Abstract
Introduction:
Globally, there are 3 million people living with multiple sclerosis (PLW-MS). A large proportion of PLW-MS have abnormal vestibular function tests that suggest central vestibular lesions. Yet, data regarding vestibular-ocular control in PLW-MS is limited. Thus, we aimed to further characterize compensatory saccade (CS) behavior in PLW-MS.
Methods:
We analyzed video head impulse data from four groups of six age- and sex-matched adults: people living with mild MS (PLW-mild-MS, people living with moderate MS (PLW-moderate-MS), people living with unilateral vestibular deafferentation (PLW-UVD), and healthy controls (HC).
Results:
PLW-moderate-MS had lower lateral canal vestibulo-ocular reflex (VOR) gain bilaterally compared to PLW-mild MS (p < 0.001), HC (p < 0.001), and PLW-UVD (p < 0.001). CS frequency was higher for impulses towards the less affected side in PLW-moderate-MS versus the more (p = 0.01) and less (p < 0.001) affected sides in PLW-mild-MS. CS latency was shorter (p < 0.001) and CS peak velocity was lower (p < 0.001) with impulses towards the more affected side versus the less affected side in PLW-moderate-MS. However, CS peak velocity with impulses towards each side was similar in PLW-mild-MS (p = 0.12). Gaze position error (GPE) was larger after impulses towards the more affected side versus the less affected side in PLW-moderate-MS (p < 0.001) and PLW-mild-MS (p < 0.001). MS-related disability was moderately associated with VOR gain (p < 0.001) and GPE (p < 0.001). Additionally, we identified micro-saccades and position correcting saccades that were uniquely employed by PLW-MS as compensatory gaze stabilizing strategies.
Conclusions:
In PLW-MS, the characteristics of compensatory oculomotor behavior depend on the extent of residual VOR gain.
Keywords: Vestibulo-ocular reflex, Video head impulse test, Multiple sclerosis
1. Introduction
Worldwide, there are nearly three million people living with multiple sclerosis (PLW-MS) [1], a central nervous system disease characterized by the demyelination and inflammation of neurons throughout the central nervous system [2]. One-third of PLW-MS experience vertigo [3], and two-thirds of those individuals have abnormalities in vestibular evoked myogenic potentials that suggest demyelination in central otolithic pathways [4]. Likewise, abnormally low vestibular-ocular reflex (VOR) gain or the presence of overt compensatory saccades bilaterally on video head impulse testing (vHIT) is strongly suggestive of abnormal conveyance of semicircular canal afference from brainstem nuclei in PLW-MS [5–7].
Infratentorial involvement is common in PLW-MS as prior research shows that over 70% of PLW-MS have brainstem lesions on magnetic resonance imaging and nearly 90% have abnormal brainstem reflexes [8]. The majority of PLW-MS also have signs of cerebellar involvement [9]. Brainstem and cerebellar lesions result in oculomotor dysfunction in up to 80% of PLW-MS [10], which, in turn, is associated with greater disability [11]. Additionally, PLW-MS have been shown to have impaired visual acuity during head movement [12], and worse functional performance of the VOR has also been associated with increased MS-related disability [13]. Visual function is equivocally ranked with gait as being important to PLW-MS, particularly those with more advanced disease [14]. This coupled with the interaction between vestibular and visual function during dynamic activities, suggests that further attention to visual-vestibular capacity in PLW-MS is warranted.
A compensatory saccade (CS) is a high acceleration eye rotation that repositions the eye in the direction opposite a head rotation, and, thus, is a strategy for reducing gaze position error (GPE) and gaze instability [15]. During VOR testing using vHIT, PLW-MS have been shown to recruit compensatory saccades (CSs) that are more frequent and of a reduced latency relative to healthy controls [15]. Additionally, asymmetrical VOR gains have been reported in PLW-MS who have brainstem lesions [16,17]. However, the characteristics of CSs recruited by PLW-MS who have differing levels of disease-related disability have not yet been described, and whether CSs represent an effective strategy for reducing GPE in PLW-MS has not yet been examined. Thus, we sought to 1) contrast vHIT results of PLW-MS who have differing levels of disease severity with those of people living with unilateral vestibular deafferentation (PLW-UVD) and healthy controls (HC), 2) compare the results for rightward and leftward impulses within and between the groups, 3) examine whether MS-related disability was associated with vestibulo-ocular and compensatory oculomotor function, and 4) characterize vHIT abnormalities that are unique to PLW-MS.
2. Methods
2.1. Participants
We analyzed baseline data for a subset of 24 adults that was previously collected [12,18]. This sample included four groups of age- and sex-matched adults: 6 people living with mild MS (PLW-mild-MS: Extended Disability Status Scale [EDSS] [19] score = 2.0 to 3.5) [12], 6 people living with moderate MS (PLW-moderate-MS: EDSS = 3.0 to 4.5) [12], 6 people who were status-post unilateral vestibular schwannoma resection (PLW-UVD) [18], and 6 HC [12] (See Table 1).
Table 1.
Participant characteristics.
| Variable | HC (n = 6) | PLW-UVD (n = 6) | PLW-mild-MS (n = 6) | PLW-moderate-MS (n = 6) |
|---|---|---|---|---|
|
| ||||
| Agea | 57.2 (16.6) | 52.3 (14.7) | 56.2 (12.9) | 54.8 (14.1) |
| EDSSb | NA | NA | 2.8 (2.5–3.0) | 4.0 (4.0–4.0) |
| ABC Scale (Average)b | NA | 70 (52–85) | 89 (74–92) | 66 (41–89) |
| DHI (Total)b | NA | 28 (18–58) | 37 (28–42) | 52 (32–65) |
HC = healthy control. PLW-UVD = unilateral vestibular deafferentation. PLW-mild-MS = people living with mild multiple sclerosis. PLW-moderate-MS = people living with moderate multiple sclerosis. EDSS = Expanded Disability Severity Scale. ABC Scale = Activities-specific Balance Confidence Scale. DHI = Dizziness Handicap Inventory.
These data are presented as the mean (standard deviation).
These data are given as the median (interquartile range).
2.2. Inclusion and exclusion criteria
All PLW-MS had a neurologist-confirmed diagnosis of MS, in addition to a self-reported symptom of dizziness (Dizziness Handicap Inventory [20]), evidence of impaired balance (Dynamic Gait Index [21] score < 19 or Activities Balance Confidence Scale [22] average score < 80%), and/or a history of ≥2 falls in the past year.
Each potential participant participated in a thorough clinical examination during which we obtained a medical history and conducted objective testing including video-oculography for spontaneous nystagmus, post-head-shaking nystagmus, oculomotor function (i.e. saccades and smooth pursuit, VOR suppression), a clinical yaw head impulse test, and positional testing to rule out benign paroxysmal positional vertigo [23]. The purpose of this examination was to rule out co-morbid peripheral vestibular dysfunction and/or other central nervous system disorders. Testing of PLW-MS was conducted while their disease was stable. PLW-MS who had a concurrent peripheral vestibular dysfunction, another central or peripheral system disorder besides MS, and/or EDSS scores >6.0 were excluded. None of the PLW-MS had clinical evidence of internuclear ophthalmoplegia (INO).
The PLW-UVD were tested during the chronic stage of recovery (> 6 weeks) to permit adequate compensation [24] (Mean [SD]: 89.31 [211.06] days). Individuals with UVD and concurrent neurologic disorders were excluded. The HC were age- and gender-matched to PLW-moderate-MS.
2.3. Video head impulse test
The vHIT data were collected using the ICS Impulse system (Oto-metrics, Natus Medical Incorporated; Taastrup, Denmark). The raw data traces for each head impulse were post-processed using custom written software (MATLAB; Natick, MA) [15,16,26]. Each assessor delivered low amplitude (10–25°), high acceleration (4000°/s2) yaw plane head impulses to both the left and right sides while the participant viewed a target positioned on the wall at 1 m. The head movement data were collected with goggle-mounted accelerometers and eye velocity was video-recorded at 250 frames/s.
2.4. Identification of compensatory saccades
We used an acceleration threshold of 4000°/s2 to aid in identifying each CS [25,26]; however, the initiation and termination of each CS was defined using a semi-automated, iterative process. First, to identify the approximate initiation of each CS, our software identified the peak acceleration (≥ 4000°/s2) and then worked backwards to the point where the acceleration first rose above 2000°/s2 [24]. Second, to find the approximate termination of each CS, our software worked forward from the peak acceleration to find the deceleration peak, and defined the approximate CS termination point as the time after the deceleration peak when the acceleration rose above − 4000°/s2 and the eye no longer traveled in the same direction [27]. Third, to aid in delineating covert CS from slow phase eye velocity (SPEV), the deceleration peak that was used to determine the termination of the CS was automatically reset more conservatively at − 2000°/s2. The approximate termination point for a CS was defined by our software as the time when the deceleration peak was > − 2000°/s2 and the eye acceleration no longer monotonically decreased.
Once these approximate boundaries were defined, our software refined the initiation and termination of each CS. First, the eye velocity signal was processed through a zero-phase loss, 20 Hz low pass filter (MATLAB; filtfilt) to find the peak velocity between the temporary CS initiation and termination points, and the filtered velocity trace was used to define the first point where the CS velocity was > 10°/s [28]. Second, two linear fits were performed (MATLAB; polyfit) between the peak eye velocity and the first point when eye velocity was >10°/s and a point 44 ms earlier [28]. Our software used the intersection of these two linear fits to define the initiation point for each CS [28]. The termination point for each CS was defined by performing linear fits between peak eye velocity and the first point after the peak eye velocity that was < 10°/s and a point 44 ms later. Additionally, a minimum CS duration was hard-coded at 6 ms.
Throughout this process, an experienced rater (CRG) reviewed the traces with the CSs marked, assessed the trace for any artifacts or errors, and, then, confirmed the position of the finalized initiation and termination points for each CS. If the endpoint(s) for a given CS fell well outside of the expected bounds of any CS based upon visual inspection of the eye velocity, acceleration, and/or position traces, the rater used the available data to define its appropriate initiation and/or termination point(s) [25–27].
2.5. Variables of interest
VOR gain was measured by dividing the area under the desaccaded eye velocity curve, i.e., with covert CSs removed, by the area under the head velocity curve [15,25,27,29–31]. A VOR gain of 1.0 reflects the ideal compensatory SPEV response for a given head impulse velocity and normal values vary from 0.80 to 1.20 on vHIT [31]. We calculated the overall CS frequency as the total number of covert and/or overt CSs per impulse. We defined asymmetry in VOR gain as the difference in VOR gain magnitude for rightward and leftward impulses. The overall CS latency and overall CS peak velocity of all CSs that occurred during each impulse were also calculated. We examined the effectiveness of CSs in overcoming GPE by calculating the difference (in degrees) between the eye position at rest prior to the head impulse and the eye position at the conclusion of the head impulse, once head velocity reached 0°/s. GPE reflects the combined influence of both the SPEV, i.e., VOR gain, and any covert CSs, and, thus, is a more comprehensive measure of the functioning of the gaze stability system in the position domain. A GPE of 5° implies that the eye is 5° off target in the same direction of the head impulse. A single CS with an amplitude of − 5° or multiple CSs with the same cumulative amplitude in the direction of the earth-fixed visual target would perfectly accommodate this error. Detrimental effects on gaze stability occur when CSs are either under- or over-compensatory.
2.6. Statistical analysis
All analyses were conducted in R for Statistical Computing (v. 4.1.2) [32]. The a priori alpha level for all analyses was 0.05. For most metrics, the distributions significantly deviated from normality (Shapiro-Wilkes tests) and the variances were unequal (Fligner-Killeen tests); thus, between-side and between-group differences were evaluated using Kruskal-Wallis tests. The p-values for pairwise comparisons were corrected using Holm’s method. Correlations between either the EDSS score and other variables of interest were examined using Spearman’s method. Complete results are presented in Table S1 (Supplemental Content).
3. Results
VOR gains were asymmetrical for PLW-moderate-MS, PLW-mild-MS, and PLV-UVD, but symmetrical for HC (Fig. 1). Since no statistically significant between-side differences were found for HC, data for rightward and leftward impulses in HC were averaged for comparison with other groups. CS frequency, latency, and peak velocity differed across groups, depending on the VOR gain (Fig. 2). Additionally, GPE was dramatically worse for PLW-moderate-MS compared to all other groups and moderately worse in PLW-mild-MS compared to HC (Fig. 3). Since PLW-mild- and moderate-MS were found to have significant differences in CS characteristics when compared to HC, we presumed a global impact of MS on the central nervous system; subsequently, we refer to the sides with lower and higher residual VOR gain in these groups as the “more” and “less” affected sides, respectively.
Fig. 1.
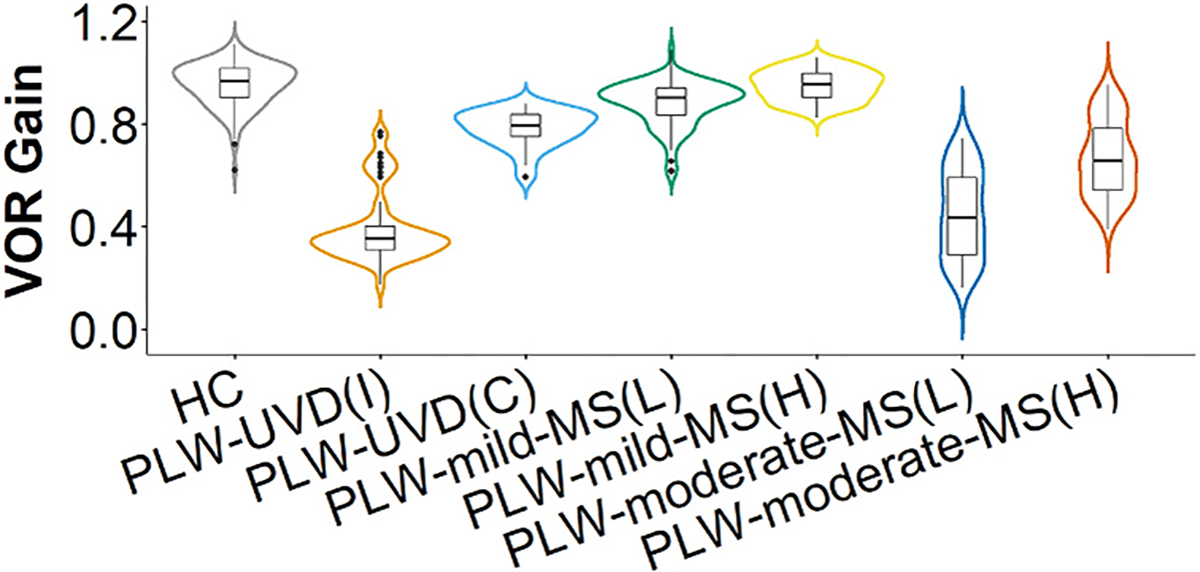
Vestibulo-ocular reflex gain. The data for vestibulo-ocular reflex (VOR) gains for each group is depicted by overlaying a box plot on a violin plot. The box plot provides a traditional visualization of the median and interquartile range and the violin plot shows the distribution of the data. HC = healthy control. PLW-UVD = people living with unilateral vestibular deafferentation. PLW-mild-MS(L) = people living with mild multiple sclerosis, lower VOR gain side. PLW-mild-MS(H) = people living with mild multiple sclerosis, higher VOR gain side. PLW-moderate-MS(L) = people living with moderate multiple sclerosis, lower VOR gain side. PLW-moderate-MS(H) = people living with moderate multiple sclerosis, higher VOR gain side.
Fig. 2.
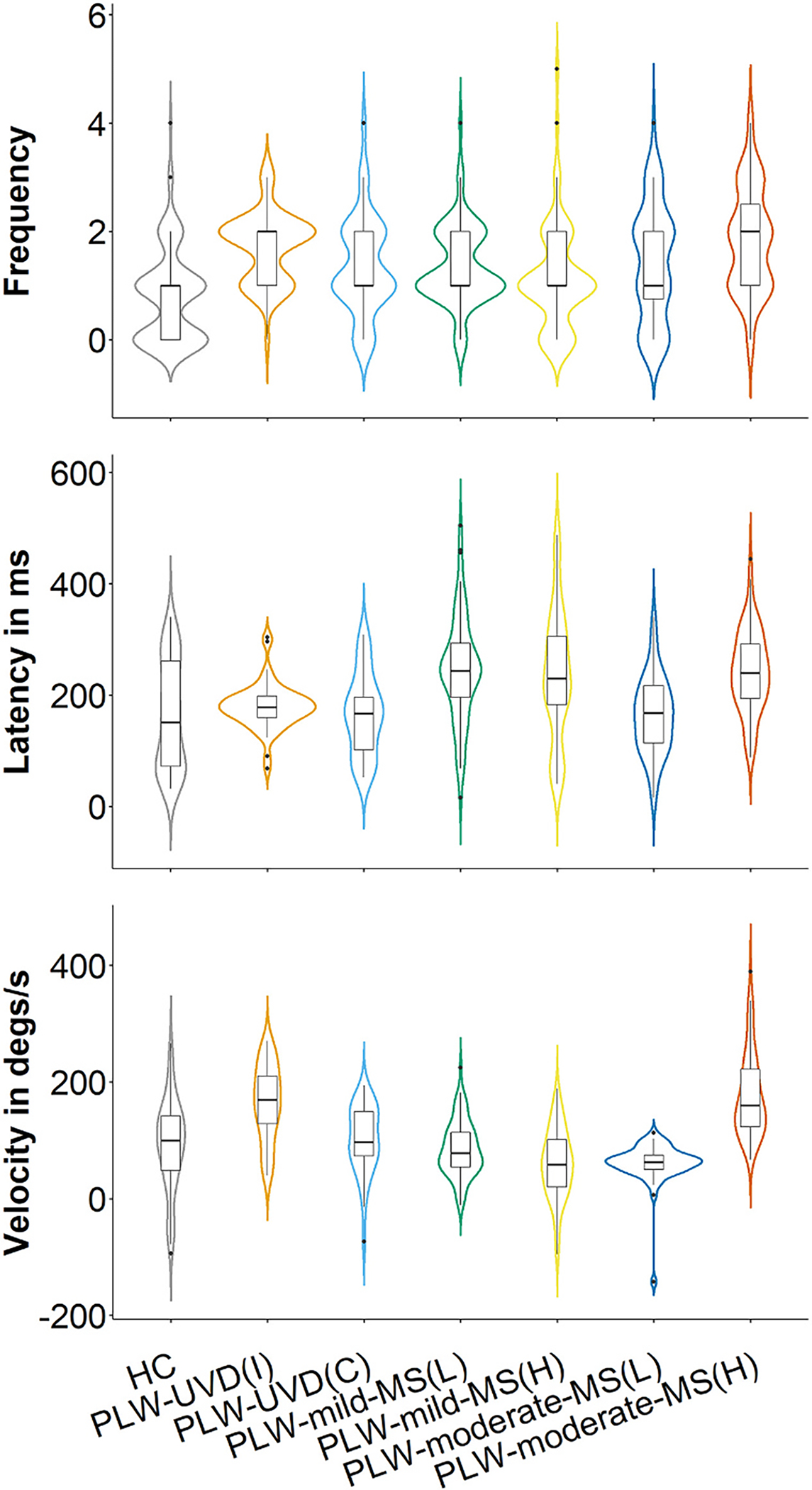
Compensatory saccade frequency, latency, and peak velocity. The data for compensatory saccade characteristics for each group is depicted by overlaying a box plot on a violin plot. The box plot provides a traditional visualization of the median and interquartile range and the violin plot shows the distribution of the data. HC = healthy control. PLW-UVD = people living with unilateral vestibular deafferentation. PLW-mild-MS(L) = people living with mild multiple sclerosis, lower VOR gain side. PLW-mild-MS(H) = people living with mild multiple sclerosis, higher VOR gain side. PLW-moderate-MS(L) = people living with moderate multiple sclerosis, lower VOR gain side. PLW-moderate-MS(H) = people living with moderate multiple sclerosis, higher VOR gain side. ms = milliseconds. Degs = degrees. s = second.
Fig. 3.
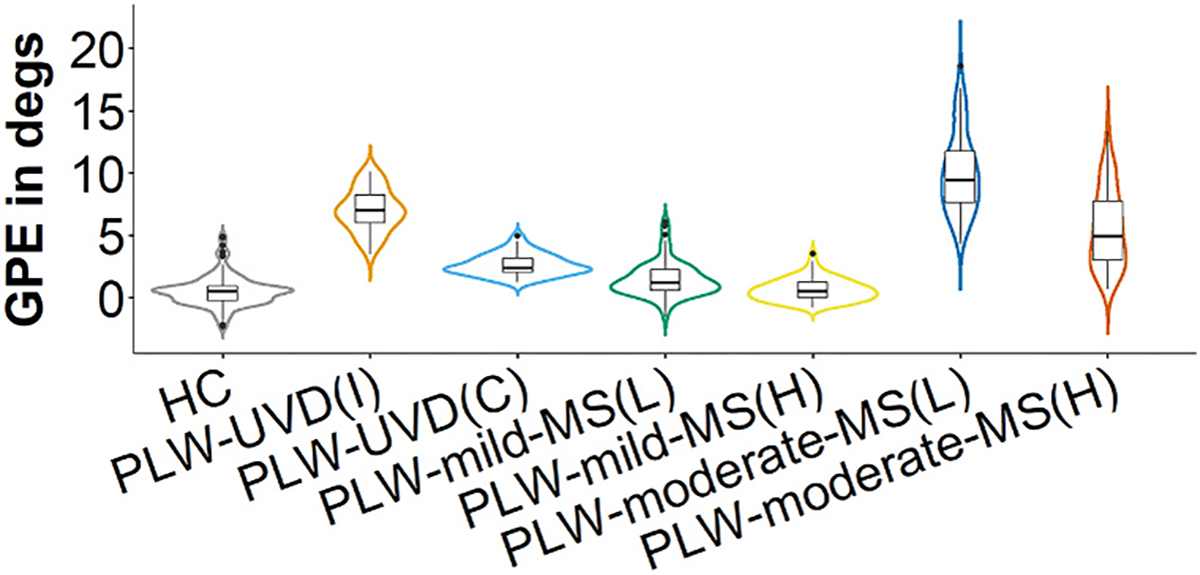
Gaze position error. The data for gaze position error (GPE) for each group is depicted by overlaying a box plot on a violin plot. The box plot provides a traditional visualization of the median and interquartile range and the violin plot shows the distribution of the data. HC = healthy control. PLW-UVD = people living with unilateral vestibular deafferentation. PLW-mild-MS(L) = people living with mild multiple sclerosis, lower VOR gain side. PLW-mild-MS(H) = people living with mild multiple sclerosis, higher VOR gain side. PLW-moderate-MS(L) = people living with moderate multiple sclerosis, lower VOR gain side. PLW-moderate-MS(H) = people living with moderate multiple sclerosis, higher VOR gain side. Degs = degrees.
3.1. Vestibulo-ocular reflex gain
VOR gain (right and left yaw) was always lower for PLW-moderate-MS than PLW-mild MS (p < 0.001), healthy controls (p < 0.001), and for contralesional head rotation in PLW-UVD (p < 0.001). All PLW-moderate-MS had asymmetrical, bilaterally reduced VOR gains (right lower than left) (p < 0.001). Although, as a group, PLW-mild-MS had VOR gains that were within normal limits, all PLW-mild-MS had asymmetrical VOR gains (two with right lower than left) compared with HC (p < 0.001).
3.2. Compensatory saccade characteristics
3.2.1. Compensatory saccade frequency
In PLW-moderate-MS (p = 0.18) and PLW-mild-MS (p = 0.33), CS frequency was similar for impulses towards the more and less affected sides. CSs were more frequently elicited with impulses towards the less affected side in PLW-moderate-MS versus the more (p = 0.01) and less (p < 0.001) affected sides in PLW-mild-MS.
Compared to HC, fewer CSs were elicited with impulses towards the more affected side (p = 0.001) but more CSs were evoked by impulses towards the less affected side (p < 0.001) in PLW-moderate-MS. Additionally, fewer CSs were elicited with impulses towards the more affected side in PLW-mild-MS compared with HC (p < 0.001), but the number of CSs evoked by impulses towards the less affected side in PLW-mild-MS was similar to that of HC (p = 0.07).
CSs were elicited as frequently with impulses towards the more affected side in PLW-moderate-MS as with ipsilesional (p = 0.57) and contralesional (p > 0.99) impulses in PLW-UVD. Similarly, CSs were elicited as frequently with impulses towards the less affected side in PLW-moderate-MS as with ipsilesional (p > 0.99) and contralesional (p = 0.09) impulses in PLW-UVD. However, fewer CSs were elicited with impulses towards the more affected (p = 0.03) and less affected (p < 0.001) side in PLW-mild-MS compared with that for ipsilesional impulses in PLW-UVD.
3.2.2. Compensatory saccade latency
In PLW-moderate-MS, CS latency was shorter for impulses towards the more affected side compared with impulses towards the less affected side (p < 0.001). However, CS latency for impulses towards each side was similar in PLW-mild-MS (p > 0.99). CS latency for impulses towards the more affected side in PLW-moderate-MS was shorter than that for impulses towards the less affected (p < 0.001) and more affected (p < 0.005) sides in PLW-mild-MS.
Compared to HC, CS latency for PLW-moderate-MS was similar for impulses towards the more affected side (p > 0.99) but shorter with impulses towards the less affected side (p < 0.001). In PLW-mild-MS, CS latency for impulses towards the more affected (p < 0.001) and less affected (p = 0.008) side was longer than that for HC.
CS latency for impulses towards the less affected side in PLW-moderate-MS was longer than that for ipsilesional (p < 0.001) and contralesional (p < 0.005) impulses in PLW-UVD. Additionally, CS latency for impulses towards the more affected side in PLW-mild-MS was longer than that for ipsilesional (p < 0.001) and contralesional (p < 0.001) impulses in PLW-UVD. Similarly, CS latency for impulses towards the less affected side gain in PLW-mild-MS was also longer than that for ipsilesional (p = 0.005) and contralesional (p = 0.01) impulses in PLW-UVD.
3.2.3. Compensatory saccade peak velocity
In PLW-moderate-MS, CS peak velocity was lower for impulses towards the more affected versus the less affected side (p < 0.001). However, CS peak velocity was similar for impulses towards both sides in PLW-mild-MS (p = 0.12). CS peak velocity for impulses towards the less affected side was higher in PLW-moderate-MS versus that for impulses towards the more affected side (p < 0.001) and less affected (p < 0.001) sides in PLW-mild-MS.
Compared to HC, CS peak velocity for impulses towards the more affected side was lower in PLW-moderate-MS (p = 0.001). Conversely, CS peak velocity for impulses towards the less affected side in PLW-moderate-MS was higher than that for HC (p < 0.001). In PLW-mild-MS, CS peak velocity for impulses towards the less affected side was lower versus that for HC (p = 0.02).
CS peak velocity for impulses towards the more affected side was lower in PLW-moderate-MS versus that for ipsilesional (p < 0.001) and contralesional (p = 0.001) impulses in PLW-UVD. Conversely, CS peak velocity for impulses towards the less affected side in PLW-moderate-MS was higher than that for contralesional impulses (p < 0.001) in PLW-UVD. In PLW-mild-MS, CS peak velocity for impulses towards the more affected side was lower versus that for ipsilesional (p < 0.001) and contralesional (p < 0.001) impulses in PLW-UVD. Additionally, CS peak velocity for impulses towards the less affected side in PLW-mild-MS was lower than that for contralesional impulses in PLW-UVD (p = 0.006).
3.3. Gaze position error
GPE was larger after impulses towards the more affected side versus impulses towards the less affected side in PLW-moderate-MS (p < 0.001) and PLW-mild-MS (p < 0.001). In PLW-moderate-MS, GPE was larger after impulses towards the more affected side versus that for impulses towards the more affected (p < 0.001) and less affected (p < 0.001) sides in PLW-mild-MS. Similarly, PLW-moderate-MS had larger GPE after impulses towards the less affected side versus that for impulses towards the more affected (p < 0.001) and less affected (p < 0.001) sides in PLW-mild-MS.
Compared to HC, GPE was larger after impulses towards the more affected (p < 0.001) and less affected (p < 0.001) sides in PLW-moderate-MS. Additionally, GPE was larger after impulses towards the more affected side (p < 0.001) in PLW-mild-MS versus that of HC.
GPE was larger after impulses towards the more affected side in PLW-moderate-MS compared with ipsilesional (p < 0.001) and contralesional (p < 0.001) impulses in PLW-UVD. Similarly, PLW-moderate-MS had larger GPE after impulses towards the less affected side versus that for ipsilesional (p = 0.004) and contralesional (p < 0.001) impulses in PLW-UVD. In PLW-mild-MS, GPE was smaller after impulses towards the more affected side versus that for ipsilesional (p < 0.001) and contralesional (p < 0.001) impulses in PLW-UVD. Similarly, PLW-mild-MS had smaller GPE after impulses towards the less affected side versus that for ipsilesional (p < 0.001) and contralesional (p < 0.001) impulses in PLW-UVD.
3.4. Association between disability and vestibular and oculomotor functions
A significant, moderate, negative association between the EDSS score and VOR gain (rho = − 0.68 [95% Confidence Interval: − 0.74 - −0.61]; p < 0.001) and a significant, moderate, positive association between EDSS score and GPE (rho = 0.69 [95% Confidence Interval: 0.62–0.75]; p < 0.001) was present in PLW-MS. However, the EDSS score was only weakly correlated with CS frequency (rho = 0.35 [95% Confidence Interval: 0.24–0.45]; p < 0.001) and CS peak velocity (rho = 0.21 [95% Confidence Interval: 0.09–0.33]; p < 0.001). The EDSS score was not significantly correlated with CS latency (rho = − 0.10 [95% Confidence Interval: − 0.23–0.02]; p = 0.112) in PLW-MS.
3.5. Unique responses to head impulses in PLW-MS
Visual analysis of the raw head and eye velocity traces led to the discovery of several novel compensatory oculomotor findings. First, PLW-moderate-MS generated slow and/or repetitive micro-saccadic eye movements that gradually brought the eyes towards the earth-fixed visual target (Fig. 4). Second, PLW-mild-MS often generated CSs that were over-compensatory for the impulse, and, then, generated another CS to correct for over-shooting the target (Fig. 5). Third, we observed that compensatory oculomotor responses were absent with impulses towards the more affected side yet were preserved with impulses towards the less affected side in one person living with moderate MS (Fig. 6).
Fig. 4.
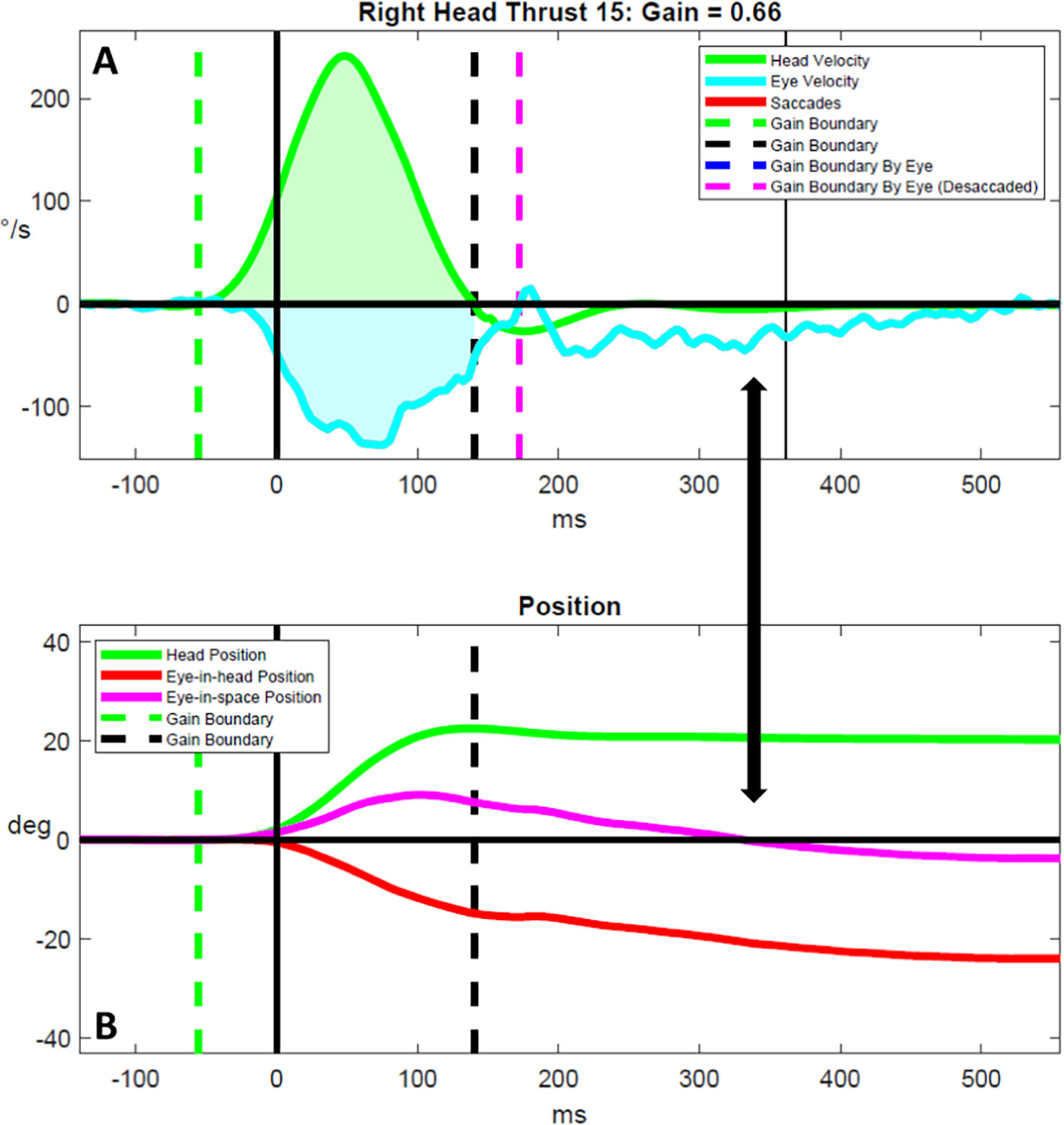
Micro-compensatory-saccadic eye movements in people living with moderate multiple sclerosis. Head and eye velocity traces are shown in (A) and head and eye position traces are shown in (B) for a representative vHIT result for a person living with moderate multiple sclerosis. The double arrow highlights possible micro-compensatory-saccadic eye movements that were of too low acceleration and velocity to be adequately detected and parsed by our custom software, despite halving the detection thresholds (A), that correspond with a slow return of the eye-in-space position to 0 degrees (B). °/s = degrees/s. deg. = degrees. ms = milliseconds.
Fig. 5.
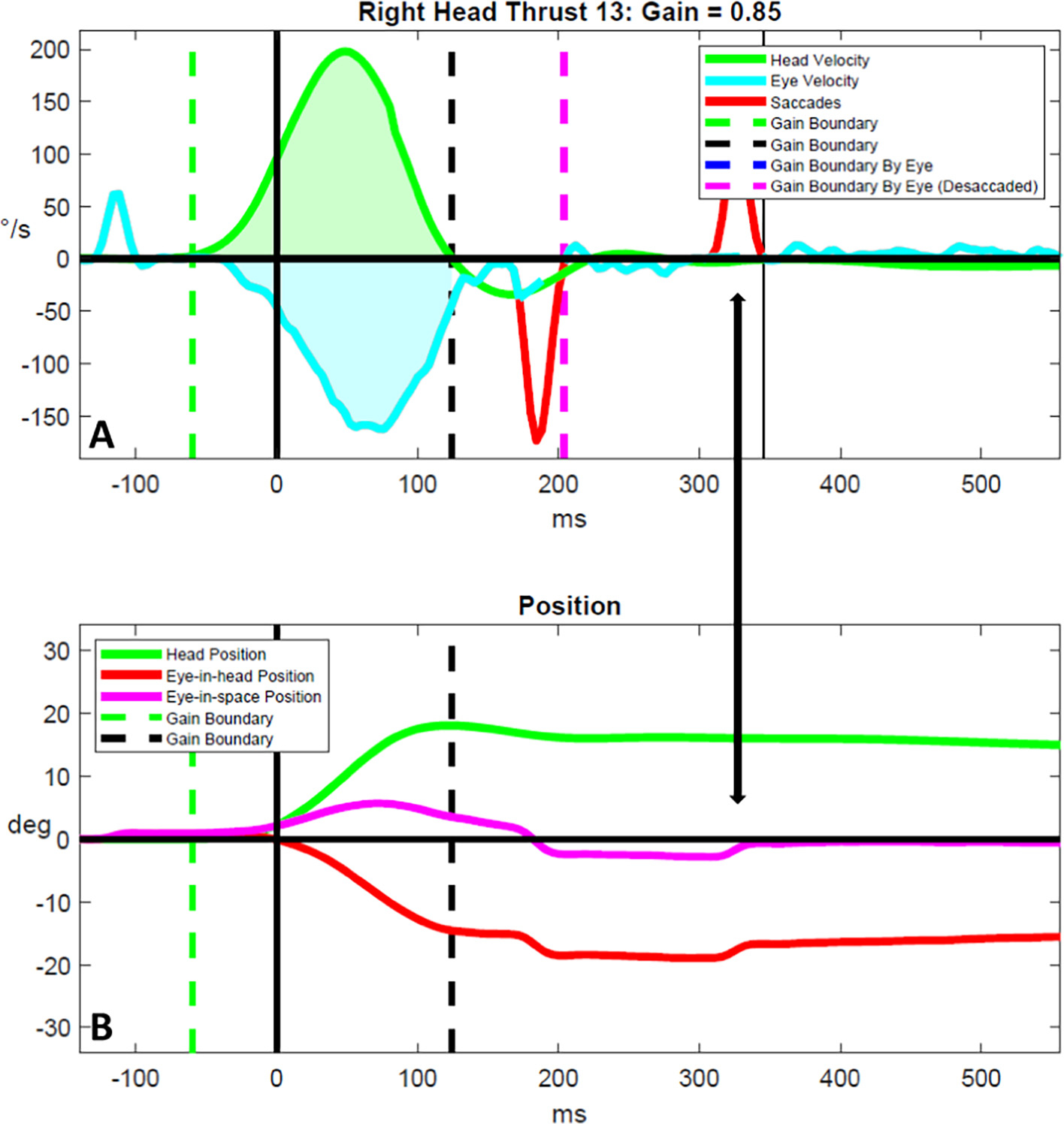
Position correcting saccades in people living with mild multiple sclerosis. Head and eye velocity traces are shown in (A) and head and eye position traces are shown in (B) for a representative vHIT result for a person living with mild multiple sclerosis. The double arrow highlights the occurrence of a position correcting saccade that is elicited in response to the head impulse and that re-adjusts eye position following an over-compensatory overt saccade. Position correcting saccades were only observed in those with mild multiple sclerosis. °/s = degrees/s. deg. = degrees. ms = milliseconds.
Fig. 6.
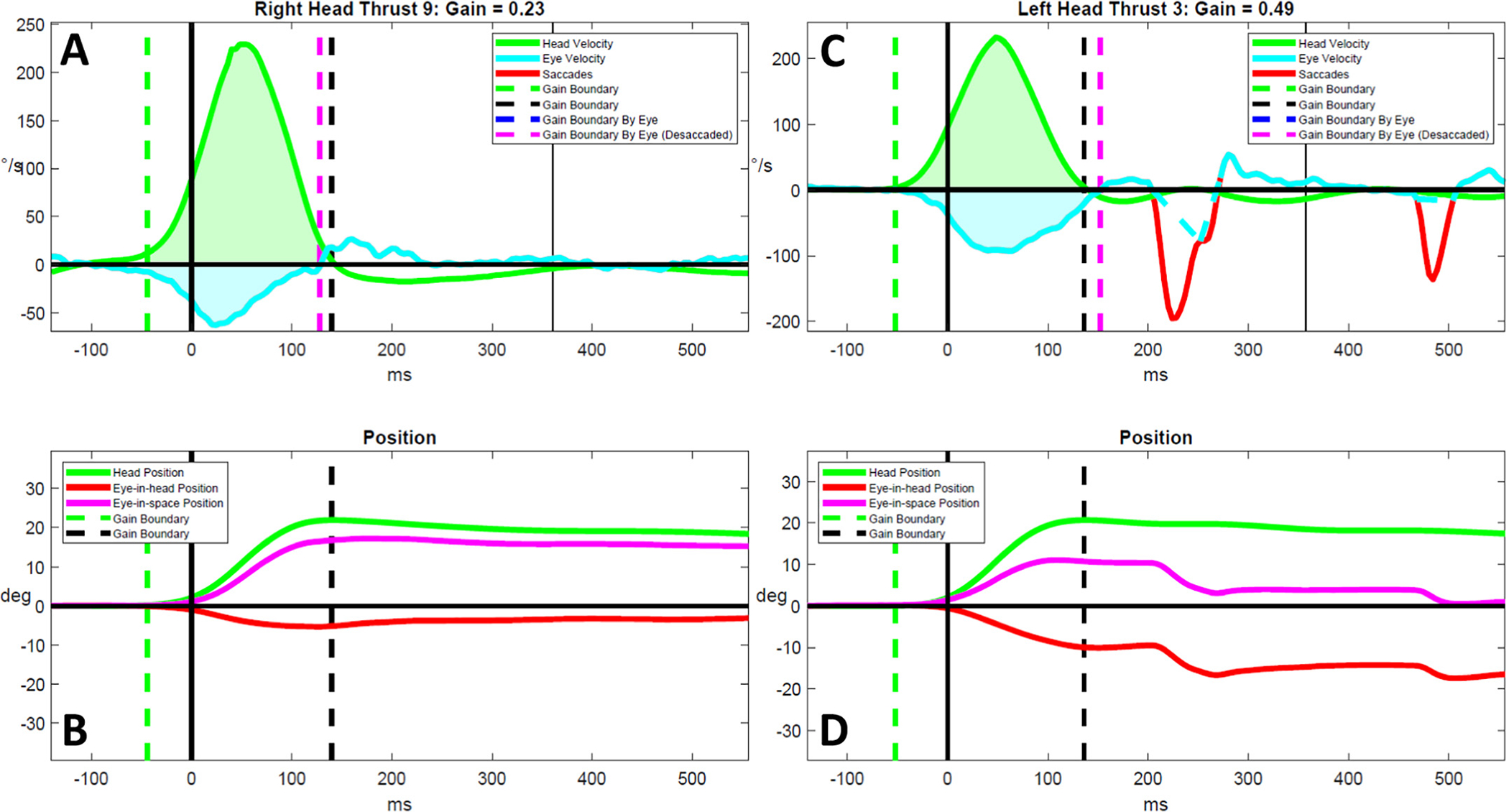
Absent compensatory saccades in a person with moderate multiple sclerosis. Head and eye velocity traces are shown in (A, C) and head and eye position traces are shown in (B, D) for representative rightward and leftward vHIT results for a person living with moderate multiple sclerosis. Although the rightward head impulse consisted of a weak slow eye velocity, no compensatory saccade response occurs (A). However, delayed overt compensatory saccades eventually resolve gaze position error by 500 milliseconds during a leftward head impulse (B). °/s = degrees/s. deg. = degrees. ms = milliseconds.
4. Discussion
We analyzed gaze stabilization mechanisms and function in a cohort of PLW-MS who have different levels of MS-related disability. Overall, the VOR was severely deficient and compensatory gaze stabilization mechanisms were ineffective in PLW-moderate-MS compared to PLW-mild-MS, HC, and PLW-UVD. Dramatic differences in responses to impulses towards the more affected versus the less affected side distinguished PLW-moderate-MS from PLW-mild-MS. We also identified associations between the EDSS score and vestibular and oculomotor control that highlight the importance of gaze stabilization and coordination of eye movements to overall function in PLW-MS. Additionally, examination of the raw vHIT traces led to the identification of vHIT abnormalities that appear to be unique to PLW-MS.
4.1. Vestibulo-ocular reflex gains
The reduced VOR gains in PLW-moderate-MS were below expectations for age-based normative data [31] and were significantly lower than the VOR gains measured in HC. Though our findings regarding VOR gains in PLW-mild-MS align with previous publications, the VOR gains we measured in PLW-moderate-MS were much lower than those reported previously [5,6]. In fact, the asymmetrical, bilateral VOR deficiencies that we identified in PLW-moderate-MS are consistent with prior reports of vestibulo-ocular abnormalities in PLW-MS who have known brainstem lesions [16,17]. We also found asymmetrical, bilateral reduction in VOR function in PLW-UVD, which has been reported [33] and is believed to be related to central vestibular compensation. The VOR deficiency of PLW-moderate-MS was significantly more pronounced than that of PLW-UVD; thus, one might expect PLW-moderate-MS to have similar difficulty with gaze instability as persons with bilateral vestibular hypofunction. Additionally, we identified a moderate association between the EDSS score and VOR gain. Consistent with prior studies linking oculomotor dysfunction with disability in PLW-MS [11], our data suggest that vestibulo-ocular deficiency also plays a significant role in functioning for PLW-MS.
4.2. Characteristics of compensatory saccades
Whether CSs were employed by PLW-MS in response to impulses depended on whether impulses were directed towards the more or less affected side. Since CSs were infrequent, and often absent, in PLW-moderate-MS for impulses towards the more affected side, persons with more advanced MS would be expected to benefit less from saccadic suppression of the degraded visual image, particularly during rapid movements towards their more affected side [34]. However, given that CSs were recruited with equal frequency for head impulses towards either side in PLW-mild-MS, saccadic visual suppression should be at least partially preserved in persons with less severe MS. Degradation in visual acuity during head movement (due to oscillopsia) has been correlated with performance on balance and gait tests in PLW-MS [12] and persons with vestibular dysfunction [35], respectively, and may increase the risk of falling.
Few CSs were generated by HC, PLW-UVD for impulses towards the contralesional side, and for impulses towards the more and less affected sides in PLW-mild-MS (mean VOR gains = 0.97, 0.80, 0.90, and 0.95, respectively); however, CSs were more frequent in PLW-moderate-MS during impulses towards the side with higher VOR gain and in PLW-UVD for ipsilesional impulses (mean VOR gains = 0.66 and 0.35, respectively). These data suggest that VOR gain must be substantially reduced in persons with PLW-UVD and PLW-MS before CSs are elicited at significantly higher rates to augment gaze stabilization. Additionally, our results demonstrate that CS frequency is not increased in general in PLW-MS as has been suggested [15]. Our finding of a weak association between EDSS score and the frequency of CSs appears to be contrary to what might be expected; however, we believe this analysis was confounded by CS frequency being low in PLW-mild-MS owing to minimal effects of MS on CS generation and CS frequency being suppressed in PLW-moderate-MS due to severe disruption in neural transmission within saccade pathways.
Given the effects of demyelination on the nervous system, PLW-MS may be expected to show evidence of delayed processing within vestibular pathways or decreased eye velocity. Indeed, contrary to prior research [15], our data show that the latency of CSs in PLW-mild-MS was significantly longer than that of HC and PLW-UVD. Additionally, we found evidence that the peak velocity of CSs in PLW-mild-MS was significantly lower than that of HC and PLW-UVD. Although PLW-moderate-MS recruited CSs as early as PLW-UVD, CS peak velocity was much lower for PLW-moderate-MS compared to PLW-UVD. This suggests that the brain could only generate a weak oculomotor response to compensate for the GPE signal. The lack of an association between MS-related disability and the generation or execution of CSs provides further evidence of confounding by minimal effects of MS on CS generation in PLW-mild-MS and suppression of CSs in PLW-moderate-MS due to severe disruption in neural transmission.
4.3. Effectiveness of compensatory saccades
PLW-moderate-MS were eight- to ten-fold less effective in recruiting and executing CSs to minimize GPE than PLW-mild MS. The fact that PLW-mild-MS also had significantly greater GPE compared to HC, suggests that vestibular-oculomotor pathways may be affected in persons with less advanced MS. Our results align with those of previous studies of PLW-MS [12,15]; however, the magnitude of GPE is much larger for our participants, particularly for PLW-moderate-MS. In fact, PLW-moderate-MS had GPE that exceeds that for persons with bilateral vestibular hypofunction [29]. Our finding that the EDSS score was moderately associated with GPE extends prior work that demonstrates associations between MS-related disability and oculomotor [11] and vestibular [13] function, and provides further evidence that vestibulo-ocular deficiency is a significant factor in functional ability for PLW-MS.
4.4. Influence of possible brainstem lesions on Vestibulo-ocular function
The etiology for the large, asymmetrical VOR gain deficiency in PLW-moderate-MS in this study is not known. These individuals may have had cranial nerve VIII root entry zone lesions; however, those with concurrent peripheral vestibular dysfunction were excluded. Alternatively, several of our findings suggest that the PLW-moderate-MS may have had occult unilateral or bilateral INO. First, the pattern of VOR deficiency that we measured in PLW-moderate-MS has been reported in PLW-MS who have INO [16,17]. Second, we found similar levels of deficiency in VOR function for impulses towards the more affected side in PLW-moderate-MS compared with ipsilesional impulses in PLW-UVD; yet, CSs occurred less frequently in PLW-moderate-MS. This is contrary to evidence of preservation of CSs in the setting of complete vestibular loss [36]. Thus, the failure of CS triggering, which putatively arises from cervical somatosensory afference [37], suggests that another pathophysiological process interfered with the generation of CSs in PLW-moderate-MS. Third, taken together, our findings that the peak velocity of CSs was significantly slower for CSs evoked by impulses towards the more affected side (right) and that peak velocities were similar for CSs triggered by impulses towards the less affected side (left) in PLW-moderate-MS and ipsilesional impulses in PLW-UVD imply that adduction of the right eye may have been slowed during rightward impulses in PLW-moderate-MS. Fourth, the findings of substantially worse GPE in PLW-moderate-MS compared with PLW-UVD, despite both groups having similar VOR gains also suggests the presence of additional mitigating pathology affecting vestibulo-ocular functioning.
4.5. Unique findings on video head impulse testing
We observed several unique phenomena in the vHIT results of PLW-MS that have not been reported previously. First, PLW-moderate-MS appeared to generate repetitive micro-CSs in addition to typical CSs as a strategy to stabilize gaze. Although these eye movements appeared saccadic in nature, the vast majority were not detected by our signal processing algorithm due to the fact that eye accelerations did not exceed the detection threshold (2000°/s2). Detection was not improved when reducing this threshold by half (1000°/s2). Thus, further refinement of saccade detection methods [38,39] is needed to more fully characterize the physiologic and/or pathophysiologic mechanisms underlying this unique compensatory gaze stabilization strategy. Second, PLW-mild-MS demonstrated a tendency to generate CSs that were over-compensatory for the head impulse. Subsequently, during trials in which CSs would over-shoot the earth-fixed target, PLW-mild-MS generated position correcting saccades that were used to re-direct the eyes back towards the target to correct for this over-compensation. Finally, vHIT results, as are shown in Fig. 6, may provide evidence for the effects of INO on gaze stabilization. In this participant, nearly all of the impulses towards the more affected (right) side resulted in a marked increase in GPE yet CSs were not elicited within 500 ms from the start of the impulse; however, overt CSs were robust for all impulses towards the less affected (left) side. We posit that a finding of absent or markedly deficient CSs in the presence of severely reduced VOR gain may be pathognomonic for INO; however, further investigation using six-canal vHIT in persons with medial longitudinal fasciculus lesions is needed to test this hypothesis.
4.6. Limitations
Though all of the PLW-moderate-MS had evidence on vHIT that may suggest brainstem or cerebellar involvement, we cannot corroborate these findings with supporting evidence from imaging. Thus, we cannot draw sound conclusions regarding whether the differences between those with mild and moderate MS are attributable to differences in the extent and/or location of central nervous system lesions. Specifically, since INO was not detected on the clinical examination, we cannot speak to whether any PLW-MS had lesions of the medial longitudinal fasciculus. Additionally, despite the fact that all PLW-MS underwent a thorough clinical examination that included binocular video-oculography to rule out the presence of concomitant peripheral vestibular system abnormalities, we can only relate our findings to the VOR pathways in general and cannot make any inferences regarding whether the abnormalities were associated with peripheral, central, or mixed vestibular dysfunction.
5. Conclusions
PLW-MS were found to have unique responses to vHIT compared to PLW-UVD and HC that were not apparent during the clinical examination. The characteristics of compensatory oculomotor responses to vHIT depended on the extent of residual VOR gain. In addition to well-defined CSs, PLW-moderate-MS generated repetitive micro-CSs and PLW-mild-MS utilized position correcting saccades to compensate for low VOR gains. Further research is needed to examine the relationship between the extent and location of central nervous system lesions and the characteristics of CSs in PLW-MS and to determine whether and how clinicians should use vHIT to differentiate between PLW-UVD and PLW-MS and to characterize the severity of disease in PLW-MS.
Supplementary Material
Acknowledgements
The authors acknowledge Yoav Gimmon, PT, PhD, Jennifer Millar, MS, PT, Annie Fangman, PT, DPT, and Lindsey Agnew, BS for their help in data collection.
Funding
Dr. Grove was supported by funding awarded to Dr. Schubert (Department of Defense, Congressionally Directed Medical Research Programs W8lXWH-l7-CTRR-CTA). Dr. Schubert was funded by the Department of Defense under the Neurosensory and Rehabilitation Research Award Program (Grant award # W81XWH-15–1-0442). Dr. Dibble was funded by the Multiple Sclerosis Society (NMSS RG-1701–26763). Dr. Loyd received salary supported in part by the Foundation for Physical Therapy Research New Investigator Fellowship Training Initiative (NIFTI). Dr. Wagner was supported in part by a promotion of doctoral studies scholarship provided by the Foundation for Physical Therapy Research. These funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Declaration of Competing Interest
The authors report no competing interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jns.2022.120411.
Data availability
The dataset will be made available upon written request to the corresponding author.
References
- [1].Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, et al. , Rising prevalence of multiple sclerosis worldwide: insights from the atlas of MS, third edition, Mult. Scler. 26 (14) (2020. Dec) 1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amatya B, Khan F, Galea M, Rehabilitation for people with multiple sclerosis: an overview of Cochrane reviews, Cochrane Database Syst. Rev. 1 (1) (2019. Jan 14), CD012732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Di Stadio A, Dipietro L, Ralli M, Greco A, Ricci G, Bernitsas E, The role of vestibular evoked myogenic potentials in multiple sclerosis-related vertigo. A systematic review of the literature, Mult. Scler. Relat. Disord. 28 (2019. Feb) 159–164. [DOI] [PubMed] [Google Scholar]
- [4].Crnošija L, Krbot Skorić M, Gabelić T, Adamec I, Habek M, Vestibular evoked myogenic potentials and MRI in early multiple sclerosis: validation of the VEMP score, J. Neurol. Sci. 372 (2017. Jan 15) 28–32. [DOI] [PubMed] [Google Scholar]
- [5].Ertugrul G, Konuskan B, Solmaz I, Anlar B, Aksoy S, Vestibulo-ocular reflex involvement in childhood-onset multiple sclerosis, Mult. Scler. Relat. Disord. 44 (2020. Sep), 102329. [DOI] [PubMed] [Google Scholar]
- [6].Pavlović I, Ruška B, Pavićić T, Krbot Skorić M, Crnošija L, Adamec I, et al. , Video head impulse test can detect brainstem dysfunction in multiple sclerosis, Mult. Scler. Relat. Disord. 14 (2017. May) 68–71. [DOI] [PubMed] [Google Scholar]
- [7].Serra A, Derwenskus J, Downey DL, Leigh RJ, Role of eye movement examination and subjective visual vertical in clinical evaluation of multiple sclerosis, J. Neurol. 250 (5) (2003. May) 569–575. [DOI] [PubMed] [Google Scholar]
- [8].Magnano I, Pes GM, Pilurzi G, Cabboi MP, Ginatempo F, Giaconi E, et al. , Exploring brainstem function in multiple sclerosis by combining brainstem reflexes, evoked potentials, clinical and MRI investigations, Clin. Neurophysiol. 125 (11) (2014. Nov) 2286–2296. [DOI] [PubMed] [Google Scholar]
- [9].Weier K, Penner IK, Magon S, Amann M, Naegelin Y, Andelova M, et al. , Cerebellar abnormalities contribute to disability including cognitive impairment in multiple sclerosis, PLoS One 9 (1) (2014. Jan 22), e86916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Reulen JP, Sanders EA, Hogenhuis LA, Eye movement disorders in multiple sclerosis and optic neuritis, Brain 106 (Pt 1) (1983. Mar) 121–140. [DOI] [PubMed] [Google Scholar]
- [11].Graves J, Balcer LJ, Eye disorders in patients with multiple sclerosis: natural history and management, Clin. Ophthalmol. 4 (2010. Dec 6) 1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Loyd BJ, Agnew L, Fangman A, Thackeray A, Peterson DS, Schubert MC, et al. , Characterizing gaze and postural stability deficits in people with multiple sclerosis, Mult. Scler. Relat. Disord. 55 (2021. Oct), 103205. [DOI] [PubMed] [Google Scholar]
- [13].Mañago MM, Schenkman M, Berliner J, Hebert JR, Gaze stabilization and dynamic visual acuity in people with multiple sclerosis, J. Vestib. Res. 26 (5–6) (2016) 469–477. [DOI] [PubMed] [Google Scholar]
- [14].Heesen C, Bohm J, Reich C, Kasper J, Goebel M, Gold SM, Patient perception¨ of bodily functions in multiple sclerosis: gait and visual function are the most valuable, Mult. Scler. 14 (7) (2008. Aug) 988–991. [DOI] [PubMed] [Google Scholar]
- [15].Garg H, Dibble LE, Schubert MC, Sibthorp J, Foreman KB, Gappmaier E, Gaze stability, dynamic balance and participation deficits in people with multiple sclerosis at fall-risk, Anat. Rec. (Hoboken) 301 (11) (2018. Nov) 1852–1860. [DOI] [PubMed] [Google Scholar]
- [16].Aw ST, Chen L, Todd MJ, Barnett MH, Halmagyi GM, Vestibulo-ocular reflex deficits with medial longitudinal fasciculus lesions, J. Neurol. 264 (10) (2017. Oct) 2119–2129. [DOI] [PubMed] [Google Scholar]
- [17].Lee SH, Kim SH, Kim SS, Kang KW, Tarnutzer AA, Preferential impairment of the Contralesional posterior Semicircular Canal in Internuclear Ophthalmoplegia, Front. Neurol. 8 (2017. Sep 22) 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Millar JL, Gimmon Y, Roberts D, Schubert MC, Improvement after vestibular rehabilitation not explained by improved passive VOR gain, Front. Neurol. 11 (2020. Feb 20) 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kurtzke JF, Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS), Neurology 33 (11) (1983. Nov) 1444–1452. [DOI] [PubMed] [Google Scholar]
- [20].Jacobson GP, Newman CW, The development of the dizziness handicap inventory, Arch. Otolaryngol. Head Neck Surg. 116 (4) (1990. Apr) 424–427. [DOI] [PubMed] [Google Scholar]
- [21].Shumway-Cook AWM, Motor Control: Translating Research into Clinical Practice, 4th ed., Lippincott, Williams & Wilkins, Baltimore, MD, 2011. [Google Scholar]
- [22].Powell LE, Myers AM, The activities-specific balance confidence (ABC) scale, J. Gerontol. A Biol. Sci. Med. Sci. 50A (1) (1995. Jan) 28. [DOI] [PubMed] [Google Scholar]
- [23].Loyd BJ, Fangman A, Peterson DS, Gappmaier E, Schubert MC, Thackery A, et al. , Rehabilitation to improve gaze and postural stability in people with multiple sclerosis: study protocol for a prospective randomized clinical trial, BMC Neurol. 19 (1) (2019. Jun 10), 119–z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mantokoudis G, Schubert MC, Tehrani AS, Wong AL, Agrawal Y, Early adaptation and compensation of clinical vestibular responses after unilateral vestibular deafferentation surgery, Otol. Neurotol. 35 (1) (2014. Jan) 148–154. [DOI] [PubMed] [Google Scholar]
- [25].Anson ER, Bigelow RT, Carey JP, Xue QL, Studenski S, Schubert MC, et al. , VOR gain is related to compensatory saccades in healthy older adults, Front. Aging Neurosci. 24 (8) (2016. Jun) 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Anson ER, Bigelow RT, Carey JP, Xue QL, Studenski S, Schubert MC, et al. , Aging increases compensatory saccade amplitude in the video head impulse test, Front. Neurol. 7 (2016. Jul 18) 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wagner AR, Schubert MC, Evidence a shared mechanism mediates ipsi- and contralesional compensatory saccades and gait after unilateral vestibular deafferentation, J. Neurophysiol. 123 (4) (2020. Apr 1) 1486–1495. [DOI] [PubMed] [Google Scholar]
- [28].Colagiorgio P, Versino M, Colnaghi S, Quaglieri S, Manfrin M, Zamaro E, et al. , New insights into vestibular-saccade interaction based on covert corrective saccades in patients with unilateral vestibular deficits, J. Neurophysiol. 117 (6) (2017. Jun 1) 2324–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schubert MC, Hall CD, Das V, Tusa RJ, Herdman SJ, Oculomotor strategies and their effect on reducing gaze position error, Otol. Neurotol. 31 (2) (2010. Feb) 228–231. [DOI] [PubMed] [Google Scholar]
- [30].Mantokoudis G, Tehrani AS, Wozniak A, Eibenberger K, Kattah JC, Guede CI, et al. , VOR gain by head impulse video-oculography differentiates acute vestibular neuritis from stroke, Otol. Neurotol. 36 (3) (2015. Mar) 457–465. [DOI] [PubMed] [Google Scholar]
- [31].McGarvie LA, MacDougall HG, Halmagyi GM, Burgess AM, Weber KP, Curthoys IS, The video head impulse test (vHIT) of Semicircular Canal function - age-dependent normative values of VOR gain in healthy subjects, Front. Neurol. 6 (2015. Jul 8) 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].R Core Team. R, A language and environment for statistical computing, in: R Foundation for Statistical Computing, 2021. [Google Scholar]
- [33].Mahfuz MM, Millar JL, Schubert MC, Repeated video head impulse testing in patients is a stable measure of the passive vestibulo-ocular reflex, J. Otolaryngol. 16 (3) (2021. Jul) 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Macdougall HG, Curthoys IS, Plasticity during vestibular compensation: the role of saccades, Front. Neurol. 3 (2012. Feb 28) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Grove CR, Whitney SL, Pyle GM, Heiderscheit BC, Instrumented gait analysis to identify persistent deficits in gait stability in adults with chronic vestibular loss, JAMA Otolaryngol. Head Neck Surg. 147 (8) (2021. August 01) 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Weber KP, Aw ST, Todd MJ, McGarvie LA, Curthoys IS, Halmagyi GM, Horizontal head impulse test detects gentamicin vestibulotoxicity, Neurology 72 (16) (2009. Apr 21) 1417–1424. [DOI] [PubMed] [Google Scholar]
- [37].Iwasaki S, Kamogashira T, Fujimoto C, Kabaya K, Kinoshita M, Yamasoba T, The role of neck input in producing corrective saccades in the head impulse test, Front. Neurol. 13 (2022. May 17), 881411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McCamy MB, Otero-Millan J, Leigh RJ, King SA, Schneider RM, Macknik SL, et al. , Simultaneous recordings of human microsaccades and drifts with a contemporary video eye tracker and the search coil technique, PLoS One 10 (6) (2015. Jun 2), e0128428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Otero-Millan J, Castro JL, Macknik SL, Martinez-Conde S, Unsupervised clustering method to detect microsaccades, J. Vis. 14 (2) (2014. Feb 25) 18, 10.1167/14.2.18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset will be made available upon written request to the corresponding author.


