Abstract
Background
Nipple-sparing mastectomy is associated with a higher risk of mastectomy skin-flap necrosis than conventional skin-sparing mastectomy. There are limited prospective data examining modifiable intraoperative factors that contribute to skin-flap necrosis after nipple-sparing mastectomy.
Methods
Data on consecutive patients undergoing nipple-sparing mastectomy between April 2018 and December 2020 were recorded prospectively. Relevant intraoperative variables were documented by both breast and plastic surgeons at the time of surgery. The presence and extent of nipple and/or skin-flap necrosis was documented at the first postoperative visit. Necrosis treatment and outcome was documented at 8–10 weeks after surgery. The association of clinical and intraoperative variables with nipple and skin-flap necrosis was analysed, and significant variables were included in a multivariable logistic regression analysis with backward selection.
Results
Some 299 patients underwent 515 nipple-sparing mastectomies (54.8 per cent (282 of 515) prophylactic, 45.2 per cent therapeutic). Overall, 23.3 per cent of breasts (120 of 515) developed nipple or skin-flap necrosis; 45.8 per cent of these (55 of 120) had nipple necrosis only. Among 120 breasts with necrosis, 22.5 per cent had superficial, 60.8 per cent had partial, and 16.7 per cent had full-thickness necrosis. On multivariable logistic regression analysis, significant modifiable intraoperative predictors of necrosis included sacrificing the second intercostal perforator (P = 0.006), greater tissue expander fill volume (P < 0.001), and non-lateral inframammary fold incision placement (P = 0.003).
Conclusion
Modifiable intraoperative factors that may decrease the likelihood of necrosis after nipple-sparing mastectomy include incision placement in the lateral inframammary fold, preserving the second intercostal perforating vessel, and minimizing tissue expander fill volume.
This study evaluated the association between intraoperative technical and patient variables and the development of mastectomy skin-flap necrosis in a prospective cohort of patients undergoing nipple-sparing mastectomy. Modifiable intraoperative factors that may decrease the likelihood of necrosis after nipple-sparing mastectomy included incision placement in the inframammary fold, preservation of the second intercostal perforating vessels, and minimizing tissue expander fill volume.
Introduction
Nipple-sparing mastectomy (NSM) is increasingly being performed with the intent of obtaining superior cosmetic outcomes1–3, but it is associated with a higher risk of mastectomy skin-flap necrosis4. Patients who undergo NSM have a significantly higher risk of this complication compared with those having skin-sparing mastectomy (SSM), as shown in a previous study with prospectively collected data5. Although most cases of mastectomy skin-flap necrosis are classified as superficial and will heal uneventfully, some patients develop more serious complications. In the short term, these include wound infections requiring hospital admission or reoperation, and may ultimately result in implant loss. In the long term, skin-flap necrosis can cause skin contracture and asymmetry of the reconstructed breast. Few studies6,7 have prospectively examined predictors of skin-flap necrosis after NSM. Retrospective reports8,9 have consistently suggested that patient-related factors affect the risk, among these size and ptosis of the breasts, as well as previous radiation therapy. Reports on modifiable intraoperative variables, such as incision placement and dissection technique, are heterogeneous, making practice improvement difficult. Identification of predictors of necrosis allows implementation of strategies to mitigate the risk of complications. In the present study, intraoperative practices that have been suggested to contribute to mastectomy skin-flap necrosis in the literature were documented prospectively, and their association with the development of necrosis after NSM analysed.
Methods
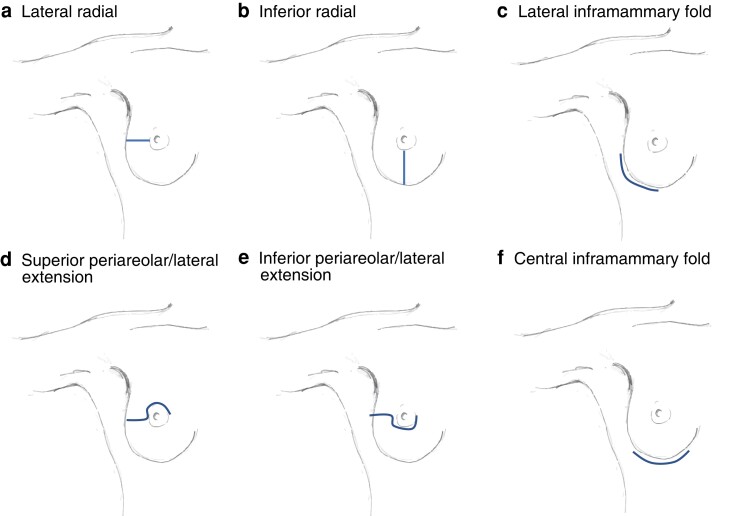
In 2018, the breast surgical oncology, and plastic and reconstructive services at Memorial Sloan Kettering Cancer Center (MSKCC) implemented a collaborative quality improvement initiative aiming to identify modifiable intraoperative risk factors for mastectomy skin-flap necrosis after NSM. A list of variables presumed to affect skin-flap necrosis was constructed based on published literature and clinical experience. This included dissection technique, use of tumescence solution for nipple–areolar complex or skin-flap dissection, blue dye injection technique, use of nipple-delay procedure, incision location and length (Fig. 1), use of separate axillary incisions, visualization and preservation of the second intercostal perforators, breast specimen weight, tissue expander position and saline fill volume, and assessment of nipple–areolar complex and overall skin perfusion at the end of the breast surgery portion of the procedure. The plastic surgeons also documented their assessment of skin perfusion at the beginning of their portion of the procedure as well as their assessment of the presence and location of areas of exposed dermis. At MSKCC, use of indocyanine green and laser-assisted fluorescence angiography (SPY) for assessment of mastectomy skin perfusion is at the discretion of the plastic surgeon. As the practice is variable across the plastic surgery service, it was not assessed in the present study.
Fig. 1.
Nipple-sparing mastectomy incision placement
a Lateral radial, b inferior radial, c lateral inframammary fold, d superior periareolar/lateral extension, e inferior periareolar/lateral extension, and f central inframammary fold. Reproduced with permission from N. Kinoti-Metz (Studio Parallel, Brooklyn, NY, USA).
The collaborative group also developed postoperative forms to capture the presence and extent of skin-flap necrosis (including necrosis of the nipple–areolar complex) occurring by the first postoperative visit approximately 2 weeks after surgery. The severity of skin-flap necrosis was assessed using elements of the Skin Ischaemia and Necrosis (SKIN) score, a validated tool developed by Lemaine et al.4. The SKIN score captures the depth as well as surface area of mastectomy skin-flap necrosis. SKIN score is based on the following observations: category A, no evidence of necrosis; category B, colour change of skin flap suggesting impaired perfusion or ischaemic injury; category C, partial-thickness skin-flap necrosis resulting in at least epidermal sloughing; and category D, full-thickness necrosis. Each category also includes an assessment of extent of necrosis ranging from 0, 1–10, 11–30, and over 30 per cent of the nipple–areolar complex or skin surface area.
This study was approved by the MSKCC Institutional Review Board. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consecutive prospectively identified patients who underwent NSM for both prophylactic and therapeutic indications between April 2018 and December 2020 were included. Both breast and plastic surgery teams completed intraoperative forms on the day of surgery. The plastic surgery team completed the postoperative form documenting extent and degree of skin-flap necrosis at the first postoperative visit, as well as treatment and outcomes of necrosis at the 8–10-week postoperative visit. Preoperative patient factors were collected by chart review.
Analyses were carried out on a per-breast or per-patient basis depending on the variable. Patient characteristics were summarized using frequency and percentage for categorical variables. Clinical and intraoperative variables associated with skin-flap necrosis were identified by univariate analysis, using χ2 test or Fisher's test for categorical variables, and the Wilcoxon rank sum test for continuous variables. Intraoperative variables significant at the type I error rate of 0.05 were then included in a multivariable logistic regression analysis, with the final model chosen by backward selection. Statistical analysis was conducted using R 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
During the study interval, 299 patients underwent 515 NSM procedures (Table 1). Of these, 54.8 per cent (282 of 515) were prophylactic and 45.2 per cent were therapeutic procedures. Median patient age was 43 (range 22–73) years, and median BMI was 22.3 (i.q.r. 20.6–24.7) kg/m2. Some 98.0 per cent of patients were non-smokers, 26.4 per cent had a history of breast surgery, and 3.3 per cent had undergone radiation therapy for breast cancer. The majority (85.4 per cent) underwent tissue expander reconstruction, 5.2 per cent of reconstructions were direct to implant, and 8.3 per cent underwent autologous flap reconstruction. Fifteen breast surgeons and 10 plastic surgeons participated in the study.
Table 1.
Clinical characteristics of patients with and without mastectomy skin-flap necrosis after nipple-sparing mastectomy
| All patients (n = 299) |
Necrosis absent (n = 228) |
Necrosis present (n = 71) |
P† | |
|---|---|---|---|---|
| Age (years), median (range) | 43 (22–73) | 43 (22–3) | 42 (22–68) | 0.800‡ |
| BMI (kg/m2) | 0.130 | |||
| < 25 | 234 (78.3) | 183 (80.3) | 51 (72) | |
| ≥ 25 | 65 (21.7) | 45 (19.7) | 20 (28) | |
| Smoking status | 0.600 | |||
| Non-smoker | 293 (98.0) | 224 (97.8) | 69 (97) | |
| Smoker | 6 (2.0) | 4 (2.2) | 2 (3) | |
| Hypertension | 14 (4.7) | 6 (2.6) | 8 (11) | 0.006 |
| Diabetes | 2 (0.7) | 2 (0.9) | 0 (0) | > 0.900 |
| Steroid use | 42 (14.0) | 36 (15.8) | 6 (8) | 0.120 |
| Previous radiation for breast cancer | 10 (3.3) | 5 (2.2) | 5 (7) | 0.060 |
| Previous breast surgery | 79 (26.4) | 64 (28.1) | 14 (20) | 0.140 |
| Previous cosmetic breast surgery* | 22 (7.4) | 16 (7.0) | 6 (8) | 0.700 |
| Neoadjuvant chemotherapy | 51 (17.1) | 39 (17.1) | 12 (17) | > 0.900 |
| Ptosis | 0.300 | |||
| None | 38 (14.8) | 30 (15.3) | 8 (13) | |
| Grade 1 | 98 (38.1) | 79 (40.3) | 19 (31) | |
| Grade 2 | 106 (41.2) | 78 (39.8) | 28 (46) | |
| Grade 3 | 15 (5.9) | 9 (4.6) | 6 (10) | |
| Unknown | 42 | 32 | 10 |
Values are n (%) unless otherwise indicated. *A total of 21 augmentations and one reduction. †χ2 test or Fisher's exact test, except ‡Wilcoxon rank sum test; unknowns were not included in calculation.
By 2 weeks after operation, 23.3 per cent of breasts (120 of 515) had developed mastectomy skin-flap necrosis, of which 45.8 per cent (55 of 120) had nipple necrosis only. Among breasts with necrosis, 27 of 120 (22.5 per cent) were classified as having SKIN score category B (superficial necrosis), 73 (60.8 per cent) as having SKIN score category C (partial necrosis), and 20 (16.7 per cent) as having SKIN score category D (full-thickness necrosis) (Table 2). The extent of necrosis was variable; most cases of category B necrosis involved 1–10 per cent of the surface area of the mastectomy skin flap. Although the majority healed without intervention, 10.0 per cent (12 of 120) required debridement in the operating room, 6.7 per cent (8 of 120) experienced nipple loss, and 7.5 per cent (9 of 120) resulted in implant loss that occurred between 1 and 22 weeks after surgery (Table 3). Among the nine cases of implant loss, eight were attributed to wound-healing complications resulting from skin-flap necrosis. By 8–10 weeks, 84.2 per cent of breasts with necrosis (101 of 120) had healed completely; one patient with bilateral superficial necrosis was lost to follow-up. Thirteen breasts with the most severe category of necrosis, SKIN score category D4, were examined further. Clinical and intraoperative variables are shown in Supplementary Table S1. Compared with other categories of necrosis, breasts with D4 necrosis showed higher rates of in-office and operating room debridement, infection, and implant loss. At 8 weeks, only 46 per cent of breasts with D4 necrosis had healed completely (Table 3).
Table 2.
Severity of necrosis based on SKIN score
| SKIN score* | Breasts with necrosis (n = 120) |
|---|---|
| B1 | 1 (0.8) |
| B2 | 23 (19.2) |
| B3 | 3 (2.5) |
| C2 | 50 (41.7) |
| C3 | 17 (14.2) |
| C4 | 6 (5.0) |
| D2 | 5 (4.2) |
| D3 | 2 (1.7) |
| D4 | 13 (10.8) |
Values are n (%). *Highest score for necrosis involving skin flap or nipple–areolar complex. Skin Ischaemia and Necrosis (SKIN) score: A, no evidence of necrosis; B, colour change of skin flap suggesting impaired perfusion or ischaemic injury; C, partial-thickness necrosis resulting in at least epidermal sloughing; D, full-thickness necrosis. Extent of necrosis (% surface area covered): 1, < 1%; 2, 1–10%; 3, 11–30%; 4, > 30%.
Table 3.
Mastectomy skin-flap necrosis management
| All breasts with necrosis (n = 120) | SKIN score B1–D3 (n = 107) | SKIN score D4 (n = 13) | |
|---|---|---|---|
| Topical agents (bacitracin, betadine, Xeroform®) | 25 (20.8) | 22 (20.6) | 3 (23) |
| Hyperbaric oxygen | 33 (27.5) | 29 (27.1) | 4 (31) |
| Infection | 17 (14.2) | 12 (11.2) | 5 (38) |
| Antibiotics only | 10 (8.3) | 8 (7.5) | 2 (15) |
| Debridement and antibiotics | 6 (5.0) | 3 (2.8) | 3 (23) |
| Debridement in office | 9 (7.5) | 5 (4.7) | 4 (31) |
| Debridement in operating room | 12 (10.0) | 6 (5.6) | 6 (46) |
| Implant loss | 9 (7.5) | 6 (5.6) | 3 (23) |
| Nipple loss | 8 (6.7) | 5 (4.7) | 3 (23) |
| Nipple depigmentation | 3 (2.5) | 3 (2.8) | 0 |
| Necrosis completely healed at 8 weeks after surgery | 101 (84.2) | 94 (87.9) | 6 (46) |
Values are n (%). Xeroform® (Cardinal Health, Dublin, OH, USA).
Variables associated with the development of skin-flap necrosis were examined. In univariate analysis, intraoperative variables associated with a higher likelihood of necrosis included non-lateral inframammary fold placement of the incision (P < 0.001), having a nipple-delay procedure (P = 0.026), sacrificing (versus sparing or not visualizing) the second intercostal perforator (P = 0.038), specimen weight over 400 g (P < 0.001), erythematous appearance of skin flaps at start of plastic surgery procedure (P = 0.030), increasing tissue expander fill volume (P < 0.001), subpectoral tissue expander placement (P = 0.01), number of procedures performed by surgeon (P = 0.006), and exposed dermis in the upper or lower outer quadrant of the skin flap (P < 0.05).
In multivariable regression analysis, significant intraoperative variables that predicted necrosis included nipple-delay procedure (P = 0.033), sacrificing the second intercostal perforator (versus not visualizing or sparing it) (P = 0.006), larger tissue expander fill volume (P < 0.001), erythematous appearance of skin flaps at start of plastic surgery procedure (P = 0.007), exposed dermis in the lower outer quadrant (P = 0.005), and non-lateral inframammary fold incision placement (P = 0.003) (Table 4).
Table 4.
Intraoperative variables associated with nipple and skin-flap necrosis after nipple-sparing mastectomy
| Overall (n = 515) |
Necrosis absent (n = 395) |
Necrosis present (n = 120) |
Univariable P† |
Multivariable regression analysis | ||
|---|---|---|---|---|---|---|
| OR* | P | |||||
| Nipple delay performed# | 11 (2.2) | 5 (1.3) | 6 (5.0) | 0.026 | 9.03 (1.28, 87.7) | 0.033 |
| Unknown | 11 | 11 | 0 | |||
| Nipple tumescence | 287 (57.2) | 225 (58.9) | 62 (51.7) | 0.200 | ||
| Unknown | 13 | 13 | 0 | |||
| Skin-flap tumescence | 8 (1.6) | 6 (1.6) | 2 (1.7) | > 0.900 | ||
| Unknown | 11 | 11 | 0 | |||
| Skin-flap dissection technique | > 0.900 | |||||
| Electrocautery | 487 (96.6) | 371 (96.6) | 116 (96.7) | |||
| Sharp | 17 (3.4) | 13 (3.4) | 4 (3.3) | |||
| Unknown | 11 | 11 | 0 | |||
| Nipple–areolar complex dissection technique | 0.800 | |||||
| Electrocautery | 113 (23.1) | 85 (22.8) | 28 (23.7) | |||
| Sharp | 377 (76.9) | 287 (77.2) | 90 (76.3) | |||
| Unknown | 25 | 23 | 2 | |||
| Blue dye injection | 0.140 | |||||
| Intraparenchymal | 213 (44.3) | 166 (46.0) | 47 (39.2) | |||
| Superficial subareaolar | 61 (12.7) | 40 (11.1) | 21 (17.5) | |||
| Not injected | 207 (43.0) | 155 (42.9) | 52 (43.3) | |||
| Unknown | 34 | 34 | 0 | |||
| Incision location | < 0.001 | |||||
| Lateral radial | 94 (19.9) | 66 (18.3) | 28 (25.0) | 1.00 (reference) | ||
| Inferior radial | 13 (2.7) | 5 (1.4) | 8 (7.1) | 0.56 (0.03, 5.16) | 0.600 | |
| Lateral IMF | 206 (43.6) | 181 (50.1) | 25 (22.3) | 0.35 (0.17, 0.7) | 0.003 | |
| Superior periareolar/lateral extension | 51 (10.8) | 30 (8.2) | 21 (18.8) | 1.59 (0.65, 3.92) | 0.300 | |
| Inferior periareolar/lateral extension | 51 (10.8) | 32 (8.9) | 19 (17.0) | 1.24 (0.52, 2.93) | 0.600 | |
| Central IMF | 58 (12.3) | 47 (13.0) | 11 (9.8) | 0.54 (0.19, 1.39) | 0.200 | |
| Unknown | 42 | 34 | 8 | |||
| Incision length (cm), median (i.q.r.) | 10 (8–12) | 10 (8–12) | 10 (8–12) | 0.800‡ | ||
| Unknown | 7 | 7 | 0 | |||
| Axillary incision for SLNB | 123 (26.1) | 98 (27.1) | 25 (22.7) | 0.400 | ||
| Unknown | 43 | 33 | 10 | |||
| Second intercostal perforator | 0.04 | |||||
| Not visualized | 215 (44.1) | 168 (45.0) | 47 (40.9) | 1.00 (reference) | ||
| Preserved | 233 (47.7) | 181 (48.5) | 52 (45.2) | 1.73 (0.99, 3.08) | 0.059 | |
| Sacrificed | 40 (8.2) | 24 (6.4) | 16 (13.9) | 3.57 (1.44, 8.88) | 0.006 | |
| Unknown | 27 | 22 | 5 | |||
| Specimen weight (g) | < 0.001 | |||||
| < 300 | 227 (48.2) | 181 (50.1) | 46 (41.8) | |||
| 300–400 | 116 (24.6) | 97 (26.9) | 19 (17.3) | |||
| > 400 | 128 (27.2) | 83 (23.0) | 45 (40.9) | |||
| Unknown | 44 | 34 | 10 | |||
| Skin-flap perfusion at start of plastic surgery procedure | 0.030 | |||||
| Erythematous | 34 (7.6) | 19 (5.7) | 15 (13.2) | 1.00 (reference) | ||
| Slightly dusky | 22 (4.9) | 16 (4.8) | 6 (5.3) | 0.31 (0.07, 1.21) | 0.100 | |
| Good | 394 (87.6) | 301 (89.6) | 93 (81.6) | 0.31 (0.13, 0.73) | 0.007 | |
| Unknown | 65 | 59 | 6 | |||
| Reconstruction type | 0.056 | |||||
| Autologous flap | 43 (8.4) | 27 (6.9) | 16 (13.3) | |||
| Direct implant | 27 (5.3) | 23 (5.9) | 4 (3.3) | |||
| Tissue expander | 440 (86.3) | 340 (87.2) | 100 (83.3) | |||
| Unknown | 5 | 5 | 0 | |||
| Expander fill volume (ml), median (i.q.r.) | 200 (150–300) | 200 (150–250) | 240 (180–300) | < 0.001‡ | ||
| Expander fill volume (ml) | < 0.001 | |||||
| ≤ 100 | 63 (13.4) | 52 (14.2) | 11 (10.6) | 1.00 (reference) | ||
| 101–200 | 175 (37.2) | 151 (41.1) | 24 (23.1) | 1.23 (0.46, 3.69) | 0.700 | |
| 201–300 | 189 (40.1) | 130 (35.4) | 59 (56.7) | 4.23 (1.69, 12.1) | 0.004 | |
| > 300 | 44 (9.3) | 34 (9.3) | 10 (9.6) | 1.97 (0.55, 7.18) | 0.300 | |
| Unknown | 44 | 28 | 16 | |||
| Expander location | 0.010 | |||||
| Prepectoral | 215 (48.4) | 177 (51.8) | 38 (37.3) | |||
| Subpectoral | 229 (51.6) | 165 (48.2) | 64 (62.7) | |||
| Axillary procedure | 0.900 | |||||
| None | 171 (33.2) | 129 (32.7) | 42 (35.0) | |||
| SLNB | 293 (56.9) | 226 (57.2) | 67 (55.8) | |||
| SLNB/ALND | 47 (9.1) | 36 (9.1) | 11 (9.2) | |||
| ALND | 4 (0.8) | 4 (1.0) | 0 | |||
| Duration of operation (min), median (i.q.r.) | 189 (162–220) | 188 (165–212) | 198 (158–252) | 0.070 | ||
| Breast surgeon experience (no. of procedures performed)** | 0.006 | |||||
| ≤ 25 | 94 (18.3) | 61 (15.4) | 33 (28.5) | |||
| 26–48 | 117 (22.7) | 97 (24.6) | 20 (16.7) | |||
| 49–120 | 304 (59.0) | 237 (60.0) | 67 (55.8) | |||
| Exposed dermis present | 99 (22.0) | 72 (21.4) | 27 (23.5) | 0.600 | ||
| Unknown | 64 | 59 | 5 | |||
| Location exposed dermis | ||||||
| UIQ | 6 (1.2) | 3 (0.8) | 3 (2.5) | 0.200 | ||
| LIQ | 16 (3.1) | 13 (3.3) | 3 (2.5) | 0.800 | ||
| UOQ | 19 (3.7) | 10 (2.5) | 9 (7.5) | 0.033 | ||
| LOQ | 18 (3.5) | 9 (2.3) | 9 (7.5) | 0.024 | 5.76 (1.75, 20.3) | 0.005** |
| Central | 49 (9.5) | 42 (10.6) | 7 (5.8) | 0.056 | ||
Values are n (%) unless otherwise indicated; *values in parentheses are 95% confidence intervals. Clinical variables are not shown; #analysis limited by small number of patients undergoing nipple delay; **location exposed dermis LOQ was used as binary variable in MVA. IMF, intramammary fold; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; UIQ, upper inner quadrant; LIQ, lower inner quadrant; UOQ, upper outer quadrant; LOQ, lower outer quadrant. †χ2 test or Fisher's exact test, except ‡Wilcoxon rank sum test.
The relationship between incision placement and incision length was examined further, with the hypothesis that incisions placed in the inframammary fold would be wider. It was found that, compared with radial or periareolar incisions, inframammary fold incisions had a median length of 11 cm compared with 8 cm for those in non-inframammary fold positions (P < 0.001). As breasts with necrosis had significantly greater fill volumes than those without necrosis (median 240 (i.q.r. 180–300) versus 200 (150–250 ml; P < 0.001), an analysis of tissue expander fill volume was also conducted. Tissue expander fill volume was categorized based on a 100-ml incremental increase. Breasts with a fill volume of 201–300 ml had four times the odds of skin-flap necrosis compared with those with 100 ml or less (OR 4.23, 95 per cent c.i. 1.69 to 12.1; P = 0.004).
Discussion
The rate of mastectomy skin-flap necrosis was 23.3 per cent after NSM in this prospective study, with 45.8 per cent of instances being nipple-only necrosis. In 83.3 per cent of patients, the necrosis was classified as superficial or partial thickness, and few cases resulted in reoperation or nipple loss (10.0 and 6.7 per cent respectively). Implant loss occurred in nine breasts, eight of which had experienced skin-flap necrosis. Modifiable intraoperative factors significantly associated with nipple and skin-flap necrosis were non-lateral inframammary fold incisions, sacrificing the second intercostal perforator vessels, and large tissue expander fill volumes.
As the use of NSM has increased1,2, particular attention has been paid to ischaemic complications at the nipple–areolar complex. Necrosis occurring elsewhere on the mastectomy flap may, however, also cause wound-healing complications, scarring, and eventual distortion of the reconstructed breast. These complications adversely affect cosmetic outcomes and patient satisfaction10. An even more concerning result is the progression to infection or exposure of the underlying implant, which can lead to reoperations and may delay adjuvant therapy. In this study, the overall rate of mastectomy skin-flap necrosis was 23.3 per cent after NSM. This is higher than reported for SSM5, and also higher than the rates of 9–13 per cent reported in previous retrospective studies9,11–13 of skin-flap necrosis after NSM. This difference in reported outcomes may be explained by variation in mastectomy technique and skin-flap thickness, but also by the differences inherent to prospective versus retrospective data collection. Among published retrospective reports, there is no consistent definition of skin-flap necrosis, and documentation is also variable, particularly in cases of superficial necrosis that heal without intervention. These limitations make accurate comparison across studies challenging. Two other studies have examined nipple and skin-flap necrosis in prospective NSM cohorts, and also reported higher rates of mastectomy skin-flap necrosis. A prospective study by Odom et al.14, which examined skin perfusion patterns and complications in 79 patients who underwent NSM, reported necrosis complications in 26 per cent of patients, in line with the present findings. The French multi-institutional prospective MAPAM trial7 included 59 women who underwent NSM, and reported nipple–areolar complex necrosis in nine patients, a rate of 15 per cent. Necrosis elsewhere in the breast was not reported and would presumably increase this rate. Given these relatively high rates of necrosis compared with those for SSM, it is important to identify and implement strategies to mitigate this complication.
Three technical aspects of the NSM procedure were identified that can be modified to reduce the risk of nipple and skin-flap necrosis: incision placement in the lateral inframammary fold, sparing the second intercostal perforator, and limiting tissue expander fill volume. The present findings also provide insight into incision location and length, and its impact on nipple and skin-flap necrosis. Patients with incisions placed in the lateral inframammary region of the breast (Fig. 1f) had significantly lower rates of skin-flap necrosis. The inframammary fold position also correlated with longer incisions, with a median length of 11 cm compared with 8 cm in non-inframammary fold placement (P < 0.001). This may explain the association with lower rates of flap necrosis, as there is likely to be less tension placed on the skin flap during dissection through a longer incision owing to improved exposure, particularly as the dissection approaches the boundaries of the breast. Although the literature on this topic is heterogeneous, the findings of this study are consistent with a number of previously published retrospective studies9,15,16 showing that incision placement in the inframammary fold is associated with a lower risk of skin necrosis. In a retrospective evaluation of 500 breasts after NSM, Colwell et al.9 found that inferolateral inframammary fold placement of the incision was significantly associated with a lower risk of complications, including the nipple–areolar complex and skin-flap necrosis. Other studies examining isolated necrosis of the nipple–areolar complex reported a similar association with the inframammary fold approach, with a decreased risk of necrosis. When thinking in terms of necrosis limited to the nipple–areolar complex, there is further support for inframammary fold incision placement. Daar and colleagues16 undertook a meta-analysis examining incision placement and its association with necrosis at the nipple–areolar complex; among 4645 NSMs, periareolar incisions were associated with a higher rate of nipple–areolar complex necrosis than inframammary fold incision placement. Carlson et al.17 examined risk factors for nipple ischaemia after NSM in 71 consecutive procedures, and found that partial nipple necrosis occurred in 28 per cent and was associated with periareolar incisions (OR 9.69, 95 per cent c.i. 1.57 to 59.77; P = 0.014). These results endorse consideration of the inframammary fold approach when feasible from the perspective of both the breast surgical oncologist and plastic surgeon.
The impact of preservation of the second intercostal perforator on incidence of skin-flap necrosis after NSM is unclear. The breast is perfused by arteries originating from the lateral thoracic and internal mammary vessels. The dominant blood supply to the breast comes from the internal mammary perforating vessels in 68–74 per cent of breasts18. Based on cadaveric and chest wall perfusion studies, there is evidence supporting the concept of a principal perforating vessel usually located in the second or third intercostal space18. This vessel courses obliquely toward the nipple–areolar complex, where it joins radiating tributaries of the lateral thoracic artery around the nipple. In the context of NSM, active preservation of this vessel to reduce nipple–areolar complex necrosis has been suggested19. For the purposes of the present study, this dominant vessel was referred to as the ‘second intercostal perforator’. There was a significantly lower rate of nipple and skin-flap necrosis in the breasts when the second intercostal perforator was visualized and spared, or not visualized, than when this vessel was sacrificed. This finding also highlights the importance of preservation of the subcutaneous blood supply during mastectomy flap dissection.
Intuitively, greater tissue expander fill volume contributes directly to pressure on mastectomy skin flaps and affects flap perfusion. Odom et al.14 examined perfusion of the mastectomy flap before and after placement of tissue expanders or implants after NSM, and found a decrease in perfusion by 36 per cent compared with before prosthesis placement, indicating that expander fill volume can immediately affect skin perfusion and likely the development of necrosis.14 Here, higher rates of nipple and skin-flap necrosis were observed in breasts with larger tissue expander fill volumes. Breasts with a fill volume of 201–300 ml had four times the odds of skin-flap necrosis than those with a fill volume below 100 ml (P = 0.004). In reality, the decision regarding how much to fill expanders initially, or whether to place immediate implants, is multifactorial and should take into consideration mastectomy skin-flap perfusion and nipple position. In this study, larger fill volumes also correlated with larger mastectomy weights in univariate analysis. In these instances, the skin envelope usually requires a greater fill volume to appropriately position the nipple. If the fill is low and the nipple is malpositioned, subsequent revision can be challenging. Plastic surgeons at MSKCC carefully assess the fill during NSM, balancing fill volume with risk of necrosis, and appropriate nipple position.
This study found that clinical assessment of skin-flap perfusion by the plastic surgeon at the start of the reconstructive procedure was associated with the risk of developing necrosis. Rates of necrosis were significantly lower in patients with good versus erythematous flaps, suggesting that the visual impression of flap perfusion is likely to provide a reliable estimation of flap perfusion, which should be considered when determining the tissue expander fill volume. Whether this assessment is improved further by use of intraoperative SPY was not captured in this data set owing to significant variability in its use.
Nipple delay is thought to increase the blood supply to the nipple–areolar complex; however, in a meta-analysis20 including 101 patients undergoing nipple delay, rates of necrosis in the nipple–areolar complex or mastectomy skin flap ranged from 0 to 16 per cent, comparable to those reported in the literature for procedures in which no nipple delay was performed. There were very few nipple-delay procedures in the present series (2 per cent) and, although breasts undergoing the nipple-delay procedure were significantly more likely to experience skin-flap necrosis, these results are likely biased, as the procedure would have been done in higher-risk patients. Given the small numbers, meaningful conclusions regarding its efficacy cannot be drawn.
Several parameters thought to affect mastectomy skin-flap necrosis were not found to be significant in this study. Rates of necrosis did not differ significantly based on injection of blue dye in the subareolar space or intraparenchymally. Similarly, use of tumescence solution, sharp dissection versus use of electrocautery, or use of a separate axillary incision for the sentinel lymph node biopsy procedure were not significant factors contributing to skin-flap necrosis. Breast surgeon volume was also examined, and a small but not statistically significantly lower risk of skin necrosis for surgeons with larger NSM volumes was found in the multivariable analysis.
Strengths of this study include its large size, prospective data collection, and use of a validated tool to document the presence and extent of necrosis. Currently, comparison of necrosis rates in the literature is difficult because of a lack of standardized definitions of skin necrosis. Use of the SKIN score should allow more accurate comparisons of severity of necrosis in future studies. A limitation of this study is that NSM was performed by a number of different breast surgeons (15) and plastic surgeons (10) with variable procedure volumes. All participants, however, undertake a high volume of breast surgery and breast reconstruction respectively, and these results may not reflect the outcomes of lower-volume surgeons. Additionally, analyses of some variables found to be significant, such as hypertension, exposed dermis location, and nipple delay, were based on small numbers of patients, limiting conclusions regarding the impact on necrosis.
In a prospective cohort of patients having NSM with comprehensive documentation of intraoperative technique, 23.3 per cent of breasts had mastectomy skin-flap necrosis, with approximately half of cases being nipple-only necrosis. Modifiable intraoperative factors that decreased the likelihood of necrosis included incision placement in the lateral inframammary fold, preservation of the second intercostal perforating vessels, and minimizing tissue expander fill volume.
Supplementary Material
Acknowledgements
T.-A.M. and J.A.N. are joint first authors of this article. The study research has not previously been preregistered in an independent, institutional registry.
Contributor Information
Tracy-Ann Moo, Breast Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Jonas A Nelson, Plastic and Reconstructive Surgical Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Varadan Sevilimedu, Biostatistics Service, Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Jillian Charyn, Breast Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Tiana V Le, Breast Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Robert J Allen, Jr, Plastic and Reconstructive Surgical Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Babak J Mehrara, Plastic and Reconstructive Surgical Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Andrea V Barrio, Breast Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Deborah M Capko, Breast Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Melissa Pilewskie, Breast Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Department of Surgery, University of Michigan, Ann Arbor, Michigan, USA.
Alexandra S Heerdt, Breast Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Audree B Tadros, Breast Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Mary L Gemignani, Breast Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Monica Morrow, Breast Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Virgilio Sacchini, Breast Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Funding
The preparation of this study was funded in part by National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748 to MSKCC.
Author contributions
Tracy-Ann Moo (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), Virgilio Sacchini (CRediT contribution not specified), Jonas Nelson (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), Varadan Sevilimedu (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing—original draft, Writing—review & editing), Jillian Charyn (Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing—original draft, Writing—review & editing), Tiana Le (Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing—original draft, Writing—review & editing), Robert Allen (Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Visualization, Writing—original draft, Writing—review & editing), B Mehrara (Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Visualization, Writing—original draft, Writing—review & editing), Andrea V. Barrio (Conceptualization, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing—original draft, Writing—review & editing), Deborah Capko (Conceptualization, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing—original draft, Writing—review & editing), Melissa Pilewskie (Conceptualization, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing—original draft, Writing—review & editing), Alexandra Heerdt (Conceptualization, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing—original draft, Writing—review & editing), Audree Tadros (Conceptualization, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing—original draft, Writing—review & editing), Mary Gemignani (Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Visualization, Writing—original draft, Writing—review & editing), Monica Morrow (Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing—original draft, Writing—review & editing), and V. Sacchini (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing).
Disclosure
B.J.M. reports receipt of an investigator-initiated grant from Pfizer, Regeneron, and Puretech, receipt of royalty payments from Puretech, and an advisory position with Mediflix. All other authors declare no other conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
The data and methods used in the analysis, and materials used to conduct the research, will be made available upon request to the authors.
References
- 1. Valero MG, Muhsen S, Moo TA, Zabor EC, Stempel M, Pusic A et al. Increase in utilization of nipple-sparing mastectomy for breast cancer: indications, complications, and oncologic outcomes. Ann Surg Oncol 2020;27:344–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coopey SB, Tang R, Lei L, Freer PE, Kansal K, Colwell AS et al. Increasing eligibility for nipple-sparing mastectomy. Ann Surg Oncol 2013;20:3218–3222 [DOI] [PubMed] [Google Scholar]
- 3. Young WA, Degnim AC, Hoskin TL, Jakub JW, Nguyen MD, Tran NV et al. Outcomes of > 1300 nipple-sparing mastectomies with immediate reconstruction: the impact of expanding indications on complications. Ann Surg Oncol 2019;26:3115–3123 [DOI] [PubMed] [Google Scholar]
- 4. Lemaine V, Hoskin TL, Farley DR, Grant CS, Boughey JC, Torstenson TA et al. Introducing the SKIN score: a validated scoring system to assess severity of mastectomy skin flap necrosis. Ann Surg Oncol 2015;22:2925–2932 [DOI] [PubMed] [Google Scholar]
- 5. Matsen CB, Mehrara B, Eaton A, Capko D, Berg A, Stempel M et al. Skin flap necrosis after mastectomy with reconstruction: a prospective study. Ann Surg Oncol 2016;23:257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wagner JL, Fearmonti R, Hunt KK, Hwang RF, Meric-Bernstam F, Kuerer HM et al. Prospective evaluation of the nipple–areola complex sparing mastectomy for risk reduction and for early-stage breast cancer. Ann Surg Oncol 2012;19:1137–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Houvenaeghel G, Cohen M, Dammacco MA, D'Halluin F, Regis C, Gutowski M et al. Prophylactic nipple-sparing mastectomy with immediate breast reconstruction: results of a French prospective trial. Br J Surg 2021;108:296–301 [DOI] [PubMed] [Google Scholar]
- 8. Gould DJ, Hunt KK, Liu J, Kuerer HM, Crosby MA, Babiera G et al. Impact of surgical techniques, biomaterials, and patient variables on rate of nipple necrosis after nipple-sparing mastectomy. Plast Reconstr Surg 2013;132:330e–338e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colwell AS, Tessler O, Lin AM, Liao E, Winograd J, Cetrulo CL et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg 2014;133:496–506 [DOI] [PubMed] [Google Scholar]
- 10. Santanelli F, Longo B, Sorotos M, Farcomeni A, Paolini G. Flap survival of skin-sparing mastectomy type IV: a retrospective cohort study of 75 consecutive cases. Ann Surg Oncol 2013;20:981–989 [DOI] [PubMed] [Google Scholar]
- 11. Warren Peled A, Foster RD, Stover AC, Itakura K, Ewing CA, Alvarado M et al. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 breasts. Ann Surg Oncol 2012;19:3402–3409 [DOI] [PubMed] [Google Scholar]
- 12. Wang F, Peled AW, Garwood E, Fiscalini AS, Sbitany H, Foster RD et al. Total skin-sparing mastectomy and immediate breast reconstruction: an evolution of technique and assessment of outcomes. Ann Surg Oncol 2014;21:3223–3230 [DOI] [PubMed] [Google Scholar]
- 13. Petit JY, Veronesi U, Orecchia R, Luini A, Rey P, Intra M et al. Nipple-sparing mastectomy in association with intra operative radiotherapy (ELIOT): a new type of mastectomy for breast cancer treatment. Breast Cancer Res Treat 2006;96:47–51 [DOI] [PubMed] [Google Scholar]
- 14. Odom EB, Parikh RP, Um G, Kantola SW, Cyr AE, Margenthaler JA et al. Nipple-sparing mastectomy incisions for cancer extirpation prospective cohort trial: perfusion, complications, and patient outcomes. Plast Reconstr Surg 2018;142:13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park S, Yoon C, Bae SJ, Cha C, Kim D, Lee J et al. Comparison of complications according to incision types in nipple-sparing mastectomy and immediate reconstruction. Breast 2020;53:85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daar DA, Abdou SA, Rosario L, Rifkin WJ, Santos PJ, Wirth GA et al. Is there a preferred incision location for nipple-sparing mastectomy? A systematic review and meta-analysis. Plast Reconstr Surg 2019;143:906e–919e [DOI] [PubMed] [Google Scholar]
- 17. Carlson GW, Chu CK, Moyer HR, Duggal C, Losken A. Predictors of nipple ischemia after nipple sparing mastectomy. Breast J 2014;20:69–73 [DOI] [PubMed] [Google Scholar]
- 18. Palmer JH, Ian Taylor G. The vascular territories of the anterior chest wall. Br J Plast Surg 1986;39:287–299 [DOI] [PubMed] [Google Scholar]
- 19. Swistel A, Small K, Dent B, Cohen O, Devgan L, Talmor M. A novel technique of preserving internal mammary artery perforators in nipple sparing breast reconstruction. Plast Reconstr Surg Glob Open 2014;2:e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karian LS, Therattil PJ, Wey PD, Nini KT. Delay techniques for nipple-sparing mastectomy: a systematic review. J Plast Reconstr Aesthet Surg 2017;70:236–242 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and methods used in the analysis, and materials used to conduct the research, will be made available upon request to the authors.