Abstract
Drug overdoses from opioids and stimulants are a major cause of mortality in the United States. It is unclear if there are stable sex differences in overdose mortality for these drugs across states, whether these differ across the lifespan, and if so, whether they can be accounted for by different levels of drug misuse. This was a state-level analysis of epidemiological data on overdose mortality, across 10-year age bins (age range: 15–74), using the CDC WONDER platform for decedents in the United States in 2020–1. The outcome measure was rate of overdose death (per 100,000) for: synthetic opioids (e.g., fentanyl), heroin, psychostimulants with potential for misuse (e.g., methamphetamine), and cocaine. Multiple linear regressions controlled for ethnic-cultural background, household net worth, and sex-specific rate of misuse (from NSDUH, 2018-9). For all these drug categories, males had greater overall overdose mortality than females, after controlling for rates of drug misuse. The mean male/female sex ratio of mortality rate was relatively stable across jurisdictions: synthetic opioids (2.5 [95% CI, 2.4–7]), heroin, (2.9 [95% CI, 2.7–3.1], psychostimulants (2.4 [95% CI, 2.3–5]), and cocaine (2.8 [95% CI, 2.6–9]). With data stratified in 10-year age bins, the sex difference generally survived adjustment (especially in the 25–64 age range). Results indicate that males are significantly more vulnerable than females to overdose deaths caused by opioid and stimulant drugs, taking into account differing state-level environmental conditions and drug misuse levels. These results call for research into diverse biological, behavioral, and social factors that underlie sex differences in human vulnerability to drug overdose.
Subject terms: Risk factors, Outcomes research
Introduction
Overdose deaths due to opioids (such as fentanyl and heroin), or stimulant drugs (such as methamphetamine and cocaine) have increased in recent years in the United States [1–3]. This increase has accelerated since the onset of the COVID-19 pandemic (March 2020 and thereafter) for fentanyl and its analogs and stimulants such as methamphetamine, but not heroin, prescription opioids, or methadone [4–7]. This was due in part to increases in the distribution of synthetic opioids such as fentanyl, and their use to lace other drugs, including illicit preparations disguised as prescription opioid analgesics, stimulant medications, or benzodiazepines [6, 8, 9].
Prevention of overdose death requires the identification of vulnerability factors, which are thought to include sex and age [6]. Regarding sex as a biological variable, data from rodent models are inconclusive due to conflicting results: some such studies have shown that female rats escalate their intake of opioids or stimulant drugs more than male rats [10], but a large number of other studies have not [11]. Similarly, some studies in human cohorts have shown that females escalated their intake of opioids or stimulant drugs more rapidly than males [12–15], but several other studies have not [16–18]. A recent comprehensive review did not find robust sex differences in vulnerability to craving and relapse [11]. Nonetheless, as reviewed below, epidemiological data have suggested a sex difference in risk of overdose death, and those findings need to be followed up on.
Regarding age, studies have suggested typical trajectories in which misuse of opioids or stimulant drugs begins in late adolescence or young adulthood and escalates thereafter [18, 19]. However, the onset of misuse may also occur later in the lifespan, and can be triggered iatrogenically [20, 21]. Recent data also point to an increase in older adults (aged 55 and older) seeking first-time treatment for OUD [22].
Epidemiological studies show that males tend to have greater overdose mortality compared to females [23]. Thus, a recent report for 2019–20, for 25 states and the District of Columbia, showed an increase in overdose mortality for all drugs combined, with a greater rate in males versus females [24]. Similarly, a US nation-level analysis showed an increase in overdose mortality from synthetic opioids (thus including fentanyl but not heroin) and psychostimulants (including methamphetamine but not cocaine) from March 2018 to March 2021, with highest mortality rates in males (especially from minoritized groups, e.g., African-American) [4]. A nation-level report on CDC data from 2017 to 2018 showed that males compared to females had greater overdose mortality for prescription opioids specifically, and for all opioids combined [6].
Here we examined sex differences in greater depth across the lifespan and determined whether their magnitude is consistent across state-level US jurisdictions, which exhibit a broad range of major demographic and socioeconomic variables, levels of misuse, and illicit drug market factors [25]. We examined overdose mortality separately for specific categories of opioid and stimulant drugs, due to their differing mechanisms of toxicity [26]. Acute overdose mortality from opioids (mu-opioid receptor agonists) is primarily attributed to respiratory depression mediated by brainstem nuclei [27, 28]. Overdose mortality from fentanyl may be greater than that for heroin, due to greater potency, faster onset of action driven by high lipophilicity, and higher likelihood to trigger chest rigidity [29–31]. It is not clear whether there are generalizable sex differences in sensitivity to respiratory depressant effects of mu-opioid agonists in humans [32–35]. Epidemiological data do not directly address this question, but could provide evidence against it (or in favor of additional explanations) if the sex difference in overdose mortality is not consistent across state-level jurisdictions.
Acute overdose mortality by methamphetamine and cocaine in humans is primarily attributed to cardiovascular and neurological events such as malignant arrhythmias, strokes, or seizures [36, 37]. Some, but not all, human studies reported that females had either more prolonged or more pronounced cardiovascular effects than males after administration of stimulant drugs [38–41]. Again, epidemiological data cannot directly address questions of biological vulnerability, but could constrain hypotheses, depending on whether the sex difference in overdose mortality is consistent across states that differ in major demographic, socioeconomic and drug use factors.
In addition to sex differences in direct pathophysiological effects of the drug, factors that could account for stable variations in rates of overdose include family and social interactions and related trauma [42, 43], propensity toward risky behaviors (e.g., injecting alone, taking large doses, or using untrusted suppliers) [44, 45], and propensity to seek treatment [46–50]. We expect that differences in these gendered behavioral factors would be largely consistent across drug categories and states in the US. Other contributing factors which could differ by state within the US include ethnic-cultural background, socioeconomic variables, illicit drug supply, and availability of evidence-based care [8, 51–54]. Using state-level nationally representative CDC data for 2020–1, the goal of this study was therefore to determine the extent to which sex differences in overdose mortality vary in four mutually exclusive drug categories (synthetic opioids, heroin, psychostimulants such as methamphetamine, and cocaine) and state, accounting for age. Different patterns of sex variability in overdose mortality would suggest (without proving or ruling out) different emphases on possible levels of causation.
Methods
This was a study of de-identified publicly available data on “multiple cause mortality”, from the CDC WONDER platform for the years 2020-1 (https://wonder.cdc.gov/mcd.html), based on death certificates in the United States. Overdose mortality data that were “suppressed” (due to n < 10 per cell) or deemed “unreliable” were considered missing and were excluded from analysis. Data were analyzed from October 2022 to February 2023. This study followed STROBE reporting guidelines.
Data set
The main analyzed outcome variable was crude death rate per 100,000 population for Drug Poisonings (ICD-10 codes for overdoses; including unintentional X40-X44, suicide X60-X64, homicide X85, and undetermined intents Y10–Y14). Data were obtained for four mutually exclusive categories: synthetic opioids other than methadone (“synthetic opioids” hereafter; this predominantly reflects fentanyl and its analogs, but also compounds from other chemical scaffolds; T40.4), [55] heroin (T40.1), psychostimulants with potential for misuse (which predominantly reflects methamphetamine; T43.6) and for cocaine (T40.5). Data were stratified by sex and by six consecutive 10-year age bins (i.e., 15–24, 25–34, 35–44, 45–54, 55–64 and 65–74) obtained for 51 jurisdictions (50 states and the District of Columbia) for 2020–1 [56].
Nomenclature
This manuscript uses the terms “male” and “female” for data analyses [57], as this is the terminology currently used in death certificates, the root document for mortality data in CDC WONDER [58]. For clarity, we use the term “stimulant drugs” to encompass compounds such as methamphetamine and cocaine overall and use the term “psychostimulants” for the CDC overdose category (T43.6) that predominantly reflects methamphetamine. As stated above, cocaine overdoses (T40.5) are analyzed separately.
Data analyses
Analyses were carried out with GraphPad Prism V.9, using jurisdiction-level data. Univariate analyses included Spearman correlations on overdose mortality data (crude death rate per 100,000 population) for males and females, per jurisdiction. Mortality and misuse ratios in males/females were compared with Wilcoxon tests. Univariate mixed-effects ANOVAs (sex x age bin) then analyzed log-transformed overdose mortality rate across the lifespan, stratified in six consecutive 10-year age bins; significant effects were followed with post-hoc Bonferroni tests. Normality of the outcome variable was examined with the D’Agostino-Pearson test (p = 0.05 level), followed by visual examination of quantile-quantile plots, when necessary.
Separately for each drug category (all ages combined in the range 15–74 years), multivariable analyses were carried out with multiple linear (least squares) regressions, to assess the effect of sex and selected potential covariates on the outcome, log-transformed death rates. Thus, the independent variables included: sex (M/F), percent of the population who is white, percent of the population who is black (from the 5-year American Community Survey for 2020; https://worldpopulationreview.com/states/states-by-race; eTable 3), and median household net worth in 2020 (www.census.gov/data/tables/2020/demo/wealth/state-wealth-asset-ownership.html; eTable 4). Data from the National Survey on Drug Use and Health (NSDUH) for 2018–9 were used as an estimate of misuse of relevant drugs (https://rdas.samhsa.gov/#/survey/NSDUH-2018-2019-RD02YR). NSDUH is a nationally representative survey of non-institutionalized persons 12 years and older, using stratified probability sampling (based on n = 67,791 and n = 67,625 completed interviews in 2018 and 2019, respectively). Full methodology for NSDUH can be found at: www.samhsa.gov/data/sites/default/files/reports/rpt34659/NSDUHmrbDCFR2019.pdf. Thus, the multiple linear regressions for overdose mortality by synthetic opioids and heroin controlled for the rate of past-year misuse of opioids, by state and sex (item “OPINMYR” in NSDUH). Likewise, the regressions for overdose mortality for psychostimulants or cocaine controlled for the rate of past-year misuse of these drugs, by sex (items “AMMEPDPYMU” and “COCMYR” in NSDUH, respectively). Using NSDUH data, we were not able to perform state- and sex-specific analyses of relevant substance use disorders (as opposed to misuse), because of frequent data suppression after stratification. The overdose mortality data were also stratified by 10-year age bins (overall range: 15–74 years), and multiple regressions were run separately for each age bin.
In the ANOVAs and multiple regressions, outliers for the outcome variable (log overdose rates) were removed, using Grubbs test at the p = 0.05 alpha level of significance (i.e., greater than ±1.96 standard deviations from the mean as the standard). Also, if there were <10 jurisdictions without overdose mortality data available for both males and females for an age bin and drug category, the data set was excluded from analysis, to maintain representativeness. The overall alpha level of significance was set at the p = 0.05 level, two-tailed.
In a sub-group analysis, we examined analogously overdoses that involved both synthetic opioids and psychostimulants (a common poly-drug pattern of overdose) (Supplement). [3, 59]. In a sensitivity analysis, we determined whether findings were affected by the use of crude versus age-adjusted rates of mortality, focusing on the synthetic opioid category.
Results
Overall overdose mortality profiles (age range 15–74)
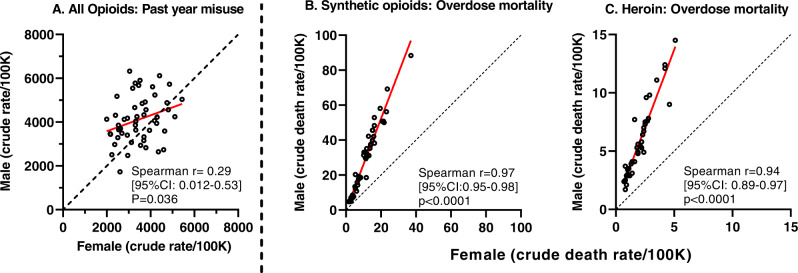
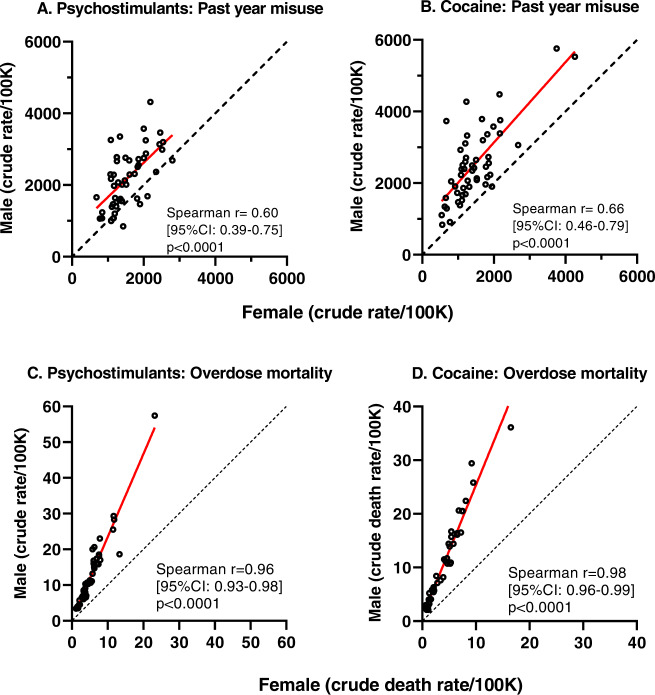
With ages combined (overall range 15–74 years), there was a broad range of overdose mortality rates for the four drug categories, across jurisdictions (Figs. 1; middle and right panels and 2; lower panels, and eTable 1). There were positive Spearman correlations, nearing unity, in the rate of overdose mortality for males and females in the same jurisdiction, for each of the drug categories (Spearman correlations ranged from +0.94 to +0.97; all p values were <0.0001) (see middle and right panels of Fig. 1 for synthetic opioids and heroin, and lower panels of Fig. 2 for psychostimulants and cocaine). Simple linear regressions provided a strong fit for these data (R2 values of 0.95, 0.88, 0.92 and 0.95, for synthetic opioids, heroin, psychostimulants and cocaine respectively). The mean ratios for mortality in males/females (all ages combined) were in the 2.4–9 range: ratios were 2.5 (95% CI: 2.4–7) for synthetic opioids, 2.9 (95% CI: 2.7–3.1) for heroin, 2.4 (95% CI: 2.3–5) for psychostimulants and 2.8 (95% CI: 2.6-9) for cocaine.
Fig. 1. Opioid misuse and overdose mortality.
A Past-year misuse of any opioid in males and females, all ages combined. B, C Overdose mortality due to synthetic opioids and heroin in males and females; all ages combined. For all panels, each symbol (open circles) is one jurisdiction. Note different axis ranges in the panels. Spearman correlations for males and females in each jurisdiction are included. The red line indicates the best-fit simple linear regression; the dashed black line indicates identity.
Fig. 2. Psychostimulant and cocaine misuse and overdose mortality.
A, B Past-year misuse of psychostimulants and cocaine in males and females, all ages combined. C, D Overdose mortality due to psychostimulants and cocaine in males and females, all ages combined. For all panels, each symbol (open circles) is one jurisdiction. Note different axis ranges in the panels. Spearman correlations for males and females in each jurisdiction are included. The red line indicates the best-fit simple linear regression. The dashed black line indicates identity.
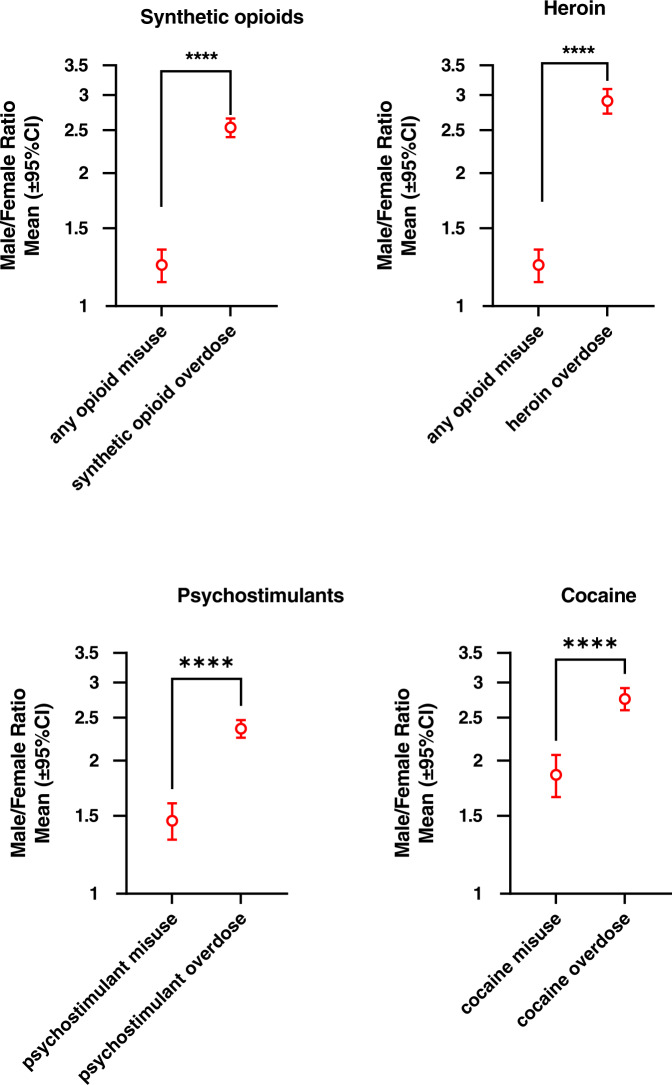
In contrast, the correlations for past-year drug misuse between males and females within jurisdictions were of smaller magnitude, ranging from Spearman r value of 0.29 for opioids, to 0.60 for psychostimulants, and 0.66 for cocaine) (Figs. 1 left panel and 2; upper panels). female ratios/female ratios for misuse were significantly smaller than the male/female ratios for overdose mortality, for each of these drug categories (see Fig. 3).
Fig. 3. Male/female ratios for past-year misuse of relevant substances and for overdose mortality (shown in logarithmic axis).
Data were analyzed with Wilcoxon’s tests, followed by Bonferroni correction where appropriate (i.e., opioid misuse data were used both in panels for synthetic opioids and heroin). P values are shown as **** (p = 0.0001) and *** (p = 0.001). Jurisdictions with missing data were excluded from the analysis.
Univariate analyses of overdose mortality across the lifespan
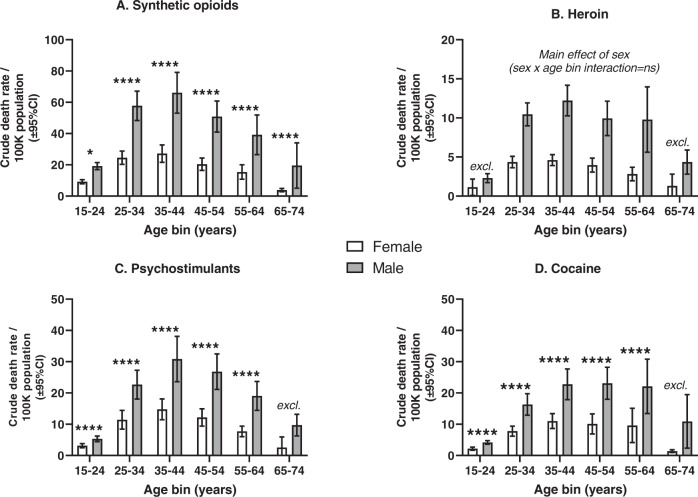
Separate 2 × 2 ANOVAs (sex x age bin) were carried out for each drug category. For synthetic opioids, psychostimulants and cocaine, there were significant main effects of sex and age bin, and also sex by age bin interaction (see Fig. 4 and eTables 2 and 5). For heroin, there were significant main effects of sex and age bin, but the interaction did not reach significance. Post-hoc Bonferroni tests for sex at each age bin showed that with few exceptions (typically for the youngest and oldest age bins, where the lowest rates were observed), males had greater mortality than females. Deviations from normality were rare, based on D’Agostino-Pearson tests, followed by examination of quantile-quantile plots. Outliers in the outcome variable were rare, and their removal from the ANOVA did not substantially affect the results (e.g., for synthetic opioids in the 65–74 age bin; not shown).
Fig. 4. Overdose mortality in males and females, for consecutive 10-year age bins.
A–D These show data for synthetic opioids, heroin, psychostimulants and cocaine, respectively. Note the different Y-axis ranges in the different panels. Means and 95% CI are calculated across 51 jurisdictions. Log –transformed overdose data were analyzed with univariate mixed-effects effects ANOVAs (sex × age bin). Bonferroni post-hoc tests for sex at each age bin are shown with black brackets (* is p < 0.05; **** is p < 0.0001). The label “excl.” indicates that the age bin was excluded from ANOVA analysis due to insufficient available data (see Methods and eTable 2). Full ANOVA table is present in the Supplement (eTable 5).
Multivariable analyses
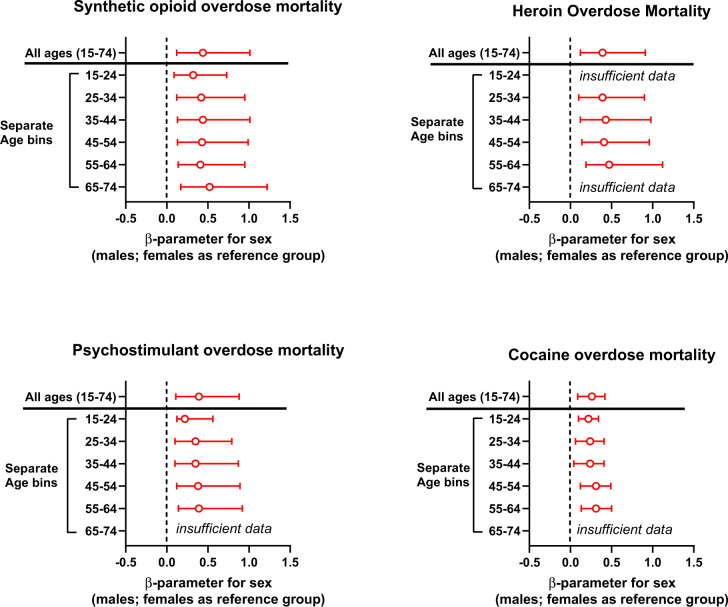
Multiple linear regressions were conducted for each drug category separately, with all ages combined (age range 15–74). Sex remained a significant factor for overdose mortality for all drug categories, after adjustment for the jurisdiction-level sex-specific rate of misuse of the relevant drugs, and for demographic variables (distribution of major ethnic-cultural groups and median household net worth) (see eTable 6, and Fig. 5).
Fig. 5. Sex as a factor in overdose mortality after adjustment for levels of drug misuse and demographic variables, from multiple linear regressions.
The outcome was log-transformed overdose mortality per 100,000 population. Data shown are β(beta)-parameter for sex as a factor in overdose mortality. The multiple regressions were adjusted for jurisdiction-specific levels of misuse of specific drugs in males and females, and for three demographic variables (%White, %Black, and net household assets; eTables 3 and 4). Full regression data are presented in the Supplement (eTables 6 and 7).
Similar multiple linear regressions were then conducted, after stratification into 10-year age bins. For all drug categories and age bins, sex remained a significant factor in overdose mortality rates after adjustment. Of note, that included the 55–64 age bin (see eTable 7, and Fig. 5). List-wise removal of the outliers in the outcome variable did not substantially affect the results of the relevant multiple regression (e.g., for synthetic opioids in the 65–74 age bin; not shown).
Subgroup analysis of overdose mortality that involved both synthetic opioids and psychostimulants
CDC WONDER can present different drugs as multiple causes of mortality. In a subgroup analysis, we examined overdose mortality due to a commonly reported pattern of polydrug use: synthetic opioids and psychostimulants (i.e., ICD-10 codes T40.4, and T43.6 reported together). [3, 59, 60]. These overdoses also showed a similar profile as above, with males having greater mortality rates than females, in several age bins (see eFig. 1, eTables 8 and 9).
Sensitivity analysis for age-adjusted rates of overdose mortality
Age-adjusted data of overdoses for synthetic opioids (age range: 15-74) showed a mean male/female ratio of 2.48 (95% CI: 2.36–60), and a near unity correlation for male and female rates across jurisdictions (Spearman r = 0.98; p < 0.0001) (data not shown). These findings for age-adjusted rates were highly congruent with those for crude rates (for comparison, see Figs. 1 and 3).
Summary of nation-level trends
We found that nation-level sex ratios in overdose mortality were generally congruent with state-level findings shown above. For example, for synthetic opioids at the national level in the age range 15–74 years, the male/female ratio of overdose mortality was 2.7 (i.e., 37.4/100,000 in males, and 13.9/100,000 in females; see Fig. 3 for comparison).
Discussion
This was a nationally representative state- and age-specific examination of differences between males and females in overdose mortality due to opioid and stimulant drugs, for 2020–1. The study showed that for these drug categories, across the lifespan, after controlling for sex-specific levels of past-year drug misuse and for major demographic factors, males died from overdoses at an ~2–3 greater rate than females. Studies from several other industrialized countries do show greater drug-induced mortality for males versus females [61, 62]. However, to our knowledge, current comparative data for these drugs, stratified by age, are not published for a broad set of countries.
For synthetic opioids, the effect of sex on overdose mortality survived adjustment in all the 10-year age bins studied (overall range 15–74 years). For the other drug categories under study (heroin, psychostimulants, and cocaine), this effect of sex survived adjustment in age bins in the 25–64 age range. Therefore, for all the drugs studied here, greater overdose mortality rates were observed in males versus females in the 55–64 age bin, considered to be in the post-menopausal range for the population [63].
The robustness of the overall sex difference in overdose mortality, even after controlling for different propensity to misuse, and across state-level jurisdictions, shows that males in the US are reliably at greater risk of fatal overdose than females. Mechanistic explanations cannot be directly derived from these epidemiological data, but some processes can be accorded greater likelihood of accounting for most of the sex difference. Sex-specific biological vulnerability to the direct toxic effects of the drugs, for example, cannot be ruled out. However, this sex-specific vulnerability would have to be shared across mu-opioid agonists and stimulant drugs, whose toxic effects are highly distinct from each other, in terms of pharmacodynamic targets and pathophysiological mechanisms. Even within these major drug categories, the pharmacodynamics and pathophysiological effects of specific mu-opioid agonists such as fentanyl versus morphine derivatives such as heroin [30, 31], and methamphetamine versus cocaine [64, 65], are not identical.
There are sex differences in the propensity to development of substance-use disorders (SUDs), but they appear unlikely to account for the observed difference in mortality rates. Nation-level data from the 2021 NSDUH [66] show that the overall male/female ratio in the prevalence of any past-year SUD (all drugs combined) was 1.3 (9.8% of males versus 7.4% of females)—a considerably smaller sex difference than the one we observed in mortality rates. We noted earlier that other, more culture-bound differences between men and women could include differential propensity toward risky behaviors, above and beyond any risk inherent in misusing an opioid or stimulant—e.g., injecting alone [44, 45], taking large doses, or using untrusted suppliers.
Males, compared to females, have been shown to have a greater propensity for other risky behaviors, with associated morbidity and mortality. For example, one study found that males were 2-3 fold more likely than females to be responsible for motor collisions, but were not more likely to be passive victims of motor collisions [67]. This is consistent with recent nationally representative US data sets (from 2020) showing that male drivers who died as a result of traffic accidents had a greater likelihood of engaging in risky behaviors such as speeding, compared to female drivers [68]. We suspect that this type of sex difference is a valuable starting point for the exploration of both a major mechanism and a modifiable risk factor in overdose deaths, but this explanation does not exclude important roles for other biological, behavioral, or social factors. For example, at the biological level, the X-chromosome contains a large number of genes and micro-RNA that are involved in neuronal and glial functions, the regulation of which could differ in males and females [69–71]. At the behavioral and social levels, patterns of initial opioid exposure could differ in men and women (for example due to medical factors, including pain), and could affect the trajectory of opioid use and overdose risk [72].
Limitations
We focused on drug categories that greatly contribute to overdose deaths in the US at this time [3, 4, 73], but there is a need for similar age-related examination of sex differences in overdose death from prescription opioids, benzodiazepines, and alcohol [6, 74]. We cannot fully exclude the possibility that some of the sex differences in overdose mortality or misuse are due to reporting biases [75, 76]. In addition, state- and sex-level data on drug misuse from NSDUH for years after 2019 are not currently publicly available. It should also be noted that the opioid misuse data in NSDUH (item OPINMYR) can encompass other compounds, in addition to synthetic opioids and heroin. Another limitation is due to prior reports that use of illicit drugs overall can be underestimated in population-based surveys [77]. Finally, the coding of gender identity in national mortality data is not currently routine [58], and these data sets do not provide information specifically for transgender or non-binary persons. This is an important issue, because of the risks of SUD and overdose in transgender, non-binary, and/or gender-expansive communities [78, 79].
Conclusions
We performed a state-level analysis of recent nationally representative data and found that males, compared to females, have a significantly greater rate of overdose mortality from synthetic opioids, heroin, and stimulant drugs such as methamphetamine and cocaine. An important finding in our study is that greater mortality in males was evident even after controlling for levels of misuse, at the state level. These sex differences were observed across the lifespan (e.g., in the range of 25–64 years of age), and were stable across states that have broad range of environmental variables. These findings call for mechanistically-based research on risk and interventions for severe OUD outcomes in both males and females. Furthermore, these findings underscore that sex differences are important targets for investigation at multiple biological and behavioral levels, potentially leading to optimized prevention and intervention approaches to mitigate risk of overdose mortality, at different stages across the lifespan.
Supplementary information
Author contributions
All authors contributed to the writing and editing process, including text and data analysis quality, and approved submission of the manuscript. All authors agree to be accountable for all aspects of the manuscript, including accuracy and integrity, and investigation and resolution of any potential discrepancies.
Funding
This work was supported by NIDA U01DA053625 (ERB), NIDA 1RO1DA048301-01A1 (RZG) and NIDA 1RO1DA049547 (NAK), and by Intramural Research Program funds of NIDA (YS and DHE).
Competing interests
YS is an Associate Editor in Neuropsychopharmacology. The other authors have nothing to disclose.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-023-01601-8.
References
- 1.Brown KG, Chen CY, Dong D, Lake KJ, Butelman ER. Has the United States reached a plateau in overdoses caused by synthetic opioids after the onset of the COVID-19 pandemic? Examination of Centers for Disease Control and Prevention Data to November 2021. Front Psychiatry. 2022; https://www.frontiersin.org/articles/10.3389/fpsyt.2022.947603/full. [DOI] [PMC free article] [PubMed]
- 2.Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013-9. Morbidity Mortal Wkly Rep. 10.15585/mmwr.mm7006a4. [DOI] [PMC free article] [PubMed]
- 3.Kariisa M, Seth P, Scholl L, Wilson N, Davis NL. Drug overdose deathsinvolving cocaine and psychostimulants with abuse potential among racial andethnic groups - United States, 2004-2019. Drug Alcohol Depend. 2021;227:109001. [DOI] [PubMed]
- 4.Han B, Einstein EB, Jones CM, Cotto J, Compton WM, Volkow ND. Racial and ethnic disparities in drug overdose deaths in the US during the COVID-19 Pandemic. JAMA Netw Open. 2022;5:e2232314. doi: 10.1001/jamanetworkopen.2022.32314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glicksberg L, Dempsey SK, Casey BK. Heroin and fentanyl in Dallas county: a 5-year retrospective review of toxicological, seized drug, and demographical data. J Forensic Sci. 2022; 10.1111/1556-4029.15155. [DOI] [PubMed]
- 6.Wilson N, Kariisa M, Seth P, Smith HT, Davis NL. Drug and opioid-involved overdose deaths - United States, 2017-8. Morbidity Mortal Wkly Rep. 2020;69:290–97.. doi: 10.15585/mmwr.mm6911a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krawczyk N, Rivera BD, Basaraba C, Corbeil T, Allen B, Schultebraucks K, et al. COVID-19 complications among patients with opioid use disorder: a retrospective cohort study across five major NYC hospital systems. Addiction. 2022;118:857–69. [DOI] [PMC free article] [PubMed]
- 8.Kilmer B, Pardo B, Pujol TA, Caulkins JP. Rapid changes in illegally manufactured fentanyl products and prices in the United States. Addiction. 2022 doi: 10.1111/add.15942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palamar JJ, Cottler LB, Goldberger BA, Severtson SG, Grundy DJ, Iwanicki JL, et al. Trends in characteristics of fentanyl-related poisonings in theUnited States, 2015-2021. Am J Drug Alcohol Abuse. 2022;48:471–80. [DOI] [PMC free article] [PubMed]
- 10.Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- 11.Nicolas C, Zlebnik NE, Farokhnia M, Leggio L, Ikemoto S, Shaham Y. Sex differences in opioid and psychostimulant craving and relapse: a critical review. Pharmacol Rev. 2022;74:119–40.. doi: 10.1124/pharmrev.121.000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–72. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abus Treat. 1993;10:63–6. doi: 10.1016/0740-5472(93)90100-G. [DOI] [PubMed] [Google Scholar]
- 14.Anglin MD, Hser YI, McGlothlin WH. Sex differences in addict careers. 2. Becoming addicted. Am J Drug Alcohol Abus. 1987;13:59–71. doi: 10.3109/00952998709001500. [DOI] [PubMed] [Google Scholar]
- 15.McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers–implications for treatment and prognosis. Am J Addict. 1999;8:300–11. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- 16.Butelman ER, Chen CY, Conybeare RA, Brown KG, Fry RS, Kimani R, et al. Are trait impulsivity and exposure to cannabis or alcohol associated with the age of trajectory of cocaine use? A gender-specific dimensional analysis in humans with cocaine dependence diagnosis. Exp Clin Psychopharmacol. 2019: 10.1037/pha0000314. [DOI] [PubMed]
- 17.Stoltman JJ, Woodcock EA, Lister JJ, Greenwald MK, Lundahl LH. Exploration of the telescoping effect among not-in-treatment, intensive heroin-using research volunteers. Drug Alcohol Depend. 2015;148:217–20. doi: 10.1016/j.drugalcdep.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodcock EA, Lundahl LH, Stoltman JJ, Greenwald MK. Progression to regular heroin use: examination of patterns, predictors, and consequences. Addic Behav. 2015;45:287–93. doi: 10.1016/j.addbeh.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butelman ER, Chen CY, Brown KG, Kreek MJ. Escalation of drug use in persons dually diagnosed with opioid and cocaine dependence: gender comparison and dimensional predictors. Drug Alcohol Depend. 2019;205:107657. doi: 10.1016/j.drugalcdep.2019.107657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch A, Arndt S, Acion L. Late- and typical-onset heroin use among older adults seeking treatment for opioid use disorder. Am J Geriatr Psychiatry. 2021;29:417–25.. doi: 10.1016/j.jagp.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Hansen JL, Heilig M, Kalso E, Stubhaug A, Knutsson D, Sandin P, et al. Problematic opioid use among osteoarthritis patients with chronic post-operative pain after joint replacement: analyses from the BISCUITS study. Scand J Pain. 2023;23:353–63. doi: 10.1515/sjpain-2022-0137. [DOI] [PubMed] [Google Scholar]
- 22.Huhn AS, Strain EC, Tompkins DA, Dunn KE. A hidden aspect of the U.S. opioid crisis: Rise in first-time treatment admissions for older adults with opioid use disorder. Drug Alcohol Depend. 2018;193:142–47.. doi: 10.1016/j.drugalcdep.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spencer MR, Warner M, Cisewski JA, Miniño A, Dodds D, Perera J, et al. Estimates of drug overdose deaths involving fentanyl, methamphetamine, cocaine, heroin, and oxycodone: United States, 2021. Vital Statistics Rapid Release; vol. 27. Hyattsville, MD: National Center for Health Statistics; 2023. 10.15620/cdc:125504.
- 24.Kariisa M, Davis NL, Kumar M, Seth P, Mattson CL, Chowdhury F, et al. Vital signs: drug overdose deaths, by selected sociodemographic and social determinants of health characteristics—25 states and the District of Columbia, 2019–20. 2022. https://www.cdc.gov/mmwr/volumes/71/wr/mm7129e2.htm. [DOI] [PMC free article] [PubMed]
- 25.D.E.A. Drug Enforcement Administration 2020 National Drug Threat Assessment. 2021. https://www.dea.gov/sites/default/files/2021-02/DIR-008-21%202020%20National%20Drug%20Threat%20Assessment_WEB.pdf.
- 26.Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baldo BA, Rose MA. Mechanisms of opioid-induced respiratory depression. Arch Toxicol. 2022;96:2247–60. doi: 10.1007/s00204-022-03300-7. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Kim DI, Oh TG, Pao GM, Kim JH, Palmiter RD, et al. Neural basis of opioid-induced respiratory depression and its rescue. Proc Natl Acad Sci USA. 2021;118:e2022134118. doi: 10.1073/pnas.2022134118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Britch SC, Walsh SL. Treatment of opioid overdose: current approaches and recent advances. Psychopharmacology. 2022;239:2063–81.. doi: 10.1007/s00213-022-06125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly E, Sutcliffe K, Cavallo D, Ramos-Gonzalez N, Alhosan N, Henderson G. The anomalous pharmacology of fentanyl. Br J Pharmacol. 2021;10.1111/bph.15573. [DOI] [PubMed]
- 31.Torralva PR, Janowsky A. Noradrenergic mechanisms in fentanyl-mediated rapid death explain failure of naloxone in the opioid Crisis. J Pharmacol Exp Ther. 2019; 10.1124/jpet.119.258566. [DOI] [PMC free article] [PubMed]
- 32.Roy S, Bruehl S, Feng X, Shotwell MS, Van De Ven T, Shaw AD, et al. Developing a risk stratification tool for predicting opioid-related respiratory depression after non-cardiac surgery: a retrospective study. BMJ Open. 2022;12:e064089. doi: 10.1136/bmjopen-2022-064089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khanna AK, Bergese SD, Jungquist CR, Morimatsu H, Uezono S, Lee S, et al. Prediction of opioid-induced respiratory depression on inpatient wards using continuous capnography and oximetry: an international prospective, observational trial. Anesth Analg. 2020;131:1012–24.. doi: 10.1213/ANE.0000000000004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garrett J, Vanston A, Ogola G, da Graca B, Cassity C, Kouznetsova MA, et al. Predicting opioid-induced oversedation in hospitalised patients: a multicentre observational study. BMJ Open. 2021;11:e051663. doi: 10.1136/bmjopen-2021-051663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dahan A, Sarton E, Teppema L, Olievier C. Sex-related differences in the influence of morphine on ventilatory control in humans. Anesthesiology. 1998;88:903–13. doi: 10.1097/00000542-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Bachi K, Mani V, Jeyachandran D, Fayad ZA, Goldstein RZ, Alia-Klein N. Vascular disease in cocaine addiction. Atherosclerosis. 2017;262:154–62.. doi: 10.1016/j.atherosclerosis.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayanthi S, Daiwile AP, Cadet JL. Neurotoxicity of methamphetamine: main effects and mechanisms. Exp Neurol. 2021;344:113795. doi: 10.1016/j.expneurol.2021.113795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, et al. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology. 1996;125:346–54. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- 39.Evans SM, Haney M, Fischman MW, Foltin RW. Limited sex differences in response to “binge” smoked cocaine use in humans. Neuropsychopharmacology. 1999;21:445–54. doi: 10.1016/S0893-133X(98)00120-1. [DOI] [PubMed] [Google Scholar]
- 40.Singha AK, McCance-Katz EF, Petrakis I, Kosten TR, Oliveto A. Sex differences in self-reported and physiological response to oral cocaine and placebo in humans. Am J Drug Alcohol Abus. 2000;26:643–57. doi: 10.1081/ADA-100101900. [DOI] [PubMed] [Google Scholar]
- 41.Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, et al. Gender differences in response to intranasal cocaine administration to humans. Biol Psychiatry. 1996;39:147–8. doi: 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 42.Luthar SS, Gushing G, Rounsaville BJ. Gender differences among opioid abusers: pathways to disorder and profiles of psychopathology. Drug Alcohol Depend. 1996;43:179–89. doi: 10.1016/S0376-8716(96)01310-5. [DOI] [PubMed] [Google Scholar]
- 43.Bagley SM, Gai MJ, Earlywine JJ, Schoenberger SF, Hadland SE, Barocas JA. Incidence and characteristics of nonfatal opioid overdose among youths aged 11 to 24 years by sex. JAMA Netw Open. 2020;3:e2030201. doi: 10.1001/jamanetworkopen.2020.30201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norton A, Hayashi K, Johnson C, Choi J, Milloy MJ, Kerr T. Injecting drugs alone during an overdose crisis in Vancouver, Canada. Harm Reduct J. 2022;19:125. doi: 10.1186/s12954-022-00701-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho JY. Cycles of gender convergence and divergence in drug overdose mortality. Popul Dev Rev. 2020;46:443–70.. doi: 10.1111/padr.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaplovitch E, Gomes T, Camacho X, Dhalla IA, Mamdani MM, Juurlink DN. Sex differences in dose escalation and overdose death during chronic opioid therapy: a population-based cohort study. PloS one. 2015;10:e0134550. doi: 10.1371/journal.pone.0134550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serdarevic M, Striley CW, Gurka KK, Leeman RF, Cottler LB. Sex differences in prescription opioid use patterns assessed through a community engagement program in Florida. Drug Alcohol Depend. 2019;204:107568. doi: 10.1016/j.drugalcdep.2019.107568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hser YI, Anglin MD, McGlothlin W. Sex differences in addict careers. 1. Initiation of use. Am J Drug Alcohol Abus. 1987;13:33–57. doi: 10.3109/00952998709001499. [DOI] [PubMed] [Google Scholar]
- 49.Goetz TG, Becker JB, Mazure CMWomen. opioid use and addiction. FASEB J. 2021;35:e21303. doi: 10.1096/fj.202002125R. [DOI] [PubMed] [Google Scholar]
- 50.Antoine D, Heffernan S, Chaudhry A, King V, Strain EC. Age and gender considerations for technology-assisted delivery of therapy for substance use disorder treatment: a patient survey of access to electronic devices. Addic Disord Treat. 2016;15:149–56.. doi: 10.1097/ADT.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shover CL, Falasinnu TO, Dwyer CL, Santos NB, Cunningham NJ, Freedman RB, et al. Steep increases in fentanyl-related mortality west of the Mississippi River: recent evidence from county and state surveillance. Drug Alcohol Depend. 2020;216:108314. doi: 10.1016/j.drugalcdep.2020.108314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabatabai M, Cooper RL, Wilus DM, Edgerton RD, Ramesh A, MacMaster SA, et al. The effect of naloxone access laws on fatal synthetic opioid overdose fatality rates. J Prim Care Community Health. 2023; 10.1177/21501319221147246. [DOI] [PMC free article] [PubMed]
- 53.Kerridge BT, Chou SP, Pickering RP, Ruan WJ, Huang B, Jung J, et al. Changes in the prevalence and correlates of cocaine use and cocaine use disorder in the United States, 2001-2 and 2012-3. Addic Behav. 2019;90:250–57. doi: 10.1016/j.addbeh.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Kerridge BT, Saha TD, Chou SP, Zhang H, Jung J, Ruan WJ, et al. Gender and nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions—III. Drug Alcohol Depend. 2015;156:47–56. doi: 10.1016/j.drugalcdep.2015.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skolnick P. Treatment of overdose in the synthetic opioid era. Pharmacol Ther. 2021;233:108019. doi: 10.1016/j.pharmthera.2021.108019. [DOI] [PubMed] [Google Scholar]
- 56.Ahmad FB, Rossen LM, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics. 2021. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm.
- 57.Clayton JA, Tannenbaum C. Reporting sex, gender, or both in clinical research? JAMA. 2016;316:1863–64. doi: 10.1001/jama.2016.16405. [DOI] [PubMed] [Google Scholar]
- 58.Haas AP, Lane AD, Blosnich JR, Butcher BA, Mortali MG. Collecting sexual orientation and gender identity information at death. Am J Public Health. 2019;109:255–59.. doi: 10.2105/AJPH.2018.304829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hedegaard H, Miniño AM, Warner M. Co-involvement of opioids in drug overdose deaths involving cocaine and psychostimulants. NCHS Data Brief No. 406. 2021 https://stacks.cdc.gov/view/cdc/103966. [PubMed]
- 60.Ciccarone D. The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Curr Opin Psychiatry. 2021;34:344–50.. doi: 10.1097/YCO.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tweed EJ, Miller RG, Schofield J, Barnsdale L, Matheson C. Why are drug-related deaths among women increasing in Scotland? A mixed-methods analysis of possible explanations. Drugs. 2022;29:62–75. doi: 10.1080/09687637.2020.1856786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:102–23. doi: 10.2471/BLT.12.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38:425–40. doi: 10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dietrich JB. Alteration of blood-brain barrier function by methamphetamine and cocaine. Cell Tissue Res. 2009;336:385–92. doi: 10.1007/s00441-009-0777-y. [DOI] [PubMed] [Google Scholar]
- 65.Kohno M, Link J, Dennis LE, McCready H, Huckans M, Hoffman WF, et al. Neuroinflammation in addiction: a review of neuroimaging studies and potential immunotherapies. Pharmacol Biochem Behav. 2019;179:34–42. doi: 10.1016/j.pbb.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2021 National Survey on Drug Use and Health. 2022. https://www.samhsa.gov/data/sites/default/files/reports/rpt39443/2021NSDUHFFRRev010323.pdf.
- 67.Ferreira AI, Martinez LF, Guisande MA. Risky behavior, personality traits and road accidents among university students. Eur J Educ Psychol. 2009;2:79–98. doi: 10.30552/ejep.v2i2.23. [DOI] [Google Scholar]
- 68.IIHS. Fatality Facts 2020: Males and females. 2022. https://www.iihs.org/topics/fatality-statistics/detail/males-and-females#yearly-snapshot. 2023.
- 69.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- 70.Pinheiro I, Dejager L, Libert C. X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. Bioessays. 2011;33:791–802. doi: 10.1002/bies.201100047. [DOI] [PubMed] [Google Scholar]
- 71.Deng X, Berletch JB, Nguyen DK, Disteche CM. X chromosome regulation: diverse patterns in development, tissues and disease. Nat Rev Genet. 2014;15:367–78. doi: 10.1038/nrg3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Darnall BD, Stacey BR. Sex Differences In Long-term Opioid Use: Cautionary Notes For Prescribing In Women. Arch Intern Med. 2012;172:431–32. doi: 10.1001/archinternmed.2011.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daiwile AP, Jayanthi S, Cadet JL. Sex differences in methamphetamine use disorder perused from pre-clinical and clinical studies: potential therapeutic impacts. Neurosci Biobehav Rev. 2022;137:104674. doi: 10.1016/j.neubiorev.2022.104674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Donnell J, Tanz LJ, Gladden RM, Davis NL, Bitting J. Trends in and characteristics of drug overdose deaths involving illicitly manufactured fentanyls—United States, 2019–20 2021; 10.15585/mmwr.mm7050e3. https://www.cdc.gov/mmwr/volumes/70/wr/mm7050e3.htm. [DOI] [PMC free article] [PubMed]
- 75.Shai D. Problems of accuracy in official statistics on drug-related deaths. Int J Addic. 1994;29:1801–11. doi: 10.3109/10826089409128258. [DOI] [PubMed] [Google Scholar]
- 76.Thompson K, Barocas JA, Delcher C, Bae J, Hammerslag L, Wang J, et al. The prevalence of opioid use disorder in Kentucky’s counties: a two-year multi-sample capture-recapture analysis. Drug Alcohol Depend. 2023;242:109710. doi: 10.1016/j.drugalcdep.2022.109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reuter P, Caulkins JP, Midgette G. Heroin use cannot be measured adequately with a general population survey. Addiction. 2021;116:2600–09. doi: 10.1111/add.15458. [DOI] [PubMed] [Google Scholar]
- 78.Paschen-Wolff MM, Kidd JD, Paine EA. The state of the research on opioid outcomes among lesbian, gay, bisexual, transgender, queer, and other sexuality- and gender-diverse populations: a scoping review. LGBT Health. 2023;10:1–17. doi: 10.1089/lgbt.2022.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Restar AJ, Jin H, Ogunbajo A, Goedel WC, Millett G, Sherwood J, et al. Prevalence and risk factors of nonmedical prescription opioid use among transgender girls and young women. JAMA Netw Open. 2020;3:e201015. doi: 10.1001/jamanetworkopen.2020.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.