Abstract
In the present work a simple enzymatic approach (Novozym 435) for transesterification to synthesize pyrrole esters was reported. To generate the best reaction conditions, which resulted in the optimum yield of 92%, the effects of lipase type, solvent, lipase load, molecular sieves, substrate molar ratio of esters to alcohol, reaction temperature, reaction duration, and speed of agitation were evaluated. The range of alcohols was assessed under optimal circumstances. The spectrum observations conclusively demonstrated that the compounds could be generated with high yield under the circumstances utilized for synthesis. The odor characteristics of the pyrrolyl esters obtained were examined by gas chromatography–mass spectrometry-olfactometry (GC–MS–O). Among them, compounds of benzhydryl 1H-pyrrole-2-carboxylate (3j), butyl 1H-pyrrole-2-carboxylate (3k) and pentyl 1H-pyrrole-2-carboxylate (3l) present sweet and acid aroma. In addition, the thermal degradation process was further studied using the Py–GC/MS (pyrolysis–gas chromatography/mass spectrometry), TG (thermogravimetry), and DSC (differential scanning calorimeter) techniques. The outcomes of the Py–GC/MS, TG, and DSC techniques show that they have excellent thermal stability.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13065-023-01039-5.
Keywords: Pyrrolyl esters, Novozym 435, Transesterification, GC–MS–O, Py–GC/MS, TG
Introduction
Esters are often utilized in the food, fragrance, cosmetic, coating, and pharmaceutical sectors as flavoring and aroma ingredients [1–3]. Heteroaryl esters, which are essential building blocks for organic synthesis and could create interesting building blocks for the creation of diverse functionalized products, are described in more detail below [4–9]. Particularly, pyrrole esters, which could be characterized by special aromatic organoleptic qualities, have found use in bioactive compounds. As a result, making pyrrole esters has garnered more interest [10]. Thus, there has been a growing interest in preparing pyrrole esters. Chemical synthesis is an alternate technique for producing pyrrole esters, however it has disadvantages including high temperature, poor yield, and catalyst residue. However, biocatalytic synthesis of flavor esters is becoming more and more popular in the flavor and fragrance industries, due to the advantages of selectivity and specificity, reusability, mild conditions and much purer products [11–15]. Esterification, interesterification, thioesterification, and transesterification processes are produced by enzymes such lipases and esterases and are catalyzed by the ping-pong bibi mechanism, ternary complex ordered bibi mechanism, or ternary complex random bibi mechanism [16–19]. A variety of functional groups, halides, and linear as well as cyclic alkyl alcohols are allowed under this procedure. For instance, several citronellyl esters [20, 21], propyl benzoate [22], adipate ester [15], ethyl hexanoate [23], monoterpenic esters [24], aliphatic esters [25], benzyl benzoate [26], pentyl valerate [27] were efficiently synthesized with the assistant of enzymes catalysis. Immobilized lipases are harmless, biodegradable catalysts that speed up chemical processes at low temperatures and pressures. Herein we report a new approach to obtain a series of pyrrole esters using methyl pyrrole-carboxylate and simple alcohols via Novozym 435-catalyzed esterification with n-hexane as the solvent. A variety of functional groups, halides, and linear as well as cyclic alkyl alcohols are allowed under this approach. In furthermore, gas chromatography–mass spectrometry-olfactometry (GC–MS–O) might be used to evaluate the olfactory properties and potential of the compounds as flavors or perfumes. In addition, the thermal behaviors of the pyrrole esters under high temperatures were investigated using the pyrolysis–gas chromatography/mass spectrometry (Py–GC/MS), thermogravimetry (TG), and differential scanning calorimeter (DSC) techniques [28–30].
In this paper, we provide the enzymatic production, odor characteristic, and thermal behaviors of new pyrrole ester derivatives. The effects of several variables such as kind of lipase, different solvents, molecular sieves load, biocatalyst load, substrate concentration, reaction temperature, time, speed of agitation and rate of reaction were systematically investigated to generate the best reaction conditions. The compatibility of various alcohols with this protocol is evaluated. GC–MS–O was also used to analyze the pyrrole esters' olfactory properties. By using Py–GC/MS and TG, the heat stability of the flavoring compounds was evaluated. These findings provide direction for the creation of efficient pyrrole flavoring agents.
Results and discussion
Effect of the lipase on lipase-catalyzed transesterification
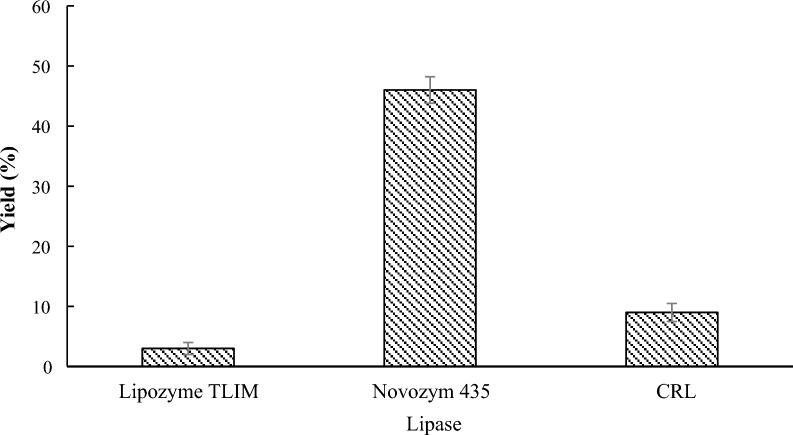
To identify the optimal conditions, methyl 1H-pyrrole-2-carboxylate (1a) and benzyl alcohol (2a) were designed as model substrates for the initial investigation. For the first lipase-catalyzed transesterification of 1a with 2a, three available commercially encapsulated lipases, namely Lipozyme TLIM, Novozym 435, and CRL, were used. Other input investigations were 5:1 (1a/2a) reactant molar ratio, lipase load of 6 mg/mL, stirrer speed of 150 r/min, molecular sieve load of 1 g, 40 °C reaction temperature, and a 24 h reaction time. According to Fig. 1, Lipozyme TLIM provided the lowest contents of benzyl 1H-pyrrole-2-carboxylate (3a), accounting for less than 3%. Compound 3a has a lower amount of 9% according to CRL. Under the catalysis of Novozym 435, compound 3a was generated in the maximum concentration which showed much higher activity (46% yield) in toluene than Lipozyme TLIM and CRL. It suggested that Novozym 435 was efficient for catalyzing the transesterification of methyl 1H-pyrrole-2-carboxylate with alcohol.
Fig. 1.
Effect of the lipase on lipase-catalyzed transesterification. Reaction conditions: methyl 1H-pyrrole-2-carboxylate 1a (1.0 mmol), benzyl alcohol 2a (0.2 mmol), 60 mg lipase, 10 mL toluene, 1.0 g molecular sieves, 40 °C, 150 rpm, 24 h
Effect of the solvent on lipase-catalyzed transesterification
The activity of three lipases is influenced by the various catalytic characteristics [28]. Candida antarctica lipase B (CALB) functioned as the source of Novozym 435. It was formerly believed that Novozym 435 was an all-purpose lipase [31]. The efficiency and stability of enzymes are substantially impacted by the reaction medium [32]. In various solvent media, lipases frequently displayed varying positional selectivity and catalytic activity, which were frequently connected to the solvent characteristic [33]. Here, the lipase Novozym 435 was used to study the effects of solvents, eight kinds of solvent, including toluene, 1,4-Dioxane, CH3CN, DMF, n-Hexane, ethanol, isooctane and DMSO, were used as the reaction medium to carry out this reaction (Table 1). Table 1 demonstrates that the n-Hexane significantly outperformed the competition, yielding the required product 3a in a yield of 61% isolated (Table 1, entry 5). The target compound 3a was produced by the isooctane in a 44% isolated yield (Table 1, entry 8). Unfortunately, 1,4-Dioxane, CH3CN, DMF, ethanol and DMSO cannot be used in this reaction, product 3a was not being found.
Table 1.
Effect of the solvent on lipase-catalyzed transesterification of methyl 1H-pyrrole-2-carboxylate with benzyl alcohol in n-Hexane
| Entry | Lipase | Solvent | 3a (%) |
|---|---|---|---|
| 1 | Novozym 435 | Toluene | 46 |
| 2 | Novozym 435 | 1,4-Dioxane | 0 |
| 3 | Novozym 435 | CH3CN | 0 |
| 4 | Novozym 435 | DMF | 0 |
| 5 | Novozym 435 | n-Hexane | 61 |
| 6 | Novozym 435 | Ethanol | 0 |
| 7 | Novozym 435 | DMSO | 0 |
| 8 | Novozym 435 | Isooctane | 44 |
Reaction conditions: methyl 1H-pyrrole-2-carboxylate 1a (1.0 mmol), benzyl alcohol 2a (0.2 mmol), 60 mg Novozym 435, 10 mL solvent, 1.0 g molecular sieves, 40 °C, 150 rpm, 24 h
Effect of the lipase load on lipase-catalyzed transesterification
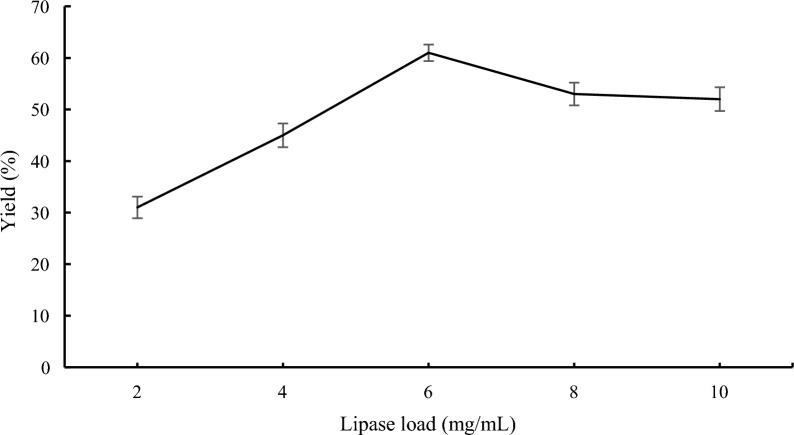
For an industrial application to be effective, lipase load is a critical component. High lipase load may shorten reaction time and increase the reaction rate. Nevertheless, the cost of lipase increased as its supply increased and was high. So, the impact of Novozym 435 load from 2 to 10 mg/mL on the composition of compound 3a was evaluated under 5:1 (1a/2a) reactant molar ratio, stirrer speed of 150 r/min, molecular sieves of 1 g, 40 °C reaction temperature, and a 24 h reaction time in n-Hexane. It can be seen from the Fig. 2 that the transformation rose from 31 to 61% when the catalyst amount was raised from 2 to 6 mg/mL. If the lipase load was from 8 to 10 mg/mL, the amount of compound 3a was slightly reduced. The lipase aggregation at the reaction interface reduced the effective lipase concentration and indeed the region of electrode contact area, may be the origin of this occurrence [34]. The ideal lipase load for Novozym 435 was determined to be 6 mg/mL after considering the price of lipase and the presence of component 3a.
Fig. 2.
Effect of the lipase load on lipase-catalyzed transesterification. Reaction conditions: methyl 1H-pyrrole-2-carboxylate 1a (1.0 mmol), benzyl alcohol 2a (0.2 mmol), 1.0 g molecular sieve, 150 rpm, 40 °C, 24 h, n-Hexane (10 mL)
Effect of the molecular sieves on lipase-catalyzed transesterification
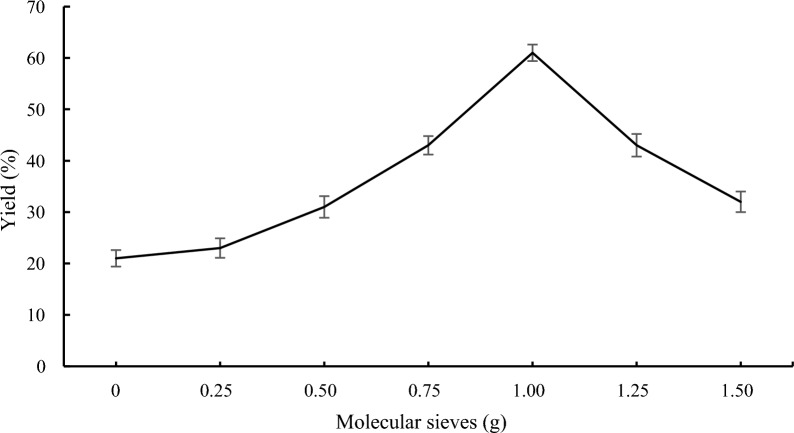
The reaction mixture including alcohol and methyl 1H-pyrrole-2-carboxylate was made using dehydrated n-hexane. Therefore, the effect of molecular sieves from 0 to 1.5 g on the content of compound 3a was investigated. As shown in Fig. 3, the conversion increased from 22 to 61% when the molecular sieves were raised from 0 to 1.0 g. The synthesis of pyrrole ester then gradually decreased when the molecular sieve concentration was raised further. The addition of molecular sieves often increases the conversion of the equilibrium [35]. However, there have also been several reports of unfavorable consequences, such as the breakdown of unstable compounds [36, 37].
Fig. 3.
Effect of the molecular sieves on lipase-catalyzed transesterification. Reaction conditions: methyl 1H-pyrrole-2-carboxylate 1a (1.0 mmol), benzyl alcohol 2a (0.2 mmol), 60 mg Novozym 435, n-Hexane (10 mL), 40 °C, 150 rpm, 24 h
Effect of the molar ratio on lipase-catalyzed transesterification
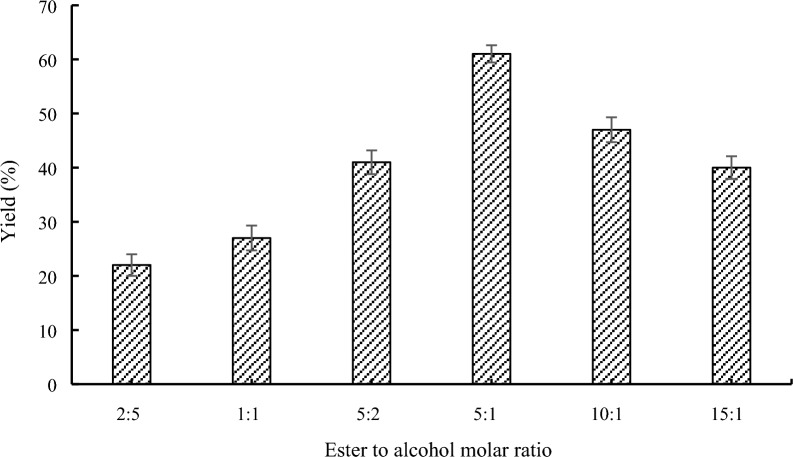
The influence of substrate molar ratio was frequently cited as a notable feature in the behavior of enzymes involved in synthesis [38]. Hence, at various molar ratios (1a/2a), the transesterification of methyl 1H-pyrrole-2-carboxylate (1a) with benzyl alcohol (2a) was investigated. Figure 4 shows the impact of molar ratio on the transesterification of 1a with 2a. The yield of compound 3a was 22% at the molar ratio of 2:5. After changing the molar ratio was adjusted from 2:5 to 5:1, the production of compound 3a raised from 22 to 61%, demonstrating that an increase in 1a might cause the reaction thermodynamic equilibrium to move in favor of 3a pyrrole ester, and enhance the iterate of 1a. The 5:1 molar ratio, which had the greatest conversion rate, was determined as the best molar ratio for further research. But with the subsequent rise in the molar ratio to 10:1 and 15:1, the yield dropped slightly to 47% and 40%, respectively. This is so that when the molar ratio reached a 5:1 ratio, the reactions equilibria would be equivalent. As a result, a rise in 1a may cause the reaction equilibrium to shift in the other way, decreasing conversion [39]. Consequently, the following tests were optimized using a molar ratio of 5:1.
Fig. 4.
Effect of the molar ratio on lipase-catalyzed transesterification. Reaction conditions: 60 mg Novozym 435, 1.0 g molecular sieve, 40 °C, 150 rpm, 24 h, n-Hexane (10 mL)
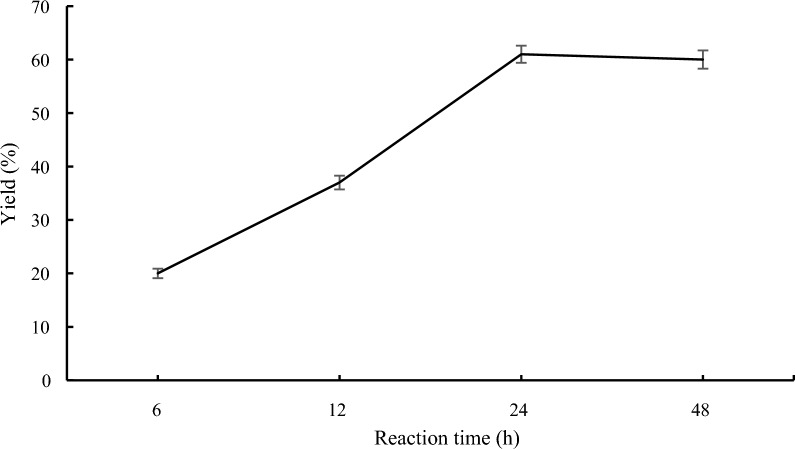
Effect of the reaction time on lipase-catalyzed transesterification
The optimum ester synthesis was time-dependent, as originally described. The yield of ethyl ferulate under Novozym’s catalysis steadily rose as the reaction period was extended [39]. In spite of this, the hydrogel-bound lipase of P. aeruginosa MTCC-4713 generated the greatest amount of ester during the 6 h synthesis of methyl acrylate, before steadily declining after that [40]. Therefore, the transesterification of pyrrole ester with benzyl alcohol was studied at different reaction time. It can be seen from the Fig. 5, when the reaction time was raised from 6 to 24 h, the translation improved from 20 to 61%. The yield significantly decreased to 60% with the additional increase in reaction time to 48 h. Therefore, the reaction time for the ideal reaction circumstances was determined to be 24 h.
Fig. 5.
Effect of the reaction time on lipase-catalyzed transesterification. Reaction conditions: methyl 1H-pyrrole-2-carboxylate 1a (1.0 mmol), benzyl alcohol 2a (0.2 mmol), 60 mg Novozym 435, 1.0 g molecular sieve, 40 °C, 150 rpm, n-Hexane (10 mL)
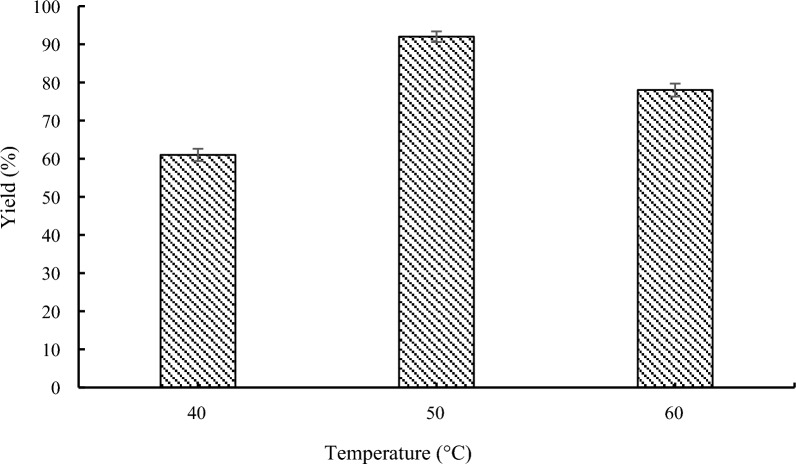
Effect of the temperature on lipase-catalyzed transesterification
Lipase activity and reaction rate were both impacted by reaction temperature. Although increasing temperature sped up reaction rate and lowered reaction time, but it also decreased lipase activity [28]. As shown in Fig. 6, the translation rate has risen from 61 to 92% as the temperature rose from 40 to 50 °C. When the temperature rose from 50 to 60 °C, the conversion was dramatically reduced. The yield of compound 3a was 78% at 60 °C. This suggested that because of the lengthy reaction time at high temperatures, the enzyme may undergo thermal denaturation. For the progressively advanced, the reaction temperature was maintained at 50 °C to prevent thermal inactivation of the enzyme and to achieve high conversion.
Fig. 6.
Effect of the temperature on lipase-catalyzed transesterification. Reaction conditions: methyl 1H-pyrrole-2-carboxylate 1a (1.0 mmol), benzyl alcohol 2a (0.2 mmol), 60 mg Novozym 435, 1.0 g molecular sieve, 150 rpm, 24 h, n-Hexane (10 mL)
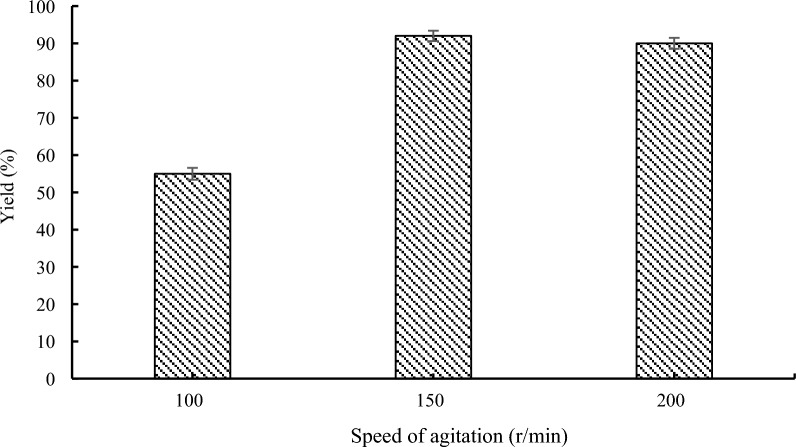
Effect of the agitation speed on lipase-catalyzed transesterification
Reaction time and rate were altered by appropriate agitation occurring at an ideal pace. Experiments were conducted at agitation rates ranging from 100 to 200 rpm to evaluate the impact of agitation speeds. As shown in Fig. 7, when converting between 100 and 150 rpm, the conversion progressively rises from 55 to 92%. The layer surrounding the solid lipase particles was reduced with an increase in agitation speed, which reduced the mass transfer resistance. Even yet, the speed was only slightly reduced when it was raised to 200 rpm. Since there was only a slight difference between stirrer speeds of 150 and 200 rpm, 150 rpm was chosen to minimize energy usage.
Fig. 7.
Effect of the agitation speed on lipase-catalyzed transesterification. Reaction conditions: methyl 1H-pyrrole-2-carboxylate 1a (1.0 mmol), benzyl alcohol 2a (0.2 mmol), 60 mg Novozym 435, 1.0 g molecular sieve, 50 °C, 24 h, n-Hexane (10 mL)
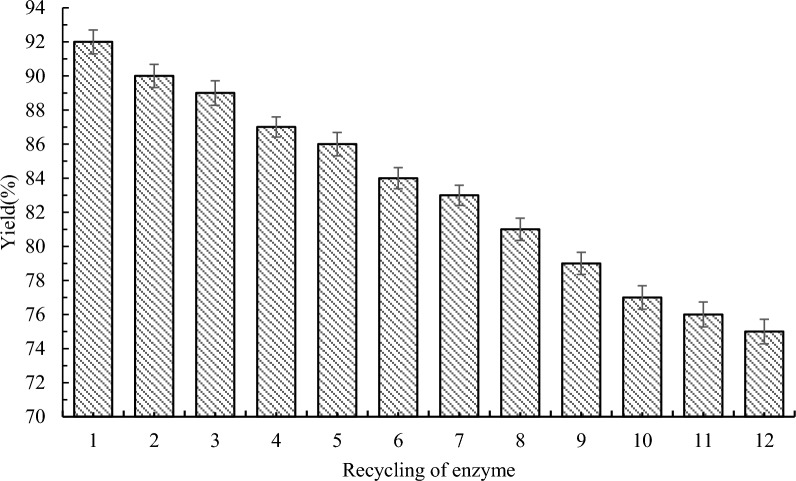
Reusability of enzymes
It’s common knowledge that one of the most important advantages or benefits of lipases is the reusability of enzymes. In this part, the product 3a was continuous obtained by using lipase Novozym 435, which we can analyze its economic viability and potential for reuse. Then we used filter paper to collect enzyme particles under the optimized conditions. After that, n-Hexane was used to clean the enzyme particles. Recycling enzyme particles were utilized to synthesize 3a after drying. The recycling of Novozym 435 in the esterification process was studied by reusing the immobilized enzyme in 12 cycles in n-Hexane at 50 °C. And the experiment was repeated three times. As shown in Fig. 8, the bar chart indicated that the yield of 3a was reduced with the times of increasingly using by Novozym 435. Interestingly, we found that the yield of desired product 3a was still at 75%, even though Novozym 435 had been used 12 cycles. Thus, the using of enzymatic esterification process shows excellent economic property and potential for reutilizing.
Fig. 8.
Reusability of enzymes. Reaction conditions: methyl 1H-pyrrole-2-carboxylate 1a (1.0 mmol), benzyl alcohol 2a (0.2 mmol), 60 mg Novozym 435, 1.0 g molecular sieve, 50 °C, 150 rpm, 24 h, n-Hexane (10 mL)
Substrate scope
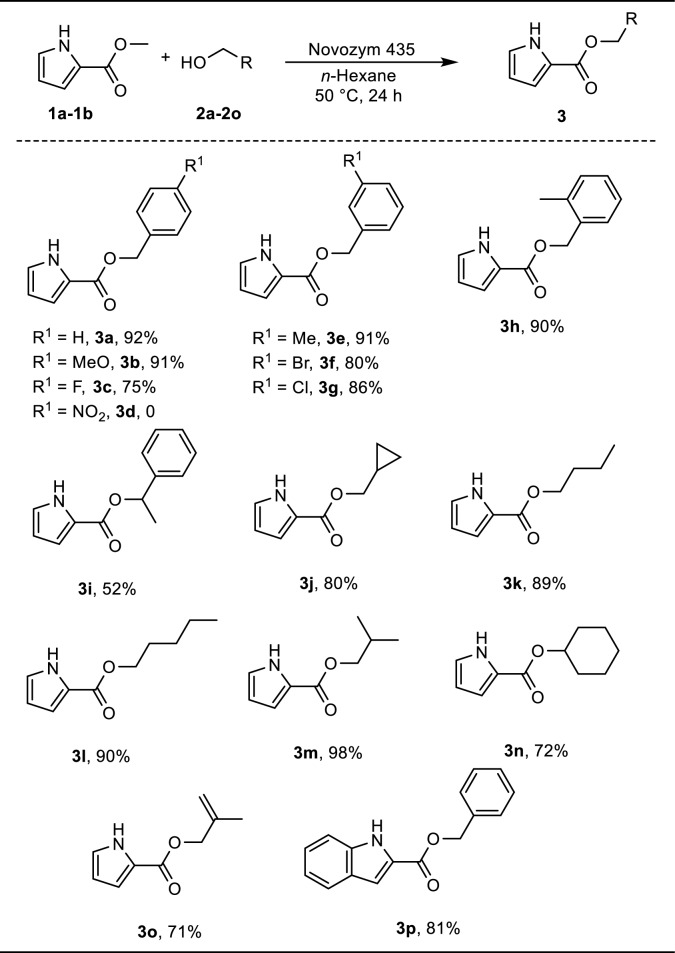
With the optimized circumstances, we assessed the scope of the substrate. It can be seen from the Table 2 that different aromatic and primary alcohols (2b–2o) were chosen to react with (1a) with optimal reaction conditions. With moderate to good yields of 75–92%, the reaction of 1a and benzylic alcohols having alkyl, methoxy and halo substituents at the o-, m-, and p- positions led to the formation of the desired compounds 3a–3h. Unfortunately, the reaction of 1a and 2d p-nitrobenzyl alcohol cannot give product 3d. The yield of products 3c, 3d 3f and 3g with electron withdrawing substituents on the benzene ring were lower than that of products 3a, 3b and 3e with electron donating substituents. It might be electron withdraw groups have a negative on reaction yield. Importantly, (R)-( +)-1-phenylethanol reacted smoothly with 1a, 52% of the target product, 3i, was successfully created. But the yield of 3i was lower than 3a, it might because the spatial hindrance of alpha-substituted benzyl alcohols resulted low yield. Gratifyingly, the reaction could also be applied to a range of cyclic and acyclic aliphatic alcohols with 1a, which gave products 3j–3n with 72–98% isolated yields. In addition, methallyl alcohol was well tolerated, giving 3o in 71% yield. It is interesting that when methyl 1H-indole-2-carboxylate (1b) and benzyl alcohol were combined, the desired product 3p was produced in 81%.
Table 2.
Substrate scope of alcohols for transesterification reactions
Reaction conditions: methyl 1H-pyrrole-2-carboxylate 1a (1.0 mmol), benzyl alcohol 2a (0.2 mmol), Novozym 435 60 mg, 1.0 g molecular sieves, and n-Hexane (10 mL) for 24 h, 50 °C
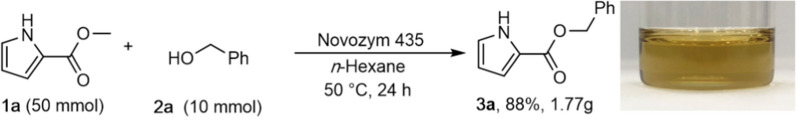
As shown in Scheme 1, large-scale (10 mmol) experiments between methyl 1H-pyrrole-2-carboxylate 1a (50 mmol, 6.25 g) and benzyl alcohol 2a (10 mmol, 1.08 g) were conducted to further evaluate the superlative scalability of this established method. These experiments produced the desired 3a with an excellent yield of 88%. (1.77 g).
Scheme 1.
Gram scale transesterification
The thermal behaviors of compounds
As shown in Table 3, compounds 3j, 3k and 3l possessed strong characteristic aroma such as sweet, herbs, fruity and acid. Studying the chemicals produced under heated conditions was therefore also crucial. The pyrolysis conditions included an oxidizing atmosphere (91% nitrogen, 9% oxygen) and temperatures between 30 and 900 °C. Furthermore, the pyrolysis gas chromatograms obtained under influence of heat are shown in Fig. 9 and the peak position areas of the pyrolysis products are listed in Table 4. As shown in Table 4, the evaporative yield of compound 3j in an oxidizing environment at high temperatures is 96.48%. Compound 3h was also the most stable material tested, releasing just 3.52% of its breakdown at high temperatures in oxidizing environments. When the volatile pyrolysis product of 3j was examined, the main byproducts were cyclopropyl carbinol (1.97%) and pyrrole (1.55%). Under extreme heat in an oxidizing atmosphere, the evaporative yields of compound 3k and compound 3l are 95.15% and 94.93%. Pyrrole (1.87%) and 1-butanol (2.98%) made up most of the pyrolysis products of 3k. Pyrole (1.91%) and 1-pentanol (3.16%) made up most of the pyrolysis products of 3l. It is important to note that several pyrolysis byproducts of the compound 3k and 3l contained the distinguishable flavour components, including pyrrole, 1-butanol and 1-pentanol. They are all appropriate to use as flavor and aroma mediators. For more characterization data of compounds see Additional file 1.
Table 3.
Odor description of compounds
| Entry | Compound | Odor description |
|---|---|---|
| 1 | benzhydryl 1H-pyrrole-2-carboxylate (3j) | Sweet, acid |
| 2 | butyl 1H-pyrrole-2-carboxylate (3k) | Sweet, herbs, acid |
| 3 | pentyl 1H-pyrrole-2-carboxylate (3l) | Sweet, fruity, acid |
Fig. 9.
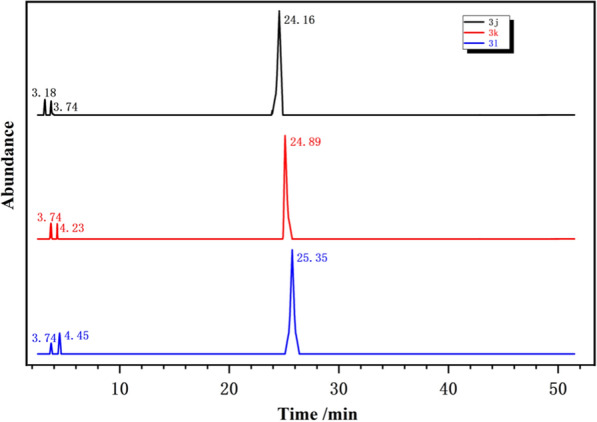
Pyrolysis-GC/MS total ion chromatogram of 3j, 3k and 3l at 30–900 °C
Table 4.
Pyrolysis products of 3j, 3k and 3l (TIC area %)
| Sample | Time | Compound | Area % |
|---|---|---|---|
| benzhydryl 1H-pyrrole-2-carboxylate (3j) | 3.18 | Cyclopropyl carbinol | 1.97 |
| 3.74 | pyrrole | 1.55 | |
| 24.16 | 3j | 96.48 | |
| butyl 1H-pyrrole-2-carboxylate (3k) | 3.74 | pyrrole | 1.87 |
| 4.23 | 1-butanol | 2.98 | |
| 24.89 | 3k | 95.15 | |
| pentyl 1H-pyrrole-2-carboxylate (3l) | 3.74 | pyrrole | 1.91 |
| 4.45 | 1-pentanol | 3.16 | |
| 25.35 | 3l | 94.93 |
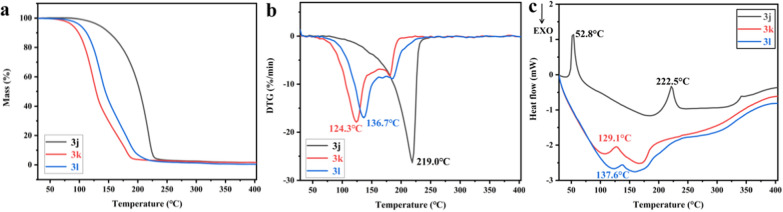
Figure 10a, b shows the TG, DTG, and DSC profiles of the title compounds with 30 and 400 °C at a rate of heating of 10 °C min−1. Compounds 3j, 3k, and 3l decomposed at conditions varying from 70.9 to 250.0, 62.2 to 220.0, and 65.7 to 230.0 °C, respectively, as shown in Fig. 10a, b. And it shows that 3k and 3l had the greatest rate of disintegration at 124.3 and 136.7 °C with starting mass reductions of 41.92% and 38.16%. The samples then showed a consistent and uniform mass loss pattern, with overall weight losses of 98.43% and 99.65%. Most of the thermal mass loss (80.42%) occurred at 219.0 °C, with an ultimate total weight loss of 98.41%, based to both the TG and DTG curves of 3j. Due to the chemical structure of 3j includes cyclopropyl while the structures of 3k and 3l contain straight-chain carbon, most of the thermal mass loss in 3j was higher than in 3k and 3l, which may have been due to distinct stabilizations. These three compounds were found to be steady at ambient temperature by the TG and DTG testing.
Fig. 10.
Thermal degradation curve of compounds 3j, 3k and 3l, a TGA, b DTG and c DSC
Figure 10c shows the DSC individuals for 3j–3l that were examined in an environment of air. Equipment was used to capture the highest temperature of DSC curves and the change in enthalpy of the samples. The Tonset, Tpeak, and Tend temperatures were similar to those discovered using DTG graphs. With the DSC graph of 3j, there is a clear, significant endothermic peak at 52.8 °C. The sharp peak indicates a melting-point of 3j, as there is no weight loss at 52.8 °C, on the other hand. Additionally, it demonstrated that the Tpeak of 3j–3l in DSC curves occurred in the major mass loss areas there, suggesting that the samples may have vaporized or broken down during the endothermic phase. Overall, the DSC and TG-DTG results agreed with the other and showed that the samples were steady at room temperature. The information regarding the outcomes based on the graphs shown in Table 5.
Table 5.
Thermal analysis data of compounds 3j, 3k and 3l
| Compound | DSC | TG-DTG | Mass loss/% | |||||
|---|---|---|---|---|---|---|---|---|
| Tmelt/°C | Tonset/°C | Tpeak/°C | Tend/°C | ∆H/kJ mol−1 | Tp/°C | Trange/°C | ||
| 3j | 52.8 | 206.9 | 222.5 | 229.2 | 92.6 | 219.0 | 70.9–250.0 | 98.41 |
| 3k | – | 111.5 | 129.1 | 144.5 | 54.4 | 124.3 | 62.2–220.0 | 98.43 |
| 3l | – | 124.5 | 137.6 | 146.9 | 25.81 | 136.7 | 65.7–230.0 | 99.65 |
Conclusion
In conclusion, a simple enzymatic approach to synthesis pyrrole esters with commercially accessible alcohols was developed. The effects of lipase type, solvent, lipase load, molecular sieves, reaction time, substrate molar ratio, reaction temperature and speed of agitation on the amount of product were investigated to give optimal reaction conditions. The optimized conditions were set as molar ratio of 5:1 (methyl 1H-pyrrole-2-carboxylate/ benzyl alcohol, 1a/2a), Novozym 435 of 6 mg/mL, stirrer speed of 150 r/min, molecular sieves of 1 g, 50 °C reaction temperature, and a 24 h reaction time in n-Hexane leading to a high yield of 92%. A wide variety of alcohols are well tolerated during the transformation using Novozym 435, and the characterization of their structure was determined by 1H NMR, 13C NMR, HRMS, and IR. The findings of the odor study revealed that compounds 3j, 3k, and 3l exhibit scents that are typically distinct from those of the related methyl 1H-pyrrole-2-carboxylate and alcohols, including sweet, herbal, fruity, and acidic notes. The Py–GC/MS was used to examine the pyrolysis byproducts of the three flavoring compounds 3j, 3k, and 3l in oxidative environments. The evaporative yields of compounds 3j, 3k and 3l were 96.48%, 95.15% and 94.93%, respectively. The TG analysis result showed that the main mass change stage of 3j, 3k and 3l were between 62.2 and 250.0 °C with mass decreased by 98.41%, 98.43% and 99.65% of the total mass, respectively. As seen by the Tpeak of 3j, 3k and 3l in DSC curves, either evaporation or breakdown of the compounds 3j, 3k and 3l took place during in the thermal decomposition phase. They have good thermal stability under certain temperature.
Supplementary Information
Additional file 1: 1. Experimental. 2. Optimization details. 3. General procedure for the transesterification. 4. Characterization data for the transesterification products. 5. Reference. 6. Copy of 1H and 13C NMR spectra of compounds 3a-3p. 7. Copy of Gas chromatography-mass spectrometry ion flow chromatograms of compounds 3a-3p.
Acknowledgements
Not applicable.
Abbreviations
- GC–MS–O
Gas chromatography–mass spectrometry–olfactometry
- Py–GC/MS
Pyrolysis–gas chromatography/mass spectrometry
- TG
Thermogravimetry
- DSC
Differential scanning calorimeter
- CRL
Candida Rugosa lipase
- TLIM
Thermomyces lanuginosus, and immobilized on a non-compressible silica gel carrier
- DMF
N,N-dimethylformamide
- DMSO
Dimethyl sulfoxide
- NMR
Nuclear magnetic resonance
Author contributions
JH: first draft, revision, editing, conceptualization, and methodology in writing. MZ: writing: first draft, revision, and editing, investigation, formal analysis, and validation. YZ: data curation and investigation. XZ: data curation and investigation. XJ: methodology and investigation. MZ: review and editing and supervision. ML: review and editing and investigation.
Funding
We would like to thanks Natural Science Foundation of Henan Province [232300421257] and Henan Agricultural University [30500845] provided funding for this work.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abdul Rahman MB, Zaidan UH, Basri M, Hussein MZ, Rahman RNZA, Salleh AB. Enzymatic synthesis of methyl adipate ester using lipase from Candida rugosa immobilized on Mg, Zn and Ni of layered double hydroxides (LDHs) J Mol Catal B Enzym. 2008;50:33–39. doi: 10.1016/j.molcatb.2007.09.020. [DOI] [Google Scholar]
- 2.Dhake KP, Thakare DD, Bhanage BM. Lipase: a potential biocatalyst for the synthesis of valuable flavour and fragrance ester compounds. Flavour Fragr J. 2013;28:71–83. doi: 10.1002/ffj.3140. [DOI] [Google Scholar]
- 3.Larios A, García HS, Oliart RM, Valerio-Alfaro G. Synthesis of flavor and fragrance esters using Candida antarctica lipase. Appl Microbol Technol. 2004;65:373–376. doi: 10.1007/s00253-004-1602-x. [DOI] [PubMed] [Google Scholar]
- 4.Liu B, Hu F, Shi BF. Recent advances on ester synthesis via transition-metal catalyzed C–H functionalization. ACS Catal. 2015;5:1863–1881. doi: 10.1021/acscatal.5b00050. [DOI] [Google Scholar]
- 5.Evans RW, Zbieg JR, Zhu SL, Li W, MacMillan DWC. Simple catalytic mechanism for the direct coupling of α-carbonyls with functionalized amines: a one-step synthesis of Plavix. J Am Chem Soc. 2013;135:16074–16077. doi: 10.1021/ja4096472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayout I, Bouzemi N, Guo N, Mao X, Serra S, Riva S, Secundo F. Natural flavor ester synthesis catalyzed by lipases. Flavour Frag J. 2020;35:209–218. doi: 10.1002/ffj.3554. [DOI] [Google Scholar]
- 7.Tan Y, Siebert K. Quantitative structure−activity relationship modeling of alcohol, ester, aldehyde, and ketone flavor thresholds in beer from molecular features. J Agr Food Chem. 2004;52:3057–3064. doi: 10.1021/jf035149j. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, Zhao J, Liu X, Zhang C, Zhao Z, Li X, Sun B. Flavor mystery of Chinese traditional fermented baijiu: the great contribution of ester compounds. Food Chem. 2022;369:130920–130932. doi: 10.1016/j.foodchem.2021.130920. [DOI] [PubMed] [Google Scholar]
- 9.Worzakowska M. Synthesis, characterization, and thermal properties of new flavor compounds. J Therm Anal Calorim. 2014;116:727–736. doi: 10.1007/s10973-013-3541-1. [DOI] [Google Scholar]
- 10.Artico M, Silvestri R, Pagnozzi E. Structure-based design, synthesis, and biological evaluation of novel pyrrolyl aryl sulfones: HIV-1 non-nucleoside reverse transcriptase inhibitors active at nanomolar concentrations. J Med Chem. 2000;43:1886–1891. doi: 10.1021/jm9901125. [DOI] [PubMed] [Google Scholar]
- 11.Chowdary GV, Prapulla SG. Optimization of synthesis of ethyl isovalerate using Rhizomucor miehei lipase. Appl Biochem Micro. 2003;39:243–248. doi: 10.1021/jm9901125. [DOI] [PubMed] [Google Scholar]
- 12.Santaniello E, Casati S, Ciuffreda P. Lipase-catalyzed deacylation by alcoholysis: a selective, useful transesterification reaction. Curr Org Chem. 2006;10:1095–1123. doi: 10.2174/138527206777698110. [DOI] [Google Scholar]
- 13.Khan NR, Rathod VK. Enzyme catalyzed synthesis of cosmetic esters and its intensification: a review. Process Chem. 2015;50:1793–1806. doi: 10.1016/j.procbio.2015.07.014. [DOI] [Google Scholar]
- 14.Petersson AEV, Gustafsson LM, Nordblad M. Wax esters produced by solvent-free energy-efficient enzymatic synthesis and their applicability as wood coatings. Green Chem. 2005;7:837–843. doi: 10.1039/B510815B. [DOI] [Google Scholar]
- 15.Chaibakhsh N, Abdul Rahman MB, Abd-Aziz S. Optimized lipase-catalyzed synthesis of adipate ester in a solvent-free system. J Ind Microbiol Biot. 2009;36:1149–1155. doi: 10.1007/s10295-009-0596-x. [DOI] [PubMed] [Google Scholar]
- 16.Vosmann K, Wiege B, Weitkamp P, Weber N. Preparation of lipophilic alkyl (hydroxy)benzoates by solvent-free lipase-catalyzed esterification and transesterification. Appl Microbiol Biot. 2008;80:29–36. doi: 10.1007/s00253-008-1534-y. [DOI] [PubMed] [Google Scholar]
- 17.Kirpluksa M, Pomilovskisab R, Vanagsa E, Abolinsa A, Mierinab I, Fridrihsone A. Influence of different synthesis conditions on the chemo-enzymatic epoxidation of tall oil fatty acids. Process Biochem. 2022;122:38–49. doi: 10.1016/j.procbio.2022.08.024. [DOI] [Google Scholar]
- 18.Meng Z, Geng WX, Li JW, Yang ZQ, Jiang J, Wang XG, Liu YF. Enzymatically catalyzed synthesis of anti-blooming agent 1,3-Dibehenoyl-2-oleoyl glycerol in a Solvent-Free system: optimization by response surface methodology. J Agric Food Chem. 2013;61:10798–10806. doi: 10.1021/jf4031844. [DOI] [PubMed] [Google Scholar]
- 19.Remonattoa D, Fantattob RR, Pietrob CLR, Monti R, Oliveirac JV, Velosode A, Bassana PJC. Enzymatic synthesis of geranyl acetate in batch and fed-batch reactors and evaluation of its larvicidal activity against Rhipicephalus (Boophilus) microplus. Process Biochem. 2022;120:287–300. doi: 10.1016/j.procbio.2022.06.012. [DOI] [Google Scholar]
- 20.Lozano P, Piamtongkam R, Kohns K. Ionic liquids improve citronellyl ester synthesis catalyzed by immobilized Candida antarctica lipase B in solvent-free media. Green Chem. 2007;9:780–784. doi: 10.1039/B617444B. [DOI] [Google Scholar]
- 21.Paroul N, Grzegozeski LP, Chiaradia V. Solvent-free production of bioflavors by enzymatic esterification of citronella (Cymbopogon winterianus) essential oil. Appl Biochem Biotech. 2012;166:13–21. doi: 10.1007/s12010-011-9399-4. [DOI] [PubMed] [Google Scholar]
- 22.Jawale PV, Bhanage BM. Synthesis of propyl benzoate by solvent-free immobilized lipase-catalyzed transesterification: optimization and kinetic modeling. Bioproc Biosyst Eng. 2021;44:369–378. doi: 10.1007/s00449-020-02448-9. [DOI] [PubMed] [Google Scholar]
- 23.Chowdary GV, Prapulla SG. Enzymatic synthesis of ethyl hexanoate by transesterification. Int J Food Sci Tech. 2003;38:127–133. doi: 10.1046/j.1365-2621.2003.00653.x. [DOI] [Google Scholar]
- 24.Adarme CAA, Leão RAC, Souza SP. Continuous-flow chemo and enzymatic synthesis of monoterpenic esters with integrated purification. Mol Catal. 2018;453:39–46. doi: 10.1016/j.mcat.2018.04.007. [DOI] [Google Scholar]
- 25.Mahapatra P, Kumari A, Garlapati VK. Enzymatic synthesis of fruit flavor esters by immobilized lipase from Rhizopus oligosporus optimized with response surface methodology. J Mol Catal B-Enzym. 2009;60:57–63. doi: 10.1016/j.molcatb.2009.03.010. [DOI] [Google Scholar]
- 26.Meneses AC, Balen M, Andrade JE. Enzymatic synthesis of benzyl benzoate using different acyl donors: comparison of solvent-free reaction techniques. Process Biochem. 2020;92:261–268. doi: 10.1016/j.procbio.2020.01.018. [DOI] [Google Scholar]
- 27.Rahman INA, Attan N, Mahat NA. Statistical optimization and operational stability of Rhizomucor miehei lipase supported on magnetic chitosan/chitin nanoparticles for synthesis of pentyl valerate. Int J Boil Macromol. 2018;115:680–695. doi: 10.1016/j.ijbiomac.2018.04.111. [DOI] [PubMed] [Google Scholar]
- 28.Scholze B, Hanser C, Meier D. Characterization of the water-insoluble fraction from fast pyrolysis liquids (pyrolytic lignin): Part II. GPC, carbonyl goups, and 13C-NMR. J Anal Appl Pyrol. 2001;60:387–400. doi: 10.1016/S0165-2370(00)00173-X. [DOI] [Google Scholar]
- 29.Gerber L, Eliasson M, Trygg J, Moritz T, Sundberg B. Multivariate curve resolution provides a high-throughput data processing pipeline for pyrolysis–gas chromatography/mass spectrometry. J Anal Appl Pyrol. 2012;95:95–100. doi: 10.1016/j.jaap.2012.01.011. [DOI] [Google Scholar]
- 30.Li R, Yin X, Zhang S, Yang J, Zhao M. Preparation and pyrolysis of two Amadori analogues as flavor precursors. J Anal Appl Pyrol. 2021;160:105357–105367. doi: 10.1016/j.jaap.2021.105357. [DOI] [Google Scholar]
- 31.Zhang Y, Wang X, Zou S, Xie D, Jin Q, Wang X. Synthesis of 2- docosahexaenoylglycerol by enzymatic ethanolysis. Biores Technol. 2018;251:334–340. doi: 10.1016/j.biortech.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Zhang DH, Chen N, Zhi GY. Synthesis of benzyl cinnamate by enzymatic esterification of cinnamic acid. Biores Technol. 2015;198:256–261. doi: 10.1016/j.biortech.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 33.Duan Z, Du W, Liu D. The solvent influence on the positional selectivity of Novozym 435 during 1,3-diolein synthesis by esterification. Biores Technol. 2010;23:2568–2571. doi: 10.1016/j.biortech.2009.11.087. [DOI] [PubMed] [Google Scholar]
- 34.Liu M, Fu J, Teng Y, Zhang Z, Zhang N, Wang Y. Fast production of diacylglycerol in a solvent free system via lipase catalyzed esterification using a bubble column reactor. J Am Oil Chem Soc. 2016;93:637–648. doi: 10.1007/s11746-016-2804-y. [DOI] [Google Scholar]
- 35.Mustafa UU. A study on the lipase catalyzed esterification in organic solvent. Trends J Agric Forest. 1998;22:1697–1702. [Google Scholar]
- 36.Kuwabara K, Watanabe Y, Adachi S, Nakanishi K, Matsuno R. Synthesis of 6-o-unsaturated acyl-ascorbates by immobilized lipase in acetone in the presence of molecular sieve. Biochem Eng J. 2003;16:17–22. doi: 10.1016/S1369-703X(03)00020-2. [DOI] [Google Scholar]
- 37.Sonwalker RD, Chen CC, Ju LK. Roles of silica gel in poly-condensation of lactic acid in organic solvent. Bioresour Technol. 2003;87:69–73. doi: 10.1016/S0960-8524(02)00197-9. [DOI] [PubMed] [Google Scholar]
- 38.Guillen M, Benaiges MD, Valero F. Biosynthesis of ethyl butyrate by immobilized recombinant Rhizopus oryzae lipase expressed in Pichia pastoris. Biochem Eng J. 2012;65:1–9. doi: 10.1016/j.bej.2012.03.009. [DOI] [Google Scholar]
- 39.Li C, Sun J, Li T, Liu SQ, Huang D. Chemical and enzymatic synthesis of a library of 2-phenethyl esters and their sensory attributes. Food Chem. 2014;154:205–210. doi: 10.1016/j.foodchem.2013.12.102. [DOI] [PubMed] [Google Scholar]
- 40.Kanwar SS, Gehlot S, Verma ML, Gupta R, Kumar Y, Chauhan GS. Synthesis of geranyl butyrate with the poly (acrylic acid-co-hydroxy propyl methacrylate-cl-ethylene glycol dimethacrylate) hydrogel immobilized lipase of Pseudomonas aeruginosa MTCC-4713. J Appl Polym Sci. 2008;110:2681–2692. doi: 10.1002/app.28241. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: 1. Experimental. 2. Optimization details. 3. General procedure for the transesterification. 4. Characterization data for the transesterification products. 5. Reference. 6. Copy of 1H and 13C NMR spectra of compounds 3a-3p. 7. Copy of Gas chromatography-mass spectrometry ion flow chromatograms of compounds 3a-3p.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.