Abstract
Lotus (Nelumbo spp.) is an important aquatic ornamental genus in the family Nelumbonaceae comprising only 2 species: Nelumbo lutea with yellow flowers and Nelumbo nucifera with red or white flowers. The petal color variations between these 2 species have previously been associated with the potential activities of FLAVONOL SYNTHASE (FLS) and MYB5. However, the underlying genetic mechanisms of flower color divergence within the N. nucifera species remain unclear. Here, quantitative trait locus mapping led to the identification of MYB5, a candidate gene controlling petal color in N. nucifera. Genotyping of 213 natural lotus accessions revealed an 80 kb presence/absence variant (PAV) of the NnMYB5 gene that is associated with petal color variation. Transcriptome analysis, dual-luciferase, and yeast 1-hybrid assays showed that NnMYB5 could directly activate the anthocyanin transporter gene GLUTATHIONE S-TRANSFERASE2 (NnGST2). Heterologous expression of NnGST2 in Arabidopsis (Arabidopsis thaliana) and its overexpression in lotus petals induced anthocyanin accumulation. Deletion of the 80 kb PAV within NnMYB5 inactivated NnGST2 expression and blocked anthocyanin accumulation in white N. nucifera petals. In contrast, the anthocyanin deficiency of N. lutea occurred due to pseudogenized NlMYB5 alleles. Our results establish a regulatory link between NnMYB5 and NnGST2 in petal anthocyanin accumulation and demonstrate the independent mechanisms controlling flower coloration in Nelumbo.
An 80 kb presence/absence variant of NnMYB5 controls the petal color in Nelumbo nucifera by regulating the anthocyanin transporter GLUTATHIONE S-TRANSFERASE2 expression.
Introduction
Petal color is typically generated by variously accumulated pigments, which predominantly include anthocyanins. Anthocyanins are a group of ubiquitously occurring polyphenolic plant pigments that produce diverse color phenotypes and antioxidant properties against various stresses (Tanaka et al. 2008). The anthocyanin biosynthesis is a dynamic branch of the flavonoid biosynthetic pathway that is catalyzed by a series of highly conserved enzymes, including PHENYLALANINE AMMONIA-LYASE (PAL), CHALCONE SYNTHASE (CHS), CHALCONE ISOMERASE (CHI), FLAVONOID 3′-MONOOXYGENASE (F3′H), FLAVONOID 3′, 5′-HYDROXYLASE (F3′5H), FLAVANONE 3-HYDROXYLASE (F3H), DIHYDROFLACONOL 4-REDUCTASE (DFR), ANTHOCYANIDIN SYNTHASE (ANS), and UDP-GLUCOSE:FLAVONOID 3-O-GLUCOSYLTRANSFERASE (UFGT), and has extensively been characterized in plants. Moreover, its biosynthesis is regulated by various transcription factors (TFs), such as MYBs and MYB/BASIC HELIX-LOOP-HELIX (bHLH)/WD40 (MBW) protein complexes (Xu et al. 2015).
The cytosolic surface of the endoplasmic reticulum (ER) acts as the site of anthocyanin biosynthesis, which subsequently is transported and stored in the vacuole. Several anthocyanin transporters have been isolated, such as GLUTATHIONE S-TRANSFERASE (GST) (Zhao 2015). Plant GSTs is a gene superfamily encoding multifunctional enzymes that recruit the tripeptide glutathione as coenzymes or cosubstrate for cellular activity (Vaish et al. 2020). Plant GSTs are divided into 9 subfamilies, with the plant specific phi (F) subfamily members being predominantly involved in anthocyanin transport (Edwards and Dixon 2005). The first anthocyanin-associated GST was reported in maize (Zea mays) (Marrs et al. 1995). The maize bronze-2 (bz2) mutants accumulate anthocyanin in the cytoplasm, where it is rapidly oxidized and polymerized, leading to brown rather than red kernels. Functional characterization of this mutant gene revealed that ZmBZ2 encodes a phi GST transporter. Subsequent studies have characterized anthocyanin GST transporters in other plants, such as ANTHOCYANIN9 (AN9), TRANSPARENT TESTA 19 (TT19), and REDUCED ANTHOCYANINS IN PETIOLES (RAP) in petunia (Petunia hybrida), Arabidopsis (Arabidopsis thaliana), and common strawberry (Fragaria ananassa), respectively (Alfenito et al. 1998; Kitamura et al. 2004; Luo et al. 2018).
Lotus (Nelumbo spp.) is an aquatic primitive eudicot plant that belongs to the family Nelumbonaceae (Shen-Miller 2007). Due to its high ornamental values, such as unique fragrance, bright petal color, and variable flower shape, lotus is considered as one of the most popular traditional flowers in China. The genus Nelumbo contains only 2 species, American lotus (Nelumbo lutea Willd.) and Asian lotus (Nelumbo nucifera Gaertn.) (Lin et al. 2019), and flower color is the most distinctive phenotypic variation between the 2 species, with the former only having yellow flowers, while the latter has white or red flowers. Numerous lotus cultivars with dual color petals have been developed by interspecific and intraspecific hybridization (Lin et al. 2019).
Similar to other ornamentals, anthocyanins are the major pigments determining the red lotus flower color (Yang et al. 2009). Anthocyanin content is positively associated with petal redness, and the red lotus cultivars predominantly accumulate anthocyanins compared with the pink varieties, while no anthocyanin is accumulated in the white and yellow cultivars (Deng et al. 2013). Deciphering the mechanisms of inter- and intraspecies pigment divergence in Nelumbo is currently a trending research topic. A recent integrated metabolite and transcriptome study between the yellow and white flower lotus cultivars identified 18 candidate genes potentially associated with the yellow color formation, such as FLAVONOL SYNTHASE (FLS) gene, which encodes a crucial flux enzyme in the flavonol biosynthesis pathway (Wu et al. 2022). The competition for substrate between FLSs and other biosynthetic enzymes contributes to the high quercetin derivatives in the yellow flowers of N. lutea (Liu, Wang, et al. 2022). Allelic variation in NnMYB5 has previously been reported to likely contribute to flower color difference between N. lutea and N. nucifera (Sun et al. 2016). A comparative genomic analysis revealed structural variations (SVs) adjacent to NnMYB5 between the 2 Nelumbo species, and transient expression of this gene induced anthocyanin accumulation in lotus petal (Zheng et al. 2022).
The genetic basis of color differentiation within the N. nucifera species has previously been explored. Comparative proteomic analysis of red and white N. nucifera cultivars identified 4 enzymes involved in anthocyanin biosynthesis and its trafficking pathway, including F3H, ANS, UFGT, and GST (Deng et al. 2015). Moreover, the study observed different methylation intensities on the promoter of ANS gene, which potentially resulted in different flower coloration between the red and white N. nucifera cultivars. The flower color of a single lotus cultivar is stable under different external environmental conditions and can stably be inherited by offspring, which suggest that flower color differentiation within N. nucifera is predominantly regulated by genetic factors. However, the genetic basis underlying the regulation of color diversity in N. nucifera is yet to be reported.
In this study, we conducted quantitative trait locus (QTL) mapping using an F2 population derived from crosses between the red and white petal cultivars to identify the genetic loci controlling petal color divergence in N. nucifera. Utilizing the recently reported high-density genetic linkage maps (Liu, Zhang, et al. 2022), a QTL with high logarithm of odds (LOD) value on linkage group 3 (LG3) was detected. After bulk sequencing and gene expression analysis, a putative petal color regulator gene, designated as NnMYB5, was identified. Comparative genomic analysis revealed that an 80 kb presence/absence causal variant harboring the NnMYB5 gene was responsible for color diversity between the red and white cultivars. Further analyses identified an anthocyanin transporter NnGST2 as the downstream target gene of NnMYB5. Transgenic assays in Arabidopsis and lotus confirmed the role of NnGST2 in anthocyanin transport and accumulation. Our finding sheds light on the molecular mechanism of flower color diversity in N. nucifera and provides molecular markers for flower color improvement during lotus breeding.
Results
NnMYB5 is a candidate regulator of petal color variation in N. nucifera
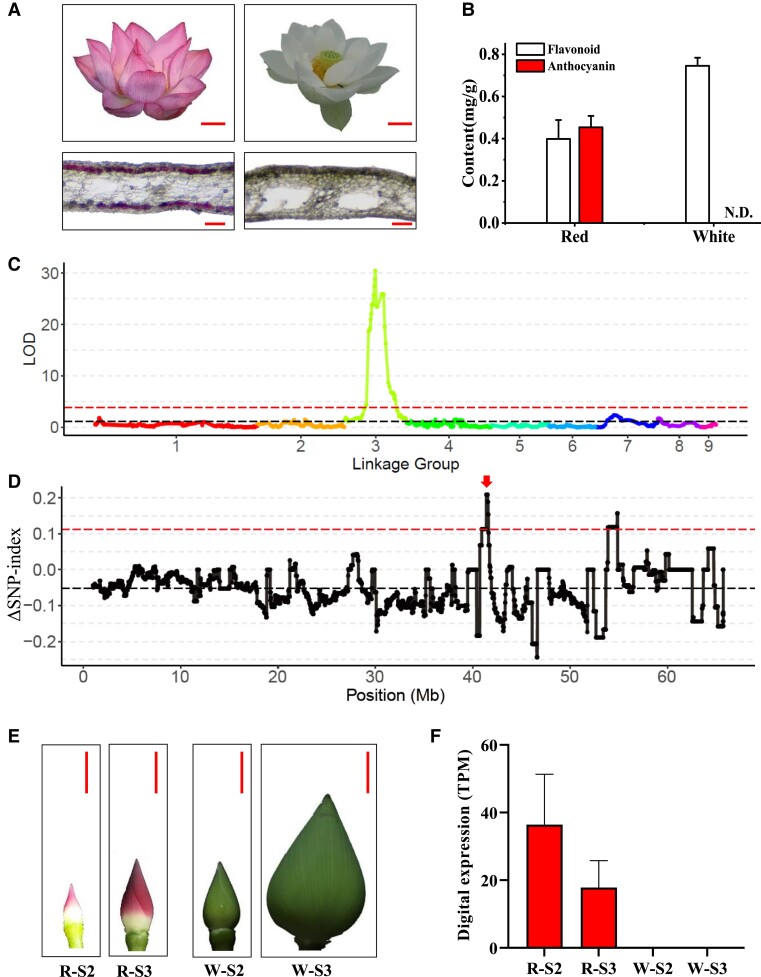
A QTL mapping of an F2 population derived from a cross between the white flower cultivar ‘BG’ and red flower cultivar ‘WR1' was conducted to identify putative genes controlling the flower color phenotype in N. nucifera (Fig. 1A). High anthocyanin content was detected in red lotus petals but with no accumulation in white petals (Fig. 1B). Of the 125 F2 individuals, 95 exhibited red flower phenotype, while the remaining (30 individuals) had white flowers, showing a segregation ratio of 3:1 (χ2 test P = 0.796) for a single-gene controlled trait. Using the recently reported high-density genetic linkage map constructed with this F2 population (Liu, Song, et al., 2022), a QTL on LG3 at the 22.87 to 60.57 cM region was found to be significantly associated with flower color (Fig. 1C). The phenotypic variation explained by the QTL was 85% (30.41 LOD). When anchored on the lotus reference genome, the QTL interval covered 27.75 Mb region on megascaffold 5 from 24.15 to 51.90 Mb (Fig. 1C). To narrow down the interval, 25 red and 23 white lotus accessions were grouped into 2 pools for bulked segregant analysis (BSA). After calculating allele frequencies for the 2 pools in all polymorphic sites across megascaffold 5, the QTL interval was narrowed to a 1 Mb region within the range of 40.75 to 41.75 Mb and a 2 Mb region within the range of 52.89 to 54.89 Mb (Fig. 1D).
Figure 1.
QTL mapping of petal color trait in N. nucifera. A) Flowers of the parental genotype in the mapping population. The upper images show the paternal flower ‘WR1' (left) and maternal flower ‘BG’ (right). Scale bar = 3 cm. The lower images are petal sections showing pigment accumulation in the abaxial and adaxial epidermis of ‘WR1' petals, but not in the ‘BG' petals. Scale bar = 300 μm. B) The anthocyanin and other flavonoid contents in the red and white petals. N.D., not detected. C) Mapping of major QTL for petal color phenotype on LG3. The black and red dashed line represent median of LOD and significant limit of LOD threshold of 3.895 at P = 0.05 (1,000 permutation), respectively. D) ΔSNP index across megascaffold 5 between the white and red lotus accessions. The black dots show the mean ΔSNP in a 1 Mb sliding window with 20 kb increments. The black dashed line represents the mean ΔSNP across the region, while the red dashed lines represent 95% CI. Red arrow shows the target interval related to petal color. E) Petal developmental stages used for high-throughput RNA-seq. R-S2 and R-S3 (W-S2 and W-S3) represent petals from N. nucifera cv. ‘QX’ (‘ZGWS’) flowers at developmental stages 2 and 3, respectively. Scale bar = 1 cm. The images in panels A) and E) were digitally extracted for comparison. F) The digital expression profiling of candidate NnMYB5 (NNU_03972) gene between the ‘QX' and ‘ZGWS' petals at 2 stages. TPM, transcripts per kilobase of exon model per million mapped reads. Data are mean ± Sd (n = 3).
Forty-one and 39 annotated genes were identified in the candidate regions, and their expression profiles were determined at the R-S2 and R-S3 or W-S2 and W-S3 developmental stages in the red or white flower petals, respectively, using the RNA-seq TPM data (Fig. 1E). Interestingly, all the 80 genes showed no differential expression patterns between the 2 different petal phenotypes. Consequently, the candidate interval was expanded by 250 kb at both ends, and the expression profiles of genes in the 4.0 Mb region (40.50 to 42.00 Mb and 52.64 to 55.14 Mb) were analyzed. As a result, 62 and 48 genes were detected in the target regions, of which, NNU_03972 in the first interval, annotated as NnMYB5, showed high expression levels in red petal, but with no expression in the white petals (Fig. 1F and Supplemental Fig. S2). NnMYB5 has been demonstrated to be involved in lotus petal color variation, whose natural variation coincided with petal color, and the function of NnMYB5 in anthocyanin accumulation was validated by overexpression in Arabidopsis and transient expression in lotus (Sun et al. 2016; Gao et al. 2022). Based on its unique profile in the red petals and previous reports, NnMYB5 was deemed as a key candidate gene controlling petal color diversity in N. nucifera.
An 80 kb presence/absence variant (PAV) harboring NnMYB5 is associated with the petal color phenotype in N. nucifera
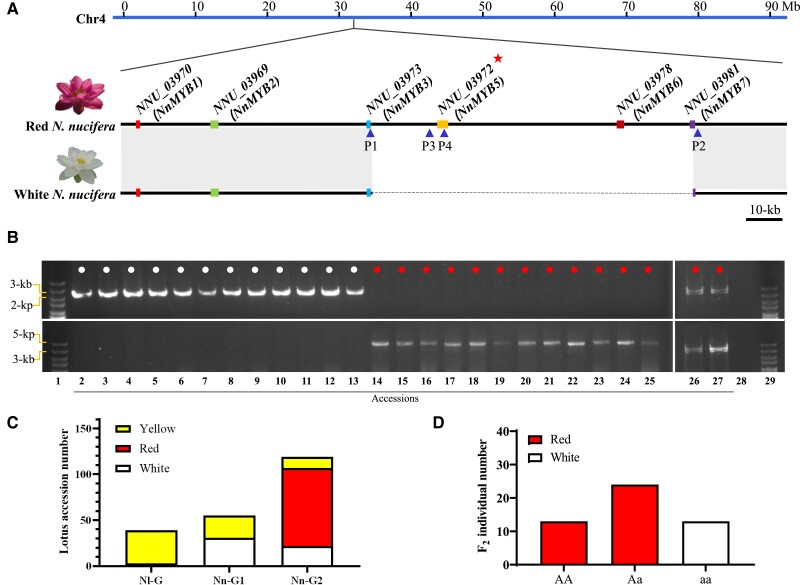
The candidate NnMYB5 gene located at 41.91 Mb on megascaffold 5, corresponding to the 33.77 Mb region on Chr 4 in the reference genome of the red flower N. nucifera, occurred within a 158 kb region containing a 6-member MYB gene cluster, including NnMYB2 (NNU_03970), NnMYB3 (NNU_03969), NnMYB4 (NNU_03973), NnMYB5 (NNU_03972), NnMYB6 (NNU_03978), and NnMYB7 (NNU_03981) (Fig. 2A). Subsequently, the resequencing data of 213 natural lotus accessions was analyzed to determine sequence variation of NnMYB5 between the red and white flower cultivars. The BLAST analysis against the resequenced lotus genomes revealed 3 NnMYB5 sequence genotypes, designated Nl-G, Nn-G1, and Nn-G2. The Nl-G genotype was a pseudogene caused by single-nucleotide polymorphism (SNPs) and small insertions and deletions (InDels) specific in N. lutea, and 92.31% of accessions with this genotype had yellow flowers. The Nn-G1 genotype was due to the absence of NnMYB5 caused by the 80 kb deletion, and this genotype occurred in N. nucifera with white (56.36%) or yellow (43.64%) flowers. The Nn-G2 genotype contained a functional NnMYB5 gene without the 80 kb deletion and was present in the red, white, and yellow N. nucifera flowers in proportions of 71.43%, 18.49%, and 10.08%, respectively (Fig. 2B). These results strongly suggested that the absence of NnMYB5 could result in the lack of anthocyanin accumulation in flower petals. Genotyping showed that the yellow flower lotus accessions were hybrids of N. nucifera and N. lutea. Further sequence variation analysis between the red and white N. nucifera revealed the presence of an 80 kb PAV on Chr 4 of the lotus genome, which harbored 3 MYB genes, including NnMYB5, NnMYB6, and partial NnMYB7 (Fig. 2A). Gene expression analysis showed that NnMYB5 was specifically expressed in the red lotus petals (Supplemental Fig. S3).
Figure 2.
An 80 kb PAV on Chr 4 controlling petal color divergence in N. nucifera. A) Physical maps of the 80 kb PAV between lotus cultivars with white or red flowers on Chr 4 showing physical locations of members of the MYB gene cluster marked in different colored boxes. The dashed line shows the 80 kb deletion in lotus cultivars with white flower. NnMYB5 is highlighted with red asterisk. Blue triangles at the bottom represent location where the 2 pairs of primers were developed. B) Genotyping of N. nucifera accessions to verify the association between PAV and petal color. Red dots indicate N. nucifera accessions with red flower, and white dots indicate accessions with white flower. DNA fragments on the upper and lower were amplified with the P1/P2 and P3/P4 primer pairs, respectively. C) Distribution of 213 lotus accessions with different petal colors based on the 3 genetic variations at the PAV locus. Nl-G represents genotype from N. lutea, while Nn-G1 or Nn-G2 represents N. nucifera genotypes harboring or lacking the 80 kb PAV, respectively. D) Genotype distribution of 50 F2 individuals derived from a cross between ‘BG’ and ‘WR1.'
To determine the association between PAV and anthocyanin deficiency in lotus petals, a pair of primers covering this PAV was designed in its flanking regions for PCR amplification, followed by Sanger sequencing for PAV sequence confirmation. In addition, another primer pair was designed to amplify the NnMYB5 genomic sequence in 14 red and 12 white lotus cultivars. PCR results showed that all 12 white flower accessions contained this 80 kb deletion of the PAV region, while the red cultivars contained an 80 kb homozygous or heterozygous PAV segment (Fig. 2C and Supplemental Table S2). Moreover, PCR screening of 50 segregating F2 population offspring derived from ‘BG’ and ‘WR1' revealed 3 genotypes, designated AA, Aa, and aa with a segregation ratio of 1:2:1 (Fig. 2D). The white individuals had aa genotype, while red individuals had Aa or AA genotypes (Supplemental Fig. S4). These results supported the hypothesis that the flower color variation in N. nucifera was affected by the 80 kb PAV harboring the NnMYB5 gene.
NnMYB5 targets and interacts with the anthocyanin transporter NnGST2 gene in N. nucifera
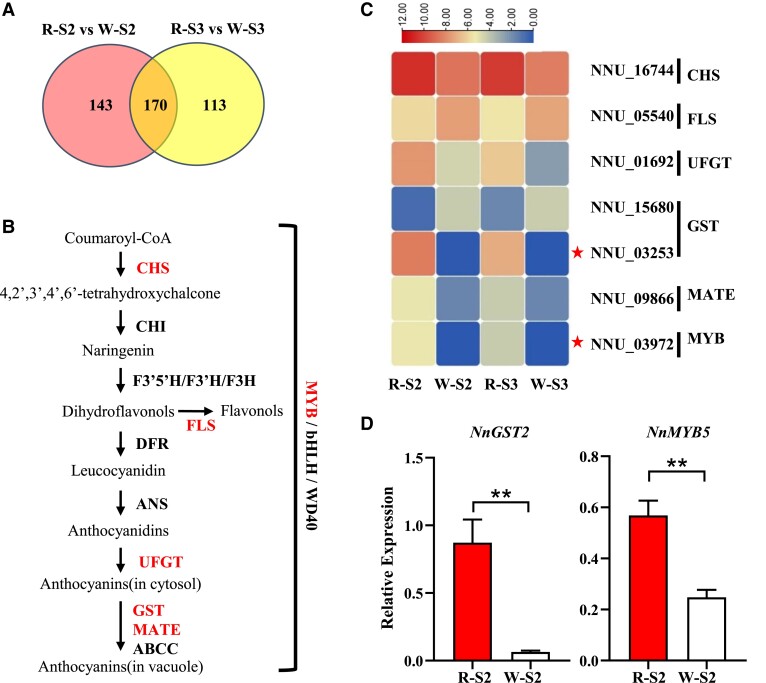
Comparative transcriptome analysis between the red and white petals was conducted to explore the regulatory mechanism of the 80 kb PAV in N. nucifera petal color variation. As a result, 283,294,832 clean reads were obtained from 12 petal libraries. The mapping ratio of each sample against the reference genome ranged from 90.46% to 92.82% (Supplemental Table S3). A total of 313 DEGs were identified between the R-S2 and W-S2 stages, including 137 up- and 176 downregulated genes. Similarly, 283 DEGs were identified between the R-S4 and W-S4, including 124 up- and 159 downregulated genes. Among the DEGs between the red and white petals at the 2 stages, 170 genes were commonly shared, with 87 and 83 exhibiting up- and downregulated expression, respectively (Fig. 3A and Supplemental Table S4). Gene Ontology (GO) enrichment showed that the 170 commonly shared genes were enriched in biological processes, such as anthocyanin containing compound biosynthesis, as well as functional molecular categories related to secondary metabolite transport, such as drug transmembrane transporter activity (Supplemental Fig. S5).
Figure 3.
Transcriptome profiles of genes putatively involved in the anthocyanin biosynthetic pathway. A) Venn diagram showing the number of overlapping DEGs between the ‘QX' and ‘ZGWS' lotus petals in 2 developmental stages. R-S2 and R-S3 (W-S2 and W-S3) represent petals from N. nucifera cv. ‘QX’ (‘ZGWS’) flowers at developmental stages 2 and 3, respectively. B) Schematic diagram of anthocyanin biosynthesis pathway. Genes highlighted in red were differentially expressed between the 2 developmental stages. C) Expression patterns of anthocyanin-related DEGs. The red asterisks indicate candidate genes analyzed in this study. The color scale indicates the log2FC values of DEGs. D) RT-qPCR expression patterns of candidate genes in petal at the S2 developmental stage. Data are mean ± Sd (n = 3). Double asterisks indicate statistical significance at P < 0.01 (t test).
Most anthocyanin biosynthesis genes showed no significant differential expression between the red and white petals (Fig. 3, B and C). The CHS gene (NNU_16744) that catalyzes the first committed step in the flavonoid biosynthetic pathway displayed significantly higher expression levels in the red lotus petals. Conversely, the FLS gene (NNU_05540), which controls the flux of flavonol rather than the anthocyanin biosynthetic pathway, showed significantly lower expression levels in the red lotus petals. One UFGT gene (NNU_01692) was considerably upregulated in the red lotus petals relative to white petals. Three anthocyanin transporter genes, including 2 GSTs, NNU_15680 and NNU_03253 as well as a MATE transporter TT12 gene (NNU_09866), were differentially expressed between the red and white lotus petals. The expression level of an anthocyanin transporter gene NNU_03253, named NnGST2, corresponded with that of NnMYB5, with both being highly expressed in the red petals, but absent in the white petals. Previous experiments showed that the expression of NnGST2 was upregulated by overexpression of NnMYB5 in Arabidopsis (Sun et al. 2016). In addition, NnGST2 was likely to be involved in petal color variation based on allele-specific expression in F1 hybrids (Gao et al. 2022). Based on these results, NnGST2 was designated as a candidate downstream target gene of NnMYB5 (Fig. 3D).
The candidate NnGST2 gene is an anthocyanin-related GST putative ortholog in N. nucifera
GSTs are ubiquitous multifunctional plant enzymes that are encoded by a large gene family. In this study, 67 GSTs were identified in the N. nucifera genome, which were phylogenetically grouped in 9 classes, including phi (F), tau (U), lambda (L), zeta (Z), dehydroascorbate reductase (DHAR), glutathionyl hydroquinone reductase (GHR), theta (T), tetrachloro-hydroquinone dehalogenase (TCHQD), and elongation factor 1Bγ (EF1Bγ) (Supplemental Figure S6A). Of these 9 GST categories, members of the phi class have previously been associated with the transport and metabolism of secondary compounds, such as anthocyanins, and 10 phi class NnGSTs were detected in this study (Supplemental Fig. S6A).
Expression profiling of these 10 NnGSTs in the petiole, leaf, petal, pistil, stamen, and rhizome tissues of the red lotus using a previously reported RNA-seq FPKM data revealed that NnGST1, NnGST4, NnGST7, and NnGST8 were predominantly expressed in root, pistil, stamen, and petiole tissues, respectively (Supplemental Figure S6B). NnGST3 was mainly expressed in pistil, petiole, and leaf tissues, while NnGST6, NnGST9, and NnGST10 were highly expressed in the lotus petiole, petal, and root organs. NnGST5 showed preferential expression in the lotus petal, stamen, and root tissues. Notably, NnGST2 was highly expressed specifically in the lotus petals, suggesting its potential regulatory function in the lotus petal color formation.
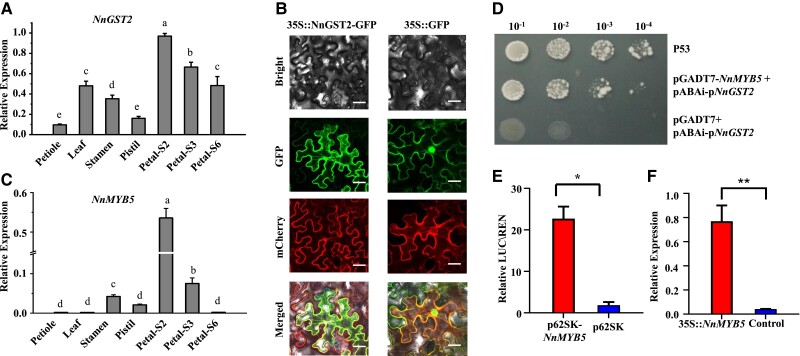
RT-qPCR analysis to validate the tissue expression profiles of NnGST2 and determine its biological function in petal color formation revealed that the candidate gene was highly expressed in the lotus petals at early developmental stages before decreasing in later stages, which was consistent with anthocyanin accumulation patterns (Fig. 4A). Subcellular localization of NnGST2 showed that green GFP signal of 35S::NnGST2-GFP fusion protein overlapped with the red mCherry signal, which indicated ER localization of NnGST2 (Fig. 4B).
Figure 4.
Functional analysis of NnGST2. A) RT-qPCR expression profiles of NnGST2 in different lotus flower tissues. Different alphabetical letters indicate statistical significance at P < 0.05 (1-way ANOVA with Tukey's test). Data are mean ± Sd (n = 3). S2 and S3 were N. nucifera cv. ‘QX’ petals at stages 2 and 3 as shown in Fig. 1E, while S6 was petal from first day opening flower. B) Subcellular localization of NnGST2 in N. benthamiana leaf. Leaves expressing 35S::GFP were used as the control. The green fluorescent protein (GFP) and ER located mCherry proteins are stained with green and red hues, respectively. C) RT-qPCR expression profiles of NnMYB5 in different lotus flower tissues. Different alphabetical letters indicate statistical significance at P < 0.05 (1-way ANOVA with Tukey's test). Data are mean ± Sd (n = 3). D) The Y1H assay showing the interaction between NnMYB5 and the NnGST2 promoter. From left to right, the yeast concentration at OD600 = 0.1, 0.01, 0.001, and 0.0001, respectively. P53 was used as positive control and pGADT7 + pABAi-pNnGST2 as negative control. E) Dual-luciferase assay showing NnMYB5 activation capacity on the NnGST2 promoter. LUC\REN: the ratio of firefly luciferase activities to renilla luciferase activities. p62SK was negative control. Single asterisk (*) indicates statistical significance at P < 0.05 (t test). Data are mean ± Sd (n = 3). F) Transcript levels of NnGST2 in transgenic petals expressing 35S::NnMYB5. Double asterisk (**) indicates statistical significance at P < 0.01 (t test). Data are mean ± Sd (n = 3).
NnMYB5 is a transcriptional activator of NnGST2
Transcriptional regulators, such as MYBs, bHLH, WRKY, and WD40 proteins, play pivotal roles in the biosynthesis of anthocyanins. Pearson correlation analysis revealed a highest coefficient of r = 0.95 between the expression levels of NnGST2 and NnMYB5 (Supplemental Fig. S7). Moreover, similar trends in the expression patterns of NnMYB5 and NnGST2 were observed (Fig. 4, A and C), with the former showing predominant expression in lotus petals at the S2 stage, which coincided with rapid anthocyanin accumulation.
Yeast 1-hybrid (Y1H) assay to verify the regulatory role of NnMYB5 revealed that it could bind to the NnGST2 promoter (Fig. 4D). Dual-luciferase assays in Nicotiana benthamiana leaf showed that NnMYB5 could significantly induce the expression of firefly luciferase driven by the NnGST2 promoter, which demonstrated its capacity to induce the expression of NnGST2 (Fig. 4E). Moreover, transient expression of NnMYB5 in lotus petals could upregulate the transcript levels of NnGST2, which was consistent with the transactivation capacity of NnMYB5 on the NnGST2 promoter (Figs. 4F and Supplemental Fig. S8). Further Y1H screening assay to determine the potential role of NnMYB5 in the regulation of other anthocyanin biosynthesis genes revealed the NnFLS gene as its likely target, and LUC assay indicated that NnMYB5 could inhibit NnFLS transcription (Supplemental Fig. S9).
Ectopic overexpression of NnGST2 promotes anthocyanin accumulation in the Arabidopsis tt19 mutants
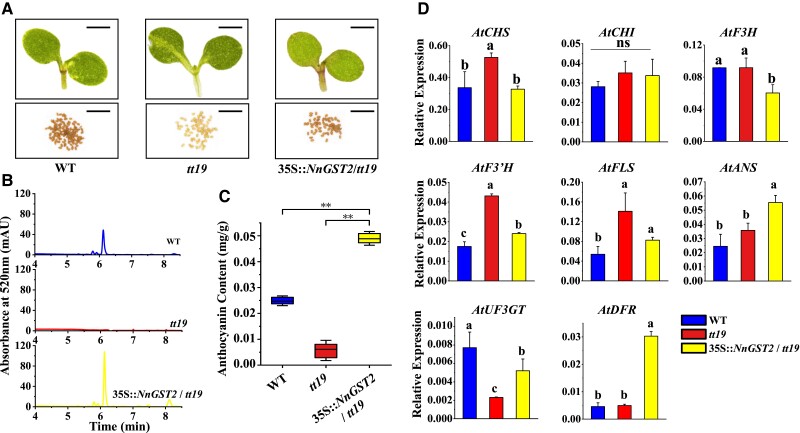
Complementation assay to validate the functional role of NnGST2 in anthocyanin transport was carried out in Arabidopsis tt19 harboring a mutant anthocyanin transporter GST-TT19, and 3 distinct 35S::NnGST2 transgenic lines with similar phenotypes were obtained. Notably, a green hypocotyl of stem in the tt19 mutant was observed in the 5-d-old Arabidopsis seedlings, while the 35S::NnGST2/tt19 transgenic line could not only rescue the red pigment phenotype but also produce normal seeds color in tt19 (Fig. 5A). HPLC results showed significantly higher anthocyanin content in the 35S::NnGST2/tt19 line than in the wild-type (WT) or tt19 (Fig. 5, B and C). Moreover, expression profiling of structural anthocyanin biosynthetic genes in the WT, tt19 mutant, and transgenic lines indicated that the early biosynthesis genes, such as AtCHS, AtCHI, and AtF3H, had similar expression profiles, while late biosynthesis genes leading to other metabolic branches, such as AtF3H and AtFLS, had decreased expression profiles in the 35S::NnGST2/tt19 lines (Fig. 5D). In contrast, the expression of key anthocyanin biosynthesis genes, such as AtDFR, AtANS, and AtUF3GT, was upregulated in the transgenic lines.
Figure 5.
NnGST2 rescues anthocyanin deficiency in Arabidopsis tt19 mutants. A) Phenotypes of 7-d-old WT, tt19, and 35S::NnGST2/tt19 Arabidopsis seedlings and their corresponding mature seeds. Images were digitally extracted for comparison. Scale bar = 500 µm. B) HPLC chromatograms of anthocyanin profile in the WT, tt19, and 35S::NnGST2/tt19 seedlings. C) Anthocyanin contents in the WT, tt19, and 35S::NnGST2/tt19 seedlings calculated based on the peak area method. Double asterisks (**) indicate statistical significance at P < 0.01 (t test). Data are mean ± Sd (n = 3). The boxplot elements are defined as follows: centerline, median; box limits, upper and lower quartiles; and whiskers, 1.5× interquartile range. D) The expression of anthocyanin biosynthesis genes in the WT, tt19, and 35S::NnGST2/tt19 transgenic lines. Different alphabetical letters show significant differences at P < 0.05 (1-way ANOVA with Tukey's test). Data are mean ± Sd (n = 3).
Transient expression of NnGST2 alters lotus petal coloration
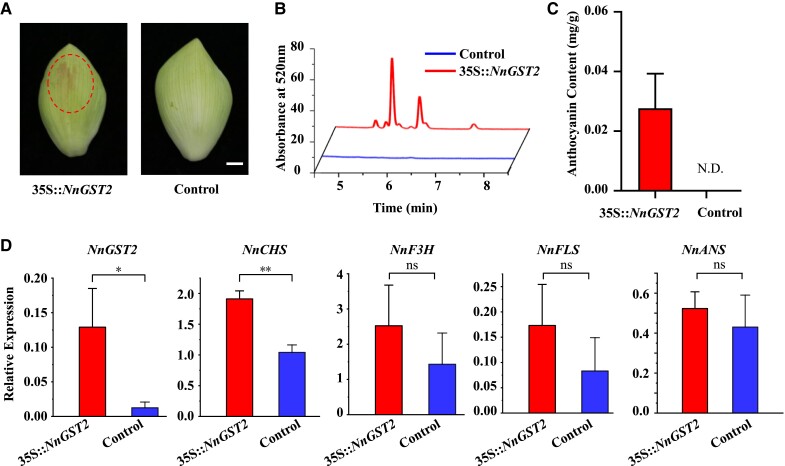
Transient expression of NnGST2 in the white lotus petals revealed that petals turned red after infiltration with 35S::NnGST2, while no pigment accumulation was observed in the control petals (Fig. 6A). Similarly, anthocyanin content and NnGST2 transcript abundance in the infiltrated sites were significantly higher than those of control petals (Fig. 6, C and D). Moreover, the transcript levels of NnCHS were upregulated in the 35S::NnGST2 infiltrated petals, whereas the expression levels of NnFLS, NnANS, and NnF3H displayed no significant differences (Fig. 6D). These results demonstrated the critical role of NnGST2 in anthocyanin accumulation and lotus petal coloration, potentially by altering both anthocyanin transport and biosynthesis processes.
Figure 6.
Transient expression of NnGST2 induces anthocyanin accumulation in the white lotus petals. A) The phenotype of lotus petal transiently overexpressing NnGST2. Scale bar = 1 cm. B) HPLC chromatogram of anthocyanin profile in the control and petals transiently overexpressing 35S::NnGST2. C) Anthocyanin content in the control and transgenic petals overexpressing 35S::NnGST2. N.D., not detected. D) Transcript levels of anthocyanin biosynthesis genes in the control and petals overexpressing 35S::NnGST2. Data are mean ± Sd (n = 3). Asterisk (*) indicates statistical significance at P < 0.05, and double asterisk (**) indicates statistical significance at P < 0.01 (t test) in C) and D). ns, no significant difference.
Discussion
A PAV of NnMYB5 is associated with petal color in N. nucifera
Anthocyanins are pigments responsible for the red flower color in N. nucifera. The anthocyanin biosynthesis is genetically controlled by structural and regulatory genes, with MYB TFs as the core transcriptional regulators. Natural variations in MYB genes have been previously demonstrated to alter the color phenotypes of organs in numerous plants. For example, a 487 bp deletion in the promoter of PpMYB10.1 was shown to be associated with the flesh color around the peach stone (Guo et al. 2020). An insertional long terminal repeat (LTR) retrotransposon upstream of the MdMYB1 gene could be attributed to the red apple skin color (Zhang et al. 2019). Natural SNP variations in the MYB recognition site at the DFR promoter were reported to cause differential DFR gene expression and distinct anthocyanin accumulation in fruits of Solanum species (Wang, Lu, et al. 2022). In this study, a petal color QTL harboring an MYB gene cluster was identified, among which only NnMYB5 gene was differentially expressed between the white and red lotus petals, suggesting its potential role in the N. nucifera petal color variation (Fig. 1).
SVs are DNA sequence rearrangements across or within genomes that range from SNPs to large-scale PAVs and inversions or translocations. Large SVs are not easily detectable, and they have greater influence on the expression and function gene compared with SNPs or small InDels (Chiang et al. 2017). Here, comparative genomic analysis identified an 80 kb PAV containing a NnMYB5 gene, which was highly associated with N. nucifera petal color variation (Fig. 2). This result is consistent with several recent studies demonstrating the effects of large SVs on the phenotypic variations within and between plant species. For example, a 1.7 Mb inversion was reported to cause the flat peach fruit trait by activating the OVATE FAMILY PROTEIN1 (PpOFP1) gene located around its breakpoints (Zhou et al. 2021). Similarly, a large inversion SV at the I locus associated with the regulation of seed coat color by silencing the expression of anthocyanin biosynthetic CHS gene during soybean domestication has recently been reported (Xie et al. 2019). Moreover, a pan-genome analysis of 29 soybean accessions verified the association between the inversion SV and yellow seed coat color, and several additional SV events accompanying the inversion and CHS gene duplication in different haplotypes were detected (Liu et al. 2020). This study reports the occurrence and effects of a large PAV on the regulation of lotus petal color.
Two independent allelic variants of MYB5 results in intra- and interspecies petal color diversity in Nelumbo
The lack of anthocyanin accumulation in the white petals of N. nucifera and yellow petals of N. lutea is potentially associated with distinct genetic mechanisms between the 2 species. The allelic variants of MYB5 have been implicated in the color differentiation between the 2 Nelumbo species, and multiple SNPs and InDels have been detected in the coding and promoter regions of MYB5 gene in N. nucifera and N. lutea (Sun et al. 2016; Zheng et al. 2022). A premature translational termination of MYB5 due to SNPs in its exons caused nonfunctional alleles with no capacity to regulate anthocyanin biosynthesis pathway in N. lutea (Sun et al. 2016). A recent transient expression assay demonstrated the role of NnMYB5 in the regulation of anthocyanin accumulation in lotus (Zheng et al. 2022). In this study, pigment deficiency in the white N. nucifera cultivars was shown to be caused by the deletion of a large genomic fragment harboring the crucial NnMYB5. The petal color difference between the white N. nucifera and yellow N. lutea was attributed to a deletion of NnMYB5 and SNP/InDel mutations in NlMYB5 that affected the downstream regulatory network. The 80 kb deletion was detected in some heterozygous hybrid red flower cultivars. Hybrids of white N. nucifera and N. lutea exhibited yellow flowers, which supported the conclusion that MYB5 mutations in N. lutea and deletion in the white N. nucifera cultivars were both functional and not complementary. Consistently, 2 independent mutations in the strawberry FaMYB10-2 gene were also shown to cause natural variation in fruit skin and flesh color (Castillejo et al. 2020). These results provide vital information for future lotus genetic improvement.
NnMYB5-mediated activation of NnGST2 regulates anthocyanin transport in N. nucifera
GSTs are crucial regulators of anthocyanin translocation from the ER into vacuoles (Zhao 2015). The subcellular localization assay of NnGST2 showed GFP signal in the ER (Fig. 4). Transgenic expression in the Arabidopsis tt19 mutants and lotus petals for functional validation of NnGST2 further demonstrated its involvement in anthocyanin transport and accumulation, with predominant expression in the red cultivars indicating its role in N. nucifera petal color divergence (Figs. 5 and 6). A recent study on the allele-specific expression analysis in F1N. nucifera and N. lutea hybrids also predicted the GST2 gene, designated as GSTF11, to be the key hub gene controlling color divergence between the 2 Nelumbo species (Gao et al. 2022). Overall, these findings indicated that anthocyanin translocation play vital roles in lotus color formation.
A previous gene coexpression analysis showed that both cis- and trans-acting regulatory capacity of GSTF11 could contribute to color divergence in N. nucifera and N. lutea (Gao et al. 2022). In contrast to interspecies sequence variations, no intraspecies SNPs or InDels were detected on the genomic region of GST2 in N. nucifera, suggesting the trans regulatory effects, especially due to TFs that regulate the intraspecies expression levels of NnGST2. MYBs have extensively been reported to regulate anthocyanin accumulation through transcriptional activation or suppression of pathway structural genes (Xu et al. 2015). However, increasing evidence shows that the MYB TF family is also involved in the anthocyanin transport. For example, a key strawberry fruit pigmentation RAP (reduced anthocyanin in petioles) gene that encodes a GST transporter was shown to act downstream of the fruit-specific FvMYB10 gene (Luo et al. 2018). In apple, an MdMYB1 protein could directly bind to the promoter of MdGSTF6 and activate its expression (Jiang et al. 2019). In addition, an anthocyanin biosynthetic MYB activator, LEGUME ANTHOCYANIN PRODUCTION 1 (LAP1), could bind to the MtGSTF7 promoter and activate its expression in Medicago (Wang, Chen, et al. 2022). In this study, dual-luciferase and Y1H assays revealed that NnGST2 was directly targeted and interacted with NnMYB5, which is a key of anthocyanin biosynthetic pathway regulator in lotus petal (Fig. 4). These findings revealed that the NnMYB5-activated NnGST2 gene could regulate anthocyanin transport in the red N. nucifera petals, which comprehensively demonstrated the molecular regulatory mechanism of anthocyanin accumulation in lotus.
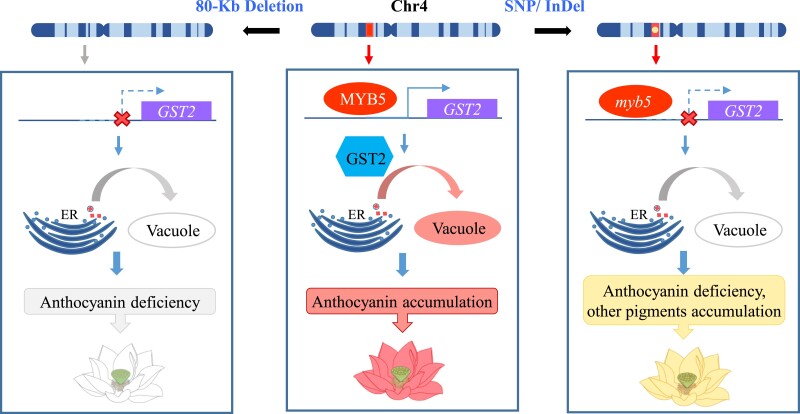
In summary, this study reports a PAV harboring NnMYB5 that controls petal color variation between the white and red N. nucifera. Missing of NnMYB5 that failed to activate NnGST2 is responsible for the observed anthocyanin deficiency in the white lotus petals. Ectopic overexpression of NnGST2 in Arabidopsis and transient expression in lotus validated its role in anthocyanin translocation. This study uncovered the molecular mechanism underlying petal color differentiation within the N. nucifera species and demonstrated the role of 2 independent NnMYB5 mutations in the control of petal color variation in the genus Nelumbo (Fig. 7).
Figure 7.
Hypothetical model illustrating the genetic mechanism underlying petal color variation in Nelumbo. In N. nucifera cultivars with red flower, MYB5 activates the transcription of GST2, which transports anthocyanin from the ER to the vacuole. In N. nucifera cultivars with white flower, deletion of a large 80 kb PAV region harboring the functional MYB5 gene causes failure of GST2 activation, consequently resulting in pigment deficiency. In N. lutea, SNPs in the MYB5 exon cause a truncated protein, thereby blocking anthocyanin accumulation. The red boxes in Chr 4 represent the presence of MYB5, while the yellow circle shows SNPs in MYB5. The gray arrow under Chr 4 shows the absence of MYB5. The solid blue arrow represents success transcription of GST2, and the dashed ones together with the red crosses represent failing to activate the transcription of GST2. The gray curved arrows showed no anthocyanin transport from the ER to the vacuole.
Materials and methods
Plant materials
N. nucifera cultivars (cv.) ‘Qiuxing’ (‘QX’) with red flower and ‘Zhigao Wushang’ (‘ZGWS’) with white flower were cultivated at the Wuhan Botanical Garden of the Chinese Academy of Sciences (Wuhan, China). Fresh petals were used to prepare tissue sections for observation with a Phenix PH100 microscope equipped with a Phenix MC-D500U camera. Flower development was divided into 4 stages (S1 to S4) based on color changes in the red flower bud prior to blooming (Supplemental Fig. S1). The S2 flower developmental stage was characterized with rapid petal anthocyanin accumulation, which peaked at S3 in the red lotus, while no anthocyanin accumulation was detected throughout flower development in white lotus. Petal samples from flower buds at S2 and S3 stages were collected in 3 independent biological replicates and then immediately frozen in liquid nitrogen and stored at −80 °C for flavonoid extraction, genomic DNA, and total mRNA isolation. Arabidopsis (A. thaliana) and N. benthamiana plants were cultivated in an incubator at 23 °C with 16 h light and 8 h dark cycle.
Linkage mapping and BSA
A total of 125 individuals in an F2 population derived from a cross between N. nucifera cultivars (cv.) ‘Baige’ (‘BG,’ female parent, white flower) and ‘Winter Red 1' (‘WR1,' male parent, red flower) were used to identify the QTL for lotus petal color by composite interval method as previously described using the WinQTL Cartographer 2.5 software (Liu et al. 2022). The significant limit of detection (LOD) threshold was determined with 1,000 permutations.
Two DNA pools for BSA were constructed using a mixture of 25 red and 23 white individuals from the F2 population. High-quality SNPs and InDels were used here as previously reported (Liu et al. 2022). Difference in allele frequencies between the 2 bulks was computed using a 1 Mb sliding window with 20 kb increments in a customized Perl script.
Evaluation of NnMYB5 gene SVs among lotus accessions
To explore the genetic variations in NnMYB5 gene between the red and white lotus flowers, small SVs (SNPs and InDels) in the genic and upstream sequence regions of NnMYB5 in 240 lotus accessions were analyzed using the previously reported genome resequencing data retrieved from NCBI under the BioProject number PRJNA749672, and highly-quality SNPs were obtained (Zheng et al. 2022). For analysis of large genetic variations, the genomes of 213 lotus accessions having pronounced flower color trait were assembled into contigs individually. Then, the NnMYB5 gene sequences including their 2 kb promoters were searched in the lotus genome with the BLAST criteria of e-value ≤ 1e−6 and sequence identity ≥ 99%. Large SVs were manually detected based on sequence alignments in the BLAST results.
PCR validation of the 80 kb PAV between the white and red flower lotus cultivars
The genomic DNA was isolated from fresh leaves of 14 red and 12 white N. nucifera cultivar samples using DNAsecure Plant Kit (TIANGEN Biotech Co., Ltd., Beijing, China) following the manufacturer's instructions. PCR was performed with PrimeSTAR Max DNA Polymerase (Takara Bio., Shiga, Japan) to detect PAVs of NnMYB5. A pair of primers, P1/P2 (Supplemental Table S1), located at each end of the PAV, was designed to assay the white flower lotus accessions lacking the NnMYB5 gene, while a pair of primers, P3/P4 (Supplemental Table S1), located in the promoter and second exon regions of NnMYB5, was designed to assay the red flower lotus accessions harboring the NnMYB5 gene. As a result, 2 white flower lotus accessions with positive P1/P2 primer amplifications were detected and subsequently sequenced to verify the 80 kb deletion.
HPLC quantitation of anthocyanins and total flavonoids
Total flavonoids were extracted according to previously reported methods with minor modification (Chen et al. 2013). Briefly, ∼1 g fresh petal sample was ground in liquid nitrogen and then extracted twice with 10 mL of extracting solution (methanol/water/formic acid = 70:28:2, v:v:v). After centrifugation at 10,000 rpm for 10 min, the supernatant was collected and filtered through a 0.22 μm membrane prior to HPLC analysis.
Chromatographic separation was performed on a liquid chromatography (Accela 1250) system with a SunFire C18 column (150 mm × 4.6 mm, 3.5 μm, Waters, MA, United States) following the protocol described by Yang et al. (2009). Anthocyanin and other flavonoids were detected at wavelengths of 520 and 350 nm, respectively. Petunidin-3-O-glucoside and isorhamnetin-3-O-rutinoside purchased from Yuanye Bio-Technology Co., Ltd. (Shanghai, China) were used to generate standard curves for quantification of anthocyanin and other flavonoids.
RNA extraction, library preparation, and sequencing
Petals of white and red flower lotus cultivars were harvested in 3 biological replicates at S2 and S3 developmental stages for transcriptome analysis. Total RNA was extracted from each sample using the fast plant RNA kit for polysaccharide and polyphenolic rich (Zoman Biotechnoligy, Beijing, China). The RNA quality was determined by agarose gel, while RNA concentration was detected using a NanoDrop 2000 spectrophotometer (Thermo Scientific, MA, United States). cDNA libraries were constructed and sequenced by Nextomics Biosciences Co., Ltd. (Wuhan, China).
Gene expression analysis
Clean reads obtained after discarding low-quality reads were mapped to the lotus reference genome by HISAT2 (Ming et al. 2013; Pertea et al. 2016). Gene expression levels were quantified by transcripts per million reads (TPM) using StringTie. Differentially expressed genes (DEGs) were identified using the DESeq2 R package based on |log2FC| ≥ 1 and false discovery rate (FDR) < 0.05 filter criteria. GO and Kyoto Encyclopedia of Genes and Genome (KEGG) enrichment analysis were performed with the “topGO” R package and KEGG database, respectively.
RT-qPCR analysis was used to validate the expression levels of selected genes from the transcriptome data. The first-strand cDNA was synthesized using the TransScript II One-Step gDNA Removal and cDNA Synthesis SuperMix Kit (TransGen, Beijing, China). The gene-specific primers used for RT-qPCR were designed using Premier 6 (Supplemental Table S1), and the RT-qPCR reactions in 3 biological and 3 technical replicates were performed on a StepOnePlus Real-Time PCR System (Applied Biosystems, MA, United States) with NnACTIN as a reference gene. The reaction condition was as follows: 1 cycle of 30 s at 94 °C, followed by 40 cycles of 5 s at 94 °C, 15 s at 58 °C, and 10 s at 72 °C. The relative expression levels were calculated using the 2−ΔCt method and normalized using NnACTIN (gene ID NNU_24864).
Phylogenetic analysis
The BLAST module in the TBtools software was used to retrieve GST genes in the N. nucifera genome (Chen et al. 2020; Shi et al. 2020). The A. thaliana and maize (Z. mays) GST proteins were downloaded from NCBI (https://www.ncbi.nlm.nih.gov/) and used as query sequences. The conserved domains of candidate genes were analyzed in the online conserved domain database (CDD) and protein classification server (https://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml) to validate a total of 67 identified NnGSTs. The amino acid sequences of 62 AtGSTs, 20 ZmGSTS, and 67 NnGSTs were aligned with ClustalW, and the phylogenetic tree was constructed using MEGA7 software with the Neighbor-Joining (NJ) method (Thompson et al. 2002; Kumar et al. 2016).
Subcellular localization assay
The NnGST2 coding region was cloned into pMDC43 vector to construct a 35S::NnGST2-GFP recombinant vector. The pMDC43 vector was used as a negative control and P53 as a positive control. An ER marker fused with a red fluorescent protein mCherry was used to determine the cellular ER location. The vectors were then transformed into Agrobacterium tumefaciens GV3101 strain and then infiltrated in N. benthamiana leaves. GFP was excited by the 488 nm laser line and was detected at 495 to 545 nm and mCherry 552 nm laser line and was detected at 600 to 650 nm using a confocal laser scanning microscopy (Leica TCS SP8, Wetzlar, Germany).
Overexpression of NnGST2 in Arabidopsis tt19 mutant
The A. tumefaciens GV3101 strain containing 35S::NnGST2-GFP was introduced into A. thaliana tt19 mutant line. T1 seeds were screened on 1/2 MS media containing 50 mg/L kanamycin. The NnGST2 gene primer was used to PCR amplify the genomic DNA (gDNA) isolated from seedlings to validate positive transgenic lines. The resulting positive T2 generation lines were collected and used for phenotypic evaluation, anthocyanin quantification, and gene expression analysis.
Transient expression of NnGST2 in lotus petals
A. tumefaciens GV3101 strain harboring 35S::NnGST2-GFP was incubated and resuspended to a final optical density of OD600 of 0.8 in infiltration buffer (10 mM MES, 100 µM AS, and 10 mM MgCl2) and then kept at room temperature for 2 h before injection in the petals of flower buds at developmental stages (S3 to S4). Empty pMDC43 vector was used as a control experiment. The injection experiment was conducted in the evening (5 PM to 7 PM) under good weather conditions and petals collected after 3 d for anthocyanin quantification and gene expression analysis.
Y1H assay
The 2 kb promoter regions of NnGST2, NnFLS, NnF3H, NnANS, and NnCHS were cloned into the pAbAi vector to generate bait constructs (Clontech, United States), while NnMYB5 gene was cloned into the pGADT7 vector (Clontech, United States). The bait vector was digested with BstBⅠ and transformed into yeast strain Y1HGold to generate the bait reporter construct. Then, the recombinant pGADT7-NnMYB5 was transformed into the positive bait reporter strain and grown on SD/-Leu/AbA (200 ng/mL) plates for 3 d. The empty pGADT7 and p53-AbAi + pGADT7-53 vectors were used as negative and positive controls, respectively.
Dual-luciferase reporter assay
The coding region of NnMYB5 was cloned into pGreenII 62-SK, while the promoter sequence of anthocyanin-related structural genes, including NnGST2, NnFLS, NnF3H, NnANS, and NnCHS, was inserted into pGreenII 0800-LUC. The empty p62SK vector was used as a negative control. Both constructs were individually transformed into A. tumefaciens GV3101 using the electro-transformation method. The A. tumefaciens solution with plasmid was injected into N. benthamiana leaves with a needleless syringe. The Firefly luciferase (LUC) and Renilla luciferase (REN) activities were analyzed 3 d after infiltration using the Dual-Luciferase Reporter Assay System (E1910 and E1960, Promega, Madison, WI, United States). Three biological replicates were performed for each treatment.
Accession numbers
The raw RNA-seq sequencing reads are submitted to NCBI Sequence Read Archive (SRA) database under the accession number PRJNA758182. The accession numbers of NnMYB5 and NnGST2 are ALU11263.1 and XP_010270745.1
Supplementary Material
Acknowledgments
We thank Dr. Kang Chunying from the College of Horticulture and Forestry Sciences, Huazhong Agricultural University (Wuhan, China), for kindly donating the Arabidopsis tt19 mutant seeds.
Contributor Information
Juan Liu, Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, Hubei 430074, China; Aquatic Plant Research Center, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, Hubei 430074, China.
Yuxin Wang, Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, Hubei 430074, China; College of Life Science, University of Chinese Academy of Sciences, 19A Yuquanlu, Beijing 100049, China.
Xianbao Deng, Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, Hubei 430074, China; Aquatic Plant Research Center, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, Hubei 430074, China.
Minghua Zhang, Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, Hubei 430074, China; College of Life Science, University of Chinese Academy of Sciences, 19A Yuquanlu, Beijing 100049, China.
Heng Sun, Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, Hubei 430074, China; Aquatic Plant Research Center, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, Hubei 430074, China.
Lei Gao, Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, Hubei 430074, China.
Heyun Song, Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, Hubei 430074, China; College of Life Science, University of Chinese Academy of Sciences, 19A Yuquanlu, Beijing 100049, China.
Jia Xin, Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, Hubei 430074, China; College of Life Science, University of Chinese Academy of Sciences, 19A Yuquanlu, Beijing 100049, China.
Ray Ming, Center for Genomics and Biotechnology, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China.
Dong Yang, Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, Hubei 430074, China; Aquatic Plant Research Center, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, Hubei 430074, China.
Mei Yang, Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, Hubei 430074, China; Aquatic Plant Research Center, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, Hubei 430074, China.
Author contributions
M.Y. and D.Y. conceptualized the study and designed the experiments. D.Y., X.D., M.Z., Hen.S., L.G., Hey.S., and J.X. performed the experiments. M.Y., J.L., and Y.W. wrote the manuscript. L.G. and R.M. provide constructive advice for the manuscript. All authors discussed the results and commented on the manuscript.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Flower development and anthocyanin accumulation in the red and white lotus flowers.
Supplemental Figure S2. The expression patterns of 110 genes in the 2 target intervals between the red and white petals at developmental stages 2 (S2) and stage 3 (S3).
Supplemental Figure S3. Digital expression levels of 3 genes in the 80 kb PAV in red petals at different developmental stages.
Supplemental Figure S4. PCR genotype verification of the F2 individuals used in this study.
Supplemental Figure S5. GO enrichment of the 170 DEGs.
Supplemental Figure S6. Phylogenetic analysis and expression patterns of GSTs in N. nucifera.
Supplemental Figure S7. Pearson correlation analysis between candidate TFs and NnGST2.
Supplemental Figure S8. Transient expression of NnMYB5 induces anthocyanin accumulation in the white lotus petals.
Supplemental Figure S9. The Y1H and dual-luciferase reporter assay (LUC) validation of NnMYB5 regulatory activity on 4 anthocyanin biosynthesis genes.
Supplemental Table S1. Primers used in this study.
Supplemental Table S2. List of natural lotus accessions tested in Fig. 2B.
Supplemental Table S3. Summary statistics of clean reads and RNA-seq mapping results.
Supplemental Table S4. List of the 170 overlapping DEGs between the white and red petal at developmental stages 2 and 3.
Supplemental Table S5. Gene ID and accession numbers of GSTs used for phylogenetic analysis.
Funding
This research was financially supported by the Biological Resources Programme, Chinese Academy of Sciences (KFJ-BRP-007-009) and the National Natural Science Foundation of China (Grant No.32070336).
References
- Alfenito MR, Souer E, Goodman CD, Buell R, Mol J, Koes R, Walbot V. Functional complementation of anthocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell 1998:10(7):1135–1149. 10.1105/tpc.10.7.1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillejo C, Waurich V, Wagner H, Ramos R, Oiza N, Muñoz P, Triviño JC, Caruana J, Liu Z, Cobo N, et al. . Allelic variation of MYB10 is the major force controlling natural variation in skin and flesh color in strawberry (Fragaria spp.) fruit. Plant Cell 2020:32(12):3723–3749. 10.1105/tpc.20.00474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020:13(8):1194–1202. 10.1016/j.molp.2020.06.009 [DOI] [PubMed] [Google Scholar]
- Chen S, Xiang Y, Deng J, Liu Y, Li S. Simultaneous analysis of anthocyanin and non-anthocyanin flavonoid in various tissues of different lotus (Nelumbo) cultivars by HPLC-DAD-ESI-MS(n). PLoS One 2013:8(4):e62291. 10.1371/journal.pone.0062291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Scott AJ, Davis JR, Tsang EK, Li X, Kim Y, Hadzic T, Damani FN, Ganel L, Montgomery SB, et al. . The impact of structural variation on human gene expression. Nat Genet. 2017:49(5):692–699. 10.1038/ng.3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Chen S, Yin X, Wang K, Liu Y, Li S, Yang P. Systematic qualitative and quantitative assessment of anthocyanins, flavones and flavonols in the petals of 108 lotus (Nelumbo nucifera) cultivars. Food Chem. 2013:139(1–4):307–312. 10.1016/j.foodchem.2013.02.010 [DOI] [PubMed] [Google Scholar]
- Deng J, Fu Z, Chen S, Damaris RN, Wang K, Li T, Yang P. Proteomic and epigenetic analyses of lotus (Nelumbo nucifera) petals between red and white cultivars. Plant Cell Physiol. 2015:56(8):1546–1555. 10.1093/pcp/pcv077 [DOI] [PubMed] [Google Scholar]
- Edwards R, Dixon DP. Plant glutathione transferases. In: Sies H, Packer L, editors. Methods in enzymology. San Diego (CA): Academic Press; 2005. p. 169–186 [DOI] [PubMed] [Google Scholar]
- Gao Z, Yang X, Chen J, Rausher MD, Shi T. Expression inheritance and constraints on cis- and trans-regulatory mutations underlying lotus color variation. Plant Physiol. 2022:191(3):1662–1683. 10.1093/plphys/kiac522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Deng C, Li Y, Zhu G, Fang W, Chen C, Wang X, Wu J, Guan L, Wu S, et al. . An integrated peach genome structural variation map uncovers genes associated with fruit traits. Genome Biol. 2020:21(1):58. 10.1186/s13059-020-02169-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Chen M, He N, Chen X, Wang N, Sun Q, Zhang T, Xu H, Fang H, Wang Y, et al. . MdGSTF6, activated by MdMYB1, plays an essential role in anthocyanin accumulation in apple. Hortic Res. 2019:6(1):40. 10.1038/s41438-019-0118-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Shikazono N, Tanaka A. TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J. 2004:37(1):104–114. 10.1046/j.1365-313X.2003.01943.x [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016:33(7):1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Zhang C, Cao D, Damaris RN, Yang P. The latest studies on Lotus (Nelumbo nucifera)-an emerging horticultural model plant. Int J Mol Sci. 2019:20(15):3680. 10.3390/ijms20153680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Du H, Li P, Shen Y, Peng H, Liu S, Zhou GA, Zhang H, Liu Z, Shi M, et al. . Pan-genome of wild and cultivated soybeans. Cell 2020:182(1):162–176.e113. 10.1016/j.cell.2020.05.023 [DOI] [PubMed] [Google Scholar]
- Liu Y, Song H, Zhang M, Yang D, Deng X, Sun H, Liu J, Yang M. Identification of QTLs and a putative candidate gene involved in rhizome enlargement of Asian lotus (Nelumbo nucifera). Plant Mol Biol. 2022:110(1–2):23–36. 10.1007/s11103-022-01281-w [DOI] [PubMed] [Google Scholar]
- Liu J, Wang Y, Zhang M, Wang Y, Deng X, Sun H, Yang D, Xu L, Song H, Yang M. Color fading in lotus (Nelumbo nucifera) petals is manipulated both by anthocyanin biosynthesis reduction and active degradation. Plant Physiol Biochem. 2022:179(1):100–107. 10.1016/j.plaphy.2022.03.021 [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhang D, Liu F-L, Liu Z, Wang X, Yang Y, Li S-S, Li H, Tian D, Wang L. Quercetin-derivatives paint the yellow petals of American lotus (Nelumbo lutea) and enzymatic basis for their accumulation. Horticultural Plant J. 2022:9(1):169–182. 10.1016/j.hpj.2022.02.001 [DOI] [Google Scholar]
- Luo HF, Dai C, Li YP, Feng J, Liu ZC, Kang CY. Reduced anthocyanins in petioles codes for a GST anthocyanin transporter that is essential for the foliage and fruit coloration in strawberry. J Exp Bot. 2018:69(10):2595–2608. 10.1093/jxb/ery096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs KA, Alfenito MR, Lloyd AM, Walbot V. A glutathione S-transferase involved in vacuolar transfer encoded by the maize gene Bronze-2. Nature 1995:375(6530):397–400. 10.1038/375397a0 [DOI] [PubMed] [Google Scholar]
- Ming R, VanBuren R, Liu Y, Yang M, Han Y, Li LT, Zhang Q, Kim MJ, Schatz MC, Campbell M, et al. . Genome of the long-living sacred lotus (Nelumbo nucifera Gaertn. Genome Biol. 2013:14(5):R41. 10.1186/gb-2013-14-5-r41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016:11(9):1650–1667. 10.1038/nprot.2016.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Miller J. Sacred lotus, the long-living fruits of China antique. Seed Sci Res. 2007:12(3):131–143. 10.1079/SSR2002112 [DOI] [Google Scholar]
- Shi T, Rahmani RS, Gugger PF, Wang M, Li H, Zhang Y, Li Z, Wang Q, Van de Peer Y, Marchal K, et al. . Distinct expression and methylation patterns for genes with different fates following a single whole-genome duplication in flowering plants. Mol Biol Evol. 2020:37(8):2394–2413. 10.1093/molbev/msaa105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S-S, Gugger PF, Wang Q-F, Chen J-M. Identification of a R2R3-MYB gene regulating anthocyanin biosynthesis and relationships between its variation and flower color difference in lotus (Nelumbo Adans.). Peerj. 2016:4(1):e2369. 10.7717/peerj.2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Sasaki N, Ohmiya A. Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J. 2008:54(4):733–749. 10.1111/j.1365-313X.2008.03447.x [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics. 2002:Chapter 2:Unit 2.3. https://doi:10.1002/0471250953.bi0203s00 [DOI] [PubMed] [Google Scholar]
- Vaish S, Gupta D, Mehrotra R, Mehrotra S, Basantani MK. Glutathione S-transferase: a versatile protein family. 3 Biotech. 2020:10(7):321. 10.1007/s13205-020-02312-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen X, Luo S, Ma W, Li N, Zhang W, Tikunov Y, Xuan S, Zhao J, Wang Y, et al. . Discovery of a DFR gene that controls anthocyanin accumulation in the spiny Solanum group: roles of a natural promoter variant and alternative splicing. Plant J. 2022:111(4):1096–1109. 10.1111/tpj.15877 [DOI] [PubMed] [Google Scholar]
- Wang R, Lu N, Liu C, Dixon RA, Wu Q, Mao Y, Yang Y, Zheng X, He L, Zhao B, et al. . MtGSTF7, a TT19-like GST gene, is essential for accumulation of anthocyanins, but not proanthocyanins in Medicago truncatula. J Exp Bot. 2022:73(12):4129–4146. 10.1093/jxb/erac112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wu S, Shi Y, Jiang L, Yang J, Wang X, Zhu K, Zhang H, Zhang J. Integrated metabolite profiling and transcriptome analysis reveal candidate genes involved in the formation of yellow Nelumbo nucifera. Genomics 2022:114(6):110513. 10.1016/j.ygeno.2022.110513 [DOI] [PubMed] [Google Scholar]
- Xie M, Chung CY, Li MW, Wong FL, Wang X, Liu A, Wang Z, Leung AK, Wong TH, Tong SW, et al. . A reference-grade wild soybean genome. Nat Commun. 2019:10(1):1216. 10.1038/s41467-019-09142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Dubos C, Lepiniec L. Transcriptional control of flavonoid biosynthesis by MYB–bHLH–WDR complexes. Trends Plant Sci. 2015:20(3):176–185. 10.1016/j.tplants.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Yang R-Z, Wei X-L, Gao F-F, Wang L-S, Zhang H-J, Xu Y-J, Li C-H, Ge Y-X, Zhang J-J, Zhang J. Simultaneous analysis of anthocyanins and flavonols in petals of lotus (Nelumbo) cultivars by high-performance liquid chromatography-photodiode array detection/electrospray ionization mass spectrometry. J Chromatogr A. 2009:1216(1):106–112. 10.1016/j.chroma.2008.11.046 [DOI] [PubMed] [Google Scholar]
- Zhang L, Hu J, Han X, Li J, Gao Y, Richards CM, Zhang C, Tian Y, Liu G, Gul H, et al. . A high-quality apple genome assembly reveals the association of a retrotransposon and red fruit colour. Nat Commun. 2019:10(1):1494. 10.1038/s41467-019-09518-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. Flavonoid transport mechanisms: how to go, and with whom. Trends Plant Sci. 2015:20(9):576–585. 10.1016/j.tplants.2015.06.007 [DOI] [PubMed] [Google Scholar]
- Zheng P, Sun H, Liu J, Lin J, Zhang X, Qin Y, Zhang W, Xu X, Deng X, Yang D, et al. . Comparative analyses of American and Asian lotus genomes reveal insights into petal color, carpel thermogenesis and domestication. Plant J. 2022:110(5):1498–1515. 10.1111/tpj.15753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Ma R, Gao L, Zhang J, Zhang A, Zhang X, Ren F, Zhang W, Liao L, Yang Q, et al. . A 1.7-Mb chromosomal inversion downstream of a PpOFP1 gene is responsible for flat fruit shape in peach. Plant Biotechnol J. 2021:19(1):192–205. 10.1111/pbi.13455 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.