Abstract
Introduction
Snakebites are a neglected tropical disease. In many areas, envenoming incidence and antivenom administration rates are unknown. This study compared antivenom (AV) availability to rates of envenoming and recommendations to treat (RTT) in South Africa.
Methods
This retrospective study identified, extracted, and reviewed all cases of envenoming (snake bites and spits) reported to the Poisons Information Helpline of the Western Cape of South Africa (PIHWC) from June 1, 2015 to May 31, 2020 by public hospitals in the Western Cape. A standardized interview was administered to the pharmacies of the 40 hospitals in winter and summer to determine how many vials of monovalent and polyvalent AV they had on hand at the time of the call and their expiration dates. Descriptive analysis was used to compare rates of envenoming and recommendations to treat to antivenom stock in winter and summer and by hospital type and location.
Results
Public hospitals reported 300 envenomings, 122 from snakes. The PIHWC recommended antivenom administration in 26% of cases (N = 32). All hospital pharmacies queried answered our questions. Our study demonstrates urban district hospitals have higher ratios of AV vials compared to mean annual rates of envenoming and RTT than rural district hospitals at both the winter and summer timepoints.
Conclusion
This study evaluates antivenom supply and demand in a province of South Africa. The findings suggest South African urban hospitals have a relative excess of antivenom, and thus more capacity to meet demand, than their rural counterparts. It supports consideration of a redistribution of antivenom supply chains to match seasonal and local rates of envenoming. It indicates a need for higher quality, prospective data characterizing envenoming incidence and treatment.
Keywords: Snake bites, Antivenins, Antidotes, Global health, Toxicology
African relevance
-
•
Snakebite is a frequent yet neglected disease in Africa; the World Health Organization (WHO) has recently declared it a category A neglected tropical disease.
-
•
African countries including South Africa face significant financial constraints; the timely and appropriate treatment of envenoming patients stands to improve health outcomes and economics.
-
•
A redistribution of antivenom supply chains to match seasonal and local rates of envenoming should be considered.
Introduction
Snakebite envenoming is recognized as a significant cause of morbidity and mortality in low and middle-income countries. Recently, the World Health Organization (WHO) declared it a category A neglected tropical disease and launched a strategic plan to halve deaths and disabilities caused by envenoming by 2030 [1,2]. There is a call for renewed action to end what the Wellcome Trust has termed “one of the world's biggest hidden health crises” [3]. Efforts to improve the care of snakebite victims, however, are hindered by a critical lack of reliable data describing envenoming incidence and antivenom availability [4,5]. Reports describing mortality vary significantly and are frequently based on modelled data. Different sources report global snakebite incidences of 1.2 to 5.5 million persons per year, for example [6], [7], [8], [9]. In Sub-Saharan Africa, not only are rates of envenoming unknown, but there is scant quantitative data describing antivenom (AV) availability and utilization. Antivenom is reportedly frequently unavailable when medically indicated, especially in rural settings, but there is very little published data quantifying current antivenom distribution and use [10], [11], [12]. Given the difficulty in improving envenoming outcomes without accurate information on incidence and treatment rates, multiple experts and nongovernmental organizations have called for the publication of higher quality data describing snakebite incidence and antivenom availability [2,6,9,10,13,14].
Members of our workgroup staff the Poisons Information Helpline of the Western Cape of South Africa (PIHWC) and provide clinical support to physicians who treat snakebite victims throughout South Africa. We have published previously on envenoming incidence as reported to the PIHWC, not only for snakes, but also spiders and scorpions [15], [16], [17], [18]. We have not, however, characterized antivenom availability. It is our experience that antivenom is frequently unavailable when indicated, especially in rural settings; the degree to which antivenom availability is mismatched to demand, however, is unknown. The aim of this study was to compare antivenom availability to rates of envenoming and recommendations to treat in a single region of Sub-Saharan Africa, the Western Cape province of South Africa.
Methods
Study design
This study consisted of a retrospective arm to determine the incidence of envenoming and recommendations to treat, as well as a prospective arm to determine antivenom availability. Ethical approval for this project was granted by the Health Research Ethics Committee of Stellenbosch University (Ref:X21/03/004).
Study setting
The Western Cape's public hospital system consists of 33 District Hospitals, four Regional Hospitals (excluding specialised hospitals e.g., maternity hospital), and three Central/ Tertiary Hospitals. The smallest, first-line hospitals in both rural and urban settings are termed district level hospitals. Health facilities utilize a referral network system to transfer patients to higher levels of care when necessary (from district to regional to tertiary) [19]. In this study, hospitals within Cape Town were considered urban, and hospitals outside of Cape Town rural. Note that the designation of “rural” describes the hospital's location only, not its resources or ability to care for poisoned patients.
The PIHWC is a joint telephone service provided by the Tygerberg Poisons Information Centre and the Red Cross War Memorial's Children Hospital Poisons Information Centre, both situated in Cape Town [20]. The telephonic service provides free toxicology advice to healthcare workers and members of the public. It is available at all hours. Calls are captured on the electronic AfriTox TeleLog database in real time and there is a retrospective quality control system in place [21]. All data entered into AfriTox Telelog is reviewed by a database manager at the poison centre who refers potential errors back to the original data collector. Data is modified pending a case review by all parties. The PIHWC manage approximately 5000 human-related calls per annum and does not routinely record clinical outcomes [20].
Study population
All cases of snake envenoming (bites and spits) reported to the PIHWC from 1 June 2015 to 31 May 2020 by public hospitals in the Western Cape were included in the retrospective arm of the study. Data were extracted from the electronic AfriTox TeleLog Database [21]. Abstractors documented from which hospital the call originated, the date of the call, whether the case was a snake bite or spit, and whether the PIHWC recommended treatment with polyvalent or monovalent antivenom (the two forms of antivenom available in South Africa). Data abstraction was performed by members of the PIHWC. All abstractors followed the same stepwise approach to identify cases. There were no cases with missing data. No cases were excluded from our review. All cases were reviewed to ensure no calls were counted twice (e.g. in case of a patient being transferred between facilities).
The pharmacies of all 40 public hospitals in the Western Cape were called to determine antivenom availability. A structured interview was used (Appendix 1). The pharmacies were initially called in winter (21–28 June 2021) and again from 23 August to 3 September 2021. Pharmacies were called twice to ensure the results were reliable. The questionnaire was repeated in summer (23–29 March 2022) to correspond with the peak snakebite season. Appendix 2 includes a dosing schedule for polyvalent and monovalent antivenoms, the per vial cost of both antivenoms, and the species from which polyvalent antivenom was derived. Both polyvalent and monovalent antivenom expire after three years.
Analysis
Descriptive analysis was performed. The location of every public hospital in the Western Cape was identified to determine which hospitals were urban and which were rural. Antivenom vials included un-expired monovalent and polyvalent antivenom.
Ratios were calculated to compare the number of antivenom vials available (as reported by the pharmacies in 2021 and 2022) to the mean annual (annualized) number of envenoming calls per hospital (as recorded in the electronic database between 2015 and 2020). Ratios were also calculated that compared antivenom availability to the mean annual recommendation to treat. Ratios are presented by hospital type (district versus regional or tertiary), and location (urban or rural).
The ratios were then themselves compared: we contrasted ratios by hospital type (district versus all types of hospitals) and location (urban or rural). For example, the ratio of annualized antivenom availability to recommendations to treat in urban hospitals was compared to the same ratio (antivenom availability versus recommendations to treat) in rural hospitals.
The study includes five years of call volume because envenoming as reported to the PIHWC was a relatively rare event. Therefore, to characterize annual incidence most accurately, it was necessary to average across multiple years: the mean annual number of calls (or recommendation to treat, etc.) describes the mean number of calls per year based on the five years of data reviewed. The analysis is presented as a ratio to give a comparative, qualitative sense of antivenom availability versus demand. This approach was selected in response to the limitations described in the discussion below.
Results
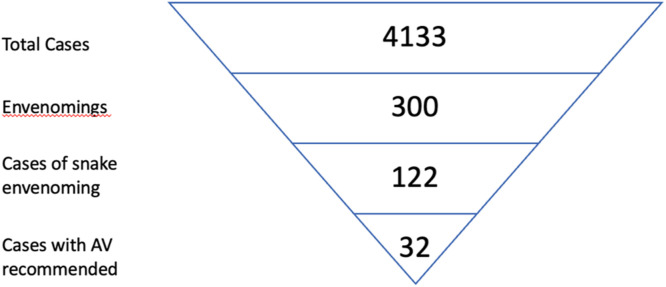
There were 4133 toxicology-related cases reported to the PIHWC by public hospitals in the Western Cape over the study period. Of these, 300 cases involved envenoming, of which 122 cases resulted from snakes (bites n = 113, spits n = 9, mean per year=24.4). There were no cases with missing data and no cases were excluded. The highest number of snake envenoming reported from a single hospital was 16 cases; 13 hospitals did not report any snake envenoming. Ninety cases (73.8%) were reported from rural hospitals (meaning outside of Cape Town) and 80 (65.6%) were from district-level hospitals. The specific snake was mentioned in 37 cases, with the cape cobra (Naja nivea, n = 14) and the puff adder (Bitis arietans, n = 13) most commonly involved. The PIHWC recommended antivenom administration in 32 cases (26% of cases) over the five year period (a mean of 6.4 times a year). No data were available on clinical outcomes such as death or time to antivenom administration. In our experience, however, envenoming by Naja Nivea is associated with the most morbidity and mortality in the Western Cape. Fig. 1 demonstrates visually the breakdown of cases from the Western Cape.
Fig. 1.
Visual representation of cases reported to the PIHWC from public hospitals in the Western Cape.
We had a 100% response rate from all 40 public hospital pharmacies at all timepoints. There was a total of 394 antivenom vials at the winter timepoint and 395 in summer. Of these vials, only three were monovalent at both timepoints. The amount of antivenom available per hospital ranged from none (two hospitals) to 24 vials (two hospitals). All vials of antivenom were unexpired at all timepoints. When asked what challenges inhibited pharmacies’ ability to stock antivenom, 15 (37.5%) pharmacies reported they had no issues stocking and 5 (12.5%) pharmacies reported they never stock antivenom (two urban and three rural hospitals). There were 13 (32.5%) pharmacies who noted that AV's short expiry date limited their ability to consistently stock this medication, and 22.5% (N = 9) reported cold chain and supply chain problems.
Tables 1 and 2 report the average annual number of calls, average annual recommendations to treat, total number of antivenom vials available, antivenom availability versus mean annual calls (envenoming incidence), and annualized recommendations to treat. The two tables compare data between rural and urban settings at the level of all hospitals, and at the district hospital level only. Table 1 reports winter data, and Table 2 summer data.
Table 1.
Ratio of antivenom availability in summer per annual snake envenoming reported to and recommendation to treat by the Poisons Information Helpline of the Western Cape of South Africa (PIHWC).
| Calls (mean per annum) | Recommendation to treat (mean per annum) |
Antivenom vials (n) | Antivenom vials per mean annual calls | Antivenom vials per mean recommendation to treat | ||
|---|---|---|---|---|---|---|
| All hospitals | Rural | 18.0 | 4.8 | 292 | 16.22 | 60.83 |
| Urban | 6.4 | 1.6 | 103 | 16.09 | 64.38 | |
| District-level hospitals | Rural | 13.6 | 3.0 | 257 | 18.90 | 85.67 |
| Urban | 2.2 | 0.2 | 68 | 30.91 | 340 |
Table 2.
Ratio of antivenom availability in winter per annual snake envenoming reported to and recommendation to treat by the Poisons Information Helpline of the Western Cape of South Africa (PIHWC).
| Calls (mean per annum) | Recommendation to treat (mean per annum) |
Antivenom vials (n) | Antivenom vials per mean annual calls | Antivenom vials per mean recommendation to treat | ||
|---|---|---|---|---|---|---|
| All hospitals | Rural | 18.0 | 4.8 | 276 | 15.33 | 57.50 |
| Urban | 6.4 | 1.6 | 118 | 18.44 | 73.75 | |
| District-level hospitals | Rural | 13.6 | 3 | 229 | 16.84 | 76.33 |
| Urban | 2.2 | 0.2 | 73 | 33.18 | 365 |
At the summer timepoint (Table 1), a mean of 16 vials of antivenom were available at both urban and rural hospitals for each snake envenoming reported to the PIHWC. The mean vials available per treatment recommendation from the PIHWC was 61 in rural areas and 64 in urban areas. The ratio of antivenom availability during the winter timepoints is available in Table 2. At both timepoints there was greater antivenom relative to annual calls in urban versus rural hospitals at the district level, but not the level of all hospitals. Urban hospitals had greater antivenom availability compared to recommendations to treat than their rural counterparts at the district and all hospital level at both timepoints.
Discussion
Our data suggests a high ratio of antivenom availability to snake envenoming rate and recommendation to treat as reported to the PIHWC. The availability of antivenom per annual reported snake envenoming should be interpreted with care, however. The data does not indicate that hospitals have a surplus of antivenom. A value of 16.22 vials per annualized call (as noted in rural hospitals in summer for example), suggests that there are an expected 16 vials of antivenom for every case reported. As noted previously, the overwhelming majority of antivenom in this study was polyvalent, and dosing for polyvalent antivenom varies significantly. Cytotoxic snakes are treated with an initial dose of five to ten vials, while neurotoxic snakes are treated with eight to twelve vials [22]. Severe envenoming from a black mamba (Dendroaspis polylepsis) may require an initial twenty vials [22]. The dosing schedule for polyvalent and monovalent antivenom and the cost per vial is included in appendix 2. Furthermore, it is very likely that the rates of envenoming reported to the PIHWC significantly underestimate the true burden of disease (see “Limitations” below). Note that all data describing antivenom (availability and recommendation to treat) includes both polyvalent and monovalent antivenom; we found that monovalent antivenom (used only for Boomslang (Dispholidus typus) envenoming) was rarely stocked and used. The exclusion or inclusion of monovalent antivenom data did not alter our findings.
Most compellingly, this study demonstrates a higher ratio of antivenom availability to recommendation to treat and envenoming incidence, and thus more capacity to meet demand, in urban versus rural areas. The difference between urban and rural antivenom availability exists mostly at the level of the district hospital. Urban district hospitals have the highest ratios of antivenom vials per mean annual recommendation to treat. It has been hypothesized that the difference in antivenom availability between urban and rural hospitals at the level of the district hospital (Tables 1 and 2) could be because rural hospitals simply refer snakebite victims to regional hospitals rather than treat at the district level.
It is alternatively possible that the discrepancy between urban and rural hospitals results from rural hospitals being unable to afford adequate antivenom stock or because distribution systems do not effectively reach smaller hospitals. It is also possible that rural hospitals simply exhaust their supplies more rapidly because they treat a higher incidence and/or greater severity of envenoming than urban hospitals. Our data does not speak to the cause of this discrepancy, but as it is generally accepted that most snakebites occur in rural areas, the mismatch between urban and rural supply warrants further investigation [10]. There do not exist provincial policies indicating how many vials of antivenom should be stocked at a hospital at any given point in time, however, our study and data could inform this question in the future.
Limitations
Our study has two significant limitations. The first is that we did not have concurrent data on antivenom availability versus envenoming incidence and recommendation to treat. As the records in AfriTox Telelog did not record whether hospitals had antivenom available when a recommendation is made to treat, we did not have access to historical information regarding antivenom availability. We therefore called every public hospital in the Western Cape to determine antivenom availability and compared the hospital's antivenom stock to their mean annual case load.
Our second limitation was that we suspect the rates of envenoming as reported to the PIHWC underestimate the true burden of disease. We suspect rates are underreported for three reasons. Firstly, hospitals in South Africa are not required to report envenoming [23]. Secondly, snakebites are endemic to South Africa and local physicians are in our experience generally comfortable with managing snakebites [24]. They may only call if they have an unusual case or if they are for some reason relatively unfamiliar with snakebites. Thirdly, many physicians in the Western Cape are also simply not aware that the poison information centre exists as a clinical resource and therefore do not call. An underestimation of envenoming is consistent with previous publications which argue envenoming incidence as reported to healthcare facilities in Africa underrepresents the actual burden of disease [13,14]. Our study also only evaluated the burden of disease at public, not private hospitals. Future prospective studies that include both sectors are necessary to more accurately characterize envenoming rates.
It is in response to these to limitations that we present our data as a ratio and a mean annual value. Our analysis does not describe clinical outcomes or speak to how disparities in antivenom stocking impact patient care. Rather, it gives a comparative, qualitative sense of antivenom availability versus demand by location.
Other limitations include the fact that data describing envenoming incidence and recommendations to treat were retrospective. Nevertheless, data were obtained by a standardized registry tool and no information was missing. Also, while the questionnaire administered to the pharmacies was consistent, the individuals with whom researchers spoke were not; heads of pharmacy departments were reached at some hospitals, more junior employees at others. However, all individuals were able to answer our survey questions and were familiar with hospital stocking.
Conclusion
Urban district-level hospitals in the Western Cape of South Africa demonstrate a higher ratio of antivenom availability to recommendations to treat and envenoming incidence, and thus more capacity to meet demand, compared to their rural counterparts. This work supports consideration of a redistribution of antivenom supply chains to match seasonal and local rates of envenoming. The collection of higher quality, prospective data characterizing the incidence of snakebite envenoming and the relationship between envenoming incidence and antivenom availability is indicated.
Dissemination of results
The results of this study were shared with all staff members of the Poisons Information Helpline of the Western Cape during informal research and staff meetings.
Author's contribution
Authors contributed as follow to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content: NN contributed 40%; AdP, CM, and SW 15% each; and DJvH, COH, and AL contributed 5% each. All authors approved the version to be published and agreed to be accountable for all aspects of the work.
Declaration of Competing Interest
The authors declared no conflicts of interest.
Acknowledgements
We would like to extend our heartfelt thanks to the community of staff and patients at the public hospitals and Poisons Information Helpline of the Western Cape without whom this research would not have been possible. We are also eternally grateful to Drs Mark Mycyk and Helmuth Reuter for their guidance and support of this research.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.afjem.2023.08.002.
Appendix. Supplementary materials
References
- 1.World Health Organization . Report of the tenth meeting of the WHO strategic and technical advisory group for neglected tropical diseases. World Health Organization; Geneva: 2017. [Google Scholar]
- 2.Williams D.J., Faiz M.A., Abela-Ridder B., Ainsworth S., Bulfone T.C., Nickerson A.D., et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wellcome Trust. Snakebite, https://wellcome.org/what-we-do/our-work/snakebite; [Accessed 6 December 2022].
- 4.Benjamin J.M., Abo B.N., Brandehoff N. Review article: snake envenomation in Africa. Curr Trop Med Rep. 2020;7:1–10. doi: 10.1007/s40475-020-00198-y. [DOI] [Google Scholar]
- 5.World Health Organization . Fourteenth Meeting of the strategic and technical advisory group for neglected tropical diseases. World Health Organization; Geneva: 2021. [Google Scholar]
- 6.Longbottom J., Shearer F.M., Devine M., Alcoba G., Chappuis F., Weiss D.J., et al. Vulnerability to snakebite envenoming: a global mapping of hotspots. Lancet. 2018;392:673–684. doi: 10.1016/S0140-6736(18)31224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chippaux J.P. Snake-bites: appraisal of the global situation. Bull World Health Organ. 1998;76:515–524. [PMC free article] [PubMed] [Google Scholar]
- 8.Kasturiratne A., Wickremasinghe A.R., de Silva N., Gunawardena N.K., Pathmeswaran A., Premaratna R., et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . World Health Organization; Geneva: 2019. Snakebite envenoming A strategy for prevention and control. [Google Scholar]
- 10.Gutiérrez J.M. Improving antivenom availability and accessibility: science, technology, and beyond. Toxicon. 2012;60:676–687. doi: 10.1016/j.toxicon.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Habib A.G., Musa B.M., Iliyasu G., Hamza M., Kuznik A., Chippaux J.-P. Challenges and prospects of snake antivenom supply in sub-Saharan Africa. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chippaux J.P., Habib A.G. Antivenom shortage is not circumstantial but structural. Trans R Soc Trop Med Hyg. 2015;109:747–748. doi: 10.1093/TRSTMH/TRV088. [DOI] [PubMed] [Google Scholar]
- 13.Chippaux J.P. Estimate of the burden of snakebites in sub-Saharan Africa: a meta-analytic approach. Toxicon. 2011;57:586–599. doi: 10.1016/j.toxicon.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Chippaux J.P. Estimating the global burden of snakebite can help to improve management. PLoS Med. 2008;5:e221. doi: 10.1371/journal.pmed.0050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marks C.J., Muller G.J., Sachno D., Reuter H., Wium C.A., du Plessis C.E., et al. The epidemiology and severity of scorpion envenoming in South Africa as managed by the Tygerberg Poisons Information Centre over a 10-year period. Afr J Emerg Med. 2019;9:21–24. doi: 10.1016/J.AFJEM.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Plessis C.E. 2019. Venomous spider bites in South Africa: epidemiology and clinical features.https://scholar.sun.ac.za/handle/10019.1/106053 [Accessed 6 December 2022] [Google Scholar]
- 17.Veale D.J.H., Wium C.A., Müller G.J. Toxicovigilance. I: a survey of acute poisonings in South Africa based on Tygerberg Poison Information Centre data. S Afr Med J. 2013;103:293–297. doi: 10.7196/SAMJ.6647. [DOI] [PubMed] [Google Scholar]
- 18.Tygerberg Poison Information Centre . 2014. Tygerberg poison information centre annual report 2014.https://docplayer.net/63719300-Tygerberg-poison-information-centre-annual-report.html [Accessed 6 December 2022] [Google Scholar]
- 19.Western Cape Government Department of Health and Wellness . 2022. Annual Report 2021-2022.https://www.westerncape.gov.za/documents/annual_reports [Accessed 6 December 2022] [Google Scholar]
- 20.Du Plessis C.E., Mohamed F., Stephen C.R., Reuter H., Voigt G., van Hoving D.J., et al. A retrospective review of calls to the Poisons Information Helpline of the Western Cape during the first 6 months of the COVID-19 pandemic in South Africa. S Afr J Infect Dis. 2022;37 doi: 10.4102/sajid.v37i1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephen C.R., Leyser S., Roberts J.C., Mohamed F., Balme K.H., Curling L.E., et al. AfriTox (R) TeleLog: the development of a novel web-based data collection tool for poison centre call data in South Africa. Clin Toxicol. 2016;54:344–519. doi: 10.3109/15563650.2016.1165952. [DOI] [Google Scholar]
- 22.Müller G., Modler H., Wium C., Veale D., Marks C. Snake bite in southern Africa: diagnosis and management. Contin Medical Educ. 2012;30:362–381. [Google Scholar]
- 23.Wood D., Sartorius B., Hift R. Estimating the Burden of Snakebite on Public Hospitals in KwaZulu Natal, South Africa. Wilderness Environ Med. 2016;27:53–61. doi: 10.1016/j.wem.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Wood D., Sartorius B., Hift R. Snakebite in north-eastern South Africa: clinical characteristics and risks for severity. South African Family Pract. 2016;58:62–67. doi: 10.1080/20786190.2015.1120934. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.