Abstract
PCR amplification technology for the detection of epizootic hemorrhagic disease virus (EHDV) ribonucleic acid in cell culture and clinical specimens was developed. With oligoribonucleotide primers selected from genome segment 10 of EHDV serotype 1 (EHDV-1), which codes for two nonstructural proteins (NS3 and NS3a), the PCR-based assay resulted in a 535-bp PCR product. RNAs from North American EHDV-1 prototype, EHDV-2 prototype, and a number of EHDV field isolates, including the Central African isolates of EHDV-5 and EHDV-318 propagated in cell cultures, were detected by this PCR-based assay. The specific 535-bp PCR products were visualized onto agarose gels, and the identity of the PCR products was confirmed by chemiluminescent hybridization with a 352-bp internal probe. The sensitivity of the EHDV PCR assay was increased by chemiluminescent hybridization; by this EHDV-NS3 PCR, 10 fg of EHDV RNA was detected (equivalent to 600 viral particles). Amplification product was not detected when the PCR-based assay was applied to RNAs from North American bluetongue virus prototype serotypes 2, 10, 11, 13, and 17; total nucleic acid extracts from uninfected BHK-21 cells; or unfractionated blood from calves and deer that were EHDV seronegative and virus isolation negative. The described EHDV PCR-based assay with primers derived from segment 10 of EHDV-1 resulted in detection of EHDV RNA from blood and tissues collected from calves and deer with natural and experimental EHDV infections and provides a valuable tool to study the epidemiology of EHDV infection in susceptible ruminants.
Epizootic hemorrhagic disease virus (EHDV) is a double-stranded RNA (dsRNA) orbivirus in the family Reoviridae and is related to bluetongue virus (BTV) (6, 7, 9). EHDV causes an often fatal hemorrhagic infection in North American white-tailed deer (Odocoileus virginianus) (8, 14, 16, 22, 25, 26). The association of EHDV with clinical hemorrhagic disease in sheep and cattle is rare, but the infection is typically asymptomatic (2, 10, 19). Ten serotypes of EHDV are distributed worldwide (11), but only EHDV serotypes 1 and 2 (EHDV-1 and EHDV-2, respectively) are enzootic in North America (8, 13, 28, 29); EHDV-5 and EHDV-318 are enzootic in Africa (20).
EHDV has a genome composed of 10 dsRNA segments (12). The genome segments code for viral proteins (VP) (15). The nonstructural proteins NS1, NS2, and NS3 are encoded by genome segments 6, 8, and 10, respectively, and were found to be highly conserved (15, 16, 17, 36). Segment 2 (L2) codes for the major structural protein of the outer coat, VP2, and is associated with serotype specificity (4, 5) and induction of neutralizing antibody (17). Genome segment 3 (L3) codes for the structural protein VP3. VP7 is encoded by genome segment 7 (17). Genome segments coding for NS1 and NS2 of EHDV-2 (Alberta strain) were successfully used as serogroup-specific probes for the detection of cell culture-adapted EHDV isolates (1–4, 33). In previous studies, PCR amplification technology was developed and evaluated for detection of EHDV in cell culture and clinical samples based on the NS1 genome of EHDV-2 (1–3, 34, 35). The nonstructural protein 1 (NS1) gene was targeted for development of a single PCR amplification with chemiluminescent hybridization (1–3). A nested EHDV-PCR was also developed and evaluated for detection of EHDV in cell culture and in the biting midge based on sequence analysis of genome segment 6, which encodes NS1 of EHDV-2 (34, 35). However, no work has yet been carried out to validate the potential use of PCR for the detection of EHDV with primers derived from genome segment 10 of EHDV-1, which codes for NS3 and NS3a. Previous studies showed that genome segment 10 is conserved among serotypes of the EHDV serogroup (16, 21). Recently, we have cloned and sequenced genome segment 10 of EHDV-1 (New Jersey strain). The sequence analysis showed that this genome has a 97% nucleic acid identity compared with that of EHDV-2 (16). Therefore, it was suggested that a fragment from segment 10 of the EHDV-1 genome could be targeted and used for detection of EHDV by PCR amplification technology.
In the present investigation, we described PCR technology with primers derived from genome segment 10 of EHDV-1 (New Jersey strain) for detection of EHDV serogroups in cell culture and a variety of tissue samples.
MATERIALS AND METHODS
Virus and cells.
The North American orbiviruses of EHDV-1 and EHDV-2; five BTV prototype serotypes, i.e., 2, 10, 11, 13, and 17, which are present in the United States (Arthropod-Borne Animal Disease Research Laboratory, Laramie, Wyo.); and 12 field isolates of EHDV (National Veterinary Services Laboratories, Animal and Plant Health Inspection Service, U.S. Department of Agriculture, Ames, Iowa, and Washington Animal Disease Diagnostic Laboratory, Pullman, Wash.) were studied. The Central African isolates of EHDV-5 and EHDV-318 were also used in this study (Faculty of Veterinary Science, University of Khartoum, Khartoum, Sudan). The North American viruses were propagated and processed in our laboratory at Davis, Calif., as described previously (1). All viruses were propagated on confluent monolayers of baby hamster kidney (BHK-21) cells. The infectious material was harvested and centrifuged at 1,500 × g for 30 min, and the cell pellet was used for dsRNA extraction. The Central African isolates of EHDV RNA were extracted in Khartoum, Sudan.
Extraction of viral nucleic acid from infected cell monolayers.
The EHDV and BTV dsRNAs were extracted from infected cells as previously described (1). Total nucleic acid was ethanol precipitated. Viral dsRNA was purified by differential lithium chloride precipitation, resuspended in 100 μl of double-distilled water, and quantified with a spectrophotometer at a wavelength of 260 nm.
Experimental animals and collection of clinical samples.
Two 6- to 8-month-old calves were purchased, and after repeated clinical examinations for evidence of clinical hemorrhagic disease, each calf was subjected to virologic and serologic examination to eliminate the possibility of EHDV infection. The calves were healthy and free of EHDV infection. One calf was inoculated with EHDV-1 at 106 50% tissue culture infectious doses/ml, and the other calf was inoculated with EHDV-2 at the same dose. During the course of the experiment, the animals were housed in insect-secured enclosures and were fed a ration of concentrates and hay with water ad libitum. Blood samples were collected from the jugular veins of the experimentally infected calves as well as from clinically normal calves and deer. The clinical samples were processed as described previously (3). The processed blood samples were inoculated on BHK-21 cell monolayers for virus isolation (VI) and typed by plaque inhibition testing (27). Extraction of viral nucleic acid from the clinical samples was as previously described (3). Briefly, 250 μl of processed blood or spleen was digested with sodium dodecyl sulfate and proteinase K (Boehringer Mannheim, Indianapolis, Ind.). The samples were phenol extracted twice. Total nucleic acid was ethanol precipitated and resuspended in 20 μl of double-distilled water. Five microliters of the resuspended nucleic acid was used in the PCR assay.
Primer selection and synthesis of the probe.
Primers (24-mer each) were selected from the published sequence of segment 10 of EHDV-1 (16) and used in these PCR assays. Primers 1 and 2 (P1 and P2) were selected for the synthesis of specific EHDV PCR product. P1 included bases 233 to 256 of the positive-sense strand of genome segment 10, i.e., 5′-GGTTGCTTATGCTTCGTATGCGGA-3′. P2 included bases 735 to 758 of the complementary strand, i.e., 5′-CACGACATAGTGACCTTGGAGCTT-3′. EHDV PCR with P1 and P2 resulted in a 535-bp product. For synthesis of a probe complementary to the predicted amplified viral sequences generated by P1 and P2, oligonucleotide primers P3 and P4 were selected from the published sequence cited above. P3 and P4 were internal to the annealing sites of P1 and P2. P3 consisted of bases 324 to 347 of the positive-strand 5′-ATGCGTGTAGAGTTGACAGCGATG-3′. P4 was designed from the complementary strand between bases 653 and 676, i.e., 5′-CTCTGTCACACTCATTCGTACTGC-3′. PCR amplification with P3 and P4 resulted in a 352-bp PCR product internal to the annealing sites of P1 and P2. All primers were synthesized on a DNA synthesizer (Milligen/Biosearch, a division of Millipore, Burlington, Mass.) and purified with Oligo-Pak oligonucleotide purification columns (Glen Research Corporation, Sterling, Va.) as per the manufacturer’s instructions. The amplification product produced by P3 and P4 was purified with DNA binding beads (Mermaid Kit; Bio 101, La Jolla, Calif.) according to the manufacturer’s instructions and was used as a probe for chemiluminescent hybridization of the blotted nucleic acids (1–3).
PCR.
The PCR protocol used in this study was basically as previously described (1), except that the thermal cycling profiles were as follows: a 2-min incubation at 95°C, followed by 40 cycles of 95°C for 1 min, 55°C for 30 s, and 72°C for 45 s, and a final incubation at 60°C for 10 min. The Taq DNA polymerase was used at a concentration of 2.5 μl per reaction. All PCRs were carried out at a volume of 100 μl. Thermal profiles were performed with a Techne PHC-2 thermal cycler (Techne, Princeton, N.J.). Following amplification, 20 μl from each PCR mixture containing amplified product was loaded onto gels of 2% SeaKem agarose (FMC Bioproduct, Rockland, Maine) and electrophoresed. The gels were stained with ethidium bromide, and the expected PCR products were visualized under UV light.
Southern blot hybridization.
Southern blotting with chemiluminescent hybridization was performed with nonradiolabelled internal probe as previously described (1). After primary and secondary washes, the detection reagents (ECL System; Amersham, Arlington Heights, Ill.) were added and the membranes were sealed in Saran Wrap. The wrapped membranes were then exposed to X-ray film for 1 to 60 min with an intensifying screen.
RESULTS
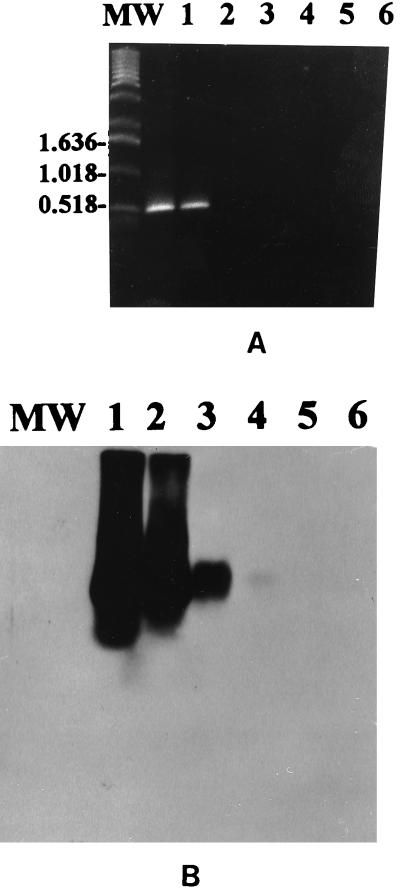
The described PCR-based assay with primers derived from segment 10 of EHDV-1 afforded sensitive and specific detection of EHDV prototype serotypes 1 and 2 and all EHDV field isolates used in this study. The specific 535-bp PCR product was visualized on an ethidium bromide-stained gel from ≥1.0 pg of RNA of U.S. EHDV prototype serotype 1 (Fig. 1A). Southern blotting with chemiluminescent hybridization detected as little as 10 fg of viral RNA target (Fig. 1B).
FIG. 1.
Sensitivity of the PCR for detection of EHDV with primers from NS3 genome sequence of EHDV-1. (A) Visualization of the 535-bp EHDV-specific PCR product on an ethidium bromide-stained agarose gel from 1.0 pg of EHDV RNA. Lanes: MW, molecular weight marker; 1 to 5, 10 pg, 1.0 pg, 100 fg, 10 fg, and 1.0 fg, respectively, of EHDV-1; 6, BHK-21 total nucleic acid extract. (B) Southern blot with chemiluminescent hybridization of the gel shown in panel A showing detection of as little as 10 fg of EHDV-1 RNA.
With 1 pg of EHDV RNA target, the 535-bp specific PCR product was detected from the 14 EHDV field isolates, including Central African isolates of EHDV-5 and EHDV-318, both with the ethidium bromide-stained agarose gel (Fig. 2A) and by chemiluminescent hybridization (Fig. 2B). The amount of 1.0 ng of RNA extracts from North American BTV-2, -10, -11, -13, and -17 and total nucleic acid extracts from uninfected BHK-21 cells failed to demonstrate PCR products or to produce hybridization signals (Fig. 3).
FIG. 2.
Visualization of the 535-bp EHDV-specific PCR product on an ethidium bromide-stained agarose gel from 1.0 pg of RNA of 14 different EHDV field isolates. Lanes: MW, molecular weight marker; 1 to 8, EHDV-1 field isolates; 9 to 12, EHDV-2 field isolates; 13 and 14, Central African isolates of EHDV-5 and EHDV-318, respectively; 15, BHK-21 total nucleic acid extract. (B) Southern blot with chemiluminescent hybridization of the gel shown in panel A.
FIG. 3.
Specificity of the PCR for RNA from the EHDV NS3 genome. Amplification product was not detected from a high concentration of 1.0 ng of BTV RNA from North American BTV prototype viruses or total nucleic acid extracts from BHK-21 cells. Lanes: MW, molecular weight marker; 1, 1.0 pg of EHDV-1; 2 to 6, BTV-2, -10, -11, -13, and -17, respectively; 7, BHK-21 total nucleic acid extract.
The specific PCR products were detected by chemiluminescent hybridization directly from unfractionated lysed blood of the calves experimentally infected with EHDV-1 and EHDV-2. EHDV RNA extracts from spleen homogenate and lung tissues from EHDV-2-infected deer were also detected. Blood samples from uninfected calves and deer failed to produce hybridization signals (Fig. 4). All EHDV isolates which were PCR positive were also EHDV positive by conventional virus isolation.
FIG. 4.
Detection of the 535-bp EHDV NS3-specific PCR product by Southern blotting with chemiluminescent hybridization from clinical samples. Lanes: MW, molecular weight marker; 1, spleen homogenate from EHDV-2-infected deer; 2, lung homogenate from EHDV-2-infected deer; 3 and 4, unfractionated lysed blood cells from a calf experimentally infected with EHDV-1; 5 and 6, unfractionated lysed blood from a calf experimentally infected with EHDV-2; 7 and 8, unfractionated lysed blood from uninfected calves; 9 and 10, unfractionated lysed blood cells from uninfected deer.
DISCUSSION
The economic importance of EHDV infection is attributed mainly to the fatal hemorrhagic disease it causes in white-tailed deer populations (8, 14, 25, 26). Even in the absence of clinical hemorrhagic disease, there is a restriction on the international trade of livestock and associated germ plasm (23). In addition, the pathological lesions caused by EHDV are undistinguishable from those caused by BTV, and hence, EHDV is of interest to veterinary diagnosticians (18, 24). Moreover, conventional virus isolation and serology are time-consuming and cumbersome (3, 24, 27). Therefore, it is becoming increasingly obvious that the development of molecular diagnostic techniques which provide rapid detection and differentiation of EHDV and BTV would be advantageous (1, 4, 5, 29, 31). In the present study, we validated the potential use of PCR technology to detect EHDV infection by using primers derived from genome segment 10 of EHDV-1, which codes for NS3 and NS3a (15–17). We also compared this EHDV PCR-based detection assay with previously reported EHDV PCR assays. In the present study, the EHDV PCR-based assay with primers derived from segment 10 of EHDV-1 reproducibly and specifically detected EHDV RNA in infected cell cultures and clinical samples. The specific 535-bp PCR products, which were visualized on an ethidium bromide-stained agarose gel or were detected by chemiluminescent hybridization, were obtained from all EHDV RNA samples tested. The PCR assay was a simple procedure that efficiently detected all EHDV serotypes and field isolates under the stringency conditions used in this study.
The sensitivity studies indicated that the PCR protocol described herein was capable of detecting an amount of 10 fg of total EHDV genomic dsRNA. The total molecular mass of the EHDV genome has been calculated to be 11.44 × 106 Da, and 10 fg of EHDV RNA corresponds to 600 viral particles (15). This EHDV PCR assay based on the segment 10 genome was found to be more sensitive than those reported by Harding et al. (13), who used EHDV segment 3, which codes for VP3, as a target genome. However, in the present study, genome segment 10 seemed to be more conserved than genome segment 3, and the sensitivity limit was 10 fg by the hybridization assay (equivalent to 600 viral particles). In a previous study, we reported an EHDV PCR using the EHDV-2 NS1 genome sequence that was found to be 10 times more sensitive than the present PCR assay, in which only 0.1 fg of EHDV RNA (equivalent to 6 viral particles) could be detected by PCR technology (1). These results confirm that the EHDV NS1 genome is more conserved than the EHDV NS3 genome. Nevertheless, the PCR based-assay described here, using primers derived from the EHDV NS3 genome, could serve as an alternative or a complement to the existing diagnostic methods currently used for detection of EHDV infection during an outbreak of the disease among susceptible animals. In addition, the concentration of Taq DNA polymerase used in this protocol was 2.5 μl, compared to 5.0 μl when the EHDV-2 NS1 PCR assay was used. This finding renders the PCR protocol reported here less expensive compared with our previously protocol for the NS1 EHDV PCR-based assay.
It is worth mentioning that EHDV segment 2 (L2), which codes for VP2, is the most variable genome and can be used for specific identification of EHDV serotypes and not serogroup alone (4, 5, 30). In previous studies, we used the VP2 genome sequence of EHDV-1 (5) and that of EHDV-2 for specific identification of EHDV-1 (5) and EHDV-2 (4), respectively.
The specificity studies indicated that the 535-bp PCR product was not amplified from a relatively high concentration of 1.0 ng of RNA from North American BTV-2, -10, -11, -13, and -17; or from total nucleic acid extracts from uninfected BHK-21 cell controls; or from blood cells from uninfected calves or deer under the stringency conditions described in this study.
The EHDV field isolates used in this study represented a range of virus isolates, which were obtained from different animal species and collected over a period of 15 years from diverse geographic locations in Central Africa (Khartoum, Sudan) and North America (including California, Georgia, Nebraska, Oregon, Missouri, Virginia, Colorado, Idaho, North Dakota, Kentucky, and New Jersey). Excellent correlation of results from ethidium bromide-stained agarose gels and chemiluminescent hybridization was obtained by this PCR-based assay. This finding suggests that tentative diagnosis of EHDV infection could be based on visualization of the amplified product on an ethidium bromide-stained agarose gel, which is a simple procedure that requires only one additional hour after amplification. Hybridization is necessary to confirm the identity of the amplified product and to increase the sensitivity of the PCR-based assay, particularly when the concentration of the amplified product is too small to be visualized on an ethidium bromide-stained agarose gel under UV light (1–5, 32, 33). In the present study, the use of nonradioactive chemiluminescent hybridization removes the hazardous and cumbersome radioactive laboratory procedures of working with 32P or 33P. Successful amplification was also obtained from whole blood, which was collected from the calves experimentally infected with North American EHDV-1 and EHDV-2 and from spleen homogenate and lung tissues from naturally infected deer. In this study, extraction of EHDV RNA from whole blood is easier and less time-consuming than the previously described method which required isolation of leukocytes prior to nucleic acid extraction (29).
It is important to include negative and positive controls in each PCR amplification to estimate the sensitivity and the specificity of the PCR assay.
In conclusion, the PCR protocol described here, using primers derived from the genome 10 sequence of EHDV-1, should provide an alternative or a complement to the methods currently used for detection of EHDV infection in cell culture and from a variety of clinical specimens. It can also be used as a valuable tool to study the epidemiology of EHDV serogroup infection in wild animals and domestic livestock.
ACKNOWLEDGMENTS
This work was supported by funds from the Livestock Disease Research Laboratory and the Dairy Food Safety Laboratory, School of Veterinary Medicine, University of California, Davis.
REFERENCES
- 1.Aradaib I E, Akita G Y, Osburn B I. Detection of epizootic hemorrhagic disease virus serotype 1 and 2 in cell culture and clinical samples using polymerase chain reaction. J Vet Diagn Invest. 1994;6:143–147. doi: 10.1177/104063879400600202. [DOI] [PubMed] [Google Scholar]
- 2.Aradaib I E, Sawyer M M, Osburn B I. Experimental infection of calves with epizootic hemorrhagic disease virus: virologic and serologic studies. J Vet Diagn Invest. 1994;6:489–492. doi: 10.1177/104063879400600416. [DOI] [PubMed] [Google Scholar]
- 3.Aradaib I E, Akita G Y, Pearson J E, Osburn B I. Comparison of polymerase chain reaction and virus isolation for detection of epizootic hemorrhagic disease virus in clinical samples from clinically infected deer. J Vet Diagn Invest. 1995;7:196–200. doi: 10.1177/104063879500700205. [DOI] [PubMed] [Google Scholar]
- 4.Aradaib I E, Wilson W C, Cheney C, Pearson J E, Osburn B I. Application of the polymerase chain reaction for specific identification of epizootic hemorrhagic disease virus serotype 2. J Vet Diagn Invest. 1995;7:388–392. doi: 10.1177/104063879500700316. [DOI] [PubMed] [Google Scholar]
- 5.Aradaib I E, McBride J W, Wilson W C, Osburn B I. Development of polymerase chain reaction for detection of epizootic hemorrhagic disease virus serotype 1. Arch Virol. 1995;40:2273–2281. doi: 10.1007/BF01323247. [DOI] [PubMed] [Google Scholar]
- 6.Aradaib I E, Brewer A W, Osburn B I. Interaction of epizootic hemorrhagic disease virus with bovine erythrocytes. Comp Immun Microbiol Infect Dis. 1996;20:281–283. doi: 10.1016/s0147-9571(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 7.Borden E C, Shope R E, Murphy F A. Physicochemical and morphological relationships of some arthropod-borne viruses to bluetongue virus-a new taxonomic group. Physicochemical and serological studies. J Gen Virol. 1971;3:261–271. doi: 10.1099/0022-1317-13-2-261. [DOI] [PubMed] [Google Scholar]
- 8.Brannian R E, Giessman N, Porath W, Hoff G L. Epizootic hemorrhagic disease in white-tailed deer from Missouri. J Wildl Dis. 1983;19:357–358. doi: 10.7589/0090-3558-19.4.357. [DOI] [PubMed] [Google Scholar]
- 9.Fenner F, Pereira H G, Porterfield J S, et al. Family and generic names for virus approved by the international committee on taxonomy of viruses. Intervirology. 1974;3:193–194. doi: 10.1159/000149755. [DOI] [PubMed] [Google Scholar]
- 10.Gibbs E P, Lawman M J P. Infection of British deer and farm animals with epizootic hemorrhagic disease of deer virus. J Comp Pathol. 1977;87:335–343. doi: 10.1016/0021-9975(77)90023-8. [DOI] [PubMed] [Google Scholar]
- 11.Gorman B M. An overview of the orbiviruses. In: Walton T E, Osburn B I, editors. Bluetongue, African horse sickness and related orbiviruses. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 335–347. [Google Scholar]
- 12.Hammami S, Osburn B I. Analysis of genetic variation of epizootic hemorrhagic disease virus and bluetongue virus field isolates by coelectrophoresis of their double-stranded RNA. Am J Vet Res. 1992;53:636–642. [PubMed] [Google Scholar]
- 13.Harding M J, Prud’homme I, Rola J, Dulac G C. Development of PCR test for the identification of North American isolates of epizootic hemorrhagic disease virus. Can J Vet Res. 1996;60:59–64. [PMC free article] [PubMed] [Google Scholar]
- 14.Hoff G L, Trainer D O. Observations on bluetongue and epizootic hemorrhagic disease virus in white-tailed deer. J Wildl Dis. 1974;10:25–31. doi: 10.7589/0090-3558-10.1.25. [DOI] [PubMed] [Google Scholar]
- 15.Huismans H, Bremer C W, Barber L T. The nucleic acid and proteins of epizootic hemorrhagic disease virus. Onderstepoort J Vet Res. 1979;46:95–104. [PubMed] [Google Scholar]
- 16.Jensen J M, Wilson W C. A model for membrane topology of the NS3 protein as predicted from the sequence of segment 10 of epizootic hemorrhagic disease virus serotype 1. Arch Virol. 1995;140:799–805. doi: 10.1007/BF01309968. [DOI] [PubMed] [Google Scholar]
- 17.Mecham J O, Dean C V. Protein coding assignments for the genomes of epizootic hemorrhagic disease virus. J Gen Virol. 1988;69:1255–1262. doi: 10.1099/0022-1317-69-6-1255. [DOI] [PubMed] [Google Scholar]
- 18.Metcalf H E, Luedke A J, Jochim M M. Epizootic hemorrhagic disease virus infection in cattle. In: Walton T E, Osburn B I, editors. Bluetongue, African horse sickness and related orbivirus. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 687–693. [Google Scholar]
- 19.Metcalf H E, Luedke A J. Bluetongue and related viruses. Bovine Pract. 1980;15:188–191. [Google Scholar]
- 20.Mohammed M E H, Mellor P. Further studies on bluetongue and bluetongue related orbiviruses in the Sudan. Epidemiol Infect. 1990;105:619–632. doi: 10.1017/s0950268800048263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nel L H, Huismans H. A comparison of different genome segments of epizootic hemorrhagic disease virus as serogroup specific probes. Arch Virol. 1990;110:103–112. doi: 10.1007/BF01310706. [DOI] [PubMed] [Google Scholar]
- 22.Nettles V F, Hylton S A, Stallknecht O, Davidson W R. Epidemiology of epizootic hemorrhagic disease in wildlife in the USA. In: Walton T E, Osburn B I, editors. Bluetongue, African horse sickness and related orbiviruses. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 238–248. [Google Scholar]
- 23.Osburn B I, Aradaib I E, Schore C. Proceedings of the 13th International Symposium of the World Association of Veterinary Microbiologist, Immunologist and Infectious Disease Specialist, Perugia, Montova, Italy. 1994. Comparison of bluetongue and epizootic hemorrhagic disease complex; pp. 219–224. [Google Scholar]
- 24.Pearson J E, Gustafson G A, Shafer A L, Alstad A D. Diagnosis of bluetongue virus and epizootic hemorrhagic disease. In: Walton T E, Osburn B I, editors. Bluetongue, African horse sickness and related viruses. CRC Press, Boca Raton, Fla. 1992. pp. 533–546. [Google Scholar]
- 25.Shope R E, MacNamara L G, Mangold R. Report on the deer mortality, epizootic hemorrhagic disease of deer. N J Outdoors. 1955;6:17–21. [Google Scholar]
- 26.Shope R E, MacNamara L G, Mangold R. A virus-induced epizootic hemorrhagic disease of the Virginia white-tailed deer (Odocoileus virginianus) J Exp Med. 1960;111:155–170. doi: 10.1084/jem.111.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stott J L, Barber T L, Osburn B I. Proceedings of the 21st Annual Meeting of the American Association of Veterinary Laboratory Diagnosticians. 1978. Serotyping bluetongue virus: a comparison of plaque inhibition and plaque neutralization methods; pp. 399–410. [Google Scholar]
- 28.Venter E H, Viljoen G J, Nel L H, Huismans H, Van Dijk A A. A comparison of different genomic probes in the detection of virus-specified RNA in Orbivirus-infected cells. J Virol Methods. 1991;32:171–180. doi: 10.1016/0166-0934(91)90048-5. [DOI] [PubMed] [Google Scholar]
- 29.Wade-Evans A M, Mertens P P C, Bostock C J. Development of polymerase chain reaction for the identification of bluetongue virus in tissue samples. J Virol Methods. 1991;30:15–24. doi: 10.1016/0166-0934(90)90040-m. [DOI] [PubMed] [Google Scholar]
- 30.Wilson W C, Fukusho A, Roy P. Diagnostic complementary DNA probe for genome segment 2 and 3 of epizootic hemorrhagic disease virus serotype 1. Am J Vet Res. 1990;51:855–860. [PubMed] [Google Scholar]
- 31.Wilson W C. Development and optimization of a hybridization assay for epizootic hemorrhagic disease viruses. J Virol Methods. 1990;30:173–181. doi: 10.1016/0166-0934(90)90018-b. [DOI] [PubMed] [Google Scholar]
- 32.Wilson W C. Detection of epizootic hemorrhagic disease virus in Culicoides variipennis (Diptera: Ceratopogonidae) J Med Entomol. 1991;28:742–744. doi: 10.1093/jmedent/28.5.742. [DOI] [PubMed] [Google Scholar]
- 33.Wilson W C, Archer J L, Chase C C L. The use of RNA detection techniques to identify bluetongue and epizootic hemorrhagic disease viruses in Culicoides variipennis. In: Walton T E, Wilson B I, editors. Bluetongue, African horse sickness and related orbiviruses. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 687–693. [Google Scholar]
- 34.Wilson W C, Chase C C L. Nested and multiplex polymerase chain reaction for the identification of bluetongue virus infection in the biting midge, Culicoides variipennis. J Virol Methods. 1993;45:39–47. doi: 10.1016/0166-0934(93)90138-h. [DOI] [PubMed] [Google Scholar]
- 35.Wilson W C. Development of nested-PCR tests based on sequence analysis of epizootic hemorrhagic disease virus non-structural protein 1 (NS1) Virus Res. 1994;31:357–365. doi: 10.1016/0168-1702(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 36.Wilson W C. Sequence analysis of the non-structural protein 2 from epizootic hemorrhagic disease virus. Virus Res. 1994;34:63–68. doi: 10.1016/0168-1702(94)90119-8. [DOI] [PubMed] [Google Scholar]