Abstract
Cerebral palsy is the most common physical disability of childhood describing a heterogeneous group of neurodevelopmental disorders that cause activity limitation, but often are accompanied by disturbances of sensation, perception, cognition, communication and behavior, or by epilepsy. Inborn errors of metabolism have been reported in the literature as presenting with features of cerebral palsy. We reviewed and updated the list of metabolic disorders known to be associated with symptoms suggestive of cerebral palsy and found more than 150 relevant IEMs. This represents the fifth of a series of articles attempting to create and maintain a comprehensive list of clinical and metabolic differential diagnosis according to system involvement.
Keywords: Cerebral palsy, Inborn errors of metabolism, Developmental delay, Motor developmental delay, Developmental regression, Spasticity, Extrapyramidal movement disorder, Ataxia, Hypertonia
1. Introduction
This is the fifth in a series of articles that aim to provide a comprehensive list of inherited metabolic diseases associated with specific signs and symptoms. The first four issues of the clinical and biochemical footprints were dedicated to inborn errors of metabolism (IEMs) associated with movement disorders [1], metabolic liver disorders [2], those with psychiatric presentations [3] and those associated with cardiovascular involvement [4]. These articles provide a rapidly accessible list that serve as an updated metabolic differential diagnosis for clinicians.
The list follows the classification utilized in the knowledge base of inborn errors of metabolism (IEMbase) [5], the Nosology of inborn errors of metabolism (IEMs) [6] and the International Classification of Inherited Metabolic Disorders (ICIMD) [7].
2. Definitions
Cerebral palsy (CP) is a well-known physically disabling condition. It affects between 2 and 3 per 1,000 live births, and is thought to be the most common cause of serious disability in childhood (Surveillance of Cerebral Palsy in Europe 2000) [8]. CP describes a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain. The motor disorders of cerebral palsy are often accompanied by disturbances of sensation, cognition, communication, and behavior, by epilepsy and by secondary musculoskeletal problems [9]. The general agreement is that CP is a heterogeneous condition in terms of etiology, severity and types of impairments. Commonly reported comorbidities include intellectual disability in 30–65%, seizure disorders in 30–50%, speech and language deficits in 40%, visual impairments in 40% and hearing problems in 5–15% of patients [10]. It disrupts the normal process of development in childhood, and it is a permanent condition, but it can have changing pattern of manifestations over time. Neurodevelopmental disabilities that do not primarily affect movement and posture are not considered CP. A child who has severely impaired cognition and no motor signs is not considered to have CP. The term non-progressive is used to denote that the pathophysiological mechanisms leading to CP are presumed to arise from a single inciting event or a series of events which are no longer active. This event can still cause changing clinical manifestations over time [9].
3. Classification
The classification of CP is made according to the motor abnormalities (tone and movement disorders – spasticity, ataxia, dystonia, athetosis), the accompanying impairments (seizures, hearing, vision impairments, attention, behavior or cognitive deficits), the anatomical and neuro-imaging findings, and the causation and timing.
The understanding of developmental neurology, including genetic and biochemical influences on brain development, is increasing rapidly, but the full understanding of causal pathways and mechanisms leading to CP remains elusive.
4. Genetic causes of CP
Prior to investigating genetic causes of CP, the most common causes were attributed to isolated, or a combination of prenatal, perinatal and postnatal risk factors, such as prematurity, birth asphyxia, low birth weight, multiple pregnancy, coexisting congenital anomalies, fetal intrauterine infection, maternal infection during labor, or placental pathology [11–13]. The International Cerebral Palsy Task Force published a list of three essential and five non-essential criteria to define an acute intrapartum hypoxic event, with the absence of any of the essential criteria strongly suggesting that CP was not caused by intrapartum hypoxia. The essential criteria include evidence of metabolic acidosis in fetal or neonatal blood and early onset of neonatal encephalopathy. In the nonessential criteria the Task Force includes evidence of an intrapartum hypoxic event, such as low fetal heart rate, low Apgar scores, multisystem presentation and early imaging abnormalities [14].
In recent years, the improved and more widely available genetic testing, have allowed an alternative hypothesis of rare genomic abnormalities causing many of the neurodevelopmental disorders, such as intellectual disabilities and autism. Previous studies based on familial forms of CP estimated that genetic variants causing CP account for about 2% of cases [15]. With the advent of affordable next generation sequencing, more genetic causes are identified, increasing this number to anywhere between 14% to 45–60% [10,16,17]. A recent study by Moreno-de-Luca studying a large cohort of pediatric and adult patients with CP, found the yield for molecular diagnosis between 10.5–32.7%, depending if patients were ascertained through a health-care based cohort (adult patients), or were from clinical laboratory referral (pediatric patients) [18]. Several monogenic disorders often present with clinical manifestations of cerebral palsy. These include several non-syndromic genetic disorders and inborn errors of metabolism. Although individually rare, these disorders are collectively common, and should be entertained when evaluating someone with the diagnosis of CP, especially since some can be treated successfully if diagnosed in early life (i.e., dopa-responsive dystonia caused by GCH1 pathogenic variants and biopterin deficiency disorders).
Early genetic studies identified a few genes that can cause idiopathic cerebral palsy, such as GAD1, KANK1, AP4M1, AP4E1, AP4B1, and AP4S1 [19–22]. With the introduction of exome sequencing (ES) more genetic causes have been found, with variable yield of a positive diagnosis. MacLennan et al. reported a 14% yield for likely pathogenic variants while investigating 98 cases with trio-ES and finding genetic variants in 57 cases. They also described 8 novel genes in CP, 5 known genetic diseases with CP as a new phenotype, and another 44% with genetic variants of lesser bioinformatic priority [16]. Schnekenberg et al. investigated seven patients with ataxic CP, using trio-ES and identified de novo mutations in four, associated with advanced paternal age, in three different genes, KCNC3, ITPR1 and SPTBN2 [23]. Kruer et al. added ADD3 as a cause of spastic CP [24]. Furthermore, several genes causing Spastic Paraplegia have been implicated in inherited forms of CP, such as NIPA1 (SPG6), SPAST (SPG4), and SPG34 [25] [21]. A study done by Takezawa et al. in 2018 analyzed trio-ES in 17 patients with CP and no specific MRI findings, and found pathogenic/likely pathogenic variants within eight genes: CTNNB1, CYP2U1, SPAST, GNAO1, CACNA1A, AMPD2, STXBP1, and SCN2A [26]. In the most recent publication by Moreno-de-Luca, the following genes were found to be present in both pediatric and adult patients with CP: MECP2, TCF4, TUBA1A, SLC2A1, KMT2A, CAMTA1, ATL1, IQSEC2, ASXL3 and L1CAM [18].
Large next generation sequencing studies have done network analysis and identified enrichment of Rho GTPase, extracellular matrix, focal adhesion and cytoskeleton pathways. In the ES analysis of 250 parent-offspring trios, Jin et al. estimated that 14% of cases could be attributed to an excess of damaging de novo or recessive variants, providing evidence for genetically mediated dysregulation of early neuronal connectivity in CP [27].
Leach et al. did a systematic review of the literature for treatable inborn errors of metabolism (IEM) presenting as CP, identifying all reports of IEMs presenting with CP symptoms before 5 years of age. They have identified 54 treatable IEMs belonging to 13 different biochemical categories. For 26 of these IEMs, a treatment is available that targets the primary underlying pathophysiology (e.g., neurotransmitter supplementation) and for 41 treatment is available to stabilize/prevent metabolic crisis [28]. Matthews et al. in 2019 reported the result of trio-ES in 49 probands presenting with atypical CP and found a molecular diagnosis in 65% of patients [29]. Patients considered eligible, had to have impaired motor function with onset at birth or within the first year of life and one or more of the following: severe intellectual disability, progressive neurological deterioration, other abnormalities on neurological examination, multiorgan disease, congenital abnormalities outside the nervous system, an abnormal neurotransmitter profile, family history, or brain imaging findings not typical for CP. The high diagnostic rate in this study can be explained by focused recruitment and strict inclusion criteria. A few other reviews have tried to present a list of neurogenic disorders causing CP, such as Lee et al. 2014, or focusing on IEMs and Hakami et al. 2019 [30] [31], but no comprehensive review and differential diagnosis in inborn errors of metabolism has been attempted before. We have done a systematic review of IEMbase and found 151 diseases that lead to CP presentation. We have summarized the most updated list of differential diagnosis of IEMs presenting with symptoms suggestive of CP in Suppl. Table 1. The relative occurrence rates of the most common symptoms associated with 151 IEMs presenting with CP phenotype, is represented as a heat map of the major motor symptoms in Figure 1.
Figure 1.
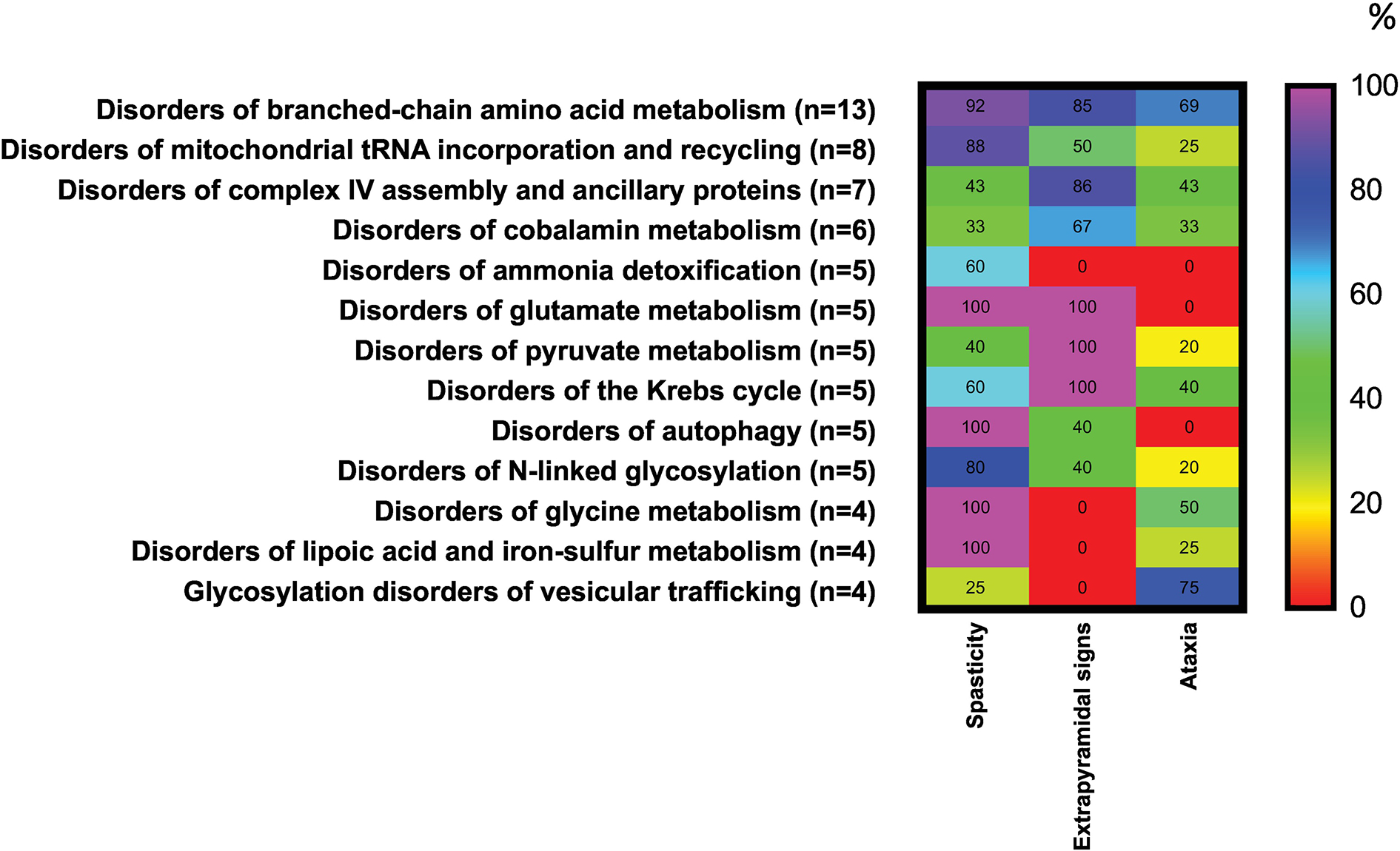
Occurrence (%) of spasticity, extrapyramidal signs or ataxia in a combination with developmental delay or impairment or regression in 13 categories of IEMs. The percentages for neurological involvement were calculated using as the denominator the total number of IEMs in each category presenting with spasticity, extrapyramidal signs or ataxia. Heat scale ranges from red (0%) for diseases with no particular symptoms reported to violet (100%) for diseases with particular symptoms occurring with highly frequency. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
5. Signs and symptoms
We categorized the signs and symptoms in two groups of disorders:
Developmental delay/motor/psychomotor
Developmental regression with motor problems; since CP by definition encompasses only permanent/non-progressive disorders, only conditions where regression starts early were included, as exact clarification of regression may not always be easy at this age in the clinical setting
All categories had as presenting symptoms predominant motor signs: ataxia, extrapyramidal signs and/or spasticity.
Only conditions with age of presentation in the first 2 years of life were included. In the category of developmental delay/motor/psychomotor retardation there were 22 conditions identified to present with ataxia as the isolated motor finding, 12 with isolated extrapyramidal disorders and 37 with only spasticity. Sixty-eight conditions can present with some combination of these three. In the last category of developmental regression there were 6 conditions presenting with some combination of the presenting movement disorder, 1 with isolated ataxia and spasticity each and 2 conditions with isolated extrapyramidal disorder (Suppl. Table 1). Given that in category 2 there were very few disorders, for the analysis of the percentage of occurrence of symptoms associated with CP phenotypes we combined the 2 categories into a single category of “developmental delay/impairment/regression”, separating them into ataxia, pyramidal signs and spasticity (Fig 1, Suppl. Table 1).
6. Diagnosis and differential diagnosis
With the recent advances in genomic analysis in CP and the growing number of IEMs that have specific treatment options, an inherited metabolic disease should be suspected and ruled out in patients presenting with CP phenotype, especially if the criteria for hypoxia are not met. Red flags should be all symptoms and signs that are not in concordance with the aforementioned essential or non-essential criteria for intrapartum hypoxia.
A list of laboratory investigations to aid in the diagnosis of the various listed IEMs is summarized in Table 1. Only 21 conditions out of the 151 can be solely diagnosed through genetic testing.
Table 1.
Biochemical investigations in cerebral palsy.
| Basic tests | Profiles | Special tests |
|---|---|---|
|
| ||
| Ammonia (B) | Acylcamitines (DBS, P) | Copper(S, U) |
| ASAT/ALAT (P) | Amino acids (P, U) | Ceruloplasmin (S) |
| Blood count | Lipid panel (S) | Carnitine (P) |
| CK (P) | Biogenic amines (CSF) | Lysosomal enzyme activity |
| Coagulation factors | Organic acids (U) | (WBC, FB) |
| Glucose (P) | Pterins (CSF) | Glutathione (RBC) |
| Lactate (P) | Sialotransferins (S) | Interferon-alpha (CSF) |
7. Treatment
It is of utmost importance to make a diagnosis of IEM in patients with CP, because some of these conditions may have treatment that could be considered curative, for example L-dopa therapy in most of the biopterin deficiencies, including dopa-responsive dystonia, caused by autosomal dominant or recessive GCH1 pathogenic variants. Other conditions may have treatment that significantly alter the course of the disease, either with specific dietary manipulation or with avoidance of acute metabolic crises (disorders of ammonia detoxification, disorders of sulfur amino acid and sulfide metabolism, branch-chain aminoacidopathies, disorders of serine and glycine metabolism, cobalamin disorders, disorders of folate and copper metabolism, pyruvate metabolism, and ketone body metabolism). Some conditions have gene therapy available, such as AADC deficiency. Also, through constant research, new therapies are developed regularly. Supplemental Table 1 includes information on primary treatment options for the list of IEMs. Finding a specific etiology, such as a diagnosis of IEM in patients diagnosed with CP is helpful in genetic counseling, especially if familial cases are present, prevent birth of a similarly affected sibling, but would also end a diagnostic odyssey and help clear any feeling of guilt in parents or health professionals involved in the delivery.
8. Conclusions
In this fifth issue from a series of educational summaries providing a comprehensive and updated list of metabolic differential diagnosis according to system involvement, we focus on IEMs presenting with CP signs and symptoms. We provide a comprehensive differential diagnosis, standard laboratory diagnostic methods and treatment approaches. The full list can be accessed at www.iembase.org/gamuts.
Supplementary Material
Supplementary Table 1. List of Inborn Errors of Metabolism that can present as cerebral palsy. It contains the gene, inheritance, clinical features, laboratory markers, treatment options if any and pertinent references for each disorder.
Footnotes
Disclosures
N.B. has nothing to disclose and no conflicts of interest.
G.H. has nothing to disclose and no conflicts of interest.
C.F. has nothing to disclose and no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ferreira CR, Hoffmann GF, Blau N, Clinical and biochemical footprints of inherited metabolic diseases. I. Movement disorders. Mol Genet Metab 127 (2019) 28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ferreira CR, Cassiman D, Blau N, Clinical and biochemical footprints of inherited metabolic diseases. II. Metabolic liver diseases. Mol Genet Metab 127 (2019) 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Horvath GA, Stowe RM, Ferreira CR, Blau N, Clinical and biochemical footprints of inherited metabolic diseases. III. Psychiatric presentations. Mol Genet Metab 130 (2020) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ferreira CR, Blau N, Clinical and biochemical footprints of inherited metabolic diseases. IV. Metabolic cardiovascular disease. Mol Genet Metab (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee JJY, Wasserman WW, Hoffmann GF, van Karnebeek CDM, Blau N, Knowledge base and mini-expert platform for the diagnosis of inborn errors of metabolism. Genet Med 20 (2018) 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ferreira CR, van Karnebeek CDM, Vockley J, Blau N, A proposed nosology of inborn errors of metabolism. Genet Med 21 (2019) 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ferreira CR, Rahman S, Keller M, Zschocke J, Group Icimd Advisory, An international classification of inherited metabolic disorders (ICIMD). J Inherit Metab Dis 44 (2021) 164–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morris C, Definition and classification of cerebral palsy: a historical perspective. Dev Med Child Neurol Suppl 109 (2007) 3–7. [DOI] [PubMed] [Google Scholar]
- [9].Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B, A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 109 (2007) 8–14. [PubMed] [Google Scholar]
- [10].Moreno-De-Luca A, Ledbetter DH, Martin CL, Genetic [corrected] insights into the causes and classification of [corrected] cerebral palsies. Lancet Neurol 11 (2012) 283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nelson KB, Causative factors in cerebral palsy. Clin Obstet Gynecol 51 (2008) 749–62. [DOI] [PubMed] [Google Scholar]
- [12].Keogh JM, Badawi N, The origins of cerebral palsy. Curr Opin Neurol 19 (2006) 129–34. [DOI] [PubMed] [Google Scholar]
- [13].Clark SM, Ghulmiyyah LM, Hankins GD, Antenatal antecedents and the impact of obstetric care in the etiology of cerebral palsy. Clin Obstet Gynecol 51 (2008) 775–86. [DOI] [PubMed] [Google Scholar]
- [14].MacLennan A template for defining a causal relation between acute intrapartum events and cerebral palsy: international consensus statement. BMJ (Clinical research ed.) 319 (1999) 1054–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rajab A, Yoo SY, Abdulgalil A, Kathiri S, Ahmed R, Mochida GH, Bodell A, Barkovich AJ, Walsh CA, An autosomal recessive form of spastic cerebral palsy (CP) with microcephaly and mental retardation. Am J Med Genet A 140 (2006) 1504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].MacLennan AH, Thompson SC, Gecz J, Cerebral palsy: causes, pathways, and the role of genetic variants. Am J Obstet Gynecol 213 (2015) 779–88. [DOI] [PubMed] [Google Scholar]
- [17].Costeff H, Estimated frequency of genetic and nongenetic causes of congenital idiopathic cerebral palsy in west Sweden. Ann Hum Genet 68 (2004) 515–20. [DOI] [PubMed] [Google Scholar]
- [18].Moreno-De-Luca A, Millan F, Pesacreta DR, Elloumi HZ, Oetjens MT, Teigen C, Wain KE, Scuffins J, Myers SM, Torene RI, Gainullin VG, Arvai K, Kirchner HL, Ledbetter DH, Retterer K, Martin CL, Molecular Diagnostic Yield of Exome Sequencing in Patients With Cerebral Palsy. JAMA 325 (2021) 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lynex CN, Carr IM, Leek JP, Achuthan R, Mitchell S, Maher ER, Woods CG, Bonthon DT, Markham AF, Homozygosity for a missense mutation in the 67 kDa isoform of glutamate decarboxylase in a family with autosomal recessive spastic cerebral palsy: parallels with Stiff-Person Syndrome and other movement disorders. BMC Neurol 4 (2004) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lerer I, Sagi M, Meiner V, Cohen T, Zlotogora J, Abeliovich D, Deletion of the ANKRD15 gene at 9p24.3 causes parent-of-origin-dependent inheritance of familial cerebral palsy. Hum Mol Genet 14 (2005) 3911–20. [DOI] [PubMed] [Google Scholar]
- [21].Moreno-De-Luca A, Helmers SL, Mao H, Burns TG, Melton AM, Schmidt KR, Fernhoff PM, Ledbetter DH, Martin CL, Adaptor protein complex-4 (AP-4) deficiency causes a novel autosomal recessive cerebral palsy syndrome with microcephaly and intellectual disability. J Med Genet 48 (2011) 141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Abou Jamra R, Philippe O, Raas-Rothschild A, Eck SH, Graf E, Buchert R, Borck G, Ekici A, Brockschmidt FF, Nothen MM, Munnich A, Strom TM, Reis A, Colleaux L, Adaptor protein complex 4 deficiency causes severe autosomal-recessive intellectual disability, progressive spastic paraplegia, shy character, and short stature. Am J Hum Genet 88 (2011) 788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Parolin Schnekenberg R, Perkins EM, Miller JW, Davies WI, D’Adamo MC, Pessia M, Fawcett KA, Sims D, Gillard E, Hudspith K, Skehel P, Williams J, O’Regan M, Jayawant S, Jefferson R, Hughes S, Lustenberger A, Ragoussis J, Jackson M, Tucker SJ, Nemeth AH, De novo point mutations in patients diagnosed with ataxic cerebral palsy. Brain 138 (2015) 1817–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kruer MC, Jepperson T, Dutta S, Steiner RD, Cottenie E, Sanford L, Merkens M, Russman BS, Blasco PA, Fan G, Pollock J, Green S, Woltjer RL, Mooney C, Kretzschmar D, Paisan-Ruiz C, Houlden H, Mutations in gamma adducin are associated with inherited cerebral palsy. Ann Neurol 74 (2013) 805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gee HY, Zhang F, Ashraf S, Kohl S, Sadowski CE, Vega-Warner V, Zhou W, Lovric S, Fang H, Nettleton M, Zhu JY, Hoefele J, Weber LT, Podracka L, Boor A, Fehrenbach H, Innis JW, Washburn J, Levy S, Lifton RP, Otto EA, Han Z, Hildebrandt F, KANK deficiency leads to podocyte dysfunction and nephrotic syndrome. J Clin Invest 125 (2015) 2375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Takezawa Y, Kikuchi A, Haginoya K, Niihori T, Numata-Uematsu Y, Inui T, Yamamura-Suzuki S, Miyabayashi T, Anzai M, Suzuki-Muromoto S, Okubo Y, Endo W, Togashi N, Kobayashi Y, Onuma A, Funayama R, Shirota M, Nakayama K, Aoki Y, Kure S, Genomic analysis identifies masqueraders of full-term cerebral palsy. Ann Clin Transl Neurol 5 (2018) 538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jin SC, Lewis SA, Bakhtiari S, Zeng X, Sierant MC, Shetty S, Nordlie SM, Elie A, Corbett MA, Norton BY, van Eyk CL, Haider S, Guida BS, Magee H, Liu J, Pastore S, Vincent JB, Brunstrom-Hernandez J, Papavasileiou A, Fahey MC, Berry JG, Harper K, Zhou C, Zhang J, Li B, Zhao H, Heim J, Webber DL, Frank MSB, Xia L, Xu Y, Zhu D, Zhang B, Sheth AH, Knight JR, Castaldi C, Tikhonova IR, Lopez-Giraldez F, Keren B, Whalen S, Buratti J, Doummar D, Cho M, Retterer K, Millan F, Wang Y, Waugh JL, Rodan L, Cohen JS, Fatemi A, Lin AE, Phillips JP, Feyma T, MacLennan SC, Vaughan S, Crompton KE, Reid SM, Reddihough DS, Shang Q, Gao C, Novak I, Badawi N, Wilson YA, McIntyre SJ, Mane SM, Wang X, Amor DJ, Zarnescu DC, Lu Q, Xing Q, Zhu C, Bilguvar K, Padilla-Lopez S, Lifton RP, Gecz J, MacLennan AH, Kruer MC, Mutations disrupting neuritogenesis genes confer risk for cerebral palsy. Nat Genet 52 (2020) 1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Leach EL, Shevell M, Bowden K, Stockler-Ipsiroglu S, van Karnebeek CD, Treatable inborn errors of metabolism presenting as cerebral palsy mimics: systematic literature review. Orphanet J Rare Dis 9 (2014) 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Matthews AM, Blydt-Hansen I, Al-Jabri B, Andersen J, Tarailo-Graovac M, Price M, Selby K, Demos M, Connolly M, Drogemoller B, Shyr C, Mwenifumbo J, Elliott AM, Lee J, Ghani A, Stockler S, Salvarinova R, Vallance H, Sinclair G, Ross CJ, Wasserman WW, McKinnon ML, Horvath GA, Goez H, van Karnebeek CD, Tide Bc United for Metabolic Diseases, the Causes Study, Atypical cerebral palsy: genomics analysis enables precision medicine. Genet Med 21 (2019) 1621–1628. [DOI] [PubMed] [Google Scholar]
- [30].Lee RW, Poretti A, Cohen JS, Levey E, Gwynn H, Johnston MV, Hoon AH, Fatemi A, A diagnostic approach for cerebral palsy in the genomic era. Neuromolecular Med 16 (2014) 821–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hakami WS, Hundallah KJ, Tabarki BM, Metabolic and genetic disorders mimicking cerebral palsy. Neurosciences (Riyadh) 24 (2019) 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. List of Inborn Errors of Metabolism that can present as cerebral palsy. It contains the gene, inheritance, clinical features, laboratory markers, treatment options if any and pertinent references for each disorder.


