Abstract
Background
Hereditary and wild-type transthyretin-mediated (ATTRv and ATTRwt) amyloidoses result from the misfolding of transthyretin and aggregation of amyloid plaques in multiple organ systems. Diagnosis of ATTR amyloidosis is often delayed due to its heterogenous and non-specific presentation. This review investigates the association of musculoskeletal (MSK) manifestations with ATTR amyloidosis and the delay from the onset of these manifestations to the diagnosis of ATTR amyloidosis.
Methods
This systematic review utilized Medline and EMBASE databases. Search criteria were outlined using a pre-specified patient, intervention, comparator, outcome, time, study (PICOTS) criteria and included: amyloidosis, ATTR, and MSK manifestations. Publication quality was assessed utilizing Joanna Briggs Institute (JBI) critical appraisal checklists.
The search initially identified 7,139 publications, 164 of which were included. PICOTS criteria led to the inclusion of epidemiology, clinical burden and practice, pathophysiology, and temporality of MSK manifestations associated with ATTR amyloidosis. 163 publications reported on ATTR amyloidosis and MSK manifestations, and 13 publications reported on the delay in ATTR amyloidosis diagnosis following the onset of MSK manifestations.
Results
The MSK manifestation most frequently associated with ATTR amyloidosis was carpal tunnel syndrome (CTS); spinal stenosis (SS) and osteoarthritis (OA), among others, were also identified. The exact prevalence of different MSK manifestations in patients with ATTR amyloidosis remains unclear, as a broad range of prevalence estimates were reported. Moreover, the reported prevalence of MSK manifestations showed no clear trend or distinction in association between ATTRv and ATTRwt amyloidosis.
MSK manifestations precede the diagnosis of ATTR amyloidosis by years, and there was substantial variation in the reported delay to ATTR amyloidosis diagnosis. Reports do suggest a longer diagnostic delay in patients with ATTRv amyloidosis, with 2 to 12 years delay in ATTRv versus 1.3 to 1.9 years delay in ATTRwt amyloidosis.
Conclusion
These findings suggest that orthopedic surgeons may play a role in the early diagnosis of and treatment referrals for ATTR amyloidosis. Detection of MSK manifestations may enable earlier diagnosis and administration of effective treatments before disease progression occurs.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-023-06853-5.
Introduction
Background
Systemic amyloidoses are protein-misfolding diseases characterized by the aggregation and deposition of amyloid plaques in multiple organ systems [1, 2]. Transthyretin-mediated (ATTR) amyloidosis is caused by misfolding of the precursor protein transthyretin (TTR) [1, 2]. There are two types of ATTR amyloidoses, variant (ATTRv) [also known as hereditary or hATTR] and wild-type (ATTRwt) [2, 3]. In ATTRv amyloidosis, point variants in the TTR gene lead to destabilization and dissociation of TTR from its native tetrameric conformation, and subsequent aggregation as amyloid fibrils [4]. In ATTRwt amyloidosis, wild-type, non-variant TTR dissociates, and amyloid aggregation occurs [4]. ATTRwt and ATTRv amyloidoses overlap in their clinical presentation, and therefore, definitive distinction relies on TTR gene sequencing in suspected patients [2]. ATTRv amyloidosis affects approximately 50,000 people worldwide. While the exact prevalence of ATTRwt amyloidosis is unknown, it is thought to be more prevalent than ATTRv amyloidosis [1, 5].
ATTR amyloidosis is a heterogeneous, multisystem disease in which a significant proportion of patients develop a mixed phenotype of polyneuropathy (PN) and cardiomyopathy (CM) [2, 5, 6]. The disease is rapidly progressive; ATTRv and ATTRwt amyloidoses have a median survival of 4.7 years and 3.6 years after diagnosis, respectively, and disease progression substantially negatively impacts quality of life [5, 7, 8]. Diagnosis can be difficult or delayed due to the heterogenous, non-specific nature of ATTR amyloidosis and symptom overlap with other diseases [9–11]. Various musculoskeletal (MSK) manifestations, such as carpal tunnel syndrome (CTS), spinal stenosis (SS), osteoarthritis (OA), and others, have been reported in patients with ATTR amyloidosis [1]. Importantly, these MSK manifestations have been shown to precede the diagnosis of the disease by years [1, 4, 11].
Rationale
The typical patient journey before being diagnosed with ATTR amyloidosis is lengthy and involves consulting numerous physicians from different specialties [2, 11]. Consequently, ATTR amyloidosis may remain undetected, and treatment is often delayed until the disease progresses to an advanced stage. This diagnostic delay increases patient disability and morbidity, whereas earlier therapeutic intervention can attenuate disease progression and worsening in patient quality of life. [2]. Enabling earlier diagnosis of ATTR amyloidosis is critical to improving overall patient prognosis [1]. Various MSK manifestations have been reported in the literature to be associated with ATTR amyloidosis. Additionally, certain manifestations, such as CTS, symptoms of which can also be caused by the PN of ATTR amyloidosis, are already included among the early signs, which are considered ‘red flags’ for the disease.
This systematic review was conducted to investigate the association between ATTR amyloidosis and MSK manifestations, and to investigate the temporal association between the onset of MSK manifestations and ATTR amyloidosis diagnosis.
Methods
Search strategy and criteria
The protocol for this systematic review is registered on the international prospective register of systematic reviews (PROSPERO) from the National Institute for Health Research Database (www.crd.york.ac.uk/prospero; protocol no. CRD42022310956), and the PRISMA statement was adhered to [12].
An electronic database search was run on November 3, 2021 across two databases in Ovid®: Medline and EMBASE. No restriction on publication year was applied. Search strategies are detailed in Supplement 1.
Gray literature searches included hand searches of previously published systematic reviews and a review of conference proceedings from 2019 to 2021. Independent hand searches of conference proceedings were conducted for the American Association for Hand Surgery (AAHS), American Society for Surgery of the Hand (ASSH), European Society of Cardiology (ESC), European ATTR amyloidosis meeting (EU-ATTR), Federation of European Societies for the Surgery of the Hand (FESSH), International Federation of Societies for Surgery of the Hand (IFSSH), International Society of Amyloidosis (ISA), and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Conferences of interest that were not independently hand searched, given that the EMBASE electronic database search already captured their proceedings, included the American College of Cardiology (ACC), Heart Failure Society of America (HFSA), and the Peripheral Nerve Society (PNS).
Inclusion and exclusion criteria
The inclusion and exclusion criteria were pre-defined in a patient, intervention, comparator, outcome, time, study (PICOTS) table during protocol development (Supplement 2). These included outcomes related to the epidemiology, pathophysiology, temporal association (the time from the diagnosis of the MSK manifestation(s) to the diagnosis of ATTR amyloidosis), clinical burden, and current clinical practice related to MSK manifestations associated with ATTR amyloidosis. Publications reporting data only from patients diagnosed with amyloidoses other than ATTR amyloidosis were excluded, as were publications reporting on outcomes related to MSK manifestations outside of an ATTR amyloidosis context and/or publications reporting separately on either ATTR amyloidosis or MSK manifestations. Case series were included, while case reports involving individual patients were excluded [13]; for the list of those case reports by MSK manifestation, refer to Supplement 3.
All abstracts and full texts included were screened by two separate reviewers. Conflicts on inclusion or exclusion were resolved by a third senior reviewer.
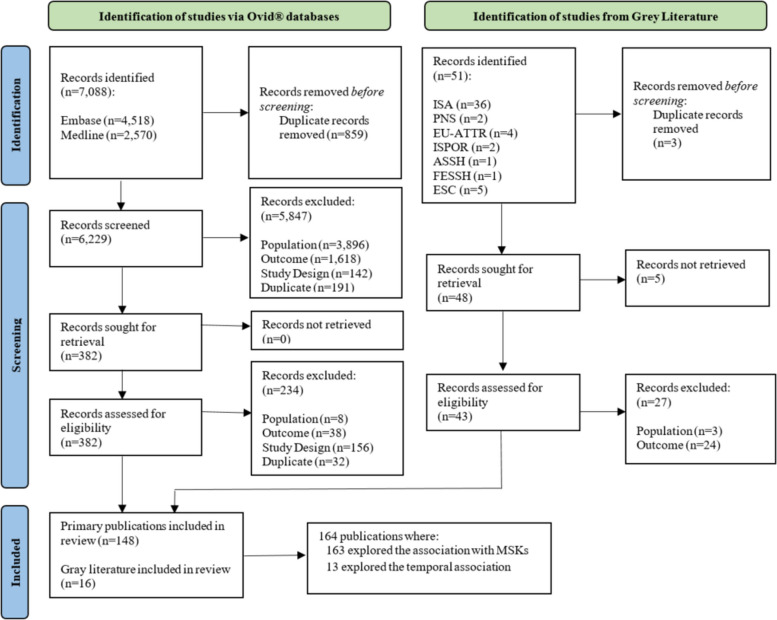
Of the 7,139 publications identified, 164 publications were included in the analysis, as shown in the PRISMA diagram, (Fig. 1). Importantly, authors of the publications included approached the association between MSK manifestations and presence of ATTR amyloidosis differently. For example, some authors investigated MSK manifestations in patients with a confirmed diagnosis of ATTR amyloidosis, whereas other authors investigated the presence of ATTR amyloidosis in patients who had undergone treatment for MSK manifestations or who were diagnosed with a MSK manifestation, presented in Table 1.
Fig. 1.
PRISMA flow diagram of the study identification and screening process. ASSH = American Society for Surgery of the Hand; ESC = European Society of Cardiology; EU-ATTR = European transthyretin-mediated amyloidosis meeting; FESSH = Federation of European Societies for Surgery of the Hand; n = number; ISA = International Society of Amyloidosis; ISPO = International Society for Pharmacoeconomics and Outcomes Research; PNS = Peripheral Nerve Society; SLR = systematic literature review
Table 1.
Cross-tabulation of the number of publications investigating the association between ATTR amyloidosis and MSK manifestations, and the direction of the association reported
| MSK manifestation reported in the included publications | Total number of publications identified | Number of publications that reported ATTR amyloidosis in patients with MSK manifestations | Number of publications that reported MSK manifestations in patients with ATTR amyloidosis |
|---|---|---|---|
| Publications where one MSK manifestation was reported | |||
| Carpal tunnel syndrome | 109 | 22 | 87 |
| Spinal stenosis | 9 | 2 | 7 |
| Osteoarthritis | 8 | 8 | 0 |
| Trigger finger | 1 | 1 | 0 |
| Publications where more than one MSK manifestation was reported | |||
| Carpal tunnel syndrome and spinal stenosis | 20 | 3 | 17 |
| Carpal tunnel syndrome, spinal stenosis, and trigger finger | 2 | 0 | 2 |
| Carpal tunnel syndrome, spinal stenosis, and osteoarthritis | 2 | 0 | 2 |
| Carpal tunnel syndrome, spinal stenosis, and biceps tendon rupture | 2 | 2 | 0 |
| Hip arthroplasty and knee arthroplasty (osteoarthritis) | 2 | 0 | 2 |
| Othera | 8 | 2 | 6 |
aATTR amyloidosis in patients where more than one musculoskeletal manifestation was reported and multiple musculoskeletal manifestations in patients with ATTR amyloidosis, refer to Table 7
One hundred sixty-three publications examined the association between MSK manifestations and ATTR amyloidosis (Fig. 2 provides an overview of studies and Tables 2, 3, 4, 5, 6 and 7 provide study details), and 13 publications investigated the temporal association between MSK manifestations and ATTR amyloidosis (Fig. 3 with study details reported in Table 8). One publication reported only on the temporal delay and did not report on the association between MSK manifestations and ATTR amyloidosis.
Fig. 2.
Number of publications reporting on various MSK manifestations associated with ATTR amyloidosis. Eight other publications reported on the association between ATTR amyloidosis and several different MSK manifestations in various combinations, the details of which are reported in Table 7
Table 2.
Carpal tunnel syndrome in patients with ATTR amyloidosis
| Publication | Study design | Research population | Prevalence | Additional statistics | Information relating to and/or confirming diagnosisa |
|---|---|---|---|---|---|
| Carpal tunnel syndrome in ATTR amyloidosis | |||||
| Abe et al., 2021 [14] | Case Series | 90 patients with ATTR amyloidosis (type unspecified) | 7.8% with CTS | NR |
MSK biopsy and staining (tenosynovial tissue within the transverse carpal ligament) Other biopsy and staining MS Autopsy |
| 44 patients with ATTRv amyloidosis | 18.2% with CTS | NR | |||
| 106 patients with ATTRwt amyloidosis | 34.9% with CTS | NR | |||
| Akinboboye et al., 2015 [15] | Cross-sectional survey | 14 patients with ATTRv amyloidosis (Val122Ile point mutation) | 14% with CTS | NR |
Gene testing and/or point mutation testing in patients Point mutations: Val122Ile |
| Ando et al., 2021 [16] | Case series | 1,937 patients with ATTR amyloidosis (type unspecified) | ATTRwt: 17% with CTS | NR |
MSK biopsy and staining Other biopsy and staining Myocardium, submucosa, skin (with fat) Gene and/or point mutation testing Point mutations: (V30M, p.TTR, V50M, P24S, A25T, V28M, V28S, V30A, F33V, A36D, A45D, G47V, G47R, T49I, T49S, S50I, S50R, G53E, E54L, L55P, T59R, T60A, E61K, S77Y, K80R, G83R, E89K, E89Q, V94G, A97G, R104H, I107V, Y114C, Y114S, and V122I) MS |
| ATTRv: 6.8% with CTS | NR | ||||
| Arevalo et al., 2020 [17] | Database/registry | 12,745 patients with cardiac ATTR amyloidosis (type unspecified) | 60 (0.5%) with CTS | Multivariate linear regression adjustment for confounders of age, gender, race, hypothyroidism, and diabetes mellitus showed a significant relationship between cardiac ATTR amyloidosis and CTS OR=4.31, 2.46–7.56 (OR 95% CI), p<0.001 | NR |
| Bishop et al., 2018 [18] | Case series | 52 patients with ATTR amyloidosis (type unspecified) | 27 (51.9%) with CTS | NR |
Cohort 1: Biopsy and staining, MS |
| 67 patients with ATTRv amyloidosis | NR | 4.57 (RR) with 2.58–8.09 (95% CI) of CTS leading to ATTR diagnostic delay |
Cohort 2: Biopsy and staining |
||
| Bukhari et al., 2020 [19] | Case series | 45 patients with ATTR amyloidosis (type unspecified) | 62% with CTS, p<0.01 | NR |
LV wall thickening 99mPYP/DPD imaging |
| Bukhari et al., 2021 [20] | Case series | 125 Caucasian patients with ATTR amyloidosis (94% ATTRwt; 6% ATTRv) | 76% with bilateral CTS | NR |
99mPYP/DPD imaging Gene and/or point mutation testing Point mutation s: pV50M, pT80A |
| 20 African American patients with ATTR amyloidosis (25% ATTRv; 75% ATTRwt) | 81% with bilateral CTS | NR | |||
| Cappellari et al., 2009 [21] | Case series | 14 patients with ATTRv amyloidosis | 1 with CTS | NR |
Biopsy and staining Gene and/or point mutation testing Point mutations: Val30Met, Phe64Leu, Asn124Ser, Glu89Gln, Val122Ile, Ile107Phe, Thr49Ala, Ser50Arg |
| ss Cappellari et al., 2011 [22] | Case series | 17 patients with ATTRv amyloidosis | 3 (17.6%) with CTS | NR |
Biopsy and staining Gene and/or point mutation testing Point mutation s: p.Val30Met, p.Arg34Thr, p.Thr49Ala, p.Ser50Arg, p.Phe64Leu, p.Glu89Gln, and p.Ile107Phe |
| Cappelli et al., 2021 [23] | Cross-sectionala | 168 patients with ATTR amyloidosis (type unspecified) | 122 (73%) with CTS | NR |
Point mutation testing: Point mutations: le68leu, Val122ile, other (unspecified) |
| Cerudelli et al., 2019 [24] | Case series | 81 patients with ATTR amyloidosis (type unspecified) | 20% with CTS | NR | 99mPYP/DPD imaging |
| Chen et al., 2021 [25] | Case series | 29 patients with ATTRv amyloidosis | 17 (57.5%) with CTS | Prevalence by age: 15 (62.5%) aged 61(51–70) years old; 2 (40%) aged 42(30–48%) years old |
Biopsy and staining LV wall thickening Point mutation testing |
| Choi et al., 2020 [26] | Case series | 135 patients with ATTR amyloidosis (type unspecified) | 15 with CTS | NR | NR |
| Cipriani et al., 2019 [27] | Case series | 18 patients with ATTRwt amyloidosis | 12 (66%) with CTS | NR |
Biopsy and staining Gene and/or point mutation testing |
| Cortese et al., 2014 [28] | Case series | 150 patients with ATTRv amyloidosis | 41% with CTS | NR |
Gene and/or point mutation testing Point mutations: Val30Met, Glu89Gln, Phe64Leu, Ile68Leu |
| Minutoli et al., 2010 [29] | Case series | 16 patients with ATTRv amyloidosis | 3 (18.8%) with CTS | NR |
LV wall thickening Gene and/or point mutation testing Point mutations: Glu89Gln, Phe64Leu, Thr49A, Gly6Ser 99mPYP/DPD imaging |
| Du et al., 2021 [30] | Case series | 54 patients with ATTRv amyloidosis | 14 (25.9%) with CTS | NR |
Biopsy and staining Gene and/or point mutation testing Point mutations: Val30Met, Ala97Ser |
| 12 patients with ATTRv amyloidosis (Val30Met point mutation) | 2 (16.7%) with CTS | NR | |||
| 7 patients with ATTRv amyloidosis (Ala97Ser point mutation) | 4 (57.1%) with CTS | NR | |||
| Durmus et al., 2014 [31] | Case series | 14 patients with ATTRv amyloidosis | 5 with CTS | NR |
Gene and/or point mutation testing Point mutations: Val30Met, Glu89Gln, Gly53Glu, Glu74Gly, Gly47Glu, Glu109Gly |
| Durmus et al., 2012 [32] | Case series | 10 patients with ATTRv amyloidosis | 1 with CTS | NR |
Gene and/or point mutation testing Point mutations: Glu89Gln |
| Erdogan et al., 2020 [33] | Database/registry | 44 patients with ATTRv amyloidosis | 10 with CTS | NR |
Biopsy and staining Gene and/or point mutation testing Point mutations: Val30Met, Glu89Gln, Gly47Ala, Gly47Glu, Gly53Glu, Glu54Gly, Val32Ala, Asp18Asn and Ala45Thr. |
| Eriksson et al., 2009 [34] | Case series | 33 patients with ATTR amyloidosis (ATTRv and ATTRwt) | 3 with CTS (all ATTRwt) | NR |
MSK biopsy and staining with Congo red (unspecified tissues obtained at CTRS) Gene and/or point mutation testing |
| Gabrovesk et al., 2019 [35] | Case series | 96 patients with ATTR amyloidosis (76% ATTRv [Val122Ile]; 22% ATTRwt; 1% ATTRv [Asp18Asn]; 1% ATTRv [Glu54Gln]) | 46% with CTS | NR |
Biopsy and staining Gene and/or point mutation testing Point mutations: Val122Ile |
| Gagliardi et al., 2018 [36] | Case series | 82 patients with ATTRwt amyloidosis | 30 (37%) with CTS | NR |
Biopsy and staining LV wall thickening Gene and/or point mutation testing Point mutations: Ile68Leu |
| 67 patients with ATTRv amyloidosis | 29 (43%) with CTS | NR | |||
| Galat et al., 2016 [37] | Case series | 17 patients with ATTR amyloidosis (13 ATTRwt; 1 ATTRv; 3 unspecified) | 5 with CTS | NR |
Biopsy and staining Gene and/or point mutation testing Point mutations: Val122I 99mPYP/DPD imaging |
| Gawor et al., 2018 [38] | Case series | 4 patients with ATTRv amyloidosis (3 Phe33Leu; 1 Ala81Val) | 2 with CTS | NR |
LV wall thickening Gene and/or point mutation testing Point mutations: Phe33Leu |
| Gawor et al., 2019 [39] | Case series | 6 patients with ATTRwt amyloidosis | 4 (75%) with CTS | NR |
LV wall thickening Gene and/or point mutation testing 99mPYP/DPD imaging |
| Gawor et al., 2020 [40] | Case series | 58 patients with ATTRv amyloidosis | 4 with CTS | NR | NR |
| Gentile et al., 2020 [41] | Case series | 9 patients with ATTRv amyloidosis | 6 (67%) with CTS | NR |
Gene and/or point mutation testing Point mutations: V122I and E89Q 99mPYP/DPD imaging |
| Goena et al., 2021 [42] | Case series | 181 patients with suspected ATTR amyloidosis (type unspecified) | NR | CTS as a predictor of ATTR diagnosis: OR=15.02; 95% CI (3.66–61.65); p<0.001 | 99mPYP/DPD imaging |
| Gospodinova et al., 2015 [43] | Cross-sectional | 40 patients with ATTRv amyloidosis (Glu89Gln point mutation) | 17 (42.5%) with CTS | NR | NR |
| Hewitt et al., 2020 [44] | Case series | 15 patients with ATTRv amyloidosis (T60A point mutation) | 5 (33%) with CTS | NR |
Biopsy and staining Point mutation testing Point mutations: T60a |
| Hussain et al., 2019 [45] | Case series | 12 patients with ATTRv amyloidosis | 1 with CTS | NR |
Biopsy and staining Point mutation testing 99mPYP/DPD imaging |
| Jercan et al., 2020 [46] | Case series | 23 patients with ATTRv amyloidosis (18 with Glu54Gln point mutation) | 7 (53%) with CTS | NR |
Point mutation testing Point mutations: Glu54Gln |
| Kaku et al., 2020 [47] | Case series | 92 patients with ATTRv amyloidosis | 72% with CTS | NR | NR |
| Kalinoski-Dubose et al., 2020 [48] | Case series | 26 patients with ATTRv amyloidosis (male) | 84% with CTS | NR |
LV wall thickening Point mutation testing Point mutations: V122I |
| 12 patients with ATTRv amyloidosis (female) | 36% with CTS | NR | |||
| Karam et al., 2019 [49] | Case series | 23 patients with ATTRv amyloidosis | 17 with CTS | Age of CTS symptom onset 48.5(36–63) years old |
Point mutation testing Point mutations: Val30Met, Glu89Gln, Gly53Glu, Glu54Gly, Gly47Glu |
| Keller et al., 2021 (hATTR Compass study) [50] | Database/registry | 28 patients with ATTRv amyloidosis (“rare unspecified” point mutations | 30% with CTS | NR |
Point mutation testing Point mutations: p.V142I, p.T80A, unspecified |
| 466 patients with ATTRv amyloidosis (V142I point mutation) | 23% with CTS | NR | |||
| 15 patients with ATTRv amyloidosis (T80A point mutation) | 44% with CTS | NR | |||
| Kessler et al., 2019 [51] | Cross-sectional survey | 68 patients with ATTR amyloidosis (type unspecified) | 5 (5%) with CTS | 17 patients received their diagnosis within 6 months of initial symptoms, average of 3.6 years until diagnosis reached |
MSK biopsy and staining Other biopsy and staining Gene and/or point mutation testing 99mPYP/DPD imaging |
| Khella et al., 2021 [52] | Database/registry | 345 patients with ATTRv amyloidosis | 90 (26%) with CTS | NR | Point mutation testing |
| Khella et al., 2021 [53] | Database/registry | 14 patients with ATTRv amyloidosis (p.V142l) | 22% with CTS | NR |
Point mutation testing Point mutations: p.V142I |
| Khella et al., 2021 [54] | Database/registry | 37 patients with ATTRv amyloidosis (p.V50M) | 15% with CTS | NR | Point mutation testing |
| 42 patients with ATTRv amyloidosis (p.T80A) | 35% with CTS | NR | Point mutations: p.V50M, p.T80A | ||
| Khella et al., 2021 (hATTR Compass) [55] | Database/registry | 321 patients with ATTRv amyloidosis (V142l/V122 point mutations) | 20% with CTS | NR | NR |
| Kristen et al., 2010 [56] | Case series | 24 patients with ATTRwt amyloidosis | 2 (8.3%) with CTS | NR | Gene testing |
| Kristen et al., 2016 [57] | Case series | 191 patients with ATTRwt amyloidosis | 87 (48.3%) with CTS | NR | Gene testing |
| La Malfa et al., 2019 [58] | Case seriesb | 21 patients with ATTRwt amyloidosis | 9.0% with CTS | NR | 99mPYP/DPD imaging |
| Longhi et al., 2014 [59] | Case series | 260 patients with ATTR amyloidosis (type unspecified) | 90 (35.0%) with CTS (18 ATTRwt; 72 ATTR unspecified) | NR | NR |
| Longhi et al., 2015 [60] | Case series | 5 patients with ATTRwt amyloidosis | 2 with CTS | NR |
LV wall thickening Gene testing 99mPYP/DPD imaging |
| Luigetii et al., 2012 [61] | Case series | 15 patients with ATTRv amyloidosis | 13 (86%) with CTS | Delay of 4.3±2.4 years from ATTR symptom onset to ATTR diagnosis |
Biopsy and staining (Abdominal fat, sural nerve) Point mutation testing Point mutations: p.Val30Met, p.Phe64Leu, p.Ala120Ser |
| Malladi et al., 2019 [62] | Database/registry | 562 patients with ATTRv amyloidosis | 110 (20%) with CTS | NR |
Point mutation testing Point mutations: Val122Ile, Val30Met, and Thr60Ala |
| Martone et al., 2019 [63] | Case series | 70 black patients with ATTRv amyloidosis (V122l point mutation) | 64% with CTS | NR |
Biopsy and staining Point mutation testing Point mutations: V122I 99mPYP/DPD imaging |
| 19 Caucasian patients with ATTRv amyloidosis (V122l point mutation) | 31% with CTS | NR | |||
| Merli et al., 2019 [64] | Case series | 11 patients with amyloidosis (8 ATTRwt; 3 types unspecified) | 6 with CTS | NR | 99mPYP/DPD imaging |
| Milandri et al., 2016 [65] | Case series | 109 patients with ATTRv amyloidosis (Glu89Gln point mutation) | >50.0% with CTS | NR | NR |
| Nakagawa et al., 2016 [66] | Case series | 31 patients with ATTRwt amyloidosis | 17 (55.0%) with CTS | NR |
MSK biopsy and staining (Tenosynovial tissue) Biopsy and staining Gene testing 99mPYP/DPD imaging |
| Ng et al., 2020 [67] | Case series | 11 patients with ATTRv amyloidosis (p.Ala117Ser) | 3 with CTS | NR | NR |
| Oike et al., 2021 [68] | Case series | 113 patients with ATTRwt amyloidosis | 40 (47.0%) with CTS | NR |
Biopsy and staining Gene testing 99mPYP/DPD imaging |
| Papoutsidakis et al., 2017 [69] | Case series | 17 patients with ATTR amyloidosis (type unspecified) | 8 (47.0%) with CTS | NR | 99mPYP/DPD imaging |
| Pastorelli et al., 2016 [70] | Case series | 20 patients with ATTRwt amyloidosis | 15 (75.0%) with CTS | NR | NR |
| Patel et al., 2021 [71] | Cross-sectionalb | 107 patients with ATTRwt amyloidosis | 38% with CTS | NR | LV wall thickening |
| Peltier et al., 2020 [72] | Case series | 9 patients with ATTRv amyloidosis (V142l point mutation) | 1 (11.0%) with CTS | NR |
Point mutation testing Point mutations: V122I |
| Peltier et al., 2021 [73] | Case series | 18 patients with ATTRv amyloidosis (V142l point mutation) | 11 (61.0%) with CTS | NR |
Point mutation testing Point mutations: p.V142I 99mPYP/DPD imaging |
| Pinney et al., 2011 [74] | Case series | 55 patients with ATTRwt amyloidosis | 24 (43.6%) with CTS | 7.04(0.54–8.41) survival from symptom onset; 4.58(0.07–5.41) survival from diagnosis |
MSK biopsy and staining (carpal tunnel tissue) |
| Plante-Bordeneuve et al., 2019 [75] | Case series | 28 patients with ATTRv amyloidosis (asymptomatic) | 5 with CTS (and subsequent CTRS) | NR |
MSK biopsy and staining (carpal tunnel nerve) Other biopsy and staining Point mutations: Val30Met 99mPYP/DPD imaging |
| Quarta et al., 2013 [76] | Case series | 190 patients with ATTRv amyloidosis (Ile68Leu point mutation) | 35.0% with CTS | NR |
Gene and/or point mutation testing Point mutations: Ile68Leu |
| Quarta et al., 2017 [77] | Case series | 97 patients with ATTRwt amyloidosis | 45 (46.0%) with CTS | NR |
LV wall thickening 99mPYP/DPD imaging |
| Ruiz Hueso et al., 2021 [78] | Cross-sectional | 13 patients with amyloidosis (84.4% ATTRwt; 7.7% ATTRv; 7.7% non-ATTR) | 30.0% with CTS (amyloidosis type not specified) | NR |
Gene and/or point mutation testing 99mPYP/DPD imaging |
| Russo et al., 2011 [79] | Case series | 23 patients with ATTRv amyloidosis (13 Glu89Gln; 8 Phe64Leu; 2 Thr49Ala) | 3 with CTS | NR |
Gene and/or point mutation testing Point mutations: Glu89Gln, Phe64Leu, Thr49Ala |
| Russo et al., 2012 [80] | Case series | 18 patients with ATTRv amyloidosis | 3 (16.7%) with CTS | NR |
LV wall thickening Point mutation testing 99mPYP/DPD imaging |
| Russo et al., 2019 [81] | Database/registry | 260 patients with ATTRv amyloidosis | 73 with CTS | NR |
MSK biopsy and staining Point mutation testing Gene mutations: Phe64Leu, Val30Met, Glu89Gln |
| Salvalaggio et al., 2021 [82] | Cross-sectionalb | 62 patients with ATTRv amyloidosis | 49 (79.0%) with CTS | NR |
Gene and/or point mutation testing Point mutations: Phe64Leu, Val30Met, Glu89Gln, Ile68Leu, Thr49Ala, Tyr78Phe, Ala120Ser, Ala36Pro, Arg34Thr, Glu62Lys, Gly47Ala |
| Salvi et al., 2012 [83] | Database/registryb | 131 patients with ATTRv amyloidosis | 46 (35.1%) with CTS | 3(0–13) years delay between clinical ATTRv onset and ATTR diagnosis |
Point mutation testing Point mutations: 30 Met, 68 Leu, 34 Thr, 89 Glu, 49 Ala, 34 Thr, 36 Pro, 50 Arg, 47 Arg, 54 Lys, 23 Asn, 53 Ala, 30 Ala, 33 Val, 50 Ser, 14 Leu, 88 Arg, 59 Lys, 54 Gln |
| Saturi et al., 2020 [84] | Database/registry | 4,418 patients with ATTRv amyloidosis | 18.6% males with CTS | NR | NR |
| 15.5% females with CTS | NR | ||||
| Shah et al., 2020 [85] | Case series | 130 patients with ATTRv amyloidosis | 22.3% with CTS | NR |
Point mutation testing Point mutations: V142I/V122I |
| Shah et al., 2021 [86] | Case series | 397 patients with ATTRv amyloidosis | 24.0% with CTS | NR | Point mutation testing |
| Shah et al., 2021 [87] | Case series | 586 patients with ATTRv amyloidosis | 25.0% with CTS | NR |
Point mutation testing Point mutations: p.V142I/V122 |
| Silva-Hernández et al., 2020 [88] | Case series | 30 patients with ATTRv amyloidosis | 15 (20.0%) with CTS | NR | NR |
| Slama et al., 2021 [89] | Database /registry | 4,815 patients with ATTR amyloidosis (type not specified) | 18.8% with CTS | NR | NR |
| Soper et al., 2021 [90] | Database/registry | 32 patients with ATTRv amyloidosis (V142l) | 10 (31.0%) with CTS | NR | NR |
| Sousa Paiva et al., 2021 [91] | Case series | 30 patients with ATTR amyloidosis (25 ATTRwt; 5 ATTRv [3 Val50Met; 2 Val142lle]) | 8 (26.7%) with CTS | NR |
Biopsy and staining (endomyocardial tissues) Gene and/or point mutation testing Point mutations: Val50Met and Val142lle |
| Svendsen et al., 1998 [92] | Cross-sectional | 25 patients with ATTRv amyloidosis | 9 (36.0%) with CTS | NR |
MSK biopsy and staining with Congo red (synovial specimen) LV wall thickening Point mutation testing Autopsy |
| Tzagournissakis et al., 2015 [93] | Case series | 17 patients with ATTRv amyloidosis (Met30) | 4 with CTS | NR |
Point mutation testing Point mutations: Met30 |
| Tzagournissakis et al., 2020 [94] | Case series | 10 patients with ATTRv amyloidosis (p.Val114Ala) | 10 (100.0%) with CTS | NR |
Biopsy and staining Point mutation testing: p.Val114Ala |
| Warner et al., 2019 [95] | Case series | 32 patients with ATTR amyloidosis (type unspecified) | 31.0% with CTS | NR |
Biopsy and staining LV wall thickening 99mPYP/DPD imaging |
| Yamada et al., 2020 [96] | Cross-sectional | 129 patients with ATTRwt amyloidosis | 57 (54.0%) with CTS | NR |
Biopsy and staining with Congo red (endomyocardial tissues) Gene testing 99mPYP/DPD imaging |
| Yamashita et al., 2020 [97] | Case series | 1,937 patients with amyloidosis (13.4% ATTRv; 14.3% ATTRwt; 4.6% ATTR type unspecified) | In 5.6% of total amyloidosis patients (including non-ATTR patients) the initial manifestation of disease was CTS | Point mutations: V30M from an endemic area (7.4%), V30M from a non-endemic area (51.2%), and non-V30M (41.4%) | |
| Zadok et al., 2020 [98] | Case series | 26 patients with ATTR amyloidosis (type unspecified) | 62.0% with CTS | NR | NR |
| Zampino et al., 2021 [99] | Case series | 56 patients with ATTRv amyloidosis | 31 patients with V122l point mutation: 30 (97.0%) with CTS | CTS symptoms preceded ATTR diagnosis by >7years in 30% of patients with V1221 |
Biopsy and staining with Congo red (skin) Point mutation testing Point mutations: V122I |
| 12 patients with V30M point mutation: 7 (57.0%) with CTS | CTS symptoms preceded ATTR diagnosis by >7years in 29% of patients with V30M | ||||
| 13 patients with L58H point mutation: 10 (77.0%) with CTS | CTS symptoms preceded ATTR diagnosis by >7years in 30% of patients with L58H | ||||
| Zivkovic et al., 2020 [100] | Case series | 7 patients with ATTRwt amyloidosis | 100.0% with CTS | NR | NR |
aBiopsy and staining of tissues described as other refers to instances where diagnosis was confirmed through staining of non-MSK biopsied tissues, such as endomyocardial tissue
bData from patients with amyloidosis was compared to control patients in these publications
AS Aortic stenosis, ATTR Transthyretin-mediated amyloidosis, ATTRv Hereditary transthyretin amyloidosis, ATTRwt Wild-type transthyretin amyloidosis; confidence interval, CTS Carpal tunnel syndrome, DPD 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid, LV Left ventricular, MS Mass spectrometry, MSK Musculoskeletal, NR Not reported, OR Odds ratio: PYP = technetium-99m pyrophosphate, RR Risk ratio, SSA Senile systemic amyloidosis, TTR Transthyretin
Table 3.
ATTR amyloidosis in patients with carpal tunnel syndrome
| Publication | Study design | Research population | Prevalence | Additional statistics | Information relating to and/or confirming diagnosisa |
|---|---|---|---|---|---|
| ATTR amyloidosis in carpal tunnel syndrome | |||||
| Bäcker et al., 2021 [101] | Case series | 699 patients undergoing CTRS | 10 (1.4%) with amyloidosis (type unspecified) | NR | MSK biopsy and staining with Congo red (tenosynovium within the carpal tunnel obtained at CTRS) |
| Bastkjær et al., 2020 [102] | Case series | 100 patients with CTS | 13 (13.0%) with ATTR amyloidosis (type unspecified) | NR | MSK biopsy and staining with Congo red (Tenosynovial and fatty tissue) |
| Breda et al., 1993 [103] | Case series | 98 patients with CTS | 12 (12.2%) with amyloidosis (type unspecified) | NR |
MSK biopsy and staining (tenosynovial and flexor retinaculum tissues obtained at CTRS) Staining method NR |
| Fernandez et al., 2017 [104] | Case series | 147 patients with CTS | 29 (19.7%) with amyloidosis (type unspecified) | NR | MSK biopsy and staining with Congo red (carpal transverse ligament) |
| Fosbol et al., 2019 [105] | Database/registry | 56,032 patients undergoing CTRS | NR | CTS was associated with a future diagnosis of amyloidosis (type unspecified): HR of 12.2 (95% CI: 4.37–33.60), p <0.0001 | NR |
| Gioeva et al., 2013 [106] | Case series | 98 patients who underwent CTS biopsy | 98 (100.0%) with ATTR amyloidosis (11 ATTRv, 70 ATTRwt, 17 ATTR unspecified due to lack of genomic DNA available for testing) | NR |
MSK biopsy and staining with Congo red (tissues of the transversal carpal ligament) Gene and/or point mutation testing Point mutations: p.G6S & p.M13I |
| Hahn et al., 2018 [107] | Database/registry | 582 patients with CTS | 68 (11.7%) with ATTR amyloidosis (type unspecified) | NR |
MSK biopsy and staining (tenosynovial and flexor retinaculum tissues obtained at CTRS) Stanning method NR |
| Hansen et al., 2020 [108] | Case series | 182 patients undergoing CTRS | 25 (14.0%) with ATTR amyloidosis (type unspecified) | NR |
MSK biopsy and staining with Congo red (unspecified tissues obtained at CTRS) Gene and/or point mutation testing 99mPYP/DPD imaging MS |
| Itzhaki et al., 2020 [109] | Case series | 36 patients with history of CTRS | 16 (44.5%) with ATTR amyloidosis (type unspecified) | NR |
Biopsy and staining with Congo red (endomyocardial tissues) LV wall thickening Gene and/or point mutation testing 99mPYP/DPD imaging |
| Milandri et al., 2020 [110] | Database/registry | 57 patients with history of CTRS | 25 (43.9%) with ATTRv amyloidosis and 27 (47.4%) with ATTRwt amyloidosis | Among ATTRv patients, history of CTRS was a strong predictor of later cardiac involvement (positive predictive value 92.0% [95% CI 74.0–99.0%]) |
LV wall thickening Point mutation testing: Glu89Gln (28.0% of ATTRv), Ile68Leu (48.0% of ATTRv), Val30Met (0.0% of ATTRv), other (24.0%) 99mPYP/DPD imaging |
| Among ATTRwt patients, history of CTS was associated with an increased risk of death (HR 3.63, [95% CI 1.27–10.3]) | |||||
| Nakamichi et al., 1996 [111] | Case series | 108 patients with a history of CTRS | 6 (5.6%) with ATTR amyloidosis (type unspecified) | NR | MSK biopsy and staining with Congo red (tissues of the transversal carpal ligament obtained at CTRS) |
| Reyes et al., 2017 [112] | Cross-sectional | 58 patients undergoing CTRS | 5 (8.6%) with ATTR amyloidosis (type unspecified) | NR |
MSK biopsy and staining with Congo red (tenosynovial tissues obtained at CTRS) MS 99mPYP/DPD imaging |
| Samões et al., 2017 [113] | Case series | 16 patients with history of CTRS | 14 (87.5%) with ATTRv amyloidosis | 8 (57.1%) patients developed bilateral CTS and were submitted to a second CTRS |
MSK biopsy and staining with Congo red (transverse carpal ligaments) Gene and/or point mutation testing Point mutations: V30M |
| Scott et al., 2019 [114] | Case series | 35 patients with a history of CTRS | 9 (26.0%) with amyloidosis (7 with ATTRwt amyloidosis; 2 with non-ATTR amyloidosis) | NR |
MSK biopsy and staining with Congo red (flexor tenosynovium) MS |
| Sekijima et al., 2010 [115] | Case series | 83 patients with a history of CTRS | 28 (35.0%) with ATTRwt amyloidosis |
Multivariate logistic regression showed that the prevalence of ATTRwt in the CTS group was significantly high compared to a control group, and age and male gender are independent risk factor for ATTRwt amyloidosis in patients with a history of CTRS. |
MSK biopsy and staining with Congo red (tenosynovial tissues obtained at CTRS) Gene and/or point mutation testing MS Autopsy |
| Sekijima et al., 2011 [116] | Cross-sectionalb | 100 patients with CTS undergoing CTRS | 34 (34.0%) with ATTRwt amyloidosis | Binomial logistic regression, corrected for age and sex, showed that ATTRwt amyloidosis in the idiopathic CTS group was significantly higher than that in the control group (odds ratio 15.8, 95% CI 3.29 –75.7) |
MSK biopsy and staining with Congo red (tenosynovial tissues obtained at CTRS) Gene testing |
| Stein et al., 1987 [117] | Case series | 140 CTS biopsies | 16 (11.4%) with ATTR amyloidosis (type unspecified) | NR | MSK biopsy and staining with Congo red (retinaculum flexor, perineurial fat and connective tissue, and peritendinous and synovial structures) |
| Sugiura et al., 2021 [118] | s | 79 patients with a history of CTRS | 27 (34.0%) with ATTR amyloidosis (type unspecified) | 16/27 patients with ATTR amyloidosis underwent further testing and all were suspected to have ATTRwt amyloidosis |
MSK biopsy and staining with Congo red (tenosynovial tissue within the transverse carpal ligament obtained at CTRS) LV wall thickening 99mPYP/DPD imaging |
| Uchiyama et al., 2014 [119] | Case series | 107 patients undergoing CTRS | 38 (36.0%) with ATTRwt amyloidosis | NR |
MSK biopsy and staining with Congo red (tenosynovial tissues obtained at CTRS) Other biopsy and staining |
| Vianello et al., 2021 [120] | Cross-sectional | 53 male patients with history of CTRS | 2 (4.0%) with ATTRwt amyloidosis | NR |
LV wall thickening 99mPYP/DPD imaging |
| Wininger et al., 2021 [121] | SLR (case series)c | 35 patients with CTS | 33 (94.2%) with ATTR amyloidosis (type unspecified) | NR | MSK biopsy and staining with Congo red (carpal ligament or synovium) |
| Zegri-Reiriz et al., 2019 [122] | Cross-sectional | 233 patients with history of CTRS | 2 (0.9%) with ATTRwt amyloidosis | NR |
LV wall thickening 99mPYP/DPD imaging |
aBiopsy and staining of tissues described as other refers to instances where diagnosis was confirmed through staining of non-MSK biopsied tissues, such as endomyocardial tissue
bData from patients with amyloidosis was compared to control patients in these publications
cData from case series investigation by Kyle et al. 1992, as reported in the SLR conducted by Wininger et al. 2021 on the association between amyloid deposition and MSK pathology
ATTR Transthyretin-mediated amyloidosis, ATTRv Hereditary transthyretin amyloidosis, ATTRwt Wild-type transthyretin amyloidosis, CI Confidence interval, CTS Carpal tunnel syndrome, CTRS Carpal tunnel release surgery, DPD Technetium-99m 3, 3-diphospho-1, 2-propanodicarboxylic acid, HR Hazard ratio, LV Left ventricular, MS Mass spectrometry, MSK Musculoskeletal, NR Not reported, OR Odds ratio, PYP Technetium-99m pyrophosphate
Table 4.
Spinal stenosis in patients with ATTR amyloidosis, and ATTR amyloidosis in patients with spinal stenosis
| Publication | Study design | Research population | Prevalence | Additional statistics | Information relating to and/or confirming diagnosis |
|---|---|---|---|---|---|
| Spinal stenosis in ATTR amyloidosis | |||||
| Arevalo et al., 2019 [123] | Database/registry | 1,068 patients hospitalized with cardiac amyloidosis (ATTR not specified) | 90 (8.4%) with SS | NR | NR |
| Cortese et al., 2016 [124] | Case series | 150 patients with ATTRv amyloidosis | 11 (22.0%) patients with previous diagnosis of SS | NR |
Biopsy and staining Point mutations: Val30Met (p.Val50Met) Glu89Gln (p.Glu109Gln) Phe64Leu (p.Phe84Leu) Ile68Leu (p.Ile88Leu) Thr49Ala (p.Thr69Ala) |
| ATTR amyloidosis in spinal stenosis | |||||
| D'Agostino et al., 1992 [125] | Case series | 97 patients with a history of LSS | 12 (12.0%) with amyloidosis (type unspecified) | NR | MSK biopsy and staining with Congo red (ligamentum flavum) |
| Eldhagen et al., 2021 [126] | Cross-sectional | 250 patients undergoing LSS | 93 (37.0%) with ATTR amyloidosis (type unspecified) | NR |
MSK biopsy and staining with Congo red (ligamentum flavum) LV wall thickening |
| Gagne et al., 1995 [127] | Case series | 41 patients with a history of LSS | 14 (34.0%) with ATTR amyloidosis (type unspecified) | NR | MSK biopsy and staining with Congo red (ligamentum flavum obtained at LSS) |
| Gies et al., 1996 [128] | Case series | 100 patients with SS | 5 (5.0%) with ATTR amyloidosis (type unspecified) | NR | MSK biopsy and staining with Congo red (ligamentum flavum obtained at LSS or surgery for herniated discs) |
| Godara et al., 2020 [129] | Case series | 325 patients with SS | 44 (13.0%) with ATTR amyloidosis (type unspecified) | NR | MSK biopsy and staining with Congo red (ligamentum flavum) |
| Westermark et al., 2014 [130] | Case series | 26 patients with history of LSS | 5 (19.0%) with ATTR amyloidosis (4 ATTRwt and 1 ATTR type unspecified) | NR | MSK biopsy and staining with Congo red (bone fragments, pieces of ligament and other connective tissue obtained at LSS) |
| Yanagisawa et al., 2015 [131] | Case series | 56 patients with SS | 43 (45.3%) with ATTRwt amyloidosis | NR |
MSK biopsy and staining with Congo red (ligamentum flavum) LV wall thickening MS |
ATTR Transthyretin-mediated amyloidosis, ATTRv Hereditary transthyretin amyloidosis, ATTRwt Wild-type transthyretin amyloidosis, LSS Lumbar spinal surgery, LV Left ventricular, MS Mass spectrometry, MSK Musculoskeletal, NR Not reported, SS Spinal stenosis
Table 5.
Carpal tunnel syndrome and or spinal stenosis in patients with ATTR amyloidosis
| Publication | Study design | Research population | Prevalence CTS | Prevalence SS | Additional statistics | Information relating to and/or confirming diagnosis |
|---|---|---|---|---|---|---|
| Carpal tunnel syndrome and or spinal stenosis in ATTR amyloidosis | ||||||
| Abboud et al., 2020 [132] | Case series | 46 patients with cardiac ATTR amyloidosis (type not specified) | 10.9% with CTS | 21.7% with SS | NR | 99mPYP/DPD imaging |
| Arana et al., 2021 [133] | Case series | 89 patients with ATTR amyloidosis (83 ATTRwt; 6 ATTRv) | 13 (14.8%) with unilateral CTS; 20 (22.7%) with bilateral CTS | 21 (23.9%) with SS | NR |
Gene and/or point mutation testing Point mutations: Val50Met (100% of ATTRv patients) |
| Auer-Grumbach et al., 2020 [134] | Case series | 22 patients with ATTRv amyloidosis | 11 (55.0%) with CTS | 2 (1.0%) with SSa | NR |
Biopsy (endomyocardial tissues) Point mutation testing Point mutations: Val40Ile, Arg41Gln, Val50Met, Thr69Ile, Thr80Ala, His108Arg, Val113Leu, Val114Ala, Ile127Phe, Val142Ile |
| Aus dem Siepen et al., 2019 [135] | Cross-sectional | 77 asymptomatic ATTRv (gene carriers) | 10 (13.0%) with CTS | NR | NR | NR |
| 253 patients with ATTRwt amyloidosis | 152 (60.0%) with CTS | 35 (14.0%) with SS | 32 (12.0%) with CTS and SS | |||
| 136 patients with ATTRv amyloidosis | 77 (56.0%) with CTS | 7 (5.0%) with SS | 3 (2.2%) with CTS and SS | |||
| Bhadola et al., 2020 [136] | Case series | 92 patients with ATTRv amyloidosis | 73.0% with CTS | 18.0% with SS | NR |
Point mutation testing Point mutations: V122I, T60A, V30M, L58H, F64L, Y114C, and S77Y |
| Bukhari et al., 2020 [137] | Case series | 440 patients who underwent a 99mTc-PYP skin scan (assumed by authors to be indicative of cardiac ATTR amyloidosis) | NR | NR |
OR 4.06 (2.74–5.99), p<0.0001 CTS as a predictor of a positive skin 99mPYP test; OR 2.09 (1.39–3.14), p<0.0001 SS as a predictor of a positive skin 99mPYP test |
99mPYP/DPD imaging |
| Bukhari et al., 2021 [138] | Case series | 206 patients with a positive 99mTc-PYP skin scan (assumed by authors to be indicative of cardiac ATTR amyloidosis) | NR | NR | 0.48 regression coefficient for bilateral CTS; 0.15 regression coefficient for SS | 99mPYP/DPD imaging |
| Campagnolo et al., 2020 [139] | Case series | 25 patients with ATTRwt amyloidosis | 16 with CTS | 2 with SS | NR |
Biopsy (endomyocardial and salivary gland tissues) LV wall thickening 99mPYP/DPD imaging |
| Debonnaire et al., 2021 [140] | Case series | 114 patients with ATTR amyloidosis (type not specified) | 43% with CTS | 40% with SS | NR | NR |
| Di Stefano et al., 2021 [141] | Case series | 16 patients with ATTRv amyloidosis | NR | NR | r=0.731 (p=0.0001) association between ATTR and bilateral CTS | NR |
| NR | NR | r=0.52 (p=0.040) association between ATTR and SS | ||||
| Durmus-Tekçe et al., 2015 [142] | Case series | 5 patients with ATTRv amyloidosis (Glu89Gln point mutation) | 3 with CTS | 1 with SS | NR |
Gene and/or point mutation testing Point mutations: Glu89Gln |
| Durmus-Tekçe et al., 2016 [143] | Case series | 17 patients with ATTRv amyloidosis | 3 with CTS (all Glu89Gln) | 1 with SS (Glu89Gln) | NR |
Gene and/or point mutation testing Point mutations: Val30Met, Glu89Gln, Gly53Glu, Glu54Gly, Gly47Glu |
| Huda et al., 2019 [144] |
Database/ registry |
373 patients with ATTRwt amyloidosis | NR | NR | CTS as a feature associated with ATTRwt amyloidosis: OR=5.7; 95% CI (4.3–11.8)b | NR |
| SS as a feature associated with ATTRwt amyloidosis: OR=2.1; 95% CI (1.5–3.1)b | ||||||
| Lauppe et al., 2021 [145] |
Database/ registry |
994 patients with ATTR cardiac amyloidosis (type not specified) | 167 (16.8%) with CTS | 86 (8.7%) with SS | NR | NR |
| Martyn et al., 2021 [146] |
Database/ registry |
28,825 patients with suspected cardiac ATTR amyloidosis (type not specified) | 2,463 (8.5%) with CTS | 5,874 (20.0%) with SS | NR | NR |
| Russo et al., 2020 [147] |
Database/ registry |
260 patients with ATTRv amyloidosis | 21 (8.1%) with CTS | 16 (6.2%) with SS | NR | NR |
| Russell et al., 2021 [148] | Case series | 41 patients with ATTRwt amyloidosis | 36 (88.0%) with CTS | 9 (22%) with SS (6 with history of LSS) | NR |
Biopsy (endomyocardial tissues) 99mPYP/DPD imaging |
| Other | ||||||
| George et al., 2020 [149] | Case series | 27 patients with ATTRwt amyloidosis and SS | 5 (19%) patients had also undergone CTRS | NR | NR |
MSK biopsy and staining with Congo red (tissues unspecified) Gene testing MS |
| George et al., 2021 [150, 151] | Case series | 178 patients who underwent LSS with pathology specimens and preoperative MRI | 24 (13.5%) with ATTRwt amyloidosis | NR | NR |
MSK biopsy and staining (ligamentum flavum obtained from spinal surgery) LV wall thickening MS |
| 177 patients who underwent LSS with pathology specimens and preoperative MRI | 20 (17.0%) with ATTRwt amyloidosis | NR | NR | |||
| 30 patients with surgical indication of SS | 6 (20.0%) of patients with ATTRwt amyloidosis+CTS | NR | NR | |||
| 161 patients with surgical indication of SS | 4 (16.7%) of patients with ATTRwt amyloidosis+CTS | NR | NR | |||
| Godara et al., 2021 [152] | Cross-sectional | 43 patients with ATTR amyloidosis (type not specified) who underwent LSS | 15 (35%) with CTS | NR | OR=5.4 (2.2–13.0) CTS independent predictor of ATTR ligamentum flavum deposition |
MSK biopsy and staining with Congo red (ligamentum flavum sections) Gene and/or point mutation testing 99mPYP/DPD imaging MS |
aSpinal stenosis was reported in two patients but was not documented and questioned in all patients
bfindings from a machine learning model of ATTRwt using ICD codes from US medical claims data, compared to a random cohort of HF patients matched by age, gender, and medical histories [cohort 1a (ATTRwt): N=373, cohort 1b (HF): N=373]
ATTR Transthyretin-mediated amyloidosis, ATTRv Hereditary transthyretin amyloidosis, ATTRwt Wild-type transthyretin amyloidosis, CTS Carpal tunnel syndrome, CTRS Carpal tunnel release surgery, DPD Tc-3,3-diphosphono-1,2-propanodicarboxylic acid, HF Heart failure, ICD International Classification of Diseases, IVS Intraventricular septum, LSS Lumbar spinal surgery, LV Left ventricular, MRI Magnetic resonance imaging, MS Mass spectrometry, NR Not reported, OR Odds ratio, PYP Technetium-99m pyrophosphate, R Regression, SLR Systematic literature review, SS Spinal stenosis, US United States
Table 6.
ATTR amyloidosis in patients with osteoarthritis, and ATTR amyloidosis in patients with osteoarthritis
| Publications | Study design | Population | Prevalence | Additional statistics | Information relating to and/or confirming diagnosis |
|---|---|---|---|---|---|
| ATTR amyloidosis in osteoarthritis | |||||
| Akasaki et al., 2015 [153–155] | Case series | 12 autopsy patients with OA | 12 (100%) with amyloid deposits (type unspecified) | NR |
MSK biopsy and staining with Congo red (knee cartilage) Autopsy |
| Egan et al., 1982 [156] | Cross-sectional | 18 patients with OA with history of TKA or THA | 10 (55.0%) with amyloid deposits (type unspecified) | NR |
MSK biopsy and staining with Congo red (cartilage, synovium and articular tissue of the knee and hip) ATTR amyloidosis diagnosis status because of MSK biopsy and staining NR |
| Gu et al., 2014 [157] | Cross-sectional | 36 patients with knee OA and TKA/total knee replacement | 8 (22.0%) with amyloid deposits | The mean OA duration in ATTR positive patients was 16.5 (7–30) years compared to ATTR negative patients 12.0 (5–20) years (p=0.014) | MSK biopsy staining with Congo red (synovial specimen obtained at TKA) resulting in a diagnosis of ATTRwt amyloidosis in all patients |
| Niggemeyer et al., 2011 [158] | Cross-sectional | 50 patients with end-stage hip OA who were presently undergoing THA/total hip replacement | 17 (33.0%) with amyloid deposits (type unspecified) | NR |
MSK biopsy and staining with Congo red (synovium and cartilage of the femoral head obtained at THA) ATTR amyloidosis diagnosis status because of MSK biopsy and staining NR |
| Takanashi et al., 2013 [159] | Cross-sectional | 232 patients with OA and a history of TKA/total knee joint replacement | 21 (8.1%) with amyloid deposits (type unspecified) | NR |
MSK biopsy and staining with Congo red (synovial tissue obtained at TKA) ATTR amyloidosis diagnosis status because of MSK biopsy and staining NR |
| Yanagisawa et al., 2016 [160] | Case series | 52 patients with OA and a history of TKA | 18 (35.3%) with amyloid deposits (type unspecified) in meniscus tissue | NR |
MSK biopsy and staining with Congo red (meniscus, articular cartilage, synovial membrane obtained at TKA) ATTR amyloidosis diagnosis status because of MSK biopsy and staining NR |
| 8 (29.6%) with amyloid deposits (type unspecified) in articular cartilages | NR | ||||
| 6 (17.6%) with amyloid deposits (type unspecified) in synovial membrane | NR | ||||
| Osteoarthritis in patients with ATTR amyloidosis | |||||
| Paccagnella et al., 2020 [161] | Database/registry | 29 patients with ATTR amyloidosis (20 ATTRwt; 9 unspecified) | 59% with THA | NR | NR |
| 41% with TKA | NR | ||||
| Rubin et al., 2017 [162] | Database/registry | 156 patients with cardiac ATTR amyloidosis (type unspecified) | 20 (12.8%) underwent THA | NR | NR |
| 22 (14.1%) underwent TKA | NR | ||||
ATTR Transthyretin-mediated amyloidosis, MSK Musculoskeletal, NR Not reported, OA Osteoarthritis, THA Total hip arthroplasty, TKA Total knee arthroplasty
Table 7.
ATTR amyloidosis in patients where more than one musculoskeletal manifestation was reported and multiple musculoskeletal manifestations in patients with ATTR amyloidosis
| Publication | Study design | Population | Prevalence | Additional statistics | Information relating to and/or confirming diagnosis |
|---|---|---|---|---|---|
| Publications where more than one musculoskeletal manifestation in ATTR amyloidosis was reported | |||||
| Campbell et al., 2020 [163] | Case series | 36 patients with ATTRwt amyloidosis | 52.8% with CTS | NR | Gene and/or point mutation testing: 74% of patients with ATTRv had the p.Val142Ile mutation |
| 44.4% with CTS with history of CTRS | NR | ||||
| 44.4% with SS | NR | ||||
| 33.3% with SS with history of LSS | NR | ||||
| ATTRwt: 63.8% with OA with history of JR | NR | ||||
| 27.8% with RCI | NR | ||||
| 34 patients with ATTRv amyloidosis | ATTRwt: 30.8% with RCI underwent RCR | NR | |||
| ATTRv: 85.3% with CTS | NR | ||||
| ATTRv: 47.1% with CTS underwent CTS release | NR | ||||
| ATTRv: 41.2% with SS | NR | ||||
| ATTRv: 2.9% with SS underwent laminectomy | NR | ||||
| ATTRv: 17.5% with OA | NR | ||||
| Geller et al., 2015 [164] | Case series | 99 patients with cardiac ATTR amyloidosis (type unspecified) | 30 with CTS | NR | NR |
| 20 with BTR | NR | ||||
| Gorevic et al., 2020 [165] | Case series | 31 patients with ATTRv amyloidosis (ATTRIle122 point mutation) | 38.7% with CTS | NR |
99mPYP scan MS Gene and/or point mutation testing |
| 25.8% with SS | NR | ||||
| 25.8% with OA | NR | ||||
| 63 patients with ATTRwt amyloidosis | 36.5% with CTS | NR | |||
| 23.8% with SS | NR | ||||
| 25.3% with OA | NR | ||||
| Kastritis et al., 2020 [166] | Case series | 50 patients with ATTRwt amyloidosis | 36% with CTS | NR | 99mPYP scanning and gene testing |
| 4% with SS | NR | ||||
| 14% with T | NR | ||||
| Kogan et al., 2020 [167] | Case series | 397 patients with cardiac amyloidosis (70% ATTRwt; 30% ATTRv) | 204 (51.4%) with CTS | NR | NR |
| 101 (25.4%) with SS | NR | ||||
| 94 (23.7%) with JR | NR | ||||
| 69 (17.4%) with T | NR | ||||
| 68 (17.1) with CTS+SS | NR | ||||
| 60 (15.1) with CTS+JR | NR | ||||
| 49 (12.3) CTS+T | NR | ||||
| 35 (8.8) SS+T | NR | ||||
| 33 (8.3) SS+JR | NR | ||||
| 18 (4.5) T+JR | NR | ||||
| 29 (7.3) CTS+SS+T | NR | ||||
| 22 (5.5) CTS+SS+JR | NR | ||||
| 12 (3.0) CTS+T+JR | NR | ||||
| 12 (3.0) SS+T+JR | NR | ||||
| Nativi-Nicolau et al., 2020 [168] | Case series | 6 patients with ATTRwt amyloidosis | 100% with CTS | NR | NR |
| 32% with SS | NR | ||||
| 50% with TF | NR | ||||
| Rapezzi et al., 2020 [169] | Case series | 106 patients with ATTRv amyloidosis | ATTRv: 27% with CTS | NR | NR |
| ATTRv NR with SS | NR | ||||
| ATTRv: 9% with OA | NR | ||||
| 335 patients with ATTRwt amyloidosis | ATTRwt: 40% with CTS | NR | |||
| ATTRwt: 11% with SS | NR | ||||
| ATTRwt: 15% with OA | NR | ||||
| Rubin et al., 2017 [170] | Database/registry | Patients with ATTRv amyloidosis | 33 (51.6%) with CTS | NR | Point mutation testing |
| 13 (20.3%) with SS | NR | ||||
| 7 (10.9%) with THA | NR | ||||
| 5 (7.8%) with TKA | NR | ||||
| 3 (4.7%) with RCR | NR | ||||
| Patients with ATTRwt amyloidosis | 64 (59.3%) with CTS | NR | NR | ||
| 15 (13.9%) with SS | NR | ||||
| 15 (13.9%) with THA | NR | ||||
| 20 (18.5%) with TKA | NR | ||||
| 14 (13.0%) with RCR | NR | ||||
| sss Sekijima et al., 2018 [171] | Cross-sectionala | 51 patients with ATTRwt amyloidosis | 10 (20.0%) with CTS as the initial clinical manifestation observed in patients (prior to diagnosis of ATTRwt amyloidosis) | NR |
MSK biopsy and staining (tenosynovial tissue within the transverse carpal ligament obtained at CTRS) resulting in a diagnosis of ATTRwt in two patients whose initial clinical manifestation was CTS ATTR amyloidosis diagnosis status because of MSK biopsy and staining NR for the remaining patients Staining method NR |
| 1 (2.0%) with TF as the initial clinical manifestation observed in patients (prior to diagnosis of ATTRwt amyloidosis ) | NR | ||||
| 11 (22%) with SS as a clinical manifestation present at diagnosis of ATTRwt amyloidosis | NR | ||||
| 23 (45.0%) with CTS as a clinical manifestation present at diagnosis of ATTRwt amyloidosis | NR | ||||
| Willis et al., 2021 [172] | Database/registry | 1,091 patients “at risk” for ATTRwt amyloidosis | 340 (31.0%) with CTS | NR | NR |
| 654 (60%) with OA | |||||
| Publications where ATTR amyloidosis was reported in more than one musculoskeletal manifestation | |||||
| Hara et al., 2020b [173] | Case series | 20 patients with TF | 9 (69.2%) with ATTR amyloidosis (type unspecified) | The mean number of fingers with tenosynovitis was significantly higher in amyloid-positive cases (3.8 fingers) than in amyloid-negative cases (2.0 fingers)c | MSK biopsy and staining with the direct fast scarlet method (tendon synovium tissue or flexor tendon sheath tissues obtained at TFRS) resulting in a diagnosis of ATTR (type unspecified) in all patients |
| Sood et al., 2021 [174] | Database/registry | 310 patients with ATTR (type unspecified) following CTRS | 122 (39.4%) with bilateral CTS after CTRS | NR | LV wall thickening |
| 82 (26.5%) with SS after CTRS | NR | ||||
| 4 (1.3%) with CTS+BTR | NR | ||||
| 89,981 patients with history of CTRS | NR | 0.25%, 0.21–0.29% - cumulative incidence of ATTR (type unspecified) after CTRS at 5 years | |||
| NR | 0.55%, 0.47–0.63% - cumulative incidence of ATTR (type unspecified) after CTRS at 10 years | ||||
| NR | 0.80%, 0.67–0.93% - cumulative incidence of ATTR (type unspecified) after CTRS at 15 years | ||||
| Sperry et al., 2021 [175] | Cross-sectional | 13 patients undergoing both TFRS and at least one CTRS | 2 (15.4%) with ATTR amyloidosis (type unspecified) in the CTS tenosynovial tissue but not the TF tenosynovial tissue | NR |
MSK biopsy and staining with Congo red (tenosynovial tissues obtained at TFRS and CTRS where concomitant CTS was present in patients) ATTR amyloidosis (type unspecified) diagnosis status because of MSK biopsy and staining NR |
| NR | |||||
| Sperry et al., 2018 [176] | Cross-sectional | 98 patients with history of CTRS | 1 with ATTR amyloidosis (type unspecified) and CTS | NR |
Gene and/or point mutation testing MSK biopsy and staining with Congo red (tenosynovial tissues obtained at CTRS) resulting in a diagnosis of ATTR amyloidosis (type unspecified) in all remaining patients Point mutations: Ala81Thr, Leu58His |
| 4 with ATTR amyloidosis (type unspecified) and CTS+SS | NR | ||||
| 2 with ATTR and CTS+BTR (1 ATTRv and 1 ATTRwt) | NR | ||||
| Sueyoshi et al., 2011 [177] | Case series | 54 patients with CTS | 18 (33.3%) with ATTRwt amyloidosis | NR | MSK biopsy and staining with Congo red (RC tendons, yellow ligaments, and tenosynovial tissues obtained at CTRS) resulting in a diagnosis of ATTRwt amyloidosis in 5 patients (MSK manifestation subgroup not specified) |
| 21 patients with RCT | 5 (23.8%) with ATTRwt amyloidosis | NR | |||
| 36 patients with SS | 19 (52.8%) with ATTRwt amyloidosis | NR | |||
ATTR Transthyretin amyloidosis, ATTRv Hereditary transthyretin amyloidosis, ATTRwt Wild-type transthyretin amyloidosis, BTR; CTS Carpal tunnel syndrome, CTRS Carpal tunnel release surgery, JR Joint replacement, LSS Lumbar spinal surgery, MS Mass spectrometry, MSK Musculoskeletal, NR Not reported, OA Osteoarthritis, PYP Technetium-99m pyrophosphate, RC Rotator cuff, RCI Rotator cuff injury, RCR Rotator cuff repair, RCT Rotator cuff tear, SS Spinal stenosis, T Tendon tear and tendon rupture, TF Trigger finger, TFRS Trigger finger release surgery, THA Total hip arthroplasty, TKA Total knee arthroplasty
aData from patients with ATTR amyloidosis was compared to control patients in these publications
bFor conciseness in reporting, the one publication reporting on an association between TF and ATTR amyloidosis is reported here
cThis data relates to 13 patients diagnosed with amyloidosis, where 9 were diagnosed with ATTR amyloidosis and the amyloidosis type of the remaining 4 was not specified
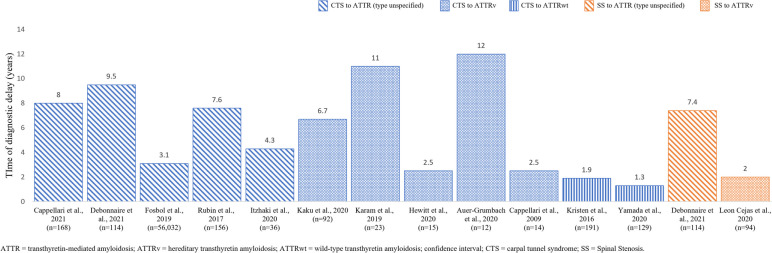
Fig. 3.
Time between MSK symptom onset and ATTR amyloidosis diagnosis
Table 8.
Temporal association between MSK manifestation onset and ATTR amyloidosis diagnosis
| Publication | Study design | Population | MSK type and prevalence | Temporal association | Information relating to and/or confirming diagnosis |
|---|---|---|---|---|---|
| Auer-Grumbach et al., 2020 [134] | Case series | 22 patients with ATTRv amyloidosis | 11 (55.0%) with CTS | 1–12 years between CTS symptom onset and diagnosis of ATTRv amyloidosis |
Biopsy (endomyocardial tissues) Point mutation testing Point mutations: Val40Ile, Arg41Gln, Val50Met, Thr69Ile, Thr80Ala, His108Arg, Val113Leu, Val114Ala, Ile127Phe, Val142Ile |
| Cappellari et al., 2009 [21] | Case series | 14 patients with ATTRv amyloidosis | 1 with CTS | Delay from CTS symptom onset to ATTR amyloidosis diagnosis: 2.5 (1–7) years |
Biopsy and staining Gene and/or point mutation testing Point mutations: Val30Met, Phe64Leu, Asn124Ser, Glu89Gln, Val122Ile, Ile107Phe, Thr49Ala, Ser50Arg |
| Cappelli et al., 2021 [23] | Cross-sectional* | 168 patients with ATTR amyloidosis (type unspecified) | 122 (73%) with CTS | Approximately 8 years between CTS symptom onset and ATTR diagnosis |
Point mutation testing: Point mutations: le68leu, Val122ile, other (unspecified) |
| Debonnaire et al., 2021 [140] | Case series | 114 patients with ATTR amyloidosis (type not specified) |
43% with CTS 40% with SS |
CTS preceded diagnosis with ATTR amyloidosis by 9.5 years; SS preceded diagnosis with ATTR amyloidosis by 7.4 years | NR |
| Fosbol et al., 2019 [105] | Database/registry | 56,032 patients undergoing CTRS | NR | The median time from CTS surgery to diagnosis of ATTR amyloidosis (type unspecified) was 3.1 years | NR |
| Hewitt et al., 2020 [44] | Case series | 15 patients with ATTRv amyloidosis (T60A point mutation) | 5 (33%) with CTS | Delay from CTS symptom onset to diagnosis of ATTRv amyloidosis: 2.5(1–7) years |
Biopsy and staining Point mutation testing Point mutations: T60a |
| Itzhaki et al., 2020 [109] | Case series | 36 patients with history of CTRS | 16 (44.5%) with ATTR amyloidosis (type unspecified) | The median time from CTS diagnosis to diagnosis of ATTR amyloidosis (type unspecified) was 4.3 years |
Biopsy and staining with Congo red (endomyocardial tissues) LV wall thickening Gene and/or point mutation testing 99mPYP/DPD imaging |
| Kaku et al., 2020 [47] | Case series | 92 patients with ATTRv amyloidosis | 72% with CTS | 31 patients underwent CTS release, average of 6.7 years from initial CTS symptoms to ATTR diagnosis | NR |
| Karam et al., 2019 [49] | Case series | 23 patients with ATTRv amyloidosis | 17 with CTS | Time taken from CTS and diagnosis 11(0–36) years |
Point mutation testing Point mutations: Val30Met, Glu89Gln, Gly53Glu, Glu54Gly, Gly47Glu |
| Kristen et al., 2016 [57] | Case series | 191 patients with ATTRwt amyloidosis | 87 (48.3%) with CTS | Delay from CTS symptom onset to diagnosis of ATTRwt amyloidosis: 22.2±2.2 months | Gene testing |
| Leon Cejas et al., 2020 [178] | Case series | 94 patients with ATTRv amyloidosis | NR | 2 years between SS symptom onset and a diagnosis of ATTRv amyloidosis |
Point mutation testing Point mutations: Val30Met (89.4%), Ala97ser (6.4%), Tyr114cys (2.1%), Ile93val (1.1%) and Ala36pro (1.1%) |
| Rubin et al., 2017 [162] | Database/registry | 156 patients with cardiac ATTR amyloidosis (type unspecified) | 22 (14.1%) underwent TKA | OA arthroplasty occurred an average of 7.6 years before cardiac ATTR amyloidosis (type unspecified) was diagnosed | NR |
| Yamada et al., 2020 [96] | Cross-sectional | 129 patients with ATTRwt amyloidosis | 57 (54.0%) with CTS | Delay from CTS symptom onset to diagnosis of ATTRwt amyloidosis: 15.5 (2–75) months |
Biopsy and staining with Congo red (endomyocardial tissues) Gene testing 99mPYP/DPD imaging |
AsTTR Transthyretin amyloidosis, ATTRv Hereditary transthyretin amyloidosis, ATTRwt Wild-type transthyretin amyloidosis, BTR; CTS Carpal tunnel syndrome, CTRS Carpal tunnel release surgery, JR Joint replacement, LSS Lumbar spinal surgery, MS Mass spectrometry, MSK Musculoskeletal, NR Not reported, OA Osteoarthritis, PYP Technetium-99m pyrophosphate, RC Rotator cuff, RCI Rotator cuff injury, RCR Rotator cuff repair, RCT Rotator cuff tear, SS Spinal stenosis, T Tendon tear and tendon rupture, TF Trigger finger, TFRS Trigger finger release surgery, THA Total hip arthroplasty, TKA Total knee arthroplasty
Assessment of study quality
A quality assessment of the included publications was performed by one reviewer (and cross-checked by a second to ensure accuracy with discrepancies settled by a third senior reviewer) using the most appropriate Joanna Briggs Institute (JBI) critical appraisal checklist. This assessment was conducted at the publication level [179].
Following the JBI quality assessment of the 163 publications examining the association between MSK manifestations and ATTR amyloidosis, 51 publications were identified as being at low risk of bias [179]. 87 publications had at least one quality domain that implied some potential bias. The most common reason was limited reporting on the method of participant selection and method of diagnosis. In the 25 remaining publications, insufficient information was reported to measure the potential risk of bias.
Of the 13 publications examining the temporal association, four were found to have a low risk of bias, seven had at least one quality domain that implied some potential bias, and in the remaining two publications, there was insufficient information reported to measure the potential risk of bias.
Data collection and data extraction
The following information from each included publication was extracted: (1) publication characteristics: title, author, publication year, study design, objectives, country, and data collection period, (2) population characteristics: ATTR amyloidosis diagnosis, MSK manifestation subgroup, sample size, and demographic data such as age and sex, (3) the direction of the association relationship (ATTR amyloidosis outcomes in patients with MSK manifestations or MSK manifestations outcomes in patients with ATTR amyloidosis), (4) outcomes as defined in the PICOTS criteria (Supplement 2). Each independent reviewer piloted the data extraction form, and discussions were held to inform any necessary refinements. Data extraction was performed by one reviewer and cross-checked by a second to ensure accuracy. Discrepancies were settled by a third senior reviewer.
Results
What evidence supports the association between ATTR amyloidosis and MSK manifestations?
Most studies reported an association between ATTR amyloidosis and CTS (Tables 2 and 3); however, SS, OA, biceps tendon rupture (BTR), rotator cuff injury (RCI), and trigger finger (TF) were also reported and those studies are detailed in Tables 4, 5, 6 and 7. The association between MSK manifestations and the presence of ATTR amyloidosis were reported bi-directionally; for example, some authors investigated CTS in patients with a confirmed diagnosis of ATTR amyloidosis (Table 2), whereas other authors investigated the presence of ATTR amyloidosis in patients who had undergone treatment for CTS (Table 3). When case series were excluded, the prevalence of CTS in patients with ATTR amyloidosis (inclusive of ATTRv and ATTRwt) ranged between 0.5 and 80% (Table 2) [17, 100], and the prevalence of ATTR amyloidosis (inclusive of ATTRv and ATTRwt) in patients with CTS and/or a history of carpal tunnel release (CTR) surgery ranged between 0.9 and 38% (Table 3) [106, 122]. The prevalence of ATTRv amyloidosis in patients with a history of CTR surgery was higher, at 87.5% [113]. Due to the heterogeneity of the studies’ methodologies and approaches, it is not possible to directly compare the prevalences reported. Two publications investigated the prevalence of ATTRv and ATTRwt amyloidoses separately in the same cohort of patients with CTS, finding that ATTRwt amyloidosis was more prevalent in both instances [106, 110].
The prevalence range for SS in patients with ATTR amyloidosis (inclusive of ATTRv and ATTRwt) was narrower than the range reported for CTS, at 8.4–22.0% [123, 124] (Table 4). As observed with CTS, the range of prevalence of ATTR amyloidosis in patients with SS was broader than the range of SS prevalence in patients with ATTR amyloidosis, at 5.0–45.3% (Table 4) [125–131]. Where patients with ATTRwt amyloidosis were the focus, the prevalence of SS ranged between 19.0 and 45.3% [130, 131]. Comparably, Cortese et al. found that in a cohort of patients with ATTRv amyloidosis, 22.0% of patients had previously been diagnosed with SS [124]. In reports where the prevalence of both CTS and SS was explored in the same cohort of patients with ATTR amyloidosis (Table 5), CTS was more prevalent than SS in patients with ATTRv amyloidosis [134–136, 143, 147], as well as in patients with ATTRwt amyloidosis [139].
Several studies investigated ATTR amyloidosis in OA [153–160], and two database/registry studies investigated the presence of OA in patients with ATTR amyloidosis [161, 162] (Table 6). The studies which investigated ATTR amyloidosis in OA explored either the prevalence of amyloid or TTR deposits in patients with OA. In three publications, the presence of amyloid deposits led to a diagnosis of ATTR amyloidosis [157, 158, 160]. For those studies which investigated ATTR amyloidosis in OA, the association between OA and ATTR amyloidosis was confirmed through the staining of biopsy samples taken from the knee and/or hip with Congo red, a standard method used to identify amyloid [153–160]. In patients biopsied during total hip arthroplasty (THA), the prevalence of amyloid deposits in the synovial membrane was 22.0%, leading to a diagnosis of ATTRwt amyloidosis in these patients [157]. In patients biopsied during total knee arthroplasty (TKA), the prevalence of amyloid deposits ranged from 8.1 to 33.0% [158, 159]. In an autopsy study by Akasaki et al., TTR amyloid deposits were present in the knee cartilage and synovial fluid in all 12 autopsies of individuals with OA; no analyses of whether systemic ATTR amyloidosis was present were conducted [153–155]. With respect to the database/registry studies which reported OA in patients with ATTR amyloidosis, the study by Paccagnella et al., reported on 29 patients with ATTR amyloidosis, finding 59% having had THA and 41% having had TKA [161]. The second study by Ruben et al., reported on 156 patients with unspecified ATTR amyloidosis with CM, finding 12.8% having had THA and 14.1% having had TKA [162].
What is the temporal association between MSK manifestation onset and ATTR amyloidosis diagnosis?
The publications reporting on the temporal association between MSK manifestation onset and a diagnosis of ATTR amyloidosis were limited to CTS, SS, and OA (Fig. 3; Table 8).
Across all CTS-focused publications, CTS symptom onset preceded a diagnosis of ATTR amyloidosis (ATTRv and ATTRwt inclusive) by up to 12 years [21, 23, 44, 47, 49, 57, 96, 105, 109, 134]. In publications reporting on ATTRv amyloidosis separately, the time between CTS symptom onset and diagnosis of ATTRv ranged from 2 to 12 years [21, 44, 47, 49, 134]. This range was 1.3 to 1.9 years in publications reporting on ATTRwt amyloidosis separately [57, 96].
Three studies investigated the temporal association between SS and ATTR amyloidosis; one reported SS symptom onset preceding a diagnosis of ATTRv amyloidosis by approximately 2 years [178], while another reported a 7.4 years delay before an ATTRwt amyloidosis diagnosis [140]. In the same cohort of patients with ATTRwt amyloidosis, CTS symptom onset occurred even earlier than SS symptom onset, preceding the diagnosis of ATTR amyloidosis by 9.5 years [140].
A single publication reported on the temporal association for OA, reporting an average of 7.6 years delay before an ATTR amyloidosis with CM diagnosis was made from OA related surgeries, TKA, and THA [162].
Discussion
Background and rationale
The ability to diagnose ATTR amyloidosis early in the disease course is critical to improving patient prognosis, and MSK manifestations may act as an early indicator of ATTR amyloidosis. This systematic review was conducted to investigate the association between ATTR amyloidosis and MSK manifestations, and to investigate the temporal association between MSK manifestation onset and ATTR amyloidosis diagnosis, in order to potentially aid clinicians in identifying and diagnosing the disease earlier. MSK manifestations, including CTS, SS, OA, among others, were found to be associated with a diagnosis of ATTR amyloidosis (Tables 2, 3, 4, 5, 6 and 7). These manifestations were reported to precede the diagnosis of ATTR amyloidosis by years and could be one of the earliest signs of the disease (Table 8). One of the major systemic manifestations of ATTR amyloidosis is CM which causes progressive heart failure, that can lead to significant morbidity and mortality [3, 10]. The number of patients with ATTRv amyloidosis with cardiomyopathy is estimated to be approximately 40,000 to 50,000 globally [10]. Although the exact prevalence of ATTRwt is not known, it is significantly more common than ATTRv, and CM is the most frequent and predominant systemic involvement in ATTRwt amyloidosis [3, 10]. Awareness of and timely detection of MSK manifestations, months or, even years ahead of the beginning of CM can lead to a significant improvement in the care of these patients [180–182].
Limitations
This systematic review is not all-encompassing, and caution should be exercised when drawing conclusions from such a heterogenous evidence base, including many studies reporting on a small number of patients. With the use of machine learning harnessing big data from registries and electronic health records and advanced statistical methodologies, it may be possible to enhance our understanding of the association between MSK manifestations and ATTR amyloidosis. For example, with the application of machine learning, Willis et al. determined which patients with heart failure were ‘at risk’ for developing ATTR amyloidosis; CTS and OA were highlighted as clinical predictive indicators of interest [172]. The potential benefit of utilizing MSK manifestations associated with ATTR amyloidosis to reduce the delay in diagnosis supports further research in the field.
The included publications were highly heterogenous in terms of how the possible association of ATTR amyloidosis with MSK manifestations was demonstrated (Tables 2, 3, 4, 5, 6 and 7). Biopsy followed by tissue staining of MSK or other specified tissues [14, 16, 18, 21, 22, 25, 27, 30, 33–37, 44, 51, 61, 63, 66, 68, 74, 75, 81, 91, 92, 94–96, 99, 101, 102, 104, 106–115, 117–119, 121, 124–131, 134, 139, 148–150, 152–160, 171, 173, 175, 177, 183] were common. However, detecting amyloid in MSK tissues alone does not necessarily mean a patient is or will be diagnosed with ATTR amyloidosis. Tc-99 m PYP/DPD scintigraphy [19, 20, 24, 29, 37, 39, 41, 42, 45, 51, 58, 60, 63, 64, 66, 68, 69, 73, 77, 78, 80, 95, 96, 109, 110, 118, 120, 122, 132, 137–139, 148, 152, 165, 166], a non-invasive diagnostic method which has been more commonly used during last several years to make a diagnosis of cardiac amyloidosis [3], was also used to confirm the disease in 30% of the publications included in this review (Table 2). Additionally, methods such as mass spectrometry were utilized to confirm that amyloid was caused by TTR [14, 16, 18, 108, 112, 114, 115, 131, 149, 152, 165]. Another significant limitation is that, although an association between MSK manifestations and ATTR amyloidosis is shown in the literature, it does not necessarily demonstrate causation in all cases. Some MSK manifestations seen in patients with (or who will be diagnosed in the future with) ATTR amyloidosis may not be caused by early amyloid deposition. It will be necessary for clinicians and future researchers to take these limitations into account.
What evidence supports the association between ATTR amyloidosis and MSK manifestations?
The current evidence supports that many MSK manifestations are associated with a diagnosis of ATTR amyloidosis. The MSK manifestation most commonly associated with ATTR amyloidosis is CTS; however, SS, OA, BTR, RCI, TF, among others, were also identified. The exact prevalence of CTS in patients with ATTR amyloidosis remains unclear, with both CTS and ATTR amyloidosis prevalence estimates reported bi-directionally having a broad range. Similarly, no clear trend was identified regarding whether the association with CTS is stronger (indicated by a higher prevalence) in patients with ATTRv or ATTRwt amyloidosis. Nonetheless, given the extent of the identified literature reporting a possible association between CTS and ATTR amyloidosis, patients with CTS may represent a population where targeted screening for ATTR amyloidosis would be valuable [184].
SS was also often associated with ATTR amyloidosis, with similar prevalence estimates identified in patients with ATTRv and ATTRwt amyloidoses. Notably, where the prevalence of CTS and SS was explored in the same patient cohorts with ATTRv or ATTRwt amyloidosis, CTS was more prevalent than SS in all reports [134–136, 139, 143, 147].
Finally, the identified evidence supports that ATTR amyloidosis may be prevalent in patients who previously underwent surgery (THA and/or TKA) for OA. TTR amyloid has been detected in the tissues from the joints of patients with OA, which may or may not be indicative of a diagnosis of ATTR amyloidosis, which was confirmed only in three publications. An interesting case series by Akasaki et al., found that all 12 OA patients who donated their knee articular cartilage for biopsy at autopsy had amyloid deposits in their tissue samples [153–155]. Although further research is needed, the findings of this publication suggest that there may be value for surgeons to consider biopsy and staining with Congo red in patients who undergo knee or hip surgery for OA.
What is the temporal association between MSK manifestation symptom onset and ATTR amyloidosis diagnosis?
The current evidence highlights that CTS and SS symptom onset can occur months to years, or even decades, before the diagnosis of ATTR amyloidosis [21, 23, 44, 47, 49, 57, 96, 105, 109, 134, 140, 162, 178].
The exact length of time that MSK manifestations precede a diagnosis of ATTR amyloidosis is unclear, with great variation reported across publications. However, the current evidence offers insight into how the temporal association between CTS symptom onset and a diagnosis of ATTR amyloidosis might differ between patients with ATTRv and ATTRwt amyloidosis. According to the current review, CTS symptom onset appears to precede a diagnosis of ATTRv amyloidosis by a substantially longer period than a diagnosis of ATTRwt amyloidosis [21, 44, 47, 49, 96, 134].
Care needs to be taken in the interpretation of the results from these studies given the variation in methodology. For example, at the time of MSK surgery, TTR amyloid deposition may not have occurred within the tissue taken for biopsy, which may confound clinical diagnosis in these patients [153, 160, 175]. Currently, there is no clear order to ATTR amyloid deposition within MSK tissues, i.e., no specific tissue has been identified as the ‘gold standard’ for early detection of ATTR amyloidosis, and biopsy results can vary according to tissue type [153, 160, 175].
Conclusion
Increased awareness of the MSK manifestations associated with ATTR amyloidosis can enable earlier diagnosis and improve outcomes, given there are effective treatments for this rapidly progressive and fatal condition. Surgeons can play a critical role in early diagnosis of ATTR amyloidosis by recognizing associated MSK manifestations. Currently available data, summarized in this first systematic review conducted on the association between MSK manifestations and ATTR amyloidosis, demonstrates that MSK manifestations can be one of the earliest signs of ATTR amyloidosis; however, it should be kept in mind that the available data is heterogenous, and the extent of the causal relationship between MSK manifestations and ATTR amyloidosis should be further investigated.
Supplementary Information
Additional file 1: Supplement 1. PICOTS criteria for study inclusion and exclusion in the SLR. Supplement 2. Ovid® search strategies for EMBASE and Medline (run on November 3rd, 2021). Supplement 3. Case studies excluded from the systematic literature with ATTR MSK manifestation
Acknowledgements
The authors would like to thank and acknowledge Louise Heron, Patrick Lavelle, and Fleur Howells from Adelphi Values PROVE™ for their support in conducting the literature review and drafting content.
Authors’ contributions
Catherine Summers and Emre Aldinc provided conceptualization and review of the protocol, results, and interpretation. Dr Courtney Campbell, Dr Finn Gustafsson, and Dr Dafang Zhang provided expert guidance including conceptualization and review of the protocol, results, and interpretation. Richard Macey, Abigail Beveridge, and Laura Marr conducted the review of the literature, developed the protocol, and drafted the manuscript. All authors were involved in the review process and approval of the manuscript content.
Funding
This systematic review was funded by Alnylam Pharmaceuticals.
Availability of data and materials
All data from the review are available within the references included in this manuscript. Only peer-reviewed data reported in published articles, and data presented at congresses and subsequently published as abstracts were used.
Declarations
Ethics approval and consent to participate
Not applicable this is a secondary analysis of primary research.
Consent for publication
Not applicable this is a secondary analysis of primary research.
Competing interests
Catherine Summers and Emre Aldinc are employees of Alnylam Pharmaceuticals. Finn Gustafsson is an adviser to Alnylam, Ionis and Pfizer. Courtney Campbell is an adviser to Alnylam and Pfizer and has received research support from Alnylam and Akari Therapeutics. Richard Macey, Abigail Beveridge, and Laura Marr are employees of Adelphi Values PROVE™. Adelphi Values PROVE™ received funding from Alnylam Pharmaceuticals for the conduct of the review, from development of the systematic review methodology through to overseeing the final formatting and manuscript submission processes.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang D, Makhni MC, Kang JD, Blazar P. Orthopaedic manifestations of amyloidosis. JAAOS-Journal of the American Academy of Orthopaedic Surgeons. 2021;29(10):e488–96. doi: 10.5435/JAAOS-D-20-01146. [DOI] [PubMed] [Google Scholar]
- 2.Muchtar E, Dispenzieri A, Magen H, Grogan M, Mauermann M, McPhail E, Kurtin P, Leung N, Buadi F, Dingli D. Systemic amyloidosis from A (AA) to T (ATTR): a review. J Intern Med. 2021;289(3):268–92. doi: 10.1111/joim.13169. [DOI] [PubMed] [Google Scholar]
- 3.Nativi-Nicolau J, Siu A, Dispenzieri A, Maurer MS, Rapezzi C, Kristen AV, Garcia-Pavia P, LoRusso S, Waddington-Cruz M, Lairez O. Temporal trends of wild-type transthyretin amyloid cardiomyopathy in the transthyretin amyloidosis outcomes survey. Cardio Oncol. 2021;3(4):537–46. doi: 10.1016/j.jaccao.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuddy SA, Falk RH. Amyloidosis as a systemic disease in context. Can J Cardiol. 2020;36(3):396–407. doi: 10.1016/j.cjca.2019.12.033. [DOI] [PubMed] [Google Scholar]
- 5.Adams D, Tournev IL, Taylor MS, Coelho T, Planté-Bordeneuve V, Berk JL, González-Duarte A, Gillmore JD, Low S-C, Sekijima Y, et al. Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: a randomized clinical trial. Amyloid. 2023;30(1):1–9. doi: 10.1080/13506129.2022.2091985. [DOI] [PubMed] [Google Scholar]
- 6.Adams D, Polydefkis M, González-Duarte A, Wixner J, Kristen AV, Schmidt HH, Berk JL, Losada López IA, Dispenzieri A, Quan D, et al. Long-term safety and efficacy of patisiran for hereditary transthyretin-mediated amyloidosis with polyneuropathy: 12-month results of an open-label extension study. Lancet Neurol. 2021;20(1):49–59. doi: 10.1016/S1474-4422(20)30368-9. [DOI] [PubMed] [Google Scholar]
- 7.Rintell D, Heath D, Braga Mendendez F, Cross E, Cross T, Knobel V, Gagnon B, Turtle C, Cohen A, Kalmykov E. Patient and family experience with transthyretin amyloid cardiomyopathy (ATTR-CM) and polyneuropathy (ATTR-PN) amyloidosis: results of two focus groups. Orphanet J Rare Dis. 2021;16(1):1–13. doi: 10.1186/s13023-021-01706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang C-C, Ueda M, Kristen AV, Tournev I, Schmidt HH, Coelho T, Berk JL, et al. Patisiran, an RNAi therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018;379(1):11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 9.Ueda M, Ando Y. Recent advances in transthyretin amyloidosis therapy. Translational Neurodegeneration. 2014;3(1):19. doi: 10.1186/2047-9158-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurer MS, Bokhari S, Damy T, Dorbala S, Drachman BM, Fontana M, Grogan M, Kristen AV, Lousada I, Nativi-Nicolau J. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circulation: Heart Failure. 2019;12(9):e006075. doi: 10.1161/CIRCHEARTFAILURE.119.006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nativi-Nicolau JN, Karam C, Khella S, Maurer MS. Screening for ATTR amyloidosis in the clinic: overlapping disorders, misdiagnosis, and multiorgan awareness. Heart Fail Rev. 2022;27(3):785–793. doi: 10.1007/s10741-021-10080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Reviews. 2021;10(1):1–11. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agha RA, Borrelli MR, Farwana R, Koshy K, Fowler AJ, Orgill DP, Zhu H, Alsawadi A, Noureldin A, Rao A. The SCARE 2018 statement: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg. 2018;60:132–6. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Abe RK, Takahashi N, Takasone Y, Yoshinaga K, Yazaki T, Kametani M, Sekijima F. Distribution of amyloidosis subtypes based on tissue biopsy site - consecutive analysis of 729 patients at a single amyloidosis center in Japan. Pathol Int. 2021;71(1):70–9. doi: 10.1111/pin.13041. [DOI] [PubMed] [Google Scholar]
- 15.Akinboboye OA, Malik K, Warner A, Karsten A, Damy V, Taylor T, Maurer HA. M. S.: DISCOVERY: A study examining the prevalence of transthyretin mutations in subjects suspected of having cardiac amyloidosis. Orphanet J Rare Dis. 2015;10(Suppl 1):O8.
- 16.Ando YY, Misumi T, Nomura Y, Sasada T, Okada K, Inoue M, Masuda Y, Ueda T, Takamatsu A, Obayashi K, Matsui K, Naiki H, Ueda H. Clinical, pathological, and proteomic characteristics of newly diagnosed amyloidosis patients: experience from a single referral center in Japan. Neurol Clin Neurosci. 2021;9(1):37–44. [Google Scholar]
- 17.Arevalo. Carpal tunnel syndrome related to cardiac amyloidosis. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain 2020 529.
- 18.Bishop EB, Fajardo EE, Barouch J, Judge LA, Halushka DP. Seven factors predict a delayed diagnosis of cardiac amyloidosis. Amyloid. 2018;25(3):174–9. doi: 10.1080/13506129.2018.1498782. [DOI] [PubMed] [Google Scholar]
- 19.Bukhari SB, Nieves A, Eisele R, Follansbee Y, Soman WP. Clinical Predictors of positive 99mTc-99m pyrophosphate scan in patients hospitalized for decompensated heart failure. J Nucl Med. 2020;61(Supplement 1):659. [Google Scholar]
- 20.Bukhari SF, Brownell S, Eisele A, Soman YS. Race-specific phenotypic and genotypic comparison of patients with Transthyretin Cardiac Amyloidosis. J Am Coll Cardiol. 2021;77(18 Supplement 1):675. [Google Scholar]
- 21.Cappellari MF, Taioli M, Cavallaro F, Ferrari T, Rizzuto S, Fabrizi NGM. Diagnostic pitfalls in transthyretin-related familial amyloid polyneuropathies (TTR-FAPS) J Peripheral Nerv Syst. 2009;14(S1):S27–8. [Google Scholar]
- 22.Cappellari MC, Ferrarini T, Cabrini M, Taioli I, Ferrari F, Merlini S, Obici G, Briani L, Fabrizi C. Variable presentations of TTR-related familial amyloid polyneuropathy in seventeen patients. J Peripheral Nerv Syst. 2011;16(2):119–29. doi: 10.1111/j.1529-8027.2011.00331.x. [DOI] [PubMed] [Google Scholar]
- 23.Cappelli FZ, Fumagalli M, Nardi C, Del Monaco G, Matucci Cerinic G, Allinovi M, Taborchi M, Martone G, Gabriele R, Ungar M, Moggi Pignone A, Marchionni A, Di Mario N, Olivotto C, Perfetto I. Tenosynovial complications identify TTR cardiac amyloidosis among patients with hypertrophic cardiomyopathy phenotype. J Intern Med. 2021;289(6):831–9. doi: 10.1111/joim.13200. [DOI] [PubMed] [Google Scholar]
- 24.Cerudelli EA, Gazzilli D, Bonacina M, Durmo M, Dondi R, Mazzoletti F, Bertagna A, Giubbini F. Transthyretin cardiac amyloidosis and aortic stenosis: in which patients we have to suspect a coexistence. Clin Translational Imaging. 2019;7(Supplement 1):100–S101. [Google Scholar]
- 25.Chen ZK, Saini JS, Tay M, Jayne Tan KSS, Chai Y, Fam JYH, Juraidah SR, Lim AR, Ng PK, Prasad ASL, Tan K, Umapathi CB, Verma T, Yong KK, Yu MH, Ng C. Hereditary Transthyretin Amyloidosis- Clinical and genetic characteristics of a Multiracial South-East Asian Cohort in Singapore. J Neuromuscul Dis. 2021;8(4):723–33. doi: 10.3233/JND-210656. [DOI] [PubMed] [Google Scholar]
- 26.Choi. “The giant awakes” – rapid increases in the diagnosis of transthyretin (ttr) amyloidosis after the attr-act trial of tafamidis. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain. 2020;29(Supplement 2):S115 529.
- 27.Cipriani AC, Civera M, De Michieli S, Cecchin L, Pilichou D, Babuin K, Marra L, Iliceto MP, Briani S, Calore C. Diagnostic approach to wild-type transthyretin cardiac amyloidosis. A single-centre experience. Eur Heart J Supplement. 2019;21(SUPPL J):J178–9. [Google Scholar]
- 28.Cortese AO, Palladini L, Milani G, Sforzini P, Perlini C, Verga S, Lavatelli L, Casarini F, Merlini SG. Clinical and demographic aspects of ATTR amyloidosis: genetic heterogeneity, prognostic markers and novel therapeutic approaches. J Peripheral Nerv Syst. 2009;14(S1):S27–8. [Google Scholar]
- 29.Minutoli FG, Di Bella M, Crisafulli G, Mure C, Militano G, Brancati V, Di Leo M, Mazzeo R, Baldari AS. Cardiac involvement in transthyretin familial amyloid polyneuropathy - comparison between 99mTc-DPD SPECT and magnetic resonance imaging. Eur J Nucl Med Mol Imaging. 2010;37(S2):S382–3. [Google Scholar]
- 30.Du KL, Wang F, Miao H, Lv Y, Zhang H, Wang W, Yuan Z, Meng Y. Hereditary transthyretin amyloidosis in mainland China: a unicentric retrospective study. Ann. 2021;8(4):831–41. doi: 10.1002/acn3.51328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durmus HM, Atmaca Z, Poda MM, Oflazer-Serdarotlu M, Deymeer P, Parman FY. Genotypic and phenotypic presentation of TTR-FAP in Turkey. J Neurol. 2015;84(14 Supplement):P2.034.
- 32.Durmus HC, Demirci A, Alaylioglu H, Gezen-Ak M, Dursun D, Gulsen Parman E. An exploratory study of cognitive involvement in Hereditary Transthyretin Amyloidosis. Acta Neurol Scand. 2021;144(6):640–6. doi: 10.1111/ane.13507. [DOI] [PubMed] [Google Scholar]
- 33.Erdogan CB, Uluc AO, Karli K, Koc N, Ozturk F, Sengun S, Secil IS, Tutuncu Y, Akalin M, Uysal MA, Ozdamar H, Parman SE. Y. Transthyretin familial amyloid polyneuropathy (TTR-FAP): A database analysis. Neurology Conference: 72nd Annual Meeting of the American Academy of Neurology, AAN. 2020;94(15 Supplement):3987.
- 34.Eriksson MB, Todorov J, Yumlu T, Schonland S, Hegenbart S, Kristen U, Dengler AV, Lohse T, Helmke P, Schmidt B, Rocken H. Prevalence of germline mutations in the TTR gene in a consecutive series of surgical pathology specimens with ATTR ayloid. Am J Surg Pathol. 2009;33(1):58–65. doi: 10.1097/PAS.0b013e3181788566. [DOI] [PubMed] [Google Scholar]
- 35.Gabrovsek AD, Estep JP, Perez J, Tang A, Hanna WHW. Transthyretin Cardiac Amyloidosis in the Black Population: a ten-year experience. J Card Fail. 2019;25(8 Supplement):6. [Google Scholar]
- 36.Gagliardi CP, Lorenzini F, Ferlini M, Salvi A, Milandri F, Quarta A, Taborchi CC, Bartolini G, Frusconi S, Martone S, Cinelli R, Foffi MM, Reggiani S, Fabbri MLB, Cataldo G, Cappelli P, Rapezzi F. Phenotypic profile of Ile68Leu transthyretin amyloidosis: an underdiagnosed cause of heart failure. Eur J Heart Fail. 2018;20(10):1417–25. doi: 10.1002/ejhf.1285. [DOI] [PubMed] [Google Scholar]
- 37.Galat AG, Bodez A, Slama D, Dijos M, Zeitoun M, Milleron DM, Attias O, Dubois-Rande D, Mohty JL, Audureau D, Teiger E, Rosso E, Monin J, Damy JL. Aortic stenosis and transthyretin cardiac amyloidosis: the chicken or the egg? Eur Heart J. 2016;37(47):3525–31. doi: 10.1093/eurheartj/ehw033. [DOI] [PubMed] [Google Scholar]
- 38.Gawor MF, Sioma M, Mazurkiewicz A, Lipowska L, Ojrzynska M, Szczygiel N, Spiewak J, Marczak M, Bilinska M, Grzybowski Z. Genetic diagnosis in transthyretin cardiac amyloidosis - A single centre experience. Kardiologia Polska. 2018;76(Supplement 1):28–9. [Google Scholar]
- 39.Gawor MS, Marczak A, Teresinska M, Grzybowski A. It is not so rare to have a rare disease: single centre experience with wild type transthyretin amyloid cardiomyopathy. Kardiologia Polska. 2019;77(Supplement 1):139–40. [Google Scholar]
- 40.Gawor. Rare transthyretin gene mutations: phe33leu, glu89lys and ala81val in patients with cardiac transthyretin amyloidosis- single centre experience. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain; 2020. p. PM091.
- 41.Gentile LDB, Minutoli G, Cucinotta F, Obici F, Mussinelli L, Arimatea R, Russo I, Toscano M, Vita A, Mazzeo G. Description of a large cohort of caucasian patients with V122I ATTRv amyloidosis: neurological and cardiological features. J Peripheral Nerv Syst. 2020;25(3):273–8. doi: 10.1111/jns.12385. [DOI] [PubMed] [Google Scholar]
- 42.Goena CA, Villanueva X, Solla I, Rengel I, Querejeta A. Are there predictor variables for the diagnosis of transthyretin cardiac amyloidosis? Eur Heart J. 2021;42(SUPPL 1):859. [Google Scholar]
- 43.Gospodinova MS, Guergueltcheva S, Kirov V, Chamova A, Todorova T, Tournev A, Denchev IS. Cardiomyopathy and peripheral polyneuropathy severity in patients with Glu89Gln mutation at the time of diagnosis. Conference: 1st European Congresson Hereditary ATTR Amyloidosis. Paris France. Orphanet J Rare Dis. 2015;10(Supplement 1):59.
- 44.Hewitt KM, Giblin M, Murphy G, Joyce L. The clinical spectrum of T60a variant Hereditary Transthyretin Amyloidosis in Ireland. J Card Fail. 2020;26(10 Supplement):33. [Google Scholar]
- 45.Hussain Y. Variable presentation of Hereditary transthyretin-mediated amyloidosis at a single Center. J Clin Neuromuscul Dis. 2021;23(1):7–17. doi: 10.1097/CND.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 46.Jercan AE, Jurcut A, Draghici R, Badelita M, Dragomir S, Dobrea M, Popescu C, Jardan M, Stoica D, Iacob E, Codita S, Stan I, Coriu C. Clinical characteristics in patients with hereditary amyloidosis with Glu54Gln transthyretin identified in the romanian population. Orphanet J Rare Dis. 2020;15(1):34. doi: 10.1186/s13023-020-1309-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaku. Neurological manifestations in patients with hereditary transthyretin amyloidosis: a major multidisciplinary center experience. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain; 2020. p. PM093.
- 48.Kalinoski-Dubose VL, Efebera S, Parikh Y, Almaani S, Sharma S, Redder N, Freimer E, Bumma M, Kahwash N, Vallakati R, Campbell AC. Phenotypically Sex Differences in Transthyretin Amyloidosis V122I Mutation Patients. Circulation Conference: American Heart Association Scientific Sessions, AHA. 2020;142(SUPPL 3):A17058-A17058.
- 49.Karam CD, Christ D, Heitner M. Carpal tunnel syndrome and associated symptoms as first manifestation of hATTR amyloidosis. Neurol. 2019;9(4):309–13. doi: 10.1212/CPJ.0000000000000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keller AS, Delgado K, Vallakati D, Akinboboye A, Towne O, Olugemo M, Narayana K. Clinical characteristics of patients with hereditary transthyretin mutations primarily associated with cardiomyopathy and other rare transthyretin mutations: insights from a genetic testing programme. Eur Heart J. 2021;42(SUPPL 1):1808. [Google Scholar]
- 51.Kessler ASY, Pollock A, Guthrie M, McCausland S. Characterizing the journey to diagnosis for patients with Transthyretin Amyloidosis and Accompanying Congestive Heart failure. J Card Fail. 2019;25(8 Supplement):60. [Google Scholar]
- 52.Khella SS, Delgado K, Marti D, Keller C, Jefferies A, Towne J, Gabriel M, Narayana A, Olugemo A. Clinical characteristics of patients with transthyretin gene mutations and polyneuropathy manifestations of hereditary transthyretin amyloidosis. J Peripheral Nerv Syst. 2021;26(3):390–1. [Google Scholar]
- 53.Khella SD, Towne U, Narayana M, Olugemo A. Hereditary transthyretin amyloidosis and other neuromuscular diseases. Eur J Neurol. 2021;28(SUPPL 1):805. [Google Scholar]
- 54.Khella SD, Gertz D, Towne M, Narayana M, Olugemo A. Characteristics of patients with p.V50M and p.T80A mutations associated with hereditary transthyretin amyloidosis. Eur J Neurol. 2021;28(SUPPL 1):422. [Google Scholar]
- 55.Keller AS, Delgado KB, Vallakati D, Akinboboye A, Marti O, Dolinsky C, Gabriel J, Narayana A, Olugemo A. Genotypic and phenotypic differences and similarities among patients with Transthyretin Amyloidosis or other inherited Cardiovascular Diseases: insights from a genetic testing program. J Am Coll Cardiol. 2021;77(18 Supplement 1):888. [Google Scholar]
- 56.Kristen AVH, Schnabel S, Rocken P, Hardt C, Altland S, Katus K, Dengler HA. TJ. Scintigraphic heart retention and annular plane systolic excursion predict outcome in patients with senile systemic amyloidosis. J Heart Lung Transplant. 2010;29(2 Supplement):S24.
- 57.Kristen AVB, Aus Dem Siepen R, Hein F, Aurich S, Riffel M, Katus J, Buss HA. Risk stratification in wild-type transthyretin amyloidosis. J Am Coll Cardiol. 2016;1:1543. [Google Scholar]
- 58.La Malfa GM, Rizzola A, Vianello G, Tini PF, Porto G, Brunelli I, Sambuceti C, Capitanio G, Canepa S. Wrist tracer uptake in transthyretin amyloidosis cardiomyopathy patients diagnosed using bone scintigraphy. G Ital Cardiol. 2019;20(12 Supplement 1):133S. [Google Scholar]
- 59.Longhi SQ, Gagliardi CC, Milandri C, Gentile A, Manuzzi N, Lorenzini L, Bartolomei M, Salvi I, Rapezzi F. Carpal tunnel syndrome in amyloidosis: prevalence, risk factors and correlation with cardiac involvement in a large cohort of 435 consecutive patients. Eur Heart J. 2014;1:1085. [Google Scholar]
- 60.Longhi SM, Gagliardi A, Lorenzini C, Saia M, Leone F, Guidalotti O, Rapezzi PLC. Coexistence of degenerative aortic stenosis and wild type transthyretin-related cardiac amyloidosis: A potentially dangerous association that can be non-invasively identified. Orphanet J Rare Dis. 2015;10(Suppl 1):P45.
- 61.Luigetti MC, Del Grande A, Bisogni A, Romano G, Marcaccio A, LoMonaco A, Laurenti M, Obici L, Merlini L, Sabatelli GM. Ttr-related amyloidneuropathy: cliniclectrophysiological and pathological findings infifteen unrelated patients. J Peripheral Nerv Syst. 2012;1:S33–4. [Google Scholar]
- 62.Malladi RT, Winder R, Dinh T, Melanson Q, Agarwal M. Alnylam Act: Heterogenous Disease Manifestations of Hereditary transthyretin-mediated amyloidosis. J Card Fail. 2019;25(8 Supplement):111–S112. [Google Scholar]
- 63.Martone RB, De Los Santos J, Gagliardi J, Caponetti C, Perfetto G, Di Mario F, Marcucci C, Rapezzi R, Maurer C, Cappelli MF. Effect of ethnic background on phenotype at presentation in V122i-related hereditary cardiac amyloidosis. Eur Heart J Supplement. 2019;21(SUPPL J):J183. [Google Scholar]
- 64.Merli EG, Antonopoulos I, Pontone A, Cicchitelli G, Amadei G, Fabbri G, Del Giudice E. Myocardial distribution of 99mTc-HMDP and regional longitudinal strain in aTTR amyloidosis cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2019;20(Supplement 1):i529. [Google Scholar]
- 65.Milandri AG, Mazzeo M, Alfonzo A, Stancanelli A, Vita C, Rapezzi G, Tournev C. Transthyretin-related amyloidosis in the mediterranean and balkan area: focus on the GLU89GLN mutation. J Peripheral Nerv Syst. 2016;21(3):281. [Google Scholar]
- 66.Nakagawa MS, Yazaki Y, Tojo M, Yoshinaga K, Doden T, Koyama T, Yanagisawa J, Ikeda S. Carpal tunnel syndrome: a common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid. 2016;23(1):58–63. doi: 10.3109/13506129.2015.1135792. [DOI] [PubMed] [Google Scholar]
- 67.Ng. The clinical course of transthyretin familial amyloid polyneuropathy with p.ala117ser mutation. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain; 2020 p. PM123.
- 68.Oike FU, Yamamoto H, Yamada E, Egashira T, Morioka K, Nishi M, Komorita M, Hirakawa T, Tabata K, Yamanaga N, Fujisue K, Hanatani K, Sueta S, Arima D, Araki Y, Takashio S, Oda S, Misumi S, Kawano Y, Matsushita H, Ueda K, Matsui M, Tsujita H. Prognostic value of left atrial strain in patients with wild-type transthyretin amyloid cardiomyopathy. ESC Heart Fail. 2021;28:28. doi: 10.1002/ehf2.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Papoutsidakis NJ, Rodonski D, Miller A. How soon is now? Delay in the utilization of technetium-99m pyrophosphate scintigraphy for the diagnosis of cardiac transthyretin amyloidosis in patients with symptoms. J Nuclear Cardiol. 2017;24(4):1485. [Google Scholar]
- 70.Pastorelli FP, Rapezzi R, Milletti C, Salvi DF. Neurophysiological assessment of senile systemic amyloidosis. J Peripheral Nerv Syst. 2016;21(S1):S23. [Google Scholar]
- 71.Patel KS, Nitsche P, Williams C, Tillin S, Captur T, Chako G, Newton L, Kennon J, Menezes S, Pugliese L, Fontana F, Treibel M, Mascherbauer TA, Moon J. AS-amyloidosis. Dual pathology or novel disease? A multimodality, multi-centre assessment across health and disease. Eur Heart J Cardiovasc Imaging. 2021;22(SUPPL 1):i459–60. [Google Scholar]
- 72.Peltier AH, Wigger R, Punnoose M, Slosky L. Spectrum of polyneuropathy observed in V122I inherited transthyretin amyloidosis patients. J Peripheral Nerv Syst. 2020;25(4):503–4. [Google Scholar]
- 73.Peltier AD, Ilieva U. Polyneuropathy characteristics in hATTR V122I patients: a multicenter perspective. J Peripheral Nerv Syst. 2021;26(3):345. [Google Scholar]
- 74.Pinney JHL, Gillmore HJ, Wechalekar JD, Gibbs A, Sattianayagam SDJ, Banypersad P, Dungu SM, Wassef J, McCarthy N, Hawkins CA, Whelan PNCJ. Senile systemic amyloidosis: A common cause of heart failure in the elderly? Heart. 2011;97:A59–A60. [Google Scholar]
- 75.Plante-Bordeneuve VG, Hebrard F, Damy B, Ayache T, Nordine S, Lefaucheur TJP. Understand the disease emergence in hereditary transthyretin amyloidosis (hATTR): A longitudinal study of asymptomatic carriers to inaugural disease manifestations. Neurology. 2019;92(15 Supplement):P1.9-035.
- 76.Quarta CL, Cappelli S, Perfetti F, Ferlini F, Perlini A, Cinelli S, Gentile MM, Merlini N, Rapezzi G. Late onset cardiomyopathy due to transthyretin Ile68Leu mutation: a cardiogenic variant of familial amyloidosis potentially mimicking sarcomeric hypertrophic cardiomyopathy. Eur Heart J. 2013;1:528. [Google Scholar]
- 77.Quarta CCT, Gonzalez-Lopez AL, Lane E, Maurer T, Whelan M, Kristen CJ, Falk A, Damy RH, Garcia-Pavia T, Merlini P, Rapezzi G, Gillmore C, Hawkins JDPN. Characterization of wild-type transthyretin amyloidosis among women: Preliminary results from an international multicenter study. Orphanet J Rare Dis. 2017;12(Suppl 1):165. [Google Scholar]
- 78.Rocio Ruiz Hueso RBC, Calvo Moron I, Bermudo Guitarte C, Aramburu Bodas C, Carmona Nimo O, Rico Corral E, Salamanca Bautista MA. Prevalence of cardiac amyloidosis in internal medicine patients with heart failure. Eur J Heart Fail. 2021;23(SUPPL 2):320. [Google Scholar]
- 79.Russo MS, Fabrizi C, Ferrarini GM, Gentile M, Vita L, Toscano G, Mazzeo AA. The transthyretin amyloidoses outcome survey (THAOS): record of patients from our site. J Peripheral Nerv Syst. 2011;3:S121.
- 80.Russo MM, Stancanelli A, Di Leo C, Gentile R, Di Bella L, Minutoli G, Baldari F, Vita S. Transthyretin-related familial amyloidotic polyneuropathy: description of a cohort of patients with Leu64 mutation and late onset. J Peripheral Nerv Syst. 2012;17(4):385–90. doi: 10.1111/j.1529-8027.2012.00436.x. [DOI] [PubMed] [Google Scholar]
- 81.Russo MM, Obici G, Pacciolla L, Fabrizi P, Cavallaro G, Sabatelli T, Luigetti M, Bisogni M, Pareyson G, Fenu D, Calabrese S, Rapezzi D, Bartolomei C, Grandis I, Gemelli M, Mauro C, Pradotto A, Santoro L, Manganelli L, Antonini F, Leonardi G, Vanoli L, My F, Gentile F, Stancanelli L, Mazzeo C, Vita A. Hattr Italian registry: preliminary data from the collaborative network of telethon gup 15010 study. J Peripheral Nerv Syst. 2019;24(Supplement 1):38–S39. [Google Scholar]
- 82.Salvalaggio AC, Cacciavillani D, Obici M, Mazzeo L, Luigetti A, Pastorelli M, Grandis F, Cavallaro M, Bisogni T, Lozza G, Gemelli A, Gentile C, Ermani L, Fabrizi M, Plasmati GM, Campagnolo R, Castellani M, Gasparotti F, Martinoli R, Padua C, Briani L. Nerve ultrasound in hereditary transthyretin amyloidosis: red flags and possible progression biomarkers. J Neurol. 2021;268(1):189–98. doi: 10.1007/s00415-020-10127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salvi FP, Plasmati F, Bartolomei R, Dall’Osso I, Rapezzi D. Genotypic and phenotypic correlation in an italian population of hereditary amyloidosis TTR-related (HA-TTR): clinical and neurophysiological aids to diagnosis and some reflections on misdiagnosis. Amyloid. 2012;19(Suppl 1):58–60. doi: 10.3109/13506129.2012.682187. [DOI] [PubMed] [Google Scholar]
- 84.Saturi GG, Caponetti C, Longhi A, Milandri S, Fabbri A, Ponziani G, Massa A, Sguazzotti P, Salvi M, Rapezzi FC. Role of gender in transthyretin-related amyloidosis: data from the thaos registry. Eur Heart J Supplement. 2020;22(SUPPL G):G18. [Google Scholar]
- 85.Shah KBB, Gabriel N, Stevenson A, Cannon M, Khella C. Genetic testing for Hereditary Attr Amyloidosis: insights from the Hattr Compass Program. J Am Coll Cardiol. 2020;75(11):744. [Google Scholar]
- 86.Shah KK, Delgado S, Keller D, Jefferies A, Towne J, Narayana M, Olugemo A. Symptom burden and clinical characteristics of patients with mutations associated with hereditary transthyretin amyloidosis: insights from referrals by cardiologists to a genetic testing programme. Eur J Heart Fail. 2021;23(SUPPL 2):245. [Google Scholar]
- 87.Shah KBB, Delgado N, Keller D, Khella A, Gabriel S, Narayana A, Olugemo A. Characteristics of patients with P.V142i mutations Associated with Hereditary Transthyretin Amyloidosis: insights from a genetic testing program. J Am Coll Cardiol. 2021;77(18 Supplement 1):742. [Google Scholar]
- 88.Silva-Hernandez LHH, Valls Carbo A, Guerrero Sola A, Montalvo-Moraleda A, Galan Davila MT. Red flags in patients with hereditary transthyretin amyloidosis at diagnosis in a non-endemic area of Spain. Neurologia (Engl Ed) 2020;04:04. doi: 10.1016/j.nrleng.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 89.Slama MC, Algalarrondo P, Lairez V, Pelcot O, Durand-Zaleski F, Lilliu I, Bartoli H, Famelart M, Fievez V, Geffroy S, de Neuville C, Rault B, Bourel C, Damy G. PCV5 clinical characteristics of patients with transthyretin amyloid cardiomyopathy (ATTR-CM) in France: EPACT, a study based on the French Nationwide Claims Database Snds. Value in Health. 2020;23(Supplement 2):486–S487. [Google Scholar]
- 90.Soper ERS, Braganza SA, Kontorovich GT, Kenny AR, Abul-Husn EE. Genomic screening identifies individuals at high risk for Hereditary Transthyretin Amyloidosis. J Personalized Med. 2021;11(1):15. doi: 10.3390/jpm11010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mariana Sousa Paiva MSPBR, Christopher Strong BLR, Pedro Lopes CS, Goncalo Cunha PL, Catarina Brizido GC, Sergio Maltes CB, Carlos Aguiar SM, Miguel Mendes CA. Tracking Transthyretin Cardiac Amyloidosis: steps towards a tailored approach. Eur J Heart Fail. 2021;23(SUPPL 2):17–8. [Google Scholar]
- 92.Svendsen IHS-H, Nordvag F. A clinical, echocardiographic and genetic characterization of a danish kindred with familial amyloid transthyretin methionine 111 linked cardiomyopathy. Eur Heart J. 1998;19(5):782–9. doi: 10.1053/euhj.1997.0841. [DOI] [PubMed] [Google Scholar]
- 93.Tzagournissakis MS, Amoiridis C, Plaitakis G, Mitsias AP. Familial amyloidotic polyneuropathy in Crete, Greece. Neurology. 2016;86(16 Supplement):P5.106.
- 94.Tzagournissakis MF, Marinis E, Mathioudakis A, Michaelidou L, Spanaki K, Tsilimbaris C, Plaitakis M, Mitsias A, Zaganas PI. High prevalence of transthyretin-related amyloidosis in Crete, Greece is due to three TTR pathogenic variants with markedly differing phenotypic presentations. Neurology. 2020;94(15 Supplement):3974. [Google Scholar]
- 95.Warner ALH, Fazeli S, Berenji F, Ghaznavi G. Echocardiographic and electrocardiographic predictors of Transthyretin Cardiac Amyloidosis. J Am Coll Cardiol. 2019;73(9 Supplement 1):794. [Google Scholar]
- 96.Yamada TT, Arima S, Nishi Y, Morioka M, Hirakawa M, Hanatani K, Fujisue S, Yamanaga K, Kanazawa K, Sueta H, Araki D, Usuku S, Nakamura H, Suzuki T, Yamamoto S, Ueda E, Kaikita M, Tsujita K. Clinical characteristics and natural history of wild-type transthyretin amyloid cardiomyopathy in Japan. ESC Heart Fail. 2020;7(5):2829–37. doi: 10.1002/ehf2.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamashita TU, Nomura M, Okada T. N.: Amyloidosis typing at amyloidosis medical practice center in Kumamoto University hospital. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain; 2020. p. PM069
- 98.Zadok. Prior carpal tunnel syndrome and early concomitant echocardiographic findings among patients with systemic amyloidosis. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain; 2020. p. PT075. [DOI] [PubMed]
- 99.Zampino SS, Vaishnav F, Judge J, Ebenezer D, Polydefkis G. Distinct clinical profiles among ATTRv genotypes. J Peripheral Nerv Syst. 2021;26(3):404. [Google Scholar]
- 100.Zivkovic et al. Electrodiagnostic and clinical features of neuropathy in patients with wtATTR. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain; 2020. p. PW081.
- 101.Backer HCG, Lentzsch SE, Freibott S, Shoap CE, Strauch S, Rosenwasser RJ. Flexor tenosynovectomy in carpal tunnel syndrome as a screening tool for early diagnosis of amyloidosis. Ir J Med Sci. 2021;28:28. doi: 10.1007/s11845-021-02832-8. [DOI] [PubMed] [Google Scholar]
- 102.Bastkjær et al. The frequency of localised and systemic amyloidosis in patients receiving carpal tunnel release. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain; 2020. p. PM056.
- 103.Breda SR, Schachenmayr HP. [Incidence of biopsy-detectable amyloid deposits in the retinaculum flexorum and in the tenosynovial tissue in carpal tunnel syndrome] Zentralbl Neurochir. 1993;54(2):72–6. [PubMed] [Google Scholar]
- 104.Fernandez Fuertes JRV, Sanchez Herraez O, Ramos Pascua S. Early diagnosis of systemic amyloidosis by means of a transverse carpal ligament biopsy carried out during carpal tunnel syndrome surgery. Med Clin (Barc) 2017;148(5):211–4. doi: 10.1016/j.medcli.2016.10.046. [DOI] [PubMed] [Google Scholar]
- 105.Fosbol ELR, Leicht R, Schou BP, Maurer M, Kristensen MS, Kober SL, Gustafsson L. Association of carpal tunnel syndrome with amyloidosis, heart failure, and adverse Cardiovascular Outcomes. J Am Coll Cardiol. 2019;74(1):15–23. doi: 10.1016/j.jacc.2019.04.054. [DOI] [PubMed] [Google Scholar]
- 106.Gioeva ZU, Meliss P, Haag RR, Axmann J, Siebert HD, Becker F, Radtke K, Rocken HG. ATTR amyloid in the carpal tunnel ligament is frequently of wildtype transthyretin origin. Amyloid. 2013;20(1):1–6. doi: 10.3109/13506129.2012.750604. [DOI] [PubMed] [Google Scholar]
- 107.Hahn KU, Melis P, Axmann RR, Siebert HD, Rocken F. [Carpal tunnel syndrome and ATTR-amyloidosis] Handchir Mikrochir Plast Chir. 2018;50(5):329–34. doi: 10.1055/a-0747-6096. [DOI] [PubMed] [Google Scholar]
- 108.Hansen FGB, Nielsen R, Petersen SK, Moeller AS, Jensen HEH, Tromborg KP, Kejlaa H, Beck G, Rojek HC, Hansen AS, Marcussen CT, Overgaard N, Abildgaard S, Mogensen N. The frequency of cardiac amyloidosis in 182 patients receiving carpal tunnel release surgery. Eur Heart J. 2020;41(SUPPL 2):2126. [Google Scholar]
- 109.Itzhaki Ben Zadok OA, Vaxman A, Eisen I, Iakobishvili A, Sagie Z, Kornowski A, Vaturi R. Prior carpal tunnel syndrome and early concomitant echocardiographic findings among patients with Cardiac Amyloidosis. J Card Fail. 2020;26(11):909–16. doi: 10.1016/j.cardfail.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 110.Milandri AF, Gagliardi A, Longhi C, Salvi S, Curti F, Foffi S, Caponetti S, Lorenzini AG, Ferlini M, Rimessi A, Mattioli P, Violante S, Rapezzi FS. Carpal tunnel syndrome in cardiac amyloidosis: implications for early diagnosis and prognostic role across the spectrum of aetiologies. Eur J Heart Fail. 2020;22(3):507–15. doi: 10.1002/ejhf.1742. [DOI] [PubMed] [Google Scholar]
- 111.Nakamichi KIT. Amyloid deposition in the synovium and ligament in idiopathic carpal tunnel syndrome. Muscle Nerve. 1996;19(10):1349–51. doi: 10.1002/(SICI)1097-4598(199610)19:10<1349::AID-MUS16>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 112.Reyes BAI, Sperry A, Shapiro B, Hanna DB, Seitz M. Carpal tunnel syndrome and amyloid cardiomyopathy. J Hand Surg. 2017;42(9 Supplement 1):31–S32. [Google Scholar]
- 113.Samoes RT, Valdrez R, Goncalves K, Melo Pires I, Martins da Silva M, Coelho A. Amyloid detection in the transverse carpal ligament of patients with hereditary ATTR V30M amyloidosis and carpal tunnel syndrome. Amyloid. 2017;24(2):73–7. doi: 10.1080/13506129.2017.1313222. [DOI] [PubMed] [Google Scholar]
- 114.Scott KLC, Renfree CR. Histopathologic evaluation of Flexor Tenosynovium in recurrent carpal tunnel syndrome. Plast Reconstr Surg. 2019;143(1):169–75. doi: 10.1097/PRS.0000000000005090. [DOI] [PubMed] [Google Scholar]
- 115.Sekijima YU, Tojo S, Sano K, Imaeda K, Kato T, Ikeda HS. Prevalence of senile systemic amyloidosis in patients with idiopathic carpal tunnel syndrome. Amyloid. 2010;17(S1):P-141.
- 116.Sekijima YU, Tojo S, Sano K, Shimizu K, Imaeda Y, Hoshii T, Kato Y, Ikeda H. High prevalence of wild-type transthyretin deposition in patients with idiopathic carpal tunnel syndrome: a common cause of carpal tunnel syndrome in the elderly. Hum Pathol. 2011;42(11):1785–91. doi: 10.1016/j.humpath.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 117.Stein KS, Linke S, Goebel RP. Chemical heterogeneity of amyloid in the carpal tunnel syndrome. Virchows Arch A Pathol Anat Histopathol. 1987;412(1):37–45. doi: 10.1007/BF00750729. [DOI] [PubMed] [Google Scholar]
- 118.Sugiura KK, Ueba H, Kubo H, Ochi T, Baba Y, Miyagawa Y, Noguchi K, Hirota T, Yamasaki T, Wada N, Nakashima N, Murakami J, Ikeuchi I, Kitaoka M. Tenosynovial and Cardiac Transthyretin amyloidosis in japanese patients undergoing carpal tunnel release. Circ Rep. 2021;3(6):338–44. doi: 10.1253/circrep.CR-21-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Uchiyama SS, Tojo Y, Sano K, Imaeda K, Moriizumi T, Ikeda T, Kato S. Effect of synovial transthyretin amyloid deposition on preoperative symptoms and postoperative recovery of median nerve function among patients with idiopathic carpal tunnel syndrome. J Orthop Sci. 2014;19(6):913–9. doi: 10.1007/s00776-014-0635-y. [DOI] [PubMed] [Google Scholar]
- 120.Vianello PFP, Canepa I, Tini M, La Malfa G. Prevalence of transthyretin amyloid cardiomyopathy in male patients who underwent bilateral carpal tunnel surgery: the actual study. Eur Heart J Supplement. 2020;22(SUPPL N):N130. doi: 10.1016/j.ijcard.2020.12.044. [DOI] [PubMed] [Google Scholar]
- 121.Wininger AEP, Le BM, Harris JT, Trachtenberg JD, Liberman BH. Musculoskeletal pathology as an early warning sign of systemic amyloidosis: a systematic review of amyloid deposition and orthopedic surgery. BMC Musculoskelet Disord. 2021;22(1):51. doi: 10.1186/s12891-020-03912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zegri-Reiriz IdH-DM FJ, Dominguez F, Salas C, de la Cuadra P, Plaza A, Krsnik I, Gonzalez-Lopez E, Garcia-Pavia P. Prevalence of Cardiac Amyloidosis in patients with carpal tunnel syndrome. J Cardiovasc Transl Res. 2019;12(6):507–13. doi: 10.1007/s12265-019-09895-0. [DOI] [PubMed] [Google Scholar]
- 123.Arevalo ABH, Murray F, Contreras S, Luo G, Ali Y. Lumbar spinal stenosis in patients with wild-type transthyretin cardiac amyloidosis. Arthritis and Rheumatology. 2019;71(Supplement 10):3807–8. [Google Scholar]
- 124.Cortese AV, Lozza E, Alfonsi A, Montini E, Moglia A, Merlini A, Obici GL. Diagnostic challenges in transthyretin amyloidosis: avoiding misdiagnosis of a treatable hereditary neuropathy. J Peripheral Nerv Syst. 2016;21(S1):S11–2. doi: 10.1136/jnnp-2016-315262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.D’Agostino ANM, Quinn MS. Lumbar spinal stenosis and spondylosis associated with amyloid deposition in the ligamentum flavum. Clin Neuropathol. 1992;11(3):147–50. [PubMed] [Google Scholar]
- 126.Eldhagen PB, Lund S, Sorensson LH, Suhr P, Westermark OB. Transthyretin amyloid deposits in lumbar spinal stenosis and assessment of signs of systemic amyloidosis. J Intern Med. 2021;289(6):895–905. doi: 10.1111/joim.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gagne F. Vertebral ligament amyloid deposits in spinal stenosis. [French] Can J Neurol Sci. 1995;22(2):164–7. doi: 10.1017/s0317167100040257. [DOI] [PubMed] [Google Scholar]
- 128.Gies UL, Schachenmayr RP. Amyloid deposits of immunohistochemically different classes in the ligamentum flavum in biopsies from patients with herniated discs or lumbar spinal stenosis. Clin Neuropathol. 1996;15(1):54–9. [PubMed] [Google Scholar]
- 129.Godara et al. Amyloid in the ligamentum flavum of patients with spinal stenosis and ATTR (wild-type) cardiac involvement. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain; 2020. p. PW089
- 130.Westermark PW, Suhr GT, Berg OB. Transthyretin-derived amyloidosis: probably a common cause of lumbar spinal stenosis. Ups J Med Sci. 2014;119(3):223–8. doi: 10.3109/03009734.2014.895786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yanagisawa AU, Sueyoshi M, Okada T, Fujimoto T, Ogi T, Kitagawa Y, Tasaki K, Misumi M, Oshima Y, Jono T, Obayashi H, Hirakawa K, Uchida K, Westermark H, Ando P, Mizuta Y. Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod Pathol. 2015;28(2):201–7. doi: 10.1038/modpathol.2014.102. [DOI] [PubMed] [Google Scholar]
- 132.Abboud AC, Nguonly A, Fiseha A, Bean N, Osborne A, Ibrahim MT, McCarthy NE, Tawakol CP, Ruberg A, Sadjadi FL, David R, Lewis WS, Januzzi GD, Gaggin JL. Care Trajectory and early clinical features among patients with 99mtc-Pyrophosphate positive transthyretin amyloid cardiomyopathy (Attr-Cm) J Am Coll Cardiol. 2020;75(11):820. [Google Scholar]
- 133.Arana XG, Villanueva C, Rengel I, Manas A, Rilo L, Solla I, Querejeta I. Transthyretin cardiac amyloidosis: what are the main clinical suspicion data? Eur J Heart Fail. 2021;23(SUPPL 2):250. [Google Scholar]
- 134.Auer-Grumbach MR, Ablasser R, Agis K, Beetz H, Duca C, Gattermeier F, Glaser M, Hacker F, Kain M, Kaufmann R, Kovacs B, Lampl GG, Ljevakovic C, Nagele N, Polzl J, Quasthoff G, Raimann S, Rauschka B, Reiter H, Skrahina C, Schuhfried V, Sunder-Plassmann O, Verheyen R, Wanschitz ND, Weber J, Windhager T, Wurm R, Zimprich R, Loscher F, Bonderman WN. Hereditary ATTR Amyloidosis in Austria: Prevalence and Epidemiological Hot Spots. J. 2020;9(7):14. doi: 10.3390/jcm9072234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aus dem Siepen FH, Prestel S, Baumgartner S, Schonland C, Hegenbart S, Rocken U, Katus C, Kristen HA. A. V.: Carpal tunnel syndrome and spinal canal stenosis: harbingers of transthyretin amyloid cardiomyopathy? Clin 2019, 108(12):1324–30. [DOI] [PubMed]
- 136.Bhadola SK, Lau MKHV. Neurological manifestations in patients with hereditary transthyretin amyloidosis: A retrospective database study based at a major multidisciplinary amyloidosis center. Neurology. 2020;94(15 Supplement):2888. [Google Scholar]
- 137.Bukhari SM, Shpilsky S, Nieves D, Soman R. Amyloidosis prediction score: a clinical model for diagnosing Transthyretin Cardiac Amyloidosis. J Card Fail. 2020;26(10 Supplement):33. [Google Scholar]
- 138.Bukhari SM, Shpilsky S, Nieves D, Bashir R, Soman Z. Development and validation of a diagnostic model and scoring system for transthyretin cardiac amyloidosis. J Investig Med. 2021;69(5):1071–2. [Google Scholar]
- 139.Campagnolo MC, Cacciavillani C, Berno M, Salvalaggio T, Cipriani A, Castellani A, Briani F. Wild-type transthyretin amyloidosis: Clinical, Neurophysiological and Imaging Profile. J Peripheral Nerv Syst. 2020;25(4):555. [Google Scholar]
- 140.Debonnaire PC, De Smet M, Trenson M, Lycke S, Demeester M, Van Droogenbroeck C, De Vriese J, Verhoeven AS, Vantomme K, Van Meirhaeghe N, Willandt J, Lambert B, de Paepe M, Delanote P, De Geeter J, Tavernier FR. Trends in diagnosis, referral, red flag onset, patient profiles and natural outcome of de novo cardiac amyloidosis and their multidisciplinary implications. Acta Cardiol. 2022;77(9):791–804. doi: 10.1080/00015385.2021.1976450. [DOI] [PubMed] [Google Scholar]
- 141.Di Stefano VT, Giustino E, Gagliardo V, Lupica A, Iacono A, Palma S, Battaglia A, Brighina GF. Bioimpedance analysis as a marker for disease progression in hereditary transthyretin amyloidosis with polyneuropathy. J Neurol Sci. 2021;429(Supplement):118376.
- 142.Durmus HM, Atmaca Z, Poda MM, Cakar M, Serdaroglu-Oflazer A, Deymeer P, Parman FY. Genotypic and phenotypic presentation of GLU89GLN mutation in Turkey. Orphanet J Rare Dis. 2015;10(Suppl 1):P33. [Google Scholar]
- 143.Durmus HC, Sahin A, Matur E, Poda Z, Altunoglu M, Oflazer-Serdaroglu U, Deymeer P, Parman F. Genotypic and phenotypic presentation of transthyretin-related familial amyloid polyneuropathy (TTR-FAP) in Turkey. J Peripheral Nerv Syst. 2017;22(3):276–7. doi: 10.1016/j.nmd.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 144.Huda AS, Castano SJ, Niyogi A, Schumacher A, Stewart J, Deo M. A machine learning model for the systematic identification of wild-type transthyretin cardiomyopathy. J Card Fail. 2019;25(8 Supplement):53–S54. [Google Scholar]
- 145.Lauppe RELH, Gerdeskold J, Rozenbaum C, Strand MH, Vakevainen AM, Kuusisto M, Gude J, Gustafsson E, Smith F. Nationwide prevalence and characteristics of transthyretin amyloid cardiomyopathy in Sweden. Open Heart. 2021;8(2):10. doi: 10.1136/openhrt-2021-001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Martyn TS, Estep J, Collier J, Kwon P, Jaber D, Hanna W, Tang M. The Use of Natural Language Processing Tools and the Integrated Electronic Medical Record (Emr) to identify patients at high-risk for Transthyretin Cardiac Amyloidosis. J Am Coll Cardiol. 2021;77(18 Supplement 1):839. [Google Scholar]
- 147.Russo MO, Bartolomei L, Cappelli I, Luigetti F, Fenu M, Cavallaro S, Chiappini T, Gemelli MG, Pradotto C, Manganelli LG, Leonardi F, My L, Sampaolo F, Briani S, Gentile C, Stancanelli L, Di Buduo C, Pacciolla E, Salvi P, Casagrande F, Bisogni S, Calabrese G, Vanoli D, Di Iorio F, Antonini G, Santoro G, Mauro L, Grandis A, Di Girolamo M, Fabrizi M, Pareyson GM, Sabatelli D, Perfetto M, Rapezzi F, Merlini C, Mazzeo G, Vita A. ATTRv amyloidosis Italian Registry: clinical and epidemiological data. Amyloid. 2020;27(4):259–65. doi: 10.1080/13506129.2020.1794807. [DOI] [PubMed] [Google Scholar]
- 148.Russell AH, Chhibber C, Korngut S, Fine L. Utility of Neuropathy Screening for Wild-Type Transthyretin Amyloidosis Patients. Can J Neurol Sci. 2021;48(5):607–15. doi: 10.1017/cjn.2020.271. [DOI] [PubMed] [Google Scholar]
- 149.George KMD, Nail RS, Yu J, Mastroianni A, Wang M, Arkun AY, Patel K, Kryzanski A, Comenzo J, Riesenburger R. Wild-type Transthyretin Amyloidosis Occurring in the Ligamentum Flavum of the Cervicothoracic spine. World Neurosurg. 2020;142:e325–30. doi: 10.1016/j.wneu.2020.06.228. [DOI] [PubMed] [Google Scholar]
- 150.George KMH, Breton NS, Cooper J, Dowd B, Nail RS, Yu J, Mastroianni A, Wang M, Godara A, Zhang A, Arkun D, Patel K, Varga AR, Soto C, Kryzanski O, Comenzo J, Riesenburger R. Increased thickness of lumbar spine ligamentum flavum in wild-type transthyretin amyloidosis. J Clin Neurosci. 2021;84:33–7. doi: 10.1016/j.jocn.2020.11.029. [DOI] [PubMed] [Google Scholar]
- 151.George KMH, Breton NS, Cooper J, Dowd B, Nail RS, Yu J, Mastroianni A, Wang M, Godara A, Zhang A, Arkun D, Patel K, Varga AR, Soto C, Kryzanski O, Comenzo J, Riesenburger R. Lumbar ligamentum flavum burden: evaluating the role of ATTRwt amyloid deposition in ligamentum flavum thickness at all lumbar levels. Clin Neurol Neurosurg. 2021;206:106708. doi: 10.1016/j.clineuro.2021.106708. [DOI] [PubMed] [Google Scholar]
- 152.Godara AR, Zhang RI, Varga DX, Fogaren C, Siddiqui T, Yu NS, Wang A, Mastroianni A, Dowd M, Nail R, McPhail TJ, Kurtin ED, Theis PJ, Toskic JD, Arkun D, Pilichowska K, Kryzanski M, Patel J, Comenzo ARR. Association between spinal stenosis and wild-type ATTR amyloidosis. Amyloid. 2021;4:1–8. doi: 10.1080/13506129.2021.1950681. [DOI] [PubMed] [Google Scholar]
- 153.Akasaki Y, Reixach N, Matsuzaki T, Alvarez-Garcia O, Olmer M, Iwamoto Y, Buxbaum JN, Lotz MK. Transthyretin deposition in articular cartilage: a novel mechanism in the pathogenesis of osteoarthritis. Arthritis & Rheumatology. 2015;67(8):2097–107. doi: 10.1002/art.39178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Akasaki YA-G, Reixach O, Buxbaum N, Iwamoto J, Lotz YMK. Transthyretin and amyloid in cartilage aging and osteoarthritis. Arthritis Rheumatol. 2014;(10):S452.
- 155.Akasaki YL, Matsuzaki MK, Alvarez-Garcia T, Okazaki O, Nakashima KY. Transthyretin and amyloid deposition in osteoarthritis. J Orthop Res Conf. 2017;35(S1):1538. [Google Scholar]
- 156.Egan MSG, Cohen DL, Segal AS. The association of amyloid deposits and osteoarthritis. Arthritis Rheum. 1982;25(2):204–8. doi: 10.1002/art.1780250214. [DOI] [PubMed] [Google Scholar]
- 157.Gu YJG, Mu P, Lu Y, Zheng JH, Sun F. Clinical and laboratory characteristics of patients having amyloidogenic transthyretin deposition in osteoarthritic knee joints. J Zhejiang Univ Sci B. 2014;15(1):92–9. doi: 10.1631/jzus.B1300046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Niggemeyer OS, Deuretzbacher J, Zustin G, Ruther J. Amyloid deposition in osteoarthritis of the hip. Arch Orthop Trauma Surg. 2011;131(5):637–43. doi: 10.1007/s00402-010-1187-z. [DOI] [PubMed] [Google Scholar]
- 159.Takanashi TM, Yazaki M, Yamazaki M, Nawata H, Katagiri M, Ikeda Y. Synovial deposition of wild-type transthyretin-derived amyloid in knee joint osteoarthritis patients. Amyloid. 2013;20(3):151–5. doi: 10.3109/13506129.2013.803190. [DOI] [PubMed] [Google Scholar]
- 160.Yanagisawa AU, Sueyoshi M, Nakamura T, Tasaki E, Suenaga M, Motokawa G, Toyoshima H, Kinoshita R, Misumi Y, Yamashita Y, Sakaguchi T, Westermark M, Mizuta P, Ando H. Knee osteoarthritis associated with different kinds of amyloid deposits and the impact of aging on type of amyloid. Amyloid. 2016;23(1):26–32. doi: 10.3109/13506129.2015.1115758. [DOI] [PubMed] [Google Scholar]
- 161.Paccagnella AB, Mattana R, Mei F, Saturi R, Massa G, Caponetti P, Sguazzotti G, Ponziani M, Gagliardi A, Longhi C, Galie S, Fanti N. Prevalence of transthyretin cardiac amyloidosis (ATTRCA) in a population of patients with hip and knee arthroplasty: could they be early signs? Eur J Nucl Med Mol Imaging. 2020;47(SUPPL 1):528. [Google Scholar]
- 162.Rubin JA, Teruya J, Castano S, Lehman A, Weidenbaum RA, Geller M, Helmke JA, Maurer S. Hip and knee arthroplasty are common among transthyretin cardiac amyloidosis patients and occur 7.6 years before cardiac amyloid diagnosis: can we identify affected patients earlier? J Card Fail. 2017;23(8 Supplement 1):27. doi: 10.1080/13506129.2017.1375908. [DOI] [PubMed] [Google Scholar]
- 163.Campbell C et al. Orthopaedics history preceding diagnosis of cardiac amyloidosis: Timing and variation by subtype. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain; 2020. p. PT119.
- 164.Geller HIT, Falk DRH. Ruptured biceps tendon: A novel non-cardiac clue to TTR cardiac amyloidosis. Circulation. 2015;132(SUPPL. 3):A12716. [Google Scholar]
- 165.Gorevic PD. Cardiac amyloidosis at Mount Sinai: review of 164 cases with special reference to Associated arthropathy, neuropathy, myopathy and gammopathy. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain; 2020. p. PT135.
- 166.Kastritis E, et al. Diagnosis and clinical characteristics of patients with wtATTR cardiomyopathy: a systemic disease beyond the heart. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain; 2020. p. PW092.
- 167.Kogan R, et al. Prevalence and clinical significance of orthopedic red flags in cardiac amyloidosis. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain; 2020. p. PT099.
- 168.Nativi-Nicolau J et al. Wild-type transthyretin, more than what meets the heart. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain; 2020. p. PW083.
- 169.Rapezzi et al. The incidence and temporal relationship of early clinical manifestations and transthyretin amyloid cardiomyopathy in tafamidis clinical trials. In: International Society of Amyloidosis vol. XVII International Symposium on Amyloidosis. Spain; 2020. p. PT086.
- 170.Rubin JA, Teruya J, Castano S, Lehman A, RA Weidenbaum, Geller M, Helmke JA, Maurer S. M. S. Hip and knee arthroplasty are common among patients with transthyretin cardiac amyloidosis, occurring years before cardiac amyloid diagnosis: can we identify affected patients earlier? Amyloid. 2017;24(4):226–230. doi: 10.1080/13506129.2017.1375908. [DOI] [PubMed] [Google Scholar]
- 171.Sekijima YY, Ueda M, Koike M, Yamada H, Ando M. First nationwide survey on systemic wild-type ATTR amyloidosis in Japan. Amyloid. 2018;25(1):8–10. doi: 10.1080/13506129.2017.1409706. [DOI] [PubMed] [Google Scholar]
- 172.Willis CK, Watanabe K, Biskupiak A, Nolen J, Blackner K, Bruno L, Ateya M, Schepart M, Nativi-Nicolau A. Screening of patients at risk for wild type Attr-Cm using a computational machine learning algorithm. J Am Coll Cardiol. 2021;77(18 Supplement 1):677. [Google Scholar]
- 173.Hara YT, Kawano Y, Hoshikawa K, Kita S. The Tenosynovitis of Fingers Associated with Transthyretin Amyloidosis. J Hand Surg Asian Pac Vol. 2020;25(3):340–4. doi: 10.1142/S2424835520500381. [DOI] [PubMed] [Google Scholar]
- 174.Sood RFK, McCreary S, Sather E, Schmitt BK, Peterson M, Lipira SL. Diagnosing systemic amyloidosis presenting as carpal tunnel syndrome: a risk nomogram to Guide Biopsy at Time of carpal tunnel release. J Bone Joint Surg Am. 2021;103(14):1284–94. doi: 10.2106/JBJS.20.02093. [DOI] [PubMed] [Google Scholar]
- 175.Sperry BWK, Gabrovsek R, Donnelly A, Kilpatrick JP, Shapiro S, Evans D, Maschke PJ, Cotta S, Nakashima C, Seitz M, Hanna W. Cardiac amyloidosis screening at trigger finger release surgery. Am J Cardiol. 2021;160:96–8. doi: 10.1016/j.amjcard.2021.08.049. [DOI] [PubMed] [Google Scholar]
- 176.Sperry BWR, Ikram BA, Donnelly A, Phelan JP, Jaber D, Shapiro WA, Evans D, Maschke PJ, Kilpatrick S, Tan SE, Rodriguez CD, Monteiro ER, Tang C, Kelly WHW, Seitz JW. W. H., Jr. Hanna, M.: Tenosynovial and Cardiac Amyloidosis in Patients Undergoing Carpal Tunnel Release. Journal of the American College of Cardiology 2018, 72(17):2040–2050. [DOI] [PubMed]
- 177.Sueyoshi TU, Jono M, Irie H, Sei H, Ide A, Ando J, Mizuta Y. Wild-type transthyretin-derived amyloidosis in various ligaments and tendons. Hum Pathol. 2011;42(9):1259–64. doi: 10.1016/j.humpath.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 178.Leon Cejas LC, Conti C, Aguirre ME, Rodriguez F, Landricina A, Pedelhez P, Cordoba R, Chaves M, Rugiero M, Marchesoni M, Pardal C, Barroso A, Reisin F. Argentinean study in transthyretin familial amyloid polyneuropathy. An old illness that we need to think. Eur J Neurol. 2020;27(Supplement 1):405. [Google Scholar]
- 179.JBI Manual for Evidence Synthesis. [https://jbi-global-wiki.refined.site/space/MANUAL].
- 180.Grogan M, Hawkins PN, Kristen AV, Berk JL, Suhr OB, Lin H, Merkel M, McManus A, Powell C, Vest J, et al. Identifying mixed phenotype: evaluating the Presence of Polyneuropathy in patients with Hereditary transthyretin-mediated amyloidosis with cardiomyopathy. J Card Fail. 2019;25(8):9–S10. [Google Scholar]
- 181.Griffin JM, Rosenthal JL, Grodin JL, Maurer MS, Grogan M, Cheng RK. ATTR Amyloidosis: current and emerging management strategies: JACC: CardioOncology State-of-the-art review. JACC CardioOncol. 2021;3(4):488–505. doi: 10.1016/j.jaccao.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D. Time Course of Common Clinical Manifestations in patients with Transthyretin Cardiac Amyloidosis: Delay from Symptom Onset to diagnosis. J Card Fail. 2018;24(2):131–3. doi: 10.1016/j.cardfail.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 183.Looi LM. Dystrophic amyloidosis: a local complication of tissue damage with heterogeneous distribution. Histopathology. 1991;19(2):169–72. doi: 10.1111/j.1365-2559.1991.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 184.Westin O, Fosbøl Emil L, Maurer Mathew S, Leicht Birgitte P, Hasbak P, Mylin Anne K, Rørvig S, Lindkær Thomas H, Johannesen Helle H, Gustafsson F. Screening for Cardiac Amyloidosis 5 to 15 years after surgery for bilateral carpal tunnel syndrome. J Am Coll Cardiol. 2022;80(10):967–77. doi: 10.1016/j.jacc.2022.06.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplement 1. PICOTS criteria for study inclusion and exclusion in the SLR. Supplement 2. Ovid® search strategies for EMBASE and Medline (run on November 3rd, 2021). Supplement 3. Case studies excluded from the systematic literature with ATTR MSK manifestation
Data Availability Statement
All data from the review are available within the references included in this manuscript. Only peer-reviewed data reported in published articles, and data presented at congresses and subsequently published as abstracts were used.