Abstract
Erythropoietin (EPO) is a hypoxia-responsive cytokine that induces neuroprotective effect in hypoxic-ischaemic, traumatic, excitotoxic and inflammatory injuries. Recently, utilizing a clinically relevant murine model of TBI and delayed hypoxemia, we have found that ongoing recombinant human EPO (rhEPO) administration influenced neurogenesis, neuroprotection, synaptic density and, behavioral outcomes early after TBI, and the impact on long-lasting outcomes 6 months after injury. We also demonstrated that the 1-month behavioral improvement was associated with mitogen-activated protein kinase (MAPK)/cAMP response element-binding protein (CREB) signaling activation and increased of excitatory synaptic density in the amygdala. However, we did not uncover which type of cells were involved in fear memory response enhancement after rhEPO treatment in the setting of TBI with delayed hypoxemia. In this report, using chemogenetic tools in our controlled cortical impact model, we were able to inactivate excitatory neurons and eliminate rhEPO-induced fear memory recall enhancement. In summary, these data demonstrate that rhEPO treatment initiated after TBI enhances contextual fear memory in the injured brain via activation of excitatory neurons in the amygdala.
Keywords: traumatic brain injury, hypoxemia, erythropoietin, designer receptors exclusively activated by designer drugs (DREADDs), fear memory
Introduction
Traumatic brain injury (TBI) affects around 1.7 million people each year in USA [18]. After the primary injury, secondary injuries, such as inflammation, hypoxia, excitotoxicity, are critical in determining the extent of injury expansion and damage to brain tissue [34]. Much of the research and studies performed in the field of TBI are focused on the secondary injury cascade in order to control neurogeneration and neuroinflammation; however, most of these studies have demonstrated limited translational success [22].
Erythropoietin (EPO) and its receptor (EPOr), expressed in neurons, astrocytes and oligodendrocytes [28], have been related with neuroprotective functions reducing apoptosis, neuroinflammation and excitotoxicity in the injured brain [5, 6, 13, 19]. Recombinant human EPO (rhEPO) administration in rodents has been associated with functional improvements of sensorimotor and spatial memory in TBI [1, 39, 40]. Recently, we have reported that rhEPO administration rescued of fear memory response 1 month after TBI with delayed hypoxemia by the mitogen-activated protein kinase (MAPK)/cAMP response element-binding protein (CREB) signaling activation in the amygdala [9]. Nevertheless, the particular cell type involved in the rhEPO’s modulation of fear memory performance after TBI is unknown.
To further understand the mechanisms by which ongoing rhEPO treatment impacted fear memory performance, we employed Designer Receptors Exclusively Activated by Designer Drugs (DREADD)-based chemogenetic tools to inhibit excitatory neurons and to activate astrocytes, also associated with fear behaviors [2, 26]. Here we present our new findings of rhEPO efficacy on neuronal activation in the setting of TBI and delayed hypoxemia.
Material and methods
Virus delivery of DREADDs and confirmation of virus expression location
All procedures were approved by the Washington University Animal Studies Committee (Protocol 19-0864) and are consistent with the National Institutes of Health guidelines for the care and use of animals. Animals were housed 5/cage and had free access to water and food with a 12-hour light/dark cycle. C57BL/6J 8 week-old male mice (Jackson Laboratory, Bar Harbor, ME) weighing 20-25 grams (g) were used. To evaluate how the ongoing rhEPO treatment impacts fear memory performance, we used DREADDs expression mediated by adeno-associated viral vectors (AAVs)-induced neuronal and astrocytes transfection [7, 30] in the basolateral amygdala (BLA). BLA glutamatergic neurons and astrocytes were transduced with an AAV8 containing a CaMKIIα [25, 36] and Gfap [2, 3, 12] promoter-driven gene that encodes the evolved human M4-muscarinic receptor fused to mCherry (hM4D(Gi)-mCherry). The hM4D(Gi) couples the Gi protein-mediated signaling to inhibit neurons and glia upon biding of clozapine-N-oxide (CNO) [7]. AAV8-CAMKII-hM4D(Gi)-mCherry and AAV8-GFAP-hM4D(Gi)-mCherry (adenovirus serotype 8, 2×1012 virus molecules per ml; Addgen, Watertown, MA) and their control viruses (AAV8-CAMKII-mCherry and AAV8-GFAP-mCherry) were used. Stereotaxic left side unilateral injections (300 nl at 100 nl min−1) were made into the BLA (anterior-posterior, −1 mm; medial-lateral, ±2.85 mm; dorsal-ventral, 5.15 mm; from bregma) 3 weeks before controlled cortical impact (CCI). The injection volume and flow rate were controlled by a syringe pump (Harvard apparatus, Holliston, MA). The needle was left in place for an additional 5 min after each injection to minimize upward flow of viral solution. The location of the virus was confirmed with immunohistochemical staining of mCherry expression after sacrifice. Animals in which mCherry was not to be primarily located in the BLA region were excluded from all analysis.
Traumatic brain injury and delayed hypoxemia
We performed CCI as previously described [8, 10, 11] three weeks after the DREADDs injection. Briefly, mice were anesthetized with 5% isoflurane at induction, followed by maintenance at 2% isoflurane for the procedure’s duration. Buprenorphine sustained release (0.5 mg/kg subcutaneously) was administered before scalp incision. The head was shaved and ear bars were used to stabilize the head within the stereotaxic frame (MyNeurolab, St. Louis, MO). Then, the craniotomy was performed over the left parietotemporal cortex using a 5-mm trephine attached to an electric drill. Then, the impactor tip was positioned over the left fronto-parietal cortex by moving it 1.5 mm anteriorly and 1.2 mm to the left from lambda. The impact was delivered at 2 mm depth (velocity 5 m/s, dwell time 100 ms). The ears bars were released immediately after the injury. All animals then received a loose fitting plastic cap secured over the craniectomy with Vetbond (3M, St. Paul, MN). The skin was closed with interrupted sutures and was treated with antibiotic ointment before the mouse was recovered from anesthesia on a warming pad. One day after surgery, animals who had undergone CCI experienced hypoxemia (8% O2, 4% CO2) for 60 min in a hypoxia chamber (Coy Laboratory, Grass Lake, MI). A mixture of N2, O2, and CO2 was utilized to maintain normocarbic hypoxemia.
Recombinant human erythropoietin treatment
Male mice were injected intraperitoneally (i.p.) with 5000 U/kg rhEPO (Amgen, Thousand Oaks, CA), the most common dose utilized in murine models [1, 9]. Mice received a total of 6 of rhEPO, with the first dose of rhEPO administrated 8 h before hypoxemia. Daily doses were given for the next 2 consecutive days, followed by weekly rhEPO injections up to 1 month. Injured animals were randomized to receive rhEPO or saline vehicle administration using computer-generated number randomization.
Clozapine-N-oxide injection
Clozapine-N-oxide (CNO) was dissolved in 0.5% of DMSO and then diluted in 0.9% saline. 3 mg/kg was injected intraperitoneal 15 min prior to contextual and cued fear test [26].
Contextual and cued fear conditioning
To study fear memory response in mice we used a fear conditioning protocol as previously describe [8, 10, 11]. Briefly, we used a 3-day fear conditioning experimental paradigm (Fig. 1). On day 1 (conditioning), mice were placed in context A for 10.5 min and received 5 tone-shock pairings (30 s tone with 0.5 mA and shock during the last 2 s). Freezing time in 30 s epochs was measured by a blinded observer. On day 2 (contextual test), mice were placed in context A for 10.5 min and freezing time was measured to assess contextual fear memory. On day 3 (cued test), mice were placed in a novel context B (checkered walls and white hard cover on the floor) to eliminate any confounding interactions of contextual fear for 10.5 min and subsequently given five 30 s tones without any shocks. On day 1, after the final tone-shock pairing, mice remained in the conditioning chamber for 30 s before being returned to their home cages. Freezing was defined as the absence of visible movement except that required for respiration.
Figure 1. Experimental design.
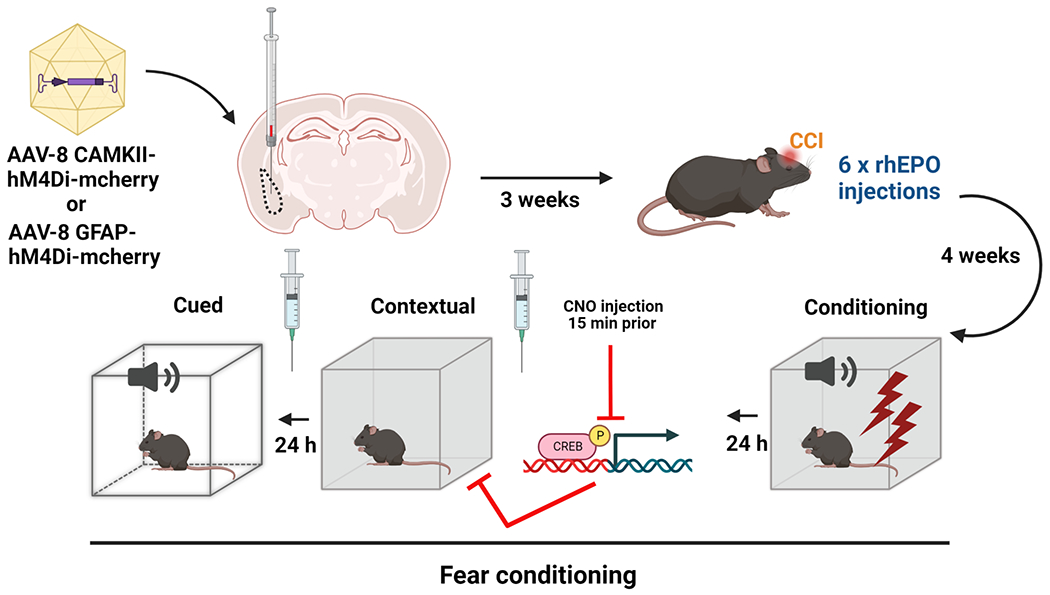
AAV8-CAMKII-hM4D(Gi)-mCherry in excitatory neurons or AAV8-GFAP-hM4D(Gi)-mCherry in astrocytes were locally injected in the BLA region three weeks before CCI. After 6 doses of rhEPO and 4 weeks post-injury, we evaluated fear memory injecting CNO (DREADDs agonist) 15 min prior to contextual and cued testing.
Tissue processing
Mice were anesthetized with isoflurane followed by transcardial perfusion with ice-cold 0.1 M heparinized phosphate-buffered saline (PBS, pH 7.4) and 4% paraformaldehyde (PFA, Sigma-Aldrich, St Louis, MO). Perfused brains were kept in 4% PFA at 4°C overnight, followed by equilibration in 30% sucrose for at least 48 h before sectioning.
Immunohistochemistry
Histological sections were examined to assess the injection site and the neuronal activation in amygdala. Brains were taken 90 min after the completion of the fear conditioning test. Fifty-μm thick serial sections were cut on a freezing microtome starting with a visibly complete corpus callosum and continuing caudally to bregma −3.08 mm. Sets of 12 cryosections spaced every 300 μm were used for immunohistochemical studies. Immunofluorescence staining was performed on free-floating sections (50-μm thick). Staining was performed on free-floating sections washed in PBS between applications of primary and secondary antibodies. Normal donkey serum (NDS, 20%) in PBS was used to block nonspecific staining for all antibodies. Slices were then incubated at 4°C overnight with the primary antibodies (Table 1). The following day, antibody binding was detected by incubating sections with Alexa fluorescence secondary antibody (Table 1) for 2 hours. Sections were mounted on glass slides in PBS, dried, and coverslipped with mounting medium for fluorescence with DAPI.
Table 1.
Overview of the primary antibodies used in the present study
| Antibody | Fluorophore | Species | Source | Product number |
|---|---|---|---|---|
| pCREB | Rabbit polyclonal | Cell Signaling | 9198 | |
| GFAP | Rabbit polyclonal | DAKO North America | Z033429-2 | |
| mCherry | Goat polyclonal | Novus Biologicals | NBP3-05558 | |
|
| ||||
| Secondary antibody | AF598 | Donkey anti-goat | Thermo Fisher | A-11055 |
| Secondary antibody | AF488 | Donkey anti-rabbit | Thermo Fisher | A-21206 |
Quantification of immunohistochemistry
Fluorescence images were taken on a Zeiss Axio Imager Z2 Fluorescence Microscope (Zeiss) with ApoTome 2 optical sectioning grid imager with 20X objective. 20 μm z stacks with 1 μm interval were obtained of the ipsilateral amygdala. The BLA of three serial dorsal amygdala slices 300 μm apart from each mouse were counted for co-localization of pCREB and AAV8-CAMKII-hM4D(Gi)-mCherry virus (Table 1). The cellular specificity of DREADDs expression was tested by immunohistochemical analysis of randomly selected areas of BLA.
Statistical analysis
All data for each animal was entered and tracked utilizing a REDCap database to maintain data integrity [20]. The histological data and behavioral data have normal distribution and repeated measures two-way ANOVA was performed. All analysis was performed with Statistica v13.3 (TIBCO software. Palo Alto, CA).
Results
Designer Receptors Exclusively Activated by Designer Drugs (DREADDs)-based chemogenetic tools to inhibit excitatory neurons and to activate astrocytes.
Previously we have been reported that rhEPO administration has been associated with improvement of fear memory in our combined injury model of TBI and delayed hypoxemia [9]. rhEPO effect was associated with MAPK signaling pathway exclusively in the amygdala [9]. However, it is unclear if MAPK signaling activation by rhEPO was specific to astrocytes, neurons, or both cell types. A growing body of literature suggests that astrocytes are involved in the modulation of neuronal activity and synaptic physiology in hippocampus-amygdala circuitry in fear memory responses [2, 26]. We next asked whether the astrocyte activation and/or glutamatergic-neuron inhibition in the amygdala could modulate the fear memory enhancement observed after rhEPO treatment. For this purpose, we delivered AAV8-CAMKII-hM4D(Gi)-mCherry in excitatory neurons and AAV8-GFAP-hM4D(Gi)-mCherry in astrocytes in the BLA region three weeks before CCI (Fig. 1). After 6 doses of rhEPO, we performed conditioning fear memory injecting CNO (DREADDs agonist) 15 min prior conditioning, contextual, and the cued text (Fig. 1). In the BLA, we observed that the hM4Di receptor was transfected in neurons and in astrocytes (Fig. 2A) with high specificity and penetrance in neurons (Fig. 2B and C). To verify that hM4Di inhibited neurons in vivo upon i.p. CNO injection (3 mg/kg), we quantified pCREB staining to evaluate neuronal activation, as previously described [37]. CNO injections dramatically decreased pCREB levels in the neurons in both CAMKII-hM4D(Gi) CCI-Veh and CCI-EPO (Fig. 2D and E). We also confirmed the transfection of the hM4Di receptor in the astrocytes of the BLA (Fig 2F) with high specificity and penetrance in astrocytes (Fig. 2G and H). The activation of the transfected astrocytes after CNO injections did not affect the neuronal pCREB activation (Fig. 2I).
Figure 2. DREADDs-based chemogenetic tools to inhibit excitatory neurons and to activate astrocytes.
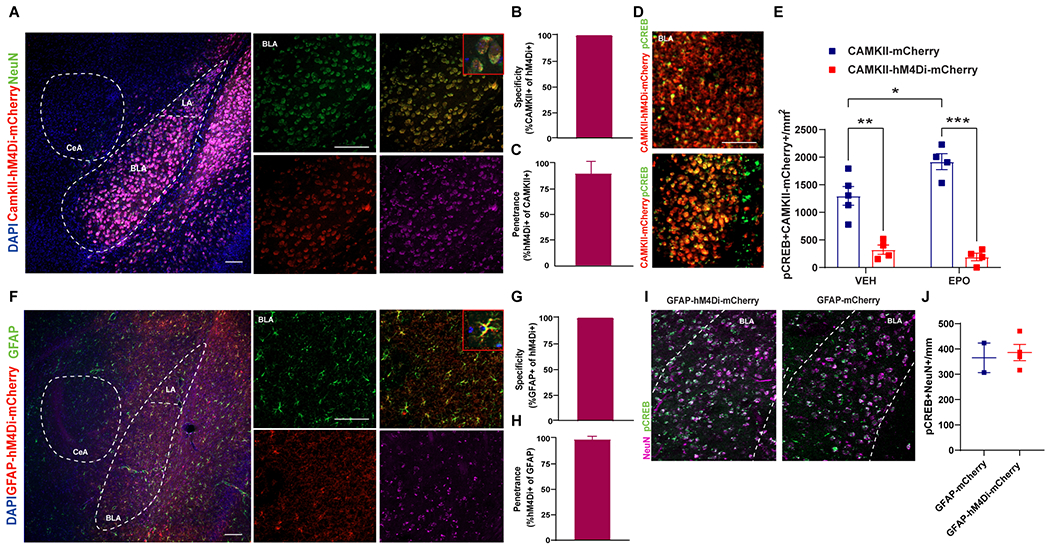
A Unilateral injection of AAV8-CAMKII-hM4D(Gi)-mCherry (red) in the BLA (3 weeks before injury) resulted in hMD4i expression in neurons (NeuN, green) with a zoomed in insert. Scale bar: 50 μm. CAMKII-hM4D(Gi)-mCherry was expressed in the (B) 100% of the neurons (662/662 neurons from 2 mice) with (C) 90% specificity (662/595 neurons from 2 mice). D Representative fluoroscopy of the BLA labeled with pCREB (green) and AAV8-CAMKII-hM4D(Gi)-mCherry (red) with a zoomed in insert. E Quantification of pCREB density in the BLA. Two-way ANOVA (DREADDs and Treatment) followed by post hoc Tukey. DREADDs F(1,13) = 103.0 p < 0.0001. Treatment F(1,13) = 3.37 p = 0.09. DREADDs*Treatment F(1,13) = 8.01 p = 0.014. *p<0.05, n=4-5 mice per group. F Unilateral injection of AAV8-GFAP-hM4D(Gi)-mCherry (red) in the BLA (3 weeks before injury) resulted in hMD4i expression in astrocytes (GFAP, green) with a zoomed in insert. Scale bar: 50 μm. GFAP-hM4D(Gi)-mCherry was expressed in the (G) 100% of the neurons (332/332 neurons from 2 mice) with (H) 98.5% specificity (332/325 neurons from 2 mice). I Representative fluoroscopy of the BLA labeled with pCREB (green) and NeuN (purple). J Quantification of pCREB positive neurons in the BLA.
rhEPO administration enhanced fear memory through neuronal activation in the amygdala
Four weeks after CCI, we performed contextual and cued-fear conditioning. No differences in behavior were observed between the GFAP-mCherry and CAMKII-mCherry control groups within rhEPO or vehicle-treated mice and these groups were combined for further analysis. In mice expressing control virus, we observed that rhEPO administration increased contextual freezing response compared with CCI-Veh (Fig. 3A). In rhEPO-treated mice, amygdala neuronal inactivation with CNO (CCI-EPO CAMKII) decreased freezing time compared with CNO-induced astrocyte activation (CCI-EPO GFAP) or control virus (CCI-EPO CONTROL, Fig. 3B). However, no differences in freezing response were observed in Veh-treated mice across the three DREADDs groups (CCI-Veh CAMKII, CCI-Veh GFAP and, CCI-Veh CONTROL, Fig. 3C). In contrast, we found no differences in cued-fear memory between the DREADDS groups in both rhEPO-treated (Fig. 3E) and untreated mice (Fig. 3F). In conclusion, hM4Di activation in excitatory amygdala neurons impaired rhEPO contextual fear memory recovery upon CNO administration.
Figure 3. rhEPO treatment enhanced contextual fear memory through excitatory neuronal activation in the amygdala.
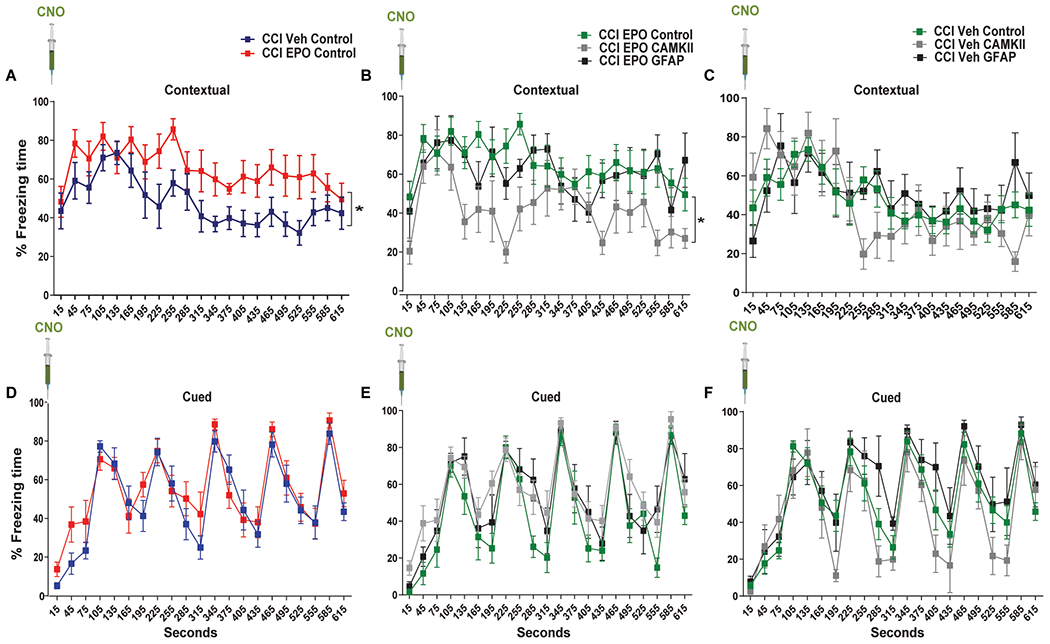
Fear conditioning quantification of percentage freezing time of (A) contextual control groups. Two-way (Treatment and Time) repeated measures ANOVA. Treatment F(1,18) = 5.09 p = 0.037. Time F(20,360) = 5.46 p < 0.0001, n = 4-5 mice per group (B) contextual CCI-EPO groups. Treatment F(2,16) = 3.87 p = 0.042. Time F(20,320) = 4.18 p < 0.0001, n = 5-9 mice per group. C contextual CCI-Veh groups. Treatment F(2,18) = 0.15 p = 0.86. Time F(20,320) = 6.81 p < 0.0001, n = 5-9 mice per group. D-F cued memory.
Discussion
Using a chemogenetic approach, we demonstrated that silencing excitatory neurons in amygdala during ongoing rhEPO treatment impaired contextual fear memory 1 month after TBI with delayed hypoxemia. These data support that chronic rhEPO administration in the setting of TBI improved fear memory performance through neuronal MAPK pathway activation in an amygdala-dependent matter.
The hippocampus-amygdala pathway have been implicated in the retrieval of contextual fear memory [32, 33]. Basal amygdala receives excitatory hippocampal inputs from contextual-responding ventral CA1 neurons that contributes to encoding fear memory for the context [23]. In our model of delayed hypoxemia after TBI, we have previously found that rhEPO treatment for 1 month influenced fear memory response through neuronal MAPK/CREB signaling activation in the amygdala [7]. Nevertheless, the mechanism what rhEPO activates hippocampal-amygdala circuit remained unclear. EPO/EPOr system is upregulated and expressed in neurons, astrocytes and oligodendrocytes upon brain injury [21, 35]. Using only CCI animals, we wanted to explore the neuronal vs astrocytes activation in the EPO effect specifically after injury by using DREADDs-based chemogenetic tools and to understand the possible mechanism which rhEPO induces fear memory enhancement. DREADDs-based chemogenetic approach can modulate excitatory neurons activity (CAMK-positive neurons) inducing alteration in behavior performance by CNO administration [41–43]. The amygdala plays an important role in the fear memory response [24]. The conditioned stimulus (e.g. tone, context) and the unconditioned stimulus (e.g. foot shock) inputs converge and associate in the BLA [24]. Then, the BLA glutamatergic neurons project directly or indirectly to the central amygdala, which is the major source of output pathways for fear responses such as freezing [17, 38]. Previously reported, we demonstrated that after rhEPO treatment induced MAPK/CREB signaling activation specifically in the amygdala associated with an increase of fear conditioning [9]. Then, we used CaMKII-driven Gi-DREADDs to inactivate excitatory neurons in the BLA in order to confirm whether rhEPO-induced fear memory enhancement is dependent on amygdala neuronal activation. We observed contextual-fear memory impairment in CCI-Veh CONTROL compare with CCI-EPO CONTROL. In the present study, silencing excitatory neurons in the amygdala decreased contextual-fear memory in EPO-DREADDs injured mice compared with EPO-CONTROL injured mice. However, we did not observe cued-fear memory impairments when we inhibited neuronal response. While contextual learning involves inputs from the hippocampus, others have reported that this specific input is not necessary for cued-fear learning [14]. After CCI, the hippocampus is highly affected by the injury with increased neuronal loss and inflammation [15, 29] that can produce deficits explicitly in contextual fear memory [9]. This finding was not reproduced in Veh-DREADDS injured mice providing evidence for rhEPO-induced neuronal activation in the amygdala as a mechanism for fear memory enhancement.
Previous studies have revealed that astrocytes are actively involved in neuronal activation and that they are necessary for plasticity and memory [2]. Unlike in neurons where Gq-DREADDs activations leads to cellular activation and Gi-DREADDs activation leads to cellular inhibition, astrocyte cellular activation can occur via either Gq- or Gi-DREADDs [16]. Gi-DREADDs activation of astrocytes in hippocampus has been shown to enhance memory associated with conditioned place preference test [27]. Surprisingly, Gq-DREADDs activation of astrocytes in amygdala induced fear memory impairment [26]. Based on these findings, we employed Gi-DREADDs-driven astrocytes activation in amygdala to induce fear memory impairment to neutralize rhEPO-induced fear memory enhancement. rhEPO fear-memory improvements were not altered by CNO Gi-DREADDs-driven astrocyte activation. Future investigation will have to address whether hippocampal neuronal inactivation may influence amygdala-dependent fear memory. The role of rhEPO administration in inhibitory neurons and/or synapsis and its impacts on fear memory are unknown. Future studies will be required to characterize the influence of rhEPO on the balance between excitation and inhibition that may regulate the plasticity of this neural network [4].
Epidemiologic studies have provided evidence of sex-dependent differences following TBI. A growing body of clinical evidence show that sex may be an important factor conferring risk from TBI, defining the underlying mechanisms using animal models remains an important goal [31]. We did not use female mice in this report to test sex-dependent differences after TBI in fear memory. However, these studies suggest that future investigations of rhEPO administration should include the interaction of age and sex on the candidate therapeutic evaluation.
Conclusions
In conclusion, our data demonstrate for first time that chemogenetic silencing of neurons in the amygdala after TBI with delayed hypoxemia impairs rhEPO-induced contextual-fear memory enhancement. This data provides an insight that injured ventral hippocampus might decrease the network excitability towards the amygdala after TBI that is restored upon rhEPO treatment. Studies on characterizing the ongoing rhEPO impact on inhibition and/or excitation balance in the brain will be essential to understanding the mechanism underlying how rhEPO modulates neuronal activation within hippocampus-amygdala circuitry associated with behavioral changes after injury. Targeting EPO/EPOr in neurons in the hippocampus-amygdala circuitry may yield promising treatment approaches in TBI.
Funding Statement
This work was supported by the National Institutes of Health (R01NS097721). Fluorescent imaging was performed on a Zeiss Axio Imager Z2 Fluorescence Microscope with ApoTome 2 optical sectioning grid imager through the use of Washington University Center for Cellular Imaging (WUCCI) supported by Washington University School of Medicine, The Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (CDI-CORE-2015-505 and CDI-CORE-2019-813) and the Foundation for Barnes-Jewish Hospital (3770 and 4642).
Abbreviations:
- TBI
traumatic brain injury
- EPO
erythropoietin
- rEPO
erythropoietin receptor
- CCI
controlled cortical impact
- MAPK/CREB
mitogen-activated protein kinase/cAMP response element-binding protein
- BLA
basolateral amygdala
- DREADDs
designer receptors exclusively activated by designer drugs
- CNO
Clozapine-N-oxide
Footnotes
Declaration of Interest: None
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
References
- [1].Adamcio B, Sargin D, Stradomska A, Medrihan L, Gertler C, Theis F, Zhang M, Muller M, Hassouna I, Hannke K, Sperling S, Radyushkin K, El-Kordi A, Schulze L, Ronnenberg A, Wolf F, Brose N, Rhee JS, Zhang W, Ehrenreich H, Erythropoietin enhances hippocampal long-term potentiation and memory, BMC Biol 6 (2008) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, Refaeli R, Horn H, Regev L, Groysman M, London M, Goshen I, Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement, Cell 174 (2018) 59–71 e14. [DOI] [PubMed] [Google Scholar]
- [3].Allen NJ, Star Power: Astrocytes Regulate Behavior, Cell 177 (2019) 1091–1093. [DOI] [PubMed] [Google Scholar]
- [4].Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK, Removing brakes on adult brain plasticity: from molecular to behavioral interventions, J Neurosci 30 (2010) 14964–14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brines M, Cerami A, Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response, J Intern Med 264 (2008) 405–432. [DOI] [PubMed] [Google Scholar]
- [6].Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A, Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury, Proc Natl Acad Sci U S A 97 (2000) 10526–10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Campbell EJ, Marchant NJ, The use of chemogenetics in behavioural neuroscience: receptor variants, targeting approaches and caveats, Brit J Pharmacol 175 (2018) 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Celorrio M, Abellanas MA, Rhodes J, Goodwin V, Moritz J, Vadivelu S, Wang L, Rodgers R, Xiao S, Anabayan I, Payne C, Perry AM, Baldridge MT, Aymerich MS, Steed A, Friess SH, Gut microbial dysbiosis after traumatic brain injury modulates the immune response and impairs neurogenesis, Acta Neuropathol Commun 9 (2021) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Celorrio M, Rhodes J, Shumilov K, Moritz J, Xiao S, Anabayan I, Sauerbeck A, Kummer T, Friess S, Recombinant human erythropoietin induces neuroprotection, activates MAPK/CREB pathway, and rescues fear memory after traumatic brain injury with delayed hypoxemia in mice, Brain Res 1795 (2022) 148074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Celorrio M, Rhodes J, Vadivelu S, Davies M, Friess SH, N-acetylcysteine reduces brain injury after delayed hypoxemia following traumatic brain injury, Exp Neurol 335 (2021) 113507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Celorrio M, Shumilov K, Payne C, Vadivelu S, Friess SH, Acute minocycline administration reduces brain injury and improves long-term functional outcomes after delayed hypoxemia following traumatic brain injury, Acta neuropathologica communications 10 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen NY, Sugihara H, Kim J, Fu ZY, Barak B, Sur M, Feng GP, Han WP, Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding, Elife 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cherian L, Goodman JC, Robertson C, Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats, J Pharmacol Exp Ther 322 (2007) 789–794. [DOI] [PubMed] [Google Scholar]
- [14].Curzon P, Rustay NR, Browman KE, Cued and Contextual Fear Conditioning for Rodents. In: Buccafusco JJ (Ed.), Methods of Behavior Analysis in Neuroscience, Boca Raton (FL), 2009. [PubMed] [Google Scholar]
- [15].Davies M, Jacobs A, Brody DL, Friess SH, Delayed Hypoxemia after Traumatic Brain Injury Exacerbates Long-Term Behavioral Deficits, J Neurotrauma 35 (2018) 790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Durkee CA, Covelo A, Lines J, Kofuji P, Aguilar J, Araque A, Gi/o protein-coupled receptors inhibit neurons but activate astrocytes and stimulate gliotransmission, Glia 67 (2019) 1076–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Duvarci S, Pare D, Amygdala Microcircuits Controlling Learned Fear, Neuron 82 (2014) 966–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gardner AJ, Zafonte R, Neuroepidemiology of traumatic brain injury, Handb Clin Neurol 138 (2016) 207–223. [DOI] [PubMed] [Google Scholar]
- [19].Grasso G, Sfacteria A, Meli F, Fodale V, Buemi M, Iacopino DG, Neuroprotection by erythropoietin administration after experimental traumatic brain injury, Brain Res 1182 (2007) 99–105. [DOI] [PubMed] [Google Scholar]
- [20].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform 42 (2009) 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hassouna I, Ott C, Wustefeld L, Offen N, Neher RA, Mitkovski M, Winkler D, Sperling S, Fries L, Goebbels S, Vreja IC, Hagemeyer N, Dittrich M, Rossetti MF, Krohnert K, Hannke K, Boretius S, Zeug A, Hoschen C, Dandekar T, Dere E, Neher E, Rizzoli SO, Nave KA, Siren AL, Ehrenreich H, Revisiting adult neurogenesis and the role of erythropoietin for neuronal and oligodendroglial differentiation in the hippocampus, Mol Psychiatry 21 (2016) 1752–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Karve IP, Taylor JM, Crack PJ, The contribution of astrocytes and microglia to traumatic brain injury, Br J Pharmacol 173 (2016) 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim WB, Cho JH, Encoding of contextual fear memory in hippocampal-amygdala circuit, Nat Commun 11 (2020) 1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kochli DE, Thompson EC, Fricke EA, Postle AF, Quinn JJ, The amygdala is critical for trace, delay, and contextual fear conditioning, Learn Memory 22 (2015) 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim DS, Fenno LE, Ramakrishnan C, Deisseroth K, Global and local fMRI signals driven by neurons defined optogenetically by type and wiring, Nature 465 (2010) 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Martin-Fernandez M, Jamison S, Robin LM, Zhao Z, Martin ED, Aguilar J, Benneyworth MA, Marsicano G, Araque A, Synapse-specific astrocyte gating of amygdala-related behavior, Nat Neurosci 20 (2017) 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nam MH, Han KS, Lee J, Won W, Koh W, Bae JY, Woo J, Kim J, Kwong E, Choi TY, Chun H, Lee SE, Kim SB, Park KD, Choi SY, Bae YC, Lee CJ, Activation of Astrocytic mu-Opioid Receptor Causes Conditioned Place Preference, Cell Rep 28 (2019) 1154–1166 e1155. [DOI] [PubMed] [Google Scholar]
- [28].Ott C, Martens H, Hassouna I, Oliveira B, Erck C, Zafeiriou MP, Peteri UK, Hesse D, Gerhart S, Altas B, Kolbow T, Stadler H, Kawabe H, Zimmermann WH, Nave KA, Schulz-Schaeffer W, Jahn O, Ehrenreich H, Widespread Expression of Erythropoietin Receptor in Brain and Its Induction by Injury, Mol Med 21 (2015) 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Parikh U, Williams M, Jacobs A, Pineda JA, Brody DL, Friess SH, Delayed Hypoxemia Following Traumatic Brain Injury Exacerbates White Matter Injury, J Neuropathol Exp Neurol (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Roth BL, DREADDs for Neuroscientists, Neuron 89 (2016) 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rubin TG, Lipton ML, Sex Differences in Animal Models of Traumatic Brain Injury, J Exp Neurosci 13 (2019) 1179069519844020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schafe GE, Nader K, Blair HT, LeDoux JE, Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective, Trends Neurosci 24 (2001) 540–546. [DOI] [PubMed] [Google Scholar]
- [33].Schafe GE, Swank MW, Rodrigues SM, Debiec J, Doyere V, Phosphorylation of ERK/MAP kinase is required for long-term potentiation in anatomically restricted regions of the lateral amygdala in vivo, Learn Mem 15 (2008) 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Simon DW, McGeachy MJ, Bayir H, Clark RS, Loane DJ, Kochanek PM, The far-reaching scope of neuroinflammation after traumatic brain injury, Nat Rev Neurol 13 (2017) 171–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Siren AL, Fasshauer T, Bartels C, Ehrenreich H, Therapeutic potential of erythropoietin and its structural or functional variants in the nervous system, Neurotherapeutics 6 (2009) 108–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K, Amygdala circuitry mediating reversible and bidirectional control of anxiety, Nature 471 (2011) 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Varela P, Escosteguy-Neto JC, Coelho CT, Mello LE, da Silveira DX, Santos-Junior JG, Chronic light deprivation inhibits appetitive associative learning induced by ethanol and its respective c-Fos and pCREB expression, Int J Neuropsychopharmacol 17 (2014) 1815–1830. [DOI] [PubMed] [Google Scholar]
- [38].Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE, Rethinking the fear circuit: The central nucleus of the amygdala is required for the acquisition, consolidation, and expression of pavlovian fear conditioning, Journal of Neuroscience 26 (2006) 12387–12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xiong Y, Lu D, Qu C, Goussev A, Schallert T, Mahmood A, Chopp M, Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice, J Neurosurg 109 (2008) 510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Xiong Y, Mahmood A, Qu C, Kazmi H, Zhang ZG, Noguchi CT, Schallert T, Chopp M, Erythropoietin improves histological and functional outcomes after traumatic brain injury in mice in the absence of the neural erythropoietin receptor, J Neurotrauma 27 (2010) 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhu H, Pleil KE, Urban DJ, Moy SS, Kash TL, Roth BL, Chemogenetic inactivation of ventral hippocampal glutamatergic neurons disrupts consolidation of contextual fear memory, Neuropsychopharmacology 39 (2014) 1880–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhu H, Roth BL, DREADD: a chemogenetic GPCR signaling platform, Int J Neuropsychopharmacol 18 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhu H, Roth BL, Silencing synapses with DREADDs, Neuron 82 (2014) 723–725. [DOI] [PMC free article] [PubMed] [Google Scholar]


