Abstract
Background and Objective
With the rising importance of precision oncology in biliary tract cancer (BTC), the aim of this retrospective single-center analysis was to describe the clinical and molecular characteristics of patients with BTC who underwent comprehensive genomic profiling (CGP) and were discussed in the CCCMunichLMU molecular tumor board (MTB).
Patients and Methods
In this single-center observational study, we included BTC patients with intrahepatic cholangiocarcinoma (iCCA), extrahepatic CCA (eCCA), and gallbladder cancer (GB), who had been discussed in the institutional MTB from May 29, 2017, to July 25, 2022. Patients were followed up until 31 January 2023. Data were retrospectively collected by review of medical charts, and MTB recommendation.
Results
In total, 153 cases were registered to the MTB with a median follow-up of 15 months. Testing was successful in 81.7% of the patients. CGP detected targetable alterations in 35.3% of our BTC patients (most commonly ARID1A/ERBB2/IDH1/PIK3CA/BRAF-mutations and FGFR2-fusions). Recommendations for molecularly guided therapy were given in 46.4%. Of those, treatment implementation of targeted therapy followed in 19.4%. In patients receiving the recommended treatment, response rate was 57% and median overall survival was 19 months (vs 8 months in the untreated cohort). The progression-free survival ratio of 1.45 suggest a clinical benefit of molecularly guided treatment.
Conclusions
In line with previous work, our series demonstrates feasibility and clinical utility of comprehensive genomic profiling in BTC patients. With the growing number of targeted agents with clinical activity in BTC, CGP should become standard of care in the management of this group of patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-023-00985-3.
Key Points
| Comprehensive genomic profiling (CGP) showed 35.3% of our biliary tract cancer cohort harbored a targetable alteration. |
| Implementation rate of all recommended targeted treatments was 19.4%, indicating the need for better translation into clinical practice. |
| CGP is necessary to offer biliary tract cancer patients better care and treatment options leading to clinical benefit. |
Introduction
Biliary tract cancer (BTC) comprises a rare and heterogenous group of cancers. Due to unspecific clinical presentation, most patients are diagnosed in a palliative setting (locally advanced or metastatic). Prognosis remains poor with a median overall survival (OS) of just 10.6 months [1, 2].
BTCs can be differentiated in intrahepatic cholangiocellular adenocarcinoma (iCCA), extrahepatic CCA (eCCA), and gallbladder cancer (GB). While commonly grouped together as one entity, there is growing evidence that each subgroup of BTC has its unique pathophysiology and harbors different genetic drivers. CGP has caused a paradigm shift in the treatment of BTC from solely chemotherapeutic regimens to molecularly guided therapies [3, 4]. Recently, a variety of therapeutically relevant genomic drivers have emerged and have already made their way into the clinic. Isocitrate dehydrogenase 1 (IDH1) mutations are found in up to 20% of BTCs, mostly in iCCA. The randomized, double-blind, and placebo-controlled phase III trial ClarIDHy showed that pre-treated patients with an IDH1 alteration had a better OS on IDH1 inhibitor ivosidenib than placebo [5]. ClarIDHy led to FDA approval of ivosidenib in IDH1-mutated BTC in August 2021.
Another common driver of BTCs, especially iCCA, are fibroblast growth factor receptor (FGFR) 2 alterations. The single-arm phase II trial FIGHT 202 investigated the effect of FGFR2 inhibition by pemigatinib, an oral FGFR1-3 inhibitor, in pre-treated BTC patients. In the patient cohort with FGFR2 rearrangements or fusions, the overall response rate (ORR) was 35.5% and the median progression-free survival (PFS) for second-line pemigatinib was 7.0 months [4]. This trial led to accelerated Food and Drug Administration (FDA) approval of pemigatinib in patients with FGFR2 fusions or rearrangements in previously treated CCA [6, 7]. In the Phase II FOENIX-CCA2 trial, the FGFR1-4 inhibitor futibatinib showed clinical benefit in pre-treated intrahepatic CCA with FGFR2 fusions or rearrangements, leading to FDA approval in September 2022, and a positive European Medicines Agency (EMA) vote recommending approval [8–11].
Patients with microsatellite instability (MSI-high) or mismatch repair deficient (dMMR) tumors have high response rates to immunotherapies and a few case reports demonstrated therapeutic efficacy of PD-1 antibodies in BTC [12, 13]. Pembrolizumab received histology-agnostic FDA approval in patients with MSI-high or dMMR tumors including BTC [14].
Further emerging targets are BRAF V600E and HER2 [15–17]. The former can be found in around 5% of BTC patients and was targeted in the phase II multicenter basket trial ROAR by a combination of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib (Dabra/Tram). In 2020, Subbiah et al. reported the trial results with an ORR of 51%, a median PFS of 9.1 months, and a median of OS of 13.5 months in patients with BTC who had received previous systemic therapy [17]. FDA approval for dabrafenib in combination with trametinib in BRAF V600E in advanced solid cancers followed in June 2022 [18].
Recently, HER2 positivity (amplification or overexpression) in BTC has also surfaced as a possible target for treatment. In a phase IIa basket trial of patients with metastatic BTC, Javle and colleagues described a cohort of patients treated with trastuzumab and pertuzumab with an ORR of 23% and a median duration of response of 10.8 months [15]. Recently, the phase IIb trial, HERIZON-BTC-01, presented at ASCO 2023 showed that zanidatamab, a HER2-targeted bispecific antibody, led to a good treatment response of 41.3% after gemcitabine-based first-line therapy [19]. Further, the HERB trial, an investigator-initiated phase II trial, as well as the SUMMIT basket trial are currently both analyzing the effect of HER2-targeted treatment on BTC. While the former investigates the benefit of trastuzumab-deruxtecan (T-DXd), an antibody drug conjugate, the latter explores the use of neratinib, an oral, irreversible pan-HER tyrosine kinase inhibitor (TKI). Both studies have shown favorable ORRs so far [16, 20].
Regarding the growing options in targeted treatment based on CGP, molecular testing is already recommended and standard practice in many clinics [21]. The updated European Society for Medical Oncology (ESMO) clinical practice guideline recommends molecular testing in metastatic BTC [22]. The Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers by the ESMO Precision Medicine Working Group has previously already listed genomic ESMO Scale for Clinical Actionability of molecular Targets (ESCAT)-Level I alterations that should be considered for testing in BTC patients [21]. To describe the real-world situation and implementation of molecular testing and targeted therapies in BTC patients, we retrospectively investigated cases that were discussed in the weekly molecular tumor boards at the Comprehensive Cancer Center Munich from May 2017 to July 2022. Here we report the results from our retrospective analysis.
Material and Methods
Molecular Tumor Board (MTB)
Since 2016, patients undergoing CGP testing are discussed in the multidisciplinary MTB. Each case is discussed individually based on patient history, current literature, and available clinical trials. Therapeutic recommendations are classified by evidence levels (either ESCAT or National Center for Tumor Diseases [NCT]). Implementation of the MTB recommendation falls under the responsibility of the primary care team.
Patients
Local standard operating procedure for BTC patients at the LMU University Hospital Munich recommends that all unresectable or metastatic patients (at intital diagnosis or disease recurrence) should undergo CGP at initial diagnosis. All patients who underwent CGP testing and subsequent MTB discussion from May 29, 2017 to July 25, 2022 were included in the retrospective analysis. Median follow-up duration was 15 months. Cut-off date for data collection was 31 January 2023.
Sequencing Assays
Next-generation sequencing (NGS) assays have evolved over the years and the panels analyzed at the Institute of Pathology at LMU Munich were adapted to include more genes and features (e.g., tumor mutational burden [TMB]). A detailed overview of the in-house assays can be found in a previous report [23]. The most frequently used NGS assays in-house were TSO500 (Illumina, DNA-level: 523 genes, RNA-level: 53 genes, signatures: TMB) and a combination of OCAplus (Ion Torrent: DNA level: 501 genes, signatures: TMB) and Archer Oncology Research (RNA-level: 74 genes). At the end of 2021 we switched to TSO500. This is of relevance as TSO500 can detect fusions and rearrangements with unknown fusion partners thus potentially identifying more therapeutically relevant alterations. CGP reports from external pathology departments, and other central screening tests such as Foundation One (FO) reports, were occasionally reviewed.
Follow-Up
All patients included in the study were followed-up retrospectively by medical chart review. Patients were followed-up for survival status until 31 January 2023. Ethics approval (Project no. 21-0869) for this analysis was granted by the ethics committee of the medical faculty (LMU Munich).
Statistical Analysis
Descriptive and statistical analysis, as well as generation of graphs, were performed with IBM SPSS Statistics version 29.0.0.0 (241) and Microsoft Excel. Survival was estimated by the Kaplan–Meier method and compared statistically using the Log-rank test. Statistical significance was determined as p < 0.05.
Results
Patient Characteristics
153 BTC patients were registered for the MTB from May 29, 2017 to July 25, 2022. Male to female ratio was around 0.9:1 with an median age at first diagnosis of 62 (range 24–81 years). In the whole cohort, median time from initial diagnosis to CGP was 155.5 days (range 0–4918 days). In patients presenting in the palliative setting, molecular testing was performed after a median of 80 days (range 2–1088 days). According to the ICD-10 coding system, we grouped the cases into iCCA, eCCA, and GB cancer. As expected, the iCCA cohort represented the largest group with 63.4%, followed by eCCA with 21.6% and GB with 15.0%. At initial diagnosis, most patients presented with UICC stage IV disease (43.1%), with the proportion of UICC stage IV growing until MTB presentation (73.2%). Before discussion in the MTB, patients received a median of one prior systemic therapy in the palliative setting (range 0–6; Table 1).
Table 1.
Baseline patient characteristics
| Characteristics | n (%) |
|---|---|
| Gender | |
| Male | 72 (47.1) |
| Female | 81 (52.9) |
| Age (years) | |
| Median | 62 |
| Range | 24–81 |
| Resection | |
| Yes | 75 (49.0) |
| No | 76 (49.0) |
| Unknown | 3 (2.0) |
| BTC subtype | |
| iCCA | 97 (63.4) |
| eCCA | 33 (21.6) |
| GB | 23 (15.0) |
| Disease stage at time of initial diagnosis | |
| UICC I | 5 (3.0) |
| UICC II | 28 (18.3) |
| UICC III | 40 (26.1) |
| UICC IV | 66 (43.1) |
| Unknown | 14 (9.2) |
| Disease stage at time of MTB presentation | |
| UICC I | 3 (2.0) |
| UICC II | 2 (1.3) |
| UICC III | 21 (13.6) |
| UICC IV | 112 (73.2) |
| Unknown | 15 (9.8) |
| Therapy lines before MTB | |
| Evaluated | 113 (73.9) |
| Missing | 40 (26.1) |
| Median | 1 |
| Minimum | 0 |
| Maximum | 6 |
| Number of therapy lines before MTB | |
| 0 | 6 (5.3) |
| 1 | 63 (55.8) |
| 2 | 32 (28.3) |
| 3 | 8 (7.1) |
| 4 | 3 (2.7) |
| 6 | 1 (0.9) |
| Survival status at last follow-up | |
| Dead | 107 (69.9) |
| Alive | 46 (30.1) |
BTC biliary tract cancer, eCCA extrahepatic cholangiocarcinoma, GB gallbladder cancer, iCCA intrahepatic cholangiocarcinoma, MTB molecular tumor board, UICC Union for International Cancer Control
Molecular Alterations
A total of 109 (71.2%) patients carried a genomic alteration in their tumor profile. Out of these 109 patients, 71 (65.1%) received a recommendation by the MTB. Subsequently, 14 patients were treated with a targeted therapy. Testing was successful in 125 (81.7%) and unsuccessful in 22 (14.4%) patients. Regarding the latter, either insufficient tumor tissue or poor DNA quality led to CGP failure. Additionally, microsatellite status (MSS) and TMB status were evaluated in 70 and 61 patients, respectively. In our cohort, no MSI case was detected and the median TMB was 3 mutations/Mb (range 0–11; Supplementary Table 1, see electronic supplementary material [ESM]).
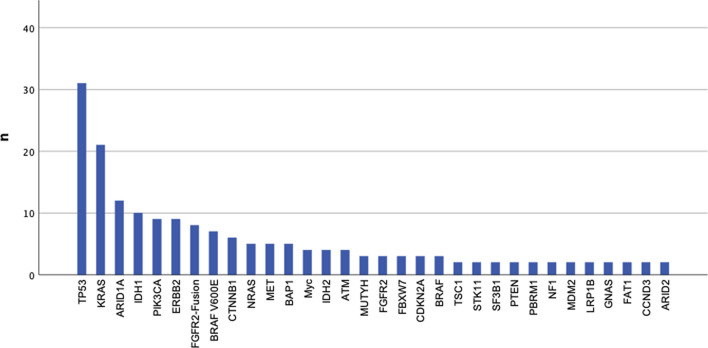
The median number of detected alterations reported on the final pathology result was two (range 1–8 alterations; Table 2). Actionability was defined according to the ESMO Precision Working Group Recommendation with ESCAT I–IV level alterations including ARID1A, IDH1, ERBB2, PIK3CA, BRAF V600E, MET, and BRCA 1/2 mutations and FGFR2 fusions [24]. In this series, 35.3% of all BTC patients had a potentially actionable mutation (Fig. 1; Table 2 and Supplementary Table 2 [see ESM]).
Table 2.
Genes more frequently altered in CGP among BTC patients
| n (%) | |
|---|---|
| Patients with alterations | |
| Yes | 109 (71.2) |
| No | 31 (20.3) |
| Unknown | 13 (8.5) |
| Number of detected alterations | |
| 1.00 | 50 (45.5) |
| 2.00 | 32 (29.1) |
| 3.00 | 13 (11.8) |
| 4.00 | 8 (7.3) |
| 5.00 | 3 (2.7) |
| 6.00 | 3 (2.7) |
| 8.00 | 1 (0.9) |
| Median | 2.00 |
| Minimum | 1.00 |
| Maximum | 8.00 |
| Targetability of all detected alterations (ESCAT tiers I–IV) | |
| ESCAT I–IV | 54 (49.5) |
| Non-actionable | 55 (50.5) |
| Frequency of actionable alterations | |
| ARID1Amut | 12 (7.8) |
| IDH1mut | 10 (6.5) |
| ERBB2mut/amp | 9 (5.9) |
| PIK3CAmut | 9 (5.9) |
| FGFR2 fusion | 8 (5.2) |
| BRAFmut V600E | 7 (4.6) |
| METmut | 5 (3.3) |
| BRCA2mut | 1 (0.7) |
BTC biliary tract cancer, CGP comprehensive genomic profiling, ESCAT ESMO Scale for Clinical Actionability of molecular Targets, ESMO European Society for Medical Oncology
Fig. 1.
All alterations detected in ≥ 2 cases, presented in decreasing order
As the molecular profile differs amongst iCCA, eCCA, and GB, we took a more comprehensive view into the different BTC subgroups. In Supplementary Table 3 (see ESM) all alterations found in > 5% are listed respectively. Regarding targetable mutations, iCCA patients carried IDH1 (10.2%), FGFR2 fusion (8.2%), and BRAF V600E (5.1%) mutations, whereas eCCA ERBB2 mutations (12.1%) occurred more frequently (Supplementary Table 3). The specific alterations within the subgroups have been described by previous groups [25]. Our cohort shows similar findings (data not shown).
Based on their molecular profile, 46.4% of the patients (n = 71) received a recommendation, with 14 of the 71 (19.4%) recommendations being enforced.
In the group of patients who received a targeted therapy based on their MTB recommendation, response rate was 57% (8 from 14 patients) and the median PFS from the start of targeted treatment was 9 months (range 1–19 months). Median duration of response (DoR) was 5.5 months. Application of targeted treatment occurred between the second and fifth line of therapy. Median duration of targeted therapy (with cut-off date on 31 January 2023) was 6 months (range 1–20 months). Treatment initiation varied from immediately after MTB recommendation to up to 2 years after MTB recommendation. Sufficient follow-up data for the calculation of PFS was available for 12 patients. PFS2 was measured from start of targeted treatment to progression of disease, and PFS1 was measured from the start of the treatment received last before targeted treatment until disease progression. The median PFS ratio (= PFS2/PFS1) was 1.45 (Table 3).
Table 3.
Patients receiving recommended molecularly guided therapy
| Mutation | Targeted therapy | Therapy line | Duration of targeted therapy (months)a | Time from MTB to treatment initiation (months) | Best treatment response | Duration of response (months) | PFS2 | PFS1 | PFS ratio (PFS2/PFS1) |
|---|---|---|---|---|---|---|---|---|---|
| ARID1A-I1691Sfs*12- deletion | Ipilimumab/Nivolumab | 3rd | 1 | 0 | PR | 1 | |||
| ATM1mut (R008H) | Olaparib | 3rd | Unknown | 4 | Unknown | ||||
| BRAFmut (V600E) | Dabra/Tram | 3rd | 17 | 24 | PR | 3 | 17 | 17 | 1 |
| BRAFmut (V600E) | Dabra/Tram | 2nd | 4 | 0 | PR | 3 | 4 | 1 | 4 |
| ERBB3mut (E928G) | Trastuzumab | 3rd | 2 | 1 | PD | 1 | 5 | 0.2 | |
| ERBB2amp | GemOx + Trastuzumab | 2nd | 14 | 1 | PR | 12 | 14 | 1 | 14 |
| FGFR2mut (C383R) | Erdafitinib | 5th | 6 | 18 | PR | 7 | 7 | 5 | 1.4 |
| FGFR2fus-BICC1 | Derazantinib | 3rd | 14 | 0 | SD | 14 | 9 | 1.56 | |
| FGFR2fus-SHANK2 | Pemigatinib | 4th | 9 | 0 | PR | 6 | 9 | 2 | 4.5 |
| FGFR2fus-PRDM | Pemigatinib | 3rd | 20 | 0 | PR | 5 | 19 | 2 | 9.5 |
| FGFR2fus-BICC1 | Pemigatinib | 2nd | 10 | 9 | PR | 7 | 9 | 20 | 0.45 |
| IDH1mut (R132L) | Ivosidenib | 4th | 3 | 20 | PD | 3 | 2 | 1.5 | |
| IDH1mut (R132C) | Ivosidenib | 3rd | 2 | 4 | PD | 1 | 3 | 0.33 | |
| IDH1mut (R132C) | Ivosidenib | 3rd | 4 | 11 | PD | 4 | 6 | 0.67 | |
| Median | 3 | 6 | 4 | 5.5 | 8 | 4 | 1.45 | ||
| Minimum | 2 | 1 | 0 | 1 | 1 | 1 | |||
| Maximum | 5 | 20 | 24 | 12 | 19 | 20 | |||
Dabra/Tram dabrafenib and trametinib, GemOx gemcitabine and oxaliplatin, MTB molecular tumor board, PD progressive disease, PFS progression-free survival, PR partial remission, SD stable disease
aLast follow-up 31 January 2023
Survival
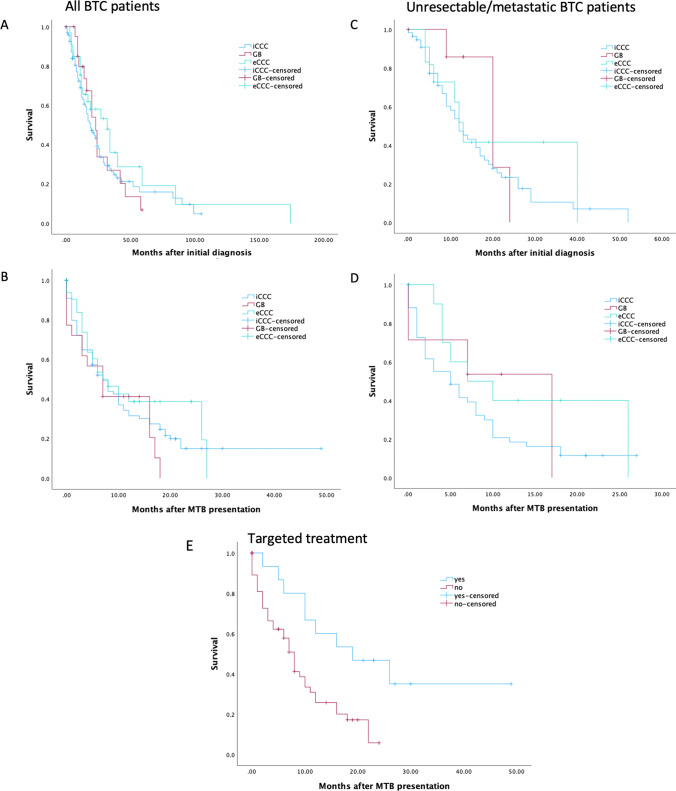
Median OS in the whole cohort was 21 months (95% CI 17.09–24.91; Fig. 2). After MTB presentation, median OS across all subtypes was 7 months (95% CI 5.15–8.86) (Fig. 2). In patients presenting with unresectable and metastasized disease stage, median OS was 13 months after initial diagnosis (95% CI 8.94–17.07) and 6 months after MTB presentation (95% CI 3.75–8.25). There was no survival difference between the BTC subgroups (Fig. 2).
Fig. 2.
Kaplan–Meier survival curves of all patients with iCCA, eCCA, and GB after initial diagnosis (A), after MTB presentation (B) and of unresectable or metastatic patients from initial diagnosis and after MTB presentation (C, D), and of BTC patients treated with and without recommended targeted therapy after MTB presentation (E, p = 0.004). BTC biliary tract cancer, eCCA extrahepatic cholangiocarcinoma, GB gallbladder cancer, iCCA intrahepatic cholangiocarcinoma, MTB molecular tumor board
We then analyzed patients that received a treatment recommendation and compared survival of patients receiving targeted therapy (n = 14) with patients who did not receive the recommended targeted therapy (n = 56). After MTB presentation, patients who went on to receive personalised treatment had a median OS of 19 months (95% CI 4.86–33.14) compared with 8 months (95% CI 6.27–9.729) in the patients without implementation of the recommended treatment (p = 0.004; Fig. 2).
Discussion
We retrospectively analyzed a real-world cohort of patients with BTC (iCCA, eCCA, GB) discussed in the MTB. Compared with epidemiological data in Europe from the ENSCCA registry [1], our BTC patient cohort presented in the MTB was younger with a median age of 62 versus 66 years.
CGP was performed after a median of 155.5 days after initial diagnosis across all stages. For advanced BTC patients, CGP testing was performed after a median of 80 days. However, it shows that CGP is still not fully standardized at our center, and internal recommendations to perform testing at initial diagnosis of unresectable BTC patients have not been implemented thoroughly. New guidelines may help reliably ensure CGP is performed in all BTC patients [22].
In our cohort, 71.2% of BTC patients had at least one genomic alteration detected in CGP and 35.3% of the detected alterations were deemed actionable. As expected, the two most common mutations across all subgroups were TP53 and KRAS. Among the therapeutically targetable alterations, IDH1, FGFR2-fusion, ARID1A, PIK3CA, and BRAF V600E were most common in iCCA, while ERBB2 and ARID1A were most common in in eCCA and GB. Those findings are consistent with previously reported data for BTC [25–28]. However, while MSI-high and TMBhigh (> 10 Mut/Mb) have been previously reported with a prevalence of approximately 1% in BTC patients, this was not seen in our MTB cohort (0 MSI cases, 1 TMBhigh case), most likely owing to the comparatively small sample size [26, 28].
Of all BTC cases discussed in the MTB, 46.4% received a targeted treatment recommendation. In line with previous reports from our group, many recommendations do not translate into clinical management [23]. Reasons behind this finding are ample. In our cohort, ten patients (17.5%) were still receiving standard treatment at the time of MTB and 15 patients (26.3%) had passed away before a switch to targeted treatment was possible. Other reasons (40%) for not implementing the suggested treatment were medical reasons (e.g., worsening health condition), different choice of treatment from the primary oncologist, further treatment externally, or missing data in patients that were lost to follow-up. Ultimately, 14 of the 71 (19.7%) treatment recommendations were put into practice, leaving 58 (80.3%) BTC patients with possible targets without a (documented) molecularly guided treatment.
The low implementation rate raises questions about the timing of CGP testing and MTB discussion and whether patients are adequately followed-up after MTB presentation. Transfer rate into clinical practice is known to be challenging, with missing clinical indication, rapid worsening of general condition due to side effects of current therapy, or progression of disease or loss to follow up being the main reasons a suggested therapy was not initiated [29]. To improve the rate of targeted treatment in BTC, awareness of CGP and MTB should be spread more widely. Guidelines for CGP testing at initial diagnosis and subsequent presentation at MTB coupled with regular follow-ups might overcome the barriers to personalized therapy [30, 31].
In the BTC patient cohort receiving molecularly guided therapy, median OS after presentation to the MTB was significantly longer than in the untreated group (19 vs 8 months; p = 0.004), suggesting a potential benefit of precision oncology in patients with advanced BTC. The survival time illustrates that there is a window of opportunity after the MTB to switch to a targeted treatment. The majority of treated actionable alterations were composed of FGFR2-fusions, and IDH1, BRAF V600E, ERBB2 mutations. Median duration of treatment was 6 months until the last follow-up with a response rate of 57% and a median PFS and duration of response (DoR) of 9 and 5.5 months, respectively. Overall survival of all patients with unresectable or metastatic BTC did not differ between the subtypes. Since comparison of PFS between targeted treatments is difficult, the PFS ratio (PFS2/PFS1, see definition above) has proven to be a useful tool to evaluate clinical success in the individual patient. A PFS ratio > 1.3 is associated with a clinical benefit [32, 33]. Here, we show a median PFS ratio of 1.54, therefore reiterating the potential clinical benefit for a targeted treatment.
Limitations to this study are its retrospective nature and analysis from only one cancer center. Due to the small sample size of patients receiving the recommended treatment, interpretation and analysis of the data must be perceived with caution. All patients undergoing CGP are presented to the MTB and this population is more likely to include younger and fitter patients as compared with the true BTC population, leading to a survival bias. Another significant limitation of this study is missing clinical data, such as tumor burden, performance status, number of metastatic lesions, or liver function values. Due to the retrospective data collection, clinical data often were not available for analysis.
In cases where treatment recommendations were followed, targeted treatment was well tolerated and patients showed clinical benefit. Early CGP testing in BTC and MTB presentation are crucial to ensure that the patients receive optimal treatment. Subsequently, a more consistent translation and follow-up of the MTB recommendations should be employed, but prospectively controlled trials are urgently needed to prove this approach is beneficial. Further, getting approval for off-label treatment from insurance companies can be challenging and requires time and energy. Therefore, CGP and treatment of advanced BTC should be centralized to institutions with experience in treating rare cancer with molecularly guided therapies.
Conclusion
Our study presents a real-world analysis of BTC patients discussed at the weekly CCCMunichLMU molecular tumor board, mirroring the shift towards CGP testing in BTC and molecularly guided therapy. At our center, CGP is already widely implemented in BTC and discussed regularly in the MTB. Several patients with actionable alterations have already profited from targeted treatment. Naturally, we intend to increase the number of patients that are offered a matched therapy.
With rapid advances and daily updates not only in cholangiocarcinoma, but in the entire field of precision oncology, a qualified translation of the large amount of CGP data into the clinical setting can only be accomplished through guidance by an expert panel. The MTB is the best solution to identify potential targeted therapies or clinical studies for patients who often have exhausted the standard therapeutic options [34]. Especially for BTC patients with unresectable or metastatic disease, CGP should be performed upfront at first diagnosis to ensure all patients can be offered the best treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
DZ: travel finance and speaker’s honoraria from AMGEN, AstraZeneca, Roche; KD: travel finance and speaker’s honoraria from AMGEN, AstraZeneca, Servier, GSK; SB: speaker’s honorarium or advisory boards for AstraZeneca, BMS, Incyte, Janssen-Cilag, MSD, Servier; research funding: Celgene; PG: advisory board AstraZeneca; DR: consulting fees from Bayer and Ipsen and lecture fee from Ipsen. CBW: honoraria from Amgen, Bayer, BMS, Chugai, Celgene, Falk, GSK, MSD, Merck, Janssen, Ipsen, Roche, Servier, SIRTeX, and Taiho; served on advisory boards for Bayer, BMS, Celgene, Janssen, MSD, Servier, Shire/Baxalta, Rafael Pharmaceuticals, RedHill, and Roche; has received travel support from Bayer, Celgene, Janssen, RedHill, Roche, Servier, and Taiho and research grants (institutional) from Roche. CBW serves as an officer for the European Society of Medical Oncology, Deutsche Krebshilfe, and Arbeitsgemeinschaft internistische Onkologie, and is a member of the European Union Commission expert group: Mission Board for cancer. Authors BR, KH, LW, MB, MH, JD, MS, SC, KN, JA, AM, FK, JW, JM, FZ, GB, EG, WK, SO, JK, MB, AM, MR declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
This retrospective chart review study involving human participants was in accordance with the principles of the Declaration of Helsinki and its later amendments. The study was approved by the local ethics committee of the Ludwig-Maximilians-University Munich (approval number: 21-0869).
Consent to participate, consent to publish
Not applicable.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by DZ, KD, and CBW. The first draft of the manuscript was written by DZ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- 1.Izquierdo-Sanchez L, Lamarca A, la Casta A, Buettner S, Utpatel K, Klümpen HJ, et al. Cholangiocarcinoma landscape in Europe: diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J Hepatol. 2022;76:1109–1121. doi: 10.1016/j.jhep.2021.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397(10272):428–444. doi: 10.1016/S0140-6736(21)00153-7. [DOI] [PubMed] [Google Scholar]
- 3.Tomczak A, Springfeld C, Dill MT, Chang DH, Kazdal D, Wagner U, et al. Precision oncology for intrahepatic cholangiocarcinoma in clinical practice. Br J Cancer. 2022;127(9):1701–1708. doi: 10.1038/s41416-022-01932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott AJ, Sharman R, Shroff RT. Precision medicine in biliary tract cancer. J Clin Oncol. 2022;40(24):2716–2734. doi: 10.1200/JCO.21.02576. [DOI] [PubMed] [Google Scholar]
- 5.Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol. 2021;7(11):1669–1677. doi: 10.1001/jamaoncol.2021.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pemazyre | European Medicines Agency [Internet]. [cited 2022 Oct 19]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/pemazyre.
- 7.Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, et al. FOENIX-CCA2: a phase II, open-label, multicenter study of futibatinib in patients (pts) with intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 gene fusions or other rearrangements. J Clin Oncol. 2020;38(15_suppl):108. doi: 10.1200/JCO.2020.38.15_suppl.108. [DOI] [Google Scholar]
- 9.FDA grants accelerated approval to futibatinib for cholangiocarcinoma | FDA [Internet]. [cited 2022 Oct 31]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-futibatinib-cholangiocarcinoma.
- 10.Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N Engl J Med. 2023;388(3):228–239. doi: 10.1056/NEJMoa2206834. [DOI] [PubMed] [Google Scholar]
- 11.Lytgobi: Pending EC decision | European Medicines Agency [Internet]. [cited 2023 Jul 8]. Available from: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/lytgobi.
- 12.Ju JY, Dibbern ME, Mahadevan MS, Fan J, Kunk PR, Stelow EB. Mismatch repair protein deficiency/microsatellite instability is rare in cholangiocarcinomas and associated with distinctive morphologies. Am J Clin Pathol. 2020;153(5):598–604. doi: 10.1093/ajcp/aqz199. [DOI] [PubMed] [Google Scholar]
- 13.Toshida K, Itoh S, Yoshizumi T, Shimagaki T, Wang H, Kurihara T, et al. Efficacy of pembrolizumab in microsatellite instability-high locally advanced cholangiocarcinoma: a case report. Clin J Gastroenterol. 2021;14(5):1459–1463. doi: 10.1007/s12328-021-01458-8. [DOI] [PubMed] [Google Scholar]
- 14.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Javle M, Borad MJ, Azad NS, Kurzrock R, Abou-Alfa GK, George B, et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021;22(9):1290–1300. doi: 10.1016/S1470-2045(21)00336-3. [DOI] [PubMed] [Google Scholar]
- 16.Harding JJ, Cleary JM, Quinn DI, Braña I, Moreno V, Borad MJ, et al. Targeting HER2 (ERBB2) mutation-positive advanced biliary tract cancers with neratinib: Results from the phase II SUMMIT ‘basket’ trial. J Clin Oncol. 2021;39(3_suppl):320. doi: 10.1200/JCO.2021.39.3_suppl.320. [DOI] [Google Scholar]
- 17.Subbiah V, Lassen U, Élez E, Italiano A, Curigliano G, Javle M, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21(9):1234–1243. doi: 10.1016/S1470-2045(20)30321-1. [DOI] [PubMed] [Google Scholar]
- 18.FDA grants accelerated approval to dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation | FDA [Internet]. [cited 2022 Oct 31]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid.
- 19.Harding JJ, Fan J, Oh DY, Choi HJ, Kim JW, Chang HM, et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol. 2023;24(7):772–782. doi: 10.1016/S1470-2045(23)00242-5. [DOI] [PubMed] [Google Scholar]
- 20.Ohba A, Morizane C, Kawamoto Y, Komatsu Y, Ueno M, Kobayashi S, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): an investigator-initiated multicenter phase 2 study (HERB trial) J Clin Oncol. 2022;40(16_suppl):4006. doi: 10.1200/JCO.2022.40.16_suppl.4006. [DOI] [Google Scholar]
- 21.Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31(11):1491–1505. doi: 10.1016/j.annonc.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;34(2):127–140. doi: 10.1016/j.annonc.2022.10.506. [DOI] [PubMed] [Google Scholar]
- 23.Heinrich K, Miller-Phillips L, Ziemann F, Hasselmann K, Rühlmann K, Flach M, et al. Lessons learned: the first consecutive 1000 patients of the CCCMunichLMU Molecular Tumor Board. J Cancer Res Clin Oncol. 2022:1–11. [DOI] [PMC free article] [PubMed]
- 24.Verdaguer H, Saurí T, Acosta DA, Guardiola M, Sierra A, Hernando J, et al. ESMO scale for clinical actionability of molecular targets driving targeted treatment in patients with cholangiocarcinoma. Clin Cancer Res. 2022;28(8):1662–1671. doi: 10.1158/1078-0432.CCR-21-2384. [DOI] [PubMed] [Google Scholar]
- 25.Bekaii-Saab TS, Bridgewater J, Normanno N. Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann Oncol. 2021;32(9):1111–1126. doi: 10.1016/j.annonc.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Jain A, Javle M. Molecular profiling of biliary tract cancer: a target rich disease. J Gastrointest Oncol. 2016;7(5):797–803. doi: 10.21037/jgo.2016.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res. 2018;24(17):4154–4161. doi: 10.1158/1078-0432.CCR-18-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silverman IM, Hollebecque A, Friboulet L, Owens S, Newton RC, Zhen H, et al. Clinicogenomic analysis of FGFR2-rearranged cholangiocarcinoma identifies correlates of response and mechanisms of resistance to pemigatinib. Cancer Discov. 2021;11(2):326–339. doi: 10.1158/2159-8290.CD-20-0766. [DOI] [PubMed] [Google Scholar]
- 29.Larson KL, Huang B, Weiss HL, Hull P, Westgate PM, Miller RW, et al. Clinical outcomes of Molecular Tumor Boards: a systematic review. JCO Precis Oncol. 2021;5:PO.20.00495. doi: 10.1200/PO.20.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subbiah V, Kurzrock R. Challenging standard-of-care paradigms in the precision oncology era. Trends Cancer. 2018;4(2):101–109. doi: 10.1016/j.trecan.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tannock IF, Hickman JA. Molecular screening to select therapy for advanced cancer? Ann Oncol. 2019;30(5):661–663. doi: 10.1093/annonc/mdz088. [DOI] [PubMed] [Google Scholar]
- 32.Mock A, Heilig CE, Kreutzfeldt S, Huebschmann D, Heining C, Schröck E, et al. Community-driven development of a modified progression-free survival ratio for precision oncology. ESMO Open. 2019;4:583. doi: 10.1136/esmoopen-2019-000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.There are no bad anticancer agents, only bad clinical trial designs--twenty-first Richard and Hinda Rosenthal Foundation Award Lecture. | Clinical Cancer Research | American Association for Cancer Research [Internet]. [cited 2023 Apr 23]. Available from: https://aacrjournals.org/clincancerres/article/4/5/1079/7593/There-are-no-bad-anticancer-agents-only-bad. [PubMed]
- 34.Luchini C, Lawlor RT, Milella M, Scarpa A. Molecular tumor boards in clinical practice. Trends Cancer. 2020;6(9):738–744. doi: 10.1016/j.trecan.2020.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).