Abstract
The presence of gap junction intercellular communication structures in bone cells has been known since the early 1970s, further confirmed by Doty and Marotti at the structural level in the 1980–1990s. Work by Civitelli, Donahue, and others showed the expression of Cx43 at the mRNA and protein levels in all bone cell types: osteoclasts (bone resorbing cells), osteoblasts (bone forming cells), and osteocytes (mature osteoblasts embedded in the bone matrix that regulate the function of both osteoclasts and osteoblasts). While Cx45, Cx46, and Cx37 were also shown to be expressed in bone cells, most studies have focused on Cx43, the most abundant member of the connexin (Cx) family of proteins expressed in bone. The role of Cx43 has been shown to be related to the formation of gap junction intercellular channels, to unopposed hemichannels, and to channel independent functions of the molecule. Cx43 participates in the response of bone cells to pharmacological, hormonal, and mechanical stimuli, and it is involved in the skeletal phenotype with old age. Human and murine studies have shown that mutations of Cx43 lead to oculodentodigital dysplasia and craniometaphyseal dysplasia, both conditions associated with abnormalities in the skeleton. However, whereas substantial advances have been made on the skeletal role of Cx43, further research is needed to understand the basis for the effects of mutated Cx43 and potential ways to prevent the effects of these mutations on bone.
Keywords: Cx43, osteoblast, osteocyte, osteoclast, bone
Introduction
Intercellular communication is required to coordinate the actions of cells within tissues and can be achieved through gap junction channels and hemichannels.1,2 These channels and hemichannels are formed by hexamers that are members of a family of membrane integral proteins named connexins (Cxs). Twenty-one members of the family of Cxs have been identified in humans, differing on sequence, molecular size (which gives rise to the name of the particular Cx), channel permeability, and cellular/tissue and subcellular localization.3–5
Cx43 is the most widespread, most abundant, and most studied member of the gap junction family of proteins.6 In bone, Cx43 is expressed in all cell types: osteoclasts (bone resorbing cells), osteoblasts (bone forming cells), and osteocytes (cells responsible for mechanical sensation and for the regulation of the differentiation and activity of the other bone cells).7–10 However, even though Cx43 expression in osteoclasts has been demonstrated and appears to regulate cell differentiation,11–13 most studies have focused on the role of the Cx in osteoblast and osteocyte differentiation, viability, and function (review in Refs.14–17). Further, Cx43 function in bone cells is not limited to intercellular communication.14 Thus, evidence shows that Cx43 hemichannels mediate the actions of hormonal, pharmacotherapeutic, and mechanical forces on osteoblasts and osteocytes. In this review, we describe the current knowledge on the actions of Cx43 and its role on bone cells.
In Vitro/Ex Vivo Studies
Studies of the past 20+ years have demonstrated a fundamental role of Cx43 in bone cell differentiation and function. Although global Cx43 deficiency in mice results in perinatal lethality due to cardiac malfunction,18 studies were possible using osteoblasts isolated from newborn Cx43−/− mice.19 These Cx43-deficient osteoblasts exhibit characteristics of dysfunctional cells, with lower type I collagen (COL1A1), alkaline phosphatase (ALP), and osteocalcin mRNA levels but similar proliferation rate when compared to wild-type osteoblastic cells.
Similar changes in osteoblast-specific genes were found in rat osteosarcoma ROS17/2.8 cells (with high Cx43 expression) overexpressing Cx45, which functions as a dominant negative for Cx43 actions.20–22 On the other hand, overexpression of Cx43 in the rat osteosarcoma cell line UMR106-1, which expresses Cx45 and very low levels of Cx43, results in increased expression of osteoblastic genes. Another study showed that transfection of Cx43 into UMR106-1 cells leads to an increase in growth rate and results in higher cell adhesion to tissue culture plates, with ALP expression and mineralization levels, both increased, suggesting that Cx43 promotes bone cell proliferation and differentiation.23
More recently, studies were carried out using the osteocytic IDG-SW3 and OCY454 cell lines. Cx43 was knocked down in these cells using the lentiviral CRISPR/Cas9 approach, to evaluate osteoblast and osteocyte gene expression and mineralization potential throughout the cellular differentiation process.24 These studies reported an increase in Cx43 levels as the differentiation took place. Further, when Cx43 was deleted, lower levels of expression of the osteoblast markers ALP and COL1A1 were detected at the osteoid-osteocyte stage when compared to control cells.
Other studies showed that Cx43-silenced osteocyte-like MLO-Y4 cells undergo spontaneous cell death in culture, as determined by increased caspase-3 activity and higher levels of apoptosis-related genes compared to scramble-silenced control cells.25 These Cx43-deficient cells also exhibit increased levels of the pro-osteoclastogenic cytokine receptor activator of NFkB signaling ligand and lower levels of the anti-osteoclastogenic cytokine osteoprotegerin compared to scramble-silenced control cells, resulting in an overall pro-osteoclastogenic state associated with increased osteoclast differentiation. In addition, Cx43 suppression in osteoblastic cell leads to increased cyclic adenosine monophosphate (cAMP) levels, promoting osteoclastogenesis in a coculture system.26
In another study, primary osteocytes isolated from 18-month-old mice with reduced Cx43 levels released less prostaglandin E2 (PGE2) to the extracellular media compared to cells obtained from 10-month-old mice.27 The decreased in PGE2 release is associated with increased muscle collagen synthesis and reduced muscle force in aged mice compared to younger mice. Overall, these studies support the conclusion that Cx43 impairment in bone cells not only affects bone but also other organs.
Exogenous Stimuli and Cx43-Mediated Cell Survival
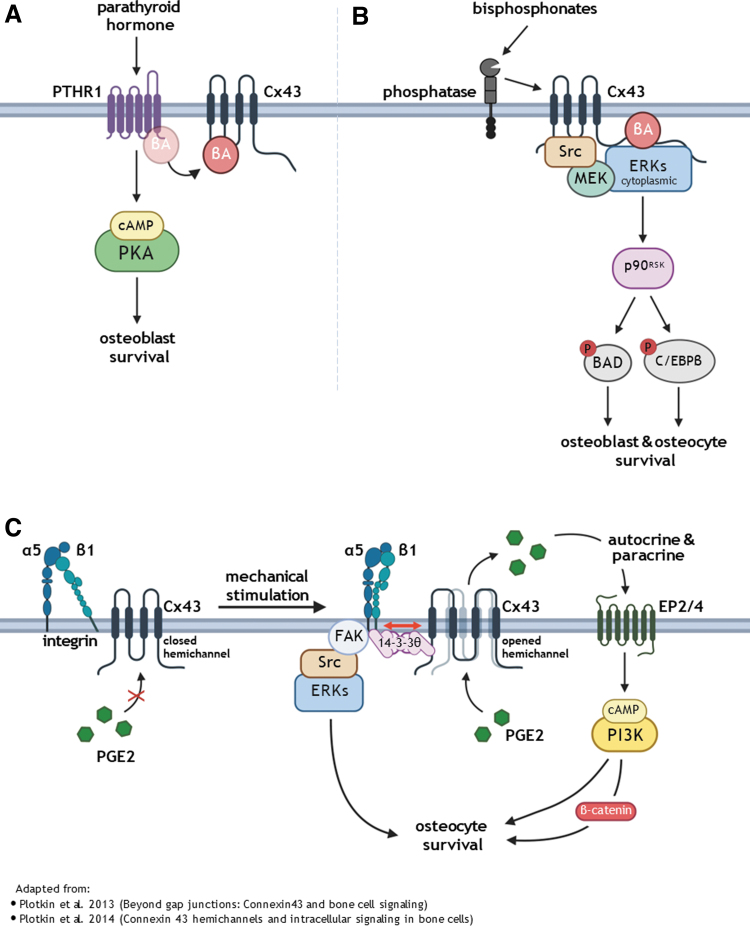
The differentiation, activity, and survival of osteoblasts and osteocytes are regulated by numerous stimuli, including pharmacologic agents, hormones, and mechanical forces.28,29 Cx43 has been shown to have a role on the response of the cells to all of these stimuli.30 In this review, we will focus on survival signals that involve pathways including Cx43 (Fig. 1).
FIG. 1.
Survival mechanisms mediated by Cx43 in response to exogenous stimuli. (A) Hormonal stimulation. PTH interacts with his membrane receptor PTHR1 activating a cAMP-dependent signaling pathway, promoting osteoblast survival. (B) Bisphosphonates bind to phosphatases on the cell membrane, stimulate Cx43 hemichannels opening followed by Cx43 interaction with Src tyrosine kinase, activated Src phosphorylates and the kinase MEK. MEK, in turn, interacts with p90RSK kinase, which targets two different proteins, the pro-apoptotic protein BAD, which becomes inactive, and C/EBPβ, which binds to and blocks pro-caspase activity. All this resulting in osteoblasts and osteocytes survival. (C) Mechanical stimulation. Integrins α5β1 on the osteocyte membrane become coupled by a mechanism involving 14-3-3θ protein, leading to Cx43 hemichannel opening and activating two downstream pathways. One pathway results in FAK/Src/ERK activation, and the second involves PGE2 release to extracellular space which acts in paracrine manner, activating membrane receptors EP2/4 and triggering the cAMP/PI3k signaling pathway resulting in the accumulation of β-catenin and Wnt pathway activation. Together, these signaling pathways prevent osteocyte apoptosis. Adapted from Refs.14,78 BAD, BCL2 associated agonist of cell death; C/EBPβ, CCAAT-enhancer-binding protein β; PGE2, prostaglandin E2; PTH, parathyroid hormone. Image made using Biorender.com
Hormonal stimulation
Decreased estrogen levels with postmenopause is known to cause osteopenia, osteoporosis, and high risk for bone fractures.31 It has been shown that low estrogen levels affect Cx43 expression and hemichannel function.32 Indeed, addition of 17β-estradiol to MLO-Y4 cell cultures results in increased Cx43 expression when compared to a nonsupplemented group. Further, estrogen withdrawal reduces the opening of hemichannels as shown by ethidium bromide dye uptake in MLO-Y4 osteocytic cells. Yet, this effect could be due to lower number of hemichannels with similar activity, or similar number of hemichannels with less activity. On the other hand, the prosurvival effect of 17β-estradiol, which does not depend on estrogen response element (ERE) transcriptional activation, does not require Cx43 expression or its function in hemichannels.33 Nevertheless, it is possible that the so-called “genotropic (ERE-mediated) effects” of estrogens require Cx43 expression and/or channel activation.
Intermittent administration of parathyroid hormone (PTH) results in decreased osteoblast and osteocyte apoptosis in vivo and in vitro (Fig. 1A).34,35 PTH, by interacting with the PTH receptor PTH1R, activates a cAMP-dependent signaling pathway, leading to phosphorylation and inactivation of the pro-apoptotic protein BCL2 associated agonist of cell death (BAD). PTH1R activation also leads to cAMP response element-binding protein (CREB)-mediated transcription of Bcl2 (a survival gene) and Runx2.34 Further studies showed that the survival effect of PTH is lost in Cx43-deficient OB-6 osteoblastic cells.36 Thus, PTH-induced cAMP signaling pathway was interrupted in cells with Cx43 knock down, with the consequent reduced expression of cAMP/CREB-target genes. Upon PTH binding, PTH1R associates with βarrestin, leading to receptor internalization and disruption of the intracellular signaling pathway triggered by the hormone. Mechanistic studies demonstrated that Cx43 (through its phosphorylated Ser368 in the C-terminal domain) interacts with βarrestin, sequestering it from its binding to PTH1R, allowing for proper downstream signal transduction. In the absence of Cx43, PTH/PTH1R signaling is abrogated, suggesting that Cx43 facilitates cAMP signaling by sequestering β arrestin, exerting a permissive role on the survival effect of PTH on osteoblasts.
The survival effect of PTH depends on permeable gap junction channels, but it is independent of hemichannel activity. Consistent with a role of Cx43 on PTH effects, the anabolic effect of intermittent PTH is attenuated in mice lacking Cx43 in osteoblastic cells.37 Similarly, lack of Cx43 in osteocytes (or absence of Cx43 C-terminus domain) leads to a suboptimal response to intermittent PTH administration in bone, with failure to increase endocortical bone formation and mechanical strength, all higher in PTH-treated control female mice.38 On the other hand, Cx43 is not required for the anabolic effect of the hormone in male mice.39
Bisphosphonates
Bisphosphonates are drugs widely used since 1970s to treat bone loss in conditions associated with low bone mass.40,41 The main action of these pharmacologic agents is the inhibition of bone resorption.42 We have shown that, in addition, bisphosphonates prevent osteoblast and osteocyte apoptosis in vitro and in vivo.43 Cx43 is an essential mediator of bisphosphonate-induced intracellular signaling through the Src/MEK/ERK pathway (Fig. 1B).33
This role of Cx43 is independent of intercellular communication but requires the opening of Cx43 hemichannels. It has been proposed that the effects of bisphosphonates on osteoblastic cells are triggered by their binding to phosphatases on the cell membrane,44 which in turn stimulates the opening of Cx43 hemichannels and their interaction with Src, a tyrosine kinase that plays a crucial role in intracellular signaling.33 Src activates the MEK/ERK1/2 pathway, which, in turn, targets the cytoplasmatic kinase p90RSK. p90RSK phosphorylates two different proteins, the pro-apoptotic protein BAD, which becomes inactive, and CCAAT-enhancer-binding protein β (C/EBPβ), which binds to and blocks pro-caspases, thus resulting in osteoblast and osteocytes survival.45
Mechanical stimulation
Mechanical loading is essential to acquire and maintain an optimal bone mass throughout life, and osteocytes are the bone cells responsible for sensing these stimuli.46,47 Cx43 hemichannels present in osteocytes act as signaling transduction mediators, leading to PGE2 release (Fig. 1C). Indeed, a correlation has been shown between the magnitude of the stimuli and hemichannel activity. Thus, when higher fluid flow shear stress is applied, it results in higher Lucifer Yellow dye uptake and increased release of PGE2.48 Once released following mechanical stimulation, PGE2 prevents the apoptotic effect of dexamethasone in osteocyte-like MLO-Y4 cells.49
Further studies uncovered the signaling pathway involved in the survival effect of mechanical stimulation in vitro. The activation of intracellular signaling begins when integrins on the osteocyte membrane become coupled as result of mechanical stimuli, leading to Cx43 hemichannel opening, and PGE2 release to the extracellular space.50 PGE2 next acts in a paracrine manner, activating membrane receptors EP2/4 triggering the cAMP/PI3K signaling pathway, which results in the accumulation of β-catenin and activation of the Wnt signaling pathway, thus preventing osteocyte apoptosis.49,51
Murine In Vivo Studies
Due to perinatal lethality, tissue-specific Cx43-deficient mice were used to establish the role of the Cx on bone cell viability in adult mice.30,52,53 These studies used different promoters aiming to target osteoblast precursors and their progeny54 and mature osteoblasts and their progeny,55,56 or osteocytes (and some mature osteoblasts).57 While some differences were found in the phenotype of these mice depending on the promoter used to target the expression of the Cre recombinase, all models showed increased periosteal apposition and endocortical resorption and reduced bone strength and, for two of the models, increased cortical osteocyte apoptosis. These features are similar to those found in aged mice, suggesting a potential role of Cx43 on the aging phenotype. Consistent with this, the expression of Cx43 is reduced in bones from aging mice,25 which also exhibit increased osteocyte apoptosis.58 Old age and Cx43 deficiency on osteocytic cells causes low levels of the pro-survival microRNA miR21 and increased PTEN protein levels, which leads to decreased Akt activation resulting in cell apoptosis.25
Studies by our group were carried out to remove Cx43 from mature osteoblasts and osteocytes (Cx43ΔOb-Ot/-) or mainly from osteocytes (Cx43ΔOt).55,57 We found that both mouse models exhibited increased osteocyte apoptosis and accumulation of empty lacunae in cortical, but not cancellus bone, in the lumbar vertebrae and femur. Similar increase in osteocyte apoptosis was reported by Donahue's group.59 In addition, the femurs of these mice showed increased total area, marrow cavity area, and cortical area, but no change in cortical thickness or bone mineral density. These bone changes are attributed to increased periosteal apposition and increased endocortical bone resorption. On the other hand, no structural or cellular changes were detected in cancellous bone. The morphological changes are seen in these mice resemble aging, with increased apoptosis and periosteal expansion.
Conversely, the increase in osteocyte apoptosis observed in aged 14-month-old mice was partially prevented in mice overexpressing Cx43 under the control of the 8 kb fragment of the DMP1 promoter (Cx43OT mice).60 While apoptosis associated with aging also occurred in the osteoblasts of both strains of mice, there was no difference between the Cx43OT mice and the wild-type mice. In addition, aged wild-type mice display decreased bone formation markers and increased resorption markers compared to young mice, but overexpression of Cx43 in Cx43OT mice led to the opposite effect: increased formation and decreased resorption markers compared to young mice.
As discussed above, Cx43 is required for the bisphosphonate actions in vitro. This was also demonstrated in in vivo experiments by our group using mice with Cx43 deleted from osteoblasts and osteocytes.55 In this experiment, mice received the glucocorticoid prednisolone to induce osteoblast and osteocyte apoptosis, together with either vehicle or the bisphosphonate alendronate. While alendronate prevented apoptosis in control mice, it was ineffective in mice lacking osteoblastic Cx43.
Similar to the tissue-specific Cx43 deletion, the role of the C-terminus Cx43 tail on bone morphology and bone cell function was examined by two different research groups using slightly different models.38,61 In one of the studies, our group showed that expression of one allele of the Cx43K258Stop gene (lacking amino acids starting from Lys 258)62 and one Cx43 floxed allele63 in female mice results in lower cancellous bone volume but higher cortical thickness compared to mice expressing wild-type Cx43.38 This phenotype is associated with decreased osteoblast number and activity. Interestingly, the consequences of expression of the truncated Cx43 contrast with those of its deletion from osteoblast/osteocytes, which lead to changes in cortical bone without affecting cancellous bone.14
However, work of Stains' group showed that expression of the same truncated Cx43 in the context of Cx43 deficiency (Cx43K258Stop/- mice) leads to a phenotype similar to the tissue-specific deletion in male mice.61 On the other hand, Cx43K258Stop/- female mice have similar changes in bone as Cx43K258Stop/flox females,38,61 indicating a sex-dependent effect of Cx43 truncation. Regarding apoptosis, no differences in cell viability (TUNEL staining) were detected in female Cx43K258Stop mice, independently of whether they expressed or not the floxed Cx43 gene in osteocytes.38 Similarly, empty lacunae (a measurement of osteocyte death) were increased in male Cx43K258Stop/- mice.61 These results indicate that, unlike its effects on bone structure, the lack of a role of Cx43 C-terminus on osteocyte viability is independent of the sex of the mice.
To tease out the contribution of gap junctions versus hemichannels on bone, Xiang's group developed two mouse models of transgenic expression of Cx43 mutants in osteocytic cells.64 These animals expressed either a Cx43 mutant in which the amino acid Arginine 76 is replaced by Tryptophan (Cx43R76W), unable to form gap junction channels but with functional hemichannels, or a truncated mutant lacking amino acids 130–136 (Cx43Δ130–136), which eliminates channel permeability for both hemichannels and gap junction channels. These mutations were targeted to osteocytes via a fragment of the DMP1 gene promoter. Adult, 4-month-old male mice expressing Cx43Δ130–136 exhibit less organized cortical bone and increased levels of cleaved caspase-3 compared to wild-type mice, consistent with increased osteocyte apoptosis.
The Cx43R76W mutant showed minimal changes in organization and cell death compared to wild type. This indicates that functional Cx43 hemichannels, and not gap junctions, are needed for osteocyte survival. In addition to these effects, expression of Cx43Δ130–136 results in increased total and bone area, cortical thickness, bone marrow cavity area, and endocortical irregularity, consistent with periosteal expansion and endocortical resorption and similar to Cx43-deficient mice. Further, Cx43Δ130–136 bones had reduced material properties.
Additional studies using the two transgenic mouse models analyzed the consequences of the mutations on bone and skeletal muscle with aging in 18-month-old male mice.27 The authors showed that the enhanced hemichannel activity in the Cx43R76W mice led to increased osteoclast surface due to higher ATP release by osteocytes. On the other hand, the impaired channel permeability in the Δ130–136 mice led to higher osteocyte apoptosis that is ascribed to reduced PGE2 levels. In the same study, it was found that osteoblast number was decreased in Cx43Δ130–136 mice with normal bone mass, and this could be explained by the impaired hemichannel activity counteracting the delay in osteoblastogenesis. In conclusion, Cx43 hemichannels are involved in bone mass regulation through their action on bone cells viability in aged mice. Additionally, the investigators showed that impairment of hemichannels caused an increase in empty lacunae and higher levels of caspase-3, hallmarks of osteocyte apoptosis.
Cx43 Mutations Associated with Bone-Related Human Disease
Human studies
Studies of the early 2000s first identified mutations of the GJA1 gene in families with ODDD (OMIM# 164200).65 By 2016, more than 76 different mutations were associated with ODDD, most of them inherited in an autosomal-dominant manner.53,66,67 ODDD is a disorder that involves craniofacial abnormalities, mandibular overgrowth, cleft palate, abnormal dentition, syndactyly, and broad tubular bones.65 In addition to the skeletal manifestations, individuals with ODDD exhibit ocular defects, and, less often, central nervous system manifestations, bladder incontinency, and skin abnormalities.67 Curiously, given the importance of the GJA1 gene expression in cardiomyocytes, heart manifestations of the disease are uncommon in ODDD individuals, potentially due to compensatory effects of other Cx expressed in the cardiac tissue.
The molecular and functional bases for ODDD-related mutations are not fully understood. Studies have shown loss of gap junction channel function, gain of hemichannel function, and improper channel trafficking, as well as trans-dominant effects on other connexins.67 Different effects on Cx43 biological function could be explained by the fact that the mutations are found in different domains of the protein, including the extracellular and intracellular domains, as well as the transmembrane regions.68 As described below, animal models for specific mutations were developed to understand the cellular and molecular mechanisms responsible for the phenotypic characteristics of ODDD patients.
In addition to ODDD, a study reported that four separate families with craniometaphyseal dysplasia (OMIM# 218400)-affected individuals exhibit a mutation leading to the change from Arginine to Glutamine in the amino acid 239 of the Cx43 gene (Cx43R239Q).69 Craniometaphyseal dysplasia is a condition associated with dysfunctional osteoblasts and osteoclasts.70 However, the cellular mechanisms associated with this Cx43R239Q recessive mutation have not been determined. Other conditions associated with GJA1 mutations include congenital heart diseases, sudden infant death syndrome (SIDS), and hereditary deafness,71 which are outside the scope of this review.
Animal models
Mouse models mimicking the human mutations have been generated and the skeletal phenotypic characteristics caused by the mutation found in mice were similar to those in humans with ODDD.53 The first report of a musculoskeletal phenotype in an animal model was published by Flenniken et al.68 These authors performed N-ethyl-N-nitrosourea mutagenesis screen and found that the G60S mutation in the GJA1 gene leads to a dominant negative disruption of gap junction assembly and function. These mice exhibit characteristics similar to individuals with ODDD, including syndactyly, dental defects, and craniofacial and ocular abnormalities. In addition, the G60S mutant mice have low bone mass and reduced bone mechanical strength.
An I130T Cx43 mutant mice was reported in 2007.72 This study focused on the cardiovascular phenotype, without analyzing the skeleton of the mice. A later study conducted in 2017 using mouse expressing the G138R Cx43 mutation showed osteopenia, reduced bone volume, and increased trabecular spacing.73 This phenotype was mainly the result of decreased number of osteoblasts. In addition to these findings, another study found that osteoblastic cells isolated from the ODDD mouse model exhibited reduced gap junction communication and impaired late stage osteoblasts differentiation.74
Further studies showed that expression of the G138R Cx43 mutant in osteochondroprogenitors (by using the Dermo1/Twist2 prompter to target the expression of the Cx43 mutant) also exhibit a skeletal phenotype.75 These mice exhibit low bone mass, craniofacial abnormalities, and a cortical bone phenotype that resembles that of Cx43-deficient mice. To our best knowledge, no studies reported the molecular mechanisms responsible for the effects of the G138R Cx43 mutation on osteoblasts. However, in the manuscript by Dobrowolski et al., the authors report that the Cx43 mutation results in loss of Cx43 phosphorylation and increased ATP-releasing activity of the mutated channels in cardiomyocytes, changes that could also be responsible for the altered osteoblastic phenotype in G138R Cx43-expressing mice.73
Conclusions
Cx43 is involved in the differentiation, activity, and survival of all bone cells: osteoblasts, osteocytes, and osteoclasts. Global Cx43 deficiency results in perinatal death, and, therefore, it is not possible to use full knockout mice to study the role of Cx43 on the adult skeleton. To overcome this obstacle, mice with deletion of Cx43 in osteoblasts/osteocytes were generated using the Cre-LoxP system.56,57,76 Interestingly, while some differences were found in the phenotype of these mice depending on the promoter used to target the expression of the Cre recombinase, all models showed increased periosteal apposition and endocortical resorption and reduced bone strength and, for two of the models, increased cortical osteocyte apoptosis.
These studies with tissue-specific deletion, together with those using Cx43 mutants lacking the C-terminus domain,38,61 with changes in osteocyte channel function,27,64 or with osteocytic Cx43 overexpression60 have allowed for a better understanding of the role of Cx43 on bone cell biology. Yet, further studies are needed to completely elucidate the mechanisms for Cx43 action, including the role of channels/hemichannels, and C-terminus domain, its subcellular localization, and the mechanisms associated for the sex-specific consequences of the truncated Cx43 molecule on bone structure.
Human studies identified mutations in GJA1, the gene encoding for Cx43, as the culprit for ODDD.67 This is a very rare condition with symptoms that might appear at birth or in infants and can worsen with aging. The life expectancy of individuals with ODDD depends on the severity of the symptoms. In particular, life expectancy is reduced if there is an involvement of the heart tissue, for example, in individuals and mice carrying the I130T Cx43 mutation.72 Due to the variability of the location and molecular effects of the mutations in the GJA1 gene, a common approach to target Cx43 to improve bone health appears unlikely. Nevertheless, approaches to block Cx43 hemichannels in bone might prove useful to prevent or reverse the skeletal complications of the disease in cases in which overactive hemichannels are shown. This could be achieved, for example, using the Cx43 hemichannel blocker antibody Cx43(M1), which was shown to impair the anabolic effect of mechanical stimulation on bone.77 Yet, further studies will be needed to test whether this antibody still blocks hemichannel activity of mutated Cx43.
Authors' Contributions
L.I.P., I.A., A.E.K., and N.S. performed bibliography search, wrote the manuscript, and reviewed the final version.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was partially supported by the grants from the National Institutes of Health (NIH) R21AG078861 and the Veterans Administration (VA) Office 1I0 1BX005154 to L.I.P. N.S. received a travel award from the Government of the Province of Santa Fe, Santa Fe, República Argentina.
References
- 1. Goodenough DA, Paul DL. Beyond the gap: Functions of unpaired connexon channels. Nat Rev Mol Cell Biol 2003;4(4):285–294. [DOI] [PubMed] [Google Scholar]
- 2. Goodenough DA, Paul DL. Gap junctions. Cold Spring Harb Perspect Biol 2009;1(1):a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar NM, Gilula NB. The gap junction communication channel. Cell 1996;84(3):381–388; doi: 10.1016/s0092-8674(00)81282-9 [DOI] [PubMed] [Google Scholar]
- 4. Friend DS, Gilula NB. Variations in tight and gap junctions in mammalian tissues. J Cell Biol 1972;53(3):758–776; doi: 10.1083/jcb.53.3.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cetin-Ferra S, Francis SC, Cooper AT, et al. . Mitochondrial connexins and mitochondrial contact sites with gap junction structure. Int J Mol Sci 2023;24(10):9036; doi: 10.3390/ijms24109036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lampe PD, Laird DW. Recent advances in connexin gap junction biology. Fac Rev 2022;11:14; doi: 10.12703/r/11-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schirrmacher K, Schmitz I, Winterhager E, et al. . Characterization of gap junctions between osteoblast-like cells in culture. Calcif Tissue Int 1992;51:285–290. [DOI] [PubMed] [Google Scholar]
- 8. Civitelli R, Beyer EC, Warlow PM, et al. . Connexin43 mediates direct intercellular communication in human osteoblastic cell networks. J Clin Invest 1993;91:1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones SJ, Gray C, Sakamaki H, et al. . The incidence and size of gap junctions between the bone cells in rat calvaria. Anat Embryol (Berl) 1993;187(4):343–352. [DOI] [PubMed] [Google Scholar]
- 10. Donahue HJ, McLeod KJ, Rubin CT, et al. . Cell-to-cell communication in osteoblastic networks: Cell line-dependent hormonal regulation of gap junction function. J Bone Min Res 1995;10(6):881–889. [DOI] [PubMed] [Google Scholar]
- 11. Ransjo M, Sahli J, Lie A. Expression of connexin 43 mRNA in microisolated murine osteoclasts and regulation of bone resorption in vitro by gap junction inhibitors. Biochem Biophys Res Commun 2003;303(4):1179–1185. [DOI] [PubMed] [Google Scholar]
- 12. Ilvesaro J, Väänänen K, Tuukkanen J. Bone-resorbing osteoclasts contain gap-junctional connexin-43. J Bone Min Res 2000;15(5):919–926. [DOI] [PubMed] [Google Scholar]
- 13. Schilling AF, Filke S, Rueger JM, et al. . Signaling via gap junctions is important for the fusion of human osteoclasts in vitro. J Bone Miner Res 2002;17(Suppl 1):S348. [Google Scholar]
- 14. Plotkin LI, Bellido T. Beyond gap junctions: Connexin43 and bone cell signaling. Bone 2013;52(1):157–166; doi: 10.1016/j.bone.2012.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moorer MC, Stains JP. Connexin43 and the intercellular signaling network regulating skeletal remodeling. Curr Osteoporos Rep 2017;15:24–31; doi: 10.1007/s11914-017-0345-4[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stains JP, Watkins MP, Grimston SK, et al. . Molecular mechanisms of osteoblast/osteocyte regulation by connexin43. Calcif Tissue Int 2014;94(1):55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loiselle AE, Jiang JX, Donahue HJ. Gap junction and hemichannel functions in osteocytes. Bone 2013;54(2):205–212; doi: 10.1016/j.bone.2012.08.132 [DOI] [PubMed] [Google Scholar]
- 18. Reaume AG, de Sousa PA, Kulkarni S, et al. . Cardiac malformation in neonatal mice lacking connexin43. Science 1995;267(5205):1831–1834. [DOI] [PubMed] [Google Scholar]
- 19. Lecanda F, Warlow PM, Sheikh S, et al. . Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol 2000;151(4):931–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koval M, Geist ST, Westphale EM, et al. . Transfected connexin45 alters gap junction permeability in cells expressing endogenous connexin43. J Cell Biol 1995;130(4):987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steinberg TH, Civitelli R, Geist ST, et al. . Connexin43 and connexin45 form gap junctions with different molecular permeabilities in osteoblastic cells. EMBO J 1994;13(4):744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lecanda F, Towler DA, Ziambaras K, et al. . Gap junctional communication modulates gene expression in osteoblastic cells. Mol Biol Cell 1998;9(8):2249–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gramsch B, Gabriel HD, Wiemann M, et al. . Enhancement of connexin 43 expression increases proliferation and differentiation of an osteoblast-like cell line. Exp Cell Res 2001;264(2):397–407. [DOI] [PubMed] [Google Scholar]
- 24. Hua R, Gu S, Jiang JX. Connexin 43 hemichannels regulate osteoblast to osteocyte differentiation. Front Cell Dev Biol 2022;10:892229; doi: 10.3389/fcell.2022.892229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davis HM, Pacheco-Costa R, Atkinson EG, et al. . Disruption of the Cx43/miR21 pathway leads to osteocyte apoptosis and increased osteoclastogenesis with aging. Aging Cell 2017;16(3):551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kawatsura R, Hara Y, Akiyama M, et al. . Gap junctional intercellular communication attenuates osteoclastogenesis induced by activated osteoblasts. Biochem Biophys Res Commun 2022;597:71–76; doi: 10.1016/j.bbrc.2022.01.118. [DOI] [PubMed] [Google Scholar]
- 27. Li G, Zhang L, Lu Z, et al. . Connexin 43 channels in osteocytes are necessary for bone mass and skeletal muscle function in aged male mice. Int J Mol Sci 2022;23(21):13506; doi: 10.3390/ijms232113506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Plotkin LI, Bruzzaniti A. Molecular signaling in bone cells: Regulation of cell differentiation and survival. Adv Protein Chem Struct Biol 2019;116:237–281; doi: 10.1016/bs.apcsb.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bellido T, Plotkin LI, Bruzzaniti A, Bone Cells. In: Basic and Applied Bone Biology. (Burr DB, Allen MR. eds.), Elsevier: London, United Kingdom; 2019; pp. 37–56. [Google Scholar]
- 30. Plotkin LI, Bonetto A.. Connexin-Mediated Signaling in Bone. In: Encyclopedia of Bone Biology. (Cardozo CP. ed.) Academic Press: Cambridge, MA; 2020. [Google Scholar]
- 31. Bouxsein ML, Pierroz DD, Glatt V, et al. . beta-Arrestin2 regulates the differential response of cortical and trabecular bone to intermittent PTH in female mice. J Bone Miner Res 2005;20(4):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma L, Hua R, Tian Y, et al. . Connexin 43 hemichannels protect bone loss during estrogen deficiency. Bone Res 2019;7:11; doi: 10.1038/s41413-019-0050-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem 2002;277(10):8648–8657. [DOI] [PubMed] [Google Scholar]
- 34. Bellido T, Ali AA, Plotkin LI, et al. . Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem 2003;278(50):50259–50272. [DOI] [PubMed] [Google Scholar]
- 35. Jilka RL, Weinstein RS, Bellido T, et al. . Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 1999;104(4):439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bivi N, Lezcano V, Romanello M, et al. . Connexin43 interacts with barrestin: A pre-requisite for osteoblast survival induced by parathyroid hormone. J Cell Biochem 2011;112(10):2920–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chung D, Castro CH, Watkins M, et al. . Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci 2006;119(Pt 20):4187–4198. [DOI] [PubMed] [Google Scholar]
- 38. Pacheco-Costa R, Davis HM, Sorenson C, et al. . Defective cancellous bone structure and abnormal response to PTH in cortical bone of mice lacking Cx43 cytoplasmic C-terminus domain. Bone 2015;81:632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pacheco-Costa R, Davis HM, Atkinson EG, et al. . Osteocytic connexin 43 is not required for the increase in bone mass induced by intermittent PTH administration in male mice. J Musculoskelet Neuronal Interact 2016;16(1):45–57. [PMC free article] [PubMed] [Google Scholar]
- 40. Cremers S, Drake MT, Ebetino FH, et al. . Pharmacology of bisphosphonates. Br J Clin Pharmacol 2019;85(6):1052–1062; doi: 10.1111/bcp.13867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Russell RG. Bisphosphonates: The first 40 years. Bone 2011;49(1):2–19. [DOI] [PubMed] [Google Scholar]
- 42. Russell RG, Watts NB, Ebetino FH, et al. . Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 2008;19(6):733–759; doi: 10.1007/s00198-007-0540-8 [DOI] [PubMed] [Google Scholar]
- 43. Plotkin LI, Weinstein RS, Parfitt AM, et al. . Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest 1999;104(10):1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lezcano V, Bellido T, Plotkin LI, et al. . Osteoblastic protein tyrosine phosphatases inhibition and connexin 43 phosphorylation by alendronate. Exp Cell Res 2014;324(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Plotkin LI, Aguirre JI, Kousteni S, et al. . Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of ERK activation. J Biol Chem 2005;280(8):7317–7325. [DOI] [PubMed] [Google Scholar]
- 46. Bass SL, Saxon L, Daly RM, et al. . The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: A Study in Tennis Players. J Bone Miner Res 2002;17(12):2274–2280; doi: 10.1359/jbmr.2002.17.12.2274 [DOI] [PubMed] [Google Scholar]
- 47. Bergmann P, Body JJ, Boonen S, et al. . Loading and skeletal development and maintenance. J Osteoporosis 2011;2011:1–15; doi: 10.4061/2011/786752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Siller-Jackson AJ, Burra S, Gu S, et al. . Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem 2008;283(39):26374–26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kitase Y, Barragan L, Jiang JX, et al. . Mechanical induction of PGE(2) in osteocytes blocks glucocorticoid induced apoptosis through both the beta-catenin and PKA pathways. J Bone Miner Res 2010;25(12):2657–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Plotkin LI, Mathov I, Aguirre JI, et al. . Mechanical stimulation prevents osteocyte apoptosis: Requirement of integrins, Src kinases and ERKs. Am J Physiol Cell Physiol 2005;289:C633–C643. [DOI] [PubMed] [Google Scholar]
- 51. Machwate M, Harada S, Leu CT, et al. . Prostaglandin receptor EP(4) mediates the bone anabolic effects of PGE(2). Mol Pharmacol 2001;60(1):36–41. [DOI] [PubMed] [Google Scholar]
- 52. Plotkin LI, Davis HM, Cisterna BA, et al. . Connexins and pannexins in bone and skeletal muscle. Curr Osteoporos Rep 2017;15(4):326–334; doi: 10.1007/s11914-017-0374-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Plotkin LI, Laird DW, Amedee J. Role of connexins and pannexins during ontogeny, regeneration, and pathologies of bone. BMC Cell Biol 2016;17(Suppl 1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chung D, Screen J, Stains JP, et al. . Osteoblast specific ablation of connexin43 (Cx43) attenuates the anabolic response to intermittent PTH (1–34) in aged mice. J Bone Min Res 2004;19(Suppl 1):S177. [Google Scholar]
- 55. Plotkin LI, Lezcano V, Thostenson J, et al. . Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. J Bone Miner Res 2008;23(11):1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang Y, Paul EM, Sathyendra V, et al. . Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS ONE 2011;6(8):e23516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bivi N, Condon KW, Allen MR, et al. . Cell autonomous requirement of connexin 43 for osteocyte survival: Consequences for endocortical resorption and periosteal bone formation. J Bone Miner Res 2012;27(2):374–389; doi: 10.1002/jbmr.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Almeida M, Han L, Martin-Millan M, et al. . Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 2007;282(37):27285–27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lloyd SA, Loiselle AE, Zhang Y, et al. . Evidence for the role of connexin 43-mediated intercellular communication in the process of intracortical bone resorption via osteocytic osteolysis. BMC Musculoskelet Disord 2014;15(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Davis HM, Aref MW, Aguilar-Perez A, et al. . Cx43 overexpression in osteocytes prevents osteocyte apoptosis and preserves cortical bone quality in aging mice. JBMR Plus 2018;2(4):206–216; doi: 10.1002/jbm4.10035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moorer MC, Hebert C, Tomlinson RE, et al. . Defective signaling, osteoblastogenesis, and bone remodeling in a mouse model of connexin43 C-terminal truncation. J. Cell Sci 2017;130(3):531–540; doi: 10.1242/jcs.197285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Maass K, Ghanem A, Kim JS, et al. . Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43. Mol Biol Cell 2004;15(10):4597–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Theis M, Speidel D, Willecke K. Astrocyte cultures from conditional connexin43-deficient mice. Glia 2004;46(2):130–141. [DOI] [PubMed] [Google Scholar]
- 64. Xu H, Gu S, Riquelme MA, et al. , Connexin 43 channels are essential for normal bone structure and osteocyte viability. J Bone Miner Res 2015;30(3):550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Paznekas WA, Boyadjiev SA, Shapiro RE, et al. . Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet 2003;72(2):408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kelly JJ, Esseltine JL, Shao Q, et al. . Specific functional pathologies of Cx43 mutations associated with oculodentodigital dysplasia. Mol Biol Cell 2016;27(14):2172–2185; doi: 10.1091/mbc.E16-01-0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Delmar M, Laird DW, Naus CC, et al. . Connexins and disease. Cold Spring Harb Perspect Biol 2018;10(9):a029348; doi: 10.1101/cshperspect.a029348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Flenniken AM, Osborne LR, Anderson N, et al. . A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development 2005;132(19):4375–4386. [DOI] [PubMed] [Google Scholar]
- 69. Hu Y, Chen IP, de Almeida S, et al. , A novel autosomal recessive GJA1 missense mutation linked to Craniometaphyseal dysplasia. PLoS One 2013;8(8):e73576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jackson WP, Hanelin J, Albright F. Metaphyseal dysplasia, epiphyseal dysplasia, diaphyseal dysplasia, and related conditions. II. Multiple epiphyseal dysplasia; its relation to other disorders of epiphyseal development. AMA Arch Intern Med 1954;94(6):886–901; doi: 10.1001/archinte.1954.00250060020002 [DOI] [PubMed] [Google Scholar]
- 71. Qiu Y, Zheng J, Chen S, et al. . Connexin mutations and hereditary diseases. Int J Mol Sci 2022;23(8):4255; doi: 10.3390/ijms23084255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kalcheva N, Qu J, Sandeep N, et al. , Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc Natl Acad Sci U S A 2007;104(51):20512–20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dobrowolski R, Sasse P, Schrickel JW, et al. . The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum Mol Genet 2008;17(4):539–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McLachlan E, Plante I, Shao Q, et al. . ODDD-linked Cx43 mutants reduce endogenous Cx43 expression and function in osteoblasts and inhibit late stage differentiation. J Bone Miner Res 2008;23(6):928–938; doi: 10.1359/jbmr.080217 [DOI] [PubMed] [Google Scholar]
- 75. Watkins M, Grimston SK, Norris JY, et al. . Osteoblast Connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol Biol Cell 2011;22(8):1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Castro CH, Stains JP, Sheikh S, et al. . Development of mice with osteoblast-specific connexin43 gene deletion. Cell Commun Adhes 2003;10(4–6):445–450. [DOI] [PubMed] [Google Scholar]
- 77. Zhao D, Riquelme MA, Guda T, et al. . Connexin hemichannels with prostaglandin release in anabolic function of bone to mechanical loading. Elife 2022;11:e74365; doi: 1110.7554/eLife.74365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Plotkin LI. Connexin 43 hemichannels and intracellular signaling in bone cells. Front Physiol 2014;5:131. [DOI] [PMC free article] [PubMed] [Google Scholar]