Abstract
Historically, disease manifestations in dogs seroreactive to Ehrlichia canis antigens by indirect immunofluorescent antibody testing have been attributed to infection with either E. canis or Ehrlichia ewingii. A 1996 study by Dawson and colleagues provided PCR evidence that healthy dogs from southeastern Virginia could be naturally infected with Ehrlichia chaffeensis. This observation stimulated us to determine which Ehrlichia spp. infected sick dogs that were referred to our hospital from the same region. Based upon PCR amplification with species-specific primers, sick dogs seroreactive to E. canis antigens were determined to be infected with four Ehrlichia species: E. canis, E. chaffeensis, E. equi, and E. ewingii. Coinfection with three Ehrlichia species (E. canis, E. ewingii, and E. equi) was documented for one dog. An additional canine pathogen presumed to be tick transmitted, Bartonella vinsonii subsp. berkhoffii, was identified in 7 of 12 dogs. Importantly, our results indicate that in naturally infected dogs, E. chaffeensis can cause severe disease manifestations that are clinically and serologically indistinguishable from disease manifestations of E. canis or E. ewingii. In addition, our findings support the efficacy of doxycycline for treatment of E. canis, E. equi, and E. ewingii infections but indicate that, based upon the persistence of E. chaffeensis DNA for 1 year following treatment, E. chaffeensis infection in dogs may be more refractory to doxycycline treatment. Undetected coinfection with Bartonella may also complicate the evaluation of treatment efficacy while resulting in disease manifestations that mimic ehrlichiosis.
Current modalities that detect Ehrlichia canis antibodies in serum samples obtained from dogs for diagnostic purposes, such as a microimmunofluorescence assay (IFA), do not facilitate differentiation of the infecting Ehrlichia species, particularly among organisms within the same genogroup. In addition to Ehrlichia muris and Cowdria ruminantium, the E. canis genogroup contains three species that are known to infect dogs: E. canis, Ehrlichia ewingii, and Ehrlichia chaffeensis (36). Although natural infection with C. ruminantium has not been reported, when dogs were experimentally infected, they developed no clinical abnormalities but remained PCR positive for periods up to 3 weeks (22). As the immunodominant antigens of E. canis and C. ruminantium contain cross-reacting epitopes, serologic differentiation of these two organisms in areas in which they coexist may not be possible (28).
The pathogenicity of E. canis, E. equi, and E. ewingii in dogs has been established through the study of both natural and experimental infections (12, 17, 24, 34, 35). E. chaffeensis, which has been isolated from patients, causes monocytic ehrlichiosis in people (7, 11, 29); however, the potential role of E. chaffeensis as a pathogen in dogs, or the role of dogs as a zoonotic reservoir for human infection, has not been clearly established. Although susceptible to infection with E. chaffeensis, experimentally infected dogs did not develop substantial clinical or hematologic abnormalities, despite seroconversion and reisolation of the organism (9). Recently, the detection of E. chaffeensis DNA by PCR amplification provided the first documentation for natural infection of dogs residing in animal shelters or in a kennel in southeastern Virginia (8). This study extends these observations by indicating that E. chaffeensis can cause severe disease manifestations in naturally infected dogs.
Current evidence indicates that one or more members of the Ehrlichia phagocytophila genogroup are responsible for causing infection in cats, dogs, horses, human beings, and small mammals in the United States as well as other regions of the world (15, 20, 26, 27, 32, 37). In 1996, Greig and colleagues (15) provided clinical, serologic, and molecular evidence that dogs were infected with a granulocytic Ehrlichia species in Minnesota and Wisconsin, the region from which the first cases of human granulocytic ehrlichiosis were identified. Subsequently, other regions where animal and human granulocytic ehrlichioses are endemic were identified. Dogs exposed to Ehrlichia equi in the northeastern United States have been shown to seroreact to E. canis antigens. In the Midwest, cross-reactivity between these two species seems less likely (15). Although E. equi is presumably an uncommon ehrlichial pathogen in the southeastern United States, its DNA was amplified from the blood of one dog in this study.
Previously, our laboratory isolated a novel Bartonella subspecies from a dog with endocarditis (5). Subsequently, the dog isolate was designated Bartonella vinsonii subsp. berkhoffii (American Type Culture Collection, type strain 51672) (23). A seroepidemiologic survey identified tick exposure as a risk factor for the presence of B. vinsonii antibodies in dog sera (30). Antibodies to B. vinsonii were found in 3.6% of serum samples from sick dogs presented to the Veterinary Teaching Hospital, and 36% of the serum samples that were known to be reactive to E. canis antigen were also reactive to B. vinsonii. Examination of sera from dogs experimentally infected with Rickettsia rickettsii or E. canis did not indicate cross-reactivity to B. vinsonii antigens. This study extends our previous observations by indicating that dogs infected with Ehrlichia species are frequently coinfected with B. vinsonii.
The purpose of this study was to determine which Ehrlichia species caused infection in naturally exposed dogs and to correlate this information with sequential evaluation of clinical, hematologic, serologic, tissue culture isolation, and PCR amplification findings. In addition, treatment outcomes were assessed on the basis of clinical response, normalization of platelet numbers, and tissue culture isolation and PCR amplification results. Because infection with B. vinsonii, a newly recognized canine pathogen, potentially influences the clinical course of canine ehrlichiosis, the dogs in our study were evaluated retrospectively for evidence of bartonella infection.
(Part of this research was presented as an abstract at the 13th Sesqui-annual Meeting of the American Society of Rickettsiology, Champion, Pa., 21 to 24 September 1997.)
MATERIALS AND METHODS
Dogs.
Twelve dogs, which were presented to the Veterinary Teaching Hospital, North Carolina State University (NCSU), for which seroreactivity to E. canis antigens was documented in conjunction with clinicopathologic abnormalities consistent with ehrlichiosis were chosen for follow-up examinations at three time points, approximately 2, 6, and 12 months after treatment. Diagnostic evaluations were performed and treatment regimens were defined by the attending clinician. Eleven of 12 dogs were treated with doxycycline hydrochloride at an approximate dosage of 5 mg/kg of body weight (range, 4.3 to 10.6 mg/kg; mean, 6.9 mg/kg) every 12 h for 14 to 28 days. Due to concurrent endocarditis, dog 9 was treated with a combination of amoxicillin and enrofloxacin. Medical records were reviewed retrospectively. Complete blood counts and serum biochemical profiles were available for all 12 dogs. Following aseptic preparation, blood obtained from the jugular vein was placed in clot tubes for serum for IFA testing and Western immunoblot analysis or in EDTA anticoagulant tubes for tissue culture isolation and DNA extraction.
Serology.
An IFA test was used to detect antibodies to E. canis (Florida), E. canis (NCSU strain DJ), E. canis (NCSU strain Jake), E. chaffeensis (Ark strain, human origin), Ehrlichia risticii, E. equi (96HE158, New York strain, human origin), and B. vinsonii subsp. berkhoffii (93-CO-1) on 30-well teflon-coated slides (16, 30). Serial twofold dilutions of sera from dogs were reacted with fluorescein isothiocyanate anti-canine immunoglobulin G conjugate (Cappel; Organon Teknika, West Chester, Pa.). Endpoint titers were determined as the last dilution at which brightly staining organisms could be detected on a fluorescence microscope with exciter and barrier filters.
Western immunoblotting.
E. canis (Florida) antigen grown in 030 cells (25) was purified by sucrose gradient centrifugation, and the protein concentration was determined (16). Dilutions made in final sample buffer at a protein concentration of 7.5 mg/ml were loaded at 20 μl per well and electrophoresed on sodium dodecyl sulfate–12% polyacrylamide precast minigels (Bio-Rad Laboratories, Rockville, Centre, N.Y.). Proteins were electrotransferred to nitrocellulose paper (0.45-μm pore size). After being blocked with 5% milk in phosphate-buffered saline, proteins were reacted with canine serum samples at a 1:100 dilution and then by peroxidase-conjugated goat anti-canine immunoglobulin G at 1:400 in 1% milk in phosphate-buffered saline. Bands were detected with the color reagent 4-chloro-1-napthol. Serum from a dog experimentally infected with E. canis (Florida) with a reciprocal titer of 10,240 was used as a positive control. Sera from uninfected laboratory-raised dogs were reacted with E. canis and normal cell antigens to detect the possibility of nonspecific binding, which was not observed.
Tissue culture isolation.
For each of the 12 dogs, 6 ml of EDTA-anticoagulated blood was collected aseptically from the jugular vein. Whole blood was spun at 1,500 × g for 5 min, the erythrocyte fraction was discarded, and the plasma was spun again for 20 min. The resulting monocyte-rich cell fractions were inoculated onto cell cultures of 030 cells (25) in 25-cm2 flasks and fed with RPMI 1640 (Gibco, Grand Island, N.Y.) containing 20% fetal bovine serum (Hyclone, Logan, Utah), l-glutamine, and sodium bicarbonate. Plates were incubated at 35°C with 5% CO2 for 8 weeks. Cellular samples of culture supernatants were tested for the presence of morulae every 2 weeks by Wright stain, by indirect immunofluorescence with monoclonal antibody obtained from D. H. Walker, Galveston, Tex., and by direct immunofluorescence with direct antiehrlichia conjugate obtained from J. E. Dawson, Centers for Disease Control and Prevention, Atlanta, Ga.
DNA extraction and nested PCR analysis.
With a commercially available QIAmp blood kit (Qiagen, Chatsworth, Calif.), DNA was extracted from 600 μl of stored EDTA–whole-blood samples that had been frozen at −70°C. Cell-culture-grown E. canis (Jake) and E. chaffeensis (obtained from J. E. Dawson, Centers for Disease Control and Prevention) were used as positive controls. A one-tube nested-PCR method was established with primers derived from the 16S rRNA gene sequences of E. canis, E. chaffeensis, E. equi, and E. ewingii (1, 8). For each dog and at each sampling point, PCR amplification was performed with broad-range Ehrlichia genus primers as well as E. canis, E. chaffeensis, E. ewingii, and E. equi species primers.
Ehrlichia-genus-specific amplification.
Amplification by PCR was performed with a 50-μl reaction mixture containing 1 μg of template DNA; 200 μM (each) dATP, dTTP, dCTP, and dGTP; 0.05 pmol (each) of the outer primers designated EHR-OUT1 and EHR-OUT2 (Table 1); 12.5 pmol (each) of the inner primers designated GE2f and EHRL3-IP2; 2 mM MgCl; and 2.5 U of Taq DNA polymerase (Promega, Madison, Wis.) in a 1× reaction buffer (50 mM KCl, 10 mM Tris HCl [pH 8.3]). The first round of amplification included denaturation at 94°C for 45 s and annealing and chain extension at 72°C for 1.5 min. The PCR cycle was repeated 20 times. The second round of amplification included denaturation at 94°C for 45 s, an annealing temperature of 50°C, and chain extension at 72°C for 1 min. This cycle was repeated 50 times and followed by a final extension of 5 min at 72°C.
TABLE 1.
Ehrlichia and Bartonella PCR primers used in this study
| Primera | Sequence (5′ to 3′) |
|---|---|
| Outside primers | |
| EHR-OUT1 | CTGGCGGCAAGCYTAACACATGCCAACATCTCACGAC |
| EHR-OUT2 | GCTCGTTGCGGGACTTAACCCAACATCTCACGAC |
| Ehrlichia genusspecific primers | |
| GE2F (9) | GTTAGTGGCATACGGGTGAAT |
| EHRL3-IP2 | TCATCTAATAGCGATAAATC |
| Ehrlichia speciesspecific primers | |
| HE3-R (8) | CTTCTATAGGTACCGTCATTATCTTCCCTAT |
| E. canis (8) | CAATTATTTATAGCCTCTGGCTATAGGAA |
| E. chaffeensis (8) | CAATTGCTTATAACCTTTTGGTTATAAATA |
| E. ewingii 2 (8) | CAATTCCTAAATAGTCTCTGACTATT |
| E. equi 3-IP2 (1) | GTCGAACGGATTATTCTTTATAGCTTG |
| Bartonella-specific primers | |
| Bh16SF (3) | AGAGTTTGATCCTGGCTCAG |
| Bh16SR (BH1) (3) | CCGATAAATCTTTCTCCCTAA |
References for the primers are given in parentheses. Primers without listed references were established in this study (EHR-OUT1, EHR-OUT2, and EHRL3-IP2). The sizes of the products produced were 152 bp with the Ehrlichia genus-specific primers, ∼395 bp with the Ehrlichia species-specific primers, and 185 bp with the Bartonella-specific primers.
Ehrlichia-species-specific amplification.
PCR amplification was performed with a 50-μl reaction volume similar to that used in the above-described procedure but with the following: 0.1 pmol of the outer primers EHR-OUT1 and EHR-OUT2 and 25 pmol of the inner primer HE3-R paired with E. canis, HE3-R paired with E. chaffeensis, HE3-R paired with E. ewingii, or HE3-R paired with E. equi 3-IP2 (Table 1). The first round of amplification included denaturation at 94°C for 45 s and annealing and chain extension at 72°C for 1.5 min. This PCR cycle was repeated 20 times. The second round of amplification included denaturation at 94°C for 45 s, annealing at 55°C for 45 s, and chain extension at 72°C for 1 min. This cycle was repeated 50 times and was followed by a final chain extension of 72°C for 5 min. All PCR products were electrophoresed through 1% agarose gels in Tris-boric acid-EDTA buffer, and the DNA fragments were visualized by ethidium bromide staining under UV fluorescence. Specificity of primer sets was established by cross-testing of canine blood samples spiked with known ehrlichial DNAs representing the species E. canis, E. chaffeensis, E. ewingii, and E. equi.
Bartonella amplification.
PCR amplification was performed with a 50-μl reaction volume similar to that used in the above-described procedure but with the following: 0.2 μM (each) primers Bh16SF and Bh16SR (Table 1) and 1.25 U of Taq polymerase. Amplification cycles included denaturation at 95°C for 30 s, annealing at 54°C for 1 min, and chain extension at 72°C for 45 s. This cycle was repeated 35 times and was followed by a final chain extension at 72°C for 5 min.
RESULTS
The signalments, historical abnormalities, and selected pretreatment clinicopathologic results for 12 dogs diagnosed with canine ehrlichiosis are summarized in Table 2. Other pertinent clinical abnormalities included proteinuria in dogs 1 and 11 (urine protein/creatinine ratios, 8.7 and 5.2, respectively). Leptospirosis with acute renal failure was diagnosed concurrently for dog 4. Immune-mediated hemolytic anemia in conjunction with a nonregenerative anemia (0.2% reticulocyte count) requiring long-term immunosuppressive drug therapy was diagnosed for dog 5, 6 months following doxycycline treatment. Canine ehrlichiosis was diagnosed for dog 5 by the referring veterinarian approximately 1 month prior to enrollment in the study, during which time thrombocytopenia persisted despite tetracycline treatment. Dog 12 had 8,030 atypical lymphocytes/μl and 584 immature cells of undetermined origin/μl in its peripheral blood. Lymphocyte subset analysis by flow cytometry identified few circulating B cells (2%) and an inversion of the CD4/CD8 ratio (ratio, 0.2; 12% CD4 cells, 60% CD8 cells). Five of 12 dogs had reciprocal IFA antibody titers of 32 or greater to B. vinsonii subsp. berkhoffii at the time of entry into the study (Table 3). Bartonella species DNA was amplified from EDTA blood samples of 7 dogs (1, 4, 7, 8, 10–12).
TABLE 2.
Signalments and historical abnormalities of and selected hematologic and biochemical values for 12 dogs with ehrlichiosis
| Dog | Age (yr) | Sex/status | Breed | Historical abnormality(ies) | Serum test resultb
|
||||
|---|---|---|---|---|---|---|---|---|---|
| % Corpuscles | No. of neutrophils/μl | No. of platelets/μl | Amt of TP/dl | Amt of Alb/dl | |||||
| 1 | 7 | M | Sharpei | Epilepsy, immune-mediated hemolytic anemia, splenectomy | 16.5 | 50,592 | 222 | 6.2 | 2.1 |
| 2 | 7 | F/S | Golden retriever | Epistaxis | 18.2 | 2,900 | 53 | NA | NA |
| 3 | 4 | M | Boykin spaniel | Epilepsy, intermittent incoordination, pyoderma | 29.7 | 7,844 | 201 | 7.7 | 2.6 |
| 4 | 2 | M | Cocker spaniel | Anterior uveitis, acute renal failure, (Leptospira grippotyphosa) | 34.6 | 16,520 | 59 | 7.8 | 2.3 |
| 5 | 6 | F/S | Miniature schnauzer | Vomiting, erythema multiforma, gastric perforation | 33.2 | 40,400 | 74 | 5.9 | 2.3 |
| 6 | 7 | F/S | Golden retriever | Chronic demodicosis, epistaxis, lymphadenopathy | 36.7 | 3,280 | 132 | 8.1 | 2.7 |
| 7 | 6 | F/S | Labrador retriever | Lethargy, anterior uveitis | 44.0 | 7,040 | 151 | 9.8 | 2.2 |
| 8 | 1 | F | German shepherd | Irregular estrus cycles, splenomegaly | 52.2 | 3,649 | 179 | NA | NA |
| 9 | 12 | F | Weimaraner | Osteomyelitis in left rear leg, Horner’s syndrome, endocarditis | 23.7 | 10,179 | 113 | 6.5 | 2.0 |
| 10 | 4 | F/S | Boykin spaniel | Epilepsy (sister of dog 3) | 44 | 5,270 | 200 | 6.0 | 2.7 |
| 11 | 7 | M/C | Mixed breed | Coughing, aspiration pneumonia, aural hemorrhage | 24.2 | 2,700 | 25 | 8.2 | 2.1 |
| 12 | 5 | M/C | Mixed breed | Splenomegaly on routine exam | 37.5 | 4,526 | 87 | 9.6 | 2.4 |
M, male; F, female; S, spayed; C, castrated.
Laboratory reference ranges were as follows: 33 to 56% corpuscles, 3,000 to 11,500 neutrophils per μl, 200 × 103 to 450 × 103 platelets per μl, 4.6 to 8.2 g of total serum protein (TP) per dl, and 2.8 to 4.4 g of serum albumin (Alb) per dl. NA, not available.
TABLE 3.
Comparative seroreactivities to three E. canis isolates, E. chaffeensis, E. ristici, E. equi, and B. vinsoniia
| Dog | Reciprocal IFA antibody titer to:
|
||||||
|---|---|---|---|---|---|---|---|
| E. canis (Florida) | E. canis (DJ) | E. canis (Jake) | E. chaffeensis | E. ristici | E. equi | B. vinsonii | |
| 1 | >10,240 | >10,240 | 640 | 1,280 | <20 | 20 | 64 |
| 2 | >10,240 | 5,120 | 1,280 | 2,560 | <20 | <20 | 32 |
| 3 | 2,560 | 2,560 | 1,280 | 2,560 | <20 | 20 | <16 |
| 4 | 5,120 | 2,560 | 640 | 1,280 | <20 | <20 | 1,024 |
| 5 | 5,120 | 1,280 | 640 | 80 | <20 | <20 | <16 |
| 6 | >10,240 | 1,280 | 160 | >10,240 | <20 | 40 | 1,024 |
| 7 | 1,280 | 640 | 320 | 1,280 | <20 | 320 | 128 |
| 8 | 160 | <20 | <20 | <20 | <20 | <20 | <16 |
| 9 | 1,280 | 1,280 | 20 | <20 | <20 | <20 | <16 |
| 10 | 320 | 640 | 160 | 160 | <20 | 20 | <16 |
| 11 | 5,120 | 2,560 | 160 | 1,280 | <20 | 40 | <16 |
| 12 | >10,240 | >10,240 | 1,280 | 5,120 | 640 | 80 | <16 |
Based upon DNA amplification by PCR with Ehrlichia species-specific primers, dogs 1 to 3 and 11 were determined to be infected with E. canis and dogs 4 to 6 were determined to be infected with E. chaffeensis. Based on DNA sequencing, dog 7 was determined to be infected with E. platys. The infecting Ehrlichia species were not identified for dogs 8 to 10 and 12.
Prior to treatment, 9 of 12 dogs were thrombocytopenic (platelet count, <200,000/μl) and 3 dogs had normal platelet numbers (Table 2). Seroreactivity to E. canis antigens was documented for 12 of 12 dogs (Table 3). Ehrlichiemia was documented for 4 of 10 dogs by tissue culture isolation and for 7 of 9 dogs by PCR amplification (Table 4). Because the diagnosis of ehrlichiosis was not initially suspected, pretreatment EDTA blood was not available for tissue culture isolation for two dogs or PCR analysis for three dogs. Cellular supernatants collected from tissue culture attempts deemed to be positive by light microscopy methods were later confirmed positive by PCR for two of four cases (dogs 2 and 3).
TABLE 4.
Summary of pre- and posttreatment platelet counts, reciprocal IFA titers, and tissue culture isolation and PCR amplification results
| Dog | Parameter | Pretreatment test resulta | Posttreatment test resulta at mo:
|
Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 6 | 8 | 12 | ||||
| 1 | Platelet count | 222 | 449 | 290 | Healthy, chronic pyoderma | ||||
| Reciprocal IFA titer | >10,240 | >10,240 | >10,240 | ||||||
| Tissue culture isolation | + | − | |||||||
| PCR amplification | +/+ | +/+ | −/− | ||||||
| Species isolated | Ec | Ec, Eew, Eeq | |||||||
| 2 | Platelet count | 53 | 556 | 539 | 255 | Healthy | |||
| Reciprocal IFA titer | >10,240 | 1,280 | 640 | 640 | |||||
| Tissue culture isolation | + | − | − | − | |||||
| PCR amplification | +/+ | −/− | −/− | −/− | |||||
| Species isolated | Ec | ||||||||
| 3 | Platelet count | 201 | 244 | 210 | 230 | Healthy, epilepsy | |||
| Reciprocal IFA titer | 5,120 | 2,560 | 1,280 | 2,560 | |||||
| Tissue culture isolation | + | − | − | − | |||||
| PCR amplification | +/+ | −/− | −/− | −/− | |||||
| Species isolated | Ec | ||||||||
| 4 | Platelet count | 59 | 450 | NA | 316 | Healthy | |||
| Reciprocal IFA titer | 5,120 | 640 | 320 | NA | |||||
| Tissue culture isolation | − | − | − | NA | |||||
| PCR amplification | +/+ | +/+ | +/+ | NA | |||||
| Species isolated | Ech | Ech | Ech | ||||||
| 5 | Platelet count | 74 | NA | 125 | 416 | Immunosuppression from IMHAb | |||
| Reciprocal IFA titer | 5,120 | 2,560 | 1,280 | 320 | |||||
| Tissue culture isolation | NA | − | − | − | |||||
| PCR amplification | NA | +/+ | +/+ | +/+ | |||||
| Species isolated | Ech | Ech | Ech | ||||||
| 6 | Platelet count | 132 | 244 | 233 | NA | Healthy | |||
| Reciprocal IFA titer | >10,240 | ND | >10,240 | >10,240 | |||||
| Tissue culture isolation | − | − | − | − | |||||
| PCR amplification | +/+ | +/+ | −/− | +/+ | |||||
| Species isolated | Ech | Ech | Ech | ||||||
| 7 | Platelet count | 151 | 204 | 121 | Healthy, owners declined sampling at 12 mo | ||||
| Reciprocal IFA titer | 10,240 | 2,560 | 2,560 | ||||||
| Tissue culture isolation | − | − | − | ||||||
| PCR amplification | +/− | −/− | +/− | ||||||
| Species isolated | Ep | ||||||||
| 8 | Platelet count | 179 | Adequate | 319 | Healthy at 6 mo, unavailable for follow-up (pregnant) | ||||
| Reciprocal IFA titer | 160 | 20 | 20 | ||||||
| Tissue culture isolation | − | − | − | ||||||
| PCR amplification | −/− | −/− | −/− | ||||||
| 9 | Platelet count | 113 | 495 | 553 | Died 10 mo posttreatment, congestive heart failure | ||||
| Reciprocal IFA titer | 1,280 | 160 | 160 | ||||||
| Tissue culture isolation | + | − | |||||||
| PCR amplification | NA | −/− | −/− | ||||||
| 10 | Platelet count | 200 | Clumping | 198 | 246 | Healthy, epilepsy | |||
| Reciprocal IFA titer | 640 | 640 | 1,280 | 320 | |||||
| Tissue culture isolation | − | − | − | − | |||||
| PCR amplification | −/− | −/− | −/− | −/− | |||||
| 11 | Platelet count | 25 | 165 | 453 | 354 | Healthy | |||
| Reciprocal IFA titer | 5,120 | 2,560 | 640 | 640 | |||||
| Tissue culture isolation | − | − | − | − | |||||
| PCR amplification | +/+ | −/− | −/− | −/− | |||||
| Species isolated | Ec | ||||||||
| 12 | Platelet count | 87 | 112 | Clumping | Healthy, owners moved to Europe 9 mo posttreatment | ||||
| Reciprocal IFA titer | >10,240 | 1,280 | 1,280 | ||||||
| Tissue culture isolation | NA | + | − | ||||||
| PCR amplification | NA | −/− | −/− | ||||||
Platelet counts (103) and reciprocal IFA titers are per microliter. “Adequate” and “clumping” indicate, respectively, that platelets were of adequate numbers for a healthy animal and that platelets clumped, making a determination of number difficult. For tissue culture isolation results, a + or − indicates that organisms were isolated or not, respectively. For PCR amplification test results, a + or − to the left of the shill indicates that Ehrlichia genus-specific DNA was or was not detected, respectively, and a + or − to the right of the shill indicates that Ehrlichia species-specific DNA was or was not detected, respectively. NA indicates that a sample was not available for testing. Species abbreviations: Ec, E. canis; Ech, E. chaffeensis; Eew, E. ewingii; Eeq, E. equi; Ep, E. platys. The species of each infecting organism was determined by PCR amplification with Ehrlichia species-specific primers for dogs 1 to 6 and 11; that of dog 7 was determined by DNA sequencing. The infecting Ehrlichia species were not identified for dogs 8 to 10 and 12.
IMHA, immunity-mediated hemolytic anemia.
Following treatment, lack of owner compliance resulted in some variation in the timing of sample collection. In addition, five dogs were not available for the entire 12-month evaluation period. Dog 9 died, dog 12 was transported to Europe, the owner of dog 8 declined blood sampling when the dog became pregnant, and the owners of dog 7 declined the 12-month evaluation since the dog was healthy. After treatment with doxycycline, clinical abnormalities, potentially attributable to ehrlichiosis, resolved in all but dog 4, which remained PCR positive for E. chaffeensis DNA and had the highest antibody titer to B. vinsonii detected among the dogs in this study (Table 3). Posttreatment, Ehrlichia species were isolated by tissue culture only from dog 12, blood from which was PCR negative. Thrombocytopenia resolved in dogs 1 to 6 and 8 to 11, but resolution was not observed for dog 7 or 12, both of which were monitored for less than a year (Table 4). Serum IFA antibody titers decreased by more than fourfold in all dogs except dogs 1 (E. canis, E. equi, and E. ewingii infected) and 6 (E. chaffeensis infected), in which stable antibody titers persisted in conjunction with positive PCR results at 6 and 12 months, respectively (Table 4).
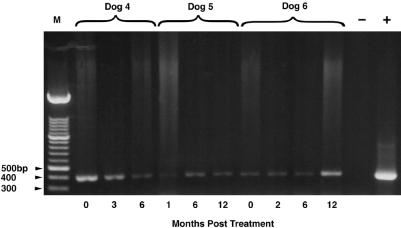
With Ehrlichia genus-specific primers, amplification products were obtained from 5 of 12 dogs (1, 4–7) at one or more times between 6 and 12 months during the posttreatment follow-up period (Table 4). Based upon species-specific PCR primers, dog 1 was infected with E. canis, E. equi, and E. ewingii; dogs 2, 3, and 11 were infected with E. canis; and dogs 4 to 6 were infected with E. chaffeensis. With the exception of dog 7 (6-month-posttreatment sample), ehrlichial speciation was successful for each sample in which an Ehrlichia genus amplicon was obtained. In all instances, species-specific primers provided reproducible results, as was best illustrated by dogs 4 to 6, from which only E. chaffeensis DNA was amplified at each datum collection point during the 12-month period of study. As the species-specific primers used in this study did not generate an amplicon, the ehrlichial-genus-positive PCR product for dog 7 was further characterized by sequencing in the laboratory of D. H. Walker, Galveston, Tex. The species was identified as Ehrlichia platys, which our primers were not expected to amplify. Ehrlichia DNA could not be amplified from the available samples for four dogs (Table 4). Based upon continued amplification of ehrlichial DNA posttreatment, all three E. chaffeensis-infected dogs (Fig. 1) appeared to remain infected or were reinfected following treatment. These dogs were generally thrombocytopenic, or their platelet counts remained in the low reference range. The PCR results from dog 1 are notable, as only E. canis was amplified from the pretreatment blood sample, whereas E. canis, E. equi, and E. ewingii were amplified from two separate blood samples obtained 6 months posttreatment, approximately 1 week apart. The second of these samples was obtained because of bacterial contamination of the initial sample obtained for ehrlichial culture. Presumably, these results reflect continued tick exposure.
FIG. 1.
PCR amplicons obtained with E. chaffeensis primers from sequential EDTA blood samples from dogs 4 to 6. Lane −, negative control blood sample; lane +, positive control (cultured E. chaffeensis); lane M, molecular size markers.
When pretreatment serum samples from these dogs were tested against antigens derived from E. canis Florida (type strain) and two NCSU E. canis isolates (DJ and Jake) or against E. chaffeensis (type strain), the seroreactive antibody titers (Table 3) did not generally facilitate differentiation of the infecting Ehrlichia species when they were compared to the PCR results (Table 4). In addition, in some instances there was substantial variation in the IFA antibody titers among the three E. canis strains used as antigens. Except with dog 12, antibodies to E. risticii were not detected. Similarly, Western immunoblot analysis with E. canis antigens did not differentiate the infecting Ehrlichia species. When results were compared across time (periods up to 13 months), there was minimal or no change in the Western blot patterns regardless of the dog’s infection status, as defined by platelet count or PCR result.
DISCUSSION
Historically, infection with Ehrlichia species has generally been considered to be host specific. For example, E. canis was thought to infect only dogs and wild carnivores and E. chaffeensis was thought to infect only deer and human beings. Recently, an isolate genetically and antigenically similar to E. canis was obtained from a veterinarian in Venezuela (31). Similarly, isolates genetically identical to E. risticii, the cause of Potomac horse fever, have been obtained from dogs (21). Recent evidence indicates that a member of the E. phagocytophila group, presumably E. equi, causes disease manifestations in cats, dogs, horses, and human beings (15). In this study, E. chaffeensis, originally isolated and characterized as a cause of human disease (7), was found to cause disease in dogs. Collectively, these observations suggest that several Ehrlichia species can be transmitted to a variety of hosts in nature. Therefore, additional efforts to define the spectrum of host susceptibility in domestic and wild animals seem appropriate.
Previously, based upon PCR amplification and DNA sequence analysis, we documented severe clinical and hematologic abnormalities due to E. ewingii infection in dogs with granulocytic ehrlichiosis from North Carolina and Virginia (14). The present study indicates that E. chaffeensis, as well as E. canis, E. equi, E. ewingii, and E. platys, can cause disease manifestations and clinicopathologic abnormalities in dogs originating from the same geographic region. Importantly, these results provide the first evidence for development of disease manifestations in dogs naturally infected with E. chaffeensis. In concert with the results derived by Dawson and Ewing (9), dogs seroreactive to E. canis antigens from this region may be infected with E. canis, E. chaffeensis, or E. ewingii. Infection with any one of these three species can cause severe disease manifestations that may be clinically, hematologically, and serologically indistinguishable from those of the other two species. The relative contribution of coinfection with three Ehrlichia species to the disease manifestations in dog 1 awaits additional studies of coinfected dogs. Collectively, these results indicate that increased utilization of molecularly based diagnostic modalities should enhance our understanding of potentially important clinical or pathologic differences associated with infection with a single Ehrlichia species or coinfection with multiple Ehrlichia species.
Serologic testing by an IFA assay was not able to consistently distinguish between infection with E. canis and that with E. chaffeensis. Similarly, when E. canis antigen was used, Western immunoblot analysis of sera from these dogs did not result in antigenic protein recognition that would facilitate diagnostic differentiation of the infecting species. Since E. ewingii has not been cultivated in an in vitro culture system, antigen from this organism was not available for comparative serologic testing. Although coinfection was not examined in this study, it is probable that coinfection with more than one Ehrlichia species further limits the utility of serologic testing for differentiation of the infecting Ehrlichia species. Recently, we have observed numerous examples of coinfection with E. canis, E. chaffeensis, and/or E. ewingii in a kennel of heavily tick-infested dogs (unpublished data).
Since readily discernible differences in IFA or Western immunoblot seroreactivity patterns to E. canis antigens do not appear to differentiate between the infecting species, molecular detection and speciation of ehrlichial DNA is necessary to determine if predictable differences in therapeutic outcomes can be further correlated with an infecting Ehrlichia species. Several factors, including anticipated duration of infection, therapeutic responsiveness (particularly to tetracycline derivatives), and zoonotic potential, emphasize the importance of determining which Ehrlichia species is causing infection and antibody reactivity to E. canis antigen in a dog. For example, E. canis causes chronic, frequently subclinical infection with the potential for the development of severe life-threatening disease manifestations (6, 14, 24) whereas E. ewingii is considered to cause polyarthritis and potentially self-limiting infection (35). Ehrlichia canis and E. chaffeensis may not be eliminated by doxycycline therapy, whereas therapeutic elimination of E. ewingii or E. equi is an expected outcome.
PCR amplification of ehrlichial DNA is gaining acceptance as an important adjunct to serologic testing for the diagnosis of canine ehrlichiosis (18, 38). In this study, to increase the sensitivity of the PCR assays, two nested techniques were used in testing, first, to determine the presence of Ehrlichia DNA (genus-specific primers) and, second, to differentiate among the various Ehrlichia species. To reduce the risk of contamination, historically associated with nested PCR, a single-tube procedure was developed for both assays by designing the outer primers with an annealing temperature substantially higher than that of the internal primers. The internal primers used for Ehrlichia species differentiation were modified slightly from the primers described by Dawson et al. (8) and Barlough et al. (1) to reduce annealing temperatures for the nested protocol. Based on the consistent amplification of only E. chaffeensis DNA at multiple time points from dogs 4 to 6 and consistently negative PCR results from dogs 8 to 10, we believe that PCR contamination and nonspecific priming are unlikely explanations for PCR evidence of coinfection in dog 1. When they were tested with blood samples spiked with DNAs of known ehrlichial species, these primer pairs did not amplify nonspecific DNA. Since E. equi is not considered to be endemic to this region, DNA was extracted from two samples on two different occasions from the dog coinfected with E. equi. Identical PCR amplicons were obtained. Since amplicons were obtained from only seven of nine pretreatment EDTA blood samples, efforts to enhance the sensitivity of detection may be warranted.
Problems recognized in dogs with increasing frequency by veterinarians are the persistence of clinical and/or hematologic abnormalities, the persistence of antibody reactivity to E. canis antigen (2), and the persistence of Ehrlichia DNA as detected by PCR following antirickettsial drug therapy (38). Documentation of one or more of these factors has caused some veterinarians to treat canine ehrlichiosis with tetracycline hydrochloride or doxycycline hydrochloride for extended periods (months to years), a less-than-optimal situation, potentially facilitating the development of drug-resistant bacteria. In other instances, alternative treatment modalities such as the administration of imidocarb diproprionate (33) have been used in an effort to obtain a satisfactory therapeutic response. Although the cause of these treatment failures is most probably multifactorial, it is of interest that all three dogs infected with E. chaffeensis in this study remained PCR positive after treatment with doxycycline at a dose and duration generally considered to be efficacious for treatment of E. canis infection. Following experimental infection with E. canis, other investigators found that three of five dogs treated with doxycycline for only 7 days failed to clear their infection (19). However, in a study of similar design from our laboratory, using experimentally infected mixed-breed dogs treated for 14 days, eight of eight dogs became culture and PCR negative following treatment (4). In the present study, all four dogs naturally infected with E. canis appeared to clear their infection, although dog 1 remained E. canis PCR positive at 6 months following the initial treatment, which may have reflected reinfection associated with continued tick exposure. In a recent study, 43 of 80 E. canis posttreatment blood samples, obtained from E. canis seroreactive dogs from Arizona and Texas, were PCR positive (38). Collectively, these results suggest that the role of drug-resistant strains of E. canis, as well as the efficacy of doxycycline for the treatment of E. chaffeensis infection in naturally infected dogs, deserves additional consideration. Due to the lack of sensitivity of tissue culture isolation, the variability in posttreatment IFA antibody responses, and the possibility of DNA persistence, unassociated with viable organisms, other modalities are needed to prove therapeutic elimination of infection.
Although this study involved a small number of dogs, Bartonella DNA was amplified from 58% of the dogs and 42% were reactive to B. vinsonii antigen, with the two highest antibody titers being found in dogs infected with E. chaffeensis. Since Amblyomma americanum ticks have been implicated in the transmission of E. chaffeensis, it is possible that this tick may cotransmit B. vinsonii. Since simultaneous infestation with more than one tick species is not unusual in dogs, controlled studies will be necessary to clarify the role of ticks in the transmission of Bartonella species. It is of interest that epistaxis, a well-recognized clinical manifestation of ehrlichiosis, has been reported in association with Bartonella henselae and Bartonella quintana infection in humans (10, 13) and B. vinsonii infection in a dog (5). The extent to which concurrent infection with B. vinsonii complicates the clinical course of ehrlichiosis in dogs deserves additional study.
ACKNOWLEDGMENTS
This research was supported by the State of North Carolina and through a grant from Fort Dodge Laboratories, Fort Dodge, Iowa.
We acknowledge the efforts of the house staff of the Veterinary Teaching Hospital, NCSU, for the clinical management of the patients and A. N. Billings, The University of Texas Medical Branch, Galveston, Texas, for performing the DNA sequencing.
REFERENCES
- 1.Barlough J E, Madigan J E, DeRock E, Bigornia L. Nested polymerase chain reaction for detection of Ehrlichia equi genomic DNA in horses and ticks (Ixodes pacificus) Vet Parasitol. 1996;63:319–329. doi: 10.1016/0304-4017(95)00904-3. [DOI] [PubMed] [Google Scholar]
- 2.Bartsch R C, Greene R T. Post-therapy antibody titers in dogs with ehrlichiosis: follow-up study on 68 patients treated primarily with tetracycline and/or doxycycline. J Vet Intern Med. 1996;10:271–274. doi: 10.1111/j.1939-1676.1996.tb02061.x. [DOI] [PubMed] [Google Scholar]
- 3.Bergmans A M, Schellekens J F P, van Embden J D A, Schouls L M. Predominance of two Bartonella henselae variants among cat-scratch disease patients in The Netherlands. J Clin Microbiol. 1996;34:254–260. doi: 10.1128/jcm.34.2.254-260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breitschwerdt E B, Hegarty B C, Hancock S I. Doxycycline hyclate treatment of experimental canine ehrlichiosis followed by challenge inoculation using two Ehrlichia canis strains. Antimicrob Agents Chemother. 1998;42:362–368. doi: 10.1128/aac.42.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breitschwerdt E B, Kordick D L, Malarkey D E, Keene B, Hadfield T L, Wilson K. Endocarditis in a dog due to infection with a novel Bartonella subspecies. J Clin Microbiol. 1995;33:154–160. doi: 10.1128/jcm.33.1.154-160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Codner E C, Farris-Smith L L. Characterization of the subclinical phase of ehrlichiosis in dogs. J Am Vet Med Assoc. 1986;189:47–50. [PubMed] [Google Scholar]
- 7.Dawson J E, Anderson B E, Fishbein J L, Sanchez C S, Goldsmith C S, Wilson K H, Duntley C W. Isolation and characterization of an Ehrlichia species from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson J E, Biggie K L, Warner C K, Cookson K, Jenkins S, Levine J, Olson J G. Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human ehrlichiosis, in dogs from southeast Virginia. Am J Vet Res. 1996;57:1175–1179. [PubMed] [Google Scholar]
- 9.Dawson J E, Ewing S A. Susceptibility of dogs to infection with Ehrlichia chaffeensis, causative agent of human ehrlichiosis. Am J Vet Res. 1992;53:1322–1327. [PubMed] [Google Scholar]
- 10.Drancourt M, Mainardi J L, Brouqui P, Bandenesch F, Carta A, Lehnert F, Etienne J, Goldstein F, Acar J, Raoult D. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med. 1995;332:419–423. doi: 10.1056/NEJM199502163320702. [DOI] [PubMed] [Google Scholar]
- 11.Dumler J S, Chen S M, Asanovich K, Trigiani E, Popov V L, Walker D H. Isolation and characterization of a new strain of Ehrlichia chaffeensis from a patient with nearly fatal monocytic ehrlichiosis. J Clin Microbiol. 1995;33:1704–1711. doi: 10.1128/jcm.33.7.1704-1711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaunt S D, Corsetvet R E, Berry C M, Brennan B. Isolation of Ehrlichia canis from dogs following subcutaneous inoculation. J Clin Microbiol. 1996;34:1429–1432. doi: 10.1128/jcm.34.6.1429-1432.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golden S E. Hepatosplenic cat-scratch disease associated with elevated anti-Rochalimaea antibody titers. Pediatr Infect Dis J. 1993;12:868–871. doi: 10.1097/00006454-199310000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Goldman E E, Breitschwerdt E B, Grindem C B, Hegarty B C, Walls J J, Dumler J S. Granulocytic ehrlichiosis in dogs from North Carolina and Virginia. J Vet Intern Med. 1998;12:61–70. doi: 10.1111/j.1939-1676.1998.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 15.Greig B, Asanovich K M, Armstrong P J, Dumler J S. Geographic, clinical, serologic and molecular evidence of granulocytic ehrlichiosis, a likely zoonotic disease in Minnesota and Wisconsin dogs. J Clin Microbiol. 1996;34:44–48. doi: 10.1128/jcm.34.1.44-48.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hegarty B C, Levy M G, Gager R F, Breitschwerdt E B. Immunoblot analysis of the immunoglobulin G response to Ehrlichia canis in dogs: an international survey. J Vet Diagn Invest. 1997;9:32–38. doi: 10.1177/104063879700900106. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal Z, Rikihisa Y. Reisolation of Ehrlichia canis from blood and tissues of dogs after doxycycline treatment. J Clin Microbiol. 1994;32:1644–1649. doi: 10.1128/jcm.32.7.1644-1649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iqbal Z, Chaichanasiriwithaya W, Rikihisa Y. Comparison of PCR with other tests for early diagnosis of canine ehrlichiosis. J Clin Microbiol. 1994;32:1658–1662. doi: 10.1128/jcm.32.7.1658-1662.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iqbal Z, Rikihisa Y. Application of the polymerase chain reaction for the detection of Ehrlichia canis in tissues of dogs. Vet Microbiol. 1994;42:281–287. doi: 10.1016/0378-1135(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 20.Johansson K E, Pettersson B, Uhlen M, Gunnarsson A, Malmqvist M, Olsson E. Identification of the causative agent of granulocytic ehrlichiosis in Swedish dogs and horses by direct solid phase sequencing of PCR products from 16S rRNA gene. Res Vet Sci. 1995;58:109–112. doi: 10.1016/0034-5288(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 21.Kakoma I, Hansen R D, Anderson B E, Hanley T A, Sims K G, Liu L, Bellamy C, Long M T, Baek B K. Cultural, molecular, and immunological characterization of the etiologic agent for atypical canine ehrlichiosis. J Clin Microbiol. 1994;32:170–175. doi: 10.1128/jcm.32.1.170-175.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly P J, Matthewman L A, Mahan S M, Semu S M, Peter T, Mason P R, Brouqui P R, Raoult D. Serologic evidence for antigenic relationships between Ehrlichia canis and Cowdria ruminantium. Res Vet Sci. 1994;56:170–174. doi: 10.1016/0034-5288(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 23.Kordick D L, Swaminathan B, Greene C T, Wilson K H, O’Connor S, Hollis D G, Matar G M, Malcom B G, Hayes P S, Hadfield T L, Breitschwerdt E B, Brenner D J. Bartonella vinsonii subsp. berkhoffii subsp. nov., isolated from dogs; Bartonella vinsonii subsp. vinsonii; and emended description of Bartonella vinsonii. Int J Syst Bacteriol. 1996;46:704–709. doi: 10.1099/00207713-46-3-704. [DOI] [PubMed] [Google Scholar]
- 24.Kuehn N F, Gaunt S D. Clinical and hematologic findings in canine ehrlichiosis. J Am Vet Med Assoc. 1985;186:355–358. [PubMed] [Google Scholar]
- 25.Levy M G, Gebhard D, Gager R, Breitschwerdt E B. Proceedings of the International Union Immunology Society, 4th International Veterinary Immunology Symposium. 1995. A newly described canine monocytoid cell line capable of supporting growth of the rickettsial parasite Ehrlichia canis, and useful for examination of host:parasite interactions; p. 274. [Google Scholar]
- 26.Lewis G E, Huxsoll D L, Ristic M, Johnson A J. Experimentally induced infection of dogs, cats, and nonhuman primates with Ehrlichia equi, etiologic agent of equine ehrlichiosis. Am J Vet Res. 1975;36:85–88. [PubMed] [Google Scholar]
- 27.Madewell B R, Gribble D H. Infection in two dogs with an agent resembling Ehrlichia equi. J Am Vet Med Assoc. 1982;180:512–514. [PubMed] [Google Scholar]
- 28.Matthewman L A, Kelly P J, Mahan S M, Semu S M, Mason P R, Bruce D, Brouqui P, Raoult D. Reactivity of sera collected from dogs in Mutare, Zimbabwe, to antigens of Ehrlichia canis and Cowdria ruminantium. Vet Rec. 1994;134:498–499. doi: 10.1136/vr.134.19.498. [DOI] [PubMed] [Google Scholar]
- 29.Paddock C D, Sumner J W, Shore G M, Bartley D C, Elie R C, Mcquade J G, Martin C R, Goldsmith C S, Childs J E. Isolation and characterization of Ehrlichia chaffeensis strains from patients with fatal ehrlichiosis. J Clin Microbiol. 1997;35:2496–2502. doi: 10.1128/jcm.35.10.2496-2502.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pappalardo B L, Correa M T, York C C, Peat C Y, Breitschwerdt E B. Epidemiologic evaluation of the risk factors associated with exposure and seroreactivity to Bartonella vinsonii in dogs. Am J Vet Res. 1997;58:467–471. [PubMed] [Google Scholar]
- 31.Perez M, Rikihisa Y, Wen B. Ehrlichia canis-like agent isolated from a man in Venezuela: antigenic and genetic characterization. J Clin Microbiol. 1996;34:2133–2139. doi: 10.1128/jcm.34.9.2133-2139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrovec M, Furlan S L, Zupanc T A, Strle F, Brouqui P, Roux V, Dumler J S. Human disease in Europe caused by a granulocytic Ehrlichia species. J Clin Microbiol. 1997;35:1556–1559. doi: 10.1128/jcm.35.6.1556-1559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price J E, Dolan T T. A comparison of the efficacy of imidocarb dipropionate and tetracycline hydrochloride in the treatment of canine ehrlichiosis. Vet Rec. 1980;107:275–277. doi: 10.1136/vr.107.12.275. [DOI] [PubMed] [Google Scholar]
- 34.Stockham S L, Tyler J W, Schmidt D A, Curtis K S. Experimental transmission of granulocytic ehrlichial organisms in dogs. Vet Clin Pathol. 1990;19:99–104. doi: 10.1111/j.1939-165x.1990.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 35.Stockham S L, Schmidt D A, Curtis K S, Schauf B G, Tyler J W, Simpson S T. Evaluation of granulocytic ehrlichiosis in dogs of Missouri including serologic status to Ehrlichia canis, Ehrlichia equi, and Borrelia burgdorferi. Am J Vet Res. 1992;53:63–68. [PubMed] [Google Scholar]
- 36.Walker D H, Dumler J S. Emergence of the ehrlichioses as human health problems. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walls J J, Greig B, Neitzel D F, Dumler J S. Natural infection of small mammal species in Minnesota with the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:853–855. doi: 10.1128/jcm.35.4.853-855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen B, Rikihisa Y, Mott J M, Greene R, Kim H-Y, Zhi N, Couto G C, Unver A, Bartsch R. Comparison of nested PCR with immunofluorescent-antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J Clin Microbiol. 1997;35:1852–1855. doi: 10.1128/jcm.35.7.1852-1855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]