Abstract
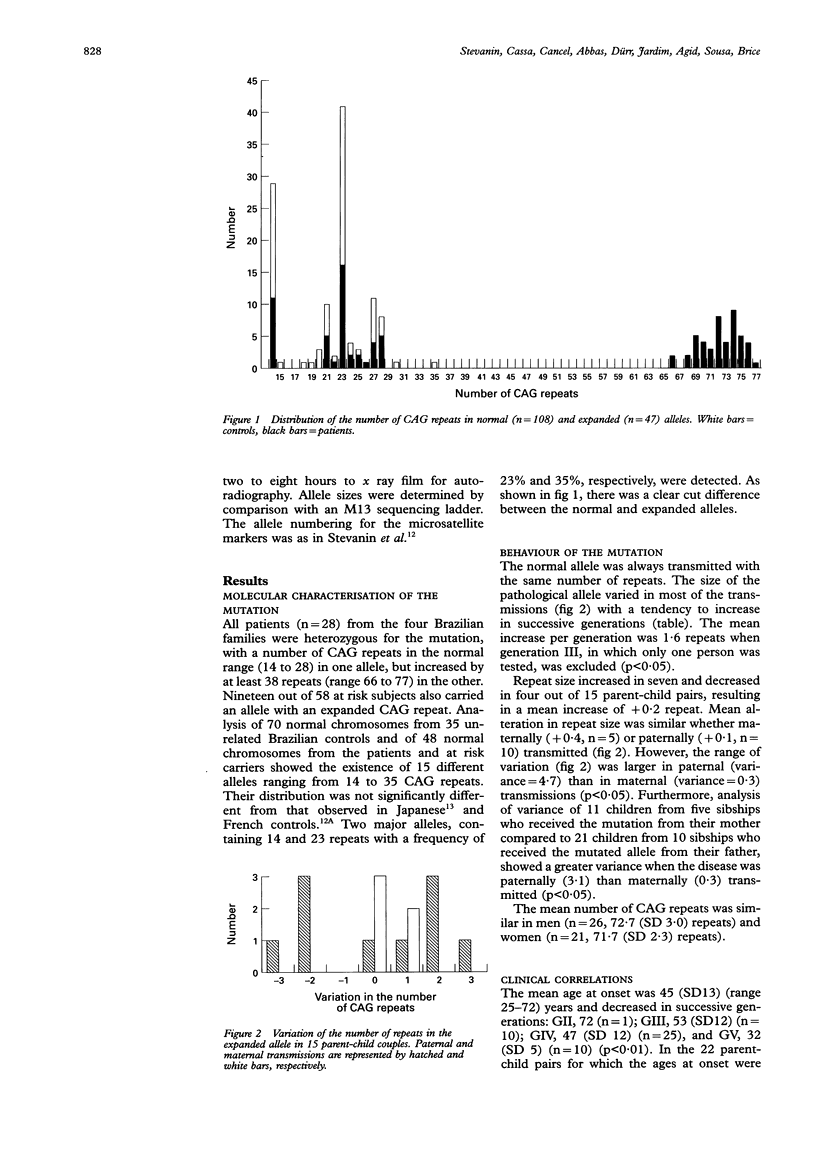
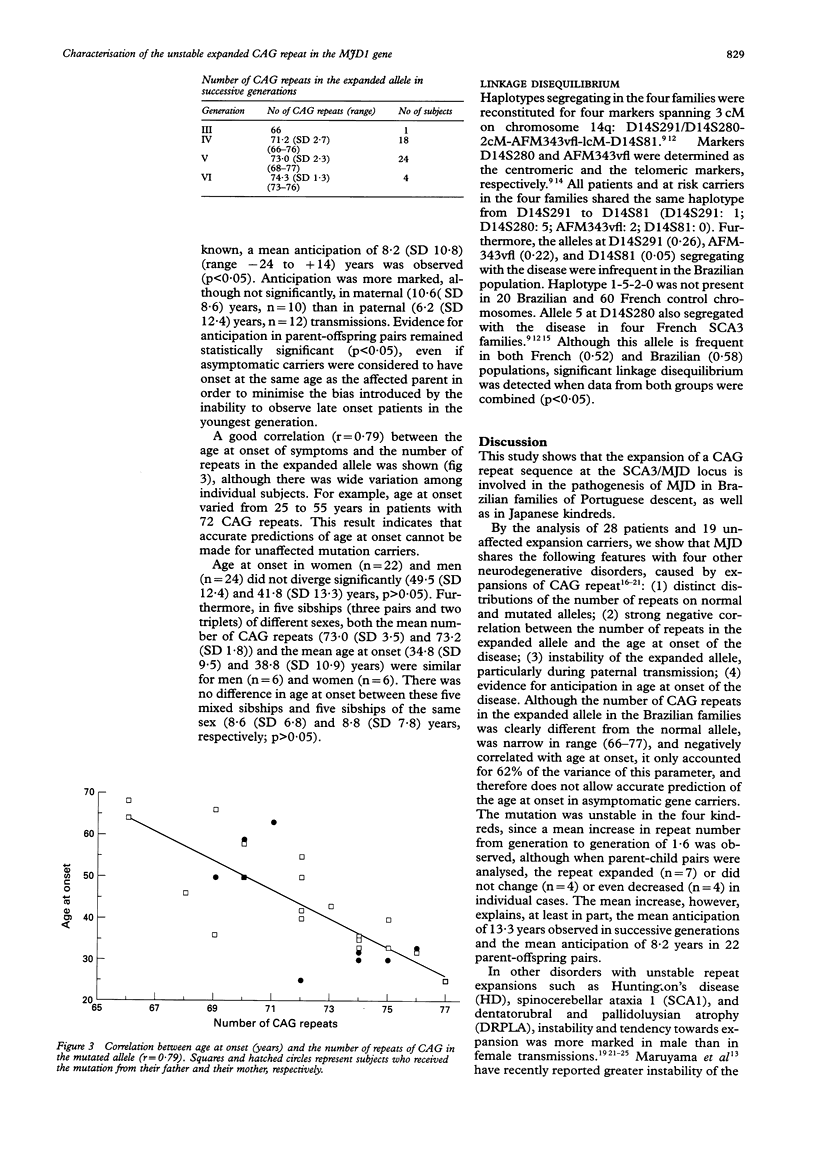
Machado-Joseph disease (MJD) is an autosomal dominant neurodegenerative disorder which has been shown to result, in Japanese families, from the expansion of a CAG repeat in the MJD1 gene on chromosome 14q. We show that the same molecular mechanism is responsible for MJD in four large Brazilian kindreds of Portuguese descent. The behaviour of the mutation was evaluated in 28 affected and 19 asymptomatic gene carriers. The number of repeats in the expanded alleles ranged from 66 to 77 with a strong negative correlation with age at onset (r=0·79). A mean 1·6 repeats increase from generation to generation correlated with clinical anticipation. Instability of the CAG repeat was bidirectional, with expansions as well as contractions, and was more marked in paternal transmissions. Finally, linkage disequilibrium was complete at locus D14S280 in the four Portuguese-Brazilian kindreds and four previously reported French families with the same mutation, which suggests the existence of a common founder.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbeau A., Roy M., Cunha L., de Vincente A. N., Rosenberg R. N., Nyhan W. L., MacLeod P. L., Chazot G., Langston L. B., Dawson D. M. The natural history of Machado-Joseph disease. An analysis of 138 personally examined cases. Can J Neurol Sci. 1984 Nov;11(4 Suppl):510–525. doi: 10.1017/s0317167100034983. [DOI] [PubMed] [Google Scholar]
- Burt T., Blumbergs P., Currie B. A dominant hereditary ataxia resembling Machado-Joseph disease in Arnhem Land, Australia. Neurology. 1993 Sep;43(9):1750–1752. doi: 10.1212/wnl.43.9.1750. [DOI] [PubMed] [Google Scholar]
- Cancel G., Dürr A., Stevanin G., Chneiweiss H., Duyckaerts C., Serdaru M., de Toffol B., Agid Y., Brice A. Is DRPLA also linked to 14q? Nat Genet. 1994 Jan;6(1):8–8. doi: 10.1038/ng0194-8. [DOI] [PubMed] [Google Scholar]
- Chung M. Y., Ranum L. P., Duvick L. A., Servadio A., Zoghbi H. Y., Orr H. T. Evidence for a mechanism predisposing to intergenerational CAG repeat instability in spinocerebellar ataxia type I. Nat Genet. 1993 Nov;5(3):254–258. doi: 10.1038/ng1193-254. [DOI] [PubMed] [Google Scholar]
- Duyao M., Ambrose C., Myers R., Novelletto A., Persichetti F., Frontali M., Folstein S., Ross C., Franz M., Abbott M. Trinucleotide repeat length instability and age of onset in Huntington's disease. Nat Genet. 1993 Aug;4(4):387–392. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- Goldberg-Stern H., D'jaldetti R., Melamed E., Gadoth N. Machado-Joseph (Azorean) disease in a Yemenite Jewish family in Israel. Neurology. 1994 Jul;44(7):1298–1301. doi: 10.1212/wnl.44.7.1298. [DOI] [PubMed] [Google Scholar]
- Harding A. E. Clinical features and classification of inherited ataxias. Adv Neurol. 1993;61:1–14. [PubMed] [Google Scholar]
- Kawaguchi Y., Okamoto T., Taniwaki M., Aizawa M., Inoue M., Katayama S., Kawakami H., Nakamura S., Nishimura M., Akiguchi I. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994 Nov;8(3):221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- Kawakami H., Maruyama H., Nakamura S., Kawaguchi Y., Kakizuka A., Doyu M., Sobue G. Unique features of the CAG repeats in Machado-Joseph disease. Nat Genet. 1995 Apr;9(4):344–345. doi: 10.1038/ng0495-344. [DOI] [PubMed] [Google Scholar]
- Koide R., Ikeuchi T., Onodera O., Tanaka H., Igarashi S., Endo K., Takahashi H., Kondo R., Ishikawa A., Hayashi T. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nat Genet. 1994 Jan;6(1):9–13. doi: 10.1038/ng0194-9. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991 Jul 4;352(6330):77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Maruyama H., Nakamura S., Matsuyama Z., Sakai T., Doyu M., Sobue G., Seto M., Tsujihata M., Oh-i T., Nishio T. Molecular features of the CAG repeats and clinical manifestation of Machado-Joseph disease. Hum Mol Genet. 1995 May;4(5):807–812. doi: 10.1093/hmg/4.5.807. [DOI] [PubMed] [Google Scholar]
- Matilla T., Volpini V., Genís D., Rosell J., Corral J., Dávalos A., Molins A., Estivill X. Presymptomatic analysis of spinocerebellar ataxia type 1 (SCA1) via the expansion of the SCA1 CAG-repeat in a large pedigree displaying anticipation and parental male bias. Hum Mol Genet. 1993 Dec;2(12):2123–2128. doi: 10.1093/hmg/2.12.2123. [DOI] [PubMed] [Google Scholar]
- Myers R. H., MacDonald M. E., Koroshetz W. J., Duyao M. P., Ambrose C. M., Taylor S. A., Barnes G., Srinidhi J., Lin C. S., Whaley W. L. De novo expansion of a (CAG)n repeat in sporadic Huntington's disease. Nat Genet. 1993 Oct;5(2):168–173. doi: 10.1038/ng1093-168. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Yanagisawa H., Ohsaki E., Shirayama T., Tadokoro K., Inoue T., Yamada M. Structure and expression of the gene responsible for the triplet repeat disorder, dentatorubral and pallidoluysian atrophy (DRPLA). Nat Genet. 1994 Oct;8(2):177–182. doi: 10.1038/ng1094-177. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Yanagisawa H., Sato K., Shirayama T., Ohsaki E., Bundo M., Takeda T., Tadokoro K., Kondo I., Murayama N. Dentatorubral and pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromosome 12p. Nat Genet. 1994 Jan;6(1):14–18. doi: 10.1038/ng0194-14. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Chung M. Y., Banfi S., Kwiatkowski T. J., Jr, Servadio A., Beaudet A. L., McCall A. E., Duvick L. A., Ranum L. P., Zoghbi H. Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993 Jul;4(3):221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. N. Machado-Joseph disease: an autosomal dominant motor system degeneration. Mov Disord. 1992;7(3):193–203. doi: 10.1002/mds.870070302. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Wakisaka A., Takada A., Yoshiki T., Ihara T., Suzuki Y., Hamada T., Iwabuchi K., Onari K., Tada J. Mapping of the gene for Machado-Joseph disease within a 3.6-cM interval flanked by D14S291/D14S280 and D14S81, on the basis of studies of linkage and linkage disequilibrium in 24 Japanese families. Am J Hum Genet. 1995 Jan;56(1):231–242. [PMC free article] [PubMed] [Google Scholar]
- Sequeiros J., Coutinho P. Epidemiology and clinical aspects of Machado-Joseph disease. Adv Neurol. 1993;61:139–153. [PubMed] [Google Scholar]
- Sequeiros J., Silveira I., Maciel P., Coutinho P., Manaia A., Gaspar C., Burlet P., Loureiro L., Guimarães J., Tanaka H. Genetic linkage studies of Machado-Joseph disease with chromosome 14q STRPs in 16 Portuguese-Azorean kindreds. Genomics. 1994 Jun;21(3):645–648. doi: 10.1006/geno.1994.1327. [DOI] [PubMed] [Google Scholar]
- Squitieri F., Andrew S. E., Goldberg Y. P., Kremer B., Spence N., Zeisler J., Nichol K., Theilmann J., Greenberg J., Goto J. DNA haplotype analysis of Huntington disease reveals clues to the origins and mechanisms of CAG expansion and reasons for geographic variations of prevalence. Hum Mol Genet. 1994 Dec;3(12):2103–2114. doi: 10.1093/hmg/3.12.2103. [DOI] [PubMed] [Google Scholar]
- Stevanin G., Cancel G., Dürr A., Chneiweiss H., Dubourg O., Weissenbach J., Cann H. M., Agid Y., Brice A. The gene for spinal cerebellar ataxia 3 (SCA3) is located in a region of approximately 3 cM on chromosome 14q24.3-q32.2. Am J Hum Genet. 1995 Jan;56(1):193–201. [PMC free article] [PubMed] [Google Scholar]
- Stevanin G., Sousa P. S., Cancel G., Dürr A., Dubourg O., Nicholson G. A., Weissenbach J., Jardim E., Agid Y., Cassa E. The gene for Machado-Joseph disease maps to the same 3-cM interval as the spinal cerebellar ataxia 3 gene on chromosome 14q. Neurobiol Dis. 1994 Nov;1(1-2):79–82. doi: 10.1006/nbdi.1994.0010. [DOI] [PubMed] [Google Scholar]
- Takiyama Y., Ikemoto S., Tanaka Y., Mizuno Y., Yoshida M., Yasuda N. A large Japanese family with Machado-Joseph disease: clinical and genetic studies. Acta Neurol Scand. 1989 Mar;79(3):214–222. doi: 10.1111/j.1600-0404.1989.tb03741.x. [DOI] [PubMed] [Google Scholar]
- Takiyama Y., Nishizawa M., Tanaka H., Kawashima S., Sakamoto H., Karube Y., Shimazaki H., Soutome M., Endo K., Ohta S. The gene for Machado-Joseph disease maps to human chromosome 14q. Nat Genet. 1993 Jul;4(3):300–304. doi: 10.1038/ng0793-300. [DOI] [PubMed] [Google Scholar]
- Telenius H., Kremer H. P., Theilmann J., Andrew S. E., Almqvist E., Anvret M., Greenberg C., Greenberg J., Lucotte G., Squitieri F. Molecular analysis of juvenile Huntington disease: the major influence on (CAG)n repeat length is the sex of the affected parent. Hum Mol Genet. 1993 Oct;2(10):1535–1540. doi: 10.1093/hmg/2.10.1535. [DOI] [PubMed] [Google Scholar]
- Twist E. C., Casaubon L. K., Ruttledge M. H., Rao V. S., Macleod P. M., Radvany J., Zhao Z., Rosenberg R. N., Farrer L. A., Rouleau G. A. Machado Joseph disease maps to the same region of chromosome 14 as the spinocerebellar ataxia type 3 locus. J Med Genet. 1995 Jan;32(1):25–31. doi: 10.1136/jmg.32.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]


