Highlights
-
•
Exercise interventions as outlined in the Scientific Exercise Guidelines should be implemented in acute SCI rehabilitation.
-
•
FES cycling and isometric training improves cardiometabolic health in SCI.
-
•
FES cycling reduces muscle atrophy and improves lean body mass.
-
•
Muscle peak torque improves with continuous resistance training.
-
•
Specific evidenced FITT prescriptions are needed for early SCI rehabilitation.
Keywords: Cardiometabolic health, Cardiorespiratory fitness, Exercise prescription, Paraplegic, Rehabilitation, Strengthening, Tetraplegic
Abstract
Objective
To determine the effect of exercise and physical activity interventions that meet current guideline recommendations on cardiorespiratory fitness, cardiometabolic health, and muscle strength in adults in the acute stage (<1 year post onset) of spinal cord injury (SCI) rehabilitation.
Data Sources
Six electronic databases (PubMed, CINAHL, SPORTDiscus, Google Scholar, National Institute Clinical Excellence, World Health Organization) were searched (January 2016-March 2022) to extend a previously published review.
Study Selection
Included studies implemented exercise interventions in the acute stage of SCI rehabilitation participants which met the exercise guidelines and measured cardiorespiratory fitness, cardiometabolic health, and strength outcomes.
Data Extraction
Titles and abstracts were screened against eligibility criteria and duplicates removed using EndNote X8. Full texts were independently assessed and results presented in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart. Data extraction was completed on included studies by 2 reviewers (L.R. and V.B.) using a modified Cochrane Group form.
Data Synthesis
Data were synthesized, appraised using the Modified Downs & Black checklist and presented in narrative and tabular format. This review was registered on PROSPERO (Register ID:CRD42021249441). Of the 1255 studies, 4 were included, featuring 108 total participants <1-year post-SCI. Functional electrical stimulation cycle ergometry reduced muscle atrophy after 3 months training and increased lean body mass after 6 months. Resistance training increased muscle peak torque, perceived muscle strength and function. Aerobic exercise interventions did not increase cardiorespiratory fitness.
Conclusions
Interventions meeting the exercise guidelines did not increase cardiorespiratory fitness but were shown to improve cardiometabolic health and perceived muscle strength and function in adults in the acute stage of SCI rehabilitation. Further empirical research using standardized outcome measures are required to explore the effectiveness of aerobic exercise and strengthening interventions in acute stage of SCI rehabilitation to support the development of exercise guidelines.
Acute spinal cord injury (SCI) results from damage to the spinal cord due to trauma or disease. SCI is often associated with high mortality and morbidity and can include significant disruption to sensory, motor, and autonomic function. Rehabilitation of SCI can be divided into 3 key phases: acute, subacute, and chronic.1 The acute phase of SCI rehabilitation usually refers to the time period up to 1 year.1,2 In the acute phase, rehabilitation focuses on optimizing neural recovery, maximizing independence, and prevention of secondary health complications.1
In 2016, the World Health Organization (WHO) Global Burden of Disease study reported that there were >27million prevalent cases of SCI globally and almost 1 million new cases of SCI per annum.3 Individuals with traumatic SCI have two and a half times greater the mortality risk due to cardiovascular disease and secondary health conditions than the general population.4,5 It is important to consider the effect of disability on individuals with SCI to ensure that effective interventions are implemented at individual and population levels.6 Physical fitness is multifaceted and includes components such as cardiorespiratory endurance, muscular endurance and strength, body composition, and flexibility.7 The promotion of regular physical activity after SCI reduces the risk of developing secondary health conditions, improves quality of life, and reduces physical and economic burden.8
The WHO Global Action Plan on Physical Activity 2018-2030 aims to reduce global prevalence of physical inactivity in adults and adolescents by 15% by 2030.9 WHO physical activity guidelines were published to increase physical activity participation in the general population including people with disabilities, and provide consistent, global recommendations.10 Despite this, Public Health England report that 43% of people with disabilities are inactive and 37%-50% of those studied complete no leisure-time physical activity.11
Several authors have since challenged the use of the WHO target of 150 minutes of weekly physical activity in SCI, arguing that guidelines should be disability specific.12,13,14 In 2017, a systematic review by van der Scheer et al highlighted exercise prescriptions at lower frequency and intensity than is outlined by the WHO guidelines were effective at improving cardiorespiratory fitness and cardiometabolic health in chronic SCI.15 From this work, new updated evidence-based scientific exercise guidelines for adults with SCI were developed to support international consistency in exercise prescription and policy development for cardiorespiratory fitness and cardiometabolic health.12 New to the guidelines were 2 different exercise prescriptions, 1 for cardiorespiratory fitness (and strengthening) and another for cardiometabolic health.
Although van der Scheer et al15 sourced 30 studies exploring exercise interventions in the acute SCI rehabilitation stage, there was insufficient high-quality evidence at that time and further research was warranted to enable an exercise guideline to be generated specifically for the acute rehabilitation population. Due to the very low incidence rate of serious adverse events, van der Scheer at al15 suggested that these guidelines might be appropriate for individuals <12 months post injury with support from a health care professional. However, a systematic review by Selph et al,16,17 exploring physical activity in a mixed wheelchair user population, found harms were reported and increased risk of autonomic dysreflexia in SCI participants. While this information was drawn from papers in the chronic SCI rehabilitation population, it corresponds with a study by Galea et al18 that evaluated functional electrical stimulation-assisted cycling (FESC) in acute traumatic SCI less than 1-year post injury. Galea et al18 outlined the harm of leg abrasion as definitely related to the exercise intervention, and hematoma over ischium and increased blood pressure during intervention as probably casually related to exercise in their study population. While there is a need for clinicians, and physiotherapists in particular, to be supporting physical activity and exercise in the acute rehabilitation phase, there is still limited guidance on how to safely prescribe exercise in this population.19
Individuals with acute SCI have differing needs to those with chronic SCI. Clinicians supporting those with acute SCI must carefully consider each individual's medical condition, unique rehabilitation goals and access to support and exercise facilities.1 There is also a need for consensus on defining and measuring physical activity in wheelchair users and clear clinical guidance on exercise frequency, intensity, timing, and type (FITT) that are both safe and effective in the acute adult SCI population.17
Therefore, this review questions “What is the effect of exercise and physical activity interventions on cardiorespiratory fitness, cardiometabolic health, and muscle strength in adults with acute SCI?”.
Methods
Ethical Approval
Ethical approval for this desk based research project was granted by the UK University of Lincoln Ethical Application System on 12/04/2021 (review reference: 2021_6564).
Search strategy
This review was registered on PROSPERO (Register ID: CRD42021249441) and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Checklist.20
The lead reviewer (L.R.) conducted a systematic search of literature published from January 2016 until March 2022, to update a previous review.15 The 30 acute rehabilitation studies highlighted by the 2017 review15 were pooled with the results from this updated search and screened against new inclusion criteria. The following 5 databases were systematically searched: PubMed (January 2016-March 2022), CINAHL (January 2016-April 2021 via EBSCO host), SPORTDiscus (January 2016-May 2021 via EBSCO host), National Institute of Clinical Excellence (May 2021), and WHO (May 2021). Google Scholar (January 2016-May 2021) was also searched to identify any additional studies from the gray literature. Search terms (outlined in Appendix 1; available online only at http://www.archives-pmr.org/) were assembled through exploring current literature in this field and using CINAHL Headings, PubMed Medical Subject Headings, and the SPORTDiscus Thesaurus.
Study eligibility
Inclusion criteria
Inclusion criteria for population: (1) adults—aged 18-79 years (men and women); and (2) participants diagnosed with SCI—acute rehabilitation timeframe (<12 months post injury), any type (traumatic, acquired), any severity (complete, incomplete), any level (tetraplegia, paraplegia).
Interventions considered for inclusion met Scientific Exercise Guidelines12 including (1) cardiorespiratory fitness—minimum of 20 min sessions twice per week moderate to vigorous intensity; (2) cardiometabolic health—minimum of 30 min sessions twice per week moderate to vigorous intensity; and (3) strengthening—minimum of 3 sets of 8-10 reps per major muscle group twice per week.
Studies with a control group of no intervention or usual rehabilitation/care featuring spinal cord injured participants were also considered for inclusion. For outcomes, quantifiable measures of cardiorespiratory fitness (ie, oxygen uptake/consumption and aerobic capacity), cardiometabolic health (vascular function, body composition, and metabolic regulation), and/or muscle strength (1 or 10 repetition max, weight lifted, or manual muscle testing) were considered for inclusion.
For settings, any acute, subacute, or community rehabilitation setting with resources to support aerobic and strength training was considered. For study design, randomized controlled trials were considered for inclusion.
Exclusion criteria
Exclusion criteria were: (1) studies with no comparison group or included a comparative intervention that was not a control or usual rehabilitation/care; (2) studies that did not sufficiently report sample or intervention details to determine inclusion; (3) participants with secondary spinal lesions (ie, as a result of multiple sclerosis) or congenital spinal/spinal cord disorders (ie, spinal cord malformation, spina bifida); (4) non-spinal cord injury participants or where the sample is mixed and SCI does not account for >75% of the sample; (5) studies including only animal data; case reports (or case series), conference abstracts, and literature reviews (systematic reviews/meta-analyses/guidelines); (6) qualitative methodology studies; (7) studies requiring translation into the English language, due to a lack of funding for language translation services.
Selection of studies
Citations were exported and duplicates removed using EndNote X8 reference management software. Titles and abstracts were initially screened against the study eligibility criteria stated above by 2 reviewers (L.R. and V.B.). A third reviewer (D.N.) was available to resolve any disagreement if required.
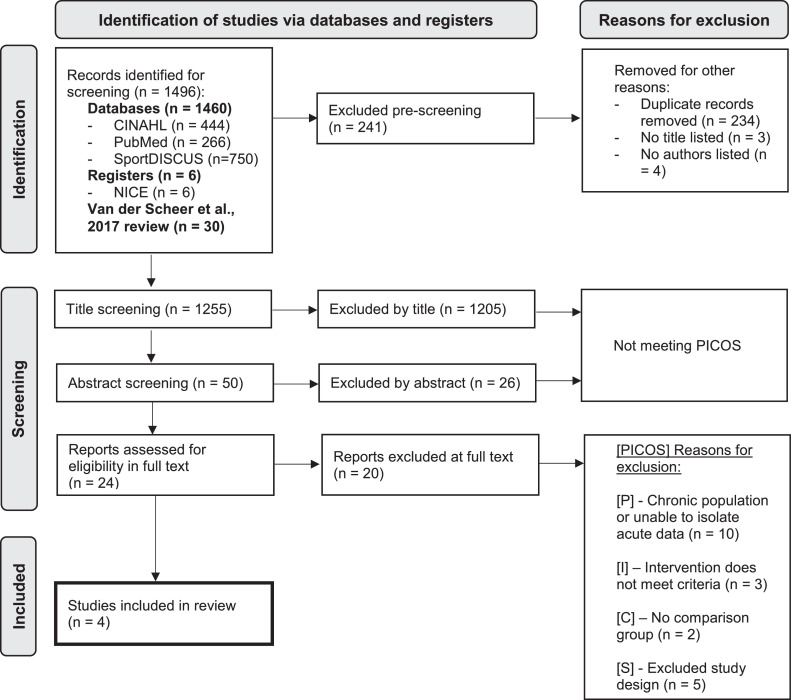
Reviewers (L.R. and V.B.) independently assessed each full text record as part of the PRISMA eligibility stage of the review. Decisions were based on the PICOS model to verify that all inclusion criteria were met. The results at each screening stage and reasons for exclusion have been presented in a PRISMA flowchart (fig 1). Any disagreements were resolved by a third reviewer (D.N.).
Fig 1.
PRISMA diagram of screening and inclusion of studies in this review.
Data extraction
To minimize bias, the guidelines of the Cochrane Handbook for Systematic Reviews of interventions outlined by Higgins et al21 was used for the data extraction and analysis processes. Data extraction was completed on included studies by the lead reviewer (L.R.) and independently checked by a second reviewer (V.B.) using a modified Cochrane Group form. Any conflicts were resolved by a third reviewer (D.N.). The following information was extracted from each article: author name, year of publication, country of study setting, patient population, intervention description, control description, outcome measures, intervention setting, study design, and findings according to each outcome.
Quality assessment
Methodological quality of included articles was assessed using the Modified Downs and Black checklist.22 A binary yes or no (or unable to detect) was applied to question 27 for consistency between reviewers (see Appendix 2 for further detail). Reviewers (L.R. and V.B.) independently assessed the risk of bias for the included studies. A third reviewer (D.N.) was available to resolve any disagreement if required.
Data synthesis strategy
Primary outcomes have been analyzed through narrative synthesis using a best evidence synthesis approach.23 Data have been grouped based on study interventions and outcomes and presented in tabular format. Although initially intended, meta-analysis was not possible due to a lack of consistency in outcome measures and the denominations used.
Results
Search results
Of the 1255 studies highlighted for title screening, 50 were screened by abstract and only 24 articles were appropriate for full text screening. After full text screening, it was unanimously decided that 4 studies were to be included in this review24, 25, 26, 27 (fig 1). One26 of the 4 included studies was previously included in the van der Scheer et al (2017) review.15
Study characteristics
Each of the 4 included studies had between 20 and 30 participants, all of which had an SCI less than 12 months prior. Our total sample size was 108 participants across all included studies, 80% of participants were men. One study24 included ambulatory participants with incomplete cervical and thoracic SCI. All other studies included participants from 9 rehabilitation settings in 6 countries. Participants were undergoing rehabilitation in specialist SCI rehabilitation units across a variety of countries, including Australia (n=4 centers), Canada (n=1), India (n=1), North America (n=1), and Norway (n=1). In 1 study,27 inpatient rehabilitation was conducted at a tertiary hospital in Turkey. Time since injury (TSI) ranged from 60.5 days to 69 days; however, 1 study27 did not specify the TSI for participants but was included as participants were recorded as being treated as in-patients after SCI. The age of participants ranged between 20 and 65 years and all intervention and control groups had a men dominance in the reported women to men participant ratio.
All training interventions met the Scientific Exercise Guidelines12 but each author implemented a different FITT (table 1). Intervention training regimes ranged between 6 weeks and 6 months in length. Four studies were randomized controlled trials and compared interventions against SCI controls, 1 study was a randomized trial comparing 2 interventions. One study reported measures of cardiorespiratory fitness compared high intensity interval training (HIIT) with moderate intensity training (MIT).24 One study reported cardiometabolic health through lean body mass (LBM) and compared functional electrical stimulation cycle ergometry (FES-CE) and functional electrical stimulation isometric contraction (FES-IC) interventions.26 Two studies explored muscle strengthening, 1 study implemented progressive muscle strength training25 and 1 implemented continuous resistance training (CRT).27
Table 1.
Summary of the characteristics of included studies
| Author | Sample | Intervention | Control | Outcome(s) | Setting | Study Design |
|---|---|---|---|---|---|---|
| Baldi et al26 | SCI (n=26; women=7; mean age 27±1 y) Injury: Frankel A or B, Cx or Tx TSI: 60.5 (±22.5) d |
Grp 1: FES-CE (3-30 min, 3 times per week; 3 months n=9; 6 months n=7) Grp 2: FES-IC (1 h, 5p/w; 3 mo n=8; 6 mo n=5) |
UC (no FES; 3 mo n=9; 6 mo n=5) |
|
Inpatient, USA | RCT (2-arm) |
| Bye et al25 | SCI (n=30; women=6; median age 46 [25-65] y) Injury: Level C1-S5; ASIA A-D TSI: 2 (1.4-3.1 IQR) mo |
Progressive strength (3 times per week, 12 wk; n=30 limbs) |
UC (no resistance strength; n=30 limbs) |
|
Inpatient, Australia (4 units) India (1 unit) | RCT |
| Wouda et al24 | SCI (n=26; women=5; mean age 41±17 y) Injury: Level Cx-Sx, ASIA A-D TSI: 69 (±29) days |
Grp 1: HIIT aerobic (35 min, 2 times per week, 12 wk; n=9) Grp 2: MIT aerobic (45 min, 3 times per week, 12 wk; n=9) |
UC (no aerobic training; n=6) |
|
Inpatient, Norway | RCT (3 parallel groups) |
| Yildirim et al27 | SCI (n=26; women=4; mean age CRT 29.6±8.5; UC 31.9±12.0 y) Injury: Level T5-L4, ASIA A-C and CE TSI: not reported |
CRT (60 min, 5 times per week, 6 wk; n=13) |
UC (no fixed mechanical strength; n=13) |
|
Tertiary rehab hospital, Turkey | RCT (cross-sectional) |
Abbreviations: ASIA, American Spinal Injury Association classification; CE, cauda equine; Cx, cervical spine; HRpeak, peak heart rate; QoL, quality of life; RCT, randomized controlled trial; Sx, sacral; TDEE, total daily energy expenditure; Tx, thoracic spine; UC, usual care.
Quality appraisal
All studies were of “good”24,25 or “fair”26,27 quality. None of the studies were fully representative of the entire population, able to blind their participants, or conceal intervention group allocation. Full criteria and scores recorded are provided in Appendix 2.
Intervention outcome
Included studies used a variety of cardiorespiratory fitness, cardiometabolic health, muscle strength, and function outcome measures with a range of denominations. Although some outcome measures used in the included studies were comparable, such as muscle strength, peak heart rate, exercise workload, exercise intensity and function, the denominations, and methods of rating (assessor rated vs self-rated) varied significantly. Inconsistency in measures, raters, and denominations of outcomes made direct comparison and meta-analysis impossible (table 2).
Table 2.
Summary of exercise interventions and cardiorespiratory, cardiometabolic, muscle strength, and function findings
| Study | Training Intervention | Findings |
|---|---|---|
| Cardiorespiratory Fitness | ||
| Wouda et al24 | HIIT - 35 min, 2p/w,12 wk vs MIT - 45 min, 3p/w, 12 wk |
|
| Cardiometabolic Health | ||
| Baldi et al26 | FES-CE - 3-30 min, 3p/w, 3 and 6 mo vs FES-IC - 1 h, 5p/w, 3 and 6 mo |
|
| Wouda et al24 | HIIT - 35 min, 2p/w, 12 wk vs MIT - 45 min, 3p/w, 12 wk |
|
| Muscle Strength | ||
| Bye et al25 | Progressive muscle strengthening (manual by therapists) - 3p/w, 12 wk |
|
| Yildirim et al27 | CRT with fixed mechanical resistance equipment - 60 min, 5p/w, 6 wk |
|
| Function | ||
| Bye et al25 | Progressive muscle strength training - 3p/w, 12 wk (n=30 limbs) |
|
| Wouda et al24 | HIIT - 35 min, 2p/w, 12 wk vs MIT - 45 min, 3p/w, 12 wk |
|
| Yildirim et al27 | CRT - 60 min, 5p/w, 6 wk |
|
Abbreviation: HRpeak, peak heart rate.
Statistically significant findings.
The study exploring cardiorespiratory fitness24 found that HIIT and MIT showed no statistically significant improvement in aerobic and physical capacity and aerobic endurance despite a 13% increase in V̇o2peak with 12 weeks HIIT (table 2).
For measures of cardiometabolic health,26 1 study exploring LBM demonstrated that after 3 months the control group had muscle atrophy of up to 12.4% LBM. This included a loss of 6.1% total LBM, 12.4% gluteal LBM, and 10.1% lower limb LBM. After 6 months, there was muscle atrophy of up to 26.8%, this included a loss of 9.5% total LBM loss, 26.8% gluteal LBM, and 21.4% lower limb LBM. FES-CE and FEC-IC significantly (P<.05) reduced muscle atrophy with 3 and 6 months training compared with control. After 6 months FES-CE training, there was a statistically significant (P<.05) increase of 7.7% gluteal LBM compared with control and a significant (P<.05) increase of 9.3% lower limb LBM compared with FES-IC and control. Conversely, 12 weeks HIIT and MIT showed no significant changes in total daily energy expenditure.24
Quantitative muscle strength measures showed no significant increase in voluntary isometric strength after 12 weeks progressive strength training, but participants perceived (via self-rating score) a clinically meaningful increase in strength.25 After 6 weeks of CRT, all measures of peak torque significantly improved. Although not primary outcomes for this review, the included studies also showed changes in functional outcomes.25,27 Participants perceived a clinically meaningful increase in physical function after 12 weeks progressive strength training and statistically significant increase in self-reported physical function after 6 weeks CRT.25,27 Distance walked, total daily steps, and fatigue ratio showed no statistically significant changes with either training intervention.24
Despite 3 of the studies reporting minor adverse events, none reported any serious harm or injuries. These minor adverse events included light-headedness in the first 2 months of FES-CE training (n=2),26 quadriceps tightening and discomfort in final week (week 12) of progressive muscle strengthening (n=1),25 and myalgia in the first session of CRT (n=3).27
Adherence to interventions was high. The FES-CE training program was well tolerated, with all participants achieving 100% attendance. Only 1 participant demonstrated 80% compliance.26 98% adherence was recorded for progressive muscle strength training.25 One study also recorded a similar number of sessions being recorded for all groups over 12 weeks (HIIT 49, MIT 53, control 55).24 Compliance for 1 study was not recorded.27
Discussion
Summary of main results
This systematic review examined the effectiveness of exercise interventions on cardiorespiratory fitness, cardiometabolic health and muscle strength after acute SCI.12 The 4 included studies implemented a range of intervention types, frequencies, and intensities and used a wide variety of outcome measures to determine efficacy. Aerobic exercise interventions did not improve measures of cardiorespiratory fitness in the acute rehabilitation SCI participants. However, significant improvements were seen in measures of cardiometabolic health with FES-CE and FES-IC as 3 months training reduced muscle atrophy and 6 months FES-CE training significantly increased LBM. Muscle peak torque also significantly improved with 6 weeks CRT and clinically meaningful improvement in perception of muscle strength was seen after 12 weeks progressive muscle strengthening. These results were mirrored by a clinically meaningful increase in function after 12 weeks progressive strengthening and significant increase in physical function with 6 weeks CRT.
Our review is consistent with the findings of van der Scheer et al15 and suggest that interventions meeting the exercise guidelines may be safe, applicable, and feasible when implemented in the acute SCI population. Although none of the included studies recorded serious adverse events and interventions were well adhered to, findings from other studies18 highlight the need to monitor blood pressure and skin condition.
Overall completeness, applicability, and certainty of results
There was insufficient evidence to complete a meta-analysis from the included studies due to the heterogeneity of interventions and outcomes. While the evidence in chronic SCI was sufficient to produce the original guidelines,12 specific studies in the acute SCI rehabilitation stage are still sparse in number and diverse in content.
Potential for variation in international and national standards of care and rehabilitation provision between sites must also be acknowledged as the included studies were conducted at multiple global sites.
A lack of consistency in reporting participant severity of injury, TSI, and standard care provision may have influenced the results of included studies. However, it is for this reason that the 2018 international guidelines were originally compiled and published. It is frequently reported that there is a time lag between research being translated into clinical practice.28 Therefore, the relatively short period of time between the guidelines being published and this review being conducted could explain the limited translation to clinical practice and limited additional research in this field as a result. The variety of exercise interventions highlights the challenges of applying these guidelines to clinical practice.
Significant improvements were reported in cardiometabolic health, peak muscle torque and perceived muscle strength. Improvements were also observed in cardiorespiratory fitness, albeit not statistically so. Our review cannot determine overall efficacy but has suggested which interventions merit further evaluation in the acute SCI rehabilitation population, particularly considering the occurrence of only minor adverse events in these studies.
Comparison with other studies and reviews
The Scientific Exercise Guidelines specify exercise prescriptions but do not stipulate the types of exercise to be used.12 SCI is a complex neurologic condition and each individual experiences differing deficits, recovery trajectories, and functional ability.1 The resultant variation may affect the potential responses to different exercise interventions, particularly in those with tetraplegia.29 Further research is needed to generate specific exercise recommendations, with reference to FITT as well as consider level and completeness of initial injury.
It has been shown that self-reported and performance-based measures are more highly correlated when examining the same domain of disability.30 Our review findings align with this, in that 1 study using manual resistance by therapists demonstrated perceived strength benefits,25 whereas another with fixed mechanical continual resistance training did not.27 Psychological and cognitive attributes, such as interaction with therapists providing the resistance, could have contributed to discrepancies between self-rated and performance-based measures and must be taken into consideration when comparing these forms of measurement30 and when designing future studies.
After initial injury, individuals with acute SCI require an initial period of stabilization and medical optimization prior to commencing early neurorehabilitation and gaining functional progressions.1,31 This review demonstrates that those in the acute rehabilitation phase are able to make positive clinically meaningful changes in strength and function by implementing the exercise guidelines. Flaccid paralysis and disuse following acute SCI is common and leads to muscular atrophy. Individuals with SCI have 35%-40% less LBM and significantly increased body fat compared with able-bodied controls, increasing their risk of developing secondary complications.32 These secondary complications, such as pressure ulcers, fractures, and deep vein thrombosis, can lead to poorer functional recovery, independence, and long-term health.18,26 Interventions such as FES-CE in the first 6 months of SCI rehabilitation has the potential to reduce muscle loss and increase LBM overall. This is particularly useful in areas which may be vulnerable to skin breakdown if there is substantial muscle loss such as the gluteal muscles and lower limb musculature.33
Targeted physical activity and exercise guidelines aim to reduce the risk of developing secondary health conditions of both the general population and those with SCI.12,9 However, guidelines are just 1 method of increasing physical activity participation34 and adherence to them can be inconsistent due to a range of factors.35 Potential barriers can include internal factors such as low self-efficacy and low motivation, which are prevalent in individuals with SCI in the first 12 months post onset.35, 36, 37 Providing access to a suitable exercise environment and equipment, education on why and how to exercise, tailored exercise prescriptions, and individualized support from qualified professionals and SCI peers are all facilitators to exercise participation which are accessible in the acute rehabilitation setting.13,35,37 Acute rehabilitation is an ideal setting to embed exercise guidelines into daily practice but must consider how to tailor to each individual to support long-term adoption and returning to the community.
Limitations
A significant limitation is the number of included studies in this review. However, its focus on an underrepresented patient population (acute SCI rehabilitation), inclusion of positive and negative outcomes, and advancement of an existing and well respected meta-analysis is a strength that justifies and counteracts this. The previously published meta-analysis was used to develop the search strategy, inclusion and exclusion criteria, alongside expert consultation. There is always the possibility that relevant studies have been missed; however, the search was repeated prior to publication. A wider pool of studies, such as non-randomized trials, involving acute SCI rehabilitation participants could have been used and would potentially have given a more complete picture of the evidence available in this field. However, including lower quality studies and alternative designs would have influenced the certainty of findings of this review. Despite the study design criteria, no studies received an “excellent” rating in the standardized quality appraisal, which could also be viewed as a limitation.
Other potential limitations to this study include the heterogeneity of participant characteristics (ie, men/women ratio, age differences, injury level, and completeness), exercise programs, injury severities, and classifications. There was an overwhelmingly higher men to women ratio in the overall sample from this review. While this produces limitations for the generalizability of these findings, it is representative of the ratio of men to women with SCI.38 The small overall data sample may also limit the strength of conclusions from this review. However, it accurately portrays the field of evidence regarding exercise in acute SCI rehabilitation, provides some recommendations for practice, and identifies directions for future study.
Recommendations for practice
Clinicians should consider implementing aerobic exercise and strengthening interventions as outlined in the Scientific Exercise Guidelines in the acute rehabilitation phase alongside usual functional training. Implementing FES cycle ergometry in the first 6 months of injury may reduce the risk of muscle atrophy and increase LBM. Progressive strength training for up to 12 weeks may increase function. Clinicians in the acute rehabilitation setting should consider providing access to appropriate exercise equipment and environments, tailored exercise education, individualized prescriptions, and peer support to work toward achieving the Scientific Exercise Guidelines. Monitoring of patients’ skin integrity and monitoring blood pressure for signs of autonomic dysreflexia should be included in treatment protocols.
Conclusions
This systematic review explored the effectiveness of exercise interventions on cardiorespiratory fitness, cardiometabolic health, and muscle strength in the acute SCI rehabilitation adult population. It was demonstrated that although prescriptions varied, some of the exercise programs meeting the Scientific Exercise Guidelines were effective at improving cardiometabolic health, peak muscle torque, perceived muscle strength, and physical function in acute SCI. Improvements were also observed in cardiorespiratory fitness, although not statistically significant. These results not only provide a good indication for further study but contribute to the evidence in this field. Further empirical research using standardized outcome measures is required to explore the effectiveness of exercise interventions in acute SCI rehabilitation to enable acute clinical guidelines development. Further research in the acute SCI rehabilitation phase may also consider using homogenous samples and exercise types to compare the different elements of FITT to enhance future clinically focused guidelines.
Acknowledgments
The authors wish to thank Professor Ros Kane and Associate Professor Ian McGonagle at the University of Lincoln for their guidance in this review. Special thanks are also given to David Whittaker, Rachel Tomasevic, and Miriam Duffy at Nottingham University Hospitals NHS Trust for their ongoing support for this research. This systematic review was undertaken as part of the Integrated Clinical Academic (ICA) programme run by the National Institute for Health Research (NIHR) and funded by the Health Education England (HEE). This award was granted to Lauren Minion as part of Masters level academic training.
Footnotes
Disclosures: The investigators have no financial or nonfinancial disclosures to make in relation to this project.
Appendix
Appendix includes supplementary materials associated with this article and can be found, in the online version, at doi:10.1016/j.arrct.2023.100278.
Appendix. Supplementary materials
References
- 1.Fehlings MG, Tetreault LA, Wilson JR, et al. A Clinical Practice Guideline for the Management of Acute Spinal Cord Injury: introduction, rationale, and scope. Glob Spine J. 2017;7(3_supplement):84S–94S. doi: 10.1177/2192568217703387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayta E, Elden H. Acute spinal cord injury: a review of pathophysiology and potential of non-steroidal anti-inflammatory drugs for pharmacological intervention. J Chem Neuroanat. 2018;87:25–31. doi: 10.1016/j.jchemneu.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 3.James SL, Bannick MS, Montjoy-Venning WC, et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Köseoǧlu BF, Safer VB, Öken Akselim S. Cardiovascular disease risk in people with spinal cord injury: is there a possible association between reduced lung function and increased risk of diabetes and hypertension? Spinal Cord. 2017;55:87–93. doi: 10.1038/sc.2016.101. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain JD, Buzzell A, Gmünder HP, et al. Comparison of all-cause and cause-specific mortality of persons with traumatic spinal cord injuries to the General Swiss Population: results from a National Cohort Study. Neuroepidemiology. 2019;52:205–213. doi: 10.1159/000496976. [DOI] [PubMed] [Google Scholar]
- 6.Cieza A, Sabariego C, Bickenbach J, Chatterji S. Rethinking disability. BMC Med. 2018;16:10–14. doi: 10.1186/s12916-017-1002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 8.Miller LE, Herbert WG. Health and economic benefits of physical activity for patients with spinal cord injury. Clin Outcomes Res. 2016;8:551–558. doi: 10.2147/CEOR.S115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . World Health Organization; Geneva: 2018. Global action plan on physical activity 2018–2030: more active people for a healthier world. [Google Scholar]
- 10.WHO. WHO 2020.pdf [Internet]. World Health Organization. 2020. Available at: https://www.who.int/health-topics/physical-activity. accessed: 06/05/2021
- 11.Withers TM, Croft L, Goosey-Tolfrey VL, Dunstan DW, Leicht CA, Bailey DP. Cardiovascular disease risk marker responses to breaking up prolonged sedentary time in individuals with paraplegia: the Spinal Cord Injury Move More (SCIMM) randomised crossover laboratory trial protocol. BMJ Open. 2018;8:1–7. doi: 10.1136/bmjopen-2018-021936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin Ginis KA, Van Der Scheer JW, Latimer-Cheung AE, et al. Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord. 2018;56:308–321. doi: 10.1038/s41393-017-0017-3. [DOI] [PubMed] [Google Scholar]
- 13.Carty C, Van Der Ploeg HP, Biddle SJH, et al. The first global physical activity and sedentary behavior guidelines for people living with disability. J Phys Act Heal. 2021;18:86–93. doi: 10.1123/jpah.2020-0629. [DOI] [PubMed] [Google Scholar]
- 14.Martin Ginis KA, Latimer-Cheung AE, West CR. Commentary on “the first global physical activity and sedentary behavior guidelines for people living with disability.”. J Phys Act Heal. 2021;18:348–349. doi: 10.1123/jpah.2020-0871. [DOI] [PubMed] [Google Scholar]
- 15.van der Scheer JW, Martin Ginis KA, Ditor DS, et al. Effects of exercise on fitness and health of adults with spinal cord injury: a systematic review. Neurology. 2017;89:736–745. doi: 10.1212/WNL.0000000000004224. [DOI] [PubMed] [Google Scholar]
- 16.Selph SS, Skelly AC, Wasson N, et al. Physical activity and the health of wheelchair users: a systematic review in multiple dclerosis, cerebral palsy, and spinal cord injury. Arch Phys Med Rehabil. 2021;102 doi: 10.1016/j.apmr.2021.10.002. 2464-81.e33. [DOI] [PubMed] [Google Scholar]
- 17.Gurwitz JH, Carlozzi NE, Davison KK, Evenson KR, Gaskin DJ, Lushniak B. National Institutes of Health Pathways to Prevention Workshop: physical activity and health for wheelchair users. Arch Rehabil Res Clin Transl. 2021;3 doi: 10.1016/j.arrct.2021.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galea MP, Panisset MG, El-Ansary D, et al. SCIPA Switch-On: a randomized controlled trial investigating the efficacy and safety of functional electrical stimulation-assisted cycling and passive cycling initiated early after traumatic spinal cord injury. Neurorehabil Neural Repair. 2017;31:540–551. doi: 10.1177/1545968317697035. [DOI] [PubMed] [Google Scholar]
- 19.Williams TL;, Smith B, Papathomas A. Physical activity promotion for people with spinal cord injury: physiotherapists’ beliefs and actions. Disabil Rehabil. 2018;40:52–61. doi: 10.1080/09638288.2016.1242176. [DOI] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:372. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 [Internet] Cochrane. 2011 www.training.cochrane.org/handbook Available at: accessed: 28/05/2021. [Google Scholar]
- 22.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Commun Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slavin RE. Best evidence synthesis: an intelligent alternative to meta-analysis. J Clin Epidemiol. 1995;48:9–18. doi: 10.1016/0895-4356(94)00097-a. [DOI] [PubMed] [Google Scholar]
- 24.Wouda MF, Lundgaard E, Becker F, Strøm V. Effects of moderate- and high-intensity aerobic training program in ambulatory subjects with incomplete spinal cord injury–a randomized controlled trial. Spinal Cord. 2018;56:955–963. doi: 10.1038/s41393-018-0140-9. [DOI] [PubMed] [Google Scholar]
- 25.Bye EA, Harvey LA, Gambhir A, et al. Strength training for partially paralysed muscles in people with recent spinal cord injury: a within-participant randomised controlled trial. Spinal Cord. 2017;55:460–465. doi: 10.1038/sc.2016.162. [DOI] [PubMed] [Google Scholar]
- 26.Baldi JC, Jackson R, Moraille R, Mysiw W. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord. 1998;36:463–469. doi: 10.1038/sj.sc.3100679. [DOI] [PubMed] [Google Scholar]
- 27.Yildirim A, Sürücü GD, Karamercan A, et al. Short-term effects of upper extremity circuit resistance training on muscle strength and functional independence in patients with paraplegia. J Back Musculoskelet Rehabil. 2016;29:817–823. doi: 10.3233/BMR-160694. [DOI] [PubMed] [Google Scholar]
- 28.Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104:510–520. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tweedy SM, Beckman EM, Geraghty TJ, et al. Exercise and sports science Australia (ESSA) position statement on exercise and spinal cord injury. J Sci Med Sport. 2017;20:108–115. doi: 10.1016/j.jsams.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Coman L, Richardson J. Relationship between self-report and performance measures of function: a systematic review. Can J Aging/La Rev Can du Vieil. 2006;25:253–270. doi: 10.1353/cja.2007.0001. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez JAS, Sharif S, Costa F, Rangel JAIR, Anania CD, Zileli M. Early management of spinal cord injury: WFNS spine committee recommendations. Neurospine. 2020;17:759–784. doi: 10.14245/ns.2040366.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hicks AL, Martin Ginis KA, Pelletier CA, Ditor DS, Foulon B, Wolfe DL. The effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: a systematic review. Spinal Cord. 2011;49:1103–1127. doi: 10.1038/sc.2011.62. [DOI] [PubMed] [Google Scholar]
- 33.van der Scheer JW, Goosey-Tolfrey VL, Valentino SE, Davis GM, Ho CH. Functional electrical stimulation cycling exercise after spinal cord injury: a systematic review of health and fitness-related outcomes. J Neuroeng Rehabil. 2021;18:1–16. doi: 10.1186/s12984-021-00882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin Ginis KA, van der Ploeg HP, Foster C, et al. Participation of people living with disabilities in physical activity: a global perspective. Lancet. 2021;398:443–455. doi: 10.1016/S0140-6736(21)01164-8. [DOI] [PubMed] [Google Scholar]
- 35.Simpson LA, Eng JJ, Tawashy AE. Exercise perceptions among people with stroke: barriers and facilitators to participation. Int J Ther Rehabil. 2011;18:520–530. doi: 10.12968/ijtr.2011.18.9.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig A, Tran Y, Guest R, Middleton J. Trajectories of self-efficacy and depressed mood and their relationship in the first 12 months following spinal cord injury. Arch Phys Med Rehabil. 2019;100:441–447. doi: 10.1016/j.apmr.2018.07.442. [DOI] [PubMed] [Google Scholar]
- 37.Cowan RE, Nash MS, Anderson KD. Exercise participation barrier prevalence and association with exercise participation status in individuals with spinal cord injury. Spinal Cord. 2013;51:27–32. doi: 10.1038/sc.2012.53. [DOI] [PubMed] [Google Scholar]
- 38.Weidner N, Rupp R, Tansey KE. Neurological aspects of spinal cord injury. Springer; 2017;. p. 10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.