Abstract
Individuals with neurologic conditions seek physical therapy services to improve mobility in their daily lives. While clinicians commonly track activity capacity, measurement of activity performance in daily life is an emerging yet unstandardized practice within routine clinical physical therapy. The purpose of this case report is to (1) provide an example of the structure, clinical reasoning, and implementation of both activity capacity and activity performance level assessments across an episode of outpatient physical therapy and (2) to describe how objective activity performance in daily life tracking supported the physical therapy intervention and education plan.
A 42-year-old woman presented to outpatient neurologic physical therapy with a rare autoimmune-mediated disorder with primary goals of independently caring for her youngest child and grandchild, walking without limitations in the home and community, participating in exercise, and returning to work due to deconditioning and dizziness. The patient participated in 12 visits across a span of 4.5 months targeting performance in daily life (steps per day), aerobic conditioning, and vestibular habituation. Activity capacity measurement served as a standardized assessment of what the patient was able to do in the clinic, and activity performance in daily life tracking via a Samsung wrist worn consumer-grade device provided a quantitative assessment of real-world daily stepping activity. Tracking of activity performance in daily life was an essential component of physical therapy management that provided an objective quantification of daily stepping activity to identify barriers and facilitators to increasing daily performance in an individual with a medical diagnosis of Susac syndrome.
KEYWORDS: Rehabilitation, Performance, Capacity, Measurement
Individuals with neurologic conditions seek physical therapy services to improve mobility in their daily lives.1 Mobility in daily life, or activity performance, is a construct within the Activity level of measurement of The World Health's Organization of Function, Disability and Health.2 This is separate from activity capacity, defined as the ability to execute an action in a controlled laboratory or clinical environment. Assessment of activity capacity has been recently adopted within clinical physical therapy with a multitude of successful efforts at the national level enhancing translation into clinical practice.3 While both activity capacity and activity performance in daily life assessments together can provide a more holistic view of the activity level, assessing the effect of rehabilitation interventions in the context of an individual's daily life should serve as a primary indicator of effectiveness of rehabilitation interventions.
A few barriers exist for the adoption of activity performance level tracking into clinical physical therapy. The first barrier includes common assumptions including (1) self-report of activity performance in daily life matches direct assessment of activity performance in daily life; (2) activity capacity level assessment (eg, gait speed) can serve as a proxy activity performance level assessment; and (3) changes in activity capacity level assessments will result in change in activity performance level assessments. Though a moderate relation between activity capacity and activity performance has been demonstrated,4 a recent analysis demonstrated that individuals engaging in routine outpatient rehabilitation improved in their activity capacity but not activity performance in daily life, highlighting the discrepancies in activity capacity and activity performance in daily life.5 It is now time to appreciate the distinct measurement properties between assessments deployed in a structured setting at an isolated point in time as compared with an assessment of what the individual does in their authentic environment throughout the day. The second barrier is the lack of affordable consumer-grade sensors sensitive to the movement characteristics of those with neurologic diagnoses.6 Wearable sensor technology is a rapidly evolving field that could afford rehabilitation clinicians the ability to quantify and monitor changes in an individual's movement in everyday life. Characteristics of movement that contribute to inaccuracy of consumer-grade devices include use of an assistive device,7, 8, 9 slowed gait speeds (<0.8 m/s),10, 11, 12, 13, 14, 15 or interruptions in continuous walking.16,17 Despite limitations in technology, activity performance level tracking is possible in many individuals, and when deployed taking into consideration these limitations, has the potential to allow more tailored interventions and educational strategies to improve activity performance in daily life and target behavioral self-modification. Behavioral self-modification strategies include a variety techniques targeting motivation, a sense of responsibility, and self-efficacy in performing exercise and activity performance in daily life.18
The purpose of this case report is to provide an example of the structure, clinical reasoning, and implementation of both activity capacity and activity performance level assessments across an episode of outpatient physical therapy and to describe how objective activity performance in daily life tracking supported the physical therapy intervention and education plan. This clinical case will serve as an example of how to assess activity performance in daily life and demonstrate how these data supported a more patient-directed, customized intervention program that prioritized behavioral self-management that could be applied to other patient populations.
Case description
Participant history and systems review
The patient was a 42-year-old woman with a diagnosis of Susac syndrome. Susac syndrome is a rare autoimmune-mediated disorder characterized by occlusions of microvessels in the brain, retina, and inner ear causing the clinical triad of encephalopathy, branch retinal artery occlusions, and hearing loss, respectively.19 Individuals can present with varied clinical presentations of central nervous system dysfunction, visual disturbances, and hearing deficits with fluctuating symptomatology.20 Data are limited regarding long-term outcomes, with some patients having irreversible damage to neurologic, auditory, and/or ocular systems,21, 22, 23 and only recently have guidelines been developed for medical management.24
The patient initially presented to the Neurology physician with vision loss, dizziness, and numbness and tingling in her distal fingers. Over the course of 9 months, the patient experienced progression and regression of her Susac syndrome symptoms, with episodic experiences of vertigo, mental fogginess, hearing loss, and balance difficulties. Magnetic resonance imaging revealed supratentorial white matter indicating lesions in the corpus callosum, pericallosal region, basal ganglia, pons, and cerebellar hemispheres. Neurology and Ophthalmology physicians closely monitored the patient with imaging and adjustment of immunosuppressive therapy. She presented to physical therapy with a referring diagnosis of Susac syndrome 9 months post-diagnosis on a stable dose of immunosuppressive agents. The patient had a past medical history of hyperlipidemia and hypercholesterolemia controlled by medication and had never received physical therapy services.
At initial physical therapy examination, the patient reported she was a mother of 3 and employed full time in a role that required frequent use of a computer. She lived in a 2-story home with her youngest daughter and her husband and had numerous other supportive family members including 2 adult children. Some of her favorite leisure time physical activities included exercising and playing basketball with her husband. The patient presented with chief complaints of brain fogginess, decreased endurance, and dizziness, which prevented her from achieving her goals of independently caring for her youngest child and grandchild, walking without limitations in the home and community, participating in exercise, and returning to work. She reported that when her symptoms were at their worst, she would spend several hours in bed until they lessened. The patient also shared feelings of frustration, anxiety, and sadness over the course of her symptoms and history of diagnosis, including a period of 5 months where she had no episodic memory. Written informed consent was obtained from the patient for publication of this retrospective case report, and Institutional Review Board approval was not applicable or required.
Activity capacity and impairment assessments
The initial examination was intentionally arranged to start with activity capacity assessments sequenced from least to most likely to increase vestibular symptomatology, specifically. The examination was arranged this way to ensure that the most provoking assessments would not cut short the comprehensive examination. Isolated impairment assessments such as strength, sensation, and proprioception were deferred, as this information likely did not have the potential to alter the plan of care, especially because the patient walked into the initial assessment appointment. Confrontational visual field testing and a single vestibular assessment were performed after the activity capacity assessments to confirm assumptions of the visual and vestibular impairments that were contributing to the functional deficits. Table 1 displays the order in which the activity capacity and impairment assessments were conducted, underlying construct assessed, the physical therapist's clinical interpretation of each assessment on the initial assessment, and the outcomes over the course of care.
Table 1.
Measure order, construct of measurement, therapist interpretation of results, and outcomes over the course of care
| Measure Order | Construct | Initial Examination | Month 1 | Month 2 | Month 4.5 |
|---|---|---|---|---|---|
| Interpretation | Interpretation | Interpretation | Interpretation | ||
|
Self-report balance confidence | 61.88 | 83.13 | Not performed, would not change plan of care | * |
| Low confidence in balance abilities25,26 | High functioning27 | N/A | N/A | ||
|
Functional strength and transitional movement capacity | Not prioritized due to time | 15.29 | 13.88 | 13.15 |
| Balance disorder28 | * | * | |||
|
Walking speed capacity | 0.79 | 1.13 | 1.14 | 1.15 |
| Slowed for age29 | Improving without explicit focus | No change | * | ||
|
Walking speed capacity | 0.95 | 1.39 | 1.68 | 1.6 |
| Slowed for age29 | Improving without explicit focus | * | No change | ||
|
Walking endurance capacity | 371 | 457 | Not prioritized for re-assessment | * |
| Below normative distance for age30 | Improving without explicit focus | N/A | N/A | ||
|
Dynamic walking capacity | 7/30 | 15/30 | 17/30 | 26/30 |
| Fall risk31 | Improving, still fall risk31 | Fall risk31 | Low fall risk31 | ||
|
Identification of central vestibular dysfunction impairment | Lack of smooth tracking, saccadic eye movement, symptom provoking | Not performed, would not change plan of care | * | * |
| Positive sign for central vestibular dysfunction | N/A | N/A | N/A | ||
|
Visual field impairment | Absent vision superior medial portion of left eye, right eye intact | Not performed, would not change plan of care | * | * |
| Unlikely to affect function | N/A | N/A | N/A |
Duplication of previous statement in prior assessment time-point. N/A refers to interpretation not applicable for the assessment time-point.
The patient was alert and oriented × 4, with report of mild memory deficits documented during a prior occupational therapy evaluation. On initial examination, the patient walked slowly on level surfaces (10 Meter Walk Test [10mWT] and Six Minute Walk Test [6MWT]), had difficulty with conditions that required head movement (functional gait assessment [FGA]), and had difficulty when turning, starting, and stopping with ambulation as demonstrated by staggering and loss of balance on these items (FGA). At the end of the initial evaluation, the patient reported less dizziness than at the beginning of the session, a sign that movement could beneficially modify her symptoms. Confrontation visual field testing revealed a superior medial quandrantanopia of the left eye that was not present with both eyes open. Smooth pursuit testing showed lack of smooth eye tracking, corrective saccades, and difficulty keeping eyes open due to symptom provocation, which the therapist interpreted as confirmatory of central vestibular dysfunction and did not pursue further vestibular testing.
Activity performance in daily life assessment
The patient's primary goals were to be independent in caring for her young child and grandchild, return to unlimited mobility in the community, participate in exercise, and return to work. The therapist used activity performance level assessments as the primary measure of clinical success to align with the patient's primary goals as well as behavioral self-management.18 After conducting activity capacity and brief vestibular system impairment assessments, the remaining portion of the initial evaluation included an iterative discussion of how activity performance in daily life assessment would be integral in achieving the patient's goals. The patient and therapist discussed how activity performance tracking would allow the patient and therapist to (1) quantify amount of movement in daily life; (2) explore barriers and facilitators to daily movement; and (3) progress the therapeutic plan aimed to reduce vestibular symptoms, improve endurance, and increase overall activity. The patient reported that she had a Samsung Galaxy Smartwatch. The patient agreed to track stepping data daily, ensuring that each day between therapy visits (7 days initially) were captured during all waking hours. Tracking for multiple days is consistent with research protocols of capture for at least 3 days15,32,33 and important because of the wide variability in stepping amounts day over day.4 The request for explicit monitoring over all days between visits was selected because of the unstable symptomatology and high-anticipated variability for this patient. The patient returned to her home environment with wearing instructions and planned to return to the next visit, a week later, with her phone to review the data collaboratively with the therapist.
Diagnosis and prognosis
There were 2 primary areas of focus for this rehabilitation episode based on findings from capacity level assessments. Based on the patient's slowed gait speed and diminished endurance as assessed by the 10mWT29,34 and 6MWT,35 respectively, initiation of an aerobic conditioning program was indicated and the patient was given a physical therapy diagnosis of hypoactivity.36 Based on the patient's limitations in any activity that required head movement, acceleration, or deceleration as assessed by the FGA, the patient was given a physical therapy diagnosis of sensory selection and weighting deficit,37,38 and a tailored vestibular habituation program was indicated. The initial examination provided critical information about the status of the patient's capacity for activity and outcomes from these assessments allowed comparison with published normative values based on the patient's age and diagnosis to inform the plan of care. Performance in daily life assessment revealed a low number of steps over the course of the initial tracking period revealing a diagnosis of hypoactivity.36 The comprehensive intervention plan included initiation of an aerobic conditioning program, daily walking program, and vestibular habituation program. Because of the patient's age, motivation for change, and social support, the patient had a positive prognosis for return to activity levels recommended for healthy adults and for reduced dizzy symptoms in daily life.
Intervention
The approach for rehabilitation over the episode of care was consistent with recommended behavioral change and self-management practices18 in order to facilitate patient's ability to manage her symptoms and achieve her goals. Consistent with this approach, appointments were initially spaced at 1 time per week to allow the patient time in the home and community environment to carryover the therapeutic activities and discover barriers that contributed to a reduction in daily activity. We note that this approach is intentionally different than the traditional 2-3 times/week over 8-week standard-of-care paradigm. All activities prescribed for the home or community environment were performed in the clinical setting prior to the patient performing independently to support greater self-efficacy to enhance adherence.39,40 Concurrent with the trialing of activities, the therapist had the patient articulate how she would monitor and ensure appropriate duration and intensity of the activities, with faded feedback provided until the patient could accurately verbalize how to self-monitor and adjust within each activity over the course of care. Each of the primary areas of the intervention were addressed at each visit, and the details for how each area was progressed are described below.
Daily stepping review and goal setting
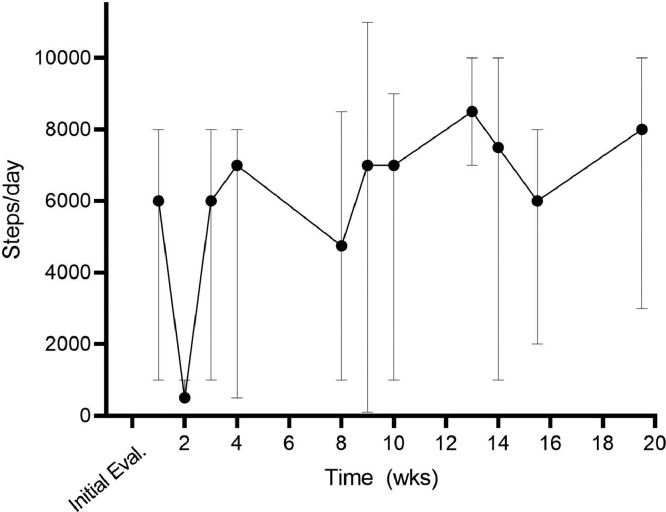
At every session, the therapist had the patient self-identify targeted daily step minimums until the next therapy visit. This allowed the patient to set realistic and achievable daily step counts based on her upcoming home and family responsibilities.41 Part of the goal setting process also included review of stepping data since the last therapy visit. The goals of this collaborative review of performance data were to identify barriers to mobility on low step count days and facilitators on high step count days, and to progress the patient to greater steps per day and to reduce variability day-over-day in stepping amount. Through these iterative and collaborative discussions, the therapist was able to support the patient in structuring a daily plan to reduce symptomatology and increase daily stepping over time, with a long-term goal of having no vestibular symptoms and progressing the patient to daily step counts described in the literature as “active”.42 Given that lack of movement made symptoms worse, a key focus was to have fewer days where there were low step count levels. The therapist capitalized on opportunities to highlight the linkage between activities included in the plan of care with the patient's goals.43 Figure 1 depicts the stepping mean range of the 7 days prior to each therapeutic visit over the episode of care as depicted on the Samsung Health phone application.
Fig 1.
Stepping data across the episode of care, as quantified by the Samsung Health device. Values are the mean of 7 days preceding the therapy visit, with error bars representing the minimum and maximum daily values within the 7-day period. Note the gradual increase over time in the mean and minimum (descending error bar) values.
Referral to other providers
Activity performance monitoring brought forth the symptom provoking effects of both patient reported anxiety and lack of sleep. On days where the symptoms were at a peak, the patient was able to recall she had not slept well the night before, or that the day was particularly stressful, which in turn had an effect on her daily performance. Collaborative activity performance data review supported the patient in identifying that anxiety (visit 4) and sleep (visit 11) as symptom provoking factors that reduced daily activity. Identifying these barriers to daily activity resulted in communication to the physician to request a referral to supportive counseling and to elicit advice on sleep hygiene. Referral back to the primary physician yielded a psychological referral and Physician recommendations for improving sleep duration and quality.
Aerobic conditioning
All exercises targeting aerobic conditioning were performed with the patient using the heart rate monitoring feature of her wrist-worn watch as a mechanism to measure intensity. Initially, the patient could only tolerate low levels of aerobic intensity due to the severity of her vestibular symptoms and so the primary goal was duration of activity. As the patient's vestibular symptoms improved, higher levels of aerobic intensity were achievable and in more dynamic environments. Over the course of care, the patient transitioned from performing low level aerobic walking tasks to incorporating interval running and basketball to achieve recommended levels of physical activity.44
Vestibular habituation program
Early on in the episode of care, slow walking and minimal amounts of daily activity were highly symptom provoking for the patient. The patient required a lot of education on central vestibular dysfunction and how habituation would target the problems she was experiencing as this treatment is counterintuitive to those experiencing severe dizziness of central origin. The patient initially targeted vestibular habituation at tolerating a greater amount of daily activity. As normal daily activities, including dressing, cooking, and cleaning, additional exercises were added to the home program. These included progressive challenges in the vestibular ocular reflex from sitting to standing to walking, walking with no head turns to walking with head turns to high level dynamic challenges including quick turning and basketball activities.
Outcomes
Over the course of care, as the patient increased consistency of daily stepping activity, improvements in capacity were noted, as demonstrated in Table 1. No adverse events occurred during the episode of care. Outcome assessments were prioritized based on their redundancy with other measures, their ability in altering the course of care, time allotted during the therapy session, and the patient's goals. For example, the Five Times Sit-to-Stand test was not prioritized on initial evaluation, as the patient did not report a primary area of concern in transferring from sitting to standing. The 6MWT was not repeated after the 1-month assessment due to the patient's improvement to age-normative range within the first month of care and due to the strong correlation between the 6MWT and 10mWT.45,46 Impairment level testing of the vestibular system was not repeated after the initial evaluation as results from this assessment would not alter the plan of care.
The episode of care was 12 visits and spanned 4 and a half months. Once the patient verbalized that she felt she had all the tools necessary to self-manage her symptoms and reported no new barriers to daily stepping, the therapist and patient determined discharge to the community was the appropriate next step. The therapist provided patient education on the signs or situations that would warrant a return to skilled physical therapy including a decline in daily activity, an increase in vestibular symptoms, or new falling episodes. The therapist discharged the patient and encouraged her to return to therapy in 6 months for evaluation of long-term carryover of activity performance goals.
The patient reported that through interventions performed in clinic combined with the use of activity performance level assessment, she was able to understand how to control her symptomatology and progress toward achieving her goals. Most notable was when the therapist would have the patient perform an activity in clinic that the patient identified as symptom provoking from her daily life, and then support the patient in using the home exercise program to return symptoms to baseline within session. This strategy brought to life how movement could control her symptoms, and that the patient was in charge of how much movement she performed. On a follow-up phone call 5 months after discharge, the patient reported she still used her watch daily to track her steps and was trying to achieve a daily output of 10,000 steps. The patient shared “You showed me the tools, but I have to use them”.
Discussion
This case report describes the use of both activity capacity and activity performance level assessment data during a routine outpatient physical therapy episode of care, and how activity performance in daily life assessment was used to provide a patient-centered intervention program that promoted behavioral self-management. This case study does not provide results of how to deliver a specified frequency, intensity, time or type of treatment, but provides an initial example in shifting the focus of aligning outcomes for clinical care to why individuals seek out rehabilitation services.1 The use of both activity capacity and activity performance level assessments provide holistic assessment across the activity level of the ICF. Activity capacity assessments provide information about what a patient is capable of, which is related to, but not predictive of activity performance.47, 48, 49 Activity capacity level assessments also provide a standardized way to assess an individual's ability to move across a variety of salient functional tasks, support the identification of movements that require further assessment, and can inform prognosis in the acute or sub-acute stages.50, 51, 52 Activity capacity level outcomes, therefore, could inform determination of frequency of therapy visit. However, activity capacity measures do not provide direct measurement of activity performance in a patient's authentic environment. This case report highlights advantages and shortcomings of activity capacity level assessments, and how they contribute to a plan of care.
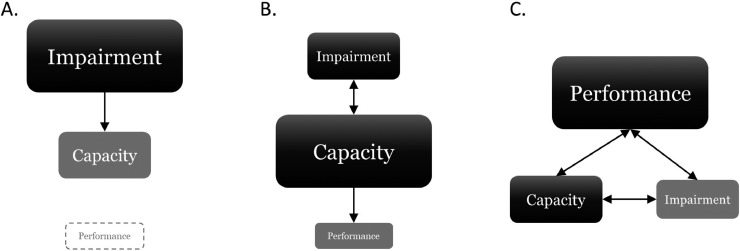
Using activity performance level assessments as a benchmark for clinical success aligns with behavioral self-management strategies, is patient-centered because of the relation to patient goals, and is complimentary to activity capacity assessments in providing information regarding activity in the real-world context. Many factors have the potential to affect activity performance outside of an individual's physical capacity including physical structures of the home and community environment, social support, transportation, cultural influences, and individual attitudes, among others. Direct assessment provides an objective means to capture data that allows exploration of facilitators to activity performance and problem solving around the barriers to activity performance. The emphasis on activity performance level assessment is in contrast to historical and contemporary approaches to clinical care, with figure 2 depicting the differences between approaches. A primary focus in historical approaches of physical therapy for both evaluation and intervention have occurred at the impairment level (fig 2A),53,54 where the assumption was made that if impairments improved, so would an individual's activity capacity. In this model, activity performance was not considered. Practice patterns have evolved to the current state, with great emphasis placed on focusing assessment and intervention on improving activity capacity, with the assumption that if activity capacity improves, so will activity performance (fig 2B).3 While activity capacity level assessments should inform clinical care decisions and be used to document changes in capacity for function, their prioritization above activity performance in daily life is questionable. The proposed approach, for which this case serves as an example, advocates performance as the primary measure for guiding or assessing the effectiveness of treatment due to its central focus within clinical care (fig 2C). Further, the proposed approach would shift the focus to maximizing activity performance in real-contexts, which has the potential to have a greater effect on overall health status.55
Fig 2.
Contributions of domains across the ICF Model that guide and prioritize evaluation and intervention. Size and shape reflects the relative importance in evaluation and clinical decision-making. (A) Historical approach where impairment is central. (B) Current approach where capacity is central. (C) Proposed approach where performance is central.
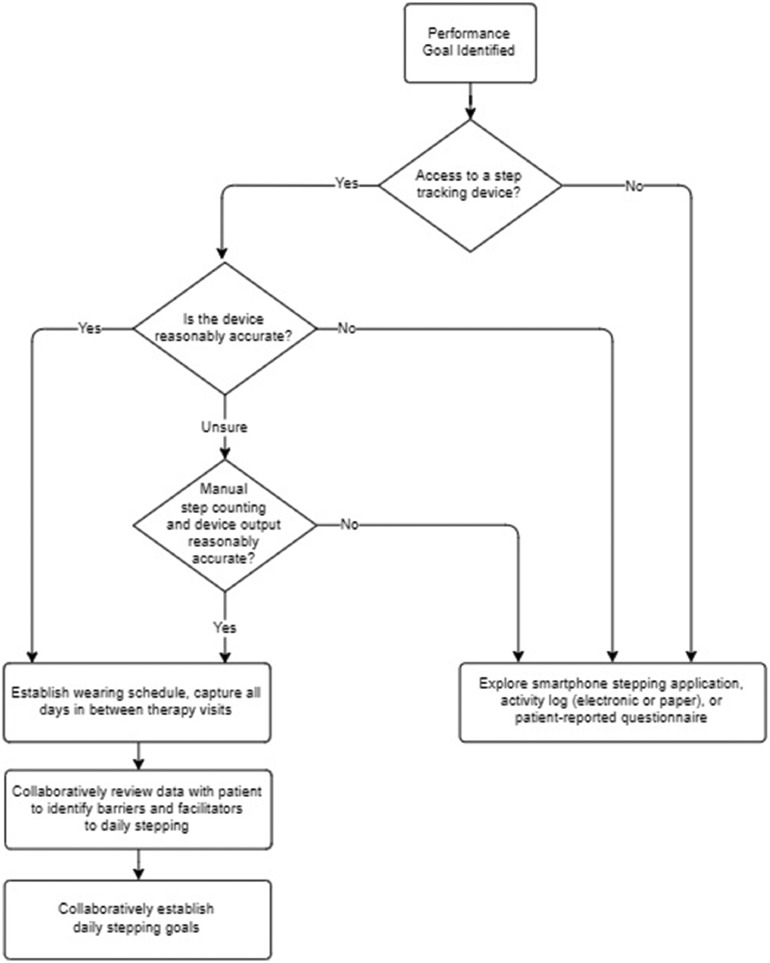
Despite the many barriers that exist in implementing activity performance monitoring into clinical practice, it is feasible with many patients. When employed in a way that takes into account limitations of the technology as described in research, activity performance monitoring can have an enormous effect on a therapeutic program. Figure 3 depicts the decision tree used to implement a lower limb activity performance-level variable (ie, daily stepping) into clinical practice by the treating clinician in partnership with the research team. The tree provides a roadmap for clinicians to follow in order to incorporate activity performance in daily life assessment with a behavioral self-management focus despite limitations of current consumer grade technology or when technology is not available. This decision tree also leverages research recommendations on minimum wear time to account for variability in daily stepping.4 This decision tree could serve as a comprehensive tool for therapists to implement activity performance assessment via either direct or self-report means (when technology not available) and engage in dialog with their patients about activity performance in daily life. A few tips to overcome barriers to capturing daily stepping due to device inaccuracies include having the patient wear the device on the ankle if they use a bilateral walking device, or performing accuracy validation comparing manual step counts to device output within the clinical environment. When direct performance monitoring is not feasible, activity logs or patient-reported questionnaires can be incorporated. Therapists should use patient-reported strategies, however, with caution, as these measures have been shown to lack consistency with more direct assessments.1,56 Incorporating lower limb activity performance monitoring into clinical practice requires therapy time to assist individuals in establishing a direct tracking mechanism, which frequently involves managing and learning new technology. When behavioral self-management is the primary focus of the rehabilitation episode, the required time to establish direct tracking is reasonable.
Fig 3.
Decision tree for implementing performance level assessments into clinical practice.
Steps-per-day is but 1 of many performance level variables studied in the literature.57, 58, 59 Steps-per-day has sufficient established psychometric and clinical utility, making it a primary variable for lower limb performance. Number of steps-per-day has also been linked mortality risk, disease incidence or risk, and has been studied in healthy individuals, at-risk populations, and those with mobility impairments,42,60,61 making steps-per-day a prime target for health promotion across populations. Further studies are required to standardize additional clinically meaningful variables to inform decision-making across the activity performance level.
Limitations
Limitations of the current case report include the potential of imprecise output of the Samsung Galaxy watch, however, device inaccuracies are likely similar across assessment intervals. While accuracy of step activity count tracking with a Samsung Galaxy smartwatch has not been established, the decision to use it was reasonable because the patient's self-selected gait speed was above thresholds where inaccuracies have been identified across consumer-grade products (>0.8 m/s)10, 11, 12, 13, 14 and because she did not use an assistive device.7, 8, 9 Other limitations include the limited generalizability of the results; however, the purpose of this manuscript is to highlight, through a case example, the use of both activity capacity and activity performance assessments in routine physical therapy, and how performance monitoring was integral in driving intervention within outpatient physical therapy. The approach used in this case report can be replicated in other patient scenarios where activity performance in daily life is the targeted outcome; however, more work is required to evaluate the effectiveness of using activity performance data to drive clinical care.
Conclusions
This complex case provides an example of the structure, clinical reasoning, and implementation of both capacity and performance level assessments across an episode of outpatient physical therapy for a patient. Assessment of activity performance in daily life created a patient-directed, customized intervention program that prioritized behavioral self-management. Without the inclusion of performance level assessment, the primary goal of behavioral self-management would have been impossible to achieve, as the exploration of barriers to behavior change would be limited without objective performance assessment.
Footnotes
The data within this manuscript were presented as a poster presentation at the 2023 Combined Sections Meeting of the American Physical Therapy Association.
List of abbreviations: 6MWT, 6 minute walk test; 10mWT, 10 meter walk test; FGA, functional gait assessment
This work was supported by the National Institutes of Health (NIH R01HD068290).
Disclosures: The investigators have no financial or nonfinancial disclosures to make in relation to this project.
References
- 1.Waddell KJ, Birkenmeier RL, Bland MD, Lang CE. An exploratory analysis of the self-reported goals of individuals with chronic upper-extremity paresis following stroke. Disabil Rehabil. 2016;38:853–857. doi: 10.3109/09638288.2015.1062926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . International Classification of Functioning, Disability, and Health: ICF. World Health Organization; Geneva, Switzerland: 2001. [Google Scholar]
- 3.Moore JL, Potter K, Blankshain K, Kaplan SL, O'Dwyer LC, Sullivan JE. A core set of outcome measures for adults with neurologic conditions undergoing rehabilitation: a clinical practice guideline. J Neurol Phys Ther. 2018;42:174–220. doi: 10.1097/NPT.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holleran CL, Bland MD, Reisman DS, Ellis TD, Earhart GM, Lang CE. Day-to-day variability of walking performance measures in individuals poststroke and individuals with Parkinson disease. J Neurol Phys Ther. 2020;44:241–247. doi: 10.1097/NPT.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang CE, Holleran CL, Strube MJ, et al. Improvement in the capacity for activity versus improvement in performance of activity in daily life during outpatient rehabilitation. J Neurol Phys Ther. 2023;47:16–25. doi: 10.1097/NPT.0000000000000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang CE, Barth J, Holleran CL, Konrad JD, Bland MD. Implementation of wearable sensing technology for movement: pushing forward into the routine physical rehabilitation care field. Sensors. 2020;20:5744. doi: 10.3390/s20205744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen RT, Korfitsen CB, Juhl CB, Andersen HB, Langberg H, Christensen J. Criterion validity for step counting in four consumer-grade physical activity monitors among older adults with and without rollators. Eur Rev Aging Phys Act. 2020;17:1. doi: 10.1186/s11556-019-0235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rozanski GM, Aqui A, Sivakumaran S, Mansfield A. Consumer wearable devices for activity monitoring among individuals after a stroke: a prospective comparison. JMIR Cardio. 2018;2:e1. doi: 10.2196/cardio.8199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke CL, Taylor J, Crighton LJ, Goodbrand JA, McMurdo MET, Witham MD. Validation of the AX3 triaxial accelerometer in older functionally impaired people. Aging Clin Exp Res. 2017;29:451–457. doi: 10.1007/s40520-016-0604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act. 2015;12:159. doi: 10.1186/s12966-015-0314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fokkema T, Kooiman TJ, Krijnen WP, Van Der Schans CP, Groot MDE. Reliability and validity of ten consumer activity trackers depend on walking speed. Med Sci Sports Exerc. 2017;49:793–800. doi: 10.1249/MSS.0000000000001146. [DOI] [PubMed] [Google Scholar]
- 12.Tophoj KH, Petersen MG, Saebye C, Baad-Hansen T, Wagner S. Validity and reliability evaluation of four commercial activity trackers’ step counting performance. Telemed J E Health. 2018;24:669–677. doi: 10.1089/tmj.2017.0264. [DOI] [PubMed] [Google Scholar]
- 13.Duclos NC, Aguiar LT, Aissaoui R, Faria C, Nadeau S, Duclos C. Activity monitor placed at the nonparetic ankle is accurate in measuring step counts during community walking in poststroke individuals: a validation study. PM & R. 2019;11:963–971. doi: 10.1002/pmrj.12080. [DOI] [PubMed] [Google Scholar]
- 14.Macko RF, Haeuber E, Shaughnessy M, et al. Microprocessor-based ambulatory activity monitoring in stroke patients. Med Sci Sports Exerc. 2002;34:394–399. doi: 10.1097/00005768-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Fulk GD, Combs SA, Danks KA, Nirider CD, Raja B, Reisman DS. Accuracy of 2 activity monitors in detecting steps in people with stroke and traumatic brain injury. Phys Ther. 2014;94:222–229. doi: 10.2522/ptj.20120525. [DOI] [PubMed] [Google Scholar]
- 16.Wendel N, Macpherson CE, Webber K, et al. Accuracy of activity trackers in Parkinson disease: should we prescribe them? Phys Ther. 2018;98:705–714. doi: 10.1093/ptj/pzy054. [DOI] [PubMed] [Google Scholar]
- 17.Maganja SA, Clarke DC, Lear SA, Mackey DC. Formative evaluation of consumer-grade activity monitors worn by older adults: test-retest reliability and criterion validity of step counts. JMIR Form Res. 2020;4:e16537. doi: 10.2196/16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobkin BH. Behavioral self-management strategies for practice and exercise should be included in neurologic rehabilitation trials and care. Curr Opin Neurol. 2016;29:693–699. doi: 10.1097/WCO.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleffner I, Duning T, Lohmann H, et al. A brief review of Susac syndrome. J Neurol Sci. 2012;322:35–40. doi: 10.1016/j.jns.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Dörr J. Encephalopathy, visual disturbance and hearing loss—recognizing the symptoms of Susac syndrome. Nat Rev Neurol. 2009;5:683–688. doi: 10.1038/nrneurol.2009.176. [DOI] [PubMed] [Google Scholar]
- 21.Vishnevskia-Dai V, Chapman J, Sheinfeld R, et al. Susac syndrome: clinical characteristics, clinical classification, and long-term prognosis. Medicine (Baltimore) 2016;95:e5223. doi: 10.1097/MD.0000000000005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aubart-Cohen F, Klein I, Alexandra JF, et al. Long-term outcome in Susac syndrome. Medicine (Baltimore) 2007;86:93–102. doi: 10.1097/MD.0b013e3180404c99. [DOI] [PubMed] [Google Scholar]
- 23.Papo T, Biousse V, Lehoang P, et al. Susac syndrome. Medicine (Baltimore) 1998;77:3–11. doi: 10.1097/00005792-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Rennebohm RM, Asdaghi N, Srivastava S, Gertner E. Guidelines for treatment of Susac syndrome—an update. Int J Stroke. 2020;15:484–494. doi: 10.1177/1747493017751737. [DOI] [PubMed] [Google Scholar]
- 25.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–M34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 26.Myers AM, Powell LE, Maki BE, Holliday PJ, Brawley LR, Sherk W. Psychological indicators of balance confidence: relationship to actual and perceived abilities. J Gerontol A Biol Sci Med Sci. 1996;51:M37–M43. doi: 10.1093/gerona/51a.1.m37. [DOI] [PubMed] [Google Scholar]
- 27.Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol A Biol Sci Med Sci. 1998;53:M287–M294. doi: 10.1093/gerona/53a.4.m287. [DOI] [PubMed] [Google Scholar]
- 28.Whitney SL, Wrisley DM, Marchetti GF, Gee MA, Redfern MS, Furman JM. Clinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the Five-Times-Sit-to-Stand Test. Phys Ther. 2005;85:1034–1045. [PubMed] [Google Scholar]
- 29.Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26:15–19. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- 30.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 31.Walker ML, Austin AG, Banke GM, et al. Reference group data for the functional gait assessment. Phys Ther. 2007;87:1468–1477. doi: 10.2522/ptj.20060344. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt AL, Pennypacker ML, Thrush AH, Leiper CI, Craik RL. Validity of the StepWatch Step Activity Monitor: preliminary findings for use in persons with Parkinson disease and multiple sclerosis. J Geriatr Phys Ther. 2011;34:41–45. doi: 10.1519/JPT.0b013e31820aa921. [DOI] [PubMed] [Google Scholar]
- 33.Roos MA, Rudolph KS, Reisman DS. The structure of walking activity in people after stroke compared with older adults without disability: a cross-sectional study. Phys Ther. 2012;92:1141–1147. doi: 10.2522/ptj.20120034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97:182–189. doi: 10.1016/j.physio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Casanova C, Celli BR, Barria P, et al. The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37:150–156. doi: 10.1183/09031936.00194909. [DOI] [PubMed] [Google Scholar]
- 36.Scheets P, Crowner N, McGee B, Norton P, Sahrmann B, Stith S. Movement System Diagnoses Neuromuscular Conditions. Washington University in St. Louis School of Medicine; 2015. p. 55.
- 37.Avers D, Wong RA. Guccione’s geriatric physical therapy. Fourth edition. Elsevier, Inc; 2020. p. 718. [Google Scholar]
- 38.Quinn L, Riley N, Tyrell CM, et al. A framework for movement analysis of tasks: recommendations from the Academy of Neurologic Physical Therapy's Movement System Task Force. Phys Ther. 2021;101:pzab154. doi: 10.1093/ptj/pzab154. [DOI] [PubMed] [Google Scholar]
- 39.Saunders DH, Greig CA, Mead GE. Physical activity and exercise after stroke: review of multiple meaningful benefits. Stroke. 2014;45:3742–3747. doi: 10.1161/STROKEAHA.114.004311. [DOI] [PubMed] [Google Scholar]
- 40.Jones F, Mandy A, Partridge C. Changing self-efficacy in individuals following a first time stroke: preliminary study of a novel self-management intervention. Clin Rehabil. 2009;23:522–533. doi: 10.1177/0269215508101749. [DOI] [PubMed] [Google Scholar]
- 41.Bailey RR. Goal setting and action planning for health behavior change. Am J Lifestyle Med. 2019;13:615–618. doi: 10.1177/1559827617729634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 43.Dusseldorp E, van Genugten L, van Buuren S, Verheijden MW, van Empelen P. Combinations of techniques that effectively change health behavior: evidence from Meta-CART analysis. Health Psychol. 2014;33:1530–1540. doi: 10.1037/hea0000018. [DOI] [PubMed] [Google Scholar]
- 44.Piercy KL, Troiano RP. Physical activity guidelines for Americans from the US department of health and human services: cardiovascular benefits and recommendations. Circ Cardiovasc Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.118.005263. [DOI] [PubMed] [Google Scholar]
- 45.Fulk GD, Echternach JL, Nof L, O'Sullivan S. Clinometric properties of the six-minute walk test in individuals undergoing rehabilitation poststroke. Physiother Theory Pract. 2008;24:195–204. doi: 10.1080/09593980701588284. [DOI] [PubMed] [Google Scholar]
- 46.Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37:75–82. doi: 10.1080/16501970410017215. [DOI] [PubMed] [Google Scholar]
- 47.Fulk GD, He Y, Boyne P, Dunning K. Predicting home and community walking activity poststroke. Stroke. 2017;48:406–411. doi: 10.1161/STROKEAHA.116.015309. [DOI] [PubMed] [Google Scholar]
- 48.Fulk GD, Lopez-Meyer P, Sazonov ES. Characterizing walking activity in people with stroke. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:5211–5214. doi: 10.1109/IEMBS.2011.6091289. [DOI] [PubMed] [Google Scholar]
- 49.Aaboud M, Aad G, Abbott B, et al. Jet reconstruction and performance using particle flow with the ATLAS Detector. Eur Phys J C Part Fields. 2017;77:466. doi: 10.1140/epjc/s10052-017-5031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bland MD, Whitson M, Harris H, et al. Descriptive data analysis examining how standardized assessments are used to guide post-acute discharge recommendations for rehabilitation services after stroke. Phys Ther. 2015;95:710–719. doi: 10.2522/ptj.20140347. [DOI] [PubMed] [Google Scholar]
- 51.Bland MD, Sturmoski A, Whitson M, et al. Prediction of discharge walking ability from initial assessment in a stroke inpatient rehabilitation facility population. Arch Phys Med Rehabil. 2012;93:1441–1447. doi: 10.1016/j.apmr.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henderson CE, Fahey M, Brazg G, Moore JL, Hornby TG. Predicting discharge walking function with high-intensity stepping training during inpatient rehabilitation in nonambulatory patients poststroke. Arch Phys Med Rehabil. 2022;103:S189–S196. doi: 10.1016/j.apmr.2020.10.127. [DOI] [PubMed] [Google Scholar]
- 53.Vaughan-Graham J, Cott C, Wright FV. The Bobath (NDT) concept in adult neurological rehabilitation: what is the state of the knowledge? A scoping review. Part I: conceptual perspectives. Disabil Rehabil. 2015;37:1793–1807. doi: 10.3109/09638288.2014.985802. [DOI] [PubMed] [Google Scholar]
- 54.Kollen BJ, Lennon S, Lyons B, et al. The effectiveness of the Bobath concept in stroke rehabilitation: what is the evidence? Stroke. 2009;40:e89–e97. doi: 10.1161/STROKEAHA.108.533828. [DOI] [PubMed] [Google Scholar]
- 55.Sim I. Mobile devices and health. N Engl J Med. 2019;381:956–968. doi: 10.1056/NEJMra1806949. [DOI] [PubMed] [Google Scholar]
- 56.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Danks KA, Pohlig R, Reisman DS. Combining fast-walking training and a step activity monitoring orogram to improve daily walking activity after stroke: a preliminary study. Arch Phys Med Rehabil. 2016;97(9 Suppl):S185–S193. doi: 10.1016/j.apmr.2016.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danks KA, Roos MA, McCoy D, Reisman DS. A step activity monitoring program improves real world walking activity post stroke. Disabil Rehabil. 2014;36:2233–2236. doi: 10.3109/09638288.2014.903303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cavanaugh JT, Ellis TD, Earhart GM, Ford MP, Foreman KB, Dibble LE. Capturing ambulatory activity decline in Parkinson's disease. J Neurol Phys Ther. 2012;36:51–57. doi: 10.1097/NPT.0b013e318254ba7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fielding RA, Guralnik JM, King AC, et al. Dose of physical activity, physical functioning and disability risk in mobility-limited older adults: results from the LIFE study randomized trial. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kraus WE, Janz KF, Powell KE, et al. Daily step counts for measuring physical activity exposure and its relation to health. Med Sci Sports Exerc. 2019;51:1206–1212. doi: 10.1249/MSS.0000000000001932. [DOI] [PMC free article] [PubMed] [Google Scholar]