Abstract
The identification of methicillin-resistant staphylococcus isolates in the clinical laboratory has typically been performed by using methods that detect phenotypic expression of resistance determinants. However, these methods may be difficult to interpret and some isolates do not express resistance until selective pressure is administered. Assays that detect genetic determinants are not subject to these limitations and have been effective in distinguishing isolates that are capable of expressing the resistance phenotype. In this study, a novel branched-DNA (bDNA) hybridization assay was used to test for the mecA gene in 416 clinical staphylococcal isolates. The results were compared with those obtained by a PCR-based assay and oxacillin disk diffusion. For 155 Staphylococcus aureus and 261 coagulase-negative Staphylococcus isolates, the bDNA assay and PCR results were 100% concordant. Among the S. aureus isolates, 20 were MecA+ and 135 were MecA−. For the coagulase-negative staphylococci, 150 were MecA+ and 111 were MecA−. The results from the genotypic detection methods were compared with those obtained by oxacillin disk diffusion. No discrepancies were detected among the S. aureus isolates; however, 10 coagulase-negative isolates were MecA+ but oxacillin sensitive and 1 isolate was MecA− but oxacillin resistant. Oxacillin resistance was induced in 6 of the 10 MecA+ isolates previously classified as oxacillin sensitive. These results suggest that the bDNA method described here is a sensitive and efficient method for detection of methicillin resistance in staphylococci and that genetic detection methods may be useful for detection of potential methicillin resistance in the clinical laboratory.
Methicillin resistance in clinical isolates of Staphylococcus is thought to occur as a combined result of the expression of the mecA gene, which codes for the cell wall surrogate enzyme penicillin binding protein (PBP) 2a or 2′ and several factors such as the fem gene series or auxiliary (aux) genes (reviewed in reference 5). In clinical laboratories, antibiotic resistance is usually detected by using methods that require a viable culture of the organism and phenotypic expression of resistance genes. However, studies indicate that there is heterogeneous expression of PBP 2a that is dependent on environmental conditions (1, 10, 16). In addition, some isolates have been shown to exhibit low- or moderate-level methicillin resistance due to overproduction of β-lactamase, modifications in the PBP binding affinities, or the presence of expression factors not related to the mecA gene (2, 9, 12, 20). Variations in laboratory reporting of high-level methicillin resistance, which requires treatment with vancomycin, may be responsible for unnecessary vancomycin usage. The current guidelines from the Centers for Disease Control and Prevention suggest restriction of vancomycin use in order to slow the occurrence of vancomycin resistance in staphylococci (4).
Molecular diagnostic assays, which detect genetic targets irrespective of expression level, have proven useful for the identification of isolates containing mecA. In recent years, several genotypic detection methods have been described (3, 7, 11, 14, 17). Most are PCR based, and some are multiplexed with broad-range 16S ribosomal DNA primers to assess the lysis efficiency of each assay. While these assays are highly sensitive and specific, we have found that they are time-consuming and PCR failures may occasionally occur due to lysis inefficiency or inhibitory substances.
In the past, assays utilizing branched-DNA (bDNA) technology have been developed to detect antibiotic resistance markers as well as pathogenic agents in clinical samples (6, 19). This assay uses multiple probes that cause an amplification of chemiluminescent signal rather than the amplification of a genetic target that is observed in PCR-based assays. To avoid the complications observed with other tests, we developed a mecA-specific assay that uses bDNA technology to detect the gene in lysates derived from bacterial colonies isolated on solid media and directly from blood cultures. The assay is performed in a 96-well microtiter plate format and takes approximately 6 h to complete, thereby allowing same-day results for cultures containing staphylococci.
(Portions of this work were presented at the Conference on Molecular Diagnostics and Therapeutics, Kananaskis, Alberta, Canada, 15–19 August 1997, and the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 28 September–1 October 1997.)
MATERIALS AND METHODS
Bacterial isolates.
Staphylococcal isolates (n = 433) were recovered from clinical samples that were taken from normally sterile anatomic sites. Consecutive isolates were recovered by inoculation onto 5% sheep erythrocyte agar plates (Becton Dickinson, Cockeysville, Md.) and incubation for 24 to 48 h at 37°C. Isolates exhibiting characteristics of gram-positive cocci by Gram stain reaction and morphology were initially identified as either Staphylococcus aureus or coagulase-negative Staphylococcus (CNS) species by using standard methods, and then all were assayed for routine antibiotic susceptibilities. With the exception of the discrepant isolates, gram-positive cocci such as Micrococcus sp. and Stomatococcus sp. were not distinguished from true staphylococci. Three bacterial cultures chosen to be controls for this study were first characterized by phenotypic detection methods, PCR (7), and by a previously described DNA hybridization assay (11). A MecA+ S. aureus (ATCC 33591), a MecA− S. aureus (ATCC 12600), and a MecA+ CNS (MC1187) were included in each assay run.
Discrepant coagulase-negative isolates were further identified by morphologic features, catalase test, and by use of the Biolog (Hayward, Calif.) Microstation system.
Phenotypic assay methods.
Isolates were emulsified in Trypticase peptone broth (Becton Dickinson) to a McFarland turbidity standard of approximately 1.0. They were then assayed for oxacillin susceptibility by using a standardized disk diffusion method as described previously (7) with the exception that Mueller-Hinton agar (MHA; Becton Dickinson) contained 2% NaCl. Positive and negative control experiments were performed for each assay. When discrepancies between phenotypic and genotypic methods occurred, the phenotypic assay was repeated in an attempt to resolve the discrepancy.
In addition, the discrepant isolates were tested for the presence of β-lactamase by the nitrocefin disk method (Cefinase; Becton Dickinson), and β-lactamase overproduction was assessed by amoxacillin-clavulanic acid (20 μg/10 μg) disk diffusion (Becton Dickinson). Next, a bacterial suspension with turbidity approximately equal to a 1.0 McFarland standard was inoculated by being swabbed onto agar medium containing 4% NaCl–6 μg of oxacillin/ml (Remel, Lenexa, Kans.), incubated at 35°C, and checked for any growth at 24 and 48 h. As a control for inhibition due to the high salt concentration, the discrepant isolates were also inoculated onto an in-house-prepared MHA medium containing 4% salt without oxacillin (Becton Dickinson).
Induction of oxacillin resistance.
Isolates that were MecA+ but sensitive to oxacillin were inoculated onto a series of MHA plates containing increasing concentrations of oxacillin. Sets of the agar plates were made by twofold dilutions of oxacillin starting at 2.0 to 0.0625 μg/ml. Two MecA− controls (ATCC 12600 and S. aureus ATCC 25923) were included to assess the media by providing an end point for MecA− isolates. Organisms were streaked for isolation on MHA containing the lowest oxacillin concentration and incubated at 37°C for 24 h. Colonies growing on the medium were inoculated onto MHA-oxacillin medium of the next highest concentration. This procedure was repeated until isolates were growing on medium containing a 2.0-μg/ml concentration of oxacillin. Subsequently, the disk diffusion assay was repeated for each isolate to assess any change in inhibition zone size (diameter).
PCR assay.
All isolates were lysed and amplified by using a multiplex PCR assay in accordance with the protocol described by Geha et al. (7).
bDNA assay.
A 617-bp amplification product for use as a positive control was created by using primers mec172 (5′-TAATAGTTGTAGTTGTCGGGTTTG-3′) and mec765 (5′-GGTTTTAAAGTGGAACGAAGGTAT-3′), which were designed from the published mecA gene sequence for S. aureus (GenBank Accession no. X52593). Each primer (0.5 μM) was included in a 50-μl PCR mixture along with 2.0 μl of S. aureus lysate, 10 mM Tris-HCl (pH 8.0), 50 mM KCl, 1.5 mM MgCl2, 1.5 μM each deoxynucleoside triphosphate and 1.25 U of Amplitaq DNA polymerase (Perkin-Elmer, Norwalk, Conn.). Amplification was performed in accordance with the following profile: initial denaturation at 94°C for 4 min, followed by 30 cycles of denaturation at 94°C for 45 s, 50°C for 45 s, and 72°C for 1 min, and then 72°C for 2 min. Subsequently, the amplification product was inserted into a plasmid vector in accordance with the manufacturer’s instructions for the TA cloning kit (Invitrogen, San Diego, Calif.). Plasmid DNA for use in the bDNA assay was isolated from one of the positive transformants and linearized with RsrII as described previously (18).
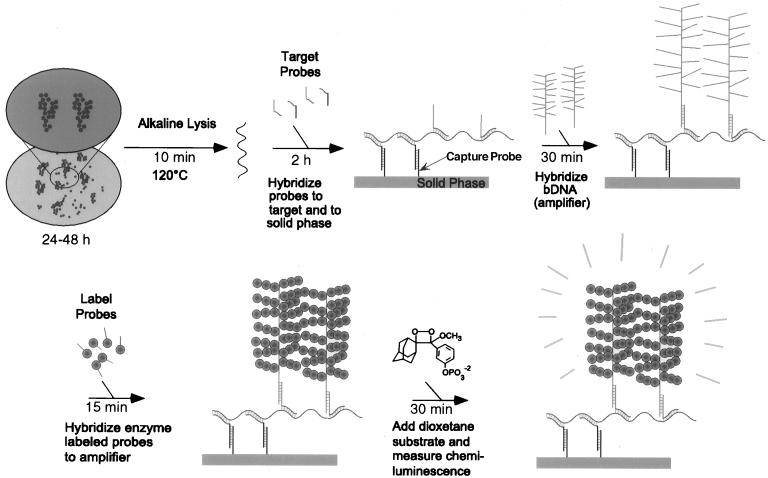
The MecA bDNA assay was performed as described in instructions provided by Chiron Diagnostics (East Walpole, Mass.) (Fig. 1). As in the phenotypic and PCR assays, both S. aureus and CNS controls were included in each run. The target-associated luminescence was then measured in a luminometer (Chiron Corporation, Emeryville, Calif.). The average relative light unit (RLU) value for each isolate was then divided by the average value obtained from the negative culture control to give a signal-to-noise (S/N) ratio. A S/N ratio of ≥3.0 indicated the presence of the mecA gene. Based on the RLU values, a coefficient of variance was determined for each sample replicate to assess the reproducibility of each assay. In the event of a positive and a negative RLU value from a set of replicates, assay of the sample was repeated.
FIG. 1.
Flow chart depicting the protocol for performance of the Chiron MecA bDNA signal amplification assay. The length of incubation time is indicated below each step.
Analytical sensitivity and specificity of the MecA bDNA assay.
Dilution studies were performed with known concentrations of MecA+ S. aureus with the intent of determining the minimum concentration of organism needed to detect the mecA gene. Eleven nonstaphylococcal isolates were assayed by using the MecA bDNA assay to detect cross-reactivity that might occur with other microbial species (Table 1).
TABLE 1.
Cross-reactivity of mecA probes as assessed by testing nonstaphylococcal clinical isolatesa
| Isolate | Mean RLU value | S/N ratio |
|---|---|---|
| Escherichia coli | 1.32 | 1.54 |
| Corynebacterium sp. | 0.94 | 1.10 |
| Klebsiella pneumoniae | 0.77 | 0.90 |
| Pseudomonas aeruginosa | 0.93 | 1.09 |
| Proteus mirabilis | 1.11 | 1.29 |
| Citrobacter freundii | 0.73 | 0.85 |
| Streptococcus pneumoniae | 1.12 | 1.30 |
| Streptococcus faecalis | 0.98 | 1.14 |
| Streptococcus bovis | 0.87 | 1.01 |
| Streptococcus agalactiae | 0.97 | 1.12 |
| Candida albicans | 0.88 | 1.10 |
The average (mean) RLU value of two replicates was divided by the average RLU value of the negative control isolate to yield the S/N ratio. A S/N value of ≥3.0 was considered positive.
RESULTS
To assess the analytical sensitivity of the MecA bDNA assay, serial dilutions of S. aureus containing the gene were analyzed by using the MecA bDNA assay. The minimum dilution from which the mecA gene was detected contained a concentration of 104 CFU/ml. No cross-reactivity with the mecA probes was detected among the 11 nonstaphylococcal isolates that were tested (Table 1). Next, the clinical sensitivity and specificity of the MecA bDNA assay were assessed in comparison to those of other methods for detection of methicillin resistance. Four hundred thirty-three clinical staphylococcal isolates were assayed by bDNA, and the results were compared with those obtained by mecA-specific PCR and oxacillin disk diffusion assays. Seventeen isolates classified as CNS were removed from the study because of PCR failure, presumably because they were not lysed by our methods. Before the results for the unknown isolates were assessed, all control results were examined to ensure the validity of each assay. Our comparison was performed with 416 isolates, including 155 S. aureus and 261 CNS isolates. The results for bDNA were all corroborated by PCR (positive and negative predictive values = 100% [each]; sensitivity and specificity = 100% [each]) (data not shown). For S. aureus, 20 isolates were MecA+ by both genetic methods; 135 isolates were MecA−. For CNS, 150 isolates were MecA+ by both genetic methods; 111 isolates were MecA−.
The bDNA and PCR results were then compared with results obtained by the oxacillin disk diffusion test. No discrepancies occurred among the S. aureus isolates. For S. aureus, all 20 MecA+ isolates were resistant by disk diffusion; 135 isolates were MecA− and sensitive by disk diffusion. For CNS isolates, 140 were MecA+ and resistant by disk diffusion; 110 were MecA− and sensitive by disk diffusion. However, 10 CNS isolates were MecA+ but were oxacillin sensitive by disk diffusion (mean zone size = 15.1 mm) and one CNS isolate was MecA− and oxacillin resistant by disk diffusion (zone size = 9 mm).
Subsequent to the initial antibiotic sensitivity testing, the discrepant CNS isolates were further identified to the species level by using the Biolog Microstation system, and the antibiotic susceptibility results were confirmed by repeating the oxacillin disk diffusion test. Among the 10 MecA+, oxacillin-sensitive isolates, 8 were identified as Staphylococcus epidermidis and two were identified as Staphylococcus hominis (Table 2). The isolate that was MecA− and oxacillin resistant was found to be Micrococcus luteus. The results of the repeated disk diffusion tests for the MecA+, oxacillin-sensitive isolates were similar to the initial test results (mean zone size = 15.6 mm). Four of the MecA+, oxacillin-sensitive staphylococcal isolates produced β-lactamase; all of these were sensitive to the amoxicillin-clavulanic acid combination (mean zone size = 27 mm). Repeat disk diffusion testing of the MecA−, oxacillin-resistant isolate also gave a similar disk zone size (9 mm) and was negative for β-lactamase production, suggesting that another mechanism was responsible for the resistant phenotype.
TABLE 2.
Results of various confirmatory tests on the discrepant CNS isolates
| No. of isolates and species identified | Results of test fora:
|
|||||
|---|---|---|---|---|---|---|
| MecA status | Repeat OX disk zone diam (mm)b | β-Lactamase production | Growth on OX-salt agar | AMOX-CLAV disk zone diam (mm)c | OX zone size after induction | |
| 57 S. hominis | + | 15 | − | NG | ND | 24 |
| 105 S. hominis | + | 11 | + | G | 25 | 13 |
| 120 S. epidermidis | + | 12 | − | NG | ND | No zone |
| 121 S. epidermidis | + | 19 | + | G | 26 | No zone |
| 133 S. epidermidis | + | 28 | − | NG | ND | NG |
| 198 S. epidermidis | + | 16 | − | NG | ND | NG |
| 265 S. epidermidis | + | 11 | − | G | ND | PPTS |
| 266 S. epidermidis | + | 13 | + | G | 27 | 9 |
| 336 S. epidermidis | + | 15 | − | G | ND | No zone |
| 412 S. epidermidis | + | 17 | + | G | 30 | No zone |
| 91 M. luteus | − | 9 | − | ND | ND | ND |
OX, oxacillin; NG, no growth; ND, not done; PPTS, pinpoint colonies growing up to the antibiotic disk.
Repeat OX disk, repeated oxacillin disk diffusion test.
AMOX-CLAV disk, amoxicillin-clavulanic acid disk diffusion test.
The discrepant isolates were then assayed by screening with the combination of 6 μg of oxacillin per ml and 4% NaCl. As a control for growth under high-salt conditions, isolates were also inoculated onto MHA medium with the addition of 4% salt but no antibiotic. For the MecA+, oxacillin-sensitive isolates, one was inhibited by the 4% salt medium at both 24 and 48 h of incubation and nine grew on the control plates. At 24 h of incubation, none of the discrepant isolates grew on the oxacillin screening medium. At 48 h of incubation, 6 of 10 MecA+, oxacillin-sensitive isolates grew, indicating that they were highly resistant to oxacillin. Interestingly, only four of the six were also positive for β-lactamase production. The isolate that was MecA− but oxacillin resistant did not grow at 24 h, but at 48 h, several tiny colonies grew on the test medium.
The ability of the MecA+, oxacillin-sensitive isolates to become phenotypically resistant during selective pressure was tested by inoculating colonies onto MHA medium with increasing concentrations of oxacillin. Neither of the MecA− control isolates grew on medium with the highest concentration (2.0 μg/ml) of oxacillin. Among the 10 MecA+, oxacillin-sensitive isolates, the 8 S. epidermidis isolates grew on medium with the final concentration of 2 μg of oxacillin per ml; however, only 6 of these 8 isolates were subsequently resistant to oxacillin by disk diffusion. Four of the six had no zone of bacterial inhibition on the media, one had a zone of 16 mm with pinpoint colonies up to the disk, and one had a zone size of 9 mm. Although the two S. hominis isolates grew on the final concentration of 2 μg/ml, they were both sensitive by repeat disk diffusion (mean zone size = 18.5 mm).
DISCUSSION
In a previous study, we evaluated a mecA-specific assay that used a paramagnetic particle-labeled probe to separate the target-probe duplexes from solution and an acridinium ester-labeled probe to detect the hybridized mecA gene (11). However, the assay was limited by low sensitivity and high concentrations of organism were needed to obtain a valid result. The MecA bDNA assay affords a significant increase in sensitivity that is probably due to the inherent signal amplification properties of the assay. When the PCR was used as the gold standard, the bDNA assay was 100% sensitive and specific for both S. aureus and CNS.
Among 416 isolates, only 11 gave discrepant results between genotypic and phenotypic assays. Of these discrepant isolates, 10 contained the mecA gene yet were phenotypically sensitive to oxacillin and 1 did not contain the mecA gene but was oxacillin resistant. Eight of these MecA+ isolates were later identified as S. epidermidis, and two were identified as S. hominis. The MecA− discrepant isolate was found to be M. luteus, and its phenotypic resistance can possibly be explained by other mechanisms not addressed in this study.
The ability of several oxacillin-sensitive isolates to become resistant after selective pressure underscores the importance of genotypic screening for antibiotic resistance markers. Presumably, such acquired resistance could occur in vivo during antibiotic therapy. Six of the 10 MecA+ discrepant isolates grew on the commercially prepared oxacillin screening medium prior to the induction experiment, 1 seemed to be inhibited by the high salt concentration, and 3 did not grow in the presence of 6 μg of oxacillin per ml. Such results are not surprising, considering that all of these isolates were sensitive to oxacillin before the induction experiment was performed. The appearance of growth after 48 h but not after 24 h might represent an induction of mecA expression during the extended incubation period. Indeed, similar results were observed by Ramotar et al. (15), with the conclusion that many mecA-positive isolates for which MICs are below breakpoint would not be detected by screening with the oxacillin-salt agar medium.
The problems associated with using phenotypic methods for identification of methicillin resistance have been well defined, and many researchers use direct detection of the mecA gene or PBP 2a as the gold standard for comparison (7, 8, 11, 13, 21). On the other hand, most phenotypic detection methods take little hands-on time and are inexpensive, thus allowing them to easily fit into the daily work flow of the clinical laboratory. Most clinical laboratories do not routinely assess the genetic status of oxacillin-resistant isolates. Isolates that contain the mecA gene yet are sensitive to oxacillin may be induced to express resistance by exposure to weak concentrations of oxacillin. Therefore, genotypic methods would be helpful in accurately assessing the potential of an isolate to become resistant during therapy. Furthermore, in light of these data, it may be possible to adjust MIC breakpoints for the coagulase-negative staphylococci to improve the sensitivity of detection of mecA-containing isolates. Additional studies are needed to determine the clinical implications of unexpressed antibiotic resistance genes within pathogenic agents and the impact of this a priori knowledge on treatment regimens.
In our hands, the MecA bDNA assay was a highly accurate alternative to other genetic detection methods and gave reproducible results for all samples, including the isolates from which the PCR assay failed to amplify the internal control. The MecA bDNA assay has also been shown to be useful for direct detection of the mecA gene in broth taken from blood culture bottles without the need for an involved DNA extraction process (22). Currently, we are investigating cost-effective methods for the integration of bDNA technology in the clinical laboratory.
ACKNOWLEDGMENTS
We thank the members of the Mayo Clinic bacteriology laboratory for isolate collection and technical support. We also thank F. R. Cockerill III for helpful suggestions.
This work was funded by a research grant from the Chiron Corporation.
REFERENCES
- 1.Annear D I. The effect of temperature on resistance of Staphylococcus aureus to methicillin and some other antibiotics. Med J Aust. 1968;1:444–446. [PubMed] [Google Scholar]
- 2.Berger-Bächi B, Strässle A, Gustafson J E, Kayser F H. Mapping and characterization of multiple chromosomal factors involved in methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:1367–1373. doi: 10.1128/aac.36.7.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carroll K C, Leonard R B, Newcomb-Gayman P L, Hillyard D R. Rapid detection of the staphylococcal mecA gene from BACTEC blood culture bottles by the polymerase chain reaction. Clin Microbiol Infect Dis. 1996;106:600–605. doi: 10.1093/ajcp/106.5.600. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Recommendations for preventing the spread of vancomycin resistance: recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC) Morbid Mortal Weekly Rep. 1995;44(No. RR-12):3–4. [PubMed] [Google Scholar]
- 5.de Lencastre H, de Jonge B L M, Mathews P R, Tomasz A. Molecular aspects of methicillin resistance in Staphylococcus aureus. J Antimicrob Chemother. 1994;33:7–24. doi: 10.1093/jac/33.1.7. [DOI] [PubMed] [Google Scholar]
- 6.Detmer J, Lagier R, Flynn J, Zayati C, Kolbert J, Collins M, Urdea M, Sanchez-Pescador R. Accurate quantification of hepatitis C virus (HCV) RNA from all HCV genotypes by using branched-DNA technology. J Clin Microbiol. 1996;34:901–907. doi: 10.1128/jcm.34.4.901-907.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geha D J, Uhl J R, Gustaferro C A, Persing D H. Multiplex PCR for identification of methicillin-resistant staphylococci in the clinical laboratory. J Clin Microbiol. 1994;32:1768–1772. doi: 10.1128/jcm.32.7.1768-1772.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerberding J L, Miick C, Liu H H, Chambers H F. Comparison of conventional susceptibility tests with direct detection of penicillin-binding protein 2a in borderline oxacillin-resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:2574–2579. doi: 10.1128/aac.35.12.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hackbarth C J, Miick C, Chambers H F. Altered production of penicillin-binding protein 2a can affect phenotypic expression of methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:2568–2571. doi: 10.1128/aac.38.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang M B, Gay T E, Baker C N, Banerjee S N, Tenover F C. Two percent sodium chloride is required for susceptibility testing of staphylococci with oxacillin when using agar-based dilution methods. Antimicrob Agents Chemother. 1993;31:2683–2688. doi: 10.1128/jcm.31.10.2683-2688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolbert C P, Connolly J E, Lee M J, Persing D H. Detection of the staphylococcal mecA gene by chemiluminescent DNA hybridization. J Clin Microbiol. 1995;33:2179–2182. doi: 10.1128/jcm.33.8.2179-2182.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDougal L K, Thornsberry C. The role of β-lactamase in staphylococcal resistance to penicillinase-resistant penicillins and cephalosporins. J Clin Microbiol. 1986;23:832–839. doi: 10.1128/jcm.23.5.832-839.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulder J G. Comparison of disk diffusion, the E test, and detection of mecA for determination of methicillin resistance in coagulase-negative staphylococci. Eur J Clin Microbiol. 1996;15:567–573. doi: 10.1007/BF01709365. [DOI] [PubMed] [Google Scholar]
- 14.Murakami K, Minamide W, Wada K, Nakamura E, Teraoka J, Watanabe S. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol. 1991;29:2240–2244. doi: 10.1128/jcm.29.10.2240-2244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramotar K, Bobroska M, Jessamine P, Toye B. Detection of methicillin resistance in coagulase-negative staphylococci initially reported as methicillin susceptible using automated methods. Diagn Microbiol Infect Dis. 1997;30:267–273. doi: 10.1016/s0732-8893(97)00248-4. [DOI] [PubMed] [Google Scholar]
- 16.Sabath L D, Wallace S J. Factors influencing methicillin resistance in staphylococci. Ann N Y Acad Sci. 1971;182:258–266. doi: 10.1111/j.1749-6632.1971.tb30662.x. [DOI] [PubMed] [Google Scholar]
- 17.Salisbury S M, Sabatini L M, Spiegel C A. Identification of methicillin-resistant staphylococci by multiplex polymerase chain reaction assay. Am J Clin Pathol. 1995;107:368–373. doi: 10.1093/ajcp/107.3.368. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 1.38–1.41. [Google Scholar]
- 19.Sanchez-Pescador R, Stempien M S, Urdea M S. Rapid chemiluminescent nucleic acid assays for detection of TEM-1 β-lactamase-mediated penicillin resistance in Neisseria gonorrhoeae and other bacteria. J Clin Microbiol. 1988;26:1934–1938. doi: 10.1128/jcm.26.10.1934-1938.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomasz A, Drugeon H B, de Lencastre H M, Jabes D, McDougall L, Bille J. New mechanism for methicillin resistance in Staphylococcus aureus: clinical isolates that lack the PBP 2a gene and contain normal penicillin-binding proteins with modified penicillin-binding capacity. Antimicrob Agents Chemother. 1989;33:1869–1874. doi: 10.1128/aac.33.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.York M K, Gibbs L, Chehab F, Brooks G F. Comparison of PCR detection of mecA with standard susceptibility testing methods to determine methicillin resistance in coagulase-negative staphylococci. J Clin Microbiol. 1996;34:249–253. doi: 10.1128/jcm.34.2.249-253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng, X., C. Kolbert, J. Arruda, P. Varga-Delmore, M. Lewis, J. Kolberg, and D. H. Persing. Unpublished data.