Abstract
Introduction: Primary cutaneous CD8+ acral T-cell lymphoproliferative disorder (CD8+ ATCLPD) is a rare form of cutaneous T-cell lymphoma that commonly presents on the acral regions of the body. We report a case of a 61-year-old man diagnosed with primary cutaneous CD8+ ATCLPD of the ear. Case presentation: A 61-year-old man presented with a non-healing, erythematous painful macule on the ear that had been evolving for the past 3 months. The lesion was resected, and histopathological examination revealed a primary cutaneous CD8+ ATCLPD with acral localization. Further investigations including CT scan of the thorax, abdomen and pelvis were done to stage the disease. The results showed no extracutaneous involvement. Conclusion: Accurate identification of primary cutaneous CD8+ ATCLPD is crucial due to its distinct prognostic and therapeutic implications compared to other CD8+ cytotoxic lymphoid proliferations. Primary cutaneous CD8+ ATCLPD can be treated conservatively and typically follows a slow clinical course, regardless of the treatment method. Understanding the clinical context, as well as the morphological and immunophenotypic characteristics, can assist in making a precise diagnosis.
Keywords: CD8-Positive, cutaneous T-cell, lymphoproliferative disorder, primary cutaneous acral
Introduction
Primary cutaneous CD8+ acral T-cell lymphoma is now classified as primary cutaneous CD8+ acral T-cell lymphoproliferative disorder (CD8+ ATCLPD), introduced in the 5th edition of WHO classification and the International Consensus classification in 2022.1,2 It typically presents as solitary or, rarely, bilateral single nodular lesions at acral sites, namely the ears, face and feet. 3 It is characterized by skin infiltration of clonal atypical CD8+ cytotoxic T-lymphocytes. Due to its infrequency and resemblance to other CD8+ lymphoid proliferations in terms of morphology and immunophenotype, identifying this condition can be difficult. However, accurately categorizing this disease is critical because of its slower clinical progression when compared to other CD8+ T-cell lymphomas. This case report focuses on a patient who presented with a painful lesion on palpation on his ear, which was initially thought to be an infection. Further examination and testing revealed a diagnosis of primary cutaneous CD8+ ATCLPD in the ear. The case highlights the importance of considering rare malignancies in the differential diagnosis of skin lesions and aim through a review of the literature to discuss the clinicopathological characteristics of this uncommon entity in this site.
Case presentation
We present the case of a 61-year-old male who presented with a 3 cm macule on his right ear that had been evolving for 3 months. The patient did not report any significant medical history and had no symptoms other than mild pain on palpation. Initial physical examination revealed a solitary, pinkish-red, non-blanchable lesion with indistinct borders. No regional lymphadenopathy was noted. The patient was referred to a dermatologist for further evaluation. The dermatologist conducted a thorough clinical examination and considered various differential diagnoses, including an infectious or inflammatory process, as well as neoplastic conditions such as basal cell carcinoma and melanoma. The lesion was excised, and histopathological analysis revealed dense dermal infiltrates of medium-sized atypical lymphoid cells with small nucleoli. Mitotic figures were sparse (1-2 mitoses/10 HPF). There was no angiocentric and angiodestructive growth pattern. There was no epidermotropism. The epidermis was separated from the infiltrate by a grenz zone (Figure 1). Immunohistochemical staining showed a predominance of CD8+ T-cells, consistent with a diagnosis of primary cutaneous CD8+ ATCLPD of the ear. Tumor cells were negative for CD4, TdT, CD20, CD30, CD10, Bcl6, CD56, Granzyme B, and perforin. CD68 was focally positive with dot-like perinuclear staining. The Ki67 expression was low <5% (Figure 2). Chromogenic in situ hybridization was used to test for Epstein-Barr virus (EBV) using the EBER probe and revealed negative. Further evaluation with a computed tomography scan of the chest, abdomen, and pelvis was performed, and no evidence of systemic involvement was found. The patient is doing well after 2 years of follow-up, with no signs of disease recurrence or progression on extra-cutaneous sites.
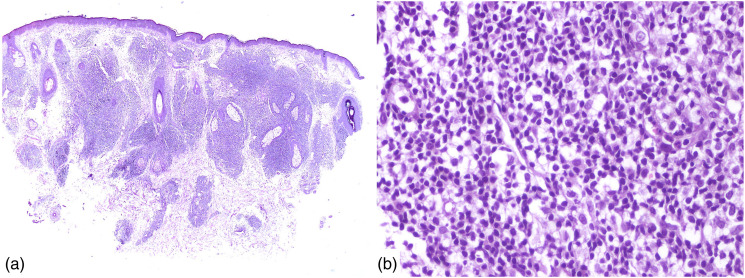
Figure 1.
(a)/Low power, H&E ×20: Skin tissue showing a thinned-out epidermis and dense infiltration of dermis by lymphoid cells (b)/High power, H&E ×100: Dense infiltration with moderately atypical lymphoid cells.
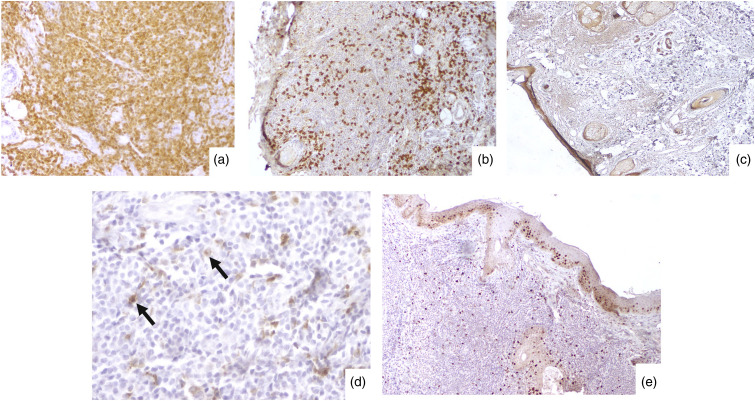
Figure 2.
Immunohistochemical staining: (a)/CD8 highlighting the neoplastic cells (original magnifications ×400); (b)/Neoplastic cells negative for CD20 (original magnification ×40); (c)/Neoplastic cells negative for TdT (original magnification ×20); (d)/Tumor cells focally positive for CD68 with a dot-like staining (original magnification ×40); (e)/Low Ki-67 proliferation index (original magnification ×40).
Discussion
Our patient had a slowly increasing erythematous lesion localized to the external ear with no systemic involvement, and a benign clinical course.
Primary cutaneous CD8+ ATCLPD most commonly affects adults over the age of 50, with a slight male predominance. 4 The majority of cases are characterized by a single, slowly developing nodule.5,6 There have been reports of bilateral, symmetrical, and recurrent disease,4,7 but without prior patches or plaques or recurrences.8,9 Although most of instances involve the ear, 10 other peripheral areas, including the nose, hands, and feet, have also been reported.4,7,8 One recent case reported a novel entity hitherto-undescribed of primary cutaneous CD8+ cutaneous lymphomas of T-cell lineage which is characterized by multifocal cutaneous localization that presented on the legs, later involving the ears bilaterally, with multiple lesions on each ear. 3 These results indicate that a local, antigenic stimulation localized to the ear is improbable. 9 There has also been suggestion that primary cutaneous CD8+ ATCLPD might be a reactive process due to the consistently indolent clinical course; nonetheless, the frequent monoclonality and aberrant loss of T-cell antigens support these lesions being a real lymphoproliferative condition. 9
Petrella et al., 11 described a diffuse proliferation of monomorphic, intermediate-sized CD8+ T-cells in the dermis and subcutis. Each example showed a clear grenz zone free of epidermotropism. The neoplastic cells exhibited lymphoblast-like nuclei that were irregular. CD3, CD8, T-cell receptor-F1, and T-cell intracytoplasmic antigen-1 were expressed in immunoperoxidase studies with a low proliferation index (10%). There was a varied loss of several T-cell antigens but no CD4, CD30, CD56, granzyme B, or Epstein-Barr virus-encoded small RNA. A monoclonal T-cell -gene rearrangement was seen in all three of the cases in the three cases that underwent molecular investigation. 11 Similarly, in our case, immunohistochemical staining showed a predominance of CD8+ T-cells, consistent with a diagnosis of primary cutaneous CD8+ ATCLPD of the ear. Tumor cells were negative for CD4, TdT, CD20, CD30, EBER, CD10, Bcl6, CD56, Granzyme B, and perforin.
Current research suggests that CD68 may serve as a selective marker to separate aggressive T-cell lymphomas with a cytotoxic CD8+ phenotype from primary cutaneous primary cutaneous CD8+ ATCLPD.12,13 Wobser et al. 12 looked into 44 cases of CD8+ cutaneous T-cell lymphomas and found 5 cases of primary cutaneous acral CD8+ T-cell lymphoproliferative disorder. All 5 primary cutaneous CD8+ ATCLPD patients showed unique, dot-like perinuclear positivity for CD68 in the Golgi zone of malignant cells during immunohistochemistry study. They concluded that CD68 with a dotlike pattern may be useful in detecting primary cutaneous CD8+ ATCLPD because none of the other CD8+ cutaneous lymphomas expressed CD68. In the present case, CD68 was focally positive with dot-like perinuclear expression.
Biopsy and histological examination are typically required to confirm the diagnosis and distinguish it from other skin diseases. Immunohistochemical and molecular studies may also be necessary to differentiate primary cutaneous CD8+ ATCLPD from other cutaneous T-cell lymphomas.
Among CD8+ variations of mycosis fungoides, primary cutaneous CD8+ ATCLPD, and type D lymphomatoid papulosis, epidermotropism is the principal morphologic marker used to identify primary cutaneous CD8+ ATCLPD from these other conditions. 6 Although epidermotropism and a single intraepidermal Pautrier collection have been seen in a few uncommon cases of primary cutaneous CD8+ ATCLPD, the majority of cases show epidermal sparing with a distinct grenz zone. 7
Despite having high-grade morphologic characteristics and a cytotoxic immunophenotype, primary cutaneous CD8+ ATCLPD always has an indolent clinical course. 10 Although there have been cases of cutaneous relapse, there are no reports of extracutaneous disease at diagnosis, and no staging investigations have revealed progression to systemic disease during follow-up durations of 3 to 168 months, regardless of the treatment technique (topical steroids, radiotherapy, surgical excision, or simple observation). 4 Patients with multifocal cutaneous disease have been treated with methotrexate, interferon, and psoralen-ultraviolet A phototherapy to reduce the chance of relapse, with varied degrees of success. 4 Our patient has just done a resection of the lesion.
Conclusion
In conclusion, our case emphasizes the critical importance of accurate diagnosis and proper management of primary cutaneous CD8+ ATCLPD. Misdiagnosis can lead to inappropriate treatment and delay in appropriate therapy. Accurate diagnosis of this rare condition requires a high index of suspicion, thorough clinical evaluation, and histopathological analysis of the tissue. Dermatologists, oncologists, and pathologists need to be aware of this rare entity and its clinical and histopathological features to avoid misdiagnosis.
Credit authorship contribution statement: All the authors read and approved the final version of the manuscript. Ghada Sahraoui (MD): conception, acquisition of clinical data, and revising the manuscript. Farah Sassi (MD): conception, acquisition of data, literature research and preparing the manuscript. Lamia Charfi (MD): revising the manuscript. Raoudha Doghri (MD): manuscript editing and revising the manuscript critically. Karima Mrad (MD): revising and accepting the final version of the manuscript critically.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor: Sassi Farah.
Provenance and peer review: Not commissioned, externally peer-reviewed.
Ethical statement
Ethical approval
Salah Azaiez Institute does not require ethical approval for reporting individual cases or case series.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
ORCID iD
Farah Sassi https://orcid.org/0000-0003-2078-6795
References
- 1.Campo E, Jaffe ES, Cook JR, et al. The international consensus classification of mature lymphoid neoplasms: a report from the clinical advisory committee. Blood 2022; 140: 1229–1253, DOI: 10.1182/blood.2022015851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022; 36: 1720–1748, DOI: 10.1038/s41375-022-01620-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrogiannis-Haliotis T, Pehr K, Roberge D, et al. Primary Cutaneous Multifocal Indolent CD8+ T-Cell Lymphoma: a novel primary cutaneous CD8+ T-cell lymphoma. Biomedicines 2023; 11: 634, DOI: 10.3390/biomedicines11020634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kluk J, Kai A, Koch D, et al. Indolent CD8-positive lymphoid proliferation of acral sites: three further cases of a rare entity and an update on a unique patient. J Cutan Pathol 2016; 43: 125–136, DOI: 10.1111/cup.12633 [DOI] [PubMed] [Google Scholar]
- 5.Skin lymphoma: the illustrated guide, 4th ed.. Hoboken, NJ: Wiley. WileyCom n.d. https://www.wiley.com/en-us/Skin+Lymphoma%3A+The+Illustrated+Guide%2C+4th+Edition-p-9781118492536 (accessed March 5, 2023). [Google Scholar]
- 6.Hathuc VM, Hristov AC, Smith LB. Primary cutaneous acral CD8+ T-cell lymphoma. Arch Pathol Lab Med 2017; 141: 1469–1475, DOI: 10.5858/arpa.2017-0230-RA [DOI] [PubMed] [Google Scholar]
- 7.Greenblatt D, Ally M, Child F, et al. Indolent CD8+lymphoid proliferation of acral sites: a clinicopathologic study of six patients with some atypical features. J Cutan Pathol 2013; 40: 248–258, DOI: 10.1111/cup.12045 [DOI] [PubMed] [Google Scholar]
- 8.Kempf W, Kazakov DV, Cozzio A, et al. Primary cutaneous CD8+ small- to medium-sized lymphoproliferative disorder in extrafacial sites. Am J Dermatopathol 2013; 35: 159–166, DOI: 10.1097/DAD.0b013e31825c3a33 [DOI] [PubMed] [Google Scholar]
- 9.Wobser M, Petrella T, Kneitz H, et al. Extrafacial indolent CD8-positive cutaneous lymphoid proliferation with unusual symmetrical presentation involving both feet. J Cutan Pathol 2013; 40: 955–961, DOI: 10.1111/cup.12213 [DOI] [PubMed] [Google Scholar]
- 10.Beltraminelli H, Mã¼llegger R, Cerroni L. Indolent CD8+ lymphoid proliferation of the ear: a phenotypic variant of the small-medium pleomorphic cutaneous T-cell lymphoma? J Cutan Pathol 2010; 37: 81–84, DOI: 10.1111/j.1600-0560.2009.01278.x [DOI] [PubMed] [Google Scholar]
- 11.Petrella T, Maubec E, Cornillet-Lefebvre P, et al. Indolent CD8-positive Lymphoid Proliferation of the Ear. Am J Surg Pathol 2007; 31: 1887–1892, DOI: 10.1097/PAS.0b013e318068b527 [DOI] [PubMed] [Google Scholar]
- 12.Wobser M, Roth S, Reinartz T, et al. CD68 expression is a discriminative feature of indolent cutaneous CD8-positive lymphoid proliferation and distinguishes this lymphoma subtype from other CD8-positive cutaneous lymphomas. Br J Dermatol 2015; 172: 1573–1580, DOI: 10.1111/bjd.13628 [DOI] [PubMed] [Google Scholar]
- 13.Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization Classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia 2022; 36: 1703–1719, DOI: 10.1038/s41375-022-01613-1 [DOI] [PMC free article] [PubMed] [Google Scholar]