Abstract
Skin biopsies of 36 patients with erythema migrans and acrodermatitis chronica atrophicans (ACA) before therapy and those of 8 patients after therapy were examined for Borrelia burgdorferi DNA by PCR. Skin biopsies of 27 patients with dermatological diseases other than Lyme borreliosis and those of 10 healthy persons were examined as controls. Two different primer sets targeting 23S rRNA (PCR I) and 66-kDa protein (PCR II) genes were used. PCR was performed with freshly frozen tissue (FFT) and paraffin-embedded tissue (PET). For FFT specimens of erythema migrans, 73% were positive by PCR I, 79% were positive by PCR II, and 88% were positive by combining PCR I and II. For PET specimens, PCR was less sensitive (PCR I, 44%; PCR II, 52%). For FFT specimens of ACA, PCR I was positive for two of five patients and PCR II was positive for four of five patients. B. burgdorferi was cultured from 79% of the erythema migrans specimens but not from any of the ACA lesions. Elevated B. burgdorferi antibodies were detected in sera of 74% of erythema migrans patients and 100% of ACA patients. All urine samples were negative by PCR II, whereas PCR I was positive for 27%. However, hybridization of these amplicons was negative. Sequencing of three amplicons identified nonborrelial DNA. In conclusion, urine PCR is not suitable for the diagnosis of skin borreliosis. A combination of two different primer sets achieves high sensitivity with skin biopsies. In early erythema migrans infection, culture and PCR are more sensitive than serology.
Laboratory diagnosis of Lyme borreliosis (LB), especially in the early stage of infection, is still unreliable. Routine diagnosis is done by serological detection of Borrelia burgdorferi-specific antibodies, but the sensitivity of this method during early infection is low and antibody concentrations decrease only slowly after therapy (1, 16, 17). Cultivation of B. burgdorferi from body fluids is slow and inefficient, indicating a need for new diagnostic methods. Since PCR has proved to be a sensitive and fast method for the diagnosis of microorganisms that are difficult to culture, this new technology has been applied to LB in recent years (11, 15, 20, 22, 25, 44). Under experimental conditions, the sensitivity of PCR is extremely high, and DNA of a single gene copy can be detected. However, application with clinical specimens has presented problems with inconsistent results (27, 30–32, 34). The type of specimen, tissue or body fluid, best suited for optimal detection of DNA is not known. There is no agreement on the selection of primers. The genetic variations of B. burgdorferi subspecies make the construction of primer sets that can detect all pathogenic subspecies even more difficult (3, 5, 53).
In contrast to skin biopsies, urine is easy to collect. Recently, promising results were published about the detection of borrelial DNA in the urine of patients with neuroborreliosis and Lyme arthritis (2, 6, 34, 42). However, the diagnostic value of urine PCR in early infection is still unclear, and published results are controversial (18, 19, 22, 23).
The purpose of this study was to determine the diagnostic value of PCR with skin biopsies and urine samples from patients with skin borreliosis.
MATERIALS AND METHODS
Patients.
All patients were examined in the Dermatological Department of the Technical University of Munich, Munich, Germany. We examined 31 patients with early-stage skin borreliosis and 5 patients with late-stage skin borreliosis before therapy, 8 patients after antibiotic treatment (5 patients were treated with ceftriaxone [2 g/day intravenously], and 3 patients were treated with doxycycline [200 mg/day orally]), 27 patients with dermatological diseases other than LB, and 10 healthy control patients. Skin biopsies from patients and healthy controls were obtained between 1992 and 1995. Urine samples from 32 patients with skin borreliosis, from 8 patients with dermatological diseases other than LB, and from 8 healthy controls were collected at the same time as the biopsy and serum samples.
Patients with early-stage skin borreliosis were divided into two subgroups based on the duration of the skin lesion. A total of 15 patients had lesions lasting less than 4 weeks, referred to as erythema migrans (EM), and 2 of these patients had multiple erythemata migrantia (MEM). The two MEM patients were biopsied twice, yielding a total of 17 freshly frozen EM biopsies examined. A total of 16 patients had lesions lasting longer than 4 weeks, referred to as erythema chronicum migrans (ECM). Diagnosis of skin borreliosis was based on five criteria as follows: (i) history of a tick bite, (ii) typical skin lesions diagnosed by experienced dermatologists (H.H. and H.B.), (iii) histological examination with perivascular lymphohistiocytic infiltration, (iv) serological detection of elevated B. burgdorferi immunoglobulin M (IgM) and/or IgG antibodies by enzyme-linked immunosorbent assay (ELISA) and confirmed by Western blotting and (v) cultivation of B. burgdorferi. Patients presenting with a typical clinical picture and meeting one other criterion were included in the study. In the EM group, 10 of 15 (67%) patients could recall a tick bite and 10 of 15 (67%) patients had elevated B. burgdorferi antibodies. In the ECM group, 5 of 16 (31%) patients could recall a tick bite and 13 of 16 (81%) patients had elevated B. burgdorferi antibodies. The late-stage skin borreliosis group consisted of five patients with acrodermatitis chronica atrophicans (ACA). All were seropositive for B. burgdorferi-specific IgG antibodies. One patient with ACA had a reinfection, leading to ECM. The diagnosis of ACA was histologically confirmed by typical dense perivascular lymphocytic infiltrates with moderate-to-rich admixture of plasma cells, degeneration of collagen, and reduction and fragmentation of elastic fibers. The 27 patients with dermatological diseases other than LB had the following diagnoses: tick bite reaction or granuloma, lymphocytic infiltration, cellulitis, erythema nodosum, hypodermitis and skin changes due to chronic venous insufficiency, vasculitis, purpura, and pityriasis rosea.
Serology.
Sera were examined for B. burgdorferi IgM and IgG antibodies by indirect flagellum ELISA (Lyme borreliosis ELISA kit; Dako, Glostrup, Denmark) and μ-capture-ELISA (IDEIA Borrelia burgdorferi IgM; Dako Ltd., Ely, United Kingdom) according to the manufacturer’s instructions. Positive and borderline results were confirmed by Western blotting (Marblot; Mar Dx/Viramed, Munich, Germany). Interpretative criteria were used as suggested by the manufacturer.
Skin biopsies.
Six-millimeter punch biopsies were taken from the margin of the skin lesion and divided into three parts. One part was formalin fixed and paraffin embedded (PET) for histological examination. The second part was inoculated immediately into modified Barbour-Stoenner-Kelly-H (BSK-H) medium (Sigma, Deisenhofen, Germany) and incubated at 32°C for up to 6 weeks (33). Cultures were examined weekly by dark-field microscopy. The third part of the biopsy was immediately frozen (freshly frozen tissue [FFT]) and stored at −70°C until PCR was performed.
Deparaffination of PET.
From each paraffin block, 10 slices (15 μm each) were cut by microtome. The blade was shifted after each block to prevent cross-contamination between the samples. Deparaffination was performed by a modified method as described previously (55).
DNA preparation of skin biopsies.
FFT as well as deparaffinated specimens was suspended in 100 to 500 μl of lysis buffer (50 mM Tris, 1 mM EDTA, 0.5% Tween 20 [pH 8.5]) containing a final concentration of 1 mg of proteinase K (Boehringer, Mannheim, Germany) per ml. After overnight incubation at 55°C, the samples were boiled at 95°C for 10 min for inactivation of proteases. Samples were centrifuged for 20 min at 13,600 × g. The supernatant was used immediately as the PCR template or stored at −20°C for later use.
DNA preparation from urine samples.
Urine samples (20 ml each) were centrifuged at 3,800 × g for 15 min, and the sediment was resuspended in 2 ml of supernatant and stored at −20°C. DNA extraction from urine was performed with glassmilk (Geneclean II kit; BIO 101, Inc., Dianova, Hamburg, Germany) according to the manufacturer’s instructions. Eluted DNA was used as the template for PCR or stored at −20°C.
PCR.
B. burgdorferi DNA was identified by using two primer sets targeting one of the two different chromosomal sequences of the 23S rRNA and the 66-kDa protein genes.
(i) 23S rRNA gene PCR (PCR I).
Primers JS1 (5′-AGA AGT GCT GGA GTC GA-3′) and JS2 (5′-TAG TGC TCT ACC TCT ATT AA-3′) were directed against the chromosomal 23S rRNA gene, producing a 259-bp amplicon that was confirmed with probe FS1 (5′-AGT CTG TTT AAA AAG GCA-3′) by Southern blotting and hybridization (44).
The reaction mixture (total volume, 25 μl) for PCR with skin biopsies contained 2.5 μl of sample template, 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 2.5 mM MgCl2, 200 μM (each) deoxynucleoside triphosphate (Pharmacia Biotech), 7.5 pmol of each primer, and 0.75 U of Taq DNA polymerase (Gibco BRL). The amplification reaction was carried out for 42 cycles in a DNA thermal cycler (Perkin-Elmer), with an amplification profile of denaturation at 94°C for 40 s, annealing at 43°C for 30 s, and extension at 72°C for 30 s. The cycles were preceded by a 5-min denaturation at 94°C and followed by a 7-min extension at 72°C. Each reaction mixture was overlaid with mineral oil.
PCR with urine samples was performed according to the method described by Maiwald et al. (23, 24). The reaction mixture (total volume, 50 μl) contained 10 μl of urine DNA template, 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 2.5 mM MgCl2, 200 μM (each) deoxynucleoside triphosphate, 25 pmol of each primer, and 1.5 U of Taq DNA polymerase. Forty-five cycles were carried out with an amplification profile of denaturation at 95°C for 45 s, annealing, and extension at 50°C for 105 s. The cycles were preceded by a 2-min denaturation at 95°C and followed by a 2-min extension at 72°C.
The efficacy of the urine DNA preparation method was evaluated by extraction and amplification of experimentally spiked urine and water (1 pg of extracted borrelial DNA/μl). Amplification results were positive.
Amplified products were analyzed by agarose gel electrophoresis (2.5% NuSieve [3:1]; FMC Bioproducts), stained with ethidium bromide in a final concentration of 0.5 μg/ml, UV illuminated, and photodocumented.
The PCR I products were analyzed by Southern blotting and hybridization as described previously (40), with nylon membranes (Boehringer Mannheim). DNA fragments were cross-linked by UV irradiation (Stratagene, Heidelberg, Germany). Hybridization was done at final concentrations of 2 pmol/ml with the digoxigenin-labeled probe FS1 and the oligonucleotide tailing kit (Biochemica Boehringer Mannheim) at 45°C for 4 h. Hybridized amplicons were detected colorimetrically with the digoxigenin nucleic acid detection kit (Biochemica Boehringer Mannheim) according to the manufacturer’s instructions.
(ii) Chromosomal 66-kDa protein gene PCR (PCR II).
The same samples as those that were used for PCR I were analyzed with a second primer set directed against the chromosomal gene coding for a 66-kDa protein, originally described by Rosa and Schwan (39), characterized by Probert et al. (35), and modified by Wienecke et al. (50). Amplification was designed as a nested PCR. Primers Bb-1 (5′-AAA ACG AAG ATA CTC GAT CTG TAA TTG C-3′) and Bb-2 (5′-TTG CAG AAT TTG ATA AAG TTG G-3′) produce a 171-bp outer amplicon, and primers Bb-3 (5′-TAA TAC GAC TCA CTA TAG GGA GAT CTG TAA TTG CAG AAA CAC CT-3′) and Bb-4 (5′-GAG TAT GCT ATT GAT GAA TTA TTG-3′) produce a 92-bp inner amplicon. PCR conditions were in accordance with those described by Wienecke et al. (50). The outer PCR contained a reaction mixture (total volume, 25 μl) of 5 μl of urine template, 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 2.5 mM MgCl2, 200 μM (each) deoxynucleoside triphosphate mixture (Pharmacia Biotech), 12.5 pmol (each) of primer Bb-1 and Bb-2, and 0.5 U of Taq DNA polymerase (Gibco BRL). Thirty cycles were carried out with an amplification profile of denaturation at 95°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 30 s. The cycles were preceded by a 5-min denaturation at 95°C. Subsequently, 0.5 μl of the product was used in a second PCR with primers Bb-3 and Bb-4 under the conditions described above. The amplification profile was identical to that described above except for annealing at 53°C and was followed by a 7-min extension at 72°C. PCR products were separated by agarose gel electrophoresis.
Sequence analysis.
Amplification products of PCR I of two skin and three urine PCR samples were sequenced for identification, according to the dideoxy method (41).
Sensitivity and specificity of PCR and contamination control.
In each PCR, negative controls containing mastermix and sterile water instead of DNA template were included. Random water controls were included in PCR I. In the nested PCR, water controls were strictly alternated with patient samples. In addition, a positive control consisting of extracted borrelial DNA was included in each PCR run. Sensitivity was determined by serial dilutions. All three subspecies, strain ACA 1 (B. afzelii), strain ZQ 1 (B. garinii), and strain B31 (B. burgdorferi sensu stricto), were detectable with a sensitivity of 2 fg with both primer systems. Sensitivity was not impaired by abundant human DNA in concentrations of 300 ng. DNA of closely related borreliae, such as B. hermsii, B. parkeri, and B. turicatae, could not be amplified. Skin and urine specimens from healthy controls were also negative. The sensitivities and specificities of the two primer sets were comparable under experimental conditions. Sequence analysis of PCR I products from skin samples showed 100% identity with the published sequence of the B. burgdorferi 23S rRNA gene.
Inhibition.
In order to exclude inhibition, all skin digests were screened for human DNA by amplification with a primer set targeting the human β2-microglobulin gene, β2-m2 (5′-ATG ATG CTG CTT ACA TGT CTC GAT-3′), and β3-m3 (5′-CAT GTG ACT TTG TCA CAG CCC AAG-3′), producing a 677-bp amplicon (14). The reaction mixture was composed of the ingredients listed above for PCR I. The annealing temperature was 49°C. If the PCR was inhibited, DNA was extracted with phenol-chloroform-isoamyl alcohol (25:24:1) to remove Taq polymerase inhibitors (47). DNA amplification was not possible because of inhibition in 9 of 131 skin samples which could not be eliminated in spite of repeated DNA extraction. However, these samples were included in the calculations of sensitivity.
Statistical analysis.
Sensitivity and specificity were calculated. McNemar’s χ2 test was applied to calculate the significance of the difference in sensitivities.
RESULTS
Amplification of DNA from skin samples.
We examined 38 skin biopsies from 36 patients with clinically defined skin lesions of early-stage (n = 33) or late-stage (n = 5) skin borreliosis. Paired FFT and PET samples from the same punch biopsy were available from 25 patients with EM or ECM and from 5 patients with ACA. Only FFT was available from eight patients. In the early-stage skin borreliosis group, 33 FFT specimens (17 EM and 16 ECM) were investigated. Two of these specimens were inhibited. The sensitivity of PCR I was 72.7% (24 of 33 specimens). The sensitivity of PCR II was 78.8% (26 of 33). With a combination of PCR I and II, B. burgdorferi DNA could be detected in 87.9% (29 of 33) of the specimens with at least one primer set. The concordance of both primer sets was 75.8% (25 of 33). The sensitivity for the EM group was 82.4% (14 of 17) by PCR I and PCR II. With at least one primer set, B. burgdorferi DNA could be detected in 94.1% of the samples (16 of 17). The sensitivity for the ECM group was 62.5% (10 of 16) by PCR I and 75% (12 of 16) by PCR II. B. burgdorferi DNA could be detected in 81.3% of the samples (13 of 16) with at least one primer set (Table 1 and Fig. 1). In the early-stage skin borreliosis group, 25 PET specimens (12 EM and 13 ECM) were examined. One ECM specimen was inhibited. The sensitivity of PCR I was 44% (11 of 25). The sensitivity of PCR II was 52% (13 of 25). In 56% (14 of 25), B. burgdorferi DNA was detected with at least one primer set. The concordance of both primer sets was 84% (21 of 25). The sensitivity for the EM group was 50% (6 of 12) by PCR I and 58.3% (7 of 12) by PCR II. In 66.6% (8 of 12), B. burgdorferi DNA was detected with at least one primer set. The sensitivity for the ECM group was 38.5% (5 of 13) by PCR I and 46.2% (6 of 13) by PCR II. In 46.2% (6 of 13), B. burgdorferi DNA was detected with at least one primer set.
TABLE 1.
Clinical characteristics and laboratory data for 36 patients with early and late skin borreliosisa
| Patient no. (gender and age) | Tick bite | Diagnosis (localization)b | Result by:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serology
|
Culture | PCR
|
|||||||||
| ELISA
|
Western
blotting
|
PET
|
FFT
|
||||||||
| IgM | IgG | IgM | IgG | PCR I | PCR II | PCR I | PCR II | ||||
| 1 (M, 46) | + | EM (back of knee) | − | − | ND | ND | ND | − | − | + | + |
| 2 (F, 75) | + | EM (back of knee) | − | − | ND | ND | + | + | + | + | + |
| 3 (M, 57) | − | EM (back of knee) | − | + | (+) | + | ND | − | − | + | + |
| 4 a (M, 41)c | + | MEM (forearm) | + | − | + | + | + | NA | NA | + | + |
| 4 b | MEM (hip) | + | NA | NA | + | + | |||||
| 5 (M, 56) | + | EM (elbow) | − | − | ND | ND | + | + | + | + | + |
| 6 (F, 51) | + | EM (mamma) | − | − | ND | ND | + | + | + | + | + |
| 7 (M, 28) | − | EM (back of knee) | + | + | + | + | ND | − | − | − | + |
| 8 (F, 27) | − | EM (back of knee) | + | − | (+) | − | ND | − | + | + | + |
| 9 (M, 51) | + | EM (thigh) | (+) | − | + | + | ND | + | + | + | − |
| 10 (M, 47) | + | EM (heel) | + | + | + | (+) | + | NA | NA | + | − |
| 11 (F, 57) | − | EM (flank) | + | − | + | + | − | + | − | + | + |
| 12 (F, 47) | + | EM (foot) | + | − | − | − | ND | + | + | − | + |
| 13 (M, 54) | − | EM (back of knee) | − | − | ND | ND | − | − | − | + | + |
| 14 a (M, 31)c | + | MEM (upper arm) | + | + | + | (+) | + | NA | NA | + | + |
| 14 b | MEM (thigh) | + | NA | NA | + | + | |||||
| 15 (M, 41) | − | EM (back knee) | + | − | + | − | + | − | + | I | I |
| 16 (F, 60) | − | ECM (thigh) | + | (+) | + | − | + | I | I | + | − |
| 17 (F, 54) | − | ECM (ear) | + | − | + | + | ND | − | − | + | + |
| 18 (F, 38) | − | ECM (thigh) | + | + | (+) | (+) | ND | − | − | − | + |
| 19 (F, 41) | − | ECM (lower leg) | − | + | (+) | + | ND | − | − | − | − |
| 20 (M, 72) | − | ECM (abdomen) | + | − | + | + | ND | − | − | − | + |
| 21 (M, 52) | − | ECM (back) | (+) | + | + | + | + | − | − | − | + |
| 22 (F, 66) | + | ECM (shoulder) | + | + | (+) | (+) | + | NA | NA | + | + |
| 23 (F, 44) | − | ECM (thigh) | + | + | + | (+) | ND | − | + | + | + |
| 24 (F, 63) | + | ECM (breast) | − | + | − | + | ND | − | − | − | − |
| 25 (M, 38) | − | ECM (abdomen) | + | + | (+) | (+) | + | + | + | + | + |
| 26 (M, 57) | + | ECM (thigh) | − | − | ND | ND | + | + | + | + | + |
| 27 (M, 58) | − | ECM (thigh) | + | + | + | (+) | + | + | + | + | + |
| 28 (F, 55) | + | ECM (abdomen) | + | − | + | − | ND | + | + | + | + |
| 29 (M, 26) | − | ECM (thigh) | − | + | − | − | − | NA | NA | + | + |
| 30 (M, 64) | − | ECM (back) | − | − | ND | ND | ND | + | + | I | I |
| 31 (F, 56) | + | ECM | − | − | ND | ND | − | NA | NA | + | + |
| 32 (M, 55) | − | ACA (lower leg) | − | + | + | + | ND | − | − | + | + |
| 33 (F, 75) | − | ACA (hand) | (+) | + | + | + | − | − | − | − | + |
| 34 (M, 52) | − | ACA (hand) | (+) | + | + | + | − | + | + | + | + |
| 35 (F, 43) | − | ACA (lower leg) | + | + | − | (+) | ND | − | − | − | + |
| 36 (M, 76) | + | ACA (lower leg) | − | + | − | + | ND | − | − | − | − |
+, positive result; (+), borderline-positive reaction; −, negative result; NA, not available; ND, not done; I, inhibition.
Clinically and histologically confirmed diagnosis.
Two biopsies were done for patient no. 4 and 14.
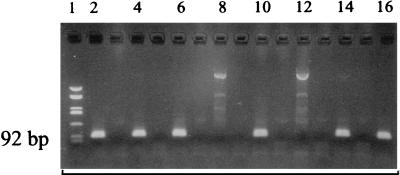
FIG. 1.
Gel electrophoresis of amplification products with the nested 66-kDa protein gene primer set (PCR II) in skin samples. Lanes: 1, molecular standard, pUC HaeIII digest (587, 458, 434, 298, 267, 257, 174, 102, 80, 18, and 11); 2, positive control, 500 fg of ACA-1 DNA; 4, PET from patient no. 2 (EM); 6, FFT from patient no. 2; 8, sample from patient with other dermatological disease; 10, FFT from patient no. 37 (ACA); 12, therapy control; 14, PET from patient no. 12 (EM); 16, PET from patient no. 9 (EM). Negative controls are shown in unlabeled lanes 3, 5, 7, 9, 11, 13, and 15.
In conclusion, there is no statistically significant difference in the sensitivities of PCR I and PCR II. However, a higher test sensitivity was achieved by using both primer sets. Overall sensitivity for the EM group was higher than that for the ECM group. The amplification sensitivity for PET is lower than that for FFT. In the late-stage skin borreliosis group, five paired PET and FFT specimens were examined. PCR I detected B. burgdorferi DNA in one PET and two FFT samples, whereas the PCR II detected B. burgdorferi DNA in one PET and four FFT specimens (Fig. 2). All biopsies taken after antibiotic treatment were negative for B. burgdorferi DNA. No borrelial DNA was detectable in 27 specimens of patients with dermatological diseases other than LB. The specificity of PCR I and PCR II was 100% for these control groups.
FIG. 2.
Results of DNA amplification by PCR I and PCR II from PET (□) and FFT (■) in skin biopsies of early- and late-stage skin borreliosis.
Comparison of PCR I and PCR II.
Discordant results were observed for the two different primer sets. With FFT, discordant results occurred in 26.3% (10 of 38) of all skin borreliosis specimens, in 24.2% (8 of 33) of early-stage skin borreliosis specimens, and in 40% (2 of 5) of late-stage skin borreliosis specimens. With PET, discordant results occurred in 16% (4 of 25) of early-stage skin borreliosis specimens.
DNA amplification from urine.
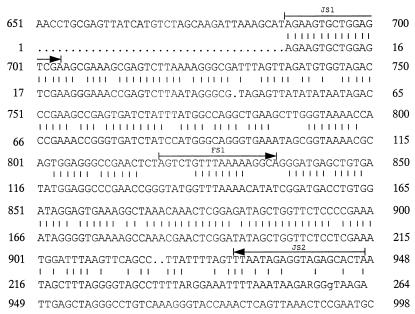
Urine specimens from 40 patients (26 with EM or ECM, 2 with ACA, 4 after therapy, and 8 with dermatological diseases other than LB) and from 8 healthy controls were examined. In 13 of 48 (27%) urine specimens, PCR I yielded a clear and intense amplicon at the same 259-bp level as the positive control amplicon in the gel. The signals were observed for all patient groups regardless of the diagnosis (7 with EM or ECM, 1 with ACA, 1 after therapy, 3 with dermatological diseases other than LB, and 1 healthy control) (Table 2 and Fig. 3). Amplification of urine samples by PCR II yielded only negative results. In order to determine the identity of the PCR I products, three samples showing distinct amplicons at 259 bp in the gel were directly sequenced. Sequencing results yielded a DNA sequence of a microorganism showing 73% identity with the 23S rRNA gene sequence of B. burgdorferi (Fig. 4). Forward primer JS1 was identical to the newly found sequence, thus explaining the strong signal in the gel. Reverse primer JS2 was only 50% homologous. Probe FS1 had only 44% homology with the central base pairs, thus explaining the negative hybridization results. The obtained sequence was most likely of bacterial origin, but the organism could not be identified with the available sequence data banks, including a bank for bacterial 23S rRNA genes. The sequence had 77% homology with Rickettsia prowazeckii and Haemophilus influenzae, which are phylogenetically distant from B. burgdorferi.
TABLE 2.
False-positive amplification results from urine samples by PCR I which could not be confirmed by hybridization or PCR II
| Diagnosis | No. of samples
positive/no. tested by:
|
||
|---|---|---|---|
| PCR I
|
PCR II | ||
| Amplicon | Hybridization | ||
| EM or ECM | 7/26 | 0/7 | 0/26 |
| ACA | 1/2 | 0/1 | 0/2 |
| Therapy controls | 1/4 | 0/1 | 0/4 |
| ODDa | 3/8 | 0/3 | 0/8 |
| Healthy controls | 1/8 | 0/1 | 0/8 |
| Total | 13/48 | 0/13 | 0/48 |
ODD, other dermatological diseases.
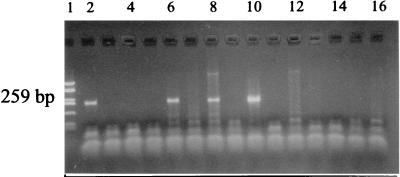
FIG. 3.
Gel electrophoresis of amplification products from urine specimens with the 23S rRNA primer set (PCR I). Lanes: 1, molecular standard, pUC HaeIII digest (587, 458, 434, 298, 267, 257, 174, 102, 80, 18, and 11); 2, positive control, 500 fg of ACA-1 DNA; 3, negative control; 4, specimen from patient no. 17 (EM); 5, specimen from patient no. 1 (EM); 6, specimen from patient no. 12 (EM); 7, specimen from patient no. 19 (ECM); 8, specimen from no. 13 (EM); 9, specimen from patient no. 15 (EM); 10, specimen from patient with other dermatological disease; 11, negative control; 12, specimen from patient no. 21 (ECM); 13, specimen from patient no. 3 (EM); 14, specimen from patient with dermatological disease other than LB; 15, specimen from patient no. 28 (ECM); 16, specimen from patient no. 38 (ACA).
FIG. 4.
Sequence of the 259-bp amplicon of urine specimens from amplification with the 23S rRNA primer set (PCR I). The upper DNA sequence is a portion of the B. burgdorferi 23S rRNA gene. The sequence shown is nucleotides 651 through 998 of the mature 23S rRNA sequence. The lower line, numbered 1 through 268, presents the sequence of the urine PCR I amplicon. Vertical bars indicate base pair homology with the borrelial gene. The sequences employed as forward (JS1) and reverse (JS2) primers and probe (FS1) are indicated by arrows.
Comparison of culture and PCR.
Skin biopsies of 21 patients (11 with EM, 8 with ECM, and 2 with ACA) were cultivated in modified BSK-H medium. B. burgdorferi could be isolated from 82% (9 of 11) of the EM lesions and 75% (6 of 8) of the ECM lesions. For early-stage skin borreliosis, 79% (15 of 19) of the cultures were positive. All culture-positive specimens were positive by PCR. In addition, B. burgdorferi DNA was also detected in FFT samples of four culture-negative biopsies. The two cultures from late-stage skin borreliosis samples were negative, but DNA amplification of FFT yielded positive results by PCR II. Cultures of the therapy control group (n = 7) and the exclusion group (n = 19) were negative, as were PCR results.
Comparison of serology and PCR.
Elevated B. burgdorferi-specific antibodies could be detected in 66.6% (10 of 15) of EM and 81.3% (13 of 16) of ECM patients. For early-stage skin borreliosis, the sensitivity of serologic antibody detection was 74.2% (23 of 31). PCR detection was superior, with a sensitivity of 87.9% (29 of 33). In this study, five EM and two ECM patients did not have elevated B. burgdorferi-specific antibodies, but PCR amplification of DNA from the skin biopsies was positive and clinical diagnosis could be confirmed. On the other hand, PCR was negative for two specimens from ECM patients with positive serological results. All patients with ACA had high concentrations of IgG antibodies. The sensitivity of the PCR was 80% (4 of 5 patients).
DISCUSSION
We demonstrated that PCR is a very sensitive and specific method for detection of B. burgdorferi in FFT specimens from patients with early-stage skin borreliosis. By PCR I and PCR II combined, B. burgdorferi DNA could be detected in 88% of the early-stage skin borreliosis biopsies. This result is in the range of previously published sensitivities of between 60 and 90% (25, 27–29, 36, 43, 44, 48, 51, 52). Despite the excellent sensitivity of PCR under experimental conditions, borrelial DNA cannot be detected by PCR in some patients with proven skin borreliosis. Taq polymerase was inhibited in two FFT samples, but borrelial DNA could be amplified in these cases when PET samples were used. In addition, borrelial DNA was not detected in PET and FFT samples from two patients with ECM, although the polymerase reaction was not inhibited in these samples. The two applied primer sets were able to detect the three known B. burgdorferi subspecies as shown with strains B31, ZQ 1, and ACA 1. It is possible that these patients were infected by strains which were not detected by either PCR I or PCR II, e.g., different subtypes of B. afzelii which are predominant in Europe (5, 45, 49). It is more likely that detection of borrelial DNA failed because either concentrations of B. burgdorferi were below the detection limit or the biopsies did not contain B. burgdorferi. The DNA detection rate decreased from 94% for EM to 81% for ECM, indicating that sensitivity decreases with the increasing duration of the infection.
The concordance between primer sets PCR I and PCR II was 72% (26 of 36) for all skin borreliosis samples. In 26% (10 of 38) samples, B. burgdorferi DNA could be detected by only one primer set. This concordance is higher than that recently reported in a study investigating synovial fluid, cerebrospinal fluid, and urine of LB patients with later stages of infection, with a concordance of only 17% with two primer sets targeting a plasmid and a chromosomal gene (34). In addition, a poor concordance of 24% for two different primer sets both targeting the plasmid OspA gene was observed for cerebrospinal fluid samples from 60 patients with neuroborreliosis. The interlaboratory reproducibility was only 26% in that study (30). The most probable explanation for the inconsistency of results is that with patients’ samples each primer system detected the target DNA of B. burgdorferi subspecies with different sensitivities despite comparable experimental sensitivities (32). This interpretation is supported by the finding that the level of concordance increases with the concentration of B. burgdorferi used to infect mice (27), suggesting that the DNA concentration is important in this respect.
To our knowledge, a comparison between paired PET and FFT samples from skin biopsies has not been published previously. Although PET and FFT samples were derived from the same punch biopsy, the sensitivity for PET with 50% (15 of 30 specimens) was much lower than that for FFT (87% [33 of 38 specimens]). This difference, which is statistically significant (P of <0.04), might be explained by the different quantities of tissue employed. The digest of FFT contained one-third of a 6-mm punch biopsy, whereas the digest of PET contained only 10 slices (15 μm) of a third of the punch biopsy. In addition, PET undergoes many processing steps, which may affect DNA to such an extent that amplification is impossible. Storage time also has a certain impact on DNA quality (12, 13, 37). Seven of eight PET samples stored less than 1 year gave positive amplification results, but only 12 of 25 stored longer than 1 year did. Therefore, we cannot recommend the use of PET for routine diagnosis of skin borreliosis. Nevertheless, PET can be valuable for resolving special questions in retrospective studies or as a substitute when FFT is not available or is inhibited.
In different studies, the rates of detection of borrelial DNA by PCR have ranged from 11 to 100% for urine samples from patients with neuroborreliosis (2, 6, 18, 19, 22, 34), from 38 to 60% for patients with Lyme arthritis (19, 34), and from 44 to 49% for patients with active LB (11, 23). We were not able to confirm the presence of borrelial DNA in the urine of patients with early-stage localized or late-stage skin borreliosis. Several possible reasons for this discrepant result were excluded. Insufficient DNA extraction from urine was ruled out, since B. burgdorferi DNA could be detected in experimentally spiked urine. Furthermore, amplification of β2-microglobulin DNA yielded positive results for urine DNA extracts, indicating that DNA was sufficiently extracted from urine samples. PCR I, which was performed as published by Maiwald et al. (23), produced a strong amplicon of 259 bp in 27% of the urine samples in our study. These amplicons were not detected by a specific hybridization probe in Southern blotting or confirmed by PCR II. Three amplicons of PCR I were sequenced. The sequence was only 73% identical to the B. burgdorferi target DNA of the 23S rRNA gene. The new sequence is most likely from a not-yet-identified organism present in urine which is not recorded in the available sequence data banks. Schmidt et al. (42), who investigated patients with early-stage skin borreliosis, reported a sensitivity of 92% for urine samples. These amplicons were neither hybridized nor sequenced.
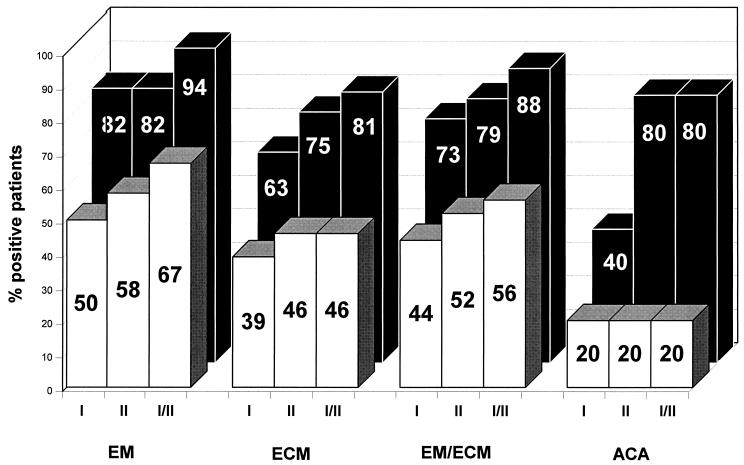
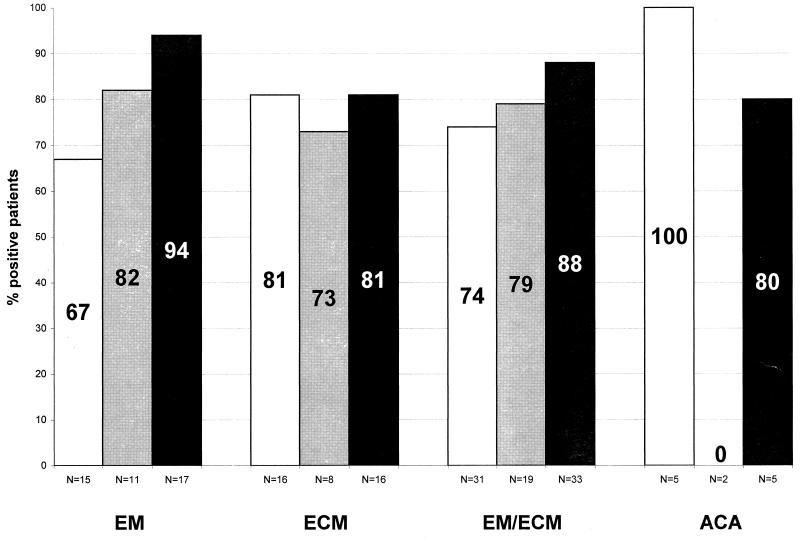
The purpose of the present study was to compare the diagnostic value of PCR with that of culture and serology (Fig. 5). At present, serology remains the main diagnostic tool for laboratory diagnosis of LB. However, in the early stage of localized skin borreliosis, only 50 to 80% of patients present with elevated B. burgdorferi-specific antibodies, depending on the duration of the infection (1, 8–10, 16, 17, 54). In this study, borrelial antibodies were elevated in 74% of the serum samples from patients with early-stage skin borreliosis. Borrelial DNA could be amplified from the skin of 88% of these patients. B. burgdorferi-specific DNA could be detected by PCR in eight specimens of patients with EM who were seronegative. This result shows that PCR is especially advantageous during the diagnostic gap of serology from the onset of infection to the development of IgM antibodies. PCR cannot replace serology because serum is much easier to obtain than a skin biopsy, but PCR can contribute to the reliability of diagnosis during early stages of infection. PCR is also a valuable tool for treatment control, as shown by eight patients with EM whose PCR results were negative after antibiotic treatment. Similar conclusions have been published by Muellegger et al. (28, 29).
FIG. 5.
Sensitivity of serology (□), culture ( ), and PCR (■) in skin borreliosis. EM/ECM, early-stage skin borreliosis; N, number of patients.
The sensitivity of PCR with two primer sets (88%) was comparable to that of the culture (79%) in early skin borreliosis (Fig. 5). Published data on the sensitivity of B. burgdorferi culture from biopsies of EM vary considerably, from 6 to 100% (4, 21, 26, 27, 44–46). Although the isolation of B. burgdorferi has become much easier during recent years, 1 to 6 weeks is required to cultivate B. burgdorferi. In contrast to culture, PCR can detect nonvital organisms; once the protocol has been established, it is a fast and easy method. Borrelial DNA can be detected by PCR in contaminated cultures in which B. burgdorferi might be overgrown by other bacteria or fungi.
In conclusion, PCR with skin biopsies is a useful tool for the diagnosis of early-stage skin borreliosis. It is fast, sensitive, and specific. In the early stage of LB, PCR might be superior to serology and culture, but during the course of infection, the sensitivity of PCR decreases and the sensitivity of serology improves. PCR of urine samples cannot be recommended for routine diagnosis of skin borreliosis.
ACKNOWLEDGMENTS
We thank Franz Hofmann for essential support and discussion, Martin Biel (Institut für Pharmakologie und Toxikologie, Technische Universität München, Munich, Germany) for sequencing the B. burgdorferi amplicon of two skin samples, and Wolfgang Ludwig (Lehrstuhl für Mikrobiologie der Technischen Universität München) for comparing the 23S rRNA gene sequence with the data in his bacterial 23S rRNA gene bank.
REFERENCES
- 1.Asbrink E. Erythema chronicum migrans Afzelius and acrodermatitis chronica atrophicans. Early and late manifestations of Ixodes ricinus-borne Borrelia spirochetes. Acta Dermato-Venereol Suppl. 1985;118:2–63. [PubMed] [Google Scholar]
- 2.Bayer M E, Zhang L, Bayer M H. Borrelia burgdorferi DNA in the urine of treated patients with chronic Lyme disease symptoms. A PCR study of 97 cases. Infection. 1996;24:347–353. doi: 10.1007/BF01716077. [DOI] [PubMed] [Google Scholar]
- 3.Belfaiza J, Postic D, Bellenger E, Baranton G, Saint Girons I. Genomic fingerprinting of Borrelia burgdorferisensu lato by pulsed-field gel electrophoresis. J Clin Microbiol. 1993;31:2873–2877. doi: 10.1128/jcm.31.11.2873-2877.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger B W, Johnson R C, Kodner C, Coleman L. Cultivation of Borrelia burgdorferifrom erythema migrans lesions and perilesional skin. J Clin Microbiol. 1992;30:359–361. doi: 10.1128/jcm.30.2.359-361.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chetcuti M, Blot M, Meyer J. Genomic variations among Borrelia afzelii strains. Med Microbiol Lett. 1994;3:423–430. [Google Scholar]
- 6.Demaerschalck I, Messaoud A B, DeKesel M, Hoyois B, Lobert Y, Hoet P, Bigaignon G, Bollen A, Godfroid E. Simultaneous presence of different Borrelia burgdorferigenospecies in biological fluids of Lyme disease patients. J Clin Microbiol. 1995;33:602–608. doi: 10.1128/jcm.33.3.602-608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duray, P. H. 1989. Clinical pathologic correlations of Lyme disease. Rev. Infect. Dis. 11(Suppl. 6):S1487–S1493. [DOI] [PubMed]
- 8.Engstrom S M, Shoop E, Johnson R C. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahrer H, van der Linden S M, Sauvain M J, Gern L, Zhioua E, Aeschlimann A. The prevalence and incidence of clinical and asymptomatic Lyme borreliosis in a population at risk. J Infect Dis. 1991;163:305–310. doi: 10.1093/infdis/163.2.305. [DOI] [PubMed] [Google Scholar]
- 10.Goodman J L, Bradley J F, Ross A E, Goellner P, Lagus A, Vitale B, Berger B W, Luger S, Johnson R C. Bloodstream invasion in early Lyme disease: results from a prospective, controlled, blinded study using the polymerase chain reaction. Am J Med. 1995;99:6–12. doi: 10.1016/s0002-9343(99)80097-7. [DOI] [PubMed] [Google Scholar]
- 11.Goodman J L, Jurkovich P, Kramber J M, Johnson R C. Molecular detection of persistent Borrelia burgdorferiin the urine of patients with active Lyme disease. Infect Immun. 1991;59:269–278. doi: 10.1128/iai.59.1.269-278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greer C E, Lund J K, Manos M M. PCR amplification from paraffin-embedded tissues: recommendations on fixatives for long-term storage and prospective studies. PCR Methods Appl. 1991;1:46–50. doi: 10.1101/gr.1.1.46. [DOI] [PubMed] [Google Scholar]
- 13.Greer C E, Peterson S L, Kiviat N B, Manos M M. PCR amplification from paraffin-embedded tissues. Anat Pathol. 1991;95:117–124. doi: 10.1093/ajcp/95.2.117. [DOI] [PubMed] [Google Scholar]
- 14.Güssow D, Rein R, Ginjaar I, Höchstenbach F, Seeman G, Kottmann A, Ploegh H L. The human β2-microglobulin gene—primary structure and definition of the transcriptional unit. J Immun. 1987;139:3132–3138. [PubMed] [Google Scholar]
- 15.Guy E C, Stanek G. Detection of Borrelia burgdorferi in patients with Lyme diseases by polymerase chain reaction. J Clin Pathol. 1991;44:610–611. doi: 10.1136/jcp.44.7.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann H. Die Borrelia burgdorferi-Infektion der Haut. Untersuchungen zum Krankheitsspektrum, zur Labordiagnostik und zur Verbreitung der Diagnostik im Saarland. Habilitation. Hamburg, Germany: Medizinische Fakultät der Universität des Saarlandes; 1991. [Google Scholar]
- 17.Hofmann H. Lyme borreliosis—problems of serological diagnosis. Infection. 1996;24:470–472. doi: 10.1007/BF01713052. [DOI] [PubMed] [Google Scholar]
- 18.Huppertz H I, Schmidt H, Karch H. Detection of Borrelia burgdorferi by nested polymerase chain reaction in cerebrospinal fluid and urine of children with neuroborreliosis. Eur J Pediatr. 1993;152:414–417. doi: 10.1007/BF01955900. [DOI] [PubMed] [Google Scholar]
- 19.Karch H, Huppertz H-I, Böhme M, Schmidt H, Wiebecke D, Schwarzkopf A. Demonstration of Borrelia burgdorferi DNA in urine samples from healthy humans whose sera contain B. burgdorferi-specific antibodies. J Clin Microbiol. 1994;32:2312–2314. doi: 10.1128/jcm.32.9.2312-2314.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller T L, Halperin J J, Whitman M. PCR detection of Borrelia burgdorferi DNA in cerebrospinal fluid of Lyme neuroborreliosis patients. Neurology. 1992;42:32–42. doi: 10.1212/wnl.42.1.32. [DOI] [PubMed] [Google Scholar]
- 21.Kuiper H, van Dam A P, Spanjaard L, de Jongh B M, Widjojokusumo A, Ramselaar T C P, Cairo I, Vos K, Dankert J. Isolation of Borrelia burgdorferifrom biopsy specimens taken from healthy-looking skin of patients with Lyme borreliosis. J Clin Microbiol. 1994;32:715–720. doi: 10.1128/jcm.32.3.715-720.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebech A-M, Hansen K. Detection of Borrelia burgdorferiDNA in urine samples and cerebrospinal fluid samples from patients with early and late Lyme neuroborreliosis by polymerase chain reaction. J Clin Microbiol. 1992;30:1646–1653. doi: 10.1128/jcm.30.7.1646-1653.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maiwald M, Stockinger C, Hassler D, von Knebel Doeberitz M, Sonntag H G. Evaluation of the detection of Borrelia burgdorferi DNA in urine samples by polymerase chain reaction. Infection. 1995;23:173–179. doi: 10.1007/BF01793860. [DOI] [PubMed] [Google Scholar]
- 24.Maiwald M, Stonkinger C, Schill M, Sonntag H G. Pro-benvorbereitungsmethoden für den Nachweis von DNA in Urinproben. Bioforum. 1994;17:232–237. [Google Scholar]
- 25.Melchers W, Meis J, Rosa P, Claas E, Nohlmans L, Koopman R, Horrevorts A, Galama J. Amplification of Borrelia burgdorferiDNA in skin biopsies from patients with Lyme disease. J Clin Microbiol. 1991;29:2401–2406. doi: 10.1128/jcm.29.11.2401-2406.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell P D, Reed K D, Vandermause M F, Melski J W. Isolation of Borrelia burgdorferifrom skin biopsy specimens of patients with erythema migrans. J Clin Pathol. 1993;99:104–107. doi: 10.1093/ajcp/99.1.104. [DOI] [PubMed] [Google Scholar]
- 27.Moter S E, Hofmann H, Wallich R, Simon M M, Kramer M D. Detection of Borrelia burgdorferi sensu lato in lesional skin of patients with erythema migrans and acrodermatitis chronica atrophicans by ospA-specific PCR. J Clin Microbiol. 1994;32:2980–2988. doi: 10.1128/jcm.32.12.2980-2988.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muellegger R R, Zoechling N, Schluepen E M, Soyer H P, Hoedl S, Kerl H, Volkenandt M. Polymerase chain reaction control of antibiotic treatment in dermatoborreliosis. Infection. 1996;24:76–79. doi: 10.1007/BF01780664. [DOI] [PubMed] [Google Scholar]
- 29.Muellegger R R, Zoechling N, Soyer H P, Hoedl S, Wienecke R, Volkenandt M, Kerl H. No detection of Borrelia burgdorferi-specific DNA in erythema migrans lesions after minocycline treatment. Arch Dermatol. 1995;131:678–682. [PubMed] [Google Scholar]
- 30.Nocton J J, Bradley J B, Rutledge B J, Persing D H, Logigian E L, Schmid C H, Steere A C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in cerebrospinal fluid in Lyme neuroborreliosis. J Infect Dis. 1996;174:623–627. doi: 10.1093/infdis/174.3.623. [DOI] [PubMed] [Google Scholar]
- 31.Nocton J J, Dressler F, Rutledge B J, Ryes P N, Persing D H, Steere A C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330:229–234. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 32.Persing D H, Rutledge B J, Rys P N, Podzorski D S, Mitchell P D, Reed K D, Liu B, Fikrig E, Malawista S E. Target imbalance: disparitiy of Borrelia burgdorferi genetic material in synovial fluid from Lyme arthritis patients. J Infect Dis. 1994;169:668–672. doi: 10.1093/infdis/169.3.668. [DOI] [PubMed] [Google Scholar]
- 33.Pollok R J, Telford III S R, Spielman A. Standardization of medium for culturing Lyme disease spirochetes. J Clin Microbiol. 1993;31:1251–1255. doi: 10.1128/jcm.31.5.1251-1255.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Priem S, Rittig M G, Kamradt T, Burmester G R, Krause A. An optimized PCR leads to rapid and highly sensitive detection of Borrelia burgdorferiin patients with Lyme borreliosis. J Clin Microbiol. 1997;35:685–690. doi: 10.1128/jcm.35.3.685-690.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Probert W S, Allsup K M, Lefebvre R B. Identification and characterization of a surface-exposed, 66-kilodalton protein from Borrelia burgdorferi. Infect Immune. 1995;63:1933–1939. doi: 10.1128/iai.63.5.1933-1939.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranki A, Aavik E, Peterson P, Schauman K, Nurmilaakso P. Successful amplification of DNA specific for Finnish Borrelia burgdorferi in erythema chronicum migrans but not in circumscribed scleroderma lesions. J Investig Dermatol. 1994;102:339–345. doi: 10.1111/1523-1747.ep12371793. [DOI] [PubMed] [Google Scholar]
- 37.Rogers B B, Alpert L C, Hine E A S, Buffone G J. Analysis of DNA in fresh and fixed tissue by the polymerase chain reaction. Am J Pathol. 1990;136:541–548. [PMC free article] [PubMed] [Google Scholar]
- 38.Rosa P A, Hogna D, Schwan T G. Polymerase chain reaction analyses identify two distinct classes of Borrelia burgdorferi. J Clin Microbiol. 1991;29:524–532. doi: 10.1128/jcm.29.3.524-532.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosa P A, Schwan T G. A specific and sensitive assay for the Lyme disease spirochete Borrelia burgdorferi using the polymerase chain reaction. J Infect Dis. 1989;160:1018–1029. doi: 10.1093/infdis/160.6.1018. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 9.34–9.45. [Google Scholar]
- 41.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt B, Muellegger R R, Stockenhuber C, Soyer H P, Hoedl S, Luger A, Kerl H. Detection of Borrelia burgdorferi-specific DNA in urine specimens from patients with erythema migrans before and after antibiotic therapy. J Clin Microbiol. 1996;34:1359–1363. doi: 10.1128/jcm.34.6.1359-1363.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz I, Bittker S, Bowen S L, Cooper D, Pavia C, Wormser G P. Polymerase chain reaction amplification of culture supernatants for rapid detection of Borrelia burgdorferi. Eur J Clin Microbiol Infect Dis. 1993;12:879–882. doi: 10.1007/BF02000415. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz I, Wormser G P, Schwartz J J, Cooper D, Weissensee P, Gazumyan A, Zimmermann E, Goldberg N S, Bittker S, Campbell G L, Pavia C S. Diagnosis of early Lyme disease by polymerase chain reaction amplification and culture of skin biopsies from erythema migrans lesions. J Clin Microbiol. 1992;30:3082–3088. doi: 10.1128/jcm.30.12.3082-3088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strle F, Cheng Y, Cimperman J, Maraspin V, Lotric-Furlan S, Nelson J A, Picken M M, Ruzic-Sabljic E, Picken R N. Persistence of Borrelia burgdorferi sensu lato in resolved erythema migrans lesions. Clin Infect Dis. 1995;21:380–389. doi: 10.1093/clinids/21.2.380. [DOI] [PubMed] [Google Scholar]
- 46.Van Dam A P, Kuiper H, Vos K, Widjojokusuko A, de Jongh B M, Spanjaard L, Ramselaar A C P, Kramer K D, Dankert J. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]
- 47.Volkenandt M, Dicker A P, Fanin R, Banerjee D, Albino A, Bertino J. Polymerase chain reaction analysis of DNA from paraffin-embedded tissue. In: White B A, editor. PCR—selected protocols and applications—1993. Totowa, N.J: Humana Press, Inc.; 1993. pp. 81–88. [DOI] [PubMed] [Google Scholar]
- 48.Von Stedingk L V, Olsson I, Hanson H S, Asbrink E, Hovmark A. Polymerase chain reaction for detection of Borrelia burgdorferi in skin lesion of early and late Lyme borreliosis. Eur J Clin Microbiol Infect Dis. 1995;14:1–5. doi: 10.1007/BF02112610. [DOI] [PubMed] [Google Scholar]
- 49.Wallich R, Helmes C, Schaible U E, Lobet Y, Moter S E, Kramer M D, Simon M K. Evaluation of genetic divergence among Borrelia burgdorferi isolates by use of OspA, fla, HSP60, and HSP70 gene probes. Infect Immun. 1992;60:4856–4866. doi: 10.1128/iai.60.11.4856-4866.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wienecke R, Neubert U, Volkenandt M. Molecular detection of Borrelia burgdorferi in formalin-fixed, parraffin-embedded lesions of Lyme disease. J Cutan Pathol. 1993;20:385–388. doi: 10.1111/j.1600-0560.1993.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 51.Wienecke R, Schlüpen E M, Zöchling N, Neubert U, Meurer M, Volkenandt M. No evidence for Borrelia burgdorferi-specific DNA in lesions of localized scleroderma. J Investig Dermatol. 1995;104:23–26. doi: 10.1111/1523-1747.ep12613456. [DOI] [PubMed] [Google Scholar]
- 52.Wienecke M, Zöchling N, Neubert U, Schlüpen E M, Meurer M, Volkenandt M. Molecular subtyping of Borrelia burgdorferi in erythema migrans and acrodermatitis chronica atrophicans. J Investig Dermatol. 1994;103:19–22. doi: 10.1111/1523-1747.ep12388947. [DOI] [PubMed] [Google Scholar]
- 53.Wilske B, Preac-Mursic V, Schierz G, Kühbeck R, Barbour A G. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]
- 54.Wilske B, Steinhuber R, Bergmeister H, Fingerle V, Schierz G, Preac-Mursic V, Vanek E, Lorbeer B. Lyme-Borreliose in Süddeutschland. Epidemiologische Daten zum Auftreten von Erkrankungsfällen sowie zur Durchseuchung von Zecken (Ixodes ricinus) mit Borrelia burgdorferi. Dtsch Med Wochenschr. 1987;112:1730–1736. doi: 10.1055/s-2008-1068320. [DOI] [PubMed] [Google Scholar]
- 55.Wright D K, Manos M M. Sample preparation from paraffin-embedded tissues. In: Innis M A, Gelfand D H, Snisky J J, White T J, editors. PCR protocols: a guide to methods and applications—1990. Berkeley, Calif: Academic Press, Inc.; 1990. pp. 153–158. [Google Scholar]