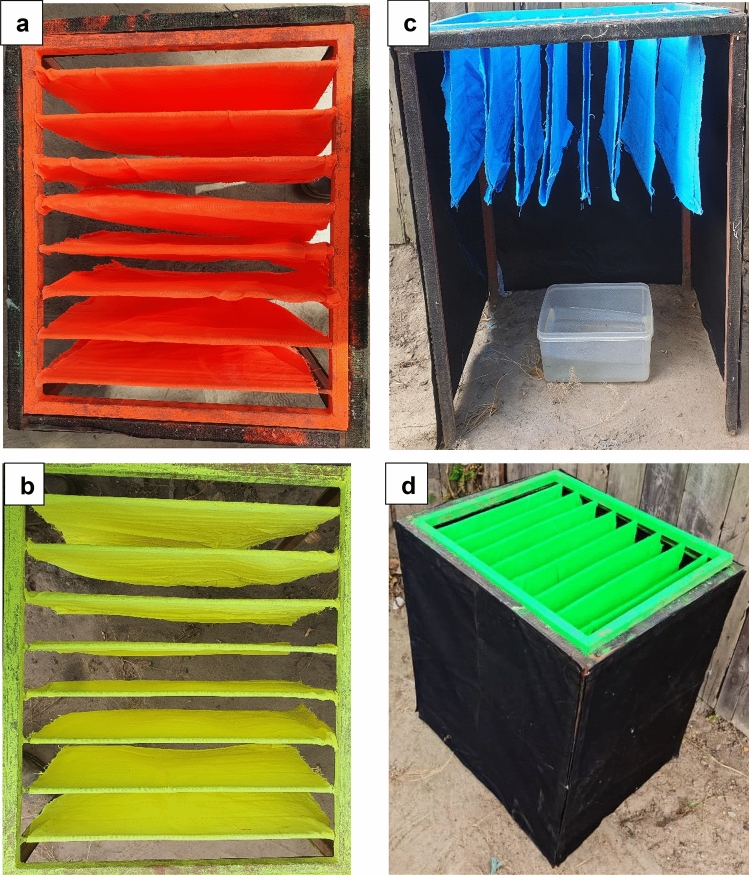
Figure 2.
The self-marking unit used previously in Saddler et al., (a,b) are respectively impregnated clothes with orange and yellow fluorescent dye. (c) The frame attached black cloth into a Velcro with pupae bowl and blue fluorescent dye. (d) The unit set up fully closed for release experiment and green fluorescent dye.
Estimates of population size
Population size for An. arabiensis was estimated as 101,886 mosquitoes while that of An. funestus was estimated as 78,991 mosquitoes. There were approximately 443 An. arabiensis and 343 An. funestus per hectare of the study area.
Discussion
This study was designed to investigate the mobility and life expectancy of two major malaria vector populations in south-eastern, Tanzania. Mosquito longevity is a critical aspect in transmission of Plasmodium falciparum that requires > 12 days to be infective17. The longer lifespan of An. funestus may have therefore contributed to its relative efficiency as a vector compared to An. arabiensis despite its comparatively low abundance. More focused attention is needed on the control of An. funestus due to its efficiency in transmitting malaria35.
Survival
Data from this study demonstrates that An. funestus survives longer, and has a 10% higher daily survival probability than An. arabiensis. These findings corroborate with earlier studies done in the Kilombero valley36,37 as well as other studies from Tanzania38 and West Africa39 that reported An. funestus having higher survival rates and lower mortality than An. arabiensis. This higher survival may be as a result of its adaptation to readily available human hosts and reported resistance9,35,40 against pyrethroid insecticides used in ITNs implemented for malaria control in this area41. These adaptations (endophily and anthrophily), also make it extremely vulnerable to control with core tools using insecticides to which An. funestus is susceptible. It should be noted that An. arabiensis in the region are also highly pyrethroid-resistant42. However, An. funestus has been shown to be more efficient in malaria transmission than An. arabiensis which may be related to differences in resistance or behaviour35 whereas laboratory studies have reported similar survivorship for An. arabiensis43,44 and An. funestus43 under a standard culturing environment.
Although, the estimated survival probability of An. funestus in this study (0.84) is 2% lower than the study conducted in the same area in the 1990’s (0.86)36 given the higher coverage (> 50%) of ITNs45 and other insecticide-based interventions than in the previous years, we hypothesize that pyrethroid resistance may be the reason for the maintained life expectancy of the species.
Changes in mosquito population survival may also be evaluated through dissecting the ovaries46 to measure the number of gonotrophic cycles that mosquitoes have undergone47 or the proportion of the proportion that have ever laid eggs48. The Detinova dissection technique is straightforward but requires dedicated technical staff, but very few people are skilled enough to routinely carry out the Polovodova technique49. Other age grading techniques include mid infra-red spectroscopy50, near-infra red spectroscopy51 as well as molecular methods such as transcription methods52. However, these more recent methods are still in development and are not used routinely53.
Dispersal
The dispersal distance of An. arabiensis and An. funestus was determined by measuring the distance travelled between the releasing point and the recapture house. An. arabiensis was found to have a similar dispersal distance to An. funestus. Similarly, Saddler et al.33 using the same MRR method, found the mean dispersal distance of An. arabiensis in Bagamoyo to be 579 m (95% CI 521–636), which is similar to that obtained for An. arabiensis in the current study. However, other researchers have reported higher dispersal distances. Wada et al. recorded individual mosquitoes travelled 5100 m within a day of being released54. Thompson et al.24 recorded An. gambiae up to 1400 m from the releasing site. But, Midega et al. found no difference in the mean dispersal distance between An. funestus and An. arabiensis along the Kenyan coast55.
Differences in dispersal distances may be due to varying geographical terrains56, density and location of human hosts, availability of sugar sources, oviposition, breeding and resting sites22 as well as environmental factors like prevailing wind direction, humidity and temperature55. During the course of the study, one of the houses located in the 4th annulus registered an unlikely large number of recaptured An. funestus (17.4%). Further, investigations showed that the house was close to a seasonal breeding site and had twice (6 members) the average number of people compared to the other households (2.8 members). There is existing evidence supporting the occurrence of higher densities of malaria vectors in households located near breeding sites57 and in those with higher number of individuals due to increased levels of carbon dioxide, a long range attractant of host-seeking mosquitoes from the presence of more residents58,59.
In the current study, females of both species (An. arabiensis and An. funestus) dispersed and recaptured at the furthest house were found 860 m from releasing site in the fourth annulus, but only a small proportion of mosquitoes (12.3%) reached this distance. Additional studies are needed with sampling more evenly distributed mosquitoes and carried out throughout the year to better understand the drivers of dispersal including population biomass and location of breeding sites in the study sites.
Release
Most MRR studies, mark adult mosquitoes that have been collected from the local area and release them at a central point31. The abundance of mosquitoes in MRR experiments is likely to be higher in houses close to the releasing point as found in our study because of the short distance between the houses and the releasing point.
In earlier investigations in Kikulukutu village, south-eastern, Tanzania60, adults of unknown age who have probably completed part of their gonotrophic cycle were released. When comparing aging mosquitoes captured and released in some studies60, results suggest the use of younger mosquitoes (pupa and larvae) as they are more likely to survive longer which increases the chance of them being recaptured61. Use of young mosquitoes of a known age also allows determination of mosquito life span.
Recapture
Most marked mosquitoes were recaptured in neighbouring houses several hours later after being released. The house closest to the releasing site which was 130 m away, had more An. funestus recaptured compared to An. arabiensis. This could be due to anthropophilic and endophilic nature of An. funestus62. Although widely used, MRR experiments are often limited by recapture rates below 5% of those released60,63. The overall recapture rate of 7.5% observed in the current study is double the average, 3% (1–9.5%) reported in the literature for Anopheles31. Fewer houses were located in the third annulus may explain why fewer mosquitoes were recaptured in the third annulus relative to the fourth annulus. Typically, more recaptures were made near the release point (first annuli) for both species and decreased as one moves further away from the releasing point. Therefore, greater sampling effort is required at greater distance from the releasing point.
The limitations to this study were refusal of some households to allow mosquito collections, resulting in unequal distribution of houses in each annulus. This was minimized by standardizing the study regions to accommodate all four annuli. Also, to simulate the natural environment, the release point was close to the natural breeding site located on the village periphery rather than centre in the study area. We did not collect data on the biomass in all houses or the location of all breeding sites in the village. A more comprehensive mapping effort at the beginning of the study would have allowed us to better understand mosquito dispersal. Another limitation was failure to get resistance profile of the two-mosquito species from the study area during the study however An. Arabiensis has been shown to be resistant to pyrethroid42.
In further studies of MRR the use of indoor resting collections and mosquito abdominal status is recommended to measure if the dyes affect the ability of mosquitoes to feed. The validity of the MRR method rests on the assumption that marked individuals behave in every respect as the unmarked ones (wild), and that both marked and unmarked mix together in a homogenous, random way. MRR includes marking mosquitoes with a fluorescent dye, a procedure that has been reported by many investigators to not affect the survival and dispersal behaviour of mosquitoes provided it is applied correctly64. The marking method was investigated during the development of the MRR method used here and found not to affect survival33 but it is not known if it can affect flight or predator response to marked insects.
Conclusion
MRR used as mosquitoes emerge from breeding sites is a simple and cost-effective method for measuring the dispersal and survival of mosquitoes. It can be deployed as part of routine entomological collections when evaluating vector control tools with active ingredients that that are designed to overcome resistance to existing classes of insecticides and consequently reduce mosquito population life expectancy when deployed at scale. This study has demonstrated that An. funestus has a substantially longer life expectancy than An. arabiensis in this setting, which may partially explain the greater efficiency of An. funestus in malaria transmission.
Methods
Study area
Three experimental phases of MRR survey were performed during the study, which was conducted at the end of the rainy season between September and October 2020 in Ikungua village in South-eastern, Tanzania65. The village had 347 houses and 984 inhabitants over 36 hectares. The village is surrounded by forests, water bodies, and agricultural areas. The temperature ranged from 23 to 32 °C during the study and the annual rainfall ranges from 1200 to 1800 ml. Residents are subsistence farmers, growing bananas and millet in the hillsides whilst growing rice in the valleys through an irrigation system, which provides breeding sites for malaria vectors. An. gambiae s.s populations have significantly declined in the study area35 leaving An. arabiensis and An. funestus as the main vectors with An. funestus mediating majority of the infections even though it is present in lower densities than An. arabiensis9. House structures in the village allow indoor entry through opened eaves, mud walls, thatch roofs, and doors not covering the whole entrance as well as windows66. National malaria control is implemented through Insecticide Treated Nets (ITNs) in the study area. Although, a field study of an indoor residual spray product was implemented in the villages shortly (about 1 year) before the study.
Mosquito preparation
Wild An. arabiensis and An. funestus pupae and larvae stage 2–4 were collected from multiple natural breeding ponds and puddles located within a one thousand meters radius from the releasing point using a larval dipper and one-millimetre bulb pipette. The colony was maintained in a field laboratory with 300 larvae per bowl reared at 25 ± 7 °C temperature and 40–99% relative humidity. These pupae and stage 4 larvae were maintained in a plastic bowl with some water and placed underneath the marking trap near a shelter in releasing area. After emergence, adult mosquitoes on their first flight out to seek for food and mate pass through pigment impregnated onto cloth strips where they would be marked with dye pigments.
Each release was conducted for 5 days consecutively with daily average of 281 pupae/larvae placed under the trap each day at 18:00 h. Recapture was conducted each night of the release and for 12 days after the last release, total 17 nights of recapture. There was then a wash out period of a further 3 days before the next round was conducted. In total, three rounds of MRR were conducted. The total mosquitoes released were: 794 in 1st, 2025 in 2nd, and 1435 in the 3rd release. A single releasing point, close to the mosquitoes’ natural breeding site was used for all of the releases (Fig. 3).
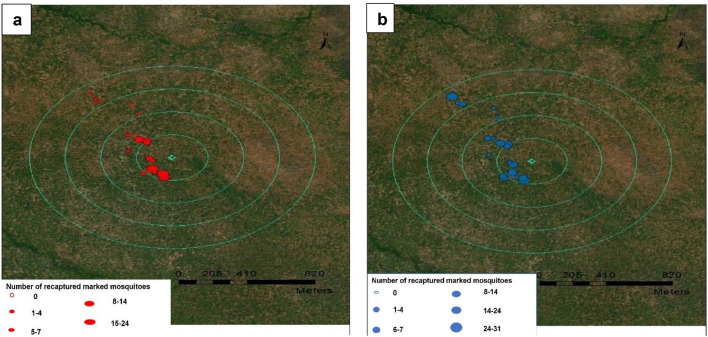
Figure 3.
Distribution of mosquito collection houses in four annuli with the dot size point indicating estimated mosquito collected in the house. (a) Presents An. arabiensis with red marker. (b) Presents An. funestus with blue marker. Base maps were provided by Open source QGIS71.
Marking unit
A self-marking unit33 was used to mark An. arabiensis and An. funestus in the field experiments (Fig. 2). There were five marking grids with a different colour used each day: pink, yellow, blue, orange, and green to distinguish each of the 5 days of release (Wtrcsv, Shenzhen Guang Chen Technology Co., China). The pigments have been shown to not affect mosquito survival33.
Recapture
Mosquitoes were recaptured from 20 houses located in four distance groups (annuli) from the releasing site; 215, 430, 645, and 860 m. Mosquito collection started on the day of the release and was conducted for 17 consecutive days between 18:00 h and 06:00 h using CDC-LT beside human-occupied bed net67 in 14 houses and paired indoor and outdoor HLC conducted by adult male volunteers29 in six houses. Collected mosquitoes were examined morphologically and identified following taxonomic keys68 and examined for fluorescent pigment using an ultraviolet light torch (21 LED 395 nm) and 10 × dissection microscope (ZEISS industrial metrology, Germany). A total number of 100 mosquitoes, 50 An. gambiae s.l. and 50 An. funestus s.l. were packed in Eppendorf tubed on silica and taken to the IHI Ifakara laboratory for PCR speciation69,70.
Analysis
Mean distance travelled (MDT), was used to describe the distance travelled by the estimate the dispersal of the released mosquitoes. This method estimates movement of adult mosquitoes against the radius of the experimental area72, with the assumption of having different densities of traps in different annuli73. As the area of the annuli increases with greater distance from the release site a correction factor (CF) for each annulus was estimated by dividing the area of the annulus by the sum of the area of all four annuli and multiplying the result by the total number of traps in the specific annuli74. Area of each annulus was calculated using half of the distance from the releasing site as the radius. Then, the number of mosquitoes recaptured in each annulus was divided by the total number of traps in the annuli and multiplied by the correction factor of the annulus to get estimated recapture (ER). Using the ER, cumulative estimated recapture (CER) was calculated. The MDT was then calculated as the sum of the product of ER and radius of the annuli over the total CER.
Survival was estimated with linear regression approach defined by Buonaccorsi et al.75 with recaptured mosquitoes adjusted in the model and for the average life expectancy was calculated according to Niebylski and Craig76.
Population size was estimated using the Lincoln Index (Eq. 1). The Fisher–Ford and Lincoln Index, are simple methods of estimating population size29 in mark-release experiments. The method assumes that: (1) marked and wild mosquitoes mix homogeneously immediately after release in a random way, (2) random dispersal of the marked and wild population without loss or gain in the population, and (3) there is a constant mortality rate among released mosquitoes. Using this method, estimates of total population size (P) are determined by the numbers of mark-released mosquitoes (a), the number captured on the subsequent occasion (n), and the number of those recaptured which had been marked (r), when r is greater than 20. In this study, it was assumed half of the marked-released mosquitoes were An. arabiensis and half were An. funestus. The analysis was done using R statistical software v4.1.177
| 1 |
P = Population size, a = Mark-released mosquitoes, n = Total captured mosquitoes, r = Mark-recaptured mosquitoes.
Equation (1) showing population size calculation.
Ethical approval
Ethical approval was granted by the Institutional Review Board of the Ifakara Health Institute (IHI) and Tanzanian National Institute of Medical Research (NIMR) (NIMR/HQ/R.8a/Vol. IX/2894). Written informed consent was obtained from household heads of the twenty houses selected for mosquito collection and from volunteers who performed HLC. The volunteers were provided doxycycline prophylaxis as per Tanzania Ministry of Health guidelines78 and were medically supervised79. I confirm that the recommended guidelines from the ministry of health were properly followed.
Acknowledgements
This study was supported financially by Imerys Minerals, 100 Mansell Ct E, Roswell, GA, 30076, USA. We would like to express our deepest gratitude to all villagers from Ikungua, especially household heads for allowing us to sample mosquitoes from their houses and volunteers who participated in the study. We also could not have achieved the aim of the project without guidance and expertise from our field staff particularly Hassani Ngonyani. We appreciate the great support received from Jason Moore, Ritha Kidyalla, Rose Philipo and Ester Giteta during the implementation of the project. Permission to publish the results of this study was granted by Tanzanian National Institute of Medical Research (NIMR) Reference NIMR/HQ/P.12 VOL.XXXVI/24.
Author contributions
W.N. conducted the study, drafted the manuscript. W.N. conducted the analysis with support from L.V. and O.G.O. N.M. collected data. C.S. and S.J.M. conceived and designed the study. S.J.M. and L.V. contributed to manuscript drafting. All authors critically revised the manuscript.
Data availability
Datasets shall be provided based on reasonable requests from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Malaria Report 2021. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021.
- 2.World Population Prospects 2022: Summary of Results. https://www.un.org/development/desa/pd/content/World-Population-Prospects-2022.
- 3.Stuck L, Chacky F, Festo C, Lutambi A, Abdul R, Greer G, Mandike R, Nathan R, Elisaria E, Yukich J. Evaluation of long-lasting insecticidal net distribution through schools in Southern Tanzania. Health Policy Plan. 2022;37:243–254. doi: 10.1093/heapol/czab140. [DOI] [PubMed] [Google Scholar]
- 4.Bisanzio D, Ally M, Ali AS, Kitojo C, Serbantez N, Kisinza WN, Magesa S, Reithinger R. Modelling insecticide resistance of malaria vector populations in Tanzania. Am. J. Trop. Med. Hygiene. 2022;20:tpmd210262. doi: 10.4269/ajtmh.21-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alegana VA, Macharia PM, Muchiri S, Mumo E, Oyugi E, Kamau A, Chacky F, Thawer S, Molteni F, Rutazanna D. Plasmodium falciparum parasite prevalence in East Africa: Updating data for malaria stratification. PLoS Glob. Public Health. 2021;1:e0000014. doi: 10.1371/journal.pgph.0000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chacky F, Runge M, Rumisha SF, Machafuko P, Chaki P, Massaga JJ, Mohamed A, Pothin E, Molteni F, Snow RW. Nationwide school malaria parasitaemia survey in public primary schools, the United Republic of Tanzania. Malar. J. 2018;17:1–16. doi: 10.1186/s12936-018-2601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Runge M, Thawer SG, Molteni F, Chacky F, Mkude S, Mandike R, Snow RW, Lengeler C, Mohamed A, Pothin E. Sub-national tailoring of malaria interventions in Mainland Tanzania: Simulation of the impact of strata-specific intervention combinations using modelling. Malar. J. 2022;21:1–17. doi: 10.1186/s12936-022-04099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Organization WH . High Burden to High Impact: A Targeted Malaria Response. World Health Organization; 2018. [Google Scholar]
- 9.Kaindoa EW, Matowo NS, Ngowo HS, Mkandawile G, Mmbando A, Finda M, Okumu FO. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south-eastern Tanzania. PLoS One. 2017;12:e0177807. doi: 10.1371/journal.pone.0177807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahamba NF, Finda M, Ngowo HS, Msugupakulya BJ, Baldini F, Koekemoer LL, Ferguson HM, Okumu FO. Using ecological observations to improve malaria control in areas where Anopheles funestus is the dominant vector. Malar. J. 2022;21:1–15. doi: 10.1186/s12936-022-04198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreppel K, Viana M, Main B, Johnson P, Govella N, Lee Y, Maliti D, Meza F, Lanzaro G, Ferguson H. Emergence of behavioural avoidance strategies of malaria vectors in areas of high LLIN coverage in Tanzania. Sci. Rep. 2020;10:1–11. doi: 10.1038/s41598-020-71187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brady OJ, Johansson MA, Guerra CA, Bhatt S, Golding N, Pigott DM, Delatte H, Grech MG, Leisnham PT, Maciel-de-Freitas R. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasit. Vectors. 2013;6:1–12. doi: 10.1186/1756-3305-6-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrett-Jones C, Shidrawi G. Malaria vectorial capacity of a population of Anopheles gambiae: An exercise in epidemiological entomology. Bull. World Health Organ. 1969;40:531. [PMC free article] [PubMed] [Google Scholar]
- 14.Milby M. Estimation of vectorial capacity: Vector survivorship. Bull. Soc. Vector Ecol. 1989;14:47–54. [Google Scholar]
- 15.Musiime AK, Smith DL, Kilama M, Rek J, Arinaitwe E, Nankabirwa JI, Kamya MR, Conrad MD, Dorsey G, Akol AM. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo, Uganda. Malar. J. 2019;18:1–9. doi: 10.1186/s12936-019-3076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith DL, Musiime AK, Maxwell K, Lindsay SW, Kiware S. A new test of a theory about old mosquitoes. Trends Parasitol. 2021;37:185–194. doi: 10.1016/j.pt.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beier JC. Malaria parasite development in mosquitoes. Annu. Rev. Entomol. 1998;43:519–543. doi: 10.1146/annurev.ento.43.1.519. [DOI] [PubMed] [Google Scholar]
- 18.Jones CM, Sanou A, Guelbeogo WM, Sagnon NF, Johnson PC, Ranson H. Aging partially restores the efficacy of malaria vector control in insecticide-resistant populations of Anopheles gambiae sl from Burkina Faso. Malar. J. 2012;11:1–11. doi: 10.1186/1475-2875-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macdonald G. The analysis of the sporozoite rate. Trop. Dis. Bull. 1952;20:49. [PubMed] [Google Scholar]
- 20.Davis, E. L., Hollingsworth, T. D., & Keeling, M. J. A novel age-structured mosquito model for assessing the mechanisms behind vector control success (2020).
- 21.Magesa S, Wilkes T, Mnzava A, Njunwa K, Myamba J, Kivuyo M, Hill N, Lines J, Curtis C. Trial of pyrethroid impregnated bednets in an area of Tanzania holoendemic for malaria. Part 2 Effects on the malaria vector population. Acta Trop. 1991;49:97–108. doi: 10.1016/0001-706X(91)90057-Q. [DOI] [PubMed] [Google Scholar]
- 22.Wu SL, Henry JM, Citron DT, Mbabazi Ssebuliba D, Nakakawa Nsumba J, Sánchez CH, Brady OJ, Guerra CA, García GA, Carter AR, et al. Spatial dynamics of malaria transmission. PLoS Comput. Biol. 2023;19:e1010684. doi: 10.1371/journal.pcbi.1010684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmes CJ, Benoit JB. Biological adaptations associated with dehydration in mosquitoes. Insects. 2019;10:375. doi: 10.3390/insects10110375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomson MC, Connor SJ, Quinones ML, Jawara M, Todd J, Greenwood BM. Movement of Anopheles gambiae sl malaria vectors between villages in The Gambia. Med. Vet. Entomol. 1995;9:413–419. doi: 10.1111/j.1365-2915.1995.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 25.Rawlings P, Curtis C, Wickramasinghe M, Lines J. The influence of age and season on dispersal and recapture of Anopheles culicifacies in Sri Lanka. Ecol. Entomol. 1981;6:307–319. doi: 10.1111/j.1365-2311.1981.tb00618.x. [DOI] [Google Scholar]
- 26.Gillies, M. T., & De Meillon, B. The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region) (1968).
- 27.Johnson BJ, Hugo LE, Churcher TS, Ong OTW, Devine GJ. Mosquito age grading and vector-control programmes. Trends Parasitol. 2020;36:39–51. doi: 10.1016/j.pt.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Benedict MQ, Charlwood JD, Harrington LC, Lounibos LP, Reisen WK, Tabachnick WJ. Guidance for evaluating the safety of experimental releases of mosquitoes, emphasizing mark-release-recapture techniques. Vector-Borne Zoonot. Dis. 2018;18:39–48. doi: 10.1089/vbz.2017.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Service M, Service M Sampling the adult resting population. Mosquito Ecol. Field Sampling Methods. 1993;20:210–290. doi: 10.1007/978-94-015-8113-4_3. [DOI] [Google Scholar]
- 30.Epopa PS, Millogo AA, Collins CM, North A, Tripet F, Benedict MQ, Diabate A. The use of sequential mark-release-recapture experiments to estimate population size, survival and dispersal of male mosquitoes of the Anopheles gambiae complex in Bana, a west African humid savannah village. Parasit. Vectors. 2017;10:1–15. doi: 10.1186/s13071-017-2310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerra CA, Reiner RC, Perkins TA, Lindsay SW, Midega JT, Brady OJ, Barker CM, Reisen WK, Harrington LC, Takken W. A global assembly of adult female mosquito mark-release-recapture data to inform the control of mosquito-borne pathogens. Parasit. Vectors. 2014;7:1–15. doi: 10.1186/1756-3305-7-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson C. The analysis of an animal population. J. Anim. Ecol. 1939;25:238–246. doi: 10.2307/1232. [DOI] [Google Scholar]
- 33.Saddler A, Kreppel KS, Chitnis N, Smith TA, Denz A, Moore JD, Tambwe MM, Moore SJ. The development and evaluation of a self-marking unit to estimate malaria vector survival and dispersal distance. Malar. J. 2019;18:1–14. doi: 10.1186/s12936-019-3077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WH Organization . Design of Epidemiological Trials for Vector Control Products: Report of a WHO Expert Advisory Group, Château de Penthes, Geneva, 24–25 April 2017. World Health Organization; 2017. [Google Scholar]
- 35.Lwetoijera DW, Harris C, Kiware SS, Dongus S, Devine GJ, McCall PJ, Majambere S. Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley, Tanzania. Malar. J. 2014;13:1–10. doi: 10.1186/1475-2875-13-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charlwood J, Smith T, Billingsley P, Takken W, Lyimo E, Meuwissen J. Survival and infection probabilities of anthropophagic anophelines from an area of high prevalence of Plasmodium falciparum in humans. Bull. Entomol. Res. 1997;87:445–453. doi: 10.1017/S0007485300041304. [DOI] [Google Scholar]
- 37.Charlwood J, Vij R, Billingsley P. Dry season refugia of malaria-transmitting mosquitoes in a dry savannah zone of east Africa. Am. J. Trop. Med. Hyg. 2000;62:726–732. doi: 10.4269/ajtmh.2000.62.726. [DOI] [PubMed] [Google Scholar]
- 38.Lines J, Lyimo E, Curtis C. Mixing of indoor-and outdoor-resting adults of Anopheles gambiae Giles sl and A. funestus Giles (Diptera: Culicidae) in coastal Tanzania. Bull. Entomol. Res. 1986;76:171–178. doi: 10.1017/S0007485300015388. [DOI] [Google Scholar]
- 39.Touré YT, Dolo G, Petrarca V, Dao A, Carnahan J, Taylor CE. Mark–release–recapture experiments with Anopheles gambiae sl in Banambani Village, Mali, to determine population size and structure. Med. Vet. Entomol. 1998;12:74–83. doi: 10.1046/j.1365-2915.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- 40.Matowo NS, Martin J, Kulkarni MA, Mosha JF, Lukole E, Isaya G, Shirima B, Kaaya R, Moyes C, Hancock PA. An increasing role of pyrethroid-resistant Anopheles funestus in malaria transmission in the Lake Zone, Tanzania. Sci. Rep. 2021;11:1–13. doi: 10.1038/s41598-021-92741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NMCP: National Malaria Strategic Plan 2014–2020. Dar es Salaam: Tanzania National Malaria Control Program 2014.
- 42.Matowo NS, Munhenga G, Tanner M, Coetzee M, Feringa WF, Ngowo HS, Koekemoer LL, Okumu FO. Fine-scale spatial and temporal heterogeneities in insecticide resistance profiles of the malaria vector, Anopheles arabiensis in rural south-eastern Tanzania. Wellcome Open Res. 2017;2:25. doi: 10.12688/wellcomeopenres.12617.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zengenene MP, Munhenga G, Chidumwa G, Koekemoer LL. Characterization of life-history parameters of an Anopheles funestus (Diptera: Culicidae) laboratory strain. J. Vector Ecol. 2021;46:24–29. doi: 10.52707/1081-1710-46.1.24. [DOI] [PubMed] [Google Scholar]
- 44.Oliver SV, Brooke BD. The role of oxidative stress in the longevity and insecticide resistance phenotype of the major malaria vectors Anopheles arabiensis and Anopheles funestus. PLoS ONE. 2016;11:e0151049. doi: 10.1371/journal.pone.0151049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yukich J, Stuck L, Scates S, Wisniewski J, Chacky F, Festo C, Kabulika G, Dimoso K, Mandike R, Greer G. Sustaining LLIN coverage with continuous distribution: The school net programme in Tanzania. Malar. J. 2020;19:1–12. doi: 10.1186/s12936-020-03222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Detinova TS, Bertram DS, WH Organization . Age-Grouping Methods in Diptera of Medical Importance, with Special Reference to Some Vectors of Malaria. World Health Organization; 1962. [PubMed] [Google Scholar]
- 47.Polovodova V. The determination of the physiological age of female Anopheles by the number of gonotrophic cycles completed. Med. Parazit. 1949;18:352–355. [Google Scholar]
- 48.Detinova T. Determination of the physiological age of the females of anopheles by the changes in the tracheal system of the ovaries. Med. Parasitol. 1945;14:25. [PubMed] [Google Scholar]
- 49.Beklemishev W, Detinova T, Polovodova V. Determination of physiological age in anophelines and of age distribution in anopheline populations in the USSR. Bull. World Health Organ. 1959;21:223. [PMC free article] [PubMed] [Google Scholar]
- 50.Siria DJ, Sanou R, Mitton J, Mwanga EP, Niang A, Sare I, Johnson PCD, Foster GM, Belem AMG, Wynne K, et al. Rapid age-grading and species identification of natural mosquitoes for malaria surveillance. Nat. Commun. 2022;13:1501. doi: 10.1038/s41467-022-28980-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lambert B, Sikulu-Lord MT, Mayagaya VS, Devine GJ, Dowell FE, Churcher TS. Monitoring the age of mosquito populations using near-infrared spectroscopy. Sci. Rep. 2018;8:5274. doi: 10.1038/s41598-018-22712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cook PE, Hugo LE, Iturbe-Ormaetxe I, Williams CR, Chenoweth SF, Ritchie SA, Ryan PA, Kay BH, Blows MW, O'Neill SL. The use of transcriptional profiles to predict adult mosquito age under field conditions. Proc. Natl. Acad. Sci. 2006;103:18060–18065. doi: 10.1073/pnas.0604875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson BJ, Hugo LE, Churcher TS, Ong OTW, Devine GJ. Mosquito age grading and vector-control programmes. Trends Parasitol. 2019;20:25. doi: 10.1016/j.pt.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Wada Y, Kawai S, Oda T, Miyagi I, Suenaga O, Nishigaki J, Omori N, Takahashi K, Matsuo R, Itoh T. Dispersal experiment of Culex tritaeniorhynchus in Nagasaki area (Preliminary report) Trop. Med. 1969;11:37–44. [Google Scholar]
- 55.Midega JT, Mbogo CM, Mwambi H, Wilson MD, Ojwang G, Mwangangi JM, Nzovu JG, Githure JI, Yan G, Beier JC. Estimating dispersal and survival of Anopheles gambiae and Anopheles funestus along the Kenyan coast by using mark–release–recapture methods. J. Med. Entomol. 2007;44:923–929. doi: 10.1093/jmedent/44.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manga L, Fondjo E, Carnevale P, Robert V. Importance of low dispersion of Anopheles gambiae (Diptera: Culicidae) on malaria transmission in hilly towns in south Cameroon. J. Med. Entomol. 1993;30:936–938. doi: 10.1093/jmedent/30.5.936. [DOI] [PubMed] [Google Scholar]
- 57.Smith T, Charlwood J, Takken W, Tanner M, Spiegelhalter D. Mapping the densities of malaria vectors within a single village. Acta Trop. 1995;59:1–18. doi: 10.1016/0001-706X(94)00082-C. [DOI] [PubMed] [Google Scholar]
- 58.Gillies M. The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): A review. Bull. Entomol. Res. 1980;70:525–532. doi: 10.1017/S0007485300007811. [DOI] [Google Scholar]
- 59.Kaindoa EW, Mkandawile G, Ligamba G, Kelly-Hope LA, Okumu FO. Correlations between household occupancy and malaria vector biting risk in rural Tanzanian villages: Implications for high-resolution spatial targeting of control interventions. Malar. J. 2016;15:1–12. doi: 10.1186/s12936-016-1268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takken W, Charlwood J, Billingsley P, Gort G. Dispersal and survival of Anopheles funestus and A. gambiae sl (Diptera: Culicidae) during the rainy season in southeast Tanzania. Bull. Entomol. Res. 1998;88:561–566. doi: 10.1017/S0007485300026080. [DOI] [Google Scholar]
- 61.Harrington LC, Buonaccorsi JP, Edman JD, Costero A, Kittayapong P, Clark GG, Scott TW. Analysis of survival of young and old Aedes aegypti (Diptera: Culicidae) from Puerto Rico and Thailand. J. Med. Entomol. 2001;38:537–547. doi: 10.1603/0022-2585-38.4.537. [DOI] [PubMed] [Google Scholar]
- 62.Soma DD, Poda SB, Hien AS, Namountougou M, Sangaré I, Sawadogo JME, Fournet F, Ouédraogo GA, Diabaté A, Moiroux N. Malaria vectors diversity, insecticide resistance and transmission during the rainy season in peri-urban villages of south-western Burkina Faso. Malar. J. 2021;20:1–11. doi: 10.1186/s12936-020-03554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Costantini C, Li SG, Torre AD, Sagnon NF, Coluzzi M, Taylor CE. Density, survival and dispersal of Anopheles gambiae complex mosquitoes in a West African Sudan savanna village. Med. Vet. Entomol. 1996;10:203–219. doi: 10.1111/j.1365-2915.1996.tb00733.x. [DOI] [PubMed] [Google Scholar]
- 64.Reisen WK, Aslamkhan M. A release—recapture experiment with the malaria vector, Anopheles stephensi Liston, with observations on dispersal, survivorship, population size, gonotrophic rhythm and mating behaviour. Ann. Trop. Med. Parasitol. 1979;73:251–269. doi: 10.1080/00034983.1979.11687255. [DOI] [PubMed] [Google Scholar]
- 65.Nambunga IH, Ngowo HS, Mapua SA, Hape EE, Msugupakulya BJ, Msaky DS, Mhumbira NT, Mchwembo KR, Tamayamali GZ, Mlembe SV. Aquatic habitats of the malaria vector Anopheles funestus in rural south-eastern Tanzania. Malar. J. 2020;19:1–11. doi: 10.1186/s12936-020-03295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Odufuwa OG, Ross A, Mlacha YP, Juma O, Mmbaga S, Msellemu D, Moore S. Household factors associated with access to insecticide-treated nets and house modification in Bagamoyo and Ulanga districts, Tanzania. Malar. J. 2020;19:1–13. doi: 10.1186/s12936-020-03303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mboera L, Kihonda J, Braks M, Knols B. Influence of centers for disease control light trap position, relative to a human-baited bed net, on catches of Anopheles gambiae and Culex quinquefasciatus in Tanzania. Am. J. Trop. Med. Hyg. 1998;59:595–596. doi: 10.4269/ajtmh.1998.59.595. [DOI] [PubMed] [Google Scholar]
- 68.Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae) Malar. J. 2020;19:70. doi: 10.1186/s12936-020-3144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am. J. Trop. Med. Hyg. 2002;66:804–811. doi: 10.4269/ajtmh.2002.66.804. [DOI] [PubMed] [Google Scholar]
- 70.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 71.Meyer D, Riechert M. Open source QGIS toolkit for the Advanced Research WRF modelling system. Environ. Model. Softw. 2019;112:166–178. doi: 10.1016/j.envsoft.2018.10.018. [DOI] [Google Scholar]
- 72.Morris C, Larson V, Lounibos L. Measuring mosquito dispersal for control programs. J. Am. Mosq. Control Assoc. 1991;7:608–615. [PubMed] [Google Scholar]
- 73.Lillie T, Kline D, Hall D. The dispersal of Culicoides mississippiensis (Diptera: Ceratopogonidae) in a salt marsh near Yankeetown, Florida. J. Am. Mosq. Control Assoc. 1985;1:463–467. [PubMed] [Google Scholar]
- 74.Vaughan JA, Noden BH, Beier JC. Population dynamics of Plasmodium falciparum sporogony in laboratory-infected Anopheles gambiae. J. Parasitol. 1992;78:716–724. doi: 10.2307/3283550. [DOI] [PubMed] [Google Scholar]
- 75.Buonaccorsi JP, Harrington LC, Edman JD. Estimation and comparison of mosquito survival rates with release-recapture-removal data. J. Med. Entomol. 2003;40:6–17. doi: 10.1603/0022-2585-40.1.6. [DOI] [PubMed] [Google Scholar]
- 76.Niebylski M, Craig G., Jr Dispersal and survival of Aedes albopictus at a scrap tire yard in Missouri. J. Am. Mosq. Control Assoc. 1994;10:339–343. [PubMed] [Google Scholar]
- 77.Tollefson M. R 4 Quick Syntax Reference. Springer; 2022. Downloading R and RStudio and Setting Up a File System; pp. 3–14. [Google Scholar]
- 78.Programme TaNMC: National Guidelines for Malaria Diagnosis and Treatment 2006. National Malaria Control Programme 2006.
- 79.Gimnig JE, Walker ED, Otieno P, Kosgei J, Olang G, Ombok M, Williamson J, Marwanga D, Abong'o D, Desai M, et al. Incidence of malaria among mosquito collectors conducting human landing catches in western Kenya. Am. J. Trop. Med. Hyg. 2013;88:301–308. doi: 10.4269/ajtmh.2012.12-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets shall be provided based on reasonable requests from the corresponding author.