Abstract
Introduction
Anticholinergic medications block the neurotransmitter acetylcholine in the brain and peripheral nervous system. Many medications have anticholinergic properties, and the cumulative effect of these medications is termed anticholinergic burden. Increased anticholinergic burden can have short-term side effects such as dry mouth, blurred vision and urinary retention as well as long-term effects including dementia, worsening physical function and falls.
Methods
We carried out a systematic review (SR) with meta-analysis (MA) looking at randomised controlled trials addressing interventions to reduce anticholinergic burden in older adults.
Results
We identified seven papers suitable for inclusion in our SR and MA. Interventions included multi-disciplinary involvement in medication reviews and deprescribing of AC medications. Pooled data revealed no significant difference in outcomes between control and intervention group for falls (OR = 0.76, 95% CI: 0.52–1.11, n = 647), cognition (mean difference = 1.54, 95% CI: −0.04 to 3.13, n = 405), anticholinergic burden (mean difference = 0.04, 95% CI: −0.11 to 0.18, n = 710) or quality of life (mean difference = 0.04, 95% CI: −0.04 to 0.12, n = 461).
Discussion
Overall, there was no significant difference with interventions to reduce anticholinergic burden. As we did not see a significant change in anticholinergic burden scores following interventions, it is likely other outcomes would not change. Short follow-up time and lack of training and support surrounding successful deprescribing may have contributed.
Keywords: older adult, falls, cognition, meta-analysis, anticholinergic medication, systematic review, older people
Key Points
This systematic review and meta-analysis synthesised interventions targeting anticholinergic burden in older people.
Results indicate no clear impact on key outcomes of anticholinergic burden score, cognition, falls or quality of life.
Short follow-up time and lack of training and support surrounding successful deprescribing may have contributed.
Future interventions should focus on key intervention components required to reduce anticholinergic burden.
Future interventions should only proceed to definitive evaluation once impact on anticholinergic burden can be demonstrated.
Introduction
Anticholinergic (AC) medications block the neurotransmitter acetylcholine in the brain and peripheral nervous system, reducing involuntary muscle contractions in areas of the body including the gastrointestinal tract, bladder and lungs. As such, they are commonly used to manage wide-ranging symptoms including irritable bowel, overactive bladder, pain and respiratory problems. However, their effects can lead to constipation, dry mouth, blurred vision, urinary retention, and impact memory and thinking. In addition, many medications used for other clinical indications such as pain, allergy and mental health conditions have AC properties that may go unrecognised by prescribers. These adverse effects are particularly troublesome for older people, especially those with existing conditions such as frailty or dementia.
Available prevalence estimates indicate that around 20% of older people are prescribed AC medications [1]. Growing evidence indicates that AC medications are associated with cognitive and physical decline in older age [2, 3]. The cumulative adverse effect of multiple AC drugs and medications with AC properties is referred to as ‘anticholinergic burden’ and is associated with potential harm. Anticholinergic burden is associated with increased risk of delirium (acute confusion) and falls, both of which are common reasons for hospital admission. Longer-term adverse effects include dementia, loss of physical function and loss of independence [3]. These outcomes are especially problematic for older people, their families and carers, and are associated with considerable cost to the health and social care systems worldwide.
There are multiple AC burden scores available such as Anticholinergic Cognitive Burden, Anticholinergic Drug Scale (ADS), Drug Burden Index (DBI) or the Anticholinergic Risk Scale. These scores identify people at risk of adverse effects from AC burden and those who may benefit from a targeted medication review, but there is no clear guidance on which one to use in routine clinical practice. Several interventions have been developed that aim to reduce anticholinergic burden for patients with the intention of preventing adverse outcomes. Interventions are typically based around initial calculation of AC burden then using this to trigger health care professionals to review medications with the aim of supervised withdrawal of any inappropriate medications (deprescribing) [4].
A 2018 systematic review of interventions to reduce anticholinergic burden in older adults reported evidence for a reduction in AC burden through targeted interventions [5]. However, the review included studies only undertaken since 2010, risking exclusion of earlier trials. Reported evidence for reduced AC burden was mainly from non-randomised studies, with attendant risk of bias, and limited evidence was reported on clinically relevant outcomes. Furthermore, no meta-analysis was performed in the review, impacting on the ability to make robust evidence statements.
Objective
The aim of this review is to synthesise the international evidence on randomised trials of interventions for reducing anticholinergic burden and related adverse outcomes in older people aged ≥ 65 years.
Methods
The review methodology followed Cochrane guidance and is reported using Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations [6] (Appendix 1). The protocol was prospectively registered with Prospero (http://www.crd.york.ac.uk/PROSPERO/; reference CRD42021279187).
Eligibility criteria
Randomised controlled trials (RCTs) and cluster RCTs of deprescribing interventions including a focus on AC burden reduction involving older adults (mean age ≥ 65), with participant use of AC medications, in all healthcare settings, were eligible for inclusion. Non-randomised intervention trials and observational studies were excluded.
Search strategy and information sources
An inclusive MEDLINE search strategy was developed with an experienced research librarian at the University of Leeds, and adapted for CINAHL, EMBASE, EMBASE Classic, Cochrane Database of Systematic Reviews, Cochrane CENTRAL (Trials) and PsycINFO. All databases were searched for English-language publications between 1946 and 22 April 2022. The search strategy for MEDLINE (Ovid) is available (Appendix 2).
Data collection process
Two independent reviewers (EB, and AA or RH) assessed titles and abstracts for potentially eligible studies and reviewed full-text papers against the eligibility criteria, with any disagreements settled by consensus discussion.
Two authors independently extracted the data from included trials using a piloted data extraction form and any disagreements were settled by consensus. Extracted data included trial setting, description of intervention, patient baseline characteristics (age, sex, comorbidities including dementia, AC burden scores and care home residence), outcomes of interest and study drop-out rates.
Data items
The primary outcomes included new diagnosis of dementia, delirium episodes and falls. Planned secondary outcomes included worsening of existing dementia; cognition; activities of daily living—basic or instrumental; quality of life; mortality; AC burden score; side effects including dry mouth, constipation and urinary retention; hospital admission; and cost-effectiveness. All outcomes were collected at each reported timepoint.
Study risk-of-bias assessment
Two independent reviewers (EB, and AA or RH) assessed risk of bias for each study using Cochrane criteria as described in the Cochrane Handbook for Systematic Reviews of Interventions [7]. We assessed included trials for adequacy of sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other sources of bias. For each domain, a judgement of low risk, high risk or unclear risk of bias was reached. Disagreements were resolved by consensus and risk of bias summary figures generated using Review Manager (RevMan) software [8].
Synthesis methods
Where required, we converted outcome data to an appropriate format for meta-analysis using established methods [7] and contacted study authors for additional information if this was not possible.
We synthesised data for meta-analysis, calculating pooled odds ratios with 95% confidence intervals for dichotomous outcomes to create summary forest plots using Mantel–Haenszel random-effects methods [8]. We calculated pooled mean differences with 95% confidence intervals for continuous outcomes using generic inverse variance random-effects modelling. Where we identified unit of analysis issues, we calculated standardised mean differences with 95% confidence intervals. In cases where the standard deviation for a study’s point estimate was missing, where this represented a small proportion of the pooled estimates, these were imputed [9]. If available data or clear trial heterogeneity precluded meta-analysis, a narrative evidence synthesis was provided. We assessed for the proportion of total variability due to between-study heterogeneity using the I2 statistic with values approaching 25%, 50% and 75% representing low, moderate and high levels, respectively. Since fewer than 10 studies were identified to provide data for each outcome, assessment for publication bias with funnel plots was not appropriate.
Results
Study selection
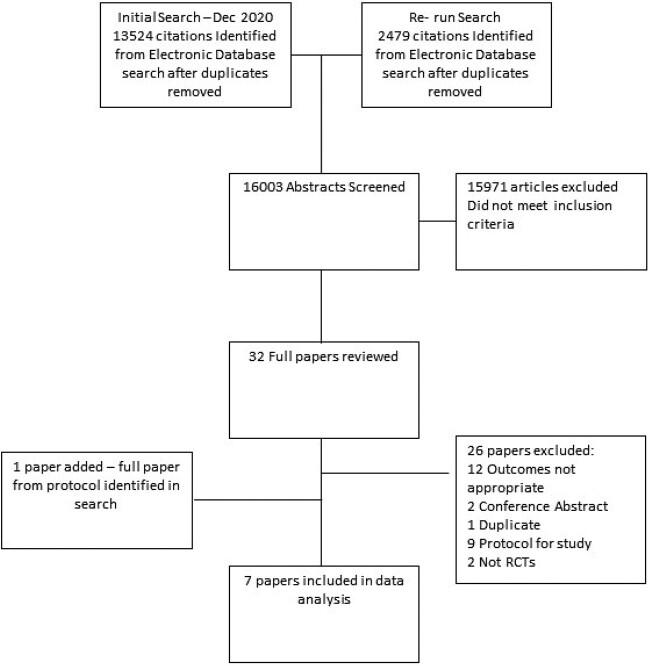
Details of the study selection are presented in Figure 1. Following detailed assessment, seven studies were eligible for inclusion in the review [10–16].
Figure 1.
Flowchart of included studies.
Study characteristics
Of the seven studies, four were RCTs [11–14] and three were cluster RCTs [10, 15, 16], with a total of 1,774 participants (study population range 49–781 participants) and mean follow-up period of 38 weeks (range 8 weeks to 4 years). Two studies were conducted in the Netherlands [14, 15], two in Australia [10, 16], one in Finland [12], one in Norway [11] and one in the United Kingdom [13]. The mean age was 80.8 years (mean age range 76.2–85.5 years) and 70% (range 61–79%) were female. Where reported (in four studies) [11–13, 15], diagnosed dementia rates ranged from 10 to 70%. Trial drop-out rates ranged from 2 to 22%.
The included studies were all community based, with two of the studies conducted in nursing homes [11, 15]. A detailed description of the interventions is provided (Table 1).
Table 1.
Characteristics of intervention
| Study | Intervention | Description and method | Provider | Setting | Frequency |
|---|---|---|---|---|---|
| Gnjidic [10] | DBI + prompt to GPs | Step 1: Interview by researcher with medication review | Researcher and phone calls to GP from pharmacologist or geriatrician | Self-care retirement villages in Australia | Once |
| Step 2: DBI calculated | |||||
| Step 3: Letter and phone call to GP to prompt medication changes. Phone call by pharmacologist or geriatrician | |||||
| Kersten [11] | Pharmacist drug review based on ADS | Step 1: MDT drug review guided by the ADS score model | Pharmacists | Nursing homes Norway | Once |
| Step 2: Advice to nursing home physician to reduce/stop or change drug | |||||
| Lampela [12] | CGA including medication review | CGA including medication review by physicians (trainees with 10+ years’ experience of GP and older adults). No formal use of an anticholinergic score. Discussed weekly with senior geriatrician | Physician (medication review), dentist, physiotherapist, nutritionist | Finland—population based | Annually 2004–2007 |
| Moga [13] | Targeted medication therapy management intervention | Medication therapy management programme | Pharmacists and physicians | UK. Alzheimer’s coordinating centre cohort | Once |
| Step 1: Label anticholinergics as potentially inappropriate | |||||
| Step 2: Evaluation of each drug risk/benefit | |||||
| Step 3: Recommendations to reduce, stop/change drugs | |||||
| Step 4: Discussion with participant at week 1 visit | |||||
| Kouladjian O’Donnell [16] | Goal-directed medication review electronic decision support system | Clinical decision support system that produces patient-specific deprescribing reports to the GP, including: | ACP undertake reports that are sent to GPs | Patients referred to pharmacist home medication reviews | Once |
| 1. Identify patient’s goals of care with medications | |||||
| 2. Calculate the DBI | |||||
| 3. Explore patient’s attitudes towards deprescribing | |||||
| 2-hour self-directed training programme for accredited clinical pharmacists (ACP) but not GP | |||||
| van der Meer [14] | MDT medication review | Step 1: Patient and pharmacist to discuss medication use | Pharmacist and GPs | Community pharmacists with GP—Netherlands | Once |
| Step 2: Pharmacist review and recommendations | |||||
| Step 3: MDT with GP and pharmacist to make action plan | |||||
| Step 4: Discussion with patient | |||||
| Step 5: Follow-up | |||||
| Wouters [15] | 3MR Multidisciplinary Multistep Medication Review | Step 1: Assessing patient perspective and medical information | Geriatricians and pharmacists | Nursing homes Netherlands | Once |
| Step 2: Review drugs using STOPP/START criteria | |||||
| Step 3: MDT meeting and pharmacotherapeutic actions | |||||
| Step 4: Execution and evaluation of actions | |||||
| Total process approximately 45 min. Brief training given |
Characteristics include intervention description and method, provider, setting and frequency.
ADS = Anticholinergic Drug Scale; CGA = comprehensive geriatric assessment; DBI = Drug Burden Index; GP = general practitioner; MDT = multi-disciplinary team; MR = medication review; STOPP = Screening Tool of Older Person’s Prescriptions; START = Screening Tool to Alert doctors to Right i.e. appropriate, indicated Treatments.
For the primary outcomes of this review, falls were reported in three studies [14–16], but data were not available for new diagnosis of dementia or delirium episodes. For secondary outcomes, anticholinergic burden was measured in all studies, cognition in five studies [11, 12, 14–16], quality of life in three studies [13–15], hospital admissions in one study [14], side effects relating to dry mouth in one study [11] and cost-effectiveness in one study [15]; data were not available for other pre-specified secondary outcomes.
Primary outcomes
Falls
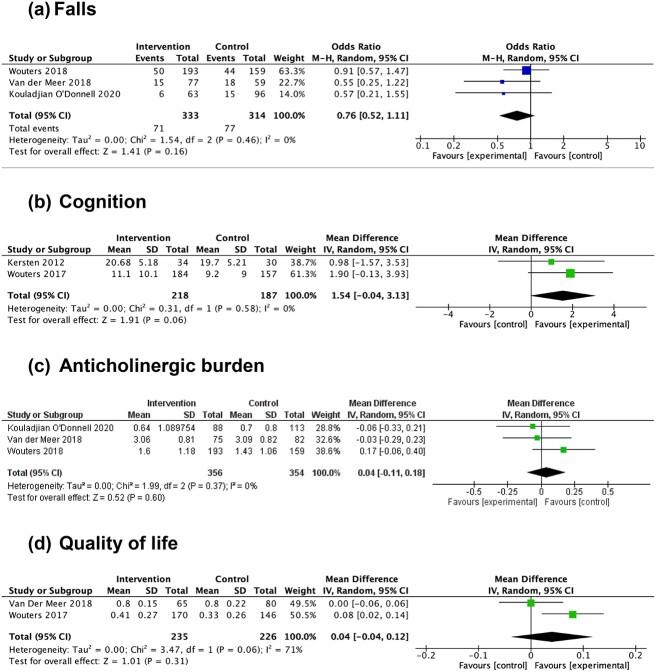
Pooled data from three trials [14–16] indicated no clear difference in odds of falling between intervention and control groups (OR 0.76, 95% CI 0.52 to 1.11, n = 647) (Figure 2a).
Figure 2.
Forest plots demonstrating at follow-up, in the intervention and the control groups: (a) pooled risk estimates of fall frequency; (b) pooled mean differences in Mini-Mental State Examination (MMSE) scores; (c) pooled mean differences in Drug Burden Index (DBI) scores; (d) pooled mean differences in EuroQol five-dimension health questionnaire (EQ-5D) scores. In (a), odds ratios less than 1 favour the intervention group; odds ratios more than 1 favour the control group. In (b, c, d), mean difference less than 0 favours the control group, mean difference more than 0 favours the intervention group. CI = confidence interval; I2 = I2 statistic; IV = inverse variance; M-H = Mantel–Haenszel method; P = P value; Random = random effects; Z = Z value.
Secondary outcomes
Cognition
Pooled data from two trials [11, 15] indicated uncertainty in the effect of interventions on cognition, based on Mini-Mental State Examination (MMSE) score (mean difference 1.54, 95% CI −0.04 to 3.13, n = 405) (Figure 2b).
One further study [12] also reported cognitive impairment using MMSE; however, the follow-up period was 4 years and therefore was not sufficiently similar to pool in the meta-analysis. There was no significant difference in MMSE at follow-up between the intervention and control groups (MMSE 23.7 and 23.7, respectively) at this timepoint.
One study [16] reported change in cognition using Mini Cog score at follow-up. There was no significant difference at follow-up between the intervention and control groups (OR −0.01, 95% CI −0.32 to 0.30).
One study [14] reported that the intervention was associated with no significant difference in individual cognitive domains (measured using the 7-minute screen (7MS)) of category fluency (unstandardised beta −0.18, 95% CI −1.55 to 1.20, n = 145), enhanced cued recall (OR 0.54, 95% CI 0.15 to 1.9), temporal orientation (OR 1.38, 95% CI 0.28 to 6.88) or clock drawing (OR 0.67, 95% CI 0.28 to 1.62) compared to the control group.
Anticholinergic burden
Synthesis of data from two trials [14, 15] identified no clear difference in post-intervention anticholinergic burden, measured using the DBI to measure exposure to anticholinergic and sedative medications (mean difference 0.04, 95% CI −0.11 to 0.18, n = 710) (Figure 2c).
One trial [10] reported that the intervention was associated with an overall increase in anticholinergic burden compared to the control group.
One trial [11] reported that the intervention was associated with a reduction in anticholinergic burden of 2 units, measured using the ADS, compared to the control group. One further trial demonstrated the intervention was associated with reduction in anticholinergic burden (intervention: mean change score 1 (standard error of mean (SEM) 0.3); control: mean change score 0.2 (SEM 0.3)).
One trial [12] demonstrated that the intervention was associated with no difference in anticholinergic burden compared to the control group using multiple different anticholinergic burden scales (Anticholinergic Drug Scale, Chew Score, Anticholinergic Cognitive Burden Scale and Rudolph’s Anticholinergic Risk Scale).
Quality of life
Synthesis of data from two trials [14, 15] indicates no clear difference in quality of life for intervention participants, measured using the EuroQol five-dimension health questionnaire (EQ-5D), compared to the control group (mean difference 0.04, 95% CI −0.04 to 0.12, n = 461) (Figure 2d).
One trial [13] reported that the intervention was associated with no difference in quality of life, measured using the Rand Short-Form 36-Item Health Survey (RAND SF36), compared to the control group: physical component summary scores: for intervention, mean change score −1.2 (±13.2); for control, mean change score 1.5 (±16.5); mental component summary scores: for intervention, mean change score 2.1 (±12.9); for control, mean change score −4.7 (±14), n = 49.
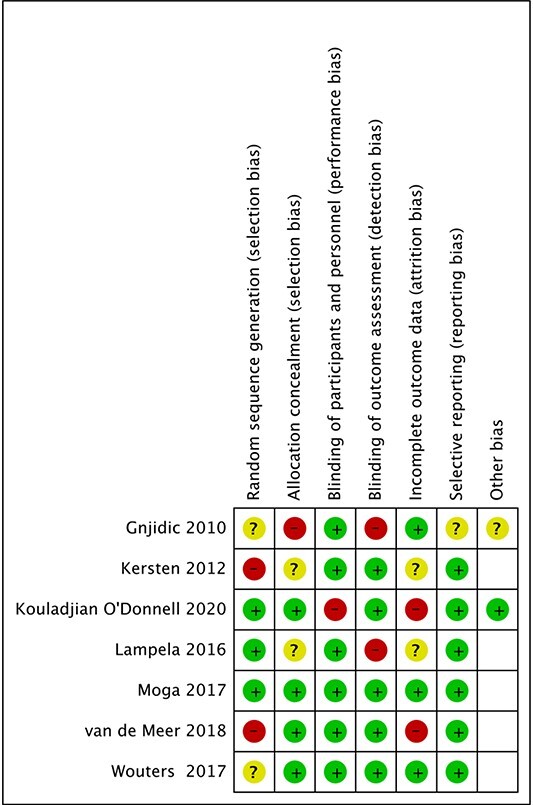
Risk of bias
We carried out a comprehensive review of risk of bias for each of the studies included using the Cochrane tool for assessing risk of bias, as summarised in Table 2. Most included studies scored low to moderate risk of bias in most domains. One study [13] scored as low risk in all domains. In the other studies, the most common domains in which they scored at high risk of bias were selection bias and detection bias. No study was assessed as at overall high risk of bias.
Table 2.
Summary of risk of bias assessment
|
|
Risk of bias assessment using the Cochrane tool [7].
Discussion
Our review has identified no clear evidence that interventions targeting AC burden in older people reduce overall AC burden score, improve cognition, impact on quality of life-related outcomes or falls. Generated evidence was from a relatively small number of trials, but included trials were generally assessed as being of low to moderate risk of methodological bias.
Key amongst these findings is that there did not appear to be a clear intervention effect on anticholinergic burden scores measured post-intervention. This is of importance as it is unlikely that downstream effects on measures of cognition or other outcomes can be generated, or attributed to the intervention, in the absence of a reduction in anticholinergic burden scores.
The challenges associated with deprescribing medicines in older people are well recognised. These include a lack of continuity in health care, time constraints during consultations, fear of the consequences of deprescribing [17, 18] and specifically in relation to anticholinergic burden, the complexity of deprescribing interventions across multiple drug classes. Alongside this, hesitancy amongst health care practitioners to deprescribe if medications were prescribed by another practitioner has been recognised and patients and relatives may also be reluctant to stop medications prescribed following a set guideline [19]. A study reporting successful deprescribing of antihypertensive medications in older adults used a clear deprescribing algorithm alongside a safety monitoring algorithm to support clinicians in the process [20]. This suggests that clearer guidance for prescribers may support deprescribing such as use of the STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right i.e. appropriate, indicated Treatments) criteria [21]. In addition, shared decision-making, person-centred care and increased communication may facilitate successful deprescribing [17].
In addition, there is evidence that certain medications with AC effects are more straightforward to deprescribe than others, with the highest success in deprescribing antihistamines and lower success deprescribing antipsychotic and antidepressant medications [22]. All studies used only single time point interventions at the beginning of the trial, which is also likely to have affected successful deprescribing, as previously demonstrated [23, 24]. Continued clinician–patient engagement would also improve effective deprescribing and subsequent reduction in AC burden scores [25]. Future work to develop and evaluate interventions to reduce anticholinergic burden in older people should aim to address these factors as key steps in intervention development.
Only one study [15] reported that intervention training was provided to staff delivering the intervention. Lack of training of those performing interventions in the remaining studies may have influenced the final outcomes. Future intervention development and evaluation should pay close attention to development of training packages that support delivery, incorporating the necessary behaviour change approaches to achieve successful deprescribing. Initial intervention feasibility testing should include a focus on fidelity of training, intervention delivery, receipt and enactment and ideally only proceed to definitive evaluation once an overall reduction in AC score can be demonstrated as it is unlikely that effects attributable to the intervention could otherwise be generated.
Limitations of the review
Although we were able to synthesise evidence for meta-analysis of outcomes, this was only possible using data from a small number of trials, resulting in considerable uncertainty in overall estimates. Consensus agreement on common data elements and reporting methods for deprescribing trials in older people and establishment of international repositories for trial data to support individual participant data meta-analysis would support generation of robust findings from future trial-based work. Use of published Core Outcome Sets may support future research [26, 27].
Five of the trials [10, 11, 13–15] had a relatively short follow-up time, so changes in outcomes such as cognition may not necessarily be expected. The selection of outcome measures for trials of AC medications and appropriate time horizon to record impact on key outcomes should be considered in future work, acknowledging the constraints of trial-based evaluations in terms of resource required for longer-term follow-up. More widespread use of routinely available data to support evaluations may help, allowing a focus on related outcomes such as hospitalisation with falls, delirium, dementia, and other health and care-related outcomes. Other limitations of the general deprescribing trial literature are also applicable to the RCTs included in the review, such as the need to consider barriers and enablers at the level of individual, practitioner and system; the need to integrate implementation into clinical decision support systems and the need to measure patient centred outcomes [28].
Conclusion
Evidence from a small number of RCTs of interventions targeting AC burden has identified no clear impact on key outcomes of AC burden score, cognition, falls or quality of life. Future interventions should focus on key intervention components required to reduce AC burden, addressing known barriers to achieving successful deprescribing. Interventions should ideally only proceed to definitive evaluation once impact on AC burden score can be demonstrated as otherwise it is implausible that intervention effect on clinical outcomes such as cognition can be generated or attributed to the intervention. Particular attention should be given to implementation and assessment of overall intervention fidelity as part of intervention optimisation. Future trials should clearly justify outcome selection and ensure that sufficient time is allocated to generate impact on outcomes such as cognition that may require a relatively long-time horizon to achieve.
Supplementary Material
Acknowledgements
We are grateful to Deirdre Andre (University of Leeds) for help with library searching. We would like to thank the authors who provided additional information and data: Sarah Hilmer and Lisa Kouladjian O’Donnell, Pasi Lampela and Helen G. van der Meer.
Contributor Information
Eve Braithwaite, Academic Unit for Ageing and Stroke Research, University of Leeds, Leeds, UK.
Oliver M Todd, Academic Unit for Ageing and Stroke Research, University of Leeds, Leeds, UK.
Abigail Atkin, Calderdale and Huddersfield NHS Foundation Trust, Huddersfield, UK.
Rachel Hulatt, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK.
Ragy Tadrous, Academic Unit for Ageing and Stroke Research, University of Leeds, Leeds, UK.
David P Alldred, School of Healthcare, University of Leeds, Leeds, UK; NIHR Yorkshire & Humber Patient Safety Translational Research Centre, Bradford, UK.
Munir Pirmohamed, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool, UK.
Lauren Walker, Department of Clinical Pharmacology, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool L69 7DE, UK.
Rebecca Lawton, Institute of Psychological Sciences, University of Leeds, Leeds, UK; Department of Quality and Safety Research, Bradford Institute for Health Research, Bradford, UK.
Andrew Clegg, Academic Unit for Ageing and Stroke Research, University of Leeds, Leeds, UK.
Declaration of Conflicts of Interest
A.C. has led the development and validation of the Anticholinergic Medication Index (ACMI) in Health Data Research UK (HDR UK) funding. The ACMI will be supplied to UK providers of electronic health record systems and related software at no cost, on the basis that a premium charge is not applied to the end user. M.P. has received partnership funding for the following: MRC Clinical Pharmacology Training Scheme (co-funded by MRC and Roche, UCB, Eli Lilly and Novartis); and a PhD studentship jointly funded by EPSRC and Astra Zeneca. He has developed an HLA genotyping panel with MC Diagnostics, but does not benefit financially from this. He is part of the IMI Consortium ARDAT (www.ardat.org). None of this funding is related to this paper.
Declaration of Sources of Funding
This research was funded by the National Institute for Health and Care Research (NIHR) Yorkshire and Humber Patient Safety Translational Research Centre (NIHR Yorkshire and Humber PSTRC). A.C. is part-funded by the NIHR Applied Research Collaboration Yorkshire & Humber, NIHR Leeds Biomedical Research Centre and Health Data Research UK, an initiative funded by UK Research and Innovation Councils, NIHR and the UK devolved administrations and leading medical research charities. The views expressed in this article are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. E.B.’s research time was funded as part of a Flexible portfolio training funded by Royal College of Physicians UK and Health Education England. O.M.T. was funded by a National Institute for Health and Care Research (NIHR) academic clinical lectureship.
Data Availability
The data used for the current study are available from the corresponding author on request.
References
- 1. Gnjidic D, Cumming RG, le Couteur DGet al. Drug burden index and physical function in older Australian men. Br J Clin Pharmacol 2009; 68: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fox C, Smith T, Maidment Iet al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: a systematic review. Age Ageing 2014; 43: 604–15. [DOI] [PubMed] [Google Scholar]
- 3. Landi F, Russo A, Liperoti Ret al. Anticholinergic drugs and physical function among frail elderly population. Clin Pharmacol Ther 2007; 81: 235–41. [DOI] [PubMed] [Google Scholar]
- 4. Reeve E, Gnjidic D, Long J, Hilmer S. A systematic review of the emerging definition of ‘deprescribing’ with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol 2015; 80: 1254–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakham A, Myint PK, Bond CM, Newlands R, Loke YK, Cruickshank M. Interventions to reduce anticholinergic burden in adults aged 65 and older: a systematic review. J Am Med Dir Assoc 2020; 21: 172–180.e5. [DOI] [PubMed] [Google Scholar]
- 6. Page MJ, Moher D, Bossuyt PMet al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372: n160. 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Higgins JPT, Thomas J. In: Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions, 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
- 8. Review Manager (RevMan) [Computer program] Version 5.4. The Cochrane Collaboration, 2020. [Google Scholar]
- 9. Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 2006; 59: 7–10. [DOI] [PubMed] [Google Scholar]
- 10. Gnjidic D, Couteur DGL, Abernethy DR, Hilmer SN. A pilot randomized clinical trial utilizing the drug burden index to reduce exposure to anticholinergic and sedative medications in older people. Ann Pharmacother 2010; 44: 1725–32. [DOI] [PubMed] [Google Scholar]
- 11. Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2013; 68: 271–8. [DOI] [PubMed] [Google Scholar]
- 12. Lampela P, Taipale H, Lavikainen P, Hartikainen S. The effect of comprehensive geriatric assessment on anticholinergic exposure assessed by four ranked anticholinergic lists. Arch Gerontol Geriatr 2017; 68: 195–201. [DOI] [PubMed] [Google Scholar]
- 13. Moga DC, Abner EL, Rigsby DNet al. Optimizing medication appropriateness in older adults: a randomized clinical interventional trial to decrease anticholinergic burden. Alzheimers Res Ther 2017; 9: 36. 10.1186/s13195-017-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meer HG, Wouters H, Pont LG, Taxis K. Reducing the anticholinergic and sedative load in older patients on polypharmacy by pharmacist-led medication review: a randomised controlled trial. BMJ Open 2018; 8: e019042. 10.1136/bmjopen-2017-019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wouters H, Scheper J, Koning Het al. Discontinuing inappropriate medication use in nursing home residents: a cluster randomized controlled trial. Ann Intern Med 2017; 167: 609–17. [DOI] [PubMed] [Google Scholar]
- 16. Kouladjian O'Donnell L, Gnjidic D, Sawan Met al. Impact of the goal-directed medication review electronic decision support system on drug burden index: a cluster-randomised clinical trial in primary care. Br J Clin Pharmacol 2021; 87: 1499–511. [DOI] [PubMed] [Google Scholar]
- 17. Doherty AJ, Boland P, Reed Jet al. Barriers and facilitators to deprescribing in primary care: a systematic review. BJGP Open 2020; 4: bjgpopen20X101096. 10.3399/bjgpopen20X101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peat G, Fylan B, Marques Iet al. Barriers and facilitators of successful deprescribing as described by older patients living with frailty, their informal carers and clinicians: a qualitative interview study. BMJ Open 2022; 12: e054279. 10.1136/bmjopen-2021-054279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Djatche L, Lee S, Singer D, Hegarty SE, Lombardi M, Maio V. How confident are physicians in deprescribing for the elderly and what barriers prevent deprescribing? J Clin Pharm Ther 2018; 43: 550–5. [DOI] [PubMed] [Google Scholar]
- 20. Sheppard JP, Burt J, Lown Met al. Effect of antihypertensive medication reduction vs usual care on short-term blood pressure control in patients with hypertension aged 80 years and older: the OPTIMISE randomized clinical trial. JAMA 2020; 323: 2039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gallagher P, Ryan C, Byrne S, Kennedy J, OMahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert Doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46: 72–83. [DOI] [PubMed] [Google Scholar]
- 22. Dharmarajan TS, Choi H, Hossain Net al. Deprescribing as a clinical improvement focus. J Am Med Dir Assoc 2020; 21: 355–60. [DOI] [PubMed] [Google Scholar]
- 23. Blum MR, Sallevelt BTGM, Spinewine Aet al. Optimizing therapy to prevent avoidable hospital admissions in multimorbid older adults (OPERAM): cluster randomised controlled trial. BMJ 2021; 374: n1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O'Mahony D, Gudmundsson A, Soiza RLet al. Prevention of adverse drug reactions in hospitalized older patients with multi-morbidity and polypharmacy: the SENATOR* randomized controlled clinical trial. Age Ageing 2020; 49: 605–14. [DOI] [PubMed] [Google Scholar]
- 25. Ravn-Nielsen LV, Duckert ML, Lund MLet al. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission: a randomized clinical trial. JAMA Intern Med 2018; 178: 375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rankin A, Cadogan CA, ın Ryan C, Clyne B, Smith SM, Hughes CM. Core outcome set for trials aimed at improving the appropriateness of polypharmacy in older people in primary care. J Am Geriatr Soc 2018; 66: 1206–12. [DOI] [PubMed] [Google Scholar]
- 27. Martin-Kerry J, Taylor J, Scott Set al. Developing a core outcome set for hospital deprescribing trials for older people under the care of a geriatrician. Age Ageing 2022; 51: afac241. 10.1093/ageing/afac241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scott IA, Reeve E, Hilmer SN. Establishing the worth of deprescribing inappropriate medications: are we there yet? Med J Aust 2022; 217: 283–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used for the current study are available from the corresponding author on request.