Abstract
Introduction:
Opioid use disorder (OUD) and psychiatric conditions commonly co-occur yet are infrequently treated with evidence-based therapeutic approaches, resulting in poor outcomes. These conditions, separately, present challenges to treatment initiation, retention, and success. These challenges are compounded when individuals have OUD and psychiatric conditions.
Methods:
Recognizing the complex needs of these individuals, gaps in care, and the potential for primary care to bridge these gaps, we developed a psychotherapy program that integrates brief, evidence-based psychotherapies for substance use, depression, and anxiety, building on traditional elements of the Collaborative Care Model (CoCM). In this article, we describe this psychotherapy program in a primary care setting as part of a compendium of collaborative services.
Results:
Patients receive up to 12 sessions of evidence-based psychotherapy and case management based on a structured treatment manual that guides treatment via Motivational Enhancement; Cognitive Behavioral Therapies for depression, anxiety, and/or substance use disorder; and/or Behavioral Activation components.
Discussion:
Novel, integrated treatments are needed to advance service delivery for individuals with OUD and psychiatric conditions and these programs must be rigorously evaluated. We describe our team’s efforts to test our psychotherapy program in a large primary care network as part of an ongoing three-arm randomized controlled trial.
Keywords: opioid use disorder, psychiatric conditions, primary care, collaborative care model
Introduction
Opioid use disorder (OUD) and psychiatric conditions commonly occur, are infrequently treated with evidence-based therapeutic approaches, and when untreated or undertreated are associated with deleterious outcomes at both the personal and societal levels (American Psychiatric Association, 2020; Anxiety & Depression Association of America, 2020; Lai et al., 2015; Seth et al., 2018). In this conceptual article, we articulate the current care gaps for individuals with OUD and co-occurring mental health problems and the potential for primary care to bridge this gap. Primary care settings are both increasingly offering medication, such as buprenorphine, for OUD and integrating mental health services utilizing models such as the Collaborative Care Model (CoCM), an integrated model of behavioral health used to enhance usual primary care (Marcotte et al., 2021). CoCM employs algorithms to guide care and involves ongoing medication management, structured longitudinal follow-up assessments, and behavioral treatment as appropriate. These strategies have been shown to be effective (Woltmann et al., 2012) and to reduce barriers to accessing care for individuals with OUD and psychiatric disorders (Kola & Kruszynski, 2010), respectively. Primary care clinics are thus well-poised to provide individuals with OUD and psychiatric conditions with accessible, evidence-based care for both conditions and to address current gaps in care in an integrated fashion.
Given the complex needs of this population, we argue that a robust psychotherapy component is needed to do this most effectively. We describe the literature that has led us to this conclusion and the psychotherapy program we developed to meet the needs of individuals with OUD and psychiatric conditions in a primary care setting as part of a compendium of collaborative services including buprenorphine and other traditional elements of CoCM. We also briefly describe our ongoing work to test this adapted model in a large primary care network as part of an ongoing randomized controlled trial (RCT).
Untreated OUD and Psychiatric Conditions
Over 2.5 million people in the United States have OUD (Seth et al., 2018), and 40% of people with OUD have a co-occurring psychiatric disorder (Grant et al., 2004; Lai et al., 2015). About 78% of people with OUD also have other active, co-occurring substance use disorders (Jones & McCance-Katz, 2019). People with OUD who also have psychiatric disorders like depression and/or anxiety experience low rates of treatment for either condition, perhaps because of unaddressed symptoms of the other. Only one in four people with OUD and one in three of people with psychiatric disorders receive treatment, and often this care is not evidence-based (American Psychiatric Association, 2020; Anxiety & Depression Association of America, 2020). An even smaller percentage of people with both OUD and psychiatric disorders receive treatment for both conditions.
Evidence supports the use of medications for OUD (MOUD) like buprenorphine to treat OUD and the CoCM to address psychiatric disorders like depression and anxiety in primary care (Wolk, Last, et al., 2021). Despite the existence of these treatments, most individuals with OUD and psychiatric disorders do not receive evidence-based care for either condition. For example, many OUD treatment programs fail to offer MOUD, instead only offering detoxification or other abstinence-only approaches (Walsh & Long, 2019). Many people with psychiatric disorders do not receive evidence-based psychosocial treatment (Kessler et al., 2005; Liberman, 2012), instead getting non-recovery-oriented and non-personalized care in an inpatient setting (Rössler & Drake, 2017). Furthermore, existing models of care fail to incorporate psychotherapy tailored for individuals with OUD and psychiatric disorders which is important for this patient population. There is a need for evidence-based, tailored, and integrated treatment of OUD and psychiatric disorders (Barry et al., 2016) delivered in accessible settings like primary care.
Complex Needs
OUD.
People with OUD and people with psychiatric conditions have complex needs that benefit from tailored treatment (Korthuis et al., 2017). People with OUD may be disengaged from the healthcare system, often due to a history of traumatic or stigmatizing healthcare (Olsen & Sharfstein, 2014), which can result in the undertreatment of medical conditions (Blanco & Volkow, 2019). They often also have social, physical, economic, and/or legal factors that may make OUD treatment initiation and retention harder (Blanco & Volkow, 2019). One study found that most people with OUD experienced unemployment, financial instability, housing instability, food insecurity and/or a lack of reliable transportation (Hooker et al., 2020), with another study finding that over 50% of people with OUD reported finances as a barrier to treatment (Novak et al., 2019). Unfortunately, these factors reduce the likelihood of initiating or staying engaged in OUD treatment, especially if these factors remain unaddressed (Hooker et al., 2020).
Psychiatric conditions.
Many patients with OUD experience comorbid psychiatric conditions, such as anxiety or depression, with estimates ranging from 40% to 80% (Grant et al., 2004; Hooker et al., 2020; Lai et al., 2015). Like people with OUD, people with psychiatric conditions are also more likely to experience poverty and housing and food insecurity (Conrad et al., 2020), which may limit their ability to initiate and remain engaged with treatment for their psychiatric conditions.
OUD + psychiatric conditions.
When individuals have both OUD and psychiatric conditions, challenges to treatment initiation, engagement, and success for each condition are compounded. Co-occurring psychiatric conditions may increase the risk of continued opioid misuse, overdose, and reduced quality of life (Erfan et al., 2010; Rounsaville, Kosten, & Kleber, 1986; Teesson et al., 2015). These individuals may also encounter barriers to treatment if treatment for their co-morbid condition is considered a contraindication (Kikkert et al., 2018). For example, some psychiatric treatment settings do not allow for opioid use of any kind, so the use of evidence based MOUD is not permitted (Mancher et al., 2019). The confluence of these realities means that only 16%-32% of individuals with OUD and co-occurring psychiatric conditions receive treatment for both conditions (Novak et al., 2019) despite evidence that suggests that concurrent treatment, including psychotherapy, confers better outcomes (Hassan et al., 2017).
Treating OUD
MOUDs, including methadone and buprenorphine, have a large and growing evidence base to support them as the most effective treatments for OUD (National Academies of Sciences Engineering Medicine, 2018). Methadone and buprenorphine are FDA-approved opioid agonists and reduce both all-cause and opioid-related mortality (Mattick et al., 2014). Despite their efficacy, only 36% of specialty substance use treatment programs offer MOUD (Mojtabai et al., 2019) and less than a quarter of people diagnosed with OUD receive one of these medications (Feder et al., 2017). Methadone is federally regulated and must be administered daily in methadone treatment facilities. Prior to December 2022, buprenorphine could only be prescribed in larger quantities (e.g., one month) by any clinician with a Drug Addiction Treatment Act (DATA) 2000 waiver (often called an “X-waiver”), a waiver that authorizes the use of buprenorphine to treat OUD in outpatient settings (Manlandro, 2005). With the passage and implementation of a federal omnibus appropriations bill in December 2022, this X-waiver requirement was eliminated (The White House, 2022).
While MOUD is effective at treating OUD, it is often prescribed without attention to the psychiatric disorders that co-occur in at least 40% of people with OUD (Lai et al., 2015). The failure to treat symptoms of co-occurring psychiatric disorders in people with OUD may contribute to worsened outcomes and PCPs frequently cite patients’ lack of psychosocial support as a barrier to prescribing buprenorphine (Hutchinson et al., 2014). Integrated treatment models addressing OUD, other active co-occurring substance use disorders, and psychiatric disorders may not only improve OUD treatment outcomes, but also improve psychiatric symptoms.
Treating Psychiatric Conditions
Treating psychiatric conditions like depression and anxiety is common in primary care, with treatment often driven by medication and connection to psychotherapy (Park & Zarate Jr, 2019). One approach that is increasingly being used to address psychiatric conditions in primary care is CoCM, which is an integrated model of behavioral health that enhances usual primary care (Bogner et al., 2016; Marcotte et al., 2021; Woltmann et al., 2012). Key aspects of CoCM include ongoing medication management and structured longitudinal follow-up assessments for patients receiving behavioral health treatment and regular psychiatric inter-specialty consultation. CoCM is typically delivered by three members of the primary care team: a behavioral health care manager, psychiatric consultant, and PCP (Marcotte et al., 2021). Using CoCM to treat depression and anxiety promotes remission, reduces symptoms (Bartels et al., 2004; Bogner et al., 2007; Bruce et al., 2004; Oslin et al., 2003; Zanjani et al., 2010), and reduces risk of all-cause mortality among older adults (Gallo et al., 2007). In 2018, the Centers for Medicare and Medicaid Services (CMS) implemented billing codes for CoCM services which may facilitate further uptake of the model (Carlo et al., 2020; Wolk, Alter, et al., 2021).
While CoCM is effective for management of mild to moderate psychiatric conditions in the general population, it has not yet been shown to be effective for treating individuals with co-occurring OUD and psychiatric conditions. Evidence suggests, however, that psychotherapy confers additional benefits for individuals on MOUD (Bickel et al., 2008; Christensen et al., 2014; Manhapra, et al., 2018; Miotto et al., 2012; Schottenfeld et al., 2005). Evidence also suggests that psychotherapy is the preferred treatment for psychiatric conditions among individuals with OUD (Bastien et al., 2021). The complex and inter-related needs of patients with OUD and psychiatric conditions suggest that psychotherapy is needed to comprehensively address the needs of these individuals (Kikkert et al., 2018). Unfortunately, however, models for effectively integrating this care, particularly in accessible contexts, are lacking.
Why Primary Care?
Primary care is among the most accessible healthcare settings. Most adults see a PCP at least once a year, and primary care is more affordable than other substance use disorder or psychiatric treatment options (Blackwell et al., 2014). Additionally, primary care practices are increasingly offering buprenorphine for treating OUD and medications and CoCM for addressing psychiatric conditions, which are effective strategies for treating each condition, respectively (Mojtabai et al., 2019; Wolk, Last, et al., 2021).
Accordingly, primary care settings are uniquely poised to address the needs of people with OUD and co-occurring psychiatric disorders in an integrated fashion, provided that appropriate clinical services are readily available in the setting. Yet few integrated models of care designed to treat both OUD and psychiatric disorders have been developed and rigorously tested in primary care (Lagisetty et al., 2017; Watkins et al., 2017), and the limited existing models fail to include a robust psychotherapy component.
CoCM for OUD and Psychiatric Conditions in Primary Care
Efforts have sought to adapt CoCM in primary care to address the needs of individuals with OUD and psychiatric conditions, but significant shortcomings exist. These efforts have sought to adapt CoCM to address OUD but have neglected to meaningfully address significant co-occurring psychiatric conditions like depression and anxiety. Instead, these trials have examined the added benefit of substance abuse counseling to medication in treating OUD, which generally has not been found to be more effective than medication alone (Fiellin et al., 2013; Ling et al., 2013; Moore et al., 2016; Watkins et al., 2017; Weiss et al., 2011). In all these studies, the psychotherapy was targeted at reducing opioid use, not at reducing symptoms of the psychiatric disorder(s).
Traditional CoCM for psychiatric conditions is not designed for individuals who also have OUD, with these models often lacking an addiction psychiatrist or care managers trained in therapy, both of which are beneficial for individuals with OUD and psychiatric conditions given their complexity (Volkow et al., 2019). When these models include psychiatrists or trained care managers, these professionals typically focus on addressing psychiatric conditions and not OUD or other currently active substance use disorders (Saldana et al., 2020). In general, literature on CoCM for OUD is lacking and there is almost no published data on CoCM for people with both OUD and psychiatric disorders. There is one clinical trial underway testing the use of collaborative care in primary care for patients with OUD and co-occurring depression and/or posttraumatic stress disorder (Meredith et al., 2021). An integrated program addressing OUD and psychiatric disorders may be more feasible for patients wishing to receive all care in one setting, has the potential to reduce opioid use and improve psychiatric symptoms, and may facilitate deployment in real world practices that often have limited resources and many competing demands.
While MOUD and CoCM are efficacious strategies for treating OUD and psychiatric disorders separately, adaptations to these models are needed to concurrently address these conditions which often exist in tandem. A model that integrates existing elements of evidence-based OUD and psychiatric treatment, separately, is a logical starting point. This approach would include initiating buprenorphine while concurrently leveraging CoCM care management, which is feasible given that both approaches already exist in primary care. The missing piece of these models, however, is the need for psychotherapy, a notable gap in existing models, designed to address the needs of individuals with OUD and psychiatric conditions.
Psychotherapy within CoCM
Recognizing the complex needs of individuals with OUD and co-occurring psychiatric conditions that are associated with a range of poor social and clinical outcomes, our team developed a treatment manual that integrates brief, evidence-based psychotherapies for substance use, depression, and anxiety (called CoCM+). This approach builds on how CoCM has been used to treat psychiatric disorders in primary care. CoCM is a well-suited foundation for this intervention given that CoCM is more developed than models for delivering MOUD (Katon et al., 1995) and some CoCMs already incorporate evidence-based OUD care by treating OUD with buprenorphine, though these models have not been tested at scale. We have tailored the model for people with both OUD and psychiatric disorders by embedding a novel psychotherapy component along with other traditional CoCM activities. This model therefore integrates two evidence-based treatments—MOUD and CoCM—by synergistically building on the strengths of each and adds to them evidence-based psychotherapies.
The novel CoCMT psychotherapy manual we developed is delivered by master’s level mental health clinicians working within the CoCM in partnership with primary care providers (PCPs) who are waivered to prescribe buprenorphine and a consulting addiction psychiatrist. The treatment manual includes brief Cognitive Behavioral Therapy (CBT) as well as Behavioral Activation (BA) components (Hedrick et al., 2003; McGregor et al., 2011), coupled with a motivational enhancement component. Our team developed this evidence-based psychotherapy manual to be tailored to the needs of patients with OUD and co-occurring psychiatric conditions like depression and anxiety. When selecting treatment models, we identified brief treatments that have demonstrated efficacy when implemented in primary care and mapped them to the most common presenting problems for our population of interest.
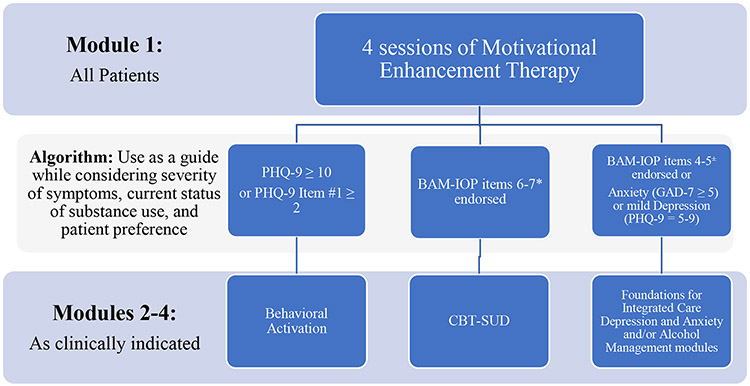
As part of CoCM+, patients receive up to 12 sessions of evidence-based psychotherapy (Miller, 1995) and case management with the care manager. The sessions can occur in person or via telehealth. The treatment manual is structured such that all patients begin with four sessions of Motivational Enhancement (MET) via Assessment with Feedback (Miller, 1995). In addition to assessment and feedback, MET sessions focus on developing and implementing a change plan. This assessment, feedback, and change planning process creates a foundation for subsequent treatment. Following the MET sessions, an algorithm (Figure 1) supports the therapist in determining next steps which may include brief CBT (Hofmann et al., 2012) or BA (Mazzucchelli et al., 2009). The algorithm utilizes scores from patient self-report measures of depression, anxiety, and concurrent substance use symptoms to suggest next steps for treatment. It is worth nothing that this algorithm should be used as a guide while considering severity of symptoms, current status of substance use, and patient preference. For patients with notable symptoms across multiple domains, the care manager in collaboration with the patient determine which domain to prioritize first, taking into consideration symptoms that are the most functionally impairing, and then may return to the algorithm to select additional modules when indicated. It is possible for patients to receive two or three modules when clinically indicated.
Figure 1.
Algorithm Guiding Selection of Psychotherapy Approach Used in Collaborative Care Model+ (CoCM+) Integrated Treatment for Comorbid Opioid Use Disorder and Psychiatric Conditions
Abbreviations: Patient Health Questionnaire (PHQ); Brief Addiction Monitor for Intensive Outpatient Programs (BAM-IOP); General Anxiety Disorder (GAD); Cognitive-Behavioral Therapy for Substance Use Disorders (CBT-SUD)
Key:
*BAM-IOP items 6-7 assess drug use in the past 30 days
±BAM-IOP items 4-5 assess alcohol use in the past 30 days
BA is recommended after MET for patients who endorse symptoms of moderate to severe depression/anhedonia on the Patient Health Questionnaire-9 (PHQ-9) (Kroenke et al., 2001). Brief CBT for substance use is recommended for patients who endorse continued substance use on the Brief Addiction Monitor-Intensive Outpatient Assessment (BAM-IOP) (Cacciola et al., 2013) and/or alcohol misuse on the BAM-IOP. During these sessions therapists complete a functional analysis and engage in problem solving and goal setting. Components of brief CBT, as outlined in the Foundations for Integrated Care program (Oslin et al.), is recommended for those reporting anxiety on the Generalized Anxiety Disorder 7-item Scale (Spitzer et al., 2006) or mild depression on the PHQ-9. The Foundations for Integrated Care is a set of open-access training manuals to aid in the integration behavioral health services into primary care (U.S. Department of Veterans Affairs) and includes brief CBT interventions for primary care patients with mild to moderate depression and anxiety (e.g., providing education about stressors like finances, physical factors like poor sleep, behavior like decreased productivity, and/or thoughts and feelings like hopelessness). The session outline is presented in Table 1. Standard CoCM practices are employed for case reviews, documentation, and billing (Carlo et al., 2020; Centers for Medicare & Medicaid Services, 2021; Press et al., 2017).
Table 1.
Session Outline
| Treatment Modules and Session Outline | |
|---|---|
| Session Topic | Session Description |
| Module 1 - Motivational Enhancement via Assessment with Feedback (All patients) | |
| Feedback | The patient and provider review the summary report of data from the first session. Feedback will be provided using MI’s Elicit-Provide-Elicit approach. |
| Change Planning | The provider and patient will discuss and complete the change plan. This discussion includes progress made and setbacks. The sessions also seek to evoke and affirm the patient’s motivations and commitment to make changes in substance use behaviors. |
| Change Planning | The provider and patient will discuss and complete the change plan. This discussion includes progress made and setbacks. The sessions also seek to evoke and affirm the patient’s motivations and commitment to make changes in substance use behaviors. |
| Finalize Change Plan | The provider and patient will identify and affirm progress. The change plan can be amended if data on anhedonia and/or substance use indicate the need for BA or CBT, respectively. |
| Module 2 - Behavioral Activation (Optional for patients with moderate to severe depression/anhedonia) | |
| Introduction to Behavioral Activation | The provider will introduce BA, provide psychoeducation about connection between activity and mood, will gain an understanding of patients’ goals and valued behaviors, and teach the patient how to monitor activities and mood. |
| Activation and Engagement * 3 sessions | The provider will continue to explore values and set activation goals. The provider and patient will work together to plan activities for the coming week and identify strategies to help with activation. |
| Module 3 – CBT-Substance Use Disorder (Optional for patients with continued substance use) | |
| Functional Analysis and Goal Setting | The provider will help the patient recognize connections between situations, thoughts/feelings, behavior, and results. The provider and patient will complete a functional analysis of the most recent episode and problem solve identified targets. |
| Progress Review and Goal Setting *3 sessions | The provider will review progress since the last session, review the risk assessment, identify high risk situations, and problem solve identified targets. |
| Module 4 - Brief CBT (Optional for patients with alcohol misuse, anxiety, or mild to moderate depression) | |
| Depression & Anxiety Management | Oslin DW, Klaus J, Ingram E, et al. Foundations for Integrated Care: Volume 2, Depression and Anxiety Management. https://www.mirecc.va.gov/cih-visn2/Documents/Provider_Education_Handouts/Vol_2_Depression_and_Anxiety_Managment.pdf Accessed January 9, 2023. |
| Alcohol Misuse | Oslin DW, Klaus J, Ingram E, et al. Foundations for Integrated Care: Volume 3, At-Risk Drinking and Alcohol Dependence Management. https://www.mirecc.va.gov/cih-visn2/foundations.asp. Accessed January 9, 2023. |
Abbreviations: motivational interviewing (MI); Behavioral Activation (BA); Cognitive Behavior Therapy (CBT)
Discussion
Novel, integrated treatments are needed to advance service delivery for individuals with OUD and psychiatric conditions in non-specialty mental health settings. We have developed a promising model and must now rigorously evaluate it. Similar models that address the same gaps in care should also be tested. Our team is testing CoCM+ as part of the Whole Health Study (WHS), a three-arm randomized controlled trial (Harris et al., 2021) in geographically diverse primary care practices affiliated with a single large health system in the mid-Atlantic region. The WHS has 3 arms; two of the arms test CoCM+, while one arm tests usual care that does not employ CoCM+. Both CoCM+ arms include a PCP waivered to prescribe buprenorphine, a mental health care manager with OUD training, and an addiction psychiatrist who the PCP can consult. One CoCM+ arm additionally includes a peer recovery specialist who leverage their lived experience with OUD to provide services including health education, recovery modeling, and crisis management (Harris et al., 2022). We anticipate that CoCM+ will yield promising outcomes for individuals with OUD and psychiatric conditions, based on the positive feedback we have received informally from PCPs and patients thus far and that we have successfully enrolled 149 patients in CoCM+, with 138 (93%) of these individuals eligible for follow-up. Rigorous effectiveness and implementation data collection are in progress. We also anticipate improvements in health services outcomes like lower rates of emergency department visits, hospital admissions, and mortality due to utilization of CoCM via primary care. We anticipate that this approach will improve outcomes for all patients but will have a disproportionately positive impact on patients of color who have historically been the most disenfranchised from treatment for OUD and co-occurring psychiatric conditions yet overrepresented in this population (McKnight-Eily et al., 2021; Novak et al., 2019).
In addition to testing integrated models of care such as the one our team has developed, more work is needed to move the field forward. We are pleased to see that one barrier to prescribing buprenorphine, the “X-waiver,” was recently eliminated. The lack of clinic infrastructure to provide MOUD still must be addressed (Jones & McCance-Katz, 2019; Olsen et al., 2021). There is also a need to increase the flexibility and amount of reimbursement for individuals with OUD and psychiatric conditions within the CoCM billing codes, such as by increasing the monthly maximum billable minutes for highly complex patients like those with comorbid OUD and psychiatric disorders and moving to a 30-day reimbursement cycle (Wolk, Alter, et al., 2021). Lastly, we must focus efforts on building the cadre of addiction psychiatrists and psychotherapists with dual expertise in addressing OUD and commonly co-occurring psychiatric conditions with evidence-based interventions given the dearth of clinicians equipped to provide this care and the ongoing, urgent need to address the opioid and overdose crisis (Olfson et al., 2020).
There is value in addressing OUD and psychiatric conditions in an integrated fashion. Doing so is compatible with CoCM, and we have found that it is possible to successfully implement our CoCM+ model. A therapeutic approach for this population must be flexible to allow for targeted treatment of the primary presenting concerns, whether those are related to substance use, depression, and/or anxiety. Our model uses an algorithm to guide treatment decisions and includes components of evidence-based treatments to best meet the complex and varied needs of individuals with OUD and psychiatric conditions and is delivered in primary care to promote accessibility. Given the considerable personal and public health impacts of OUD and co-occurring psychiatric disorders, integrated models like these are much needed and must be disseminated quickly.
Support and Role:
This study is funded through a grant from the National Institute of Mental Health to the University of Pennsylvania (UF1MH121944). The funder did not have any role in preparation of this manuscript.
Footnotes
Conflict of interest statement: None of the authors have any conflicts of interests to declare. The contents of this paper do not necessarily reflect the views of the Department of Veterans Affairs or the United State Government.
References
- American Psychiatric Association. (2020). Opioid Use Disorder. Retrieved from https://www.psychiatry.org/patients-families/addiction/opioid-use-disorder#:~:text=Effective%20treatments%20are%20available%2C%20however,use%20disorder%20receive%20specialty%20treatment.
- Anxiety & Depression Association of America. (2020). Anxiety and Depression.
- Barry DT, Cutter CJ, Beitel M, Kerns RD, Liong C, & Schottenfeld RS (2016). Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. The Journal of clinical psychiatry, 77(10), 13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels SJ, Coakley EH, Zubritsky C, Ware JH, Miles KM, Areán PA, … Costantino G (2004). Improving access to geriatric mental health services: a randomized trial comparing treatment engagement with integrated versus enhanced referral care for depression, anxiety, and at-risk alcohol use. American Journal of Psychiatry, 161(8), 1455–1462. [DOI] [PubMed] [Google Scholar]
- Bastien G, Del Grande C, Dyachenko A, Kaczorowski J, Pagé MG, Brissette S, … Jutras-Aswad D (2021). Preferences for research design and treatment of comorbid depression among patients with an opioid use disorder: A cross-sectional discrete choice experiment. Drug and Alcohol Dependence, 226, 108857. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA, Buchhalter AR, & Badger GJ (2008). Computerized behavior therapy for opioid-dependent outpatients: a randomized controlled trial. Experimental and clinical psychopharmacology, 16(2), 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell DL, Lucas JW, & Clarke TC (2014). Summary health statistics for US adults: national health interview survey, 2012. Vital and health statistics. Series 10, Data from the National Health Survey(260), 1–161. [PubMed] [Google Scholar]
- Blanco C, & Volkow ND (2019). Management of opioid use disorder in the USA: present status and future directions. The Lancet, 393(10182), 1760–1772. [DOI] [PubMed] [Google Scholar]
- Bogner HR, Joo JH, Hwang S, Morales KH, Bruce ML, Reynolds CF III, & Gallo JJ (2016). Does a depression management program decrease mortality in older adults with specific medical conditions in primary care? An exploratory analysis. Journal of the American Geriatrics Society, 64(1), 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogner HR, Morales KH, Post EP, & Bruce ML (2007). Diabetes, depression, and death: a randomized controlled trial of a depression treatment program for older adults based in primary care (PROSPECT). Diabetes care, 30(12), 3005–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce ML, Ten Have TR, Reynolds CF III, Katz II, Schulberg HC, Mulsant BH, … Alexopoulos GS (2004). Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. Jama, 291(9), 1081–1091. [DOI] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, DePhilippis D, Drapkin ML, Valadez C Jr, Fala NC, … McKay JR (2013). Development and initial evaluation of the Brief Addiction Monitor (BAM). Journal of Substance Abuse Treatment, 44(3), 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlo AD, Drake L, Ratzliff AD, Chang D, & Unützer J (2020). Sustaining the collaborative care model (CoCM): billing newly available CoCM CPT codes in an academic primary care system. Psychiatric Services, 71(9), 972–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services. (2021). Behavioral Health Integration Services. Retrieved from MLN Booklet: https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/BehavioralHealthIntegration.pdf [Google Scholar]
- Christensen DR, Landes RD, Jackson L, Marsch LA, Mancino MJ, Chopra MP, & Bickel WK (2014). Adding an Internet-delivered treatment to an efficacious treatment package for opioid dependence. Journal of Consulting and Clinical Psychology, 82(6), 964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad RC, Baum ML, Shah SB, Levy-Carrick NC, Biswas J, Schmelzer NA, & Silbersweig D (2020). Duties toward patients with psychiatric illness. Hastings Center Report, 50(3), 67–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erfan S, Hashim AH, Shaheen M, & Sabry N (2010). Effect of comorbid depression on substance use disorders. Substance abuse, 31(3), 162–169. [DOI] [PubMed] [Google Scholar]
- Feder KA, Mojtabai R, Krawczyk N, Young AS, Kealhofer M, Tormohlen KN, & Crum RM (2017). Trends in insurance coverage and treatment among persons with opioid use disorders following the Affordable Care Act. Drug and Alcohol Dependence, 179, 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, Barry DT, Sullivan LE, Cutter CJ, Moore BA, O'Connor PG, & Schottenfeld RS (2013). A randomized trial of cognitive behavioral therapy in primary care-based buprenorphine. The American journal of medicine, 126(1), 74. e11–74.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo JJ, Bogner HR, Morales KH, Post EP, Lin JY, & Bruce ML (2007). The effect of a primary care practice–based depression intervention on mortality in older adults: a randomized trial. Annals of internal medicine, 146(10), 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, … Kaplan K (2004). Prevalence and co-occurrence of substance use disorders and independentmood and anxiety disorders: Results from the national epidemiologic survey on alcohol and relatedconditions. Archives of general psychiatry, 61(8), 807–816. [DOI] [PubMed] [Google Scholar]
- Harris RA, Campbell K, Calderbank T, Dooley P, Aspero H, Maginnis J, … Bao Y (2022). Integrating peer support services into primary care-based OUD treatment: Lessons from the Penn integrated model. Paper presented at the Healthcare. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Mandell DS, Kampman KM, Bao Y, Campbell K, Cidav Z, … Lowenstein M (2021). Collaborative care in the treatment of opioid use disorder and mental health conditions in primary care: A clinical study protocol. Contemporary clinical trials, 103, 106325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AN, Howe AS, Samokhvalov AV, Le Foll B, & George TP (2017). Management of mood and anxiety disorders in patients receiving opioid agonist therapy: Review and meta-analysis. The American Journal on Addictions, 26(6), 551–563. [DOI] [PubMed] [Google Scholar]
- Hedrick SC, Chaney EF, Felker B, Liu CF, Hasenberg N, Heagerty P, … Paden G (2003). Effectiveness of collaborative care depression treatment in Veterans' Affairs primary care. Journal of General Internal Medicine, 18(1), 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, & Fang A (2012). The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cognitive therapy and research, 36(5), 427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker SA, Sherman MD, Lonergan-Cullum M, Sattler A, Liese BS, Justesen K, … Levy R (2020). Mental health and psychosocial needs of patients being treated for opioid use disorder in a primary care residency clinic. Journal of Primary Care & Community Health, 11, 2150132720932017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E, Catlin M, Andrilla CHA, Baldwin L-M, & Rosenblatt RA (2014). Barriers to primary care physicians prescribing buprenorphine. The Annals of Family Medicine, 12(2), 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, & McCance-Katz EF (2019). Characteristics and prescribing practices of clinicians recently waivered to prescribe buprenorphine for the treatment of opioid use disorder. Addiction, 114(3), 471–482. [DOI] [PubMed] [Google Scholar]
- Katon W, Von Korff M, Lin E, Walker E, Simon GE, Bush T, … Russo J (1995). Collaborative management to achieve treatment guidelines: impact on depression in primary care. Jama, 273(13), 1026–1031. [PubMed] [Google Scholar]
- Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, … Zaslavsky AM (2005). Prevalence and treatment of mental disorders, 1990 to 2003. New England Journal of Medicine, 352(24), 2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkert M, Goudriaan A, De Waal M, Peen J, & Dekker J (2018). Effectiveness of Integrated Dual Diagnosis Treatment (IDDT) in severe mental illness outpatients with a co-occurring substance use disorder. Journal of substance abuse treatment, 95, 35–42. [DOI] [PubMed] [Google Scholar]
- Kola LA, & Kruszynski R (2010). Adapting the integrated dual-disorder treatment model for addiction services. Alcoholism Treatment Quarterly, 28(4), 437–450. [Google Scholar]
- Korthuis PT, McCarty D, Weimer M, Bougatsos C, Blazina I, Zakher B, … Chou R (2017). Primary care–based models for the treatment of opioid use disorder: A scoping review. Annals of internal medicine, 166(4), 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagisetty P, Klasa K, Bush C, Heisler M, Chopra V, & Bohnert A (2017). Primary care models for treating opioid use disorders: what actually works? A systematic review. PloS one, 12(10), e0186315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai HMX, Cleary M, Sitharthan T, & Hunt GE (2015). Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: A systematic review and meta-analysis. Drug and Alcohol Dependence, 154, 1–13. [DOI] [PubMed] [Google Scholar]
- Liberman RP (2012). Recovery from schizophrenia: form follows functioning. World Psychiatry, 11(3), 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Hillhouse M, Ang A, Jenkins J, & Fahey J (2013). Comparison of behavioral treatment conditions in buprenorphine maintenance. Addiction, 108(10), 1788–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancher M, Leshner AI, National Academies of Sciences, E., & Medicine. (2019). Medications for Opioid Use Disorder in Various Treatment Settings. Medications for Opioid Use Disorder Save Lives. [PubMed] [Google Scholar]
- Manhapra A, Agbese E, Leslie DL, & Rosenheck RA (2018). Three-year retention in buprenorphine treatment for opioid use disorder among privately insured adults. Psychiatric Services, 69(7), 768–776. [DOI] [PubMed] [Google Scholar]
- Manlandro JJ (2005). Buprenorphine for office-based treatment of patients with opioid addiction. Journal of Osteopathic Medicine, 105(s63), 8–13. [PubMed] [Google Scholar]
- Marcotte LM, Reddy A, Zhou L, Razliff A, Unützer J, Chang D, & Liao JM (2021). Provision of Collaborative Care Model and General Behavioral Health Integration Services in Medicare. Psychiatric Services, 72(7), 822–825. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, & Davoli M (2014). Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews(2). [Google Scholar]
- Mazzucchelli T, Kane R, & Rees C (2009). Behavioral activation treatments for depression in adults: a meta-analysis and review. Clinical Psychology: Science and Practice, 16(4), 383–411. [Google Scholar]
- McGregor M, Lin EH, & Katon WJ (2011). TEAMcare: an integrated multi condition collaborative care program for chronic illnesses and depression. The Journal of ambulatory care management, 34(2), 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight-Eily LR, Okoro CA, Strine TW, Verlenden J, Hollis ND, Njai R, … & Thomas C (2021). Racial and ethnic disparities in the prevalence of stress and worry, mental health conditions, and increased substance use among adults during the COVID-19 pandemic—United States, April and May 2020. Morbidity and Mortality Weekly Report, 70(5), 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith LS, Komaromy MS, Cefalu M, Murray-Krezan C, Page K, Osilla KC, … & CLARO Study Group. (2021). Design of CLARO (Collaboration Leading to Addiction Treatment and Recovery from other Stresses): A randomized trial of collaborative care for opioid use disorder and co-occurring depression and/or posttraumatic stress disorder. Contemporary Clinical Trials, 104, 106354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR (1995). Motivational enhancement therapy with drug abusers: Citeseer. [Google Scholar]
- Miotto K, Hillhouse M, Donovick R, Cunningham-Rathner J, Charuvastra C, Torrington M, … Ling W (2012). Comparison of buprenorphine treatment for opioid dependence in three settings. Journal of addiction medicine, 6(1), 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojtabai R, Mauro C, Wall MM, Barry CL, & Olfson M (2019). Medication treatment for opioid use disorders in substance use treatment facilities. Health Affairs, 38(1), 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Cutter CJ, Buono FD, Barry DT, Fiellin LE, … Schottenfeld RS (2016). Cognitive behavioral therapy improves treatment outcomes for prescription opioid users in primary care buprenorphine treatment. Journal of substance abuse treatment, 71, 54–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences Engineering Medicine. (2018). Medication-Assisted Treatment for Opioid Use Disorder. [PubMed]
- Novak P, Feder KA, Ali MM, & Chen J (2019). Behavioral health treatment utilization among individuals with co-occurring opioid use disorder and mental illness: Evidence from a national survey. Journal of substance abuse treatment, 98, 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Zhang V, Schoenbaum M, & King M (2020). Buprenorphine Treatment By Primary Care Providers, Psychiatrists, Addiction Specialists, And Others: Trends in buprenorphine treatment by prescriber specialty-primary care providers, psychiatrists, and addiction medicine specialists. Health Affairs, 39(6), 984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen Y, Fitzgerald RM, & Wakeman SE (2021). Overcoming barriers to treatment of opioid use disorder. Jama, 325(12), 1149–1150. [DOI] [PubMed] [Google Scholar]
- Olsen Y, & Sharfstein JM (2014). Confronting the stigma of opioid use disorder—and its treatment. Jama, 311(14), 1393–1394. [DOI] [PubMed] [Google Scholar]
- Oslin D, Klaus J, Ingram E, DiFilippo S, Bedek K, Lantinga L, … Maisto S Foundations for Integrated Care: Volume 2, Depression and Anxiety Management. Retrieved from https://www.mirecc.va.gov/cih-visn2/Documents/Provider_Education_Handouts/Vol_2_Depression_and_Anxiety_Managment.pdf [Google Scholar]
- Oslin D, Sayers S, Ross J, Kane V, Ten Have T, Conigliaro J, & Cornelius J (2003). Disease management for depression and at-risk drinking via telephone in an older population of veterans. Psychosomatic medicine, 65(6), 931–937. [DOI] [PubMed] [Google Scholar]
- Park LT, & Zarate CA Jr (2019). Depression in the primary care setting. New England Journal of Medicine, 380(6), 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press MJ, Howe R, Schoenbaum M, Cavanaugh S, Marshall A, Baldwin L, & Conway PH (2017). Medicare payment for behavioral health integration, n Engl j Med, 376(5), 405–407. [DOI] [PubMed] [Google Scholar]
- Rössler W, & Drake R (2017). Psychiatric rehabilitation in Europe. Epidemiology and Psychiatric Sciences, 26(3), 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsaville BJ, Kosten TR, & Kleber HD (1986). Long-term changes in current psychiatric diagnoses of treated opiate addicts. Comprehensive Psychiatry, 27(5), 480–498. [DOI] [PubMed] [Google Scholar]
- Saldana L, Bennett I, Powers D, Vredevoogd M, Grover T, Schaper H, & Campbell M (2020). Scaling implementation of collaborative care for depression: adaptation of the stages of implementation completion (SIC). Administration and Policy in Mental Health and Mental Health Services Research, 47(2), 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottenfeld RS, Chawarski MC, Pakes JR, Pantalon MV, Carroll KM, & Kosten TR (2005). Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence. American Journal of Psychiatry, 162(2), 340–349. [DOI] [PubMed] [Google Scholar]
- Seth P, Scholl L, Rudd RA, & Bacon S (2018). Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. Morbidity and Mortality Weekly Report, 67(12), 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R, Kroenke K, & Williams J (2006). A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of Internal Medicine, 166, 1092–1097. [DOI] [PubMed] [Google Scholar]
- Teesson M, Marel C, Darke S, Ross J, Slade T, Burns L, … Mills KL (2015). Long-term mortality, remission, criminality and psychiatric comorbidity of heroin dependence: 11-year findings from the Australian Treatment Outcome Study. Addiction, 110(6), 986–993. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Veterans Affairs. Foundations for Integrated Care: Behavioral Health Solutions for Primary Care. Retrieved from https://www.mirecc.va.gov/cih-visn2/foundations.asp [Google Scholar]
- Volkow ND, Jones EB, Einstein EB, & Wargo EM (2019). Prevention and treatment of opioid misuse and addiction: a review. JAMA psychiatry, 76(2), 208–216. [DOI] [PubMed] [Google Scholar]
- Walsh SL, & Long KQ (2019). Deploying science to change hearts and minds: Responding to the opioid crisis. Preventive Medicine, 128, 105780. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Ober AJ, Lamp K, Lind M, Setodji C, Osilla KC, … Iyiewuare PO (2017). Collaborative care for opioid and alcohol use disorders in primary care: the SUMMIT randomized clinical trial. JAMA internal medicine, 177(10), 1480–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, … Haller DL (2011). Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Archives of general psychiatry, 68(12), 1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The White House (2022, December 30). Dr. Gupta Applauds Removal of X-Waiver in Omnibus, Urges Healthcare Providers to Treat Addiction. Retrieved from https://www.whitehouse.gov/ondcp/briefing-room/2022/12/30/dr-gupta-applauds-removal-of-x-waiver-in-omnibus-urges-healthcare-providers-to-treat-addiction/.
- Wolk CB, Alter CL, Kishton R, Rado J, Atlas JA, Press MJ, … Rosenthal LJ (2021). Improving Payment for Collaborative Mental Health Care in Primary Care. Medical care, 59(4), 324–326. [DOI] [PubMed] [Google Scholar]
- Wolk CB, Last BS, Livesey C, Oquendo MA, Press MJ, Mandell DS, … Oslin DW (2021). Addressing common challenges in the implementation of collaborative care for mental health: the Penn Integrated Care program. The Annals of Family Medicine, 19(2), 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltmann E, Grogan-Kaylor A, Perron B, Georges H, Kilbourne AM, & Bauer MS (2012). Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. American Journal of Psychiatry, 169(8), 790–804. [DOI] [PubMed] [Google Scholar]
- Zanjani F, Bush H, & Oslin D (2010). Telephone-based psychiatric referral-care management intervention health outcomes. Telemedicine and e-Health, 16(5), 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]