Abstract
Background
Graves' disease is the most common cause of hyperthyroidism. Both antithyroid medications and radioiodine are commonly used treatments but their frequency of use varies between regions and countries. Despite the commonness of the diagnosis, any possible differences between the two treatments with respect to long‐term outcomes remain unknown.
Objectives
To assess the effects of radioiodine therapy versus antithyroid medications for Graves' disease.
Search methods
We performed a systematic literature search in the Cochrane Library, MEDLINE and EMBASE and the trials registers ICTRP Search Portal and ClinicalTrials.gov. The date of the last search was September 2015 for all databases.
Selection criteria
Randomised controlled trials (RCTs) comparing the effects of radioiodine therapy versus antithyroid medications for Graves' disease with at least two years follow‐up.
Data collection and analysis
Two authors independently screened titles and abstracts for relevance. One author carried out screening for inclusion, data extraction and 'Risk of bias' assessment and a second author checked this. We presented data not suitable for meta‐analysis as descriptive data. We analysed the overall quality of evidence utilising the GRADE instrument.
Main results
We included two RCTs involving 425 adult participants with Graves' disease in this review. Altogether 204 participants were randomised to radioiodine therapy and 221 to methimazole therapy. A single dose of radioiodine was administered. The duration of methimazole medication was 18 months. The period of follow‐up was at least two years, depending on the outcome measured. For most outcome measures risk of bias was low; for the outcomes health‐related quality of life as well as development and worsening of Graves' ophthalmopathy risks of performance bias and detection bias were high in at least one of the two RCTs.
Health‐related quality of life appeared to be similar in the radioiodine and methimazole treatment groups, however no quantitative data were reported (425 participants; 2 trials; low quality evidence). The development and worsening of Graves' ophthalmopathy was observed in 76 of 202 radioiodine‐treated participants (38%) and in 40 of 215 methimazole‐treated participants (19%): risk ratio (RR) 1.94 (95% confidence interval (CI) 1.40 to 2.70); 417 participants; 2 trials; low quality evidence. A total of 35% to 56% of radioiodine‐treated participants and 42% of participants treated with methimazole were smokers, which is associated with the risk of worsening or development of Graves' ophthalmopathy. Euthyroidism was not achieved by any participant being treated with radioiodine compared with 64/68 (94%) of participants after methimazole treatment (112 participants; 1 trial). In this trial thyroxine therapy was not introduced early in both treatment arms to avoid hypothyroidism. Recurrence of hyperthyroidism (relapse) in favour of radioiodine treatment showed a RR of 0.20 (95% CI 0.01 to 2.66); P value = 0.22; 417 participants; 2 trials; very low quality evidence. Heterogeneity was high (I² = 91%) and the RRs were 0.61 or 0.06 with non‐overlapping CIs. Adverse events other than development of worsening of Graves' ophthalmopathy for radioiodine therapy were hypothyroidism (39 of 41 participants (95%) compared with 0% of participants receiving methimazole, however thyroxine treatment to avoid hypothyroidism was not introduced early in the radioiodine group ‐ 104 participants; 1 trial; very low quality evidence) and drug‐related adverse events for methimazole treatment (23 of 215 participants (11%) reported adverse effects likely related to methimazole therapy ‐ 215 participants; 2 trials; very low quality evidence). The outcome measures all‐cause mortality and bone mineral density were not reported in the included trials. One trial (174 participants) reported socioeconomic effects: costs based on the official hospital reimbursement system in Sweden for patients without relapse and methimazole treatment were USD 1126/1164 (young/older methimazole group) and for radioiodine treatment USD 1862. Costs for patients with relapse and methimazole treatment were USD 2284/1972 (young/older methimazole group) and for radioiodine treatment USD 2760.
Authors' conclusions
The only antithyroid drug investigated in the two included trials was methimazole, which might limit the applicability of our findings with regard to other compounds such as propylthiouracil. Results from two RCTs suggest that radioiodine treatment is associated with an increased risk of Graves' ophthalmopathy. Our findings suggest some benefit from radioiodine treatment for recurrence of hyperthyroidism (relapse) but there is uncertainty about the magnitude of the effect size.
Plain language summary
Radioiodine therapy versus antithyroid medications for Graves' disease
Review question
Is radioiodine treatment better than antithyroid drugs for the therapy of Graves' disease?
Background
Graves' disease is an autoimmune disorder, which means that the body's immune systems attacks the body itself by means of antibodies. These antibodies stimulate the thyroid gland to produce and secrete excessive amounts of thyroid hormones (hyperthyroidism). Graves' disease is the most common cause of hyperthyroidism. Other typical characteristics of Graves' disease are goitre and eye disease (Graves' ophthalmopathy or orbitopathy). Currently, antithyroid medications such as methimazole or propylthiouracil and radioactive iodine (radioiodine, given either in a capsule or in a tasteless solution in water) are most often used for the treatment of Graves' disease. Radioiodine destroys most cells in the thyroid gland, so that secretion of thyroid hormones is massively reduced. Antithyroid medications block the production of thyroid hormones, also leading to a decrease in the production of these hormones.
Study characteristics
We identified two randomised controlled trials (RCTs) enrolling 425 adult participants with Graves' disease. Altogether 204 participants were randomised to treatment with radioiodine and 221 to methimazole. Follow‐up in the included studies was from 2 to 21 years depending on which outcome was investigated.
Key results
Study authors reported health‐related quality of life to be similar in the radioiodine and methimazole treatment groups, however exact data were not published.
The development and worsening of Graves' opthalmopathy, which is a serious eye disease that happens in some people with Graves' disease, was observed in 361 of 1000 radioiodine‐treated persons compared with 186 of 1000 methimazole‐treated persons. A high percentage of the study participants were smokers, which is related to the risk of worsening or development of Graves' opthalmopathy.
A normal thyroid gland function (euthyroidism) was not achieved by any participant who was treated with radioiodine compared with 64 of 68 participants (94%) after methimazole treatment. In this study thyroid hormone (thyroxine) therapy was not started early in both treatment arms to accomplish euthyroidism.
A recurrence of hyperthyroidism (relapse of the disease) was seen more often after methimazole treatment, however the size of the effect is unclear because of big differences between the two included studies.
A side effect related to radioiodine therapy was an underactive thyroid gland (hypothyroidism), which was seen in 39 of 41 participants (95%) compared with 0% of participants receiving methimazole. Again, in this study thyroxine therapy, with the aim to avoid hypothyroidism, was not given early in the radioiodine group. Side effects related to methimazole treatment were seen in 23 of 215 participants (11%). The outcomes death from any cause and bone mineral density were not reported in the included studies.
One study reported socioeconomic effects: costs based on the official hospital reimbursement system in Sweden for people without relapse and methimazole treatment were between USD 1126 and USD 1164, and for radioiodine treatment USD 1862. Costs for people with relapse and methimazole treatment were between USD 1972 and USD 2284, and for radioiodine treatment USD 2760.
Quality of the evidence
The overall quality of the evidence was low to very low mainly because there were just one or two studies per outcome and the number of participants was small. Another limitation is that among antithyroid medications only methimazole was investigated, which might limit the applicability of our findings with regard to other medications such as propylthiouracil.
Currentness of evidence
This evidence is up to date as of September 2015.
Summary of findings
for the main comparison.
| Radioiodine therapy compared with antithyroid medications for Graves' disease | ||||||
|
Patient: participants with Graves' disease Settings: outpatients Intervention: radioiodine Comparison: methimazole | ||||||
| Outcomes | Assumed risk | Corresponding risk | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Methimazole | Radioiodine | |||||
|
Health‐related quality of life
[measured by the validated questionnaire SF‐36] Follow‐up: 4 years and 14 to 21 years |
See comment | See comment | See comment | 425 (2) | See comment | 2 trials assessed this outcome but no quantitative data for comparisons between intervention groups were provided; trial authors in 1 trial reported that there were no differences in the results of the SF‐36 scores between the 2 treatment groups |
| Development and worsening of Graves' ophthalmopathy [examination by ophthalmologists] Follow‐up: 2 years and 4 years | 186 per 1000 | 361 of 1000 (260 to 502) | RR 1.94 (1.40 to 2.70) | 417 (2) | ⊕⊕⊝⊝ lowa | ‐ |
|
Individuals in euthyroid state
[measured by serum thyroid hormone levels within the normal range] Follow‐up: at least 4 years |
See comment | See comment | See comment | 112 (1) | See comment | No participant who underwent radioiodine treatment achieved an euthyroid state compared to 4/68 participants not becoming euthyroid treated by methimazole (Tallstedt 1992); however, in this trial thyroxine therapy was not introduced early in both treatment arms to avoid hypothyroidism |
|
Recurrence of hyperthyroidism (relapse)
[measured by serum thyroid hormone levels above the normal range after withdrawal of methimazole or end of radioiodine treatment] Follow‐up: at least 4 years |
256 per 1000 | 51 of 1000 (3 to 680) | RR 0.20 (0.01 to 2.66) | 417 (2) | ⊕⊝⊝⊝ very lowb | ‐ |
|
Adverse events other than development or worsening of Graves' disease (a) Hypothyroidism [measured by TSH and/or thyroid hormones] (b) Drug reactions Follow‐up: (a) at least 2 years (b) at least 4 years |
See comment | See comment | See comment | (a) 104 (1) (b) 215 (2) |
a) ⊕⊝⊝⊝
very lowc b) ⊕⊝⊝⊝ very lowd |
(a) 39 of 41 participants developed hypothyroidism after radioiodine treatment for Graves' disease, compared with 0 of 65 participants receiving methimazole (Tallstedt 1992); however, in this trial thyroxine was not introduced early in both treatment groups in order to avoid hypothyroidism (b) 23 of 215 participants (11%) reported adverse effects likely related to methimazole treatment |
|
All‐cause mortality Follow‐up: at least 4 years and 14 to 21 years |
See comment | See comment | See comment | 425 (2) | See comment | No quantitative data for all‐cause mortality were reported |
|
Socioeconomic effects
[costs per patient, based on the official hospital reimbursement system in Sweden] Follow‐up: 2 years |
See comment | See comment | See comment | 112 (1) | See comment | Costs for patients without relapse and methimazole treatment were USD 1126/USD 1164 (young/older methimazole group) and for radioiodine treatment USD 1862 Costs for patients with relapse and methimazole treatment were USD 2284/1972 (young/older methimazole group) and for radioiodine treatment USD 2760 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SF‐36: 36‐item Short Form Health Status Survey; TSH: thyroid‐stimulating hormone | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Assumed risk was derived from the event rates in the comparator groups. aDowngraded by two levels: one level because of performance and detection bias and one level because of imprecision (see Appendix 12). bDowngraded by three levels: two levels because of serious inconsistency and one level because of imprecision (see Appendix 12). cDowngraded by three levels: one level because of indirectness and two levels because of imprecision (see Appendix 12). cDowngraded by three levels: one level because of risk of bias, one level because of indirectness and one level because of imprecision (see Appendix 12).
Background
Description of the condition
Epidemiology and pathogenesis of Graves' disease
Graves' disease is an autoimmune disease caused by thyroid‐stimulating hormone (TSH) receptor antibodies, which stimulate the gland to synthesise and secrete excess amounts of thyroid hormones. Graves' disease is the most common cause of hyperthyroidism. It is characterised by thyrotoxaemia, developing as a result of a complex interaction between pre‐disposing genes and environmental triggers. The annual incidence of Graves' disease is about 15 per 100,000 to 80 per 100,000 in various parts of the world (Berglund 1996; Mostbeck 1998; Tallstedt 1992; Vanderpump 1995).
Clinical manifestations of Graves' disease
The classic clinical manifestations of Graves' disease include nervousness, emotional lability, fatigue, weight loss, restless sleep, rapid heartbeat, increased bowel frequency and increased anxiety. The signs include diffuse goitre, ophthalmopathy or orbitopathy, dermopathy (pretibial or localised myxoedema), tachycardia and tremor of the outstretched hands. In some persons, particularly those with severe disease or a prolonged period before diagnosis, evidence of proximal muscle wasting may be present. Graves' ophthalmopathy has a variable clinical presentation such as eyelid retraction, proptosis, peri‐orbital oedema, chemosis and disturbances of ocular motility. In some cases, Graves' ophthalmopathy may progress to visual loss as a result of exposure to keratopathy or compressive optic neuropathy.
Diagnosis of Graves' disease
Graves' disease is an autoimmune disorder characterised by hyperthyroidism, diffuse goitre, ophthalmopathy and, rarely, dermopathy (Ginsberg 2003). The hyperthyroidism of Graves' disease may be clinically overt, biochemically apparent or subclinical. All patients exhibit a low or, in most cases, suppressed TSH. Increased 24‐hour radioiodine uptake and circulating TSH receptor antibodies can confirm the diagnosis.
Description of the intervention
Treatments for Graves' disease include antithyroid drugs, radioiodine and thyroidectomy. Currently, antithyroid drugs and radioiodine are most commonly used for the treatment of Graves' disease. Very often the choice has to be made between prolonged treatment with antithyroid drugs and lifetime therapy with thyroid hormones for thyroid failure after radioiodine therapy. In spite of almost 60 years experience in the use of antithyroid drugs and radioiodine for the treatment of Graves' disease, the treatment policy varies considerably within and between countries (Ford 1991; Wartofsky 1991). In the USA, radioiodine has been the therapy most preferred by physicians. In Europe and Japan, there has been a greater physician preference for antithyroid drugs, surgery or both (Wartofsky 1991).
Radioiodine therapy for Graves' disease
At present, radioiodine is the most commonly used treatment for Graves' disease in adults because of its proclaimed safety, ease of administration (Becker 1984), efficacy and low expense (Kendall‐Taylor 1984; Watson 1988). Radioiodine treatment is also effective for Graves' disease in children, but a significantly higher incidence of hypothyroidism compared with antithyroid drugs has been observed (Ma 2008). Definite contraindications to radioiodine therapy include pregnancy, lactation, coexisting thyroid cancer or suspicion of thyroid cancer, individuals being unable to comply with radiation safety guidelines and females planning a pregnancy within four to six months (Bahn 2011). Iodine allergy is not a contraindication to radioiodine therapy (Biersack 2007).
Antithyroid drugs for Graves' disease
Antithyroid drugs do not cure Graves' disease. Their major clinical problem is the 20% to 70% relapse of hyperthyroidism following discontinuation of treatment (Allannic 1990; Reinwein 1993; Vitti 1997). Therefore, antithyroid drugs are currently recommended for:
individuals with a high likelihood of remission (persons, especially females, with mild disease, small goitres and negative or low‐titre of thyroid receptor antibodies (TRAb));
elderly people or others with comorbidities that increase surgical risk or with limited life expectancy;
individuals with lack of access to an experienced thyroid surgeon;
persons with moderate to severe active Graves' ophthalmopathy (Bahn 2011).
Definite contraindications to long‐term antithyroid drug therapy include previous known major adverse reactions to them.
The thioamide drugs methimazole and propylthiouracil are the most often used antithyroid drugs for Graves' disease. The two drugs are actively transported into the thyroid gland and inhibit important steps in the thyroid hormone synthesis (Cooper 1984). The optimal duration of antithyroid drugs for the titration regimen is 12 to 18 months. The titration (low‐dose) regimen has fewer adverse effects than the block‐replace (high‐dose) regimen and is no less effective (Abraham 2010). Continued thyroxine treatment following initial antithyroid drug treatment does not appear to provide any benefit in terms of recurrence of Graves' disease. Immunosuppressive therapies need further evaluation. Beta‐blockers are mostly used as treatment adjuncts to offer prompt relief of the adrenergic symptoms of Graves' disease such as tremor, palpitations, heat intolerance and nervousness. Calcium channel blockers such as diltiazem can be used to reduce heart rate in persons who cannot tolerate beta‐blockers (Ginsberg 2003).
Long‐term effects of radioiodine therapy versus antithyroid drugs for Graves' disease
Once it has been established that a person is hyperthyroid and the cause is Graves' disease, the individual and the physician must choose between three initial treatment options: radioiodine therapy, antithyroid drugs or thyroidectomy. However, no treatment is perfect. Normal thyroid function for Graves' disease needs long‐term use of antithyroid drugs, and hyperthyroidism may reappear after their withdrawal. On the other hand, hypothyroidism, which needs life‐long thyroid hormone replacement, is a common consequence of radioiodine therapy. Hypothyroidism occurs in 50% to 70% of individuals after low‐dose and in 90% to 100% after high‐dose radioiodine treatment (Dunn 1964; Holm 1982). The thyroid replacement treatment approach used for radioiodine‐induced hypothyroidism seems problematic. Over‐replacement of thyroxine may cause alterations in the heart (Biondi 1993; Sawin 1994), and bones (Adlin 1991; Faber 1994), and the potency, uniformity and reproducibility of thyroxine preparations may be variable (Rennie 1997). It is reported that a significant improvement in mood and neuropsychological function occurs in adult hypothyroid patients treated with a combination of thyroxine (T4) and tri‐iodothyronine (T3) compared with a higher dose of thyroxine alone (Bunevicius 1999; Bunevicius 2000). Therefore, a therapy combining administration of T4 and T3 was recommended by these authors for people with hypothyroidism. One systematic review indicated that combined T4 and T3 treatment does not improve patients' health‐related quality of life compared with T4 alone. Sole T4 replacement may remain the drug of choice for hypothyroid patients (Ma 2009). Adding these features to possible lack of patient compliance (Canaris 2000; Jones 1982) may make the long‐term precise management of hypothyroidism problematic. Therefore, it is possible that the mode of treatment for Graves' disease may influence long‐term health‐related quality of life. In addition, the possible consequences of antithyroid drugs and radioiodine with respect to long‐term clinical outcomes are insufficiently known.
Adverse effects of the intervention
Well‐known adverse effects of radioiodine for Graves' disease include vomiting and radiation‐induced thyroiditis (Clark 1995). Patients with large goitres may have transient swelling of the goitre following therapy and some discomfort may be associated with this swelling. There may be a transient rise in free T4 and free T3 levels 7 to 10 days following radioiodine treatment, and in individuals who have been poorly controlled before radioiodine therapy there may be an exacerbation of palpitations and heart failure. Graves' ophthalmopathy is an organ‐specific autoimmune process strongly linked to Graves' disease. Radioiodine therapy for Graves' disease is associated with a small but definite increased risk of development or worsening of Graves' ophthalmopathy compared with antithyroid drugs (Acharya 2008). The major adverse consequence of radioiodine therapy is hypothyroidism, which may happen in the early post‐treatment period or many years after the patient has been rendered euthyroid (Huysmanns 1994; Sridama 1984). Other adverse effects and complications of radioiodine therapy are remarkably few.
The possible minor adverse effects of antithyroid drugs include pruritus, rash, arthralgias, fever, sore throat, mouth ulcers, nausea and jaundice. Minor side effects occur in up to 13% of persons using antithyroid drugs and resolve upon discontinuing the agent. However, adverse effects can be serious, including agranulocytosis, hepatotoxicity, aplastic anaemia and vasculitis. Potentially fatal agranulocytosis occurs in 0.2% to 0.5% of patients (Ginsberg 2003). The major clinical problem of antithyroid drugs is a high relapse of hyperthyroidism following discontinuation of treatment.
How the intervention might work
Use of radioiodine as an iodide salt exploits the mechanism of uptake of iodine by the normal cells of the thyroid gland. Radioiodine is a beta‐ and gamma‐emitting radionuclide with a physical half‐life of eight days. One‐third of the emission is beta rays with a maximum energy of 0.61 MeV, an average energy of 0.192 MeV and a range in tissue of 0.8 mm. The beta emissions of radioiodine contribute heavily to the thyroidal radiation dose. Radioiodine uptake is higher in Graves' disease than in the normal thyroid. Following uptake in the thyroid cells, part or all of the gland is destroyed by the beta rays thereby achieving a reduced production of thyroid hormones. Most individuals respond to radioiodine therapy with a normalisation of thyroid function tests and clinical symptoms within four to eight weeks.
Methimazole and propylthiouracil are commonly used antithyroid drugs for treating Graves' disease and work by blocking thyroid hormone synthesis. Propylthiouracil additionally inhibits the peripheral conversion of T4 to T3. Although propylthiouracil is more commonly used in North America, there is evidence that methimazole should be the antithyroid drug of choice (except for women who are pregnant or breastfeeding) (Cooper 1984).
Why it is important to do this review
We found two systematic reviews in relation to radioiodine therapy for Graves' disease. One is about the association of radioiodine therapy with Graves' ophthalmopathy (Acharya 2008). The results from 10 randomised controlled trials (RCTs) suggested that radioiodine therapy had an increased risk of Graves' ophthalmopathy compared with antithyroid drugs (risk ratio (RR) 4.23; 95% confidence interval (CI) 2.04 to 8.77) but compared with thyroidectomy there was no increased risk (RR 1.59; 95% CI 0.89 to 2.81). The risk of severe Graves' ophthalmopathy was also increased with radioiodine therapy compared with antithyroid drugs (RR 4.35; 95% CI 1.28 to 14.73). Steroid prophylaxis was beneficial for individuals with pre‐existing Graves' ophthalmopathy. In the second review, two prospective controlled clinical trials involving 167 children were included to assess the effects of radioiodine treatment versus antithyroid drugs or thyroidectomy for Graves' disease (Ma 2009). The conclusion was that a gland‐specific lower dosage of radioiodine treatment was potentially effective for Graves' disease in children, but was associated with a higher incidence of hypothyroidism compared with antithyroid drugs. However, the short‐ and long‐term effects of radioiodine for Graves' disease in adults were not investigated. Currently, the possible differences between radioiodine and antithyroid drugs with respect to long‐term clinical outcomes are conflicting, and not mentioned in the two systematic reviews. To resolve the conflicting evidence and help people make practical decisions, we will systematically review the clinical outcomes of patients on antithyroid drugs and radioiodine treatment.
Objectives
To assess the effects of radioiodine therapy versus antithyroid medications for Graves' disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). The follow‐up period had to be at least two years after radioiodine therapy and the completion of antithyroid medications.
Types of participants
Individuals with Graves' disease.
Types of interventions
Intervention
Radioiodine therapy.
Comparator
Antithyroid medications (e.g. methimazole or propylthiouracil).
Minimum duration of intervention
The minimum duration of intervention for antithyroid medications was one year. Radioiodine was applied with one single dose.
Exclusion criteria
We excluded participants if they had acute myocardial infarction, heart failure, hepatic failure or renal failure.
Types of outcome measures
Primary outcomes
Health‐related quality of life.
Development and worsening of Graves' ophthalmopathy.
Adverse events other than development or worsening of Graves' ophthalmopathy.
Secondary outcomes
Recurrence of hyperthyroidism (relapse).
Individuals in euthyroid state.
All‐cause mortality.
Socioeconomic effects.
Bone mineral density.
Method and timing of outcome measurement
Health‐related quality of life: evaluated by a validated instrument such as 36‐Item Short‐Form Health Survey (SF‐36) and measured at 36, 72, 108, 144 and 252 months.
Development and worsening of Graves' ophthalmopathy: defined as the deterioration of soft tissue involvement, exophthalmos (change 2 mm or mor), extraocular‐muscle involvement and visual acuity, measured at 12 and 24 months.
Adverse events other than development or worsening of Graves' ophthalmopathy: hypothyroidism (especially after radioiodine therapy) and drug toxicity, measured at any time after randomisation.
Recurrence of hyperthyroidism (relapse): defined as decreased TSH and/or FT3, FT4 or T3 above the lower limit of the reference range and measured at 3, 6, 12 and 24 months after withdrawal of antithyroid drugs.
Individuals in euthyroid state: defined as TSH, FT3 and FT4 within the reference range and measured at 3, 6, 12 and 24 months.
All‐cause mortality: defined as death from any cause during treatment and measured at any time during treatment/follow‐up.
Socioeconomic effects: defined as costs and measured at 12 and 24 months from the day of randomisation.
Bone mineral density: defined as T or Z value detected by dual‐energy X‐ray absorptiometry (DXA) and measured at 12 and 24 months from the day of randomisation.
The follow‐up of radioiodine therapy and antithyroid medications for Graves' disease had to be at least two years.
Summary of findings
We present a 'Summary of findings' table to report the following outcomes, listed according to priority.
Health‐related quality of life.
Development and worsening of Graves' ophthalmopathy.
Individuals in euthyroid state.
Recurrence of hyperthyroidism (relapse).
Adverse events other than development and worsening of Graves' ophthalmopathy.
All‐cause mortality.
Socioeconomic effects.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception to the present without language restriction:
-
Cochrane Library (2 September 2015)
Cochrane Central Register of Controlled Trials (2015, Issue 8)
Database of Abstracts of Reviews of Effect (2015, Issue 2)
Health Technology Assessment Database (2015, Issue 3)
NHS Economic Evaluation Database (2015, Issue 2)
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) 1946 to Present (2 September 2015)
EMBASE 1974 to 1 September 2015 (2 September 2015)
ClinicalTrials.gov (2 September 2015)
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (http://apps.who.int/trialsearch/) (2 September 2015), including:
Australian New Zealand Clinical Trials Registry (24 August 2015)
Chinese Clinical Trial Registry (24 August 2015)
ClinicalTrials.gov (24 August 2015)
EU Clinical Trials Register (EU‐CTR) (24 August 2015)
ISRCTN (24 August 2015)
The Netherlands National Trial Register (24 August 2015)
Brazilian Clinical Trials Registry (ReBec) (18 August 2015)
Clinical Trials Registry ‐ India (17 August 2015)
Clinical Research Information Service ‐ Republic of Korea (17 August 2015)
Cuban Public Registry of Clinical Trials (18 August 2015)
German Clinical Trials Register (10 August 2015)
Iranian Registry of Clinical Trials (4 August 2015)
Japan Primary Registries Network (17 August 2015)
Pan African Clinical Trial Registry (18 August 2015)
Sri Lanka Clinical Trials Registry (17 August 2015)
Thai Clinical Trials Register (TCTR) (18 August 2015)
We continuously applied a MEDLINE (via Ovid SP) email alert service established by the Cochrane Metabolic and Endocrine Disorders (CMED) Group to identify newly published trials using the same search strategy as described for MEDLINE (for details on search strategies, see Appendix 1). After supplying the final review draft for editorial approval, the CMED Group performed a complete updated search on all databases available at the editorial office and sent the results to the review authors. Had we identified new trials for inclusion, we would have evaluated these, incorporated the findings into our review and resubmitted another review draft (Beller 2013).
In case we detected additional relevant key words during any electronic or other searches, we would have modified the electronic search strategies to incorporate these terms and we would have documented the changes to the search strategy.
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, systematic reviews, meta‐analyses and health technology assessment reports. In addition we contacted authors of included trials to identify any further trials we may have missed.
Data collection and analysis
Selection of studies
Two review authors (CM, JWX) independently scanned the abstracts or titles, or both, of every record retrieved, to determine which trials should be assessed further. We investigated the full‐ text articles of all potentially relevant articles. We marked differences and if these studies were later included, we planned to subject the influence of the primary choice to a sensitivity analysis. If we could not resolve a disagreement, we would have categorised the trial as a 'study awaiting classification' and would have contacted the trial authors for clarification. We present an adapted Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram to show the process of trial selection (Liberati 2009).
Data extraction and management
For trials that fulfilled inclusion criteria, two review authors (SYC, JWX) independently extracted key participant and intervention characteristics. We report data on efficacy outcomes and adverse events using data extraction sheets from the CMED Group. We resolved any disagreements by discussion or, if required, by consultation with a third review author (HW) (for details see Table 2; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10).
1. Overview of study populations.
| Intervention(s) and comparator(s) | Sample sizea | Screened/eligible [N] | Randomised [N] | ITT [N] | Analysed [N] | Finishing trial [N] | Randomised finishing trial [%] | Follow‐up (extended follow‐up)b | |
| Traisk 2009 | Radioiodine | Primary endpoint was the difference in the proportion of participants with worsening or development of thyroid‐associated ophthalmopathy during a 4‐year follow‐up in the 2 groups (intention‐to‐treat analysis). A comparison (0.05, two‐tailed test) of the binomial proportions between 2 groups of 300 patients each would give more than 90% probability (power) to detect a true difference of 10% | 482/333 | ‐ | 163 | 163 | 163 | ‐ | 24 months (4 years) |
| Methimazole | ‐ | 150 | 150 | 150 | ‐ | ||||
| total: | 333 | 313 | 313 | 313 | 94 | ||||
| Tallstedt 1992e | Radioiodine | ‐ | 179 | 41 | ‐ | 39f | 39 | 95 | At least 24 months (at least 48 months; 3 years; 5 years; 14‐21 years)g |
| Methimazole | 71 | 65f | 64 | 90 | |||||
| total: | 112 | 104 | 103 | 92 | |||||
| Grand total | All radioiodine‐treated participants | 204 | |||||||
| All participants treated with methimazole | 221 | ||||||||
| All interventions | 425 | ||||||||
"‐" denotes not reported aInformation about power calculation, sample size etc. in trial publication or report. bFollow‐up under randomised conditions until end of trial or, if not available, duration of intervention; extended follow‐up refers to follow‐up of participants once the original study was terminated as specified in the power calculation. cEnrollment in the study started in May 1996. By the second half of 2002, it was obvious that the inclusion rate was too slow to obtain the full number of participants within a reasonable period of time and the study was closed in 2003. This meant that with the above specifications and the number of observations obtained so far (333 enrolled participants), the study had a power of about 70%. With the 4‐year clinical follow‐up, the study was terminated by the end of 2007. dThe cumulative drop‐out (last observation carried forward) from the ophthalmological follow‐up in the radioiodine group and the medical treatment group, respectively, was as follows: at 1 year, 3% and 1%; at 2 years, 6% and 3%; and at 3 years, 10% and 9%, respectively. At 4 years (i.e. after protocol for ophthalmological follow‐up), 20% of the participants in both groups were still followed by ophthalmologists. eParticipants were stratified into two age groups (20 to 34 years (group 1) and 35 to 55 years (group 2)). Participants in group 1 were randomised to treatment with methimazole for 18 months or subtotal thyroidectomy, participants in group 2 to methimazole, subtotal thyroidectomy or radioiodine treatment. Numbers in the table reflect the participants being treated with methimazole or radioiodine. fWith regard to occurrence of ophthalmopathy within two years after the initiation of therapy. gDifferent follow‐up times according to various publications of extended follow‐up.
ITT: intention‐to‐treat
We provide information about potentially relevant ongoing trials including trial identifier in the 'Characteristics of ongoing trials' table and in a joint appendix 'Matrix of trial endpoint (publications and trial documents)' (Appendix 5). We tried to find the protocol for each included trial and report primary, secondary and other outcomes in comparison with data in publications in a joint appendix.
We emailed all authors of included trials to enquire whether they were willing to answer questions regarding their trials; Appendix 11 shows the results of this survey. We thereafter sought relevant missing information on the trial from the primary author(s) of the article, if required.
Dealing with duplicate and companion publications
We maximised the yield of information by simultaneous evaluation of all available data in five companion papers of Tallstedt 1992 and one companion paper of Traisk 2009.
Data from clinical trial registers
In case data from included trials were available as study results in clinical trial registers such as ClinicalTrials.gov or similar sources we would have made full use of this information and extracted data. If there was also a full publication of the trial we planned to collate and critically appraise all available data. If an included trial was marked as a completed study in a clinical trial register but no additional information was available we would have added this trial to the table 'Characteristics of studies awaiting classification'.
Assessment of risk of bias in included studies
Two review authors (CM, JWX) independently assessed the risk of bias of each included trial. We resolved any disagreements by consensus, or by consultation with a third review author (HW). In cases of disagreement, we consulted the rest of the group and made a judgement based on consensus.
We used the Cochrane 'Risk of bias' assessment tool (Higgins 2011a; Higgins 2011b), and judged 'Risk of bias' criteria as either 'low', 'high' or 'unclear' risk and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), where any of the specified criteria for a judgement on 'low', unclear' or 'high' risk of bias justifies the associated categorisation.
Random sequence generation (selection bias due to inadequate generation of a randomised sequence) ‐ assessment at trial level
We described for each included trial the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing of lots, tossing a coin, shuffling cards or envelopes, and throwing dice are adequate if performed by an independent person not otherwise involved in the trial. Use of the minimisation technique was considered as equivalent to being random.
Unclear risk of bias: insufficient information about the sequence generation process.
High risk of bias: the sequence generation method was non‐random (e.g. sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number; allocation by judgement of the clinician; allocation by preference of the participant; allocation based on the results of a laboratory test or a series of tests; allocation by availability of the intervention).
Allocation concealment (selection bias due to inadequate concealment of allocations prior to assignment) ‐ assessment at trial level
We described for each included trial the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
Low risk of bias: central allocation (including telephone, interactive voice‐recorder, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
Unclear risk of bias: insufficient information about the allocation concealment.
High risk of bias: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards; alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Blinding of participants and study personnel (performance bias due to knowledge of the allocated interventions by participants and personnel during the trial) ‐ assessment at outcome level
We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self reported, investigator‐assessed or adjudicated outcome measures (see below).
Low risk of bias: blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken; no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Unclear risk of bias: insufficient information about the blinding of participants and study personnel; the trial did not address this outcome.
High risk of bias: no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of trial participants and key personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
Blinding of outcome assessment (detection bias due to knowledge of the allocated interventions by outcome assessment) ‐ assessment at outcome level
We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self reported, investigator‐assessed or adjudicated outcome measures (see below).
Low risk of bias: blinding of outcome assessment ensured, and unlikely that the blinding could have been broken; no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding.
Unclear risk of bias: insufficient information about the blinding of outcome assessors; the trial did not address this outcome.
High risk of bias: no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data (attrition bias due to amount, nature or handling of incomplete outcome data) ‐ assessment at outcome level
We described for each included trial, and for each outcome, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the number included in the analysis at each stage (compared with the number of randomised participants per intervention/comparator groups), if reasons for attrition or exclusion were reported, and whether missing data were balanced across groups or were related to outcomes. We considered the implications of missing outcome data per outcome such as high drop‐out rates (e.g. above 15%) or disparate attrition rates (e.g. difference of 10% or more between trial arms).
Low risk of bias: no missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; appropriate methods, such as multiple imputation, were used to handle missing data.
Unclear risk of bias: insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias; the trial did not address this outcome.
High risk of bias: reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; 'as‐treated' or similar analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation.
Selective reporting (reporting bias due to selective outcome reporting) ‐ assessment at trial level
We assessed outcome reporting bias by integrating the results of the appendix 'Matrix of trial endpoints (publications and trial documents)' (Boutron 2014; Jones 2015; Mathieu 2009), with those of the appendix 'High risk of outcome reporting bias according to ORBIT classification' (Kirkham 2010). This analysis formed the basis for the judgement of selective reporting.
Low risk of bias: the trial protocol is available and all of the trial's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes (ORBIT classification).
Unclear risk of bias: insufficient information about selective reporting.
High risk of bias: not all of the trial's pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the trial report fails to include results for a key outcome that would be expected to have been reported for such a trial (ORBIT classification).
Other bias (bias due to problems not covered elsewhere) ‐ assessment at trial level
Low risk of bias: the trial appeared to be free of other sources of bias.
Unclear risk of bias: insufficient information to assess whether an important risk of bias existed; insufficient rationale or evidence that an identified problem introduced bias.
High risk of bias: had a potential source of bias related to the specific trial design used; has been claimed to have been fraudulent; had some other serious problem.
We present a 'Risk of bias' graph and a 'Risk of bias' summary figure.
We distinguished between self reported, investigator‐assessed and adjudicated outcome measures.
We defined the following endpoints as self reported outcomes.
Health‐related quality of life.
Adverse events, measured by participants.
We defined the following outcomes as investigator‐assessed outcomes.
Adverse events other than development and worsening of Graves' opthalmopathy, measured by study personnel.
Development and worsening of Graves' ophthalmopathy.
All‐cause mortality.
Individuals in euthyroid state.
Recurrence of hyperthyroidism (relapse).
Bone mineral density.
Socioeconomic effects.
Summary assessment of risk of bias
Risk of bias for a trial across outcomes: some risk of bias domains like selection bias (sequence generation and allocation sequence concealment) affect the risk of bias across all outcome measures in a trial. In case of high risk of selection bias, we would have marked all endpoints investigated in the associated trial as 'high' risk. Otherwise, we planned not to perform a summary assessment of the risk of bias across all outcomes for a trial.
Risk of bias for an outcome within a trial and across domains: we assessed the risk of bias for an outcome measure including all of the entries relevant to that outcome, i.e. both trial‐level entries and outcome‐specific entries. 'Low' risk of bias was defined as low risk of bias for all key domains, 'unclear' risk of bias as unclear risk of bias for one or more key domains and 'high' risk of bias as high risk of bias for one or more key domains.
Risk of bias for an outcome across trials and across domains: these are our main summary assessments that were incorporated in our judgements about the quality of evidence in the 'Summary of finding' tables. 'Low' risk of bias was defined as most information coming from trials at low risk of bias, 'unclear' risk of bias as most information coming from trials at low or unclear risk of bias and 'high' risk of bias as a sufficient proportion of information coming from trials at high risk of bias.
Measures of treatment effect
We expressed dichotomous data as risk ratios (RR) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MDs) with 95% CI.
Unit of analysis issues
We took into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome. One trial stratified groups according to age at entry into the trial (Tallstedt 1992). One group was 20 to 34 years old and randomly assigned to treatment with methimazole or subtotal thyroidectomy, the other group was 35 to 55 years old and was randomised to methimazole therapy or radioiodine treatment. We combined both methimazole treatment arms of this trial in the associated meta‐analyses.
Dealing with missing data
If possible, we obtained missing data from trial authors and carefully evaluated important numerical data such as screened, randomly assigned participants as well as intention‐to‐treat (ITT), and as‐treated and per‐protocol populations. We investigated attrition rates (e.g. drop‐outs, losses to follow‐up, withdrawals), and we critically appraised issues concerning missing data and imputation methods (e.g. last observation carried forward (LOCF)).
In trials where the standard deviation of the outcome was not available at follow‐up, or could be recreated, we planned to standardise by the average of the pooled baseline standard deviation from those trials in which this information was reported.
Where means and standard deviations (SDs) for outcomes were not been reported and we could not receive the needed information from trial authors, we planned to impute these values by estimating the mean and variance from the median, range and the size of the sample (Hozo 2005).
We planned to investigate the impact of imputation on meta‐analyses by performing sensitivity analyses and wanted to report per outcome which trials were included with imputed SDs.
Assessment of heterogeneity
In the event of substantial clinical or methodological heterogeneity, we planned not to report trial results as the pooled effect estimate in a meta‐analysis.
We identified heterogeneity (inconsistency) by visually inspecting the forest plots and by using a standard Chi² test with a significance level of α = 0.1. In view of the low power of this test, we also considered the I² statistic, which quantifies inconsistency across trials to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003); where an I² statistic ≥ 75% indicates a considerable level of heterogeneity (Higgins 2011a).
When we found heterogeneity, we planned to determine possible reasons for it by examining individual trial and subgroup characteristics.
Assessment of reporting biases
If we included 10 studies or more investigating a particular outcome, we wanted to use funnel plots to assess small study effects. Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias. We therefore planned to interpret results carefully (Sterne 2011).
Data synthesis
Unless good evidence showed homogeneous effects across trials, we planned to primarily summarise low risk of bias data using a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration to the whole distribution of effects. In addition, we performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Quality of evidence
We present the overall quality of the evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues related not only to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results. Two review authors (CM, JWX) independently rated the quality of evidence for each outcome. We present a summary of the evidence in a 'Summary of findings' table. This provides key information about the best estimate of the magnitude of the effect, in relative terms and as absolute differences, for each relevant comparison of alternative management strategies, numbers of participants and trials addressing each important outcome and rating of overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table on the basis of methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), by means of RevMan's table editor including Appendix 12 'Checklist to aid consistency and reproducibility of GRADE assessments' (Meader 2014), to help with standardisation of the 'Summary of findings' tables. We present results for the outcomes as described in the Types of outcome measures section. If meta‐analysis was not possible, we presented the results in a narrative format in the 'Summary of findings' table. We justified all decisions to downgrade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity, and planned to carry out the following subgroup analyses with investigation of interactions.
Antithyroid medication use before and after radioiodine therapy.
Dose of radioiodine.
Age.
Gender.
Development and worsening of Graves' ophthalmopathy in individuals with or without pre‐existing eye disease.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors (when applicable) on effect sizes by restricting the analysis to the following.
Published trials.
Taking into account risk of bias, as specified in the 'Assessment of risk of bias in included studies' section.
Very long or large trials to establish the extent to which they dominate the results.
Trials using the following filters: diagnostic criteria, imputation, language of publication, source of funding (industry versus other), or country.
We also planned to the robustness of the results by repeating the analysis using different measures of effect size (RR, OR etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
For a detailed description of studies, see the 'Characteristics of included studies' and 'Characteristics of excluded studies' sections.
Results of the search
The search identified 954 records. From these, we identified 21 full‐text articles for further examination. We excluded the other records on the basis of their titles or abstracts because they clearly did not meet the inclusion criteria or were not relevant to the question under study. After screening the full text of the selected publications, two trials with eight publications met the inclusion criteria (Tallstedt 1992; Traisk 2009) (see Figure 1).
1.
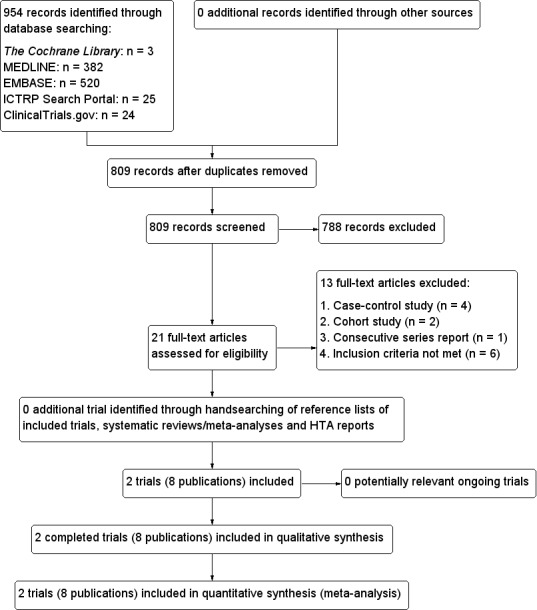
Study flow diagram.
Both trials were published in English. We contacted the authors of the included trials and received a reply from one trial author (Tallstedt 1992).
Included studies
One trial stratified groups according to age at entry into the trial (Tallstedt 1992). One group was 20 to 34 years old and randomly assigned to treatment with methimazole or subtotal thyroidectomy; the other group was 35 to 55 years old and was randomised to methimazole therapy or radioiodine treatment. We combined both methimazole treatment arms of this trial in the associated meta‐analyses.
A detailed description of the characteristics of included studies is presented elsewhere (see Characteristics of included studies and appendices). The following is a succinct overview:
Overview of study populations
We included a total of 425 participants with Graves' disease in this review; 204 participants were randomised to radioiodine and 221 to methimazole, the only drug investigated in these trials (see Table 2).
Study design and settings
The two included trials adopted a parallel‐group design and were multi‐centred, with the number of centres ranging from three (Tallstedt 1992) to eight (Traisk 2009). The trials were performed between the years 1991 and 2003.
The duration of radioiodine therapy was one day and of methimazole treatment 18 months. The duration of follow‐up was at least two years. No trial was terminated before the regular end. Both trials were conducted in Sweden and in an outpatient setting.
Participants
The two trials included participants from economically developed country; ethnic group distribution was not mentioned. The mean duration of hyperthyroidism was 11 months and reported in one trial only (Traisk 2009). Participants' gender was approximately distributed evenly in the two trials and the mean age ranged from 20 to 69 years. No trial reported on co‐morbidities of participants; smoking status ranged between 36% and 56%. Beta‐blockers were used as a co‐medication in both trials. One trial also used thyroxine after radioiodine administration (Traisk 2009).
Diagnosis
Participants were diagnosed with Graves' disease based on hyperthyroidism due to diffuse toxic goitre in the two studies. Hyperthyroidism was diagnosed based on the presence of symptoms and signs of hyperthyroidism, serum thyroid hormone levels and low thyroid‐stimulating hormone (TSH).
Interventions
No trial reported on the treatment before the start of the trial. Radioiodine was applied by the oral route in a single dose and the dosage varied between 1.4 and 4.44 MBq per gram thyroid, with an average dosage of 3.8 MBq.
Methimazole was given as 10 mg four times daily (Tallstedt 1992), or 15 mg twice daily ((Traisk 2009). For a detailed description of interventions, see Appendix 2.
Outcomes
Both trials explicitly stated primary endpoints in the publication and reported on health‐related quality of life. One trial assessed the number of participants in the euthyroid state, hypothyroidism as an adverse event and reported on socioeconomic effects (Tallstedt 1992). Both trials reported on general adverse events, the recurrence of hyperthyroidism (relapse) and on the development of Graves' ophthalmopathy. For a summary of all outcomes assessed in each study, see Appendix 5. Both trials provided a definition of most endpoint measurements.
Excluded studies
We excluded 13 publications after careful evaluation of the full text (see Characteristics of excluded studies). The reasons for exclusions were case‐control design (Berglund 1991; Chen 2005b; Mornex 1977; Patel 2006), cohort study design (Hayashi 2005; Singer 2001), consecutive series design (Berg 1996), RCTs with one‐year follow‐up only (Bartalena 1998; Chen 2005a; EI‐Kaissi 2010; Zhao 2005), a mixture of participants with Graves' disease, multinodular toxic goitre and uninodular goitre (Chen 2009), and one trial that randomised participants with recurrence of hyperthyroidism within one year after discontinuation of methimazole treatment (Azizi 2005).
Risk of bias in included studies
For details on the risk of bias of the included studies see Characteristics of included studies.
For an overview of the review authors' judgements about each risk of bias item for individual studies and across all studies see Figure 2 and Figure 3.
2.
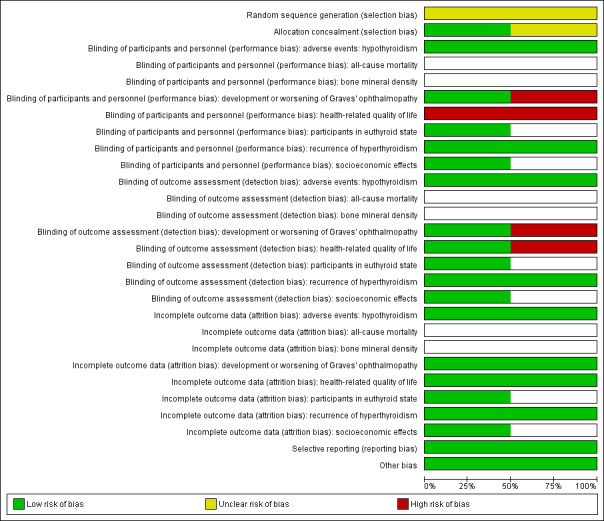
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
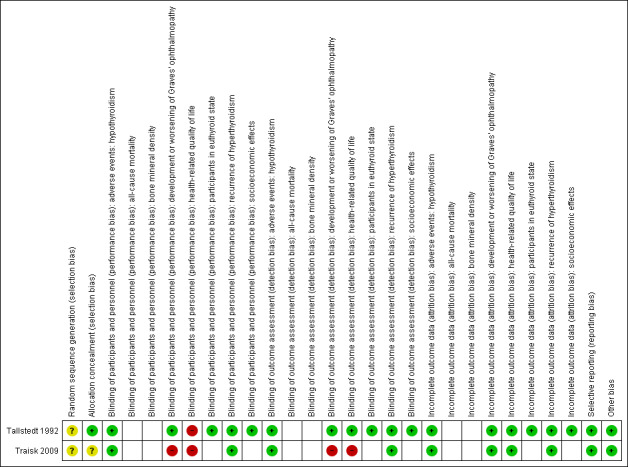
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial (blank cells indicate that the trial did not measure that particular outcome).
Allocation
Description of random sequence generation was unclear in both trials. Allocation concealment was adequately described in one trial (Tallstedt 1992).
Blinding
No trial explicitly stated that blinding of the participants and personnel was undertaken. Both trials reported that single blinding was undertaken, though it was unclear as to who was blinded and how this was achieved. Blinding of outcome assessors was undertaken in Tallstedt 1992. This resulted in a high risk of performance bias for the outcomes development and worsening of Graves' opthalmopathy (Traisk 2009), and health related‐quality of life (Tallstedt 1992; Traisk 2009). Risk of detection bias was high for the outcomes development and worsening of Graves' opthalmopathy (Traisk 2009), and health‐related quality of life (Traisk 2009).
Incomplete outcome data
One trial reported an intention‐to‐treat analysis (Tallstedt 1992). Detailed descriptions of participants' withdrawals and the reasons underpinning them were provided in both trials. One trial excluded two participants in the radioiodine group and six participants in the methimazole group with regard to occurrence of ophthalmopathy within two years after the initiation of therapy (Tallstedt 1992). In the other trial no participant was excluded from the main analysis (Traisk 2009).
Selective reporting
We rated the two trials as having low risk of reporting bias because they reported the expected outcomes. However, we did not identify trial protocols.
Other potential sources of bias
We did not detect any other potential source of bias in the two included trials.
Effects of interventions
See: Table 1
The following outcomes reflect the results of comparing radioiodine treatment with methimazole therapy.
Baseline characteristics
For details of baseline characteristics, see Appendix 3 and Appendix 4.
Primary outcomes
Health‐related quality of life
Both included trials reported on health‐related quality of life but no data for comparisons between intervention groups were provided. Follow‐up for this outcome was at least four years and 14 to 21 years.
Tallstedt 1992 reported that "no major significant differences in quality of life among the three treatments were observed. GD patients have, compared with a large Swedish reference population, diminished vital and mental quality of life aspects even many years after treatment". Health‐related quality of life also did not seem to be influenced by the thyroid hormone state of the participants. Traisk 2009 measured health‐related quality of life using the Swedish version of the SF‐36 at six time points during a 48‐month study period. In participants with and without Graves' ophthalmopathy the health‐related quality of life was similar in the radioiodine and medically treated groups. Participants who developed or had worsening of Graves' ophthalmopathy showed a decreased health‐related quality of life independent of mode of treatment. Risk of performance bias was high in both included trials. Also, one trial had a high risk of detection bias for this outcome (Traisk 2009).
Development and worsening of Graves' ophthalmopathy
Radioiodine for Graves' disease almost doubled the risk of development or worsening of Graves' ophthalmopathy(risk ratio (RR) 1.94 (95% confidence interval (CI) 1.40 to 2.70); P < 0.0001; 417 participants; 2 trials; low quality evidence (Analysis 1.1). There was a high risk of performance and detection bias for this outcome in one trial (Traisk 2009). Follow‐up for this outcome was two and four years.
1.1. Analysis.

Comparison 1 Radioiodine versus methimazole, Outcome 1 Development or worsening of Graves' ophthalmopathy.
A total of 35% to 56% of radioiodine‐treated participants and 42% of participants treated with methimazole were smokers. One study reported on the association between smoking status and development or worsening of Graves' ophthalmopathy (Traisk 2009): independent of the choice of treatment, smoking increased the risk of its worsening or development.
Adverse events
In one trial 39 of 41 participants (95%) developed hypothyroidism after radioiodine treatment for Graves' disease, compared with 0% of participants receiving methimazole (very low quality evidence) (Analysis 1.2) (Tallstedt 1992). However, in this trial, contrary to the other study (Traisk 2009), thyroxine was not introduced early in both treatment groups in order to avoid hypothyroidism. Overall risk of bias was low for this outcome.
1.2. Analysis.
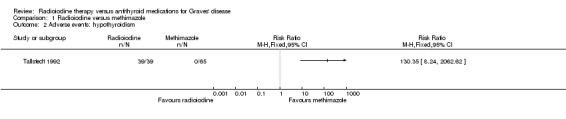
Comparison 1 Radioiodine versus methimazole, Outcome 2 Adverse events: hypothyroidism.
Altogether 23 of 215 participants (11%) treated with methimazole reported adverse effects likely related to methimazole (very low quality evidence). Risk of performance and detection bias was high for this outcome.
Follow‐up for adverse events was between at least two and at least four years. For details see Appendix 8 and Appendix 9.
Secondary outcomes
Recurrence of hyperthyroidism (relapse)
Recurrence of hyperthyroidism (relapse) in favour of radioiodine treatment had a RR of 0.20 (95% CI 0.01 to 2.66); P = 0.22; 417 participants; 2 trials; very low quality evidence (Analysis 1.3). The RRs were 0.61 and 0.06 with non‐overlapping confidence intervals (I2 = 91%). Overall risk of bias was low for this outcome and follow‐up was at least four years.
1.3. Analysis.
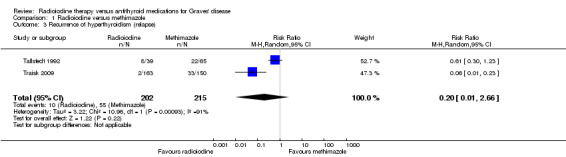
Comparison 1 Radioiodine versus methimazole, Outcome 3 Recurrence of hyperthyroidism (relapse).
Individuals in euthyroid state
No participant who underwent radioiodine treatment achieved an euthyroid state compared to 4/68 participants (6%) not becoming euthyroid who were treated with methimazole (Analysis 1.4); however, in Tallstedt 1992 thyroxine therapy was not introduced early in both treatment arms to avoid hypothyroidism with associated difficulties in achieving euthyroidism. Overall risk of bias was low for this outcome and follow‐up was at least four years.
1.4. Analysis.
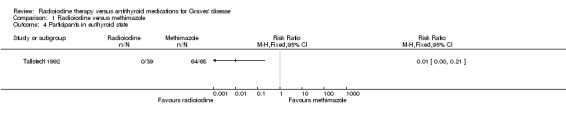
Comparison 1 Radioiodine versus methimazole, Outcome 4 Participants in euthyroid state.
All‐cause mortality
No trial reported data on all‐cause mortality. Follow‐up was at least four years and 14 to 21 years.
Socioeconomic effects
One trial reported socioeconomic effects in 174 participants (including the thyroidectomy group) (Tallstedt 1992): costs based on the official hospital reimbursement system in Sweden for patients without relapse and methimazole treatment were USD 1126/1164 (young/older methimazole group) and for radioiodine treatment USD 1862. Costs for patients with relapse and methimazole treatment were USD 2284/1972 (young/older methimazole group) and for radioiodine treatment USD 2760. Overall risk of bias was low for this outcome. Follow‐up for this outcome was two years.
Bone mineral density
No data for bone mineral density were reported
Subgroup analyses
We did not perform subgroups analyses because there were not enough included trials to estimate effects in various subgroups.
Sensitivity analyses
Sensitivity analysis was not possible due to the low number of included trials.
Assessment of reporting bias
We rated both included trials as at low risk of this bias because they reported the expected outcomes. However, no trial protocols were available.
Discussion
Summary of main results
We included two randomised controlled trials (RCTs) involving 425 adult participants with Graves' disease in this review. Altogether 204 participants were randomised to radioiodine therapy and 221 to methimazole treatment. Health‐related quality of life appeared to be similar in the radioiodine and methimazole treatment groups, however no quantitative data were reported. The only antithyroid drug investigated in the two included trials was methimazole, which might limit the applicability of our findings with regard to other compounds such as propylthiouracil. The results from the two RCTs suggest that radioiodine treatment is associated with an increased risk of Graves' opthalmopathy. Our findings suggest some benefit from radioiodine treatment for recurrence of hyperthyroidism (relapse) but there is uncertainty about the magnitude of the effect size. Adverse events other than development of worsening of Graves' ophthalmopathy for radioiodine therapy were hypothyroidism after radioiodine treatment, however thyroxine therapy to avoid anticipated hypothyroidism was not introduced early in the radioiodine group. Drug‐related adverse events for methimazole treatment were observed in 23 of 215participants (11%). The outcome measures all‐cause mortality and bone mineral density were not reported in the included trials.
Overall completeness and applicability of evidence
There are only limited data available from the two included trials comparing radioiodine therapy with methimazole for Graves' disease. Participants in both RCTs were comparable regarding gender and age. The methods for thyroid hormone measurements were the same; serum thyroxine (T4) and tri‐iodothyronine (T3) were measured by radioimmunoassay and serum thyroid‐stimulating hormone (TSH) by immuno‐radiometric assay. Inclusion and exclusion criteria were described in both trials. However, the two included trials were different in the medication before and after enrollment. In one study, radioiodine was used as the third treatment alternative (Tallstedt 1992). Apart from beta‐receptor blockers taken in both studies, Traisk 2009 used L‐thyroxine as a co‐medication 14 days after radioiodine treatment. The description of ophthalmologic outcomes varied; one study described an ophthalmopathy index score (Tallstedt 1992), the other a clinical activity score (Traisk 2009). Comorbidities were not detailed in both studies. No study reported that deaths occurred. No RCT was identified that assessed the effects of radioiodine therapy versus antithyroid medications for children with Graves' disease.
Quality of the evidence
Depending on outcome, the overall quality of evidence was low to very low, mainly because only two included studies with a limited number of participants could be evaluated. For most outcome measures risk of bias was low. However, for the outcomes health‐related quality of life as well as development and worsening of Graves' ophthalmopathy the risks of performance bias and detection bias were high in at least one of two RCTs.
Potential biases in the review process
Although we undertook a thorough literature search, there may be relevant unpublished studies or grey literature that we did not detect. Outcome reporting bias could not be thoroughly addressed because we had no access to study protocols.
Agreements and disagreements with other studies or reviews
The major clinical problem of antithyroid drugs is the 20% to 70% relapse of hyperthyroidism following discontinuation of antithyroid drugs (Allannic 1990; Reinwein 1993; Vitti 1997). Our limited data, however, suggest no substantial difference in recurrence of hyperthyroidism (relapse) between methimazole treatment and radioiodine therapy. Other authors have also reported no substantial differences in hyperthyroidism recurrence rates between these treatments after long‐term (10 years and above) continuous treatment with methimazole (Azizi 2005; Azizi 2012).
One included trial reported higher costs of radioiodine therapy for Graves' disease compared with methimazole (Tallstedt 1992). Contrarily, in Japan, radioiodine therapy was superior to the antithyroid drug therapy in terms of both cost and utility (Hayashi 2005). In the United Kingdom, the cost‐effectiveness of antithyroid drugs and radioiodine was determined for 117 patients with thyrotoxicosis. The findings indicate that the most cost‐effective primary treatment modality for hyperthyroidism is radioiodine (Patel 2006). Additionally, it was reported that radioiodine is a cost‐effective first‐line therapy in individuals with a special risk of relapse after primary conservative therapy in Germany (Dietlein 1999).
An association between radioiodine therapy for Graves' disease and the development or worsening of Graves' ophthalmopathy is often quoted. A systematic review including two RCTs, Bartalena 1998 and Tallstedt 1992, reported an increased risk of development or worsening of Graves' ophthalmopathy compared with antithyroid drugs after one‐year follow‐up (Acharya 2008). In our review, radioiodine compared with methimazole was also associated with an increased risk of Graves' ophthalmopathy. Apart from radioiodine therapy, the risk factors for the development and progression of Graves' ophthalmopathy include age, gender, genetics, smoking (Thornton 2007), thyroid dysfunction including hyperthyroidism and hypothyroidism (Kim 2004). Smoking is the most important risk factor for the occurrence and progression of Graves' ophthalmopathy (Bartalena 2012). Strong evidence for a causal association between smoking and the development of Graves' ophthalmopathy was reported in one systematic review (Thornton 2007). One of our included studies also reported on the association between smoking status and development or worsening of Graves' ophthalmopathy (Traisk 2009). Accordingly, smoking cessation should be urged for primary prevention (removal of risk factors in Graves' patients without Graves' ophthalmopathy), secondary prevention (early detection and treatment of asymptomatic or very mild Graves' ophthalmopathy) and tertiary prevention (reduction of complications and disability of overt Graves' ophthalmopathy) (Bartalena 2012).
We planned to analyse the development and worsening of Graves' ophthalmopathy in participants with or without pre‐existing eye disease caused by radioiodine or steroid use, but no trial reported suitable data.
In the two included trials with a follow‐up of at least two years, no cases of cancer were reported in both intervention groups. The incidence of thyroid cancer in a series of radioiodine‐treated patients (150 of 100,000 over a 27‐year period) did not differ substantially from the incidence in the general population (Angusti 2000). On the other hand, there are suggestions in the literature that antithyroid drugs may be carcinogens in their own right (Capen 1994; Hard 1998).
We found that no death from any cause was reported in both RCTs with at least two years of follow‐up but all‐cause mortality was not clearly addressed in either trial. However, a long‐term increase in cardiovascular and cerebrovascular deaths has been reported after radioiodine therapy (Franklyn 1998). This study also found a small increase in cancer mortality. Other long‐term studies of hyperthyroid persons have not shown a substantial increase in cancer deaths following this treatment (Ron 1998).
Authors' conclusions
Implications for practice.
Results from two randomised controlled trials in adults with Graves' disease showed that radioiodine therapy is associated with an increased risk of Graves' ophthalmopathy. Our findings suggest some benefit from radioiodine treatment for recurrence of hyperthyroidism (relapse) but there is uncertainty about the magnitude of the effect size. Hypothyroidism after radioiodine therapy was primarily seen if thyroxine treatment to avoid hypothyroidism was not given early. Drug‐related adverse events for methimazole treatment were reported in 23 of 215 participants (11%), however other antithyroid drugs were not investigated. The outcomes all‐cause mortality and bone mineral density were not reported in the included trials.
Implications for research.
More long‐term, high‐quality randomised controlled clinical trials comparing radioiodine with antithyroid medications for Graves' disease are still required. Data for children are lacking. Future trials should include all‐cause mortality, patient‐important outcome measures such as health‐related quality of life, socioeconomic effects and information on bone mineral density, as well as data on co‐medications to treat Graves' ophthalmopathy such as steroids. No long‐term data exist on other antithyroid medications such as propylthiouracil for the treatment of Graves' disease.
Notes
We have based parts of the background, the methods section, appendices, additional tables and figures 1 to 3 of this review on a standard template established by the CMED Group.
Acknowledgements
The authors wish to acknowledge Dr. Al Driedge from the Department of Nuclear Medicine/Oncology, University of Western Ontario, London, Canada for his invaluable comments, editing and formatting. We also thank Dr. Ove Torring and Dr. Danyun Chen for providing the required data for the meta‐analysis. The search strategies were designed by Maria‐Inti Metzendorf (Trials Search Co‐ordinator of the CMED Group).
Appendices
Appendix 1. Search strategies
| Cochrane Library |
| #1 [mh "Graves disease"] #2 (grave* near/7 (diseas* or thyrotoxicos* or hyperthyr* or orbitopath* or ophthalmopath*)):ti,ab,kw #3 (basedow* near/7 (diseas* or syndrom*)):ti,ab,kw #4 {or #1‐#3} #5 [mh Radiotherapy] #6 [mh "Iodine Radioisotopes"] #7 ((radioiodine or radio‐iodine) near/7 (therap* or treatment*)):ti,ab,kw #8 (radiotherap* or radio‐therap*):ti,ab,kw #9 (131‐I near/7 (therap* or treatment*)):ti,ab,kw #10 (iodine‐131 near/7 (therap* or treatment*)):ti,ab,kw #11 RAI:ti,ab,kw #12 {or #5‐#11} #13 [mh "Antithyroid Agents"] #14 ((antithyroid* or anti‐thyroid*) near/7 (therap* or treatment* or agent* or drug* or substanc* or compound*)):ti,ab,kw #15 (carbimazole* or methimazole* or methylthiouracil* or propylthiouracil* or thiouracil*):ti,ab,kw #16 [mh Carbimazole] #17 [mh Methimazole] #18 [mh Methylthiouracil] #19 [mh Propylthiouracil] #20 [mh Thiouracil] #21 {or #13‐#20} #22 #4 and #12 and #21 |
| MEDLINE (Ovid SP) |
| 1. exp Graves Disease/ 2. (grave* adj6 (diseas* or thyrotoxicos* or hyperthyr*)).tw,ot. 3. (grave* adj6 (orbitopath* or ophthalmopath*)).tw,ot. 4. (basedow* adj6 (diseas* or syndrom*)).tw,ot. 5. or/1‐4 6. exp Radiotherapy/ 7. exp Iodine Radioisotopes/tu [Therapeutic Use] 8. ((radioiodine or radio‐iodine) adj6 (therap* or treatment*)).tw,ot. 9. (radiotherap* or radio‐therap*).tw,ot. 10. (131‐I adj6 (therap* or treatment*)).tw,ot. 11. (iodine‐131 adj6 (therap* or treatment*)).tw,ot. 12. RAI.tw,ot. 13. or/6‐12 14. exp Antithyroid Agents/ 15. ((antithyroid* or anti‐thyroid*) adj6 (therap* or treatment* or agent* or drug* or substanc* or compound*)).tw,ot. 16. (carbimazole* or methimazole* or methylthiouracil* or propylthiouracil* or thiouracil*).tw,ot. 17. exp Carbimazole/ 18. exp Methimazole/ 19. exp Methylthiouracil/ 20. exp Propylthiouracil/ 21. exp Thiouracil/ 22. or/14‐21 [23‐33: Cochrane RCT Filter – sensitivity maximizing version 2008] 23. randomized controlled trial.pt. 24. controlled clinical trial.pt. 25. randomized.ab. 26. placebo.ab. 27. drug therapy.fs. 28. randomly.ab. 29. trial.ab. 30. groups.ab. 31. or/23‐30 32. exp animals/ not humans/ 33. 31 not 32 34. 5 and 13 and 22 and 33 |
| EMBASE (Ovid SP) |
| 1. exp Graves disease/
2. (basedow adj6 (diseas* or syndrom*)).tw. 3. (graves* adj6 (orbitopath* or ophthalmopath*)).tw. 4. (exophthalmic adj6 (goiter* or goitre* or hyperthyroidism*)).tw. 5. (grave* adj6 (disease* or hyperthyr* or thyrotoxicos*)).tw. 6. or/1‐5 7. exp radiotherapy/ 8. exp radioactive iodine/ 9. ((radioiodin* or radio‐iodin*) adj6 (therap* or treatment*)).tw. 10. (radiotherap* or radio‐therap*).tw. 11. ((131I or 131‐I) adj6 (therap* or treatment*)).tw. 12. RAI.tw. 13. or/7‐12 14. exp antithyroid agent/ 15. ((antithyroid* or anti‐thyroid*) adj6 (therap* or treatment* or agent* or drug* or substanc* or compound*)).tw. 16. exp carbimazole/ 17. exp thiamazole/ 18. exp methylthiouracil/ 19. exp propylthiouracil/ 20. exp thiouracil/ 21. (carbimazole* or methimazole* or methylthiouracil* or propylthiouracil* or thiouracil*).tw. 22. or/14‐21 23. 6 and 13 and 22 [24:Wong 2006– small drop in sensitivity, substantive gain in specificity filter] 24. random*.tw. or clinical trial*.mp. or exp treatment outcome/ 25. 23 and 24 26. limit 25 to embase |
| ICTRP Search Portal (Standard search) |
|
[searched as one string] grave* AND radiotherap* OR grave* AND radio therap* OR grave* AND iodin* OR grave* AND radioiodin* OR grave* AND RAI OR grave* AND 131* OR basedow* AND radiotherap* OR basedow* AND radio therap* OR basedow* AND iodin* OR basedow* AND radioiodin* OR basedow* AND RAI OR basedow* AND 131* |
| ClinicalTrials.gov (Advanced search) |
| Search Terms: (grave OR graves OR basedow OR basedows) AND (radiotherapy OR "radio therapy" OR radioiodine OR "radio iodine" OR "131" OR "131‐I" OR "RAI") |
Appendix 2. Description of interventions
| Intervention(s) [route, frequency, total dose/day] | Comparator(s) [route, frequency, total dose/day] | |
| Traisk 2009 | A single oral activity of 131I was administered using the following formula: activity (MBq) = 23.4 x thyroid mass (grams) x 120 (Gy)/estimated uptake (0 h; %) x effective half‐life (days) The thyroid mass was assessed by thyroid scintigraphy and by palpation; at day 14 after radioiodine therapy, 50 μg L‐thyroxine was added daily | Methimazole was given as 15 mg twice daily; at day 14, 50 μg L‐thyroxine was added daily; methimazole was discontinued after 18 months with an additional month of L‐thyroxine substitution of 100 μg daily, which thereafter was discontinued |
| Tallstedt 1992a | A single oral activity of 131I was administered, based on thyroid size, 24‐hour 131I uptake, and the measured effective half‐life of the isotope in the thyroid | Oral administration of 10 mg methimazole 4 times daily (40 mg/day). 3 to 5 weeks later thyroxine was added in doses between 0.1 and 0.3 mg/day (mean dose 0.17 mg/day). Methimazole and thyroxine were discontinued simultaneously after 18 months. Propranolol at a dose of 20 to 60 mg 3 to 4 times daily or 50 to 300 mg metoprolol daily in divided doses was given initially until the participants became euthyroid. In cases of adverse reactions to methimazole, the drug was exchanged with propylthiouracil (100 mg 4 times daily) |
|
aThe participants who were between 20 and 34 years old were randomly assigned to treatment with antithyroid drug plus thyroxine (young medical group) or subtotal thyroidectomy (young surgical group); the participants who were between 35 and 55 years old were randomly assigned to antithyroid drug plus thyroxine (old medical group), subtotal thyroidectomy (old surgical group) or to iodine‐131 (iodine‐131 group) 131I: iodine‐131 (radioiodine) | ||
Appendix 3. Baseline characteristics (I)
| Intervention(s) and comparator(s) | Duration of intervention (duration of follow‐up) | Description of participants | Trial period [year to year] | Country | Setting | Ethnic groups [%] | Diagnostic criteria for Graves' disease | |
| Traisk 2009 | I: radioiodine | 1 day (4 years) | Patients aged 35 to 69 years; symptomatic Graves' hyperthyroidism | Started in May 1996 and the trial was closed in 2003. With the 4‐year clinical follow‐up, the trial was terminated by the end of 2007 | Sweden | Outpatients | ‐ | Serum TSH ≤ 0.1 mIU/L and elevated triiodothyronine and/or free thyroxine, thyroid uptake of iodine‐131, and radionuclide scans compatible with Graves' disease, i.e. an even distribution of radionuclide |
| C: methimazole | 18 months (4 years) |
|||||||
| Tallstedt 1992 | I: radioiodine | 1 day (24 monthsa) | Participants aged 20 to 55 years with hyperthyroidism caused by Graves' disease | 1991 (most participants had been followed for at least 24 months) | Sweden | Outpatients | ‐ | Presence of symptoms and signs of hyperthyroidism, a diffuse goitre, elevated total and free thyroxine and total triiodothyronine concentrations in serum, detectable serum concentrations of thyrotropin‐receptor antibodies, increased uptake of iodine‐131 by the thyroid, and thyroid radionuclide scans showing a diffuse pattern of isotope uptake |
| C: methimazole | 18 months (24 monthsa) | |||||||
| ‐ denotes not reported C: comparator; I: intervention; TSH: thyroid‐stimulating hormone aDifferent follow‐up times for various outcome measures. | ||||||||
Appendix 4. Baseline characteristics (II)
| Intervention(s) and comparator(s) | Sex [female %] | Age [mean years (SD)] | Duration of disease [mean months (SD)] | Co‐medications/Co‐interventions | Comorbidities [%] | |
| Traisk 2009 | I: radioiodine | 87 | 51 (8) | 5 (4) | Beta‐blockers were used as pretreatment to radioiodine; L‐thyroxine substitution was administered at day 14 after radioiodine treatment | Current smokers: 36 |
| C: methimazole | 91 | 50 (8) | 6 (5) | At day 14, 50 μg of L‐thyroxine was added and increased to 100 μg 2 weeks later; at week 6, the dose of L‐thyroxine was adjusted to normalise the levels of serum T3 and free T4 and to bring TSH levels to less than 0.4 mIU/L; a slightly elevated serum free T4 was accepted up to 20% above the upper normal limit; beta‐blockers were used for symptomatic treatment | Current smokers: 42 | |
| Tallstedt 1992 | I: radioiodine | 85 | 45 (5) | ‐ | Unless contraindicated, propanolol or metoprolol was given to all participants; 18 participants were given more than one dose of iodine‐131 from 10 weeks to 23 months; all participants eventually had hypothyroidism and received thyroxine 0.1 ‐ 0.3 mg daily | Smoker: 56 |
| C: methimazole | 85 | 28 (4) & 45 (6) | ‐ | Thyroxine 0.1 ‐ 0.3 mg daily was added after 3 ‐ 5 weeks; propanolol 60 ‐ 240 mg daily or metoprolol 50 ‐ 300 mg daily was given initially for a few weeks; methimazole and thyroxine were discontinued simultaneously | Smoker: 42 | |
| ‐ denotes not reported BMI: body mass index; C: comparator; HbA1c: glycosylated haemoglobin A1c; I: intervention; SD: standard deviation; T3: triiodothyronine; T4: thyroxine; TSH: thyroid‐stimulating hormone | ||||||
Appendix 5. Matrix of trial endpoints (publications and trial documents)
| Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a | Trial results/ publications available in trial register Yes/No | Endpoints quoted in publication(s)b,c | Endpoints quoted in abstract of publication(s)b,c | |
| Traisk 2009 | N/T | Primary outcome measure(s): difference in the proportion of participants with worsening or development of thyroid‐associated ophthalmopathy during a 4‐year follow‐up | Primary outcome measure(s): ‐ | |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | |||
| Other outcome measure(s): recurrence of hyperthyroidism (relapse), adverse events, thyroid hormone levels, corticosteroid treatment for thyroid‐associated ophthalmopathy, health‐related quality of life | Other outcome measure(s): worsening or development of thyroid‐associated ophthalmopathy; smoking as a risk factor | |||
| Tallstedt 1992 | N/T | Primary outcome measure(s): occurrence of ophthalmopathy within 2 years after the initiation of therapye | Primary outcome measure(s): ‐ | |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | |||
| Other outcome measure(s): recurrent hyperthyroidism; predictive factors of ophthalmopathy; participants assessment of the treatment; sick leave; adverse effects; euthyroidism; health‐related quality of life; costs; laboratory assessments; TSH‐receptor autoimmunityf | Other outcome measure(s): development or worsening of Graves' ophthalmopathy | |||
| ‐ denotes not reported aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers). bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents or multiple reports of a primary trial). cOther outcome measures refer to all outcomes not specified as primary or secondary outcome measures. dRefers to outcome measures from both of the two publications of Traisk 2009. eNot explicitly stated as primary outcome but clear from paragraph 'Statistical Analysis' in the methods section of Tallstedt 1992. fRefers to outcome measures from all of the six publications of Tallstedt 1992. EMA: European Medicines Agency; FDA: Food and Drug Administration (US); mo: month(s); N/T: no trial document available; TSH: thyroid‐stimulating hormone | ||||
Appendix 6. High risk of outcome reporting bias according to ORBIT classification
| Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d | |
| Traisk 2009 | N/A | ||||
| Tallstedt 1992 | N/A | ||||
|
aClear that outcome was measured and analysed; trial report states that outcome was analysed but only reports that result was not significant.
(Classification 'A', table 2, Kirkham 2010). bClear that outcome was measured and analysed; trial report states that outcome was analysed but no results reported. (Classification 'D', table 2, Kirkham 2010). cClear that outcome was measured; clear that outcome was measured but not necessarily analysed; judgement says likely to have been analysed but not reported because of non‐significant results. (Classification 'E', table 2, Kirkham 2010). dUnclear whether the outcome was measured; not mentioned but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results. (Classification 'G', table 2, Kirkham 2010). N/A: not applicable; ORBIT: Outcome Reporting Bias In Trials | |||||
Appendix 7. Definition of endpoint measurement
| All‐cause mortality | Health‐related quality of life | Adverse events other than development and worsening of Graves' ophthalmopathy [hypothyroidism] | Recurrence of hyperthyroidism [relapse] | Development and worsening of Graves' disease | Individuals in euthyroid state | Bone mineral density | Socioeconomic effects | |
| Traisk 2009 | ‐ | Short‐Form Health Status Survey (SF‐36) | TSH was elevated and/or FT3, FT4 or T3 was below the lower limit of the reference range | ‐ | The worsening or development and improvement of ophthalmopathy met 2 of the following 4 decisive factors compared with baseline data: 1) change in exophthalmometry readings of 2 mm or more; 2) improvement or deterioration of the patient's eye movements between the 4 scoring levels (no impairment, clearly impaired, diplopia in the primary position, fixation of the globe); 3) changes of visual acuity caused by optic neuropathy; and 4) changes in 2 of the 3 ophthalmopathy activity measures (chemosis, eyelid oedema, and conjunctival redness) | ‐ | N/I | N/I |
| Tallstedt 1992 | ‐ | Short‐Form Health Status Survey (SF‐36) and quality of life 2004 (QoL 2004; not a validated instrument) | TSH was elevated and/or FT3, FT4 or T3 were below the lower limit of the reference range | TSH was decreased and/or FT3, FT4 or T3 were above the lower limit of the reference range | The eye examination by the study ophthalmologist included testing of visual acuity, tonometry, Hertel exophthalmometry, slitlamp examination and tests of extraocular‐muscle function with Lee's screen; the results of the examination were summarised as an ophthalmopathy‐index score, which was based on a modification of the American Thyroid Association's classification of ocular changes in Graves' disease; 4 categories of findings (soft tissue involvement, exophthalmos, extraocular muscle involvement and sight loss) were scored from 1 to 3 according to their severity | TSH (0.1 ‐ 4.5 mu/L), T4 (75 ‐ 150 nmol/L) and T3 (1.1 ‐ 2.5) nmol/L levels within the normal range | N/I | The costs were assessed by analysing the official hospital reimbursement system for both outpatient and inpatient costs for a period of 2 years from the day of randomisation; sick leave: number of days the participants stayed at home from work due to Graves' disease or other diseases |
| "‐" denotes not reported FT3: free triiodothyronine; FT4: free thyroxin; N/I: not investigated; T3: triiodothyronine; T4: thyroxine; TSH: thyroid‐stimulating hormone | ||||||||
Appendix 8. Adverse events except development/worsening of Graves' opthalmopathy (I)
| Intervention(s) and comparator(s) | Participants included in analysis [N] | Deaths [N] | Deaths [%] | Participants with at least one adverse event [N] | Participants with at least one adverse event [%] | Participants with at least one severe/serious adverse event [N] | Participants with at least one severe/serious adverse event [%] | |
| Traisk 2009 | I: radioiodine | 163 | ‐ | ‐ | 0 | 0 | ‐ | ‐ |
| C: methimazole | 150 | ‐ | ‐ | 12 | 8 | ‐ | ‐ | |
| Tallstedt 1992a | I: radioiodine | 39 | ‐ | ‐ | 39b | 100 | ‐ | ‐ |
| C: methimazole | 68 | ‐ | ‐ | 11 | 16 | ‐ | ‐ | |
| ‐ denotes not reported aData from up to 48 months follow‐up. bAll participants developed hypothyroidism after the initial or additional treatments and were given T4; the mean time from the initial treatment until T4 was prescribed was 3.7 months. C: comparator; I: intervention; T4: thyroxine | ||||||||
Appendix 9. Adverse events except development/worsening of Graves' opthalmopathy (II)
| Intervention(s) and comparator(s) | Participants included in analysis [N] | Participants discontinuing trial due to an adverse event [N] | Participants discontinuing trial due to an adverse event [%] | |
| Traisk 2009 | I: radioiodine | 163 | ‐ | ‐ |
| C: methimazole | 150 | ‐ | ‐ | |
| Tallstedt 1992 | I: radioiodine | 39 | ‐ | ‐ |
| C: methimazole | 68 | ‐ | ‐ | |
| ‐ denotes not reported C: comparator; I: intervention | ||||
Appendix 10. Health‐related quality of life: instruments
| Instrument | Dimensions (subscales) [no. of items] | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | Minimal important difference | |
| SF‐36 (G) | Physical functioning (PF) (10) Role‐physical (RP) (4) Bodily pain (BP) (2) General health (GH) (5) Vitality (VT) (4) Social functioning (SF) (2) Role‐emotional (RE) (3) Mental health (MH) (5) Reported health transition (RHT) (1) | Yes | 3‐, 5‐ and 6‐point Likert‐scale | Scores for dimensions
Physical component summary (PCS) Mental component summary (MCS) |
Minimum scores:
scores for dimensions/PCS/MCS:
norm‐based scale Maximum scores: scores for dimensions/PCS/MCS: norm‐based scale |
No | Higher values mean better assessment | PCS: 2 to 3 points MCS: 3 points Dimensions: PF/BT/VT: 2 points, if score < 40; 3 points, if score ≥ 40 RP: 2 points SF/MH: 3 points RE: 4 points | |
| Traisk 2009 | Quote from the publication: "Patients who had experienced development or worsening of eye problems at any time point during the 4‐year study period (TAO group) had lower QoL estimated by the SF‐36 questionnaire when no attention was paid to the mode of treatment ... throughout the whole study period, the TAO group had lower MCS and PCS for the first 3 years ... There were no differences in the results of the SF‐36 scores between the two treatment groups ... Already after 3 months, the physical component score reached the average for the Swedish reference population (score 50) and remained at this level throughout the study period (48 months). The mental component score showed some delay in both the radioiodine and medically treated groups to reach the average score of 50 not until 12 months. Thereafter, the mental component score remained rather constant for 3 years or at least until 48 months ... Comparisons between the two groups of patients without TAO showed no significant differences in QoL regardless whether they had been treated with radioiodine or ATDs ... The study also showed that QoL was rather equal in both treatment groups when no attention was paid to the possible influence of TAO ... Patients with TAO generally had significantly lower QoL scores as compared with patients without TAO at several time points, but no consistent treatment‐ or time‐related pattern could be found ... The result of this analysis showed that the SF‐36 due to its generic properties is not an optimal instrument for measuring QoL‐related issues in this population ... Although the SF‐36 is used extensively, it has not been specifically evaluated in a population of GD patients, but neither have other disease‐specific instruments." | ||||||||
| Tallstedt 1992a | Quote from publication: "The Physical Component Summary and Mental Component Summary were not significantly different among the three old‐age groups. Also the sub‐component Summaries of the Physical and Mental Component Summary were similar .... Compared to the Swedish reference population at 50‐74 years, we observed that the Vitality score was lower for all three treatment groups (p < 0.05). The medical group had a significantly lower Mental Component Summary than the reference population ... The radioiodine‐treated group had a significantly lower General Health score (p < 0.05)" | ||||||||
|
a145/179 participants with evaluated questionnaires (56 on surgery, 55 on antithyroid drugs, 34 on radioiodine); only figures are provided (Abraham‐Nordling 2005 under Tallstedt 1992). ATD: antithyroid drug; G: generic; GD: Graves' disease; QoL: quality of life; S: specific; SF: short‐form health survey | |||||||||
Appendix 11. Survey of trial investigators providing information on included trials
| Trial author contacted | Trial author replied | Trial author asked for additional information | Trial author provided data | |
| Traisk 2009 | Yes (2012) | No | No | No |
| Tallstedt 1992 | Yes (2012) | Yes (2012) | Yes (2012) | Yes (2012); trial author provided the exact number of participants in different thyroid status after treatment for hyperthyroidism |
Appendix 12. Checklist to aid consistency and reproducibility of GRADE assessments
| (1) Health‐related quality of life | (2) Development and worsening of Graves' ophthalmopathy | (3) Individuals in euthyroid state | (4) Recurrence of hyperthyroidism (relapse) | (5) Adverse events other than development and worsening of Graves' ophthalmopathy (hypothyroidism, adverse drug effects) | (6) All‐cause mortality | (7) Socioeconomic effects | ||
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | Unclear | N/A | Unclear |
| Was allocation concealment used (i.e. no potential for selection bias)? | Yes/Unclear | Yes/Unclear | Yes/Unclear | Yes/Unclear | Yes/Unclear | N/A | Yes | |
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | No (↓) | Yes/No (↓) | Yes | Yes | Yes | N/A | Yes | |
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes/No (↓) | Yes/No (↓) | Yes | Yes | Yes | N/A | Yes | |
| Was an objective outcome used? | Yes | Yes | Yes | Yes | Yes/No (↓) | N/A | Yes | |
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | Yes | Yes | Yes | N/A | Yes | |
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | Yes | Yes | Yes | N/A | Yes | |
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | Yes | Yes | Yes | N/A | Yes | |
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | Yes | Yes | N/A | Yes | |
| Inconsistencyb | Point estimates did not vary widely? | N/A | Yes | N/A | No (↓) | N/A | N/A | N/A |
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | N/A | Some | N/A | No (↓) | N/A | N/A | N/A | |
| Was the direction of effect consistent? | N/A | Yes | N/A | Yes | N/A | N/A | N/A | |
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40% to 60%), high I² > 60%)? | N/A | Low | N/A | High (↓) | N/A | N/A | N/A | |
| Was the test for heterogeneity statistically significant (P value < 0.1)? | N/A | Not statistically significant | N/A | Statistically significant (↓) | N/A | N/A | N/A | |
| Indirectnessa | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | N/A | Highly applicable |
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | N/A | Highly applicable | |
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | Yes | Yes/No (↓) | N/A | Yes | |
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | N/A | Sufficient | |
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | Yes | N/A | Yes | |
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | N/A | Yes | N/A | No (↓) | N/A | N/A | N/A |
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?e | Intermediate | Intermediate | Intermediate | Intermediate | Intermediate | N/A | Low (↓) | |
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Small (↓) | Small (↓) | Small (↓) | Small (↓) | Small (↓) | N/A | Small (↓) | |
| Was the outcome a common event (e.g. occurs more than 1/100)? | N/A | Yes | N/A | Yes | N/A | N/A | N/A | |
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | Yes | N/A | Yes |
| Was grey literature searched? | No (↓) | No (↓) | No (↓) | No (↓) | No (↓) | N/A | No (↓) | |
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | Yes | N/A | Yes | |
| There was no industry influence on studies included in the review? | Yes | Yes | Yes | Yes | Yes | N/A | Yes | |
| There was no evidence of funnel plot asymmetry? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| There was no discrepancy in findings between published and unpublished trials? | Unclear | Unclear | Unclear | Unclear | Unclear | N/A | Unclear | |
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials.
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I². cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials. eDepends on the context of the systematic review area. (↓): key item for possible downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); GRADE: Grading of Recommendations Assessment, Development and Evaluation; N/A: not applicable | ||||||||
Data and analyses
Comparison 1. Radioiodine versus methimazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Development or worsening of Graves' ophthalmopathy | 2 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.40, 2.70] |
| 2 Adverse events: hypothyroidism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Recurrence of hyperthyroidism (relapse) | 2 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 2.66] |
| 4 Participants in euthyroid state | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Tallstedt 1992.
| Methods |
Parallel randomised controlled clinical trial Superiority design |
|
| Participants |
Inclusion criteria: participants between 20 and 55 years with hyperthyroidism caused by Graves' disease and without a history of previous thyroid disease Exclusion criteria: participants who wanted treatment options other than those offered by randomisation Diagnostic criteria: diagnosis of Graves' disease: presence of symptoms and signs of hyperthyroidism, non‐nodular thyroid gland, and elevated total and free serum T4, and/or total T3 levels; if the serum T4 level was between 150 and 180 nmol/L or T3 was between 2.5 and 3.5 nmol/L, the diagnosis was supported by a TRH test. Furthermore, all participants should have a thyroid uptake of 131I that was not suppressed, a diffuse pattern of isotope uptake on the radionuclide scan and a goitre size that should enable a single dose of 131I to render the participant euthyroid or hypothyroid |
|
| Interventions |
Number of study centres: unclear Treatment before study: unclear |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details |
Run‐in period: no Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "First, is there a difference in the frequency of the development or aggravation of Graves' ophthalmopathy among young patients treated with an antithyroid drug as compared with subtotal thyroidectomy and among older patients treated with an antithyroid drug, subtotal thyroidectomy, or iodine‐131? And second, can factors that predict the development of Graves' ophthalmopathy be identified?" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Each patient was assigned a treatment group consecutively using two lists, one for each age group, on which the treatment group occurred in random order but balanced to equalize the size of the treatment groups" Quote from publication: "The patients who were between 20 and 34 years old were randomly assigned to treatment with antithyroid drug plus thyroxine (young medical group) or subtotal thyroidectomy (young surgical group); the patients who were between 35 and 55 years old were randomly assigned to antithyroid drug plus thyroxine (old medical group), subtotal thyroidectomy (old surgical group) or to iodine‐131 (iodine‐131 group)" Comment: no details on how randomisation was performed |
| Allocation concealment (selection bias) | Low risk |
Quote from the publication (Laurberg 2008): "Randomization was performed by assigning each patient a treatment group consecutively using two lists, one for each age group. On the list, each treatment group occurred in a random order but was balanced to equalize the size of the treatment groups. The lists were unavailable to the clinicians throughout the study, and randomization was performed over the phone." Comment: probably adequate concealment of allocation |
| Blinding of participants and personnel (performance bias) adverse events: hypothyroidism | Low risk |
Quote from the publication (Laurberg 2008): "Randomization was performed by assigning each patient a treatment group consecutively using two lists, one for each age group. On the list, each treatment group occurred in a random order but was balanced to equalize the size of the treatment groups. The lists were unavailable to the clinicians throughout the study, and randomization was performed over the phone." Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) development or worsening of Graves' ophthalmopathy | Low risk |
Quote from the publication (Laurberg 2008): "Randomization was performed by assigning each patient a treatment group consecutively using two lists, one for each age group. On the list, each treatment group occurred in a random order but was balanced to equalize the size of the treatment groups. The lists were unavailable to the clinicians throughout the study, and randomization was performed over the phone." Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) health‐related quality of life | High risk | Comment: self reported outcome measurement |
| Blinding of participants and personnel (performance bias) participants in euthyroid state | Low risk |
Quote from the publication (Laurberg 2008): "Randomization was performed by assigning each patient a treatment group consecutively using two lists, one for each age group. On the list, each treatment group occurred in a random order but was balanced to equalize the size of the treatment groups. The lists were unavailable to the clinicians throughout the study, and randomization was performed over the phone." Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) recurrence of hyperthyroidism | Low risk |
Quote from the publication (Laurberg 2008): "Randomization was performed by assigning each patient a treatment group consecutively using two lists, one for each age group. On the list, each treatment group occurred in a random order but was balanced to equalize the size of the treatment groups. The lists were unavailable to the clinicians throughout the study, and randomization was performed over the phone." Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) socioeconomic effects | Low risk |
Quote from the publication (Laurberg 2008): "Randomization was performed by assigning each patient a treatment group consecutively using two lists, one for each age group. On the list, each treatment group occurred in a random order but was balanced to equalize the size of the treatment groups. The lists were unavailable to the clinicians throughout the study, and randomization was performed over the phone." Comment: investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) adverse events: hypothyroidism | Low risk |
Quote from publication: "Outcomes were assessed by physicians blinded to participants' treatment assignments" Comment: investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) development or worsening of Graves' ophthalmopathy | Low risk |
Quote from publication: "Outcomes were assessed by physicians blinded to participants' treatment assignments" Comment: investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) health‐related quality of life | Low risk |
Quote from publication: "quality of life was assessed by physicians blinded to participants' treatment assignments" Comment: investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) participants in euthyroid state | Low risk |
Quote from publication: "quality of life was assessed by physicians blinded to participants' treatment assignments" Comment: investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) recurrence of hyperthyroidism | Low risk |
Quote from publication: "recurrence of hyperthyroidism was assessed by physicians blinded to participants' treatment assignments" Comment: investigator‐assessed outcome measurement Comment: low risk |
| Blinding of outcome assessment (detection bias) socioeconomic effects | Low risk |
Quote from publication: "quality of life was assessed by physicians blinded to participants' treatment assignments" Comment: investigator‐assessed outcome measurement |
| Incomplete outcome data (attrition bias) adverse events: hypothyroidism | Low risk |
Quote from publication: "outcomes data including health‐related quality of life are available for all included patients, two patients rejected the assigned treatment" Comment: no intention‐to‐treat analysis for most outcome measures |
| Incomplete outcome data (attrition bias) development or worsening of Graves' ophthalmopathy | Low risk |
Quote from publication: "outcomes data including health‐related quality of life are available for all included patients, two patients rejected the assigned treatment" Comment: no intention‐to‐treat analysis for most outcome measures |
| Incomplete outcome data (attrition bias) health‐related quality of life | Low risk |
Quote from publication: "outcomes data including health‐related quality of life are available for all included patients, two patients rejected the assigned treatment" Comment: no intention‐to‐treat analysis for most outcome measures |
| Incomplete outcome data (attrition bias) participants in euthyroid state | Low risk |
Quote from publication: "outcomes data including health‐related quality of life are available for all included patients, two patients rejected the assigned treatment" Comment: no intention‐to‐treat analysis for most outcome measures |
| Incomplete outcome data (attrition bias) recurrence of hyperthyroidism | Low risk |
Quote from publication: "outcomes data including health‐related quality of life are available for all included patients, two patients rejected the assigned treatment" Comment: no intention‐to‐treat analysis for most outcome measures |
| Incomplete outcome data (attrition bias) socioeconomic effects | Low risk |
Quote from publication: "outcomes data including health‐related quality of life are available for all included patients, two patients rejected the assigned treatment" Comment: no intention‐to‐treat analysis for most outcome measures |
| Selective reporting (reporting bias) | Low risk | Comment: identified outcomes adequately reported as compared with the description in methods |
| Other bias | Low risk | Comment: no source of other bias noted |
Traisk 2009.
| Methods |
Parallel randomised controlled clinical trial Superiority design |
|
| Participants |
Inclusion criteria: participants aged 35 to 69 years; symptomatic Graves' hyperthyroidism; confirmation of the diagnosis by serum TSH (≤ 0.1 mIU/L) and T3 and/or free T4 (elevated), thyroid uptake of iodine‐131, and radionuclide scans compatible with Graves' disease, i.e. an even distribution of the radionuclide Exclusion criteria: participants with a previous history of treatment with antithyroid drugs, iodine‐131 or thyroid surgery were excluded as well as participants with severe TAO requiring treatment with corticosteroids at the time of inclusion Diagnostic criteria: diagnosis of Graves' disease was made according to the hyperthyroidism and diffuse goitre. For the set criteria (worsening or development and improvement of TAO), 2 of the following 4 decisive factors were required (compared with baseline data): 1) change in exophthalmometry readings of 2 mm or more; 2) improvement or deterioration of the participant's eye movements between the 4 scoring levels (no impairment, clearly impaired, diplopia in the primary position, fixation of the globe); 3) changes of visual acuity caused by optic neuropathy; and 4) changes in 2 of the 3 TAO activity measures (chemosis, eyelid oedema and conjunctival redness). The participants who did not meet the criteria for improvement or worsening or development of TAO were referred to as having no change of TAO |
|
| Interventions |
Number of study centres: 4 Treatment before study: beta‐blockers |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details |
Run‐in period: no Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding (Medical Council for Research) Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "... to compare radioiodine treatment and medical therapy for long‐term worsening or development of TAO. Smoking and hypothyroidism as confounding risk factors were controlled for, and L‐thyroxine supplementation was given early, i.e. at 2 wk after the initiation of treatment for hyperthyroidism in both arms" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Patients were randomised to radioiodine and medical treatment within each center (stratified randomization). Randomization was made in blocks over time and was performed by the Oncological Centre at the Karolinska University Hospital in Stockholm" Comment: no details of the randomisation procedure provided |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "Patients were randomised to radioiodine and medical treatment within each center (stratified randomization). Randomization was made in blocks over time and was performed by the Oncological Centre at the Karolinska University Hospital in Stockholm" Comment: probably concealed allocation (by the oncological centre) |
| Blinding of participants and personnel (performance bias) adverse events: hypothyroidism | Low risk |
Quote from publication: "The study was designed as an open, randomized, prospective multicenter trial" Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) development or worsening of Graves' ophthalmopathy | High risk |
Quote from publication: "The study was designed as an open, randomized, prospective multicenter trial" Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) health‐related quality of life | High risk |
Quote from publication: "The study was designed as an open, randomized, prospective multicenter trial" Comment: self reported outcome measurement |
| Blinding of participants and personnel (performance bias) recurrence of hyperthyroidism | Low risk |
Quote from publication: "The study was designed as an open, randomized, prospective multicenter trial" Comment: investigator‐assessed outcome measurement; outcome probably not influenced by non‐blinding conditions |
| Blinding of outcome assessment (detection bias) adverse events: hypothyroidism | Low risk | Comment: no details provided; outcome probably not influenced by non‐blinding conditions |
| Blinding of outcome assessment (detection bias) development or worsening of Graves' ophthalmopathy | High risk | Comment: no details provided; investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) health‐related quality of life | High risk | Comment: self reported outcome measurement |
| Blinding of outcome assessment (detection bias) recurrence of hyperthyroidism | Low risk | Comment: no details provided; outcome probably not influenced by non‐blinding conditions |
| Incomplete outcome data (attrition bias) adverse events: hypothyroidism | Low risk | Comment: intention‐to‐treat analysis; few drop‐outs |
| Incomplete outcome data (attrition bias) development or worsening of Graves' ophthalmopathy | Low risk | Comment: intention‐to‐treat analysis; few drop‐outs |
| Incomplete outcome data (attrition bias) health‐related quality of life | Low risk | Comment: intention‐to‐treat analysis; few drop‐outs |
| Incomplete outcome data (attrition bias) recurrence of hyperthyroidism | Low risk | Comment: intention‐to‐treat analysis; few drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes adequately reported as compared with the description in methods |
| Other bias | Low risk | Comment: no source of other bias noted |
Note: where the judgement is 'Unclear' and the description is blank, the trial did not report that particular outcome
131I: radioiodine; TAO: thyroid‐associated ophthalmopathy; T4: thyroxine; T3: tri‐iodothyronine; TRH: thyrotropin‐releasing hormone
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Azizi 2005 | RCT but included participants with recurrence of hyperthyroidism after methimazole treatment for 18 months before the study |
| Bartalena 1998 | RCT but with 1‐year follow‐up only |
| Berg 1996 | Consecutive series trial |
| Berglund 1991 | Case‐control trial |
| Chen 2005a | RCT but with 1‐year follow‐up only |
| Chen 2005b | Case‐control trial |
| Chen 2009 | RCT but included participants with Graves' disease, multinodular toxic goitre and uninodular goitre |
| EI‐Kaissi 2010 | Some of the participants were randomised; the others were not randomised; with 1‐year follow‐up |
| Hayashi 2005 | Cohort trial |
| Mornex 1977 | Case‐control trial |
| Patel 2006 | Case‐control trial |
| Singer 2001 | Cohort trial |
| Zhao 2005 | RCT but with 1‐year follow‐up only |
RCT: randomised controlled trial
Differences between protocol and review
In response to the requirements of the CMED Group, we also evaluated the outcome measure development or worsening of Graves' opthalmopathy as a primary outcome.
We also planned to evaluate the effects of steroids on preventing the development and progression of Graves' opthalmopathy. However, a systematic review reported that prednisolone prophylaxis was highly effective in preventing the progression of Graves' opthalmopathy in patients with pre‐existing Graves' opthalmopathy treated by radioiodine. Therefore, we did not assess the effects of steroids on Graves' opthalmopathy in this review. Moreover, these data were not reported in the two included trials.
We did not carry out the planned subgroup analyses 'dose of radioiodine', 'age' and 'gender' because of an insufficient number of trials, no information or both.
Due to the time lag between publication of the protocol and the final review draft the whole review was completely restructured by the editorial office of the CMED Group and the newest standards were implemented.
Contributions of authors
All review authors read and approved the final review draft.
Chao Ma (CM): protocol draft, search strategy development, data analysis, data interpretation and future review update.
Jiawei Xie (JWX): trial selection and data extraction.
Hui Wang (HW): search strategy development, trial selection, data extraction and data interpretation.
Jinsong Li (JL): trial selection, data analysis and data interpretation.
Suyun Chen (SYC): acquisition of trial copies, trial selection, data extraction, data analysis and future review update.
Sources of support
Internal sources
Dr. Ma was supported by National Natural Science Fund (no. 81271612), China.
External sources
No sources of support supplied
Declarations of interest
Chao Ma: none known.
Jiawei Xie: none known.
Hui Wang: none known.
Jinsong Li: none known.
Suyun Chen: none known.
New
References
References to studies included in this review
Tallstedt 1992 {published data only}
- Abraham‐Nordling M, Törring O, Hamberger B, Lundell G, Tallstedt L, Calissendorff J, et al. Graves' disease: a long‐term quality‐of‐life follow up of patients randomized to treatment with antithyroid drugs, radioiodine, or surgery. Thyroid 2005;15(11):1279‐86. [DOI] [PubMed] [Google Scholar]
- Abraham‐Nordling M, Wallin G, Lundell G, Törring O. Thyroid hormone state and quality of life at long‐term follow‐up after randomized treatment of Graves' disease. European Journal of Endocrinology 2007;156:173‐9. [DOI] [PubMed] [Google Scholar]
- Laurberg P, Wallin G, Tallstedt L, Abraham‐Nordling M, Lundell G, Tørring O. TSH‐receptor autoimmunity in Graves' disease after therapy with anti‐thyroid drugs, surgery, or radioiodine: a 5‐year prospective randomized study. European Journal of Endocrinology 2008;158:69‐75. [DOI] [PubMed] [Google Scholar]
- Ljunggren JG, Törring O, Wallin G, Taube A, Tallstedt L, Hamberger B, et al. Quality of life aspects and costs in treatment of Graves' hyperthyroidism with antithyroid drugs, surgery, or radioiodine: results from a prospective, randomized study. Thyroid 1998;8:653‐9. [DOI] [PubMed] [Google Scholar]
- Tallstedt L, Lundell G, Tørring O, Wallin G, Ljunggren JG, Blomgren H, et al. Occurrence of ophthalmopathy after treatment for Graves' hyperthyroidism, the thyroid study group. New England Journal of Medicine 1992;326:1733‐8. [DOI] [PubMed] [Google Scholar]
- Törring O, Tallstedt L, Wallin G, Lundell G, Ljunggren JG, Taube A, et al. Graves' hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine‐‐a prospective, randomized study, thyroid study group. Journal of Clinical Endocrinology and Metabolism 1996;81:2986‐93. [DOI] [PubMed] [Google Scholar]
Traisk 2009 {published data only}
- Abraham‐Nordling M, Wallin G, Träisk F, Berg G, Calissendorff J, Hallengren B, et al. Thyroid‐associated ophthalmopathy; quality of life follow‐up of patients randomized to treatment with antithyroid drugs or radioiodine. European Journal of Endocrinology 2010;163(4):651‐7. [DOI] [PubMed] [Google Scholar]
- Träisk F, Tallstedt L, Abraham‐Nordling M, Andersson T, Berg G, Calissendorff J, et al. Thyroid‐associated ophthalmopathy after treatment for Graves' hyperthyroidism with antithyroid drugs or iodine‐131. Journal of Clinical Endocrinology and Metabolism 2009;94:3700‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Azizi 2005 {published data only}
- Azizi F, Ataie L, Hedayati M, Mehrabi Y, Sheikholeslami F. Effect of long‐term continuous methimazole treatment of hyperthyroidism: comparison with radioiodine. European Journal of Endocrinology 2005;152:695‐701. [DOI] [PubMed] [Google Scholar]
Bartalena 1998 {published data only}
- Bartalena L, Marcocci C, Bogazzi F, Manetti L, Tanda ML, Dell'Unto E, et al. Relation between therapy for hyperthyroidism and the course of Graves' ophthalmopathy. New England Journal of Medicine 1998;338:73‐8. [DOI] [PubMed] [Google Scholar]
Berg 1996 {published data only}
- Berg G, Michanek A, Holmberg E, Nyström E. Clinical outcome of radioiodine treatment of hyperthyroidism: a follow‐up study. Journal of Internal Medicine 1996;239:165‐71. [DOI] [PubMed] [Google Scholar]
Berglund 1991 {published data only}
- Berglund J, Christensen SB, Dymling JF, Hallengren B. The incidence of recurrence and hypothyroidism following treatment with antithyroid drugs, surgery or radioiodine in all patients with thyrotoxicosis in Malmö during the period 1970‐1974. Journal of Internal Medicine 1991;229:435‐42. [DOI] [PubMed] [Google Scholar]
Chen 2005a {published data only}
- Chen Y, Qiu L, Zhang CY, Long SQ, Gan XL. Therapeutic effect of 131I and prednisone on Graves' ophthalmopathy. Journal of Luzhou Medical College 2005;28:334‐6. [Google Scholar]
Chen 2005b {published data only}
- Chen DY, Chen TH. Comparison of efficacy of 131 I and antithyroid drugs in the treatment of Graves' disease in children. Chinese Journal Pediatrics 2005;43:507‐9. [PubMed] [Google Scholar]
Chen 2009 {published data only}
- Chen DY, Jing J, Schneider PF, Chen TH. Comparison of the long‐term efficacy of low dose 131I versus antithyroid drugs in the treatment of hyperthyroidism. Nuclear Medicine Communications 2009;30:160‐8. [DOI] [PubMed] [Google Scholar]
EI‐Kaissi 2010 {published data only}
- El‐Kaissi S, Bowden J, Henry MJ, Yeo M, Champion BL, Brotchie P, et al. Association between radioiodine therapy for Graves' hyperthyroidism and thyroid‐associated ophthalmopathy. International Ophthalmology 2010;30:397‐405. [DOI] [PubMed] [Google Scholar]
Hayashi 2005 {published data only}
- Hayashi K, Abe K, Sakata I, Sakaguchi C, Yamamoto K, Kosuda S. Cost‐utility analysis of antithyroid drug therapy versus 131I therapy for Graves' disease. Kakuigaku. The Japanese Journal of Nuclear Medicine 2005;42:87‐95. [PubMed] [Google Scholar]
Mornex 1977 {published data only}
- Mornex R, Quintana J, Chavrier B. Long term effects of the treatment of the Graves' disease by 4 different therapeutics (author's transl). Annales D'endocrinologie 1977;38:273‐82. [PubMed] [Google Scholar]
Patel 2006 {published data only}
- Patel NN, Abraham P, Buscombe J, Vanderpump MP. The cost effectiveness of treatment modalities for thyrotoxicosis in a U.K. center. Thyroid 2006;16:593‐8. [DOI] [PubMed] [Google Scholar]
Singer 2001 {published data only}
- Singer RB. Long‐term comparative cancer mortality after use of radio‐iodine in the treatment of hyperthyroidism, a fully reported multicenter study. Journal of Insurance Medicine (New York, NY) 2001;33:138‐42. [PubMed] [Google Scholar]
Zhao 2005 {published data only}
- Hui Zhao, Jinliang Liu, Zhiyoang Liu. Clinical observation of therapeutic effect of 131I and prednisone on Graves' ophthalmopathy. Chinese Journal of Misdiagnostics 2005;5:2801‐2. [Google Scholar]
Additional references
Abraham 2010
- Abraham P, Avenell A, McGeoch SC, Clark LF, Bevan JS. Antithyroid drug regimen for treating Graves' hyperthyroidism. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003420.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Acharya 2008
- Acharya SH, Avenell A, Philip S, Burr J, Bevan JS, Abraham P. Radioiodine therapy for Graves' disease and the effect on ophthalmopathy: a systematic review. Clinical Endocrinology 2008;69(6):943‐50. [DOI] [PubMed] [Google Scholar]
Adlin 1991
- Adlin EV, Maurer AH, Marks AD, Channick BJ. Bone mineral density in postmenopausal women treated with L‐thyroxine. American Journal of Medicine 1991;90:360‐6. [DOI] [PubMed] [Google Scholar]
Allannic 1990
- Allannic H, Fauchet R, Orgiazzi J, Madec AM, Genetet B, Lorcy Y, et al. Antithyroid drugs and Graves' disease: a prospective randomized evaluation of the efficacy of treatment duration. Journal of Clinical Endocrinology and Metabolism 1990;70: 675‐9. [DOI] [PubMed] [Google Scholar]
Angusti 2000
- Angusti T. Thyroid cancer prevalence after radioiodine treatment of hyperthyroidism. Journal of Nuclear Medicine 2000;41:1006‐9. [PubMed] [Google Scholar]
Azizi 2012
- Azizi F, Yousefi V, Bahrainian A, Sheikholeslami F, Tohidi M, Mehrabi Y. Long‐term continuous methimazole or radioiodine treatment for hyperthyroidism. Archives of Iranian Medicine 2012;15:477‐84. [PubMed] [Google Scholar]
Bahn 2011
- Bahn Chair RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American thyroid association and American association of clinical endocrinologists. Thyroid 2011;21(6):593‐646. [DOI] [PubMed] [Google Scholar]
Bartalena 2012
- Bartalena L. Prevention of Graves' ophthalmopathy. Best Practice & Research. Clinical Endocrinology & Metabolism 2012;26:371‐9. [DOI] [PubMed] [Google Scholar]
Becker 1984
- Becker DV. Choice of therapy for Graves' hyperthyroidism. New England Journal of Medicine 1984;311:464‐6. [DOI] [PubMed] [Google Scholar]
Beller 2013
- Beller EM, Chen JK, Wang UL, Glasziou PP. Are systematic reviews up‐to‐date at the time of publication?. Systematic Reviews 2013;2:36. [2046‐4053: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berglund 1996
- Berglund J, Ericsson UB, Hallengren B. Increased incidence of thyrotoxicosis in Malmo during the years 1988‐1990 as compared to the years 1970‐1974. Journal of Internal Medicine 1996;239:57‐62. [DOI] [PubMed] [Google Scholar]
Biersack 2007
- Biersack HJ, Freema LM. Clinical Nuclear Medicine. Berlin Heidelberg: Springer Verlag, 2007. [Google Scholar]
Biondi 1993
- Biondi B, Fazio S, Carella C, Amato G, Cittadini A, Lupoli G, et al. Cardiac effects of long term thyrotropin‐suppressive therapy with levothyroxine. Journal of Clinical Endocrinology and Metabolism 1993;77:334‐8. [DOI] [PubMed] [Google Scholar]
Boutron 2014
- Boutron I, Altman DG, Hopewell S, Vera‐Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled t rial. Journal of Clinical Oncology 2014;32:4120‐6. [DOI] [PubMed] [Google Scholar]
Bunevicius 1999
- Bunevicius R, Karanavicius G, Zalinkevicius R, Prange AJ Jr. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. New Zealand Medical Journal 1999;340:424‐9. [DOI] [PubMed] [Google Scholar]
Bunevicius 2000
- Bunevicius R, Prange AJ. Mental improvement after replacement therapy with thyroxine plus triiodothyronine: relationship to cause of hypothyroidism. International Journal of Neuropsychopharmacology 2000;1:167‐74. [DOI] [PubMed] [Google Scholar]
Canaris 2000
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Archives of Internal Medicine 2000;160:526‐34. [DOI] [PubMed] [Google Scholar]
Capen 1994
- Capen CC. Mechanisms of chemical injury of thyroid gland. Progress in Clinical and Biological Research 1994;387:173‐91. [PubMed] [Google Scholar]
Clark 1995
- Clark JD, Gelfand MJ, Elgazzar AH. Iodine‐131 therapy of hyperthyroidism in pediatric patients. Journal of Nuclear Medicine 1995;36:442‐5. [PubMed] [Google Scholar]
Cooper 1984
- Cooper DS. Antithyroid drugs. New England Journal of Medicine 1984;311:1353‐62. [DOI] [PubMed] [Google Scholar]
Dietlein 1999
- Dietlein M, Moka D, Dederichs B, Hunsche E, Lauterbach KW, Schicha H. Cost‐effectiveness analysis: radioiodine or antithyroid medication in primary treatment of immune hyperthyroidism. Nuclear‐Medizin 1999;38:7‐14. [PubMed] [Google Scholar]
Dunn 1964
- Dunn JT, Chapman EM. Rising incidence of hypothyroidism after radioactive iodine therapy in hyperthyroidism. New England Journal of Medicine 1964;271:1037‐42. [DOI] [PubMed] [Google Scholar]
Faber 1994
- Faber J, Galloe AM. Changes in bone mass during prolonged subclinical hyperthyroidism due to L‐thyroxine treatment: a meta‐analysis. European Journal of Endocrinology 1994;130:350‐6. [DOI] [PubMed] [Google Scholar]
Ford 1991
- Ford HC, Delahunt JW, Feek CM. The management of Graves' disease in New Zealand: results of a national survey. New Zealand Medical Journal 1991;104:251‐2. [PubMed] [Google Scholar]
Franklyn 1998
- Franklyn JA, Maisonneuve P, Sheppard MC, Betteridge J, Boyle P. Mortality after the treatment of hyperthyroidism with radioactive iodine. New England Journal of Medicine 1998;338:712‐8. [DOI] [PubMed] [Google Scholar]
Ginsberg 2003
- Ginsberg J. Diagnosis and management of Graves' disease. Canadian Medical Association Journal 2003;168(5):575‐85. [PMC free article] [PubMed] [Google Scholar]
Hard 1998
- Hard GC. Recent developments in the investigation of thyroid regulation and thyroid carcinogenesis. Environmental Health Perspectives 1998;106:427‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holm 1982
- Holm LE. Changing annual incidence of hypothyroidism after 131I therapy for hyperthyroidism, 1951–1975. Journal of Nuclear Medicine 1982;23:108‐12. [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI: 10.1186/1471-2288-5-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huysmanns 1994
- Huysmanns DA, Hermus AR, Corstens FH, Barentsz JO, Kloppenborg PW. Large compressive goiters treated with radioiodine. Annals of Internal Medicine 1994;121:757‐62. [DOI] [PubMed] [Google Scholar]
Jones 1982
- Jones SJ, Hedley AJ, Curtis B, Allisons SP, Woolfson AM, Steel R, et al. We need thyroid follow‐up registers? a cost effective study. Lancet 1982;1:1229‐33. [DOI] [PubMed] [Google Scholar]
Jones 2015
- Jones CW, Keil LG, Holland WC, Caughey MC, Platts‐Mills TF. Comparison of registered and published outcomes in randomized controlled trials: a systematic review. BMC Medicine 2015;13:282. [DOI: 10.1186/s12916-015-0520-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kendall‐Taylor 1984
- Kendall‐Taylor P, Keir MJ, Ross WM. Ablative radioiodine therapy for hyperthyroidism: long term follow up study. BMJ 1984;28(6441):361‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2004
- Kim JM, LaBree L, Levin L, Feldon SE. The relation of Graves' ophthalmopathy to circulating thyroid hormone status. British Journal of Ophthalmology 2004;88:72‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 1999;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2008
- Ma C, Kuang AR, Xie J, Liu G. Radioiodine treatment for pediatric Graves' disease. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006294.pub2] [DOI] [PubMed] [Google Scholar]
Ma 2009
- Ma C, Xie J, Huang X, Wang G, Wang Y, Wang X, et al. Thyroxine alone or thyroxine plus triiodothyronine replacement therapy for hypothyroidism. Nuclear Medicine Communications 2009;30(8):586‐93. [DOI] [PubMed] [Google Scholar]
Mathieu 2009
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977‐84. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mostbeck 1998
- Mostbeck A, Galvan G, Bauer P, Eber O, Atefie K, Dam K, et al. The incidence of hyperthyroidism in Austria from 1987 to 1995 before and after an increase in salt iodination in 1990. European Journal of Nuclear Medicine 1998;25:367‐74. [DOI] [PubMed] [Google Scholar]
Reinwein 1993
- Reinwein D, Benker G, Lazarus JH, Alexander WD. The European multicenter study group on antithyroid drug treatment. a prospective randomized trial of antithyroid drug dose in Graves' disease. Journal of Clinical Endocrinology and Metabolism 1993;6:1516‐21. [DOI] [PubMed] [Google Scholar]
Rennie 1997
- Rennie D. Thyroid storm. JAMA 1997;277:1238‐43. [PubMed] [Google Scholar]
Ron 1998
- Ron E, Doody MM, Becker DV, Brill AB, Curtis RE, Goldman MB, et al. Cancer mortality following treatment for adult hyperthyroidism. Cooperative Thyrotoxicosis Therapy Follow‐up Study Group. JAMA 1998;280(4):347‐55. [DOI] [PubMed] [Google Scholar]
Sawin 1994
- Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. New England Journal of Medicine 1994;331:1249‐52. [DOI] [PubMed] [Google Scholar]
Sridama 1984
- Sridama A, McCormick M, Kaplan EL, Fauchet R, DeGrool LJ. Long‐term follow up study of compensated low dose 131I therapy. New England Journal of Medicine 1983;311:426‐32. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Thornton 2007
- Thornton J, Kelly SP, Harrison RA, Edwards R. Cigarette smoking and thyroid eye disease: a systematic review. Eye (London, England) 2007;21:1135‐45. [DOI] [PubMed] [Google Scholar]
Vanderpump 1995
- Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty‐year follow‐up of the Whickham survey. Clinical Endocrinology 1995;43:55‐68. [DOI] [PubMed] [Google Scholar]
Vitti 1997
- Vitti P, Rago T, Chiovato L, Pallini S, Santini F. Clinical features of patients with Graves' disease undergoing remission after antithyroid drug treatment. Thyroid 1997;7:369‐75. [DOI] [PubMed] [Google Scholar]
Wartofsky 1991
- Wartofsky L, Glinoer D, Solomon B, Nagataki S, Lagasse R, Nagayama Y, et al. Differences and similarities in the diagnosis and treatment of Graves' disease in Europe, Japan, and the United States. Thyroid 1991;1:129‐35. [DOI] [PubMed] [Google Scholar]
Watson 1988
- Watson AB, Brownlie BE, Frampton CM, Turner JG, Rogers TG. Outcome following standardized 185MB dose 131I therapy for Graves' disease. Clinical Endocrinology 1988;28(5):487‐96. [DOI] [PubMed] [Google Scholar]
Wong 2006
- Wong SSL, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association 2006;94(1):41‐7. [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


