Abstract
Significance:
Reactive oxygen species (ROS) are essential in maintaining normal intestinal physiology. Inflammatory bowel disease (IBD) is a relapsing chronic inflammatory disease of the intestine that is a major risk factor for colorectal cancer (CRC). Excess ROS are widely implicated in intestinal inflammation and cancer.
Recent Advances:
Clinical data have shown that targeting ROS broadly does not yield improved outcomes in IBD and CRC. However, selectively limiting oxidative damage may improve the efficacy of ROS targeting. An accumulation of lipid ROS induces a novel oxidative cell death pathway known as ferroptosis. A growing body of evidence suggests that ferroptosis is relevant to both IBD and CRC.
Critical Issues:
We propose that inhibition of ferroptosis will improve disease severity in IBD, whereas activating ferroptosis will limit CRC progression. Data from preclinical models suggest that methods of modulating ferroptosis have been successful in attenuating IBD and CRC.
Future Directions:
The etiology of IBD and progression of IBD to CRC are still unclear. Further understanding of ferroptosis in intestinal diseases will provide novel therapies. Ferroptosis is highly linked to inflammation, cell metabolism, and is cell-type dependent. Further research in assessing the inflammatory and tumor microenvironment in the intestine may provide novel vulnerabilities that can be targeted. Antioxid. Redox Signal. 39, 551–568.
Keywords: ferroptosis, inflammatory bowel disease, IBD, CRC, colorectal cancer
Introduction
Reactive oxygen species (ROS) are a group of highly reactive molecules formed through the reduction of molecular oxygen (Sies and Jones, 2020). They include free radicals, superoxide anions (O2•−), hydroxyl (HO•), peroxyl (RO2•−), hydroperoxyl (HO2•), and alkoxyl radicals (RO•), and molecules that produce free radicals such as hydrogen peroxide (H2O2), hydroxide ion (OH−), and organic peroxides (ROOH) (Shields et al., 2021). Free radicals are short-lived and react rapidly in subcellular locations where they are produced, while nonfree radical ROS pass through membranes due to decreased reactivity (Shields et al., 2021). Excess transition metals such as cuprous copper (Cu+) or ferrous iron (Fe2+) can also react with H2O2 to generate hydroxyl radicals through the Fenton reaction (Shields et al., 2021). While many studies have focused on the detrimental effects of ROS (Alfadda and Sallam, 2012; Liu et al., 2018), it is important to understand that ROS are essential for normal physiological function and health.
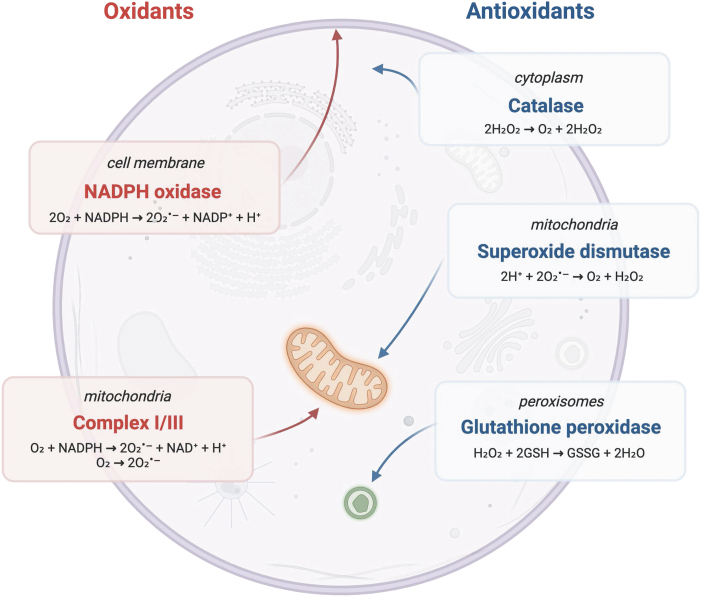
ROS are generated in subcellular compartments such as the cytoplasm, endoplasmic reticulum (ER), cell membrane, and peroxisome. The main source of ROS is the mitochondria (mtROS) by the electron transport chain (ETC) during oxidative phosphorylation (OXPHOS) (Fig. 1) (Tirichen et al., 2021). mtROS account for ∼90% of cellular ROS (Tirichen et al., 2021). During OXPHOS, O2•− is converted into H2O2 and OH− (Tirichen et al., 2021). O2•− is generated primarily in complex I at the flavin mononucleotide (FMN) site and in complex III during the Q cycle (Tirichen et al., 2021).
FIG. 1.
Main sources of oxidants and antioxidants in the cell. Redox imbalance occurs when any of these systems is compromised.
In complex I, two electrons are transferred from nicotine adenine dinucleotide (NADH) generated in the tricarboxylic acid cycle to ubiquinone (Q). These electrons can be passed on to form FMNH2 at FMN sites or circulated through a series of iron–sulfur clusters to reduce Q to uniquinol (QH2) (Tirichen et al., 2021). In complex III, O2•− is generated as a result of autooxidation of ubisemiquinone, the primary electron donor that reduces O2 to O2•− (Tirichen et al., 2021). In comparison with complex I and III, complex II generates small amounts of O2•− at the FAD site (Tirichen et al., 2021).
Mitochondria are the main source of ROS in cells; however, an important source of ROS is within the plasma membrane generated by NADPH oxidase (NOX/DUOX) enzymes (Aviello and Knaus, 2017). ROS production by innate immune cells is often a first-line defense toward pathogens (Arnold and Heimall, 2017). Innate immune cells utilize oxidative bursts to clear microbial pathogens, which is generated by the reduction of molecular oxygen to superoxide anions by NOX (Lambeth and Neish, 2014). Mutations in NOX genes and the subsequent decrease of oxidative burst capacity in innate immune cells lead to a primary immune deficiency known as chronic granulomatous disease (CGD) (Arnold and Heimall, 2017). These patients are at increased risk for infection and experience significant morbidity (Arnold and Heimall, 2017).
In addition, as part of the acquired immune response, ROS are continuously secreted by activated phagocytes to reinforce intracellular T cell signaling present at the sites of infection (Dröge, 2002). Moreover, NOX enzymes in other cell types generate ROS for the regulation of growth, survival, and apoptosis (Lambeth and Neish, 2014).
Under normal physiological conditions, ROS play an important role in regulating homeostasis. However, under conditions of decreased cellular oxygen, or hypoxia, the production of mtROS increases due to dysregulation of the ETC. The enhanced ROS in hypoxia are an essential mechanism of inducing the transcription factor hypoxia-induced factor (HIF)-1α (Guzy and Schumacker, 2006; Wang et al., 2007). HIF-1α, in turn, induces metabolic responses that normalize ROS levels and mediate redox homeostasis (Guzy and Schumacker, 2006). Physiological processes such as programmed cell death, metabolism, cell proliferation, and autophagy are regulated at the transcriptional level by ROS through modulation of chromatin dynamics and transcriptional activity (Rajendran et al., 2011).
Excess cellular ROS production has many detrimental effects, such as damaging DNA, proteins, and lipids (Pham-Huy et al., 2008). One example of this is the accumulation of phospholipid hydroperoxide (PLOOH) lipid ROS in cells, which leads to a form of iron-dependent programmed cell death called ferroptosis (Dixon et al., 2012). Ferroptosis is a nonapoptotic form of cell death that is characterized by an accumulation of lipid peroxides and reactive iron. Ferroptotic cells display morphological changes such as shrunken mitochondria with degenerated cristae and membrane rupture.
The intestine is exposed to a large number of microbes, dietary antigens, and the hypoxic nature of the gut lumen, making it necessary to have a robust mechanism to maintain physiological ROS levels. In this review, we examine the role of ROS in the intestine and in two intestinal diseases: inflammatory bowel disease (IBD) and colorectal cancer (CRC). We summarize findings that suggest ferroptosis is associated with these two disease states. Lastly, we review potential mechanisms for leveraging the role of ferroptosis in IBD and CRC as therapeutic treatments.
Intestinal Physiology
The gastrointestinal (GI) epithelium plays a crucial role in maintaining nutrient absorption, secreting entero-hormones, and serving as a physical barrier to the microbiota. In a state of homeostasis, the cellular interactions between immune cells and the GI epithelium mediate mucosal tolerance toward the microbiota (Chieppa et al., 2006; Hepworth et al., 2015; Nanno et al., 2008; Peterson and Artis, 2014). Dysregulation or damage to the intestinal barrier initiates bacteria translocation across the lamina propria eliciting antimicrobial defenses. ROS production by immune cells functions to both promote pathogen clearance and regulate intracellular signals for barrier healing and repair (Neurath, 2014). Redox imbalance promotes a sequalae of events that lead to a myriad of complications such as cancer, fibrosis, and systemic symptoms (Neurath, 2014).
In the GI tract, the primary sources of ROS are from NOX/DUOX enzymes and ETC (Aviello and Knaus, 2017). Superoxides from ETC reacts with superoxide dismutase (SOD)-2 within the mitochondrial matrix to form hydrogen peroxide, which modulates cell activity through altering levels of transcription factors, kinases, and phosphatases (Mittal et al., 2014; Tanner et al., 2011). To maintain the redox balance, both endogenous and exogenous antioxidants are necessary. Thioredoxin (Trx) and glutathione (GSH) are two major antioxidant mechanisms used by cells to protect against ROS. Trx and GSH can act as electron acceptors to limit ROS through nonenzymatic mechanisms.
In addition, Trx and GSH are essential factors in enzymatic-mediated detoxification of ROS. Glutathione peroxidase (GPX) converts GSH into glutathione disulfide (GSSG), which reduces H2O2 into water and lipid peroxides into alcohols (Ursini et al., 2022). Trx also serves as an electron donor to peroxidase to reduce ROS. Oxidized Trx and GSSG are reduced in an NADPH manner to maintain proper redox balance (Ursini et al., 2022). Other endogenous enzymatic antioxidants include SOD and catalase. SOD reacts with O2•− to form O2 and H2O2, and catalase converts H2O2 into H2O and O2 (Ighodaro and Akinloye, 2018).
Nuclear factor erythroid 2-related factor 2 (NRF2) is a transcription factor that is activated by ROS and plays a crucial role in the adaptive response to limit ROS toxicity (Mazur-Bialy and Pocheć, 2021). Under basal conditions, NRF2 binds to Kelch-like epichlorohydrin-related protein (KEAP1), resulting in the ubiquitination and subsequent degradation of NRF2. Under conditions of oxidative stress, NRF2 is stabilized and translocates to the nucleus to activate a battery of antioxidant genes (Cuadrado et al., 2019). In addition, exogenous antioxidants are another source of ROS detoxification. This category includes vitamins such as B12 and E, amino acids, peptides, fatty acids, minerals, and other bioactive natural products (Wang et al., 2020c).
ROS in Inflammation and Colon Cancer
Redox imbalance leads to insufficient microbial clearance, compromised wound healing, prolonged inflammation, and altered immune responses (Neurath, 2014). One such consequence of altered GI ROS imbalance is IBD. IBD is a group of chronic idiopathic GI inflammatory conditions that primarily include ulcerative colitis (UC) and Crohn's disease (CD). IBD is clinically characterized by symptoms such as diarrhea, nausea, vomiting, abdominal pain, bloody stool, weight loss, as well as extraintestinal symptoms such as fever, fatigue, skin rashes, and ulcers (Abraham and Cho, 2009). UC is characterized by inflammation that affects the mucosa and/or submucosa of the rectum, and in some cases may extend proximally to involve the entire colon in a continuous pattern (Abraham and Cho, 2009; Gajendran et al., 2018; Khor et al., 2011; Monteleone et al., 2006).
In contrast, CD can affect any part of the GI tract in a patchy distribution, and is often characterized by transmural inflammation, granulomas, fissuring ulceration, and a thickened submucosa (Abraham and Cho, 2009; Gajendran et al., 2018; Khor et al., 2011; Monteleone et al., 2006).
The exact cause of IBD is not fully understood, however, several ROS-related factors have been found to contribute to its pathogenesis. There is a strong genetic component to IBD. Nucleotide-binding oligomerization domain 2 (NOD2) gene, a member of the Nod-like receptor family responsible for microbial detection, was the first gene linked to IBD (Yamamoto and Ma, 2009). Alterations in mucosal bacterial recognition can lead to increased nuclear factor kappa-light-chain-enhancer of activated B cell signaling and the subsequent inflammation (Ogura et al., 2001). Host-microbiota dysregulation also contributes to the pathogenesis of IBD. While many commensal microorganisms are necessary for healthy gut homeostasis, pathogenic microbes can disrupt this balance leading to an inflammatory environment (Guan, 2019).
Environmental factors, such as diet, specifically Western diets high in polyunsaturated fatty acids (PUFAs), have been shown to contribute to the development of IBD through metabolic inflammation (Guan, 2019). Interestingly, while the rate of IBD incidence is highest among developed Western nations, the fastest acceleration of IBD incidence is among developing nations undergoing westernization (Kaplan and Windsor, 2021). In addition, abnormal immune response has been linked to the development of IBD, with CD associated with an overreactive T helper 1 (Th1) immune response leading to excessive interleukin-23/T helper 17 (IL-23/Th17) activation in response to bacterial antigens in genetically susceptible individuals (Monteleone et al., 2006; Souza and Fiocchi, 2016; Tavakoli et al., 2021). UC displays a more characteristic overreactive T helper 2 (Th2) immune response with increased IL-5 and IL-13 (Strober and Fuss, 2011). Overall, IBD is a complex, multifactorial disease with many contributing factors.
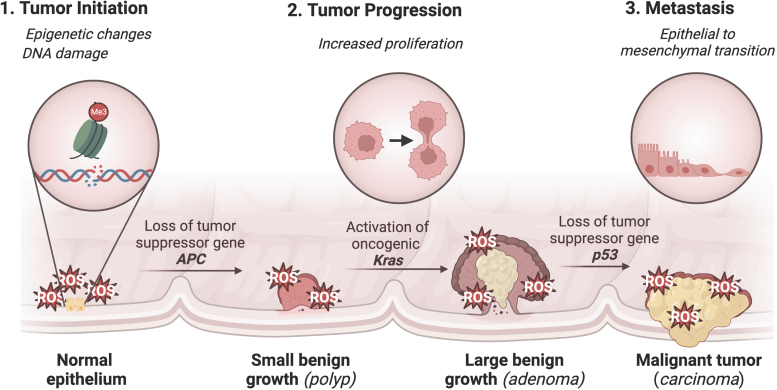
Chronic inflammation has been linked to the development of cancer, particularly CRC, which is a malignancy of the colon or rectum and one of the most common and deadly cancers in the United States (Siegel et al., 2022). IBD is major risk factor for CRC (Porter et al., 2021). Other risk factors include diets high in PUFAs, smoking, obesity, red meat, and alcohol (Hossain et al., 2022). Genetic causes of CRC include familial adenomatous polyposis and hereditary nonpolyposis colon cancer (Lynch Syndrome) (Hossain et al., 2022). The development of CRC is characterized by a well-established mutational pathway, with the earliest and most prevalent mutation involving the tumor suppressor gene APC. Subsequent mutations in KRAS and p53 lead to the development of carcinoma and metastatic cancer (Fig. 2) (Fearon and Vogelstein, 1990; Muzny et al., 2012).
FIG. 2.
Role of ROS in CRC tumorigenesis. ROS are essential in CRC tumor initiation, progression, and metastasis. Tumor initiation begins with genetic and epigenetic changes. ROS directly contribute to tumor initiation through oxidative DNA damage and the formation of mutagenic 8-oxodG, which induces guanine to thymine transversion through its ability to bond to both cytosine and adenine (Oka and Nakabeppu, 2011). In addition, ROS help drive aberrant methylation of DNA (Barnicle et al., 2017; Lucafò et al., 2021). Tumor progression involves the selective expansion of cells given a proliferative advantage. It was shown that increased H2O2 generation secondary to upregulation of NOX1 in NIH 3T3 fibroblasts led to the increased expression of cell cycle and growth genes (Arnold et al., 2001). ROS have also been shown to promote proliferation and migration of CRC cells through regulation of cell cycle progression and EMT (Zeng et al., 2021). Lastly, metastasis involves neoplastic growth at a secondary site. ROS help promote induction of EMT essential for metastasis. During EMT, epithelial-like cells lose cell–cell and cell–matrix adhesions and become more mesenchymal-like with increased motility and migratory capability (Bhatia et al., 2020). 8-oxodG, 8-oxo-7-hydro-2′-deoxyguanosine; CRC, colorectal cancer; EMT, epithelial-to-mesenchymal transition; ROS, reactive oxygen species.
In IBD and CRC, ROS play an important role in the disease pathogenesis, which has led to several human clinical trials assessing the efficacy of antioxidant therapy (Moura et al., 2015). A systematic meta-analysis review of the use of antioxidant therapy in the treatment of IBD concluded that there was not enough evidence to suggest that antioxidant therapy is effective in treating IBD. Therefore, further research is needed in this field (Table 1) (Moura et al., 2015).
Table 1.
Antioxidant Therapies Used in Preclinical Models and Clinical Trials of Inflammatory Bowel Disease
| Antioxidant | Clinical studies/animal model | Finding | Reference |
|---|---|---|---|
| HMG-CoA reductase inhibitors (statins) | • Patients with UC/CD • TNBS-induced colitis in rats • DSS-induced colitis in mice |
1. Statins reduces risk of CRC in IBD patients 2. Statins decrease risk of new-onset IBD 3. Statins ameliorate experimental colitis in rats 4. Statins ameliorate experimental colitis in mice |
Ananthakrishnan et al. (2016); Kanagarajan et al. (2008); Lei et al. (2016); Lochhead et al. (2020); Maheshwari et al. (2015); Shin et al. (2017); Ungaro et al. (2016) |
| ACE inhibitors | • Patients with UC/CD • TNBS-induced colitis in rats • AA-induced colitis in rats • DSS-induced colitis in mice • IL-10 KO-induced colitis in mice |
1. ACE-I or ARB therapy did not improve outcomes in IBD patients 2. Telmisartan ameliorates experimental colitis in rats 3. Telmisartan ameliorates experimental colitis in mice 4. Enalaprilat ameliorates experimental colitis in mice |
Arab et al. (2014); Arumugam et al. (2015); Fairbrass et al. (2021); Spencer et al. (2007); Sueyoshi et al. (2013) |
| MEL | • Patients with UC • AA-induced colitis in rats • TNBS-induced colitis in mice • DSS-induced colitis in mice |
1. Adjuvant MEL may help in sustaining remission in patients with UC 2. MEL ameliorates experimental colitis in rats 3. MEL ameliorates experimental colitis in mice |
Chojnacki et al. (2010); Esiringü et al. (2016); Nosál'ová et al. (2007); Park et al. (2015); Shin et al. (2017); Zhu et al. (2018); Zielińska et al. (2016) |
| NAC | • Patients with UC • AA-induced colitis in pigs • AA-induced colitis in rats • DSS-induced colitis |
1. NAC does not improve outcomes in patients with mild-to-moderate UC 2. NAC improves remission rates in patients with UC 3. NAC ameliorates experimental colitis in pigs 4. NAC ameliorates experimental colitis in rats 5. NAC ameliorates experimental colitis in mice |
Guijarro et al. (2008); Shirazi et al. (2021); Uraz et al. (2013); Wang et al. (2013); You et al. (2009) |
| Modified SOD | • Patients with UC • DSS-induced colitis in rats • TNBS-induced colitis in mice • DSS-induced colitis in mice |
1. SOD ameliorates colitis in patients with UC 2. SOD ameliorates experimental colitis in rats |
Hori et al. (1997); Hou et al. (2014); Ishihara et al. (2009); Suzuki et al. (2008) |
| PLC | • Patients with UC/CD • TNBS-induced colitis in rats |
1. PLC reduces mucosal inflammation in UC patients 2. PLC improves histological and endoscopic activity in UC patients 3. PLC does not improve clinical outcomes in patients with US 4. PLC ameliorates experimental colitis in rats |
Merra et al. (2012); Mikhailova et al. (2011); Scioli et al. (2014) |
| RSV | • Patients with UC • TNBS-induced colitis in rats • DSS-induced colitis in mice • IL-10 KO-induced colitis in mice |
1. RSV improved clinical colitis activity index score in UC patients 2. RSV ameliorates experimental colitis in rats 3. RSV ameliorates experimental colitis in mice |
Samsami-kor et al. (2015); Singh et al. (2012); Yao et al. (2015); Yildiz et al. (2015) |
| Vitamin E (α-tocopherol) | • Patients with UC • AA-induced colitis in rats • TNBS induced in rats • DSS-induced colitis in mice |
1. Vitamin E is safe for patients with UC 2. Vitamin E ameliorates experimental colitis in rats 3. Vitamin E ameliorates experimental colitis in mice |
Liu et al. (2021); Minaiyan et al. (2011); Mirbagheri et al. (2008); Tahan et al. (2011) |
| Vitamin C (ascorbic acid) | • Patients with CD • DSS-induced colitis in mice |
1. Vitamin C supplements improve T cell function in CD, but do not have any effects on humoral immunity 2. Vitamin C ameliorates experimental colitis in mice |
Animashaun et al. (1990); Kondo et al. (2019) |
| Selenium | • Patients with UC • DSS-induced colitis in mice |
1. Selenium supplementation improved stool frequency in patients with UC 2. Selenium-deficient diets worsen experimental colitis, while selenium supplementation ameliorates experimental colitis |
Kaushal et al. (2014); Shi et al. (2021); Stedman et al. (1997); Wu et al. (2021) |
AA, arachidonic acid; ACE, angiotensin-converting-enzyme; ARB, angiotensin receptor blocker; CD, Crohn's disease; CRC, colorectal cancer; DSS, dextran sulfate sodium; IBD, inflammatory bowel disease; IL, interleukin; KO, knockout; MEL, melatonin; NAC, N-acetylcysteine; PLC, propionyl-l-carnitine; RSV, resveratrol; SOD, superoxide dismutase; TNBS, 2,4,6-trinitrobenzenesulfonic acid; UC, ulcerative colitis.
The discrepancy between promising preclinical models and disappointing clinical trials in IBD suggests that the models currently used do not fully replicate all aspects of this complex disease. In addition, since ROS play both positive physiological roles and detrimental effects, targeting all ROS may not yield positive results. ROS can damage DNA, proteins, and lipids, and so, selectively targeting a specific aspect of ROS damage may be more effective in treating IBD. The role of antioxidants in CRC therapy is even more uncertain. Given the role of ROS in various stages of CRC progression, antioxidant agents could potentially inhibit aggressive cancer phenotypes. However, several effective chemotherapy, radiotherapy, and immunotherapy treatments rely on inducing cell death through oxidative stress (Table 2).
Table 2.
Anticancer Therapies Used for Colorectal Cancer That Rely on Reactive Oxygen Species Generating Effects
| Treatment | Type of modality | Class of drug | Mechanism of action and role of ROS | References |
|---|---|---|---|---|
| 5-Fu | Chemotherapy | Fluoropyrimidine | 5-Fu is an antimetabolite with a fluorine instead of hydrogen at the C-5 position of uracil, resulting in impaired DNA and RNA synthesis through the folate metabolic pathway. 5-Fu has been shown to also generate O2•− in CRC cell lines that regulate p53 and promote cell apoptosis. Commonly used in combination with FOLFOX or in combination with CAPOX. | Hwang et al. (2001) |
| Oxaliplatin | Chemotherapy | Platinum-based antineoplastic | Oxaliplatin inhibits DNA synthesis through crosslinking. In addition, this drug has been shown to generate ROS, resulting in the loss of mitochondrial membrane potential and GSH levels, thus triggering apoptosis. Commonly used in combination with FOLFOX. Has also been shown to promote ferroptosis by inhibiting the Nrf2 signaling pathway. | Liu and Wang (2022); Tolan et al. (2016) |
| Bevacizumab | Chemotherapy | Monoclonal antibody | Bevacizumab is a recombinant humanized monoclonal antibody against VEGF-A. This protein is crucial for promoting angiogenesis. Bevacizumab has been shown to induce oxidative cytotoxicity and apoptosis. | Özkaya and Nazıroğlu (2021) |
| Cetuximab | Chemotherapy | Monoclonal antibody | Cetuximab is a mouse/human chimeric monoclonal body directed against EGFR. Overexpression of EGFR has been associated with many cancers. Cetuximab has been shown to decrease ROS levels by inhibiting the Ras/Nox1 pathway in CRC. Interestingly, this drug has also been shown to increase the cytotoxic and ferroptosis-inducing effects of RSL3 in CRC cells. | Santoro et al. (2017); Yang et al. (2021) |
| Pembrolizumab | Immunotherapy | PD-1 inhibitor | Pembrolizumab is a therapeutic antibody targeted against PD-1 on T cells. Blocking PD-1 on T cells helps direct an immune response against cancer cells. Pembrolizumab has been shown to generate immune complexes that stimulate ROS production in granulocytes. While other checkpoint inhibitors such as nivolumab (anti PD-1) and ipilimumab (anti-CTLA-4), there are currently no available original articles describing ROS-mediated effects of these drugs. | Hock et al. (2020) |
| Radiation | Radiotherapy | N/A | Radiotherapy uses radiation to induce cell death in cancer cells through ROS generation, DNA damage, and stress response in subcellular organelles. | Kim et al. (2019) |
5-Fu, 5-fluorouracil; CAPOX, capecitabine; EGFR, epidermal growth factor receptor; FOLFOX, folinic acid and oxaliplatin; GSH, glutathione; Nrf2, nuclear factor erythroid 2-related factor 2; ROS, reactive oxygen species; RSL3, RAS-selective lethal 3; VEGF-A, vascular endothelial growth factor A.
Chemotherapeutic drugs, such as anthracyclines, platinum complexes, arsenic agents, and alkylating agents, have anticancer effects by generating high levels of ROS and/or inhibiting cellular antioxidant systems to mediate cytotoxicity in cancer cells (Loenhout et al., 2020). Radiotherapy uses ionizing radiation to induce DNA damage in cancerous tissue thus inducing cell death (Baskar et al., 2012). Immunotherapy leverages the immune system to accurately identify cancer cells and mediate cell death, with ROS playing a necessary role in coordinating the cross talk between various immune cells and creating a toxic environment for cancer cells (Wang et al., 2021). It is believed that cells with a normal level of oxidative stress would not be sufficiently affected, thus conveying a selective advantage for normal cells during oxidative treatment.
It is generally recommended that supplemental antioxidants should be avoided in cancer patients undergoing pro-oxidant therapies (Loenhout et al., 2020). A better understanding of ROS and selectively targeting specific ROS may serve to improve anticancer therapies.
Ferroptosis
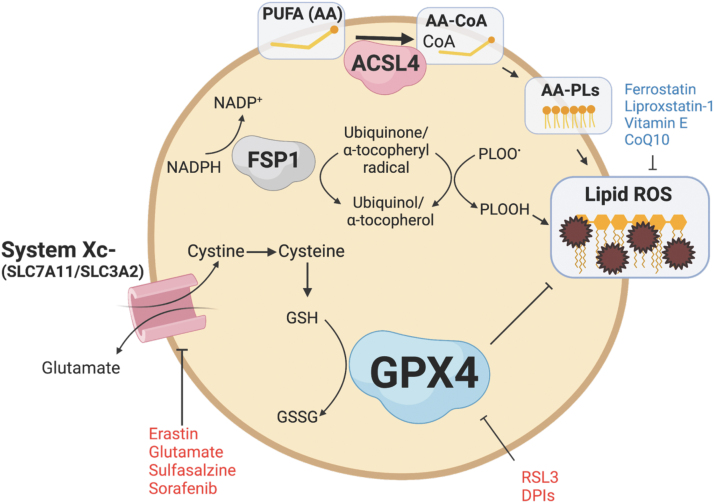
There is increasing evidence that lipid ROS and ferroptosis are implicated in the pathophysiology of IBD and CRC. The primary regulator and inhibitor of ferroptosis is the selenoprotein GPX4 (Fig. 3) (Jiang et al., 2021). GPX4 inhibits ferroptosis by reducing lipid ROS through the conversion of GSH to GSSG to maintain redox balance (Ighodaro and Akinloye, 2018). Another important inhibitor of ferroptosis is the cystine/glutamate antiporter system composed of SLC7A11 and SLC3A2. Cystine is converted to cysteine, an essential amino acid for GSH. Inhibiting GPX4 or the cystine/glutamate antiporter system can lead to an accumulation of lipid ROS and an imbalance in redox balance. Certain drugs and compounds, such as RAS-selective lethal 3 (RSL3) and erastin, can induce ferroptosis by inhibiting GPX4 or the cystine/glutamate antiporter system. Antioxidants such as ferrostatin, liproxstatin-1, vitamin E, and CoQ10 can act as inhibitors of ferroptosis (Jiang et al., 2021).
FIG. 3.
Mechanisms governing ferroptosis. Ferroptosis is an iron-dependent regulated cell death pathway based on the accumulation of lipid peroxides. The primary regulators of this pathway are GPX4 and cystine/glutamate antiporter System Xc−. GPX4 reduces cytotoxic lipid ROS, while System Xc− transports the cystine, the necessary precursor for substrates of GPX4. Additional pathways include ACSL4, which incorporates PUFA-CoA into phospholipid cell membranes, and FSP1, which reduces lipid ROS through a GPX4-independent pathway. ACSL4, acyl-CoA synthetase long-chain family member 4; FSP1, ferroptosis suppressor protein 1; GPX4, glutathione peroxidase 4; PUFA, polyunsaturated fatty acid.
Although GPX4 is the primary inhibitor of ferroptosis, other pathways contribute to regulating ferroptosis. Ferroptosis suppressor protein 1 (FSP1; also known as AIFM2) reduces lipid peroxides and prevents ferroptosis through its NADH:ubiquinone oxioreductase function (Elguindy and Nakamaru-Ogiso, 2015). FSP1 reduces ubiquione to ubiquinol, which directly reduces lipid peroxides and indirectly recovers oxidized vitamin E (Bersuker et al., 2019; Doll et al., 2019). GCH1 inhibits ferroptosis through the production of tetrahydrobiopterin (BH4) and dihydrobiopterin (BH2). BH4 traps ROS and plays a role in ubiquinone synthesis (Kraft et al., 2020). In addition, acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) are also mediators of ferroptosis.
ACSL4 incorporates long-chain PUFAs, namely arachidonic acid (20:4) and adrenic acid (22:4), with coenzyme A to form PUFA-CoA molecules, which can then be converted to phospholipids via LPCAT enzymes and thereby increasing phospholipid-PUFAs into cellular membranes (Dixon et al., 2015; Doll et al., 2017; Zou et al., 2019). Inhibiting ACSL4 through pharmacological or genetic means can alter the composition of phospholipids toward the incorporation of monounsaturated fatty acyl tails instead of long-chain PUFA tails, which can protect against ferroptosis (Doll et al., 2017).
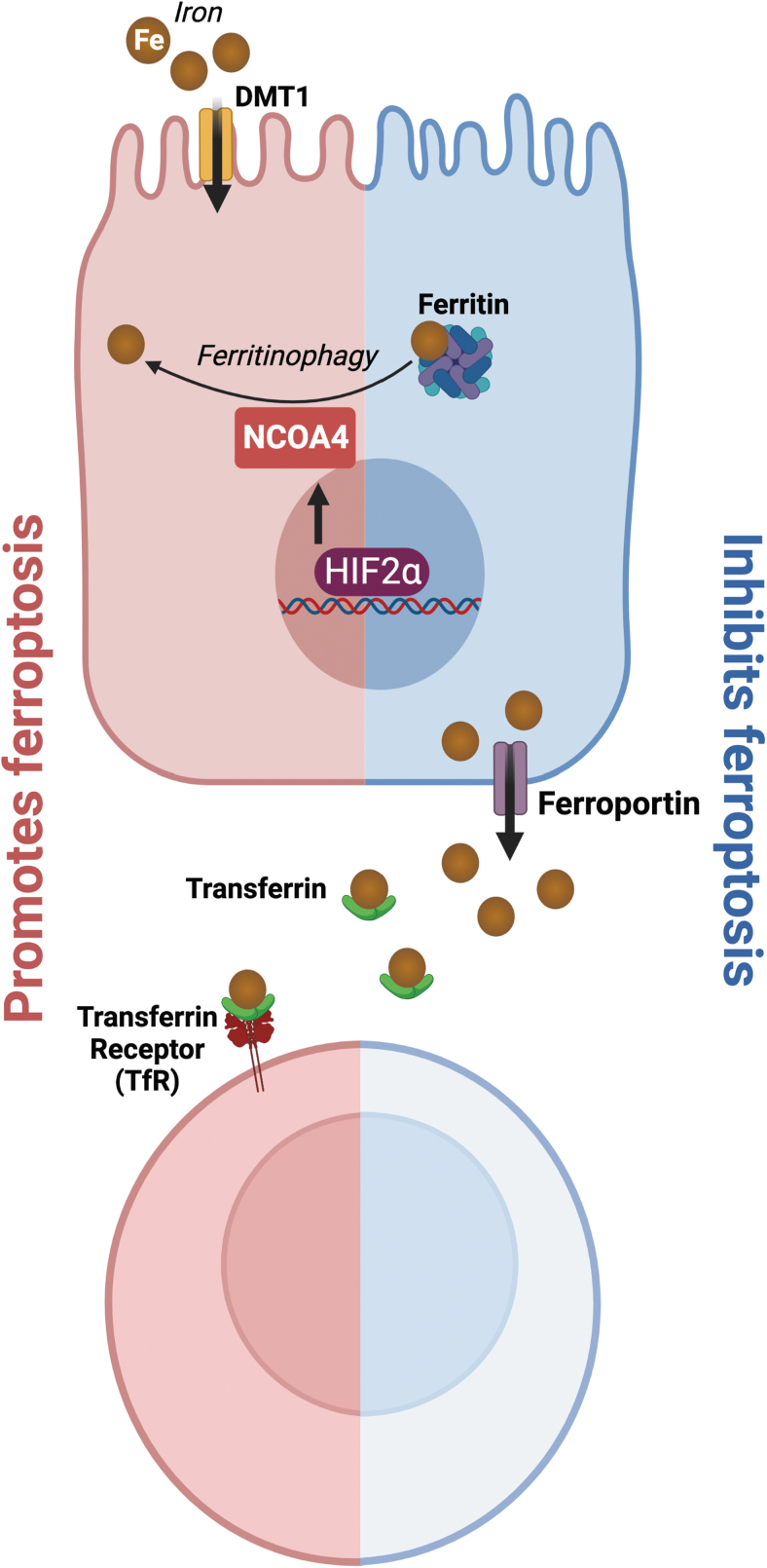
As the name implies, iron is essential in ferroptosis (Fig. 4). Iron is necessary for a multitude of redox reactions that generate cellular ROS (Jiang et al., 2021). Through the Fenton reaction, iron amplifies PLOOHs and generates additional free radicals such as PLO• and PLOO• to further oxidize lipids (Jiang et al., 2021). Iron importers, divalent metal transporter-1 (DMT1), transferrin (Tf), and transferrin receptor (TfR) promote ferroptosis, while iron storage (ferritin) and export proteins (ferroportin) inhibit ferroptosis (Jiang et al., 2021; Liang et al., 2022). Nuclear receptor coactivator 4 (NCOA4) is responsible for the degradation of ferritin through a process called ferritinophagy, which releases free iron and promotes ferroptosis (Bogdan et al., 2016; Hou et al., 2016; Mancias et al., 2014).
FIG. 4.
Role of iron metabolism in ferroptosis. Excess iron results in the Fenton reaction producing ROS. Iron transport proteins such as DMT1, transferring, and TfR promote ferroptosis. DMT1 imports iron from the apical lumen side into duodenal enterocytes. Transferrin and its receptor, TfR, mediate the transport of iron through blood. NCOA4 mediates the breakdown of ferritin to release free iron, thus promoting ferroptosis. Iron storage (ferritin) and export (ferroportin) proteins inhibit ferroptosis by sequestering iron and decreasing cellular iron levels. DMT1, divalent metal transporter-1; NCOA4, nuclear receptor coactivator 4; TfR, transferrin receptor.
In addition, HIF-2α, the master transcription factor critical for iron import, sensitizes CRC cells to ferroptosis by increasing cellular iron and altering cellular lipid composition (Singhal et al., 2021; Zou et al., 2019). HIF-2α has also been found to increase systemic iron via NCOA4-mediated intestinal ferritinophagy (Das et al., 2020). The association between HIF-2α and ferroptosis suggests a possible link between hypoxia, iron, and ferroptosis.
Identifying cellular death as ferroptosis poses many challenges. Unlike other markers of regulated cell death pathways such as capase-3 for apoptosis or RIP1/3 for necroptosis, there are no widely accepted markers of ferroptosis (Chen et al., 2016, 2021a; Ward et al., 2008). Recently, TfR1 has been proposed as a potential marker of ferroptosis, but more work is needed to understand its utility (Feng et al., 2020). Ferroptosis is typically identified by several biochemical and morphological hallmarks, including the ability to be rescued by ferroptosis inhibitors and the inability to respond to other cell death inhibitors (Chen et al., 2021a). Biochemical hallmarks include accumulation of cellular iron, induction of lipid peroxidation, and loss of antioxidant defense (Chen et al., 2021a).
Assays used to detect lipid peroxidation include the use of lipid-soluble fluorescent probes, antibodies directed against the final products of ferroptosis such as malonyl dialdehyde and 4-hydroxynonenal, and antibodies directed against oxidative DNA damage such as the phosphorylated H2A.X variant histone (γH2AX) and 8-hydroxydeoxyguanosine. Morphological features include cell enlargement, plasma membrane rupture, reduction in mitochondrial volume, disappearance of mitochondrial cristae, and increase in mitochondrial membrane density (Chen et al., 2021a).
Physiological Roles of Ferroptosis
Ferroptosis may play a physiological role in immune surveillance. It is known that other forms of regulated cell death such as apoptosis, necroptosis, and pyroptosis play a role in modulating immune responses (Green, 2019). Interferon γ (IFNγ) has been shown to downregulate the expression of system Xc− in macrophages (Sato et al., 1995). IL-4 and IL-13 suppress the expression of GPX4 in the kidney, lung, spleen, and cardiomyocytes. These same cells also exhibited upregulation of arachidonate lipoxygenase 15 (ALOX15), allowing for increased production of arachidonic acid metabolites, which serve as important inflammatory signaling molecules (Schnurr et al., 1999). GPX4 suppresses the function of LOXs and cyclooxygenases by reducing lipid peroxidation. In the context of reduced GPX4 expression, it is possible that secretion of lipid signaling molecules is altered and in turn alerts immune cells about ferroptosis-sensitive cells.
In plants, induction of ferroptosis prevents fungal Magnaporthe oryzae infection in rice through the removal of afflicted cells, thus preventing the spread of pathogen (Shen et al., 2020). It was also discovered that ferroptotic cell death in M. oryzae is necessary for the development of infection in its host (Shen et al., 2020).
Inhibition of ferroptosis plays a role in the development and homeostasis of adult tissues. GPX4 knockout embryos do not survive past midgestation (E7.5), indicating that inhibition of ferroptosis is necessary for embryonic development (Yant et al., 2003). When GPX4 is disrupted globally in adult mice via a whole-body tamoxifen-inducible Cre, mice die within 2 weeks and exhibit significant tissue injury (Angeli et al., 2014; Yoo et al., 2012). Interestingly, when both copies of GPX4 are deleted under an intestinal epithelial cell (IEC)-specific constitutive Cre driver, the mice are embryonic lethal, but ∼11% of mice were able to be rescued with the use of in utero supplementation of α-tocopherol (Mayr et al., 2020).
In addition, the intestinal GPX4 knockout pups that were rescued by α-tocopherol were born with a lower weight but regained weight by 7 weeks of age, suggesting that GPX4 is not necessary to maintain adult gut homeostasis and that there may be compensatory mechanisms in the intestines to reduce lipid ROS (Mayr et al., 2020).
Although the role of ferroptosis in normal physiology is not well understood, its implications in pathophysiological conditions are broadly studied. Ferroptosis has been linked to obesity, atherosclerosis, ischemia/reperfusion (I/R) injury, IBD, and cancer. In this section, we focus on the role of ferroptosis in IBD and CRC and how ferroptosis can be leveraged to develop therapeutics for these diseases.
Ferroptosis in IBD
There is evidence to suggest that ferroptosis may play a role in IBD; however, the specific relationship remains unclear. It is well-established that the levels of iron in the diet can have an impact on colitis in several mouse models. Studies have shown that high levels of iron supplementation induce gut dysbiosis and promote intestinal injury (Uritski et al., 2004; Werner et al., 2011; Zhang et al., 2022). In humans, high levels of dietary iron are associated with elevated risk of IBD (Kobayashi et al., 2019). In addition, levels of mucosal ROS production are correlated with disease severity, and iron chelators reduce ROS production and improve symptoms in IBD (Millar et al., 2000; Minaiyan et al., 2011).
These findings suggest that one possible mechanism by which excess iron in the intestines can lead to injury is by promoting the production of ROS through the Fenton reaction. This in turn leads to lipid peroxidation and the death of cells through a process called ferroptosis.
Ferroptosis-associated genes are elevated in IBD tissue (Xu et al., 2020). Furthermore, inhibition of ferroptosis by ferrostatin-1, liproxstatin-1, and desferoxamine reduced intestinal injury in dextran sulfate sodium (DSS)-induced murine models of colitis (Chen et al., 2020b; Wang et al., 2020b; Xu et al., 2020). Curculigoside (CUR), a botanical antioxidant, was also found to protect against ferroptosis through promoting GPX4 activity in a DSS model of colitis (Wang et al., 2020b). In an intestinal rat epithelial cell line (IEC6), CUR was shown to sensitize cells to selenium and upregulate GPX4 expression (Wang et al., 2020b). GPX4 knockdown by siRNA attenuated the protective effects of CUR against ferroptosis (Wang et al., 2020b). In IBD patients, impaired GPX4 activity and elevated markers of lipid peroxidation were present in small IECs (Mayr et al., 2020).
Similar to other mechanisms of ferroptosis, PUFAs induced inflammatory cytokine release in GPX4 knockdown IECs in response to high levels of iron, lipid peroxidation, and increased activity of ACSL4 (Mayr et al., 2020). Intestinal-specific GPX4 knockout mice fed on a PUFA-enriched Western diet displayed increased intestinal damage and symptoms similar to IBD. Together, these findings indicate that ferroptosis plays a crucial role in IBD and targeting this pathway may be a promising therapeutic approach for treating IBD (Mayr et al., 2020).
Ferroptosis in CRC
Targeting ferroptosis is a promising approach in many cancers, including CRC (Jiang et al., 2021). The first evidence that targeting ferroptosis as a therapeutic intervention in vivo was shown in mouse models of pancreatic ductal adenocarcinoma (PDAC) (Badgley et al., 2020). Cyst(e)ine is a central dependency for PDAC. Depletion of cyst(e)ine through genetic deletion of SLC7A11 or pharmacologically through cyst(e)inase induced tumor-selective ferroptosis and inhibited PDAC growth. These findings demonstrate that activating ferroptosis is a promising approach for cancer therapy (Badgley et al., 2020).
CRC accumulates large amounts of iron to meet the metabolic requirement for growth. Elevated HIF-2α levels in CRC mediate colon carcinogenesis through increased expression of the iron importer DMT1 (Xue et al., 2012). In addition, CRC activates ectopic hepcidin in the tumor microenvironment to sequester iron (Schwartz et al., 2018). Mining The Cancer Gene Atlas, GPX4 and System Xc− are highly induced in CRC (Muzny et al., 2012), potentially to inhibit iron toxicity and ferroptosis. Studies have shown that GPX4 overexpression in CRC cell lines can prevent cell death following RSL3 treatment, suggesting that ferroptosis may be a potential mechanism of cell death in CRC (Sui et al., 2018).
In vitro experiments have also demonstrated that RSL3 is able to induce ferroptosis and increase levels of ROS and cellular labile iron pools in a time- and dose-dependent manner in three CRC cell lines (Sui et al., 2018). Moreover, cisplatin induces ferroptosis, and a combination of cisplatin and erastin is synergistic in suppressing the growth of CRC cell lines suggesting that ferroptosis provides alternative mechanisms of cell death in CRC (Guo et al., 2018).
The physiological roles of ferroptosis have not been fully understood, but there is a growing body of evidence to suggest that ferroptosis plays a role in tumor suppression. Several tumor suppressors such as p53 and BAP1 can sensitize tumor cells to ferroptosis. It was discovered that acetylation of p53 lysine sites potentiates ferroptosis through suppression of System Xc− transcription (Jiang et al., 2015; Wang et al., 2016). However, the role of p53 in affecting cancer is context dependent. In CRC, p53 has also been shown to inhibit ferroptosis by blocking dipeptidyl-peptidase 4 (DPP4) activity and preventing localization to the nucleus to form the DPP4-NOX1 complex that promotes lipid peroxidation (Xie et al., 2017). Furthermore, loss of p53 in tumor-bearing mice synergistically increased the efficacy of erastin (Xie et al., 2017). Disassembly of the DPP4-NOX1 complex in the context of p53 loss restored the erastin-induced sensitivity of CRC (Xie et al., 2017).
In contrast to Jiang et al. (2015), it was found that p53 upregulates SLC7A11 expression in CRC and thus protects CRC cells from ferroptosis (Xie et al., 2017). It has also been reported that BAP1 promotes ferroptosis through downregulation of SLC7A11, one of the two subunits in system Xc− (Zhang et al., 2018). However, the exact role of tumor suppressors in modulating ferroptosis is not yet fully delineated. In addition, unlike the sufficient ferroptosis-mediated tumor suppressing activity of p53 alone, it is unclear to what extent BAP1 contributes to tumor suppression through ferroptosis (Zhang et al., 2018). The Ras mutation, one of the most common genetic mutations in CRC, is widely linked to ferroptosis (Bartolacci et al., 2022; Chen et al., 2020a; Park et al., 2018).
In Kras-mutant lung cancer, it has been reported that fatty acid synthase protects cancer cells against ferroptosis through the generation of oxidation-resistant fatty acids, while it sensitizes the tumor microenvironment to ferroptosis through producing more PUFAs (Chen et al., 2020a). Moreover, GPX4 and ACSL3 appear to be central dependencies in Kras-mutant lung cancer, highlighting the sensitivity of ferroptosis in Ras-mutant cancers (Andreani et al., 2022).
It has also been shown that during immunotherapy, IFNγ secreted from CD8+ T cells sensitizes tumor cells to ferroptosis through the downregulation of SLC3A2 and SLC7A11, resulting in reduced cystine uptake by tumor cells and the subsequent tumor cell lipid peroxidation and ferroptosis (Wang et al., 2019). Moreover, CD8+ T cell-derived IFNγ increased tumor ACSL4 expression to promote ferroptosis (Liao et al., 2022).
Several anticancer drugs have been found to inhibit CRC through a ferroptotic-dependent manner (Table 3). Apatinib induces ferroptosis in CRC cell lines through activation of the ELOVL6/ACSL4 pathway (Tian et al., 2021). A benzopyran derivative, 2-imino-6-methoxy-2H-chromene-3-carbothioamide, induces ferroptosis in CRC through downregulation of SLC7A11 (Zhang et al., 2020b). Talaroconvolutin A induces ferroptosis in CRC cells in a time- and dose-dependent manner (Xia et al., 2020). Honokiol induces ferroptosis in CRC cells through inhibiting the activity of GPX4 (Guo et al., 2020). Camellia nitidissima Chi induces ferroptosis in CRC cells through downregulation of GPX4 (Chen et al., 2021b).
Table 3.
Drugs That Have Been Found to Induce Ferroptosis in Colorectal Cancer
| Drug | Models used | Ferroptosis effect | References |
|---|---|---|---|
| Apatinib | HCT116 cells | Promotes ferroptosis through targeting ELOVL6/ACSL4 pathway. | Tian et al. (2021) |
| IMCA | DLD-1 cells HCT116 cells CRC xenograft model |
Promotes ferroptosis. Downregulates expression of SLC7A11, leading to decreased substrates necessary for GPX4 to function and thus accumulation of lipid peroxides. Also inhibits mTOR/P70S6K activity. |
Zhang et al. (2020b) |
| TalaA | HCT116 cells SW480 cells SW620 cells CRC xenograft model |
Promotes ferroptosis. Increases ROS in cells to sensitize cells to ferroptosis and also downregulates expression of SLC7A11 and upregulates expression of ALOXE3. | Xia et al. (2020) |
| Honokiol | RKO cells HCT116 cells SW480 cells HT29 cells LS174T cells HCT8 cells SW48 cells |
Promotes ferroptosis. Increases ROS and Fe2+ levels. Decreases activity of GPX4; does not affect System Xc−. | Guo et al. (2020) |
| CNC | HCT116 cells | Promotes ferroptosis. Downregulates expression of GPX4 and upregulates expression of HMOX1. | Chen et al. (2021b) |
| Sulfasalazine | HCT116 cells HT29 cells LOVO cells DLD-1 cells |
Promotes ferroptosis. Inhibits System Xc− thus preventing GSH synthesis through limiting GPX4 activity. | Ma et al. (2015) |
| BSO | HCT116 cells VACO5 cells VACO6 cells VACO8 cells |
Promotes ferroptosis. Depletes GSH in cancer cells. | Berger et al. (1994) |
| DCA | HCT116 cells HT29 cells |
Promotes ferroptosis Sequesters iron in lysosome, triggering ferroptosis. |
Sun et al. (2021) |
| Oxaliplatin | HT29 cells | Promotes ferroptosis. Inhibits Nrf2 signaling pathway. | Liu and Wang (2022) |
| Matrine | HCT116 cells | Promotes ferroptosis. Increases ROS and Fe2+ levels. Downregulates expression of GPX4 and SLC7A11. | Wang et al. (2020a) |
ACSL4, acyl-CoA synthetase long-chain family member 4; BSO, buthionine sulfoximine; CNC, Camellia nitidissima Chi; DCA, dichloracetate; GPX4, glutathione peroxidase 4; IMCA, 2-imino-6-methoxy-2H-chromene-3-carbothioamide; TalaA, talaroconvolutin A.
The role of ferroptosis in CRC is not yet fully elucidated. However, the results demonstrate that ferroptosis serves as a targetable pathway and that regulating this form of cell death may provide a new avenue of anticancer therapy.
Conclusions and Future Perspectives
ROS play a vital role in maintaining homeostasis in the body by mediating immune function, metabolism, and cellular signaling. However, an imbalance in the levels of ROS can contribute to disease states such as sepsis, atherosclerosis, and CGD. Moreover, there is growing evidence to suggest that lipid ROS specifically are implicated in IBD and CRC. Accumulation of lipid ROS results in an iron-dependent programmed cell death pathway termed ferroptosis. Although evidence of modulating ferroptosis, either induction or inhibition, is beneficial in treating preclinical models of IBD and CRC, there are still no viable pharmacological agents available to treat patients in clinical trials. The development of safe and efficacious ferroptotic modulators could serve as important means of treating IBD and CRC.
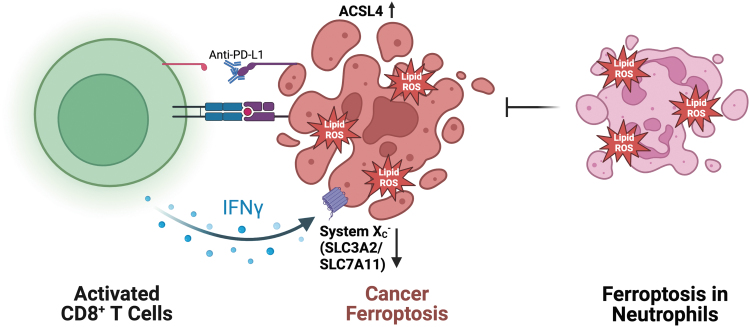
To effectively target ferroptosis in IBD and CRC, it is important to identify the specific cell types and temporal dynamics involved in the process. While inhibition of ROS in general does not provide benefits in IBD, systemic inhibition of ferroptosis could lead to similar results. Systemic cyst(e)ine depletion selectively decreased PDAC growth, and there is a growing body of evidence to suggest that other specific cell types in the tumor microenvironment affect ferroptosis sensitivity in cancer cells. It was reported that exosome-miR-522 from cancer-associated fibroblasts inhibit ferroptosis in cancer cells through inhibition of ALOX15 and lipid ROS accumulation (Zhang et al., 2020a). Likewise, the role of ferroptosis in immune cells also mediates cancer growth. Ferroptosis in immune cells has been found to be both protumorigenic and antitumorigenic (Fig. 5).
FIG. 5.
Immune-mediated mechanisms of ferroptosis. Immune checkpoint inhibitor-activated CD8+ T cells promote ferroptosis in cancer cells through IFNγ-dependent System Xc− downregulation and ACSL4 upregulation. Ferroptosis in neutrophils results in immune suppression and cancer promotion. IFNγ, interferon γ.
Liao et al. (2022) demonstrated that IFNγ secretion from CD8+ T cells mediated ferroptosis-induced tumor killing through upregulation of ACSL4. In contrast, a recent report demonstrated that ferroptosis in neutrophils results in immune suppression and promotion of tumor growth (Kim et al., 2022). The context-dependent nature of ferroptosis in cancer highlights the need to accurately identify and target specific cell types to ensure proper antitumor activity.
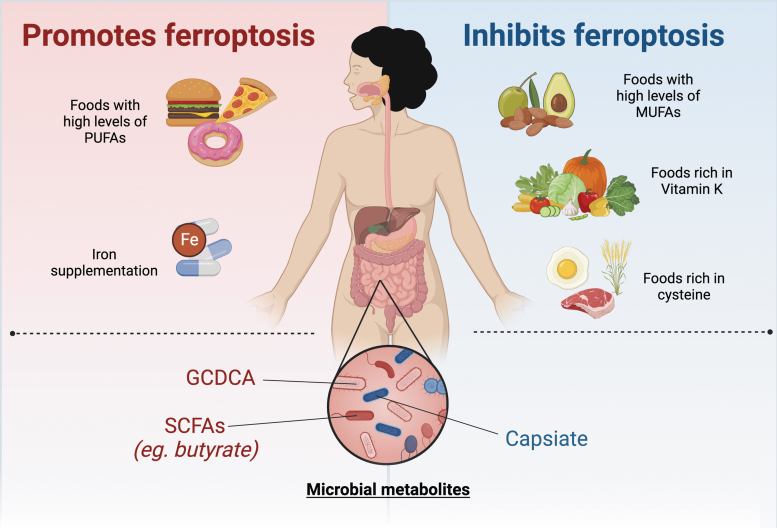
Currently, there are no pharmacological agents that target ferroptosis in humans. However, research has indicated that the gut microbiome may play a role in ferroptosis (Fig. 6). Intestinal I/R injury alters the gut microbiota and results in elevated levels of capsiate that serves to protect against ferroptosis-dependent intestinal I/R injury (Deng et al., 2021). Mice treated with antibiotics exhibited worse intestinal injuries (Deng et al., 2021). In addition, it has been demonstrated that bromoacetic acid-induced hepatic injury alters the gut microbiome and results in elevated levels of the gut microbiota metabolite glycochenodeoxycholate (GCDCA). GCDCA activates TfR-ACSL4-mediated ferroptosis to promote fatty liver disease (Liu et al., 2022). Butyrate, a short-chain fatty acid produced by gut microbiota, promotes ferroptosis through inducing NCOA4-mediated ferritinophagy (Zhao et al., 2020). A more nuanced understanding of the context-dependent nature of ferroptosis in these settings will help direct better targeted therapies in the intestine and colon.
FIG. 6.
Nonpharmacological effects on ferroptosis. Diet and microbiota have been shown to affect ferroptosis. Diets rich in PUFAs and iron supplementation promote ferroptosis, while diets rich in MUFAs, cysteine, and vitamin K inhibit ferroptosis. Gut dysbiosis affects ferroptosis in a context-dependent manner. Some microbial metabolites (GCDCA, SCFAs) have been reported to inhibit ferroptosis, while other microbial metabolites (capsiate) are able to protect against ferroptosis. GCDCA, glycochenodeoxycholate; MUFA, monounsaturated fatty acid; SCFA, short-chain fatty acid.
Lastly, modulation of diet through amino acids, vitamins, iron, and lipids also serve as a method of targeting ferroptosis (Fig. 6). In a model of cytosolic aspartate aminotransaminase (GOT1)-deficient PDAC, a diet lacking cysteine resulted in increased survival due to PDAC cell death by ferroptosis (Kremer et al., 2021). A recent study demonstrated that FSP1-dependent noncanonical vitamin K cycle protects against ferroptosis in human cancer cell lines (Mishima et al., 2022). In murine models of hemochromatosis, mice fed a high-iron diet exacerbated hepatic injury through ferroptosis (Wang et al., 2017). In addition, a high-fat diet promotes colitis-associated carcinogenesis through ferroptosis evasion in the ER stress-mediated pathway (Zhang et al., 2021).
However, it is important to note that the effect of dietary fat on ferroptosis may be specific to the type of fat consumed, as PUFAs have been shown to promote ferroptosis, while monounsaturated fatty acids have been shown to protect against ferroptosis (Magtanong et al., 2019; Yang et al., 2016).
Understanding the role of lipid ROS and ferroptosis in IBD and CRC is of central importance. As research continues to uncover the role of ferroptosis in CRC and IBD, this will open up new possibilities for treatments based on the modulation of ferroptosis.
Summary Points
ROS have physiological functions, and redox imbalance leads to pathophysiological states.
The roles of lipid ROS-induced ferroptosis in normal physiology are still relatively unclear; however, its roles in disease have been widely reported.
There is mounting evidence that ferroptosis contributes to the pathogenesis of IBD and CRC.
Inhibition of ferroptosis has been shown to be beneficial in preclinical models of IBD and CRC.
The role of ferroptosis in IBD and CRC may be leveraged for the development of new therapeutics toward these diseases.
Abbreviations Used
- 5-Fu
5-fluorouracil
- 8-oxodG
8-oxo-7-hydro-2′-deoxyguanosine
- γH2AX
phosphorylated H2A.X variant histone
- AA
arachidonic acid
- ACE
angiotensin-converting-enzyme
- ACSL4
acyl-CoA synthetase long-chain family member 4
- ALOX15
arachidonate lipoxygenase 15
- ARB
angiotensin receptor blocker
- BH2
dihydrobiopterin
- BH4
tetrahydrobiopterin
- BSO
buthionine sulfoximine
- CAPOX
capecitabine
- CD
Crohn's disease
- CGD
chronic granulomatous disease
- CNC
Camellia nitidissima Chi
- CRC
colorectal cancer
- CUR
curculigoside
- DCA
dichloracetate
- DMT1
divalent metal transporter-1
- DPP4
dipeptidyl-peptidase 4
- DSS
dextran sulfate sodium
- EGFR
epidermal growth factor receptor
- EMT
epithelial-to-mesenchymal transition
- ER
endoplasmic reticulum
- ETC
electron transport chain
- FMN
flavin mononucleotide
- FOLFOX
folinic acid and oxaliplatin
- FSP1
ferroptosis suppressor protein 1
- GCDCA
glycochenodeoxycholate
- GCH1
GTP cyclohydrolase 1
- GI
gastrointestinal
- GOT1
cytosolic aspartate aminotransaminase
- GPX
glutathione peroxidase
- GSH
glutathione
- GSSG
glutathione disulfide
- HIF
hypoxia-induced factor
- I/R
ischemia/reperfusion
- IBD
inflammatory bowel disease
- IEC
intestinal epithelial cell
- IFNγ
interferon γ
- IL
interleukin
- IMCA
2-imino-6-methoxy-2H-chromene-3-carbothioamide
- KEAP1
Kelch-like epichlorohydrin-related protein
- KO
knockout
- LOX
lipoxygenase
- LPCAT3
lysophosphatidylcholine acyltransferase 3
- MEL
melatonin
- mtROS
mitochondrial reactive oxygen species
- MUFAs
monounsaturated fatty acids
- NAC
N-acetylcysteine
- NADH
nicotine adenine dinucleotide
- NCOA4
nuclear receptor coactivator 4
- NOD2
nucleotide-binding oligomerization domain 2
- NOX/DUOX
NADPH oxidase and dual oxidase
- NRF2
nuclear factor erythroid 2-related factor 2
- OXPHOS
oxidative phosphorylation
- PDAC
pancreatic ductal adenocarcinoma
- PLC
propionyl-l-carnitine
- PLOOHs
phospholipid hydroperoxides
- PUFAs
polyunsaturated fatty acids
- Q
ubiquinone
- QH2
ubiquinol
- ROS
reactive oxygen species
- RSL3
RAS-selective lethal 3
- RSV
resveratrol
- SCFA
short-chain fatty acid
- SOD
superoxide dismutase
- TalaA
talaroconvolutin A
- Tf
transferrin
- TfR
transferrin receptor
- Th
T helper
- TNBS
2,4,6-trinitrobenzenesulfonic acid
- Trx
thioredoxin
- UC
ulcerative colitis
- VEGF-A
vascular endothelial growth factor A
Authors' Contributions
W.H. wrote the article and tables. W.H. and N.A. designed the figures. W.H. and Y.M.S. conceived the review and revised the article. All authors have read and agreed to the published version of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was funded by NIH grants: R01CA148828, R01CA245546, and R01DK095201 (Yatrik M. Shah), and UMCCC Core Grant P30CA046592 (Yatrik M. Shah). Wesley Huang was supported by NIH grant: F30DK131851.
References
- Abraham C, Cho JH. Inflammatory bowel disease. New Engl J Med 2009;361(21):2066–2078; doi: 10.1056/nejmra0804647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol 2012;2012:936486; doi: 10.1155/2012/936486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthakrishnan AN, Cagan A, Cai T, et al. Statin use is associated with reduced risk of colorectal cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2016;14(7):973–979; doi: 10.1016/j.cgh.2016.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreani C, Bartolacci C, Scaglioni PP. Ferroptosis: A specific vulnerability of RAS-driven cancers? Frontiers Oncol 2022;12:923915; doi: 10.3389/fonc.2022.923915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli JPF, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 2014;16(12):1180–1191; doi: 10.1038/ncb3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Animashaun A, Kelleher J, Heatley RV, et al. The effect of zinc and vitamin C supplementation on the immune status of patients with Crohn's disease. Clin Nutr 1990;9(3):137–146; doi: 10.1016/0261-5614(90)90045-t [DOI] [PubMed] [Google Scholar]
- Arab HH, Al-Shorbagy MY, Abdallah DM, et al. Telmisartan attenuates colon inflammation, oxidative perturbations and apoptosis in a rat model of experimental inflammatory bowel disease. PLoS One 2014;9(5):e97193; doi: 10.1371/journal.pone.0097193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold DE, Heimall JR. A review of chronic granulomatous disease. Adv Ther 2017;34(12):2543–2557; doi: 10.1007/s12325-017-0636-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold RS, Shi J, Murad E, et al. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci U S A 2001;98(10):5550–5555; doi: 10.1073/pnas.101505898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam S, Sreedhar R, Thandavarayan RA, et al. Telmisartan treatment targets inflammatory cytokines to suppress the pathogenesis of acute colitis induced by dextran sulphate sodium. Cytokine 2015;74(2):305–312; doi: 10.1016/j.cyto.2015.03.017 [DOI] [PubMed] [Google Scholar]
- Aviello G, Knaus U. ROS in gastrointestinal inflammation: Rescue or sabotage? Br J Pharmacol 2017;174(12):1704–1718; doi: 10.1111/bph.13428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgley MA, Kremer DM, Maurer HC, et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 2020;368(6486):85–89; doi: 10.1126/science.aaw9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnicle A, Seoighe C, Greally JM, et al. Inflammation-associated DNA methylation patterns in epithelium of ulcerative colitis. Epigenetics 2017;12(8):591–606; doi: 10.1080/15592294.2017.1334023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolacci C, Andreani C, Vale G, et al. Targeting de novo lipogenesis and the lands cycle induces ferroptosis in KRAS-mutant lung cancer. Nat Commun 2022;13(1):4327; doi: 10.1038/s41467-022-31963-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskar R, Lee KA, Yeo R, et al. Cancer and radiation therapy: Current advances and future directions. Int J Med Sci 2012;9(3):193–199; doi: 10.7150/ijms.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SJ, Gosky D, Zborowska E, et al. Sensitive enzymatic cycling assay for glutathione: Measurements of glutathione content and its modulation by buthionine sulfoximine in vivo and in vitro in human colon cancer. Cancer Res 1994;54(15):4077–4083. [PubMed] [Google Scholar]
- Bersuker K, Hendricks JM, Li Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019;575(7784):688–692; doi: 10.1038/s41586-019-1705-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia S, Wang P, Toh A, et al. New insights into the role of phenotypic plasticity and EMT in driving cancer progression. Front Mol Biosci 2020;7:71; doi: 10.3389/fmolb.2020.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan AR, Miyazawa M, Hashimoto K, et al. Regulators of iron homeostasis: New players in metabolism, cell death, and disease. Trends Biochem Sci 2016;41(3):274–286; doi: 10.1016/j.tibs.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Yu J, Zhang L. Necroptosis: An alternative cell death program defending against cancer. Biochim Biophys Acta 2016;1865(2):228–236; doi: 10.1016/j.bbcan.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Li X, Zhang R, et al. Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics 2020a;10(11):5107–5119; doi: 10.7150/thno.44705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Comish PB, Tang D, et al. Characteristics and biomarkers of ferroptosis. Front Cell Dev Bio 2021a;9:637162; doi: 10.3389/fcell.2021.637162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang F, Du Z, et al. Proteome analysis of Camellia nitidissima Chi revealed its role in colon cancer through the apoptosis and ferroptosis pathway. Front Oncol 2021b;11:727130; doi: 10.3389/fonc.2021.727130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang P, Chen W, et al. Ferroptosis mediated DSS-induced ulcerative colitis associated with Nrf2/HO-1 signaling pathway. Immunol Lett 2020b;225:9–15; doi: 10.1016/j.imlet.2020.06.005 [DOI] [PubMed] [Google Scholar]
- Chieppa M, Rescigno M, Huang AYC, et al. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med 2006;203(13):2841–2852; doi: 10.1084/jem.20061884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacki C, Wisniewska-Jarosinska M, Walecka-Kapica E, et al. Evaluation of melatonin effectiveness in the adjuvant treatment of ulcerative colitis. J Physiol Pharmacol 2010;62(3):327–34. [PubMed] [Google Scholar]
- Cuadrado A, Rojo AI, Wells G, et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov 2019;18(4):295–317; doi: 10.1038/s41573-018-0008-x [DOI] [PubMed] [Google Scholar]
- Das NK, Schwartz AJ, Barthel G, et al. Microbial metabolite signaling is required for systemic iron homeostasis. Cell Metab 2020;31(1):115–130.e6; doi: 10.1016/j.cmet.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng F, Zhao B-C, Yang X, et al. The gut microbiota metabolite capsiate promotes Gpx4 expression by activating TRPV1 to inhibit intestinal ischemia reperfusion-induced ferroptosis. Gut Microbes 2021;13(1):1902719; doi: 10.1080/19490976.2021.1902719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012;149(5):1060–1072; doi: 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Winter GE, Musavi LS, et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol 2015;10(7):1604–1609; doi: 10.1021/acschembio.5b00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll S, Freitas FP, Shah R, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019;575(7784):693–698; doi: 10.1038/s41586-019-1707-0 [DOI] [PubMed] [Google Scholar]
- Doll S, Proneth B, Tyurina YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 2017;13(1):91–98; doi: 10.1038/nchembio.2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 2002;82(1):47–95; doi: 10.1152/physrev.00018.2001 [DOI] [PubMed] [Google Scholar]
- Elguindy MM, Nakamaru-Ogiso E. Apoptosis-inducing factor (AIF) and its family member protein, AMID, are rotenone-sensitive NADH:ubiquinone oxidoreductases (NDH-2). J Biol Chem 2015;290(34):20815–20826; doi: 10.1074/jbc.m115.641498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiringü F, Tuğcu-Demiröz F, Acartürk F, et al. Investigation of the effect of intracolonic melatonin gel formulation on acetic acid-induced colitis. Drug Deliv 2016;23(7):2318–2326; doi: 10.3109/10717544.2014.982773 [DOI] [PubMed] [Google Scholar]
- Fairbrass KM, Hoshen D, Gracie DJ, et al. Effect of ACE inhibitors and angiotensin II receptor blockers on disease outcomes in inflammatory bowel disease. Gut 2021;70(1):218–219; doi: 10.1136/gutjnl-2020-321186 [DOI] [PubMed] [Google Scholar]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61(5):759–767; doi: 10.1016/0092-8674(90)90186-i [DOI] [PubMed] [Google Scholar]
- Feng H, Schorpp K, Jin J, et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep 2020;30(10):3411-3423.e7; doi: 10.1016/j.celrep.2020.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajendran M, Loganathan P, Catinella AP, et al. A comprehensive review and update on Crohn's disease. Dis Mon 2018;64(2):20–57; doi: 10.1016/j.disamonth.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Green DR. The coming decade of cell death research: Five riddles. Cell 2019;177(5):1094–1107; doi: 10.1016/j.cell.2019.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res 2019;2019:7247238; doi: 10.1155/2019/7247238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guijarro LG, Mate J, Gisbert JP, et al. N-Acetyl-L-cysteine combined with mesalamine in the treatment of ulcerative colitis: Randomized, placebo-controlled pilot study. World J Gastroenterol 2008;14(18):2851–2857; doi: 10.3748/wjg.14.2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Liu P, Deng G, et al. Honokiol induces ferroptosis in colon cancer cells by regulating GPX4 activity. Am J Cancer Res 2020;11(6):3039–3054. [PMC free article] [PubMed] [Google Scholar]
- Guo J, Xu B, Han Q, et al. Ferroptosis: A novel anti-tumor action for cisplatin. Cancer Res Treat 2018;50(2):445–460; doi: 10.4143/crt.2016.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: The paradox of increased reactive oxygen species during hypoxia. Exp Physiol 2006;91(5):807–819; doi: 10.1113/expphysiol.2006.033506 [DOI] [PubMed] [Google Scholar]
- Hepworth MR, Fung TC, Masur SH, et al. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria–specific CD4+ T cells. Science 2015;348(6238):1031–1035; doi: 10.1126/science.aaa4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock BD, McKenzie JL, Strother M, et al. Functional effects of immune complexes formed between pembrolizumab and patient-generated anti-drug antibodies. Cancer Immunol Immunother 2020;69(12):2453–2464; doi: 10.1007/s00262-020-02636-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori Y, Hoshino J, Yamazaki C, et al. Effect of lecithinized-superoxide dismutase on the rat colitis model induced by dextran sulfate sodium. Jpn J Pharmacol 1997;74(1):99–103; doi: 10.1254/jjp.74.99 [DOI] [PubMed] [Google Scholar]
- Hossain MdS, Karuniawati H, Jairoun AA, et al. Colorectal cancer: A review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers (Basel) 2022;14(7):1732; doi: 10.3390/cancers14071732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou CL, Zhang J, Liu XT, et al. Superoxide dismutase recombinant Lactobacillus fermentum ameliorates intestinal oxidative stress through inhibiting NF-κB activation in a trinitrobenzene sulphonic acid-induced colitis mouse model. J Appl Microbiol 2014;116(6):1621–1631; doi: 10.1111/jam.12461 [DOI] [PubMed] [Google Scholar]
- Hou W, Xie Y, Song X, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 2016;12(8):1425–1428; doi: 10.1080/15548627.2016.1187366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang PM, Bunz F, Yu J, et al. Ferredoxin reductase affects P53-dependent, 5-fluorouracil–induced apoptosis in colorectal cancer cells. Nat Med 2001;7(10):1111–1117; doi: 10.1038/nm1001-1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med 2018;54(4):287–293; doi: 10.1016/j.ajme.2017.09.001 [DOI] [Google Scholar]
- Ishihara T, Tanaka K-I, Tasaka Y, et al. Therapeutic effect of lecithinized superoxide dismutase against colitis. J Pharmacol Exp Ther 2009;328(1):152–164; doi: 10.1124/jpet.108.144451 [DOI] [PubMed] [Google Scholar]
- Jiang L, Kon N, Li T, et al. Ferroptosis as a P53-mediated activity during tumour suppression. Nature 2015;520(7545):57–62; doi: 10.1038/nature14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Stockwell BR, Conrad M. Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 2021;22(4):266–282; doi: 10.1038/s41580-020-00324-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagarajan N, Nam JH, Noah ZA, et al. Disease modifying effect of statins in dextran sulfate sodium model of mouse colitis. Inflamm Res 2008;57(1):34–38; doi: 10.1007/s00011-007-6177-4 [DOI] [PubMed] [Google Scholar]
- Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroentero 2021;18(1):56–66; doi: 10.1038/s41575-020-00360-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Kudva AK, Patterson AD, et al. Crucial role of macrophage selenoproteins in experimental colitis. J Immunol 2014;193(7):3683–3692; doi: 10.4049/jimmunol.1400347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011;474(7351):307–317; doi: 10.1038/nature10209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Hashimoto A, Markosyan N, et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature 2022;612(7939):338–346; doi: 10.1038/s41586-022-05443-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Lee S, Seo D, et al. Cellular stress responses in radiotherapy. Cells 2019;8(9):1105; doi: 10.3390/cells8091105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Ohfuji S, Kondo K, et al. Association between dietary iron and zinc intake and development of ulcerative colitis: A case–control study in Japan. J Gastroen Hepatol 2019;34(10):1703–1710; doi: 10.1111/jgh.14642 [DOI] [PubMed] [Google Scholar]
- Kondo K, Hiramoto K, Yamate Y, et al. Ameliorative effect of high-dose vitamin C administration on dextran sulfate sodium-induced colitis mouse model. Biol Pharm Bull 2019;42(6):954–959; doi: 10.1248/bpb.b18-00967 [DOI] [PubMed] [Google Scholar]
- Kraft VAN, Bezjian CT, Pfeiffer S, et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci 2020;6(1):41–53; doi: 10.1021/acscentsci.9b01063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer DM, Nelson BS, Lin L, et al. GOT1 inhibition promotes pancreatic cancer cell death by ferroptosis. Nat Commun 2021;12(1):4860; doi: 10.1038/s41467-021-24859-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen: A double-edged sword revisited. Annu Rev Pathol Mech Dis 2014;9(1):119–145; doi: 10.1146/annurev-pathol-012513-104651 [DOI] [PubMed] [Google Scholar]
- Lei A, Yang Q, Li X, et al. Atorvastatin promotes the expansion of myeloid-derived suppressor cells and attenuates murine colitis. Immunology 2016;149(4):432–446; doi: 10.1111/imm.12662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, You Z, Chen X, et al. Targeting ferroptosis in colorectal cancer. Metabolites 2022;12(8):745; doi: 10.3390/metabo12080745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao P, Wang W, Wang W, et al. CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 2022;40(4):365-378.e6; doi: 10.1016/j.ccell.2022.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Wang H. Oxaliplatin induces ferroptosis and oxidative stress in HT29 colorectal cancer cells by inhibiting the Nrf2 signaling pathway. Exp Ther Med 2022;23(6):394; doi: 10.3892/etm.2022.11321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KY, Nakatsu CH, Jones-Hall Y, et al. Vitamin E alpha- and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free Radic Biol Med 2021;163:180–189; doi: 10.1016/j.freeradbiomed.2020.12.017 [DOI] [PubMed] [Google Scholar]
- Liu S, Gao Z, He W, et al. The gut microbiota metabolite glycochenodeoxycholate activates TFR-ACSL4-mediated ferroptosis to promote the development of environmental toxin–linked MAFLD. Free Radic Biol Med 2022;193(Pt 1):213–226; doi: 10.1016/j.freeradbiomed.2022.10.270 [DOI] [PubMed] [Google Scholar]
- Liu Z, Ren Z, Zhang J, et al. Role of ROS and nutritional antioxidants in human diseases. Front Physiol 2018;9:477; doi: 10.3389/fphys.2018.00477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead P, Khalili H, Sachs MC, et al. Association between statin use and inflammatory bowel diseases: Results from a Swedish, nationwide, population-based case-control study. J Crohns Colitis 2020;15(5):757–765; doi: 10.1093/ecco-jcc/jjaa235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenhout JV, Peeters M, Bogaerts A, et al. Oxidative stress-inducing anticancer therapies: Taking a closer look at their immunomodulating effects. Antioxidants 2020;9(12):1188; doi: 10.3390/antiox9121188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucafò M, Curci D, Franzin M, et al. Inflammatory bowel disease and risk of colorectal cancer: An overview from pathophysiology to pharmacological prevention. Front Pharmacol 2021;12:772101; doi: 10.3389/fphar.2021.772101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Chen G, Wang P, et al. Xc− inhibitor sulfasalazine sensitizes colorectal cancer to cisplatin by a GSH-dependent mechanism. Cancer Lett 2015;368(1):88–96; doi: 10.1016/j.canlet.2015.07.031 [DOI] [PubMed] [Google Scholar]
- Magtanong L, Ko P-J, To M, et al. Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem Biol 2019;26(3):420-432.e9; doi: 10.1016/j.chembiol.2018.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari RA, Balaraman R, Sailor GU, et al. Protective effect of simvastatin and rosuvastatin on trinitrobenzene sulfonic acid-induced colitis in rats. Indian J Pharmacol 2015;47(1):17–21; doi: 10.4103/0253-7613.150311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancias JD, Wang X, Gygi SP, et al. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014;509(7498):105–109; doi: 10.1038/nature13148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr L, Grabherr F, Schwärzler J, et al. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn's disease. Nat Commun 2020;11(1):1775; doi: 10.1038/s41467-020-15646-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur-Bialy AI, Pocheć E. The time-course of antioxidant irisin activity: Role of the Nrf2/HO-1/HMGB1 axis. Antioxidants (Basel) 2021;10(1):88; doi: 10.3390/antiox10010088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merra G, Gasbarrini G, Laterza L, et al. Propionyl-L-carnitine hydrochloride for treatment of mild to moderate colonic inflammatory bowel diseases. World J Gastroenterol 2012;18(36):5065–5071; doi: 10.3748/wjg.v18.i36.5065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailova TL, Sishkova E, Poniewierka E, et al. Randomised clinical trial: The efficacy and safety of propionyl-l-carnitine therapy in patients with ulcerative colitis receiving stable oral treatment. Aliment Pharmacol Ther 2011;34(9):1088–1097; doi: 10.1111/j.1365-2036.2011.04844.x [DOI] [PubMed] [Google Scholar]
- Millar AD, Rampton DS, Blake DR. Effects of iron and iron chelation in vitro on mucosal oxidant activity in ulcerative colitis. Aliment Pharmacol Ther 2000;14(9):1163–1168; doi: 10.1046/j.1365-2036.2000.00828.x [DOI] [PubMed] [Google Scholar]
- Minaiyan M, Mostaghel E, Mahzouni P. Preventive therapy of experimental colitis with selected iron chelators and anti-oxidants. Int J Prev Med 2011;3(Suppl 1):S162-9. [PMC free article] [PubMed] [Google Scholar]
- Mirbagheri SA, Nezami BG, Assa S, et al. Rectal administration of D-alpha tocopherol for active ulcerative colitis: A preliminary report. World J Gastroenterol 2008;14(39):5990–5995; doi: 10.3748/wjg.14.5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima E, Ito J, Wu Z, et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 2022;608(7924):778–783; doi: 10.1038/s41586-022-05022-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal M, Siddiqui MR, Tran K, et al. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 2014;20(7):1126–1167; doi: 10.1089/ars.2012.5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone G, Fina D, Caruso R, et al. New mediators of immunity and inflammation in inflammatory bowel disease. Curr Opin Gastroenterol 2006;22(4):361–364; doi: 10.1097/01.mog.0000231808.10773.8e [DOI] [PubMed] [Google Scholar]
- Moura FA, Andrade KQ de, Santos JCF dos, et al. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol 2015;6:617–639; doi: 10.1016/j.redox.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzny DM, Bainbridge MN, Chang K, et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487(7407):330–337; doi: 10.1038/nature11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanno M, Kanari Y, Naito T, et al. Exacerbating role of Γδ T cells in chronic colitis of T-cell receptor α mutant mice. Gastroenterology 2008;134(2):481–490; doi: 10.1053/j.gastro.2007.11.056 [DOI] [PubMed] [Google Scholar]
- Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014;14(5):329–342; doi: 10.1038/nri3661 [DOI] [PubMed] [Google Scholar]
- Nosál'ová V, Zeman M, Černá S, et al. Protective effect of melatonin in acetic acid induced colitis in rats. J Pineal Res 2007;42(4):364–370; doi: 10.1111/j.1600-079x.2007.00428.x [DOI] [PubMed] [Google Scholar]
- Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 2001;411(6837):603–606; doi: 10.1038/35079114 [DOI] [PubMed] [Google Scholar]
- Oka S, Nakabeppu Y. DNA glycosylase encoded by MUTYH functions as a molecular switch for programmed cell death under oxidative stress to suppress tumorigenesis. Cancer Sci 2011;102(4):677–682; doi: 10.1111/j.1349-7006.2011.01869.x [DOI] [PubMed] [Google Scholar]
- Özkaya D, Nazıroğlu M. Bevacizumab induces oxidative cytotoxicity and apoptosis via TRPM2 channel activation in retinal pigment epithelial cells: protective role of glutathione. Graefes Arch Clin Exp Ophthalmol 2021;259(6):1539–1554; doi: 10.1007/s00417-021-05074-7 [DOI] [PubMed] [Google Scholar]
- Park S, Oh J, Kim M, et al. Bromelain effectively suppresses kras-mutant colorectal cancer by stimulating ferroptosis. Anim Cells Syst 2018;22(5):334–340; doi: 10.1080/19768354.2018.1512521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y-S, Chung S-H, Lee S-K, et al. Melatonin improves experimental colitis with sleep deprivation. Int J Mol Med 2015;35(4):979–986; doi: 10.3892/ijmm.2015.2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LW, Artis D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat Rev Immunol 2014;14(3):141–153; doi: 10.1038/nri3608 [DOI] [PubMed] [Google Scholar]
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- Porter RJ, Arends MJ, Churchhouse AMD, et al. Inflammatory bowel disease-associated colorectal cancer: Translational risks from mechanisms to medicines. J Crohns Colitis 2021;15(12):2131–2141; doi: 10.1093/ecco-jcc/jjab102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran R, Garva R, Krstic-Demonacos M, et al. Sirtuins: Molecular traffic lights in the crossroad of oxidative stress, chromatin remodeling, and transcription. J Biomed Biotechnol 2011;2011:368276; doi: 10.1155/2011/368276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsami-kor M, Daryani NE, Asl PR, et al. Anti-inflammatory effects of resveratrol in patients with ulcerative colitis: A randomized, double-blind, placebo-controlled pilot study. Arch Med Res 2015;46(4):280–285; doi: 10.1016/j.arcmed.2015.05.005 [DOI] [PubMed] [Google Scholar]
- Santoro D, Ahrens K, Vesny R, et al. Evaluation of the in vitro effect of boldo and meadowsweet plant extracts on the expression of antimicrobial peptides and inflammatory markers in canine keratinocytes. Res Vet Sci 2017;115:255–262; doi: 10.1016/j.rvsc.2017.05.021 [DOI] [PubMed] [Google Scholar]
- Sato H, Fujiwara K, Sagara J, et al. Induction of cystine transport activity in mouse peritoneal macrophages by bacterial lipopolysaccharide. Biochem J 1995;310(2):547–551; doi: 10.1042/bj3100547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurr K, Borchert A, Kuhn H. Inverse regulation of lipid-peroxidizing and hydroperoxyl lipid-reducing enzymes by interleukins 4 and 13. FASEB J 1999;13(1):143–154; doi: 10.1096/fasebj.13.1.143 [DOI] [PubMed] [Google Scholar]
- Schwartz AJ, Das NK, Ramakrishnan SK, et al. Hepatic hepcidin/intestinal HIF-2α axis maintains iron absorption during iron deficiency and overload. J Clin Invest 2018;129(1):336–348; doi: 10.1172/jci122359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scioli MG, Stasi MA, Passeri D, et al. Propionyl-L-carnitine is efficacious in ulcerative colitis through its action on the immune function and microvasculature. Clin Transl Gastroen 2014;5(3):e55; doi: 10.1038/ctg.2014.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Liang M, Yang F, et al. Ferroptosis contributes to developmental cell death in rice blast. New Phytol 2020;227(6):1831–1846; doi: 10.1111/nph.16636 [DOI] [PubMed] [Google Scholar]
- Shi C, Yue F, Shi F, et al. Selenium-containing amino acids protect dextran sulfate sodium-induced colitis via ameliorating oxidative stress and intestinal inflammation. J Inflamm Res 2021;14:85–95; doi: 10.2147/jir.s288412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields HJ, Traa A, Raamsdonk JMV. Beneficial and detrimental effects of reactive oxygen species on lifespan: A comprehensive review of comparative and experimental studies. Front Cell Dev Biol 2021;9:628157; doi: 10.3389/fcell.2021.628157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SK, Cho JH, Kim EJ, et al. Anti-inflammatory and anti-apoptotic effects of rosuvastatin by regulation of oxidative stress in a dextran sulfate sodium-induced colitis model. World J Gastroenterol 2017;23(25):4559–4568; doi: 10.3748/wjg.v23.i25.4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi KM, Sotoudeh S, Shirazi AM, et al. Effect of N-acetylcysteine on remission maintenance in patients with ulcerative colitis: A randomized, double-blind controlled clinical trial. Clin Res Hepatol Gastroenterol 2021;45(4):101532; doi: 10.1016/j.clinre.2020.08.010 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. Ca Cancer J Clin 2022;72(1):7–33; doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 2020;21(7):363–383; doi: 10.1038/s41580-020-0230-3 [DOI] [PubMed] [Google Scholar]
- Singh UP, Singh NP, Singh B, et al. Role of resveratrol-induced CD11b+ Gr-1+ myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3+ T cells and amelioration of chronic colitis in IL-10−/− mice. Brain Behav Immun 2012;26(1):72–82; doi: 10.1016/j.bbi.2011.07.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal R, Mitta SR, Das NK, et al. HIF-2α activation potentiates oxidative cell death in colorectal cancers by increasing cellular iron. J Clin Invest 2021;131(12):e143691; doi: 10.1172/jci143691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza HSP de, Fiocchi C. Immunopathogenesis of IBD: Current state of the art. Nat Rev Gastroentero 2016;13(1):13–27; doi: 10.1038/nrgastro.2015.186 [DOI] [PubMed] [Google Scholar]
- Spencer AU, Yang H, Haxhija EQ, et al. Reduced severity of a mouse colitis model with angiotensin converting enzyme inhibition. Digest Dis Sci 2007;52(4):1060–1070; doi: 10.1007/s10620-006-9124-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman JD, Spyrou NM, Millar AD, et al. Selenium supplementation in the diets of patients suffering from ulcerative colitis. J Radioanal Nucl Chem 1997;217(2):189–191; doi: 10.1007/bf02034441 [DOI] [Google Scholar]
- Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011;140(6):1756-1767.e1; doi: 10.1053/j.gastro.2011.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueyoshi R, Ignatoski KMW, Daignault S, et al. Angiotensin converting enzyme-inhibitor reduces colitis severity in an IL-10 knockout model. Digest Dis Sci 2013;58(11):3165–3177; doi: 10.1007/s10620-013-2825-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, Zhang R, Liu S, et al. RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Front Pharmacol 2018;9:1371; doi: 10.3389/fphar.2018.01371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Cheng X, Pan S, et al. Dichloroacetate attenuates the stemness of colorectal cancer cells via trigerring ferroptosis through sequestering iron in lysosomes. Environ Toxicol 2021;36(4):520–529; doi: 10.1002/tox.23057 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Matsumoto T, Okamoto S, et al. A lecithinized superoxide dismutase (PC-SOD) improves ulcerative colitis. Colorectal Dis 2008;10(9):931–934; doi: 10.1111/j.1463-1318.2008.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahan G, Aytac E, Aytekin H, et al. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid–induced ulcerative colitis in rats. Can J Surg 2011;54(5):333–338; doi: 10.1503/cjs.013610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JJ, Parsons ZD, Cummings AH, et al. Redox regulation of protein tyrosine phosphatases: Structural and chemical aspects. Antioxid Redox Signal 2011;15(1):77–97; doi: 10.1089/ars.2010.3611 [DOI] [PubMed] [Google Scholar]
- Tavakoli P, Vollmer-Conna U, Hadzi-Pavlovic D, et al. A review of inflammatory bowel disease: A model of microbial, immune and neuropsychological integration. Public Health Rev 2021;42:1603990; doi: 10.3389/phrs.2021.1603990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Li S, Ge G. Apatinib promotes ferroptosis in colorectal cancer cells by targeting ELOVL6/ACSL4 signaling. Cancer Manag Res 2021;13:1333–1342; doi: 10.2147/cmar.s274631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirichen H, Yaigoub H, Xu W, et al. Mitochondrial reactive oxygen species and their contribution in chronic kidney disease progression through oxidative stress. Front Physiol 2021;12:627837; doi: 10.3389/fphys.2021.627837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolan D, Gandin V, Morrison L, et al. Oxidative stress induced by Pt(IV) pro-drugs based on the cisplatin scaffold and indole carboxylic acids in axial position. Sci Rep 2016;6(1):29367; doi: 10.1038/srep29367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungaro R, Chang HL, Cote-Daigneaut J, et al. Statins associated with decreased risk of new onset inflammatory bowel disease. Am J Gastroenterol 2016;111(10):1416–1423; doi: 10.1038/ajg.2016.233 [DOI] [PubMed] [Google Scholar]
- Uraz S, Tahan G, Aytekin H, et al. N-acetylcysteine expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic acid-induced colitis in rats. Scand J Clin Lab Invest 2013;73(1):61–66; doi: 10.3109/00365513.2012.734859 [DOI] [PubMed] [Google Scholar]
- Uritski R, Barshack I, Bilkis I, et al. Dietary iron affects inflammatory status in a rat model of colitis. J Nutr 2004;134(9):2251–2255; doi: 10.1093/jn/134.9.2251 [DOI] [PubMed] [Google Scholar]
- Ursini F, Travain VB, Cozza G, et al. A white paper on phospholipid hydroperoxide glutathione peroxidase (GPx4) forty years later. Free Radic Biol Med 2022;188:117–133; doi: 10.1016/j.freeradbiomed.2022.06.227 [DOI] [PubMed] [Google Scholar]
- Wang H, An P, Xie E, et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology 2017;66(2):449–465; doi: 10.1002/hep.29117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu N, Jiang H, et al. Reactive oxygen species in anticancer immunity: A double-edged sword. Front Bioeng Biotechnol 2021;9:784612; doi: 10.3389/fbioe.2021.784612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tang P, Cai Q, et al. Matrine can inhibit the growth of colorectal cancer cells by inducing ferroptosis. Nat Prod Commun 2020a;15(12):1934578X20982779; doi: 10.1177/1934578x20982779 [DOI] [Google Scholar]
- Wang Q, Hou Y, Yi D, et al. Protective effects of N-acetylcysteine on acetic acid-induced colitis in a porcine model. BMC Gastroenterol 2013;13(1):133; doi: 10.1186/1471-230x-13-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q-S, Zheng Y-M, Dong L, et al. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic Biol Med 2007;42(5):642–653; doi: 10.1016/j.freeradbiomed.2006.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Liu W, Wang J, et al. Curculigoside inhibits ferroptosis in ulcerative colitis through the induction of GPX4. Life Sci 2020b;259:118356; doi: 10.1016/j.lfs.2020.118356 [DOI] [PubMed] [Google Scholar]
- Wang S-J, Li D, Ou Y, et al. Acetylation is crucial for P53-mediated ferroptosis and tumor suppression. Cell Rep 2016;17(2):366–373; doi: 10.1016/j.celrep.2016.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Green M, Choi JE, et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019;569(7755):270–274; doi: 10.1038/s41586-019-1170-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen Y, Zhang X, et al. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J Funct Food 2020c;75:104248; doi: 10.1016/j.jff.2020.104248 [DOI] [Google Scholar]
- Ward TH, Cummings J, Dean E, et al. Biomarkers of apoptosis. Br J Cancer 2008;99(6):841–846; doi: 10.1038/sj.bjc.6604519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Wagner SJ, Martínez I, et al. Depletion of luminal iron alters the gut microbiota and prevents Crohn's disease-like ileitis. Gut 2011;60(3):325; doi: 10.1136/gut.2010.216929 [DOI] [PubMed] [Google Scholar]
- Wu Z, Pan D, Jiang M, et al. Selenium-enriched Lactobacillus acidophilus ameliorates dextran sulfate sodium-induced chronic colitis in mice by regulating inflammatory cytokines and intestinal microbiota. Front Med 2021;8:716816; doi: 10.3389/fmed.2021.716816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Liu S, Li C, et al. Discovery of a novel ferroptosis inducer-talaroconvolutin A—Killing colorectal cancer cells in vitro and in vivo. Cell Death Dis 2020;11(11):988; doi: 10.1038/s41419-020-03194-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Zhu S, Song X, et al. The tumor suppressor P53 limits ferroptosis by blocking DPP4 activity. Cell Rep 2017;20(7):1692–1704; doi: 10.1016/j.celrep.2017.07.055 [DOI] [PubMed] [Google Scholar]
- Xu M, Tao J, Yang Y, et al. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis 2020;11(2):86; doi: 10.1038/s41419-020-2299-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Taylor M, Anderson E, et al. Hypoxia-inducible factor-2α activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res 2012;72(9):2285–2293; doi: 10.1158/0008-5472.can-11-3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Ma X. Role of Nod2 in the development of Crohn's disease. Microbes Infect 2009;11(12):912–918; doi: 10.1016/j.micinf.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mo J, Dai J, et al. Cetuximab promotes RSL3-induced ferroptosis by suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant colorectal cancer. Cell Death Dis 2021;12(11):1079; doi: 10.1038/s41419-021-04367-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WS, Kim KJ, Gaschler MM, et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 2016;113(34):E4966–E4975; doi: 10.1073/pnas.1603244113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yant LJ, Ran Q, Rao L, et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med 2003;34(4):496–502; doi: 10.1016/s0891-5849(02)01360-6 [DOI] [PubMed] [Google Scholar]
- Yao J, Wei C, Wang J-Y, et al. Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice. World J Gastroenterol 2015;21(21):6572–6581; doi: 10.3748/wjg.v21.i21.6572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz G, Yildiz Y, Ulutas PA, et al. Resveratrol pretreatment ameliorates TNBS colitis in rats. Recent Pat Endocr Metab Immune Drug Discov 2015;9(2):134–140; doi: 10.2174/1872214809666150806105737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-E, Chen L, Na R, et al. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radic Biol Med 2012;52(9):1820–1827; doi: 10.1016/j.freeradbiomed.2012.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y, Fu J-J, Meng J, et al. Effect of N-acetylcysteine on the murine model of colitis induced by dextran sodium sulfate through up-regulating PON1 activity. Digest Dis Sci 2009;54(8):1643–1650; doi: 10.1007/s10620-008-0563-9 [DOI] [PubMed] [Google Scholar]
- Zeng J, Li M, Xu J-Y, et al. Aberrant ROS mediate cell cycle and motility in colorectal cancer cells through an oncogenic CXCL14 signaling pathway. Front Pharmacol 2021;12:764015; doi: 10.3389/fphar.2021.764015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Deng T, Liu R, et al. CAF Secreted MiR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer 2020a;19(1):43; doi: 10.1186/s12943-020-01168-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Liu W, Liu F, et al. IMCA induces ferroptosis mediated by SLC7A11 through the AMPK/MTOR pathway in colorectal cancer. Oxid Med Cell Longev 2020b;2020:1675613; doi: 10.1155/2020/1675613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li W, Ma Y, et al. High-fat diet aggravates colitis-associated carcinogenesis by evading ferroptosis in the ER stress-mediated pathway. Free Radic Biol Med 2021;177:156–166; doi: 10.1016/j.freeradbiomed.2021.10.022 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shi J, Liu X, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol 2018;20(10):1181–1192; doi: 10.1038/s41556-018-0178-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yin L, Zeng X, et al. Dietary high dose of iron aggravates the intestinal injury but promotes intestinal regeneration by regulating intestinal stem cells activity in adult mice with dextran sodium sulfate-induced colitis. Front Vet Sci 2022;9:870303; doi: 10.3389/fvets.2022.870303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Li J, Guo W, et al. Periodontitis-level butyrate-induced ferroptosis in periodontal ligament fibroblasts by activation of ferritinophagy. Cell Death Discov 2020;6(1):119; doi: 10.1038/s41420-020-00356-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Ma Y, Ding S, et al. Effects of melatonin on intestinal microbiota and oxidative stress in colitis mice. Biomed Res Int 2018;2018:2607679; doi: 10.1155/2018/2607679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielińska M, Jarmuż A, Sałaga M, et al. Melatonin, but not melatonin receptor agonists Neu-P11 and Neu-P67, attenuates TNBS-induced colitis in mice. Naunyn Schmiedebergs Arch Pharmacol 2016;389(5):511–519; doi: 10.1007/s00210-016-1214-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Palte MJ, Deik AA, et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun 2019;10(1):1617; doi: 10.1038/s41467-019-09277-9 [DOI] [PMC free article] [PubMed] [Google Scholar]