Abstract
Background
Breast cancer is a non-communicable and common disease that accounts for a high percentage of deaths. Early diagnosis of this disease reduces the death rate. Screening methods such as digital mammography can help prevent or identify the disease earlier. Therefore, this study aims to analyze the cost-benefit of breast cancer using digital mammography.
Methods
This systematic review was conducted based on PRISMA 2020 checklist. PubMed, Scopus, Web of Science, ProQuest, Cochrane Library, and Google Scholar were searched without any time limitation on June 2022. The quality of the studies was evaluated with the CHEERS checklist. After data extraction, the results were synthesized by thematic content analysis.
Results
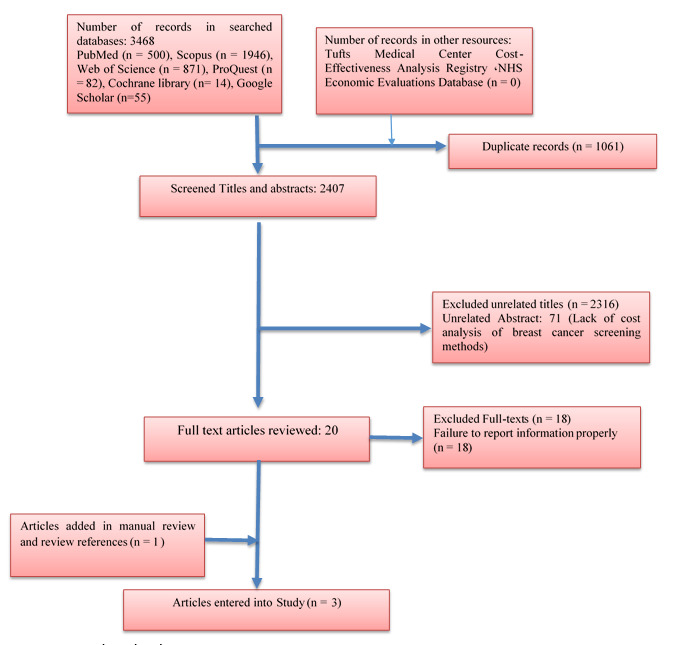
During the search, 3468 records were identified, of which 1061 were duplicates. 2407 titles and abstracts screened in terms of inclusion criteria. Finally, after studying 20 fulltexts, three of them were included in the study. The quality of these articles was scored between 10 and 16. These studies were from Spain, Denmark, and the United States from 2000 to 2019. Two studies showed that digital mammography is not as effective as other screening methods.
Conclusion
The results of this study showed that digital mammography is not very cost-benefit for the health care system. An increase in its repetition frequency imposes more costs on the health system and doesn’t have more benefits for it.
Keywords: Mammography, Breast Cancer, Cost-Benefit Analysis, Systematic Review
↑What is “already known” in this topic:
Early diagnosis of breast cancer reduces its death rate, so screening methods are a way to prevent or identify the disease earlier.
→What this article adds:
Digital mammography is not very cost-benefit for the health care system, and an increase in its repetition frequency imposes more costs on the health system and doesn’t have more benefits for it.
Introduction
Among all types of cancers, breast cancer is a non-communicable and common disease which causes a high mortality rate (1, 2). The disease ranks first among women's malignancies worldwide, and it is the main cause of women's death (3). Breast cancer and fibroids are among the tumors that are common among women and they are gradually becoming prevalent among men. If diagnosed early, the recovery and treatment process will increase significantly.
Also, this type of cancer is a general health problem in Western countries and is considered the most common type of cancer among women in the European Union. In 2018, globally, cancer and its new death cases were estimated to be around 18.1 million and 9.6 million, respectively. And one out of 5 men and one out of six women in the world will experience cancer in their lifetime, and one out of 8 men and one out of 11 women die of cancer. According to some studies, the total number of people who survive the first five years of cancer diagnosis is estimated to be 43.8 million. The global patterns show that almost half of all new cancer cases and more than half of all deaths in 2018 occurred in Asia (4).
Regarding the treatment costs of this disease, it is said in most cancers, the outpatient cost of cancer treatment gradually increases from 12 months to 3 months before death and then increases sharply two months before death (5). Breast cancer also puts a heavy economic burden on communities; the cost of treating this cancer in stages three and four in the United States in 2017 was about $127,000. Also, Stages one and two of the disease cost around $102,000 and $54.00 respectively (6). The cost of treating the disease per patient for Italy in stages one to four was estimated to be $12.000, 14.000, 15.000, and 17.000 in 2017, respectively (7). In addition, the treatment costs for stages one and two of this disease in China in 2017 were estimated to exceed $6,000, and for stages three and four, $8,000 and $12,000. The costs of these patients were higher in Taiwan in comparison to other diseases, including lung cancer and cervical cancer (8).
Early diagnosis of this disease reduces its death rate (9). Screening methods are a way to prevent or identify the disease earlier. Some cancer screening methods include self-examining breasts every month, clinical examinations, mammography, and ultrasound. Still, the usual method used for breast cancer screening is mammography, which has proven to be effective in the early diagnosis and prevention of disease progression. The use of mammography in the diagnosis or treatment of breast cancer in the early stages of cancer reduces the death rate among women aged 50 to 59 by 20% (10).
According to the results of a study conducted among Vietnamese women, the ratio of cost-effectiveness increased each year from the first mammography screening period to $3647.06 and $444.44 for women aged 50-54 and 55-59, respectively. The most effective cost belonged to the women of the 45-49 age group (11). Based on previous studies, the cost-benefit of breast cancer screening is unclear, and there is no strong evidence to show whether breast cancer screening using mammography is economically viable or not. Therefore, due to the limitations of health financing systems and the importance of improving the efficiency of resource allocation in the field of cancer economics, this study was conducted to analyze the cost-benefit of breast cancer using digital mammography as a systematic review.
Methods
This systematic review was conducted in 2022, based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (12). PubMed, Scopus, Web of Science, ProQuest, Cochrane Library, Tufts Medical Center Cost-Effectiveness Analysis Registry, NHS Economic, and Google Scholar were searched without time limitation on June 6, 2022. The main keywords searched were cost-benefit analysis, breast cancer, and mammography and their synonyms in Medical Subject Headings (MeSH) based on PICO.
P= breast cancer
I= digital mammography
C=-
O= cost-benefit
The search strategy for databases is provided in Appendix 1 . The search strategies were repeated and approved by two members of the research team. The source list of the related articles was reviewed to identify more relevant articles. The main inclusion criteria were as follows: English full-text, clinical trial studies, or decision analysis models, including cost-benefit analysis that evaluated the state of costs and implications of breast cancer screening using digital mammography, and availability to the full texts. Case studies, letters to the editor, editorials, comments, conference papers, vision, and other studies related to the evaluation of breast cancer treatment costs other than screening were excluded.
Then abstracts of all the records were imported into EndNote 8 software. After removing duplicates, the titles and abstracts were screened by two authors in the research team based on the inclusion criteria. Disputes were resolved by a third person from the research team through negotiation. Finally, the full text of the related studies was screened by two researchers, and the disagreement over the inclusion was resolved by a third person.
Evaluating the quality of studies
The included studies were evaluated by two authors using the CHEERS checklist. This checklist consists of 5 questions and 24 criteria that examine the design of each economic evaluation study in terms of title and abstract, introduction and problem statement, methodology, findings, discussion, and conclusion (13). Given the fact that the number of studies was limited, all studies identified were included in the study after Quality Assessment.
Extracting the Data
The researchers developed a data extraction form using an Excel spreadsheet. They recorded the bibliographic characteristics of each document including the first author, year of study, country, sample size, type of intervention, study model, vision, age groups, and results.
Data Analysis
In order to analyze the data, qualitative content analysis of thematic type was used based on Braun and Clark's model (14). The data analysis steps included getting to know the data, creating primary codes, searching for semantic units in the text, reviewing semantic units, defining and naming semantic units, and reporting. So, the main results regarding the cost-benefit of digital mammography for breast cancer were extracted based on this method.
Results
Figure 1 shows the study selection process.
Figure 1.
Study selection process
Descriptive results
According to Table 1, out of 24 CHEERS checklist criteria, the entered studies were scored between 10 to 16. The three studies were selected from Spain, Denmark, and the United States from 2000 to 2019 (15, 16). In two of them, the only intervention studied was digital mammography, and in the other one, the cost-benefit of MRI was compared with digital mammography ( 17).
Table 1. Details of the entered studies and their results.
| First Author (year) | Target | Country | Sample size | Type of intervention | Study-model | Perspective | Age groups | Results | Evaluating the quality of studies |
|---|---|---|---|---|---|---|---|---|---|
| Dorte Gyrd-Hansen (2000) | Cost-benefit analysis of mammography screening in Denmark | Denmark | 207 | Mammography | Discrete rankingmodeling | System Health | 50 to 74 years | A comparison of cost and willingness to pay for each of the programs suggested that net benefits are maximized when the screened person is aged 50–74 biennially. More intensive screening produces lower or similar levels of utility at a higher cost. | 14 |
| Victoria L. Mango (2019) | Cost-benefit analysis for average-risk women breast cancer screening program with MRI compared to mammography | United States of America | 5 million hypothetical women | MRI & digital mammography | Monte Carlo Simulation | System Health | Over 30 years | Baseline screening of 2.5M women with breast MRI cost $1.6 billion (B), 3× higher than baseline mammography screening ($0.54B). This yielded a cost-effective benefit compared to mammography screening in less than 6 years ($3.41B vs. $3.65B), with over a 22% cost reduction relative to mammography screening in 12 years and reaching a 38% reduction in 30 years. | 17 |
| Amparo Oltra (2007) | Cost‐benefit analysis of a follow‐up program in patients with breast cancer | Spain | 121 patients were randomized to standard clinical follow-up (n = 63) or to an intensive follow-up (n = 58) | Mammography | Randomized Prospective Study | System Health | Different age groups | The cost was € 390 per person in the clinical follow-up group versus € 1278 per person in the intensive care follow-up group. Therefore, the cost of intensive care follow-up was three times higher than clinical follow-up. | 13 |
In the study done by Mango et al. in 2019, The Monte Carlo model was used for simulation to analyze the cost-benefit of digital mammography versus MRI (17), while Hansen used discrete ranking modeling to analyze the cost-benefit of digital mammography (15). Oltra used a prospective cohort method to analyze the cost-benefit of a breast cancer screening program in both the clinical follow-up and intensive care groups, one of which was digital mammography (16). All these three studies examined cost-benefit analysis and its impact on the health care system. Two of them studied women in age groups over 30.
Analytical results
The study conducted by OLTRA et al. in 2007 was a prospective cohort method to analyze the cost-benefit of breast cancer screening in Spain. Patients were divided into two groups: clinical follow-up (n = 63) and follow-up with intensive care (n = 58). The results showed that after three years of follow-up, there were 24 recurrences of breast cancer, of which 11 were in the clinical follow-up group, and 13 cases were in the intensive care follow-up group. 7 cases of 11 clinical follow-up cases and 4 cases of 13 intensive care follow-up cases were diagnosed at pre-arranged visits. 8 out of the 11 patients in the clinical follow-up group and 9 out of 13 patients in the intensive care follow-up group had symptoms. 34 and 24 pre-arranged visits were performed for the clinical and the intensive care follow-up group, respectively. The total screening costs for the clinical follow-up group were € 24,567 versus € 74,171 for the intensive care follow-up group. The cost was € 390 per person in the clinical follow-up group versus € 1278 per person in the intensive care follow-up group. Therefore, the cost of intensive care follow-up was three times higher than the clinical follow-up.
Performing additional tests and examinations (Medical history and physical evaluation, biochemistry, hematogram, and markers of carcinoembryonic antigen (CEA) and CA15.3 at each outpatient visit with annual liver ultrasound, chest radiograph, and bone scan) and follow-up of asymptomatic patients require huge budgets for the health care system, and they have low diagnostic effectiveness. Therefore, performing complementary tests such as digital mammography during follow-up of asymptomatic patients is not economically justified and does not benefit the health care system (18 ).
The study done by Mango et al. in 2019, which compared breast cancer screening with MRI and digital mammography using the Monte Carlo simulation method, reached significant results regarding these two methods in terms of cost-benefit. The cost of initial MRI screening was $ 1.6 billion, three times the cost of mammography screening ($0.54 billion). In the next screening, MRI was more cost-effective than mammography in 24 years ($ 13.02 billion vs. $ 13.03 billion). The costs of an MRI screening program depend almost on the cost of each MRI test ($ 549.71). The second simulation model was based on the MEDICARE MRI refund process using a lower-cost MRI ($ 400). This had a cost-effective advantage over mammography screening in less than six years ($ 3.41 billion vs. $ 3.65 billion), with more than a 22% cost reduction compared to screening mammograms in 12 years and reaching a 38% reduction in 30 years. So, despite the higher initial cost of a breast MRI screening program for women at average risk, it will ultimately result in more cost savings than mammograms over time (17).
Hansen, in 2000, determined the cost-benefit of breast cancer screening with mammography using discrete ranking modeling to analyze 12 hypothetical programs. Eight of these programs were superior to the other programs in terms of reducing the risk of death from breast cancer, reducing the false-positive diagnosis of breast cancer, and the number of diagnostic tests. A program that screens people aged 50 to 74 every two years produces the highest level of utility. If the frequency of screening increases by more than 13 over a lifetime, the final desirability level decreases (15 ).
Overall, these studies showed that digital mammography is not as effective as other screening methods and doing it every two years can be cost-benefit for the healthcare system. The increasing number of them imposes the highest costs on the healthcare system, which reduces its desirability.
Discussion
This study aimed to analyze the cost-benefit of breast cancer screening with digital mammography using a systematic review method. The results of this study showed that few studies have been performed on the cost-benefit analysis of breast cancer screening in general (15-17) and screening with digital mammography (15, 18, 19). Most existing studies have analyzed the cost-effectiveness of Mammography in general (20, 21), and digital mammography (22), and made a comparison with other screening methods (23). Therefore, a systematic review study on the cost-benefit analysis of digital mammography has not been performed before, which is the subject of the present study. Studies conducted by countries such as Spain, the United States, and Denmark between 2000 and 2019 have shown that digital mammography is not very cost-benefit for the healthcare system and any increase in its frequency places a significant economic burden on The health system.
Contrary to the results of this study, Chootipongchaivat et al. In 2021, who analyzed the cost-effectiveness of mammography in Singapore, concluded that the breast cancer screening program in Singapore requires 54,158 mammograms per 100,000 women, and it has saved 1054 life years (Life Years Gained (LYG)), and 57 women have died of breast cancer. This screening program has been especially efficient at the age of 40. The ICER of this program was between 10.186 and 56.306 QALY, indicating the cost-effectiveness of this program compared to the threshold of willingness to pay 70.000 QALY. Thus, the Singapore mammography screening program is cost-effective if performed between the ages of 40 and 45, and an increase in its presence can have many benefits as well as cost-effectiveness (24).
Like the present study, Schousboe et al. 2011, stated that the existing guidelines suggest doing mammography once every one to two years at the age of 40 or 50, regardless of a person's susceptibility to breast cancer. They were looking for the cost-effectiveness of breast cancer risk factors such as age, breast density, history of breast biopsy, family history of breast cancer, and screening distance, and it was concluded that mammography screening based on female age, breast density, history of breast biopsy, family history of cancer Breast and about its benefits and potential harms, should be personalized (21). The cost-benefit review of mammography personalization has not been investigated in the present study, which could be the subject of future studies. In another study conducted by Schousboe et al. 2022, it was concluded that annual mammography is not cost-effective, and mammography can be done every two years until the age of 80. However, deaths from breast cancer have not decreased significantly, especially for women suffering from underlying diseases.
Women screened over the age of 75 should assess the potential harms of overdiagnosis versus the potential benefits of preventing death from breast cancer (20). The results of this study were different from the method used in the present study; however, the cost-benefit analysis of mammography age can also be considered by researchers to find out whether age-appropriate mammography can benefit the health system or not.
In a similar study but with a different analysis method than in the present study, Moore et al. 2009 analyzed the cost-effectiveness of MRI compared to mammography in women at high risk for breast cancer. They used the Markov model to analyze the cost-effectiveness of these two screening methods. The results showed that an MRI of the breast cancer showed 14.1 QALY at a discount of $ 18,167 compared to mammography with QALY 0.14 at a discount of $4,760 for the ages above 25 years. They conclude that while breast MRI may be useful compared to mammography screening for some high-risk women, it does not seem to be cost-effective even if you are willing to pay thresholds above QALY $ 120,000.
In a similar study but with a different analysis method than in the present study, Moore et al. analyzed the cost-effectiveness of MRI compared to mammography in women at high risk for breast cancer. They used the Markov model to analyze the cost-effectiveness of these two screening methods. The results showed that an MRI of the breast cancer showed 14.1 QALY at a discount of $ 18,167 compared to mammography with QALY 0.14 at a discount of $4,760 for the ages above 25 years. They conclude that while breast MRI may be useful compared to mammography screening for some high-risk women, it does not seem to be cost-effective even if you are willing to pay thresholds above QALY $ 120,000 ( 23).
The results of one of the studies included in the present systematic review showed that despite the higher initial cost of MRI screening programs for women at average risk, compared to mammography, it will ultimately save more costs over time. Therefore, MRI is more beneficial to the health system than digital mammography. However, due to the limited number of studies related to these results, further research is needed to confirm or refute this finding. Based on the items mentioned, follow-up programs that provide patients with easy and fast access to the health system in case of relapse symptoms or signs appear to be the most efficient care system. Studies show that primary healthcare, compared to hospital follow-up, can produce lower costs and benefit the health system (19). This can be considered by healthcare policymakers in countries.
One of the limitations of the present study was the small number of studies entered in the systematic review. The small number of these studies can be due to high costs, long time and difficulty of cost-benefit calculations compared to other methods of cost analysis. It suggested that more quality studies be conducted to confirm or reject the results of this study in the future. Considering the countless advances that have been made in molecular biology and imaging and screening methods as well as primary health care in early diagnosis of breast cancer, it suggested that more studies be done on the cost-benefit of primary health care for breast cancer and a comparison of digital mammography with other screening methods be made.
Conclusion
The results of this study showed that digital mammography is not very cost-benefit for the healthcare system, and an increase in its frequency causes a lot of costs to the health system. Therefore, it is suggested that digital mammography be performed every two years for ages over 50. However, due to the lack of evidence, it was suggested that further studies be conducted to confirm or refute these results.
Conflict of Interests
The authors declare that they have no competing interests.
Authors’ Contributions
S.Gh., A .R., M .E., and S.B. contributed in the conceptualization and design of the study. S .Gh., A .R., M .E., and S.B Searched databases and screened articles. All authors contributed in the data extraction, drafting and manuscript writing. A .R. and M.E. contributed to reviewing the manuscript. All authors approved the final version of the manuscript as submitted
Funding
This article was part of ph.D thesis (grant no: 99-1-37-17508, Ethical code: IR. IUMS. REC. 1398. 1339). That was supported by the deputy of research affiliated with Iran University of medical sciences.
Appendix 1.
Search strategies.
| Database | Search strategy |
|---|---|
| PubMed | (“Breast Neoplasm”[tiab] OR (Neoplasm[tiab] AND Breast[tiab]) OR “Breast Tumor*”[tiab] OR (Tumor*[tiab] AND Breast[tiab]) OR (Neoplasm*[tiab] AND Breast[tiab]) OR “Breast Cancer”[tiab] OR (Cancer[tiab] AND Breast[tiab]) OR “Mammary Cancer*”[tiab] OR (Cancer*[tiab] AND Mammary[tiab]) OR “Malignant Neoplasm of Breast”[tiab] OR “Breast Malignant Neoplasm*”[tiab] OR “Malignant Tumor of Breast”[tiab] OR “Breast Malignant Tumor*”[tiab] OR “Cancer of Breast”[tiab] OR “Cancer of the Breast”[tiab] OR (“Mammary Carcinoma”[tiab] AND Human[tiab]) OR (Carcinoma*[tiab] AND “Human Mammary”[tiab]) OR “Human Mammary Carcinoma*”[tiab] OR (“Mammary Carcinoma*”[tiab] AND Human[tiab]) OR (“Mammary Neoplasm*”[tiab] AND Human[tiab]) OR “Human Mammary Neoplasm*”[tiab] OR (Neoplasm*[tiab] AND “Human Mammary”[tiab]) OR (“Mammary Neoplasm*”[tiab] AND Human[tiab]) OR “Breast Carcinoma*”[tiab] OR (Carcinoma*[tiab] AND Breast[tiab])) AND (Mammograph*[tiab] OR “Digital Mammograph*”[tiab] OR “Digital Breast Tomosynthesis”[tiab] OR (“Breast Tomosyntheses”[tiab] AND Digital[tiab]) OR (“Breast Tomosynthesis”[tiab] AND Digital[tiab]) OR “Digital Breast Tomosyntheses”[tiab] OR “3D-Mammograph*”[tiab] OR “X-ray Breast Tomosynthesis”[tiab] OR (“Breast Tomosyntheses”[tiab] AND “X-ray”[tiab]) OR (“Breast Tomosynthesis”[tiab] AND “X-ray”[tiab]) OR “X ray Breast Tomosynthesis”[tiab] OR “X-ray Breast Tomosyntheses”[tiab]) AND ((Analyses[tiab] AND “Cost-Benefit”[tiab]) OR (Analysis[tiab] AND “Cost-Benefit”[tiab]) OR “Cost-Benefit Analyses”[tiab] OR “Cost Benefit Analysis”[tiab] OR (Analyses[tiab] AND “Cost Benefit”[tiab]) OR (Analysis[tiab] AND “Cost Benefit”[tiab]) OR “Cost Benefit Analyses”[tiab] OR “Cost Effectiveness”[tiab] OR (Effectiveness[tiab] AND Cost[tiab]) OR “Cost-Benefit Data”[tiab] OR “Cost Benefit Data”[tiab] OR (Data[tiab] AND “Cost-Benefit”[tiab]) OR “Cost-Utility Analysis”[tiab] OR (Analyses[tiab] AND “Cost-Utility”[tiab]) OR (Analysis[tiab] AND “Cost-Utility”[tiab]) OR “Cost Utility Analysis”[tiab] OR “Cost-Utility Analyses”[tiab] OR “Economic Evaluation”[tiab] OR “Economic Evaluations”[tiab] OR (Evaluation[tiab] AND Economic[tiab]) OR (Evaluations[tiab] AND Economic[tiab]) OR “Marginal Analysis”[tiab] OR (Analyses[tiab] AND Marginal[tiab]) OR (Analysis[tiab] AND Marginal[tiab]) OR “Marginal Analyses”[tiab] OR “Cost Benefit”[tiab] OR “Costs and Benefits”[tiab] OR “Benefits and Costs”[tiab] OR “Cost-Effectiveness Analysis”[tiab] OR (Analysis[tiab] AND “Cost-Effectiveness”[tiab]) OR “Cost Effectiveness Analysis”[tiab]) |
| Scopus | (TITLE-ABS-KEY(“Breast Neoplasm”) OR (TITLE-ABS-KEY(Neoplasm) AND TITLE-ABS-KEY(Breast)) OR TITLE-ABS-KEY(“Breast Tumor*”) OR (TITLE-ABS-KEY(Tumor*) AND TITLE-ABS-KEY(Breast)) OR (TITLE-ABS-KEY(Neoplasm*) AND TITLE-ABS-KEY(Breast)) OR TITLE-ABS-KEY(“Breast Cancer”) OR (TITLE-ABS-KEY(Cancer) AND TITLE-ABS-KEY(Breast)) OR TITLE-ABS-KEY(“Mammary Cancer*”) OR (TITLE-ABS-KEY(Cancer*) AND TITLE-ABS-KEY(Mammary)) OR TITLE-ABS-KEY(“Malignant Neoplasm of Breast”) OR TITLE-ABS-KEY(“Breast Malignant Neoplasm*”) OR TITLE-ABS-KEY(“Malignant Tumor of Breast”) OR TITLE-ABS-KEY(“Breast Malignant Tumor*”) OR TITLE-ABS-KEY(“Cancer of Breast”) OR TITLE-ABS-KEY(“Cancer of the Breast”) OR (TITLE-ABS-KEY(“Mammary Carcinoma”) AND TITLE-ABS-KEY(Human)) OR (TITLE-ABS-KEY(Carcinoma*) AND TITLE-ABS-KEY(“Human Mammary”)) OR TITLE-ABS-KEY(“Human Mammary Carcinoma*”) OR (TITLE-ABS-KEY(“Mammary Carcinoma*”) AND TITLE-ABS-KEY(Human)) OR (TITLE-ABS-KEY(“Mammary Neoplasm*”) AND TITLE-ABS-KEY(Human)) OR TITLE-ABS-KEY(“Human Mammary Neoplasm*”) OR (TITLE-ABS-KEY(Neoplasm*) AND TITLE-ABS-KEY(“Human Mammary”)) OR (TITLE-ABS-KEY(“Mammary Neoplasm*”) AND TITLE-ABS-KEY(Human)) OR TITLE-ABS-KEY(“Breast Carcinoma*”) OR (TITLE-ABS-KEY(Carcinoma*) AND TITLE-ABS-KEY(Breast))) AND (TITLE-ABS-KEY(Mammograph*) OR TITLE-ABS-KEY(“Digital Mammograph*”) OR TITLE-ABS-KEY(“Digital Breast Tomosynthesis”) OR (TITLE-ABS-KEY(“Breast Tomosyntheses”) AND TITLE-ABS-KEY(Digital)) OR (TITLE-ABS-KEY(“Breast Tomosynthesis”) AND TITLE-ABS-KEY(Digital)) OR TITLE-ABS-KEY(“Digital Breast Tomosyntheses”) OR TITLE-ABS-KEY(“3D-Mammograph*”) OR TITLE-ABS-KEY(“X-ray Breast Tomosynthesis”) OR (TITLE-ABS-KEY(“Breast Tomosyntheses”) AND TITLE-ABS-KEY(“X-ray”)) OR (TITLE-ABS-KEY(“Breast Tomosynthesis”) AND TITLE-ABS-KEY(“X-ray”)) OR TITLE-ABS-KEY(“X ray Breast Tomosynthesis”) OR TITLE-ABS-KEY(“X-ray Breast Tomosyntheses”)) AND ((TITLE-ABS-KEY(Analyses) AND TITLE-ABS-KEY(“Cost-Benefit”)) OR (TITLE-ABS-KEY(Analysis) AND TITLE-ABS-KEY(“Cost-Benefit”)) OR TITLE-ABS-KEY(“Cost-Benefit Analyses”) OR TITLE-ABS-KEY(“Cost Benefit Analysis”) OR (TITLE-ABS-KEY(Analyses) AND TITLE-ABS-KEY(“Cost Benefit”)) OR (TITLE-ABS-KEY(Analysis) AND TITLE-ABS-KEY(“Cost Benefit”)) OR TITLE-ABS-KEY(“Cost Benefit Analyses”) OR TITLE-ABS-KEY(“Cost Effectiveness”) OR (TITLE-ABS-KEY(Effectiveness) AND TITLE-ABS-KEY(Cost)) OR TITLE-ABS-KEY(“Cost-Benefit Data”) OR TITLE-ABS-KEY(“Cost Benefit Data”) OR (TITLE-ABS-KEY(Data) AND TITLE-ABS-KEY(“Cost-Benefit”)) OR TITLE-ABS-KEY(“Cost-Utility Analysis”) OR (TITLE-ABS-KEY(Analyses) AND TITLE-ABS-KEY(“Cost-Utility”)) OR (TITLE-ABS-KEY(Analysis) AND TITLE-ABS-KEY(“Cost-Utility”)) OR TITLE-ABS-KEY(“Cost Utility Analysis”) OR TITLE-ABS-KEY(“Cost-Utility Analyses”) OR TITLE-ABS-KEY(“Economic Evaluation”) OR TITLE-ABS-KEY(“Economic Evaluations”) OR (TITLE-ABS-KEY(Evaluation) AND TITLE-ABS-KEY(Economic)) OR (TITLE-ABS-KEY(Evaluations) AND TITLE-ABS-KEY(Economic)) OR TITLE-ABS-KEY(“Marginal Analysis”) OR (TITLE-ABS-KEY(Analyses) AND TITLE-ABS-KEY(Marginal)) OR (TITLE-ABS-KEY(Analysis) AND TITLE-ABS-KEY(Marginal)) OR TITLE-ABS-KEY(“Marginal Analyses”) OR TITLE-ABS-KEY(“Cost Benefit”) OR TITLE-ABS-KEY(“Costs and Benefits”) OR TITLE-ABS-KEY(“Benefits and Costs”) OR TITLE-ABS-KEY(“Cost-Effectiveness Analysis”) OR (TITLE-ABS-EY(Analysis) AND TITLE-ABS-KEY(“Cost-Effectiveness”)) OR TITLE-ABS-KEY(“Cost Effectiveness Analysis”) |
| Web of Science | (TS=(“Breast Neoplasm”) OR (TS=(Neoplasm) AND TS=(Breast)) OR TS=(“Breast Tumor*”) OR (TS=(Tumor*) AND TS=(Breast)) OR (TS=(Neoplasm*) AND TS=(Breast)) OR TS=(“Breast Cancer”) OR (TS=(Cancer) AND TS=(Breast)) OR TS=(“Mammary Cancer*”) OR (TS=(Cancer*) AND TS=(Mammary)) OR TS=(“Malignant Neoplasm of Breast”) OR TS=(“Breast Malignant Neoplasm*”) OR TS=(“Malignant Tumor of Breast”) OR TS=(“Breast Malignant Tumor*”) OR TS=(“Cancer of Breast”) OR TS=(“Cancer of the Breast”) OR (TS=(“Mammary Carcinoma”) AND TS=(Human)) OR (TS=(Carcinoma*) AND TS=(“Human Mammary”)) OR TS=(“Human Mammary Carcinoma*”) OR (TS=(“Mammary Carcinoma*”) AND TS=(Human)) OR (TS=(“Mammary Neoplasm*”) AND TS=(Human)) OR TS=(“Human Mammary Neoplasm*”) OR (TS=(Neoplasm*) AND TS=(“Human Mammary”)) OR (TS=(“Mammary Neoplasm*”) AND TS=(Human)) OR TS=(“Breast Carcinoma*”) OR (TS=(Carcinoma*) AND TS=(Breast))) AND (TS=(Mammograph*) OR TS=(“Digital Mammograph*”) OR TS=(“Digital Breast Tomosynthesis”) OR (TS=(“Breast Tomosyntheses”) AND TS=(Digital)) OR (TS=(“Breast Tomosynthesis”) AND TS=(Digital)) OR TS=(“Digital Breast Tomosyntheses”) OR TS=(“3D-Mammograph*”) OR TS=(“X-ray Breast Tomosynthesis”) OR (TS=(“Breast Tomosyntheses”) AND TS=(“X-ray”)) OR (TS=(“Breast Tomosynthesis”) AND TS=(“X-ray”)) OR TS=(“X ray Breast Tomosynthesis”) OR TS=(“X-ray Breast Tomosyntheses”)) AND ((TS=(Analyses) AND TS=(“Cost-Benefit”)) OR (TS=(Analysis) AND TS=(“Cost-Benefit”)) OR TS=(“Cost-Benefit Analyses”) OR TS=(“Cost Benefit Analysis”) OR (TS=(Analyses) AND TS=(“Cost Benefit”)) OR (TS=(Analysis) AND TS=(“Cost Benefit”)) OR TS=(“Cost Benefit Analyses”) OR TS=(“Cost Effectiveness”) OR (TS=(Effectiveness) AND TS=(Cost)) OR TS=(“Cost-Benefit Data”) OR TS=(“Cost Benefit Data”) OR (TS=(Data) AND TS=(“Cost-Benefit”)) OR TS=(“Cost-Utility Analysis”) OR (TS=(Analyses) AND TS=(“Cost-Utility”)) OR (TS=(Analysis) AND TS=(“Cost-Utility”)) OR TS=(“Cost Utility Analysis”) OR TS=(“Cost-Utility Analyses”) OR TS=(“Economic Evaluation”) OR TS=(“Economic Evaluations”) OR (TS=(Evaluation) AND TS=(Economic)) OR (TS=(Evaluations) AND TS=(Economic)) OR TS=(“Marginal Analysis”) OR (TS=(Analyses) AND TS=(Marginal)) OR (TS=(Analysis) AND TS=(Marginal)) OR TS=(“Marginal Analyses”) OR TS=(“Cost Benefit”) OR TS=(“Costs and Benefits”) OR TS=(“Benefits and Costs”) OR TS=(“Cost-Effectiveness Analysis”) OR (TS=(Analysis) AND TS=(“Cost-Effectiveness”)) OR TS=(“Cost Effectiveness Analysis”)) |
| Proquest | TI,AB,SU(“Breast Neoplasm” OR (Neoplasm AND Breast) OR “Breast Tumor*” OR (Tumor* AND Breast) OR (Neoplasm* AND Breast) OR “Breast Cancer” OR (Cancer AND Breast) OR “Mammary Cancer*” OR (Cancer* AND Mammary) OR “Malignant Neoplasm of Breast” OR “Breast Malignant Neoplasm*” OR “Malignant Tumor of Breast” OR “Breast Malignant Tumor*” OR “Cancer of Breast” OR “Cancer of the Breast” OR (“Mammary Carcinoma” AND Human) OR (Carcinoma* AND “Human Mammary”) OR “Human Mammary Carcinoma*” OR (“Mammary Carcinoma*” AND Human) OR (“Mammary Neoplasm*” AND Human) OR “Human Mammary Neoplasm*” OR (Neoplasm* AND “Human Mammary”) OR (“Mammary Neoplasm*” AND Human) OR “Breast Carcinoma*” OR (Carcinoma* AND Breast)) AND TI,AB,SU(Mammograph* OR “Digital Mammograph*” OR “Digital Breast Tomosynthesis” OR (“Breast Tomosyntheses” AND Digital) OR (“Breast Tomosynthesis” AND Digital) OR “Digital Breast Tomosyntheses” OR “3D-Mammograph*” OR “X-ray Breast Tomosynthesis” OR (“Breast Tomosyntheses” AND “X-ray”) OR (“Breast Tomosynthesis” AND “X-ray”) OR “X ray Breast Tomosynthesis” OR “X-ray Breast Tomosyntheses”) AND TI,AB,SU((Analyses AND “Cost-Benefit”) OR (Analysis AND “Cost-Benefit”) OR “Cost-Benefit Analyses” OR “Cost Benefit Analysis” OR (Analyses AND “Cost Benefit”) OR (Analysis AND “Cost Benefit”) OR “Cost Benefit Analyses” OR “Cost Effectiveness” OR (Effectiveness AND Cost) OR “Cost-Benefit Data” OR “Cost Benefit Data” OR (Data AND “Cost-Benefit”) OR “Cost-Utility Analysis” OR (Analyses AND “Cost-Utility”) OR (Analysis AND “Cost-Utility”) OR “Cost Utility Analysis” OR “Cost-Utility Analyses” OR “Economic Evaluation” OR “Economic Evaluations” OR (Evaluation AND Economic) OR (Evaluations AND Economic) OR “Marginal Analysis” OR (Analyses AND Marginal) OR (Analysis AND Marginal) OR “Marginal Analyses” OR “Cost Benefit” OR “Costs and Benefits” OR “Benefits and Costs” OR “Cost-Effectiveness Analysis” OR (Analysis AND “Cost-Effectiveness”) OR “Cost Effectiveness Analysis”) |
| Cochrane library | (“Breast Neoplasm” OR (Neoplasm AND Breast) OR “Breast Tumor*” OR (Tumor* AND Breast) OR (Neoplasm* AND Breast) OR “Breast Cancer” OR (Cancer AND Breast) OR “Mammary Cancer*” OR (Cancer* AND Mammary) OR “Malignant Neoplasm of Breast” OR “Breast Malignant Neoplasm*” OR “Malignant Tumor of Breast” OR “Breast Malignant Tumor*” OR “Cancer of Breast” OR “Cancer of the Breast” OR (“Mammary Carcinoma” AND Human) OR (Carcinoma* AND “Human Mammary”) OR “Human Mammary Carcinoma*” OR (“Mammary Carcinoma*” AND Human) OR (“Mammary Neoplasm*” AND Human) OR “Human Mammary Neoplasm*” OR (Neoplasm* AND “Human Mammary”) OR (“Mammary Neoplasm*” AND Human) OR “Breast Carcinoma*” OR (Carcinoma* AND Breast)) AND (Mammograph* OR “Digital Mammograph*” OR “Digital Breast Tomosynthesis” OR (“Breast Tomosyntheses” AND Digital) OR (“Breast Tomosynthesis” AND Digital) OR “Digital Breast Tomosyntheses” OR “3D-Mammograph*” OR “X-ray Breast Tomosynthesis” OR (“Breast Tomosyntheses” AND “X-ray”) OR (“Breast Tomosynthesis” AND “X-ray”) OR “X ray Breast Tomosynthesis” OR “X-ray Breast Tomosyntheses”) AND ((Analyses AND “Cost-Benefit”) OR (Analysis AND “Cost-Benefit”) OR “Cost-Benefit Analyses” OR “Cost Benefit Analysis” OR (Analyses AND “Cost Benefit”) OR (Analysis AND “Cost Benefit”) OR “Cost Benefit Analyses” OR “Cost Effectiveness” OR (Effectiveness AND Cost) OR “Cost-Benefit Data” OR “Cost Benefit Data” OR (Data AND “Cost-Benefit”) OR “Cost-Utility Analysis” OR (Analyses AND “Cost-Utility”) OR (Analysis AND “Cost-Utility”) OR “Cost Utility Analysis” OR “Cost-Utility Analyses” OR “Economic Evaluation” OR “Economic Evaluations” OR (Evaluation AND Economic) OR (Evaluations AND Economic) OR “Marginal Analysis” OR (Analyses AND Marginal) OR (Analysis AND Marginal) OR “Marginal Analyses” OR “Cost Benefit” OR “Costs and Benefits” OR “Benefits and Costs” OR “Cost-Effectiveness Analysis” OR (Analysis AND “Cost-Effectiveness”) OR “Cost Effectiveness Analysis”) |
Cite this article as : Ghorbani S, Rezapour A, Eisavi M, Barahman M, Bagheri Faradonbeh S. Cost-benefit Analysis of Breast Cancer Screening with Digital Mammography: A Systematic Review. Med J Islam Repub Iran. 2023 (16 Aug);37:89. https://doi.org/10.47176/mjiri.37.89
References
- 1.Ewaid SH, Al-Azzawi LHA. Breast cancer risk assessment by Gail Model in women of Baghdad. Alexandria J Med. 2017;53(2):183. [Google Scholar]
- 2.Mohammed AA. Predictive factors affecting axillary lymph node involvement in patients with breast cancer in Duhok: cross-sectional study. Ann Med Surg. 2019;44:87–90. doi: 10.1016/j.amsu.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian Z, Tang J, Yang Q, Li X, Zhu J, Wu G. Atypical ubiquitin-binding protein SHARPIN promotes breast cancer progression. Biomed Pharmacother. 2019;119:109414. doi: 10.1016/j.biopha.2019.109414. [DOI] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 5.Park M, Song I. Medical care costs of cancer in the last year of life using national health insurance data in Korea. PloS One. 2018;13(6):e0197891. doi: 10.1371/journal.pone.0197891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allaire BT, Ekwueme DU, Poehler D, Thomas CC, Guy GP, Subramanian S. et al. Breast cancer treatment costs in younger, privately insured women. Breast Cancer Res Treat. 2017;164(2):429. doi: 10.1007/s10549-017-4249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capri S, Russo A. Cost of breast cancer based on real-world data: a cancer registry study in Italy. BMC Health Serv Res. 2017;17(1):1–10. doi: 10.1186/s12913-017-2006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao XZ, Shi JF, Liu JS, Huang HY, Guo LW, Zhu XY. et al. Medical and non‐medical expenditure for breast cancer diagnosis and treatment in China: a multicenter cross‐sectional study. Asia Pac J Clin Oncol. 2018;14(3):167. doi: 10.1111/ajco.12703. [DOI] [PubMed] [Google Scholar]
- 9.Jazayeri SB, Saadat S, Ramezani R, Kaviani A. Incidence of primary breast cancer in Iran: Ten-year national cancer registry data report. Cancer Epidemiol. 2015;39(4):519. doi: 10.1016/j.canep.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Yasunaga H, Ide H, Imamura T, Ohe K. Women’s anxieties caused by false positives in mammography screening: a contingent valuation survey. Breast Cancer Res treat. 2007;101(1):59–64. doi: 10.1007/s10549-006-9270-4. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen CP, Adang EM. Cost-effectiveness of breast cancer screening using mammography in Vietnamese women. PloS One. 2018;13(3):e0194996. doi: 10.1371/journal.pone.0194996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ. et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10(1):1–19. doi: 10.1186/s13643-020-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D. et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Int J Technol Assess Health Care. 2013;29(2):117. doi: 10.1017/S0266462313000160. [DOI] [PubMed] [Google Scholar]
- 14.Clarke V, Braun V, Hayfield N. Thematic analysis. Qualitative psychology: A practical guide to research methods. 2015;222(2015):248. [Google Scholar]
- 15.Gyrd-Hansen D. Cost-benefit analysis of mammography screening in Denmark based on discrete ranking data. Int Journal Technol assess Health care. 2000;16(03):811. doi: 10.1017/s0266462300102089. [DOI] [PubMed] [Google Scholar]
- 16.Oltra A, Santaballa A, Munarriz B, Pastor M, Montalar J. Cost‐benefit analysis of a follow‐up program in patients with breast cancer: a randomized prospective study. The Breast J. 2007;13(6):571. doi: 10.1111/j.1524-4741.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 17.Mango VL, Goel A, Mema E, Kwak E, Ha R. Breast MRI screening for average‐risk women: A monte carlo simulation cost–benefit analysis. J Magn Reso Imaging. 2019;49(7):e216. doi: 10.1002/jmri.26334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilaprinyo E, Forne C, Carles M, Sala M, Pla R, Castells X. et al. Cost-effectiveness and harm-benefit analyses of risk-based screening strategies for breast cancer. PloS One. 2014;9(2):e86858. doi: 10.1371/journal.pone.0086858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen YP, Sheu ML. A cost-benefit analysis of preventive care: The case of breast cancer screening. Taiwan J Public Health. 2005;24(6):519. [Google Scholar]
- 20.Schousboe JT, Sprague BL, Abraham L, O’Meara ES, Onega T, Advani S. et al. Cost-effectiveness of screening mammography beyond age 75 years: a cost-effectiveness analysis. Ann Intern Med. 2022;175(1):11. doi: 10.7326/M20-8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schousboe JT, Kerlikowske K, Loh A, Cummings SR. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011;155(1):10–20. doi: 10.7326/0003-4819-155-1-201107050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tosteson AN, Stout NK, Fryback DG, Acharyya S, Herman BA, Hannah LG. et al. Cost-effectiveness of digital mammography breast cancer screening. Ann Intern Med. 2008;148(1):1–10. doi: 10.7326/0003-4819-148-1-200801010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore SG, Shenoy PJ, Fanucchi L, Tumeh JW, Flowers CR. Cost-effectiveness of MRI compared to mammography for breast cancer screening in a high risk population. BMC Health Serv Res. 2009;9(1):1–8. doi: 10.1186/1472-6963-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chootipongchaivat S, Wong XY, Ten Haaf, Hartman M, Tan KB, Van Ravesteyn. et al. Cost-effectiveness Analysis of Breast Cancer Screening Using Mammography in Singapore: A Modeling StudyCost-effectiveness of Breast Cancer Screening in Singapore. Cancer Epidemiol Biomarkers & Prev. 2021;30(4):653. doi: 10.1158/1055-9965.EPI-20-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]


