Abstract
Biased attention towards affective cues often co-occurs with the emergence and maintenance of internalizing disorders. However, few studies have assessed whether affect-biased attention in infancy relates to early indicators of psychopathological risk, such as negative affectivity. The current study evaluates whether negative affectivity relates to affect-biased attention in 6-month-old infants. Affect-biased attention was assessed via a free-viewing eye tracking task in which infants were presented with a series of face pairs (comprised of a happy, angry, or sad face and a neutral face). Attention was quantified with metrics of both attention orienting and attention holding. Overall, infants showed no differences in attention orienting (i.e., speed of looking) or attention holding (i.e., duration of looking) towards emotional faces in comparison to the neutral face pairs. Negative affectivity, assessed via parent report, did not relate to attention orienting but was associated with biased attention towards positive, happy faces and away from threat-cueing, angry faces in comparison to the neutral faces they were paired with. These findings suggest that negative affectivity is associated with differences in attention holding, but not initial orienting towards emotional faces; biases which have important implications for the trajectory of socioemotional development.
Keywords: negative affectivity, affect-biased attention, eye tracking, infancy, attention orienting, attention holding
Background
Attention towards faces plays a critical role in shaping how infants process the world around them and regulate their emotional experiences. Selective attentional processing of affective stimuli, also known as “affect-biased attention” (Todd et al., 2012), emerges early in life and is a central component of normative, socioemotional development. Affect-biased attention can serve an adaptive function by appropriately cueing detection and subsequent processing of affective stimuli, including signals of threat and social context (Morales et al., 2016; Petersen & Posner, 2012). However, disruptions to these attentional processes, such as over-attunement to specific affective cues, can lead to maladaptive trajectories in psychological functioning. Patterns of biased initial orienting and subsequent visual processing of emotional faces have been commonly observed in individuals with internalizing disorders, including anxiety and depression (Peckham et al., 2010). A growing body of work also suggests that these attention biases may precede the emergence of diagnosable psychopathology and are implicated in the maintenance and exacerbation of psychopathological symptoms (Joormann et al., 2007). Although these effects have been well studied in adults, far less is known about how affect-biased attention relates to key predictors of psychopathological risk in infancy, such as negative affectivity.
Attentional Processing of Emotional Faces
Broadly, attention facilitates our ability to detect salient stimuli within the environment, maintain focus to abstract relevant information, and shift gaze towards other environmental stimuli as needed (Petersen & Posner, 2012; Posner & Petersen, 1990). These attentional processes can broadly be broken down into two phases: attention orienting and attention holding (Leppänen, 2016). Attention orienting supports the detection and selection of meaningful information from competing stimuli and is typically measured by assessing the speed of orienting to a stimulus (Cohen, 1972). Attention holding on the other hand reflects the maintenance of attention over time to support detailed processing of selected information and is assessed based on the duration of looking at stimuli (Cohen, 1972). In the initial and more automatic phase of attention orienting, individuals are quicker to direct their gaze to threat cuing stimuli in their environment, such as angry or fearful faces (Morales et al., 2016). This heightened detection of threat is normative and may have an evolutionary basis because quick detection of threat mobilizes alerting mechanisms that promote safety in the face of possible danger. However, this sensitivity to threat is exacerbated amongst anxious individuals, as higher levels of anxiety in children and adults predict quicker detection of threat-cueing faces (Armstrong & Olatunji, 2012; Todd et al., 2012). In the subsequent, more voluntary phase of attention holding, individuals demonstrate sustained attention towards salient emotional stimuli that require further processing or may disengage in order to shift attention elsewhere (Petersen & Posner, 2012). Among adults with anxiety, initial facilitation of detection and orienting towards emotional faces is often followed by subsequent avoidance of emotional stimuli, also known as the vigilance-avoidance effect (see Armstrong & Olatunji, 2012 for meta-analysis and review). Adults with depression on the other hand demonstrate sustained avoidance of positive affect in comparison to non-depressed controls (see Peckham et al., 2010 for meta-analysis). Affect-biased attention is thus evident across both phases of attention orienting and holding and maintains strong links to psychopathological disorders (Armstrong & Olatunji, 2012; Van Bockstaele et al., 2014).
Development of Affect-Biased Attention
Affect-biased attention begins to develop within the first year of life. Newborns exhibit preferential attention towards faces and face-like shapes in comparison to non-face stimuli (Frank et al., 2009; Simion et al., 2001; Viola Macchi et al., 2004). This attentional attunement towards faces is highly adaptive as faces provide rich social information and infants’ attention to their caregiver’s face elicits positive parenting behaviors from caregivers (Peltola et al., 2009). Over time, visual processing of faces becomes more fine-tuned, as infants spend more time fixating on eye and mouth areas in comparison to other areas of the face by 3 to 4 months (Haith et al., 1977; Hunnius & Geuze, 2004a, 2004b). By 5 to 7 months of age, infants not only preferentially attend to faces but they are also able to discriminate between types of emotion faces, including happy, angry, fearful, and sad faces (Leppänen & Nelson, 2009; Morales et al., 2016), providing important context on caregiver state and the surrounding environment (Bornstein & Arterberry, 2003; Caron et al., 1988; Leppänen & Nelson, 2009; Nelson, 1987; Serrano et al., 1992).
Shortly after infants can discriminate between emotion faces, they also begin to exhibit patterns of preferential attention towards specific emotional faces. Infants generally are quicker to orient and demonstrate greater holding of attention on emotional faces, including both happy and angry faces, in comparison to neutral faces (Burris et al., 2017; Pérez-Edgar et al., 2017). In addition to an attention bias for emotion faces, infants also begin to demonstrate biased attention towards threat cuing stimuli. By 7 months, infants spend more time looking at fearful faces and are less likely to disengage from them in comparison to happy faces (Nelson & Dolgin, 1985; Peltola et al., 2009). A similar pattern is observed with angry faces, as infants are quicker to detect angry faces than happy faces at 8 and 14 months (LoBue & DeLoache, 2010) and dwell longer on angry faces in comparison to neutral faces (Morales et al., 2017). This heightened detection and processing of threat cues is also observable in measures of neural activity (De Haan et al., 2003; Leppänen et al., 2007; Leppänen & Nelson, 2006). For example, the negative central (Nc) component, an event related potential (ERP) signature of attentional orienting, is more pronounced to fearful faces than non-fearful faces at 7 months of age (De Haan et al., 2004; Leppänen et al., 2007; Nelson & De Haan, 1996; Peltola et al., 2009). Overall, this literature suggests that the ability to differentiate and preferentially attend to different emotion faces is well established within the first year of life.
Negative Affectivity and Affect-Biased Attention
Normative development of affect-biased attention has been well characterized in infancy. A growing literature has also explored how individual factors, such as infant temperament and other indicators of psychopathological risk, relate to individual differences in the emergence of affect-biased attention (Field & Lester, 2010; Morales et al., 2016). Developmental theories suggest that variability in early biases in infant visual exploration of emotionally salient stimuli relate to key dimensions of temperament, such as fearfulness and behavioral inhibition (Pérez-Edgar, 2018; Morales et al., 2016). These early attentional biases are then moderated by factors both internal and external to the child over time to shape the trajectory of socioemotional development (Burris et al., 2019; Field & Lester, 2010; Morales et al., 2016). However, far less is known about how other key dimensions of temperament, such as negative affectivity, relate to affect-biased attention.
Negative affectivity is conceptualized as a tendency towards experiencing negative emotions (e.g., fear, sadness, frustration, anger) more quickly and intensely (Rothbart & Bates, 2006). Negative affectivity is modestly stable over time (Lonigan et al., 2003; Putnam et al., 2008) and is also a dimensional predictor of later psychopathology, including internalizing disorders such as anxiety and depression (Lonigan et al., 2003; Kostyrka-Allchorne et al., 2020; Muris & Ollendick, 2005; Nigg, 2006). Although some studies have begun to investigate the relation between negative affectivity and affect-biased attention, few studies have explored these associations in infancy and findings have been mixed (Conejero & Rueda, 2018; Fu et al., 2020; Morales et al., 2017; Nakagawa & Sukigara, 2019; Pérez-Edgar et al., 2017; Vallorani et al., 2021). Additionally, past work in infancy has focused almost exclusively on attention towards positive, happy faces, and threat cueing angry and fearful faces, as opposed to other emotion faces which are commonly employed in the adult literature, such as sad faces.
Very few studies have looked at the association between negative affectivity and attention orienting. Fu and colleagues (2020) found evidence of an interactive effect, reporting that amongst a sample of 4- to 24-month-old infants, older infants who were higher in negative affectivity and attention control were quicker to orient to neutral faces but not happy or angry faces on a vigilance task. This suggests that infants who display this temperament profile may be more vigilant towards ambiguous, neutral faces as they age. In another study by this group, Vallorani and colleagues (2021) utilized latent profile analyses to characterize infant looking behavior in 4- to 24-month old infants across a series of tasks (i.e., modified dot-probe, overlap, and vigilance tasks). An interactive effect was observed, as infants of anxious mothers who were also higher in negative affectivity were more likely to be a part of a vigilant group, in which they were quicker to orient to both angry and happy faces.
There also is some evidence that negative affectivity may relate to patterns of attention holding. For example, the study by Vallorani and colleagues (2021) also found that 4- to 24-month-old infants who were part of a latent class that was high in maternal anxiety and infant negative affectivity displayed longer dwell times on angry but also happy faces. Pérez-Edgar and colleagues (2017) on the other hand found no effect of negative affectivity on attention holding (i.e., dwell time to angry or happy faces) in a sample of 4- to 24-month old infants participating in a modified dot-probe task. However, they did find that dwell time to angry faces influenced how long it took infants to orient to a subsequently presented probe, but only for young infants low in negative affectivity. Studies looking specifically at the ability to disengage from emotional stimuli have yielded mixed results as well. Specifically, Conejero and Rueda (2018) found that 9- to 12-month-old infants who were high in negative affectivity demonstrated difficulty disengaging from fearful faces, but not happy or neutral faces. However, when evaluating similar metrics of disengagement, both Nakagawa and Sukigara (2019) (in 6- and 12-month old infants) and Morales and colleagues (2017) (in 4- to 24-month old infants) found no significant relation between infant negative affectivity and disengagement from faces (including angry, happy, and neutral faces).
Taken together there is inconsistency in findings across the literature of how negative affectivity relates directly to both attention orienting and holding. Variance in methodological approaches evaluating affect-biased attention may also contribute to discrepancies in study findings. Most studies are designed to evaluate either attention orienting or holding, relying on either measurement of reaction time or the ability to disengage from affective cues. Tasks that measure both orienting and holding are needed because the initial phase of orienting can influence the downstream process of sustained attention (Burris et al., 2017; Burris et al., 2019). A free viewing paradigm, in which participants are presented with a display of images (e.g., emotional faces), is a methodological approach that has been commonly employed in the adult literature to capture affect-biased attention across both phases of attention (Armstrong & Olatunji, 2012; Bar-Haim et al., 2007). This approach applies minimal constraints to participant looking behaviors, as participants freely view and explore stimuli for an extended period of time, disengaging from images at their own volition. To our knowledge, free viewing tasks have not yet been used when evaluating the relation between negative affectivity and affect-biased attention in infancy. Joint assessment of attention orienting and holding through free viewing tasks may therefore offer a useful approach to disentangle how negative affectivity may relate to each attentional component.
The Current Study
The current study evaluates how negative affectivity relates to affect-biased attention in 6-month-old infants. Infants were assessed at 6 months of age because by this time, infants preferentially attend to faces in comparison other non-facial stimuli, can discriminate between differing emotion faces, and are beginning to exhibit individual differences in the visual processing of emotion faces (Burris et al., 2017; Burris et al., 2019; Field & Lester, 2010; Morales et al., 2016; Nelson & Dolgin, 1985; Peltola et al., 2009; Pérez-Edgar et al., 2017). Infant attentional biases were assessed via eye tracking in a free viewing task in which an emotional face (i.e., happy, angry, or sad face) was presented beside a neutral face for an extended period of time (i.e., 5 seconds). Affect-biased attention was characterized by metrics of attention orienting and attention holding. Attention orienting was assessed by measuring latency to the emotional face in contrast to the neutral face. Attention holding was assessed by measuring the proportion of total looking time for each emotion face (i.e., attention holding on emotional faces while also accounting for total looking time to the neutral pairs). The first study aim was to characterize affect-biased attention amongst 6-month-old infants, assessing whether infants’ attention orienting (i.e., latency difference score) and attention holding (i.e., proportion of total looking time) varied across trial types (i.e., happy, angry, and sad trials). The second study aim was to assess the relation between infant negative affect and these metrics of affect-biased attention.
Method
Participants
Participants included maternal-infant dyads, recruited as part of a longitudinal study (Davis et al., 2018). Mothers were recruited during pregnancy and initial inclusion into the parent study was based on the following criteria: a) maternal age between 18 and 45 years, b) singleton pregnancy, and c) proficiency in English. Mothers were excluded from study recruitment based on the following exclusion criteria: illicit drug use, methadone use, major health conditions involving invasive treatments (e.g., dialysis, blood transfusions, chemotherapy), past or current symptoms of psychosis or mania based on the Structured Clinical Interview (SCID) for DSM-5, and/or current participation in cognitive behavioral therapy (CBT) or interpersonal therapy (IPT). Study protocols were reviewed and received approval from the institutional review board, and mothers provided informed consent for both themselves and their infant.
Eighty-three mother-infant dyads provided ratings of infant negative affectivity and participated in the eye tracking task when infants were approximately 6 months old (M = 6.3 months, SD = 0.5, Range: 5.7 – 8.6). Two infants were age outliers (i.e., infant age > 3 SD above the mean), however removal of these participants did not change the effect size or significance of results, thus they were retained in analyses. Of those 83 infants who attempted the eye tracking task, 5 were excluded due to unsuccessful calibration and 6 were excluded due to eye-tracker malfunction, rates similar to other infant eye tracking studies (Segal et al., 2021). Thus, 72 mother-infant dyads were included in subsequent analyses (see Table 1 for demographic characteristics of the study sample).
Table 1.
Sample Characteristics (n = 72)
| Maternal Characteristics | |
|
| |
| Maternal age (M ± SD; Range) | 32.3 ± 5.1; 21.5–42.0 |
| Cohabitating (%) | 86.1 |
| Federal poverty line (FPL) (%) | |
| Poverty (<100% FPL) | 15.2 |
| Near poverty (100–200% FPL) | 16.7 |
| Household income (Median) | $90,000 |
| Years of education (%) | |
| Some high school, high school diploma, or GED | 9.7 |
| Technical or vocational school, associate degree, or some college | 27.8 |
| Bachelor’s degree or higher | 62.5 |
|
| |
| Child Characteristics | |
|
| |
| Child age at assessment (in months; M ± SD; Range) | 6.4 ± 0.6; 5.7–8.6 |
| Sex (% female) | 58.3 |
| Race/Ethnicity (%) | |
| White, Non-Hispanic/Latinx | 50.0 |
| Hispanic/Latinx | 19.4 |
| Black, Non-Hispanic/Latinx | 8.3 |
| Asian, Non-Latinx | 1.4 |
| Native American/, Non-Hispanic/Latinx | 1.4 |
| Multiracial/Multiethnic | 19.4 |
| Gestational age at birth (M ± SD; Range) | 38.8 ± 1.89; 29.4 – 41.2 |
Measures
Sociodemographic Factors
Sociodemographic characteristics, including maternal factors (age, years of education, household income, cohabitation status, race/ethnicity) and child factors (race/ethnicity) were assessed during a semi-structured interview. Child biological sex at birth (subsequently referred to as sex), gestational age at birth, and birthdate were determined from medical record abstraction. Seven participants were born preterm. Results did not change in direction or significance when preterm infants were excluded from analyses, thus these infants were retained in all subsequent analyses. Income to needs ratio (INR) was calculated by dividing household income by the federal poverty threshold for that year corresponding to household size and time of assessment (Grieger et al., 2009; U. S. Census Bureau, 2020).
Infant Negative Affectivity.
Infant negative affectivity was assessed using the Infant Behavior Questionnaire (IBQ), a standardized instrument designed to assess temperament in infancy by maternal report (Rothbart, 1981). The IBQ takes advantage of a mother’s ability to observe her children over a wide range of contexts. Given concern for the potential of maternal reporting bias, the IBQ was designed to ask about concrete infant behaviors rather than abstract judgements, as to make the responses more objective. Mothers rate their infant on each item using a 7-point Likert scale ranging from 1 (never) to 7 (always). Ratings yield composite scores: Surgency/Extraversion, Negative Affectivity, and Orienting/Regulation. Only the Negative Affectivity score was utilized in the current study analyses. The negative affectivity score on the IBQ demonstrated good internal consistency (α = .83).
Infant Attentional Processing of Emotional Faces.
Infant attentional processing of emotional faces was assessed using eye tracking technology.
Stimuli.
The stimuli consist of 18 colored photographs of faces, derived from the standardized NimStim set (Tottenham et al., 2002). Actor race and gender were held consistent across all stimuli in the 36-trial task, which was kept brief due to constraints in infants’ ability to stay seated and on task throughout the study visit. Only female faces were selected because past work demonstrates that infants display a preference for female faces over male faces by 3 to 4 months of age (Quinn et al., 2002). For actor race, only White faces were utilized because an insufficient number of stimuli were available in this face set for Black and Asian actors (4 Black, 5 Asian, and 9 White, female actors were available, and 6 unique faces were required for each emotion condition). Although this meant that the stimuli were thus not representative of the race of infant caregivers in the study sample, this limitation was unlikely to substantially impact results as the primary measure was difference in looking across faces within a face pair. Additionally, only closed mouth faces were included in the task because interrater reliability scores are higher for closed mouthed, sad faces than open mouthed, sad faces in the NimStim set (sad, closed mouth κ = .76; sad, open mouth: κ = .62; t = 4.25, p < .01) and are good for angry (κ = .78), happy (κ = .92), and neutral (κ = .86), closed mouth faces (Tottenham et al., 2009). Validity ratings provided by developers of the NimStim set were also utilized to systematically select stimuli to include in the task (i.e., the six stimuli with the highest validity ratings for a given emotion category were included) (Tottenham et al., 2009).
Each emotional face stimulus (i.e., happy, sad, or angry face) was presented simultaneously beside a neutral face from the same actor. “Emotional” face stimuli are thus defined within the context of this study as happy, sad, or angry faces, whereas “facial” stimuli also include neutral faces (Barrett et al., 2019). Because the facial expression of emotion is both culturally and contextually specific, we recognize that each of these stimuli (e.g., happy, sad, angry, and neutral facial configurations) represent an expression of each emotion type but do not necessarily reflect a universal expression of each emotional state and should thus be interpreted with this context in mind (Barret et al., 2019). We will use the phrase “happy faces” to refer to happy facial configurations, and “sad faces”, “angry faces”, and “neutral faces” to refer to sad, angry, and neutral facial configurations respectively. Additionally, “trial” type is defined by the emotional face presented during that trial (i.e., happy, angry, or sad). Six face pairs for each of the three emotion conditions were presented twice over the course of the experiment (for a total of 36 experimental trials). Face stimuli were 24 cm x 20 cm on participant monitor.
Apparatus.
Infant gaze was measured using the Tobii Pro Spectrum eye tracker, which collects data at 150 Hz, and stimuli was presented using Tobii Pro Lab software (Tobii Technology, Stockholm, Sweden). Infants were seated in a highchair throughout testing. The infant was positioned at a viewing distance of 60 cm from the Tobii Pro Spectrum monitor and eye height was aligned with the horizontal midline of the monitor. An occluder was used to obstruct the infant’s view of the experimenter and to minimize visual distractions in the testing environment. Mothers remained out of the infant’s line of sight. Infants were monitored by the experimenter via camera for the entirety of the task.
Procedure.
The eye tracker was calibrated using a standard 5-point Tobii calibration program, in which an attention grabbing, infant-friendly stimulus on a black background moved to five distinct locations on the display screen. If any of the five points were not successfully calibrated (i.e., estimates of accuracy were not generated), the experimenter recalibrated at those locations. The experimenter also used visual inspection of the calibration data to recalibrate points in which calibration was poor. Average estimated accuracy in the measurement of infant gaze was 0.49° (SD = 0.21°).
After calibration, infants were then presented with 36 experimental trials of face pairs, each presented for 5 seconds (see Figure 1). Face pairs were presented in two separate blocks of 18 trials of unique face pairs presented in a fixed randomized order, for a total of 36 trials). Stimuli were also counterbalanced to account for side presentation. Attention getters, which consisted of dynamic, infant friendly stimuli that were accompanied by sounds, were presented between trials to attract infant attention back to the center of the screen. The experimenter advanced to the next trial when the infant successfully attended to the attention getter, monitored via live gaze tracking in the experimenter monitor. The task was stopped if the infant cried excessively for 20 seconds or if the mother requested to end the task at any time.
Figure 1.
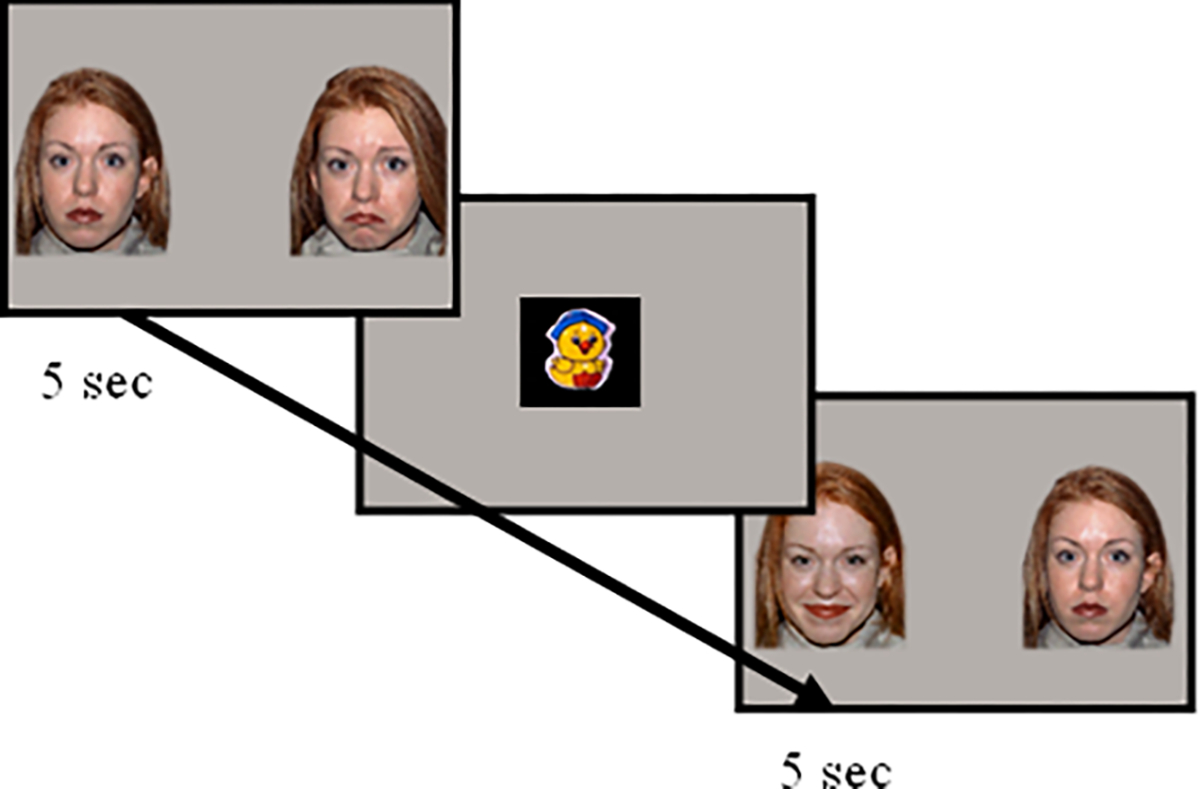
Sample Task Trials
Data Processing.
The standard Tobii Fixation Filter classification algorithm (Tobii Pro AB, 2014) was used to determine when infant fixation changed over the course of the task, with a minimum fixation duration threshold of 60 ms. When applied to the data, this classification algorithm grouped the data into separate fixations so that the spatial location of each individual fixation could be determined. The duration of each fixation was defined as the difference in time in which a fixation started and ended at a singular gaze point.
Data Cleaning.
Data were cleaned on a trial-by-trial basis. Participants provided data (i.e., looked on screen at least once during a trial) for 95.7% of all trials. Each trial was only included in further analyses if the infant fixated on screen within the first 500 ms of the trial; thus, trials in which the infant was either looking off-screen or in which gaze was not tracked during initial stimulus presentation were excluded (excluding 1.5% of all trials). As part of our a-priori analytic plan, we also established that participant data for each condition would only be used in further analyses if the participant had three or more usable trials for a given emotion category (≥ 25%). On average, 94% of trials (SD = 14%, Range 42–100%) were usable in the happy condition, 95% of trials (SD = 13%, Range 33–100%) were usable in the angry condition, and 94% of trials (SD = 16%, Range 17–100%) were usable in the sad condition. Only one participant had an insufficient number of sad trials (17%) and was thus excluded from subsequent analyses.
Areas of Interest.
Tobii Pro Lab software was used to establish areas of interest (AOIs). Oval AOIs were drawn and then adjusted to fit tightly around the actor’s face for each emotional face stimuli (i.e., happy, angry, and sad faces). Separate AOIs were drawn for each emotional face and were labeled with the emotion of the face they encompass. AOI size did not significantly vary between angry, happy, and sad trials [F(2,17) = 0.02, p = .966]. AOIs were also generated for neutral faces, with AOI dimensions held constant for the emotional face and neutral face in each face pair. To assess whether a participant fixated on a facial stimulus, Tobii Pro Lab references the spatial coordinates of each fixation and the spatial position of the areas of interest to determine whether a fixation occurred within each AOI.
Latency to Faces and Latency Difference Score.
Latency to faces and the latency to difference score were utilized as indices of attention orienting (Wieser et al., 2009). Latency was calculated by assessing the length of time which lapsed from stimulus presentation until an infant initially fixated on a given area of interest. Latency to faces was generated by computing the time from stimulus presentation to infant’s first fixation on a facial stimulus (either the emotion or the neutral face) during each trial, which was then averaged for participants within each trial type. The latency difference score was calculated by subtracting latency to the initial fixation on the neutral face from latency to first fixation on the emotional face it was paired with. A positive score thus indicates that the infant was quicker to fixate on the neutral face than the emotion face. Only trials in which infants fixated at least once on the emotional and neutral face were utilized to calculate the latency difference score. The latency difference score was used as our primary measure of attention orienting.
Total Looking Time to Faces and Proportion of Total Looking Time.
Both total looking time to faces and proportion of total looking were selected as metrics of attention holding. Total looking time was computed by summing the length of all individual fixations within a given area of interest across the course of a trial. Total looking time to faces (i.e., total amount of time fixating on facial stimuli overall) was calculated by summing the total looking time to the emotion face and the total looking time to the neutral face during each trial, and then computing average total looking time to faces within each trial type. We also computed proportion of total looking time (i.e., when infants have detected both the emotion and neutral face, how much time they spent holding attention on the emotion face in comparison to the neutral face it was presented beside) by dividing total looking time to the emotional face (i.e., happy, angry, or sad faces) by total looking time to both the emotional and neutral face during that trial. Only trials in which infants fixated at least once on the emotional and neutral face were utilized to calculate proportion of total looking time. A probability score over 50% indicates that an infant held attention more on an emotional face than its neutral pair, whereas a score of 50% indicates that the infants spent an equal proportion of time fixating on emotional and neutral faces. The proportion of total looking time was used as our primary measure of attention holding.
Statistical Analysis
Statistical analyses were conducted using the repeated measures General Linear Model module of SPSS (Version 23.0). We ran a series of general linear multivariate models with Greenhouse-Geisser correction, fitting a full model in every cell and allowing different slopes and intercepts within each cell.
Preliminary Analyses.
In preliminary analyses, we first assessed whether sociodemographic variables (i.e., child sex, child age, INR) are associated with infant looking behaviors in the general linear multivariate models. Next, we assessed whether the raw metrics of infant looking behaviors (i.e., latency to faces and total looking time to faces; repeated measure outcomes tested in separate models) differ across trial type and relate to negative affectivity in the general linear multivariate models (see additional description of tested within- and between-subjects effects below).
Testing of Study Aims.
To test study aims, two general linear multivariate models were implemented to assess whether our primary metrics of infant looking behavior (i.e., latency difference score and proportion of total looking time; repeated measure outcomes tested in separate models) differ across trial type (i.e., happy, angry, and sad trials; within-subjects factors), and whether negative affectivity predicts these looking behaviors (grand mean centered and tested as a continuous predictor). Specifically, the within-subjects effect of trial type is reported in each model to test the first study aim (i.e., testing the null hypothesis that the intercepts [centered at the average value of negative affectivity] are equal for angry, happy, and sad trials; subsequently referred to as the trial type within-subjects effect). To address the second study aim, we report whether the relation between negative affectivity and each infant looking behavior varies across trial type (i.e., testing the null hypothesis that the slopes of the linear associations between negative affectivity and the infant looking behaviors outcome are equal for angry, happy, and sad trials; subsequently referred to as the negative affectivity by trial type within-subjects effect) and whether negative affectivity predicts overall infant looking behaviors, regardless of trial type (i.e., testing the null hypothesis that the slope of the linear association between negative affectivity and the infant looking behavior outcome, averaged across the three trial types, differs from zero; subsequently referred to as the negative affectivity between-subjects effect).
Results
Preliminary Analyses
Sociodemographic Factors.
Preliminary analyses revealed that child age, child sex, and INR were not significantly associated with any of the measures of affect-biased attention (all p’s > .10; see Table S1–S3 for additional details).
Latency to Faces.
Latency to faces did not differ between happy, angry, and sad trials [within-subjects effect: F(2,138) = 0.18, p = .830, partial η2 = .003]. Additionally, negative affectivity did not relate to latency to faces [negative affectivity between-subjects effect: F(1,69) = 2.69, p = .106, partial η2 = .037; negative affectivity by trial type within-subjects interactive effect: F(2,138) = 0.18, p = .832, partial η2 = .003]. Descriptive information on latency to faces across trial types is presented in Table 2 and results from this model are represented visually in Figure 2 (descriptive information on latency to emotion faces and latency to neutral faces are presented in supplementary Table S4). These data suggest that negative affectivity does not relate to how quickly infants attended to faces in general.
Table 2.
Descriptive Statistics for Infant Attention Metrics by Trial Type
| M ± SD, Range | |
|---|---|
|
| |
| Latency to Faces (in ms) | |
|
| |
| Happy | 496.1 ± 171.0; 302.6–1410.2 |
| Angry | 494.6 ± 173.7; 294.5–1476.5 |
| Sad | 506.1 ± 189.6; 329.2–1365.6 |
|
| |
| Total Looking Time to Faces (in ms) | |
|
| |
| Happy | 2654.1 ± 757.4; 521.6–4154.1 |
| Angry | 2687.5 ± 686.1; 987.2–4270.4 |
| Sad | 2523.2 ± 757.8; 756.9–4141.4 |
|
| |
| Latency Difference Score (in ms) | |
|
| |
| Happy | 76.6 ± 1005.2; −3151.7–2510.0 |
| Angry | −46.9 ± 859.3; −2246.7–1800.6 |
| Sad | 38.4 ± 1024.7; −2613.3–2740.0 |
|
| |
| Proportion of Total Looking Time | |
|
| |
| Happy | 0.51 ± 0.10; 0.31–0.88 |
| Angry | 0.49 ± 0.10; 0.28–0.77 |
| Sad | 0.51 ± 0.10; 0.25–0.99 |
Figure 2. Negative Affectivity and Latency to Faces by Trial Type Note.
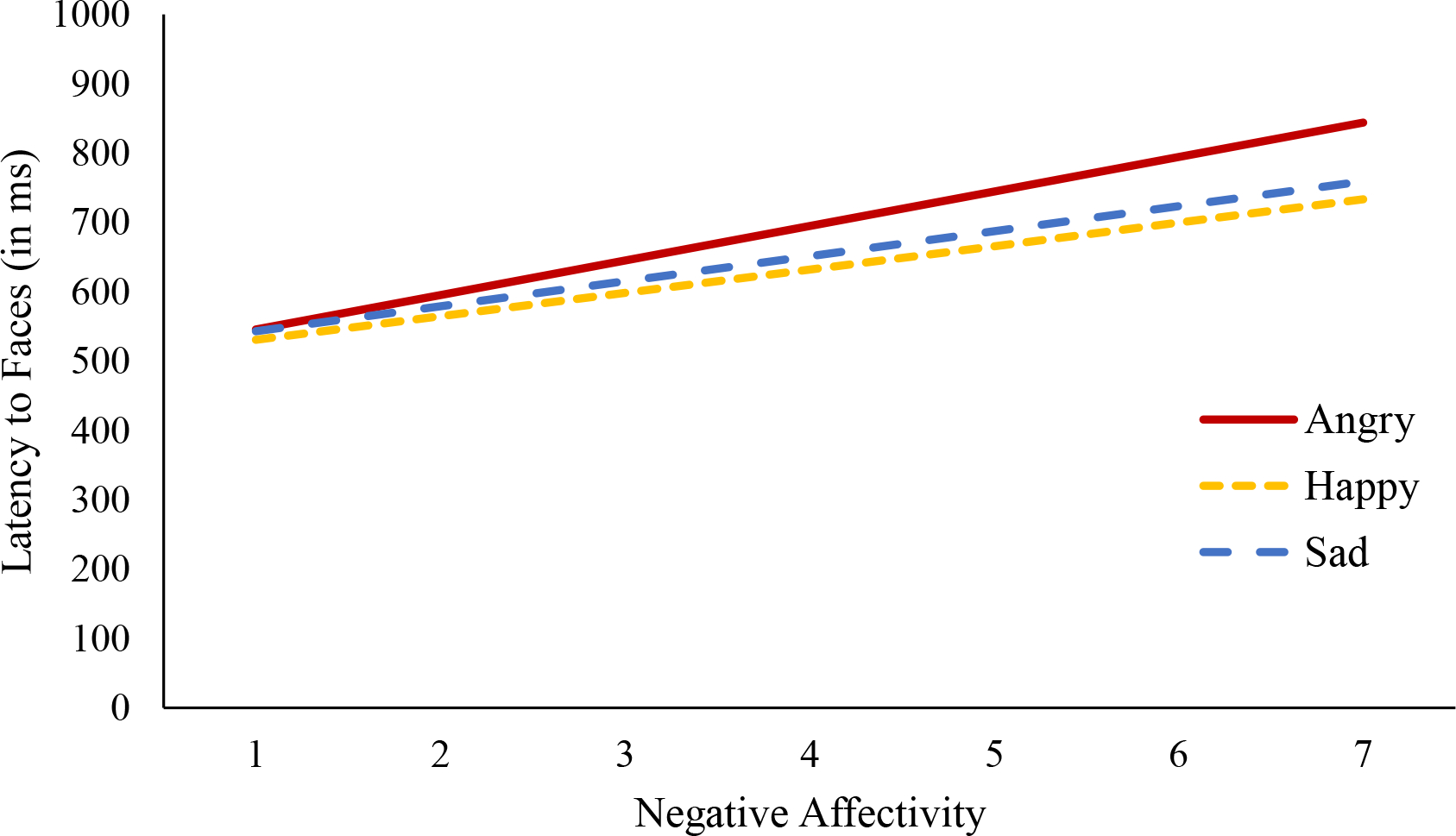
Negative affectivity was not significantly associated with latency to faces during angry (βAngry = 49.72, SE = 28.61, t = 1.74, p = .087, partial η2 = .042), happy (βHappy = 33.77, SE = 28.49, t = 1.19, p = .240, partial η2 = .020), or sad trials (βSad = 36.01, SE = 31.61, t = 1.14, p = .259, partial η2 = .018).
Total Looking Time to Faces.
The within-subjects effect of trial type on total looking time to faces (i.e., total looking time to either the emotional and neutral face within an average trial) was significant [F(2,138) = 6.49, p = .002, partial η2 = .086]. Post-hoc tests (with a Bonferroni adjusted alpha level of α = .0167) indicated that overall, infants spent less time attending to faces during sad trials in comparison to happy (p = .009) and angry trials (p = .001). Total looking time to faces did not significantly differ between happy and angry trials (p = .498). The between-subjects effect of negative affectivity on total looking time to faces was not significant [F(1,68) = 2.83, p = .097, partial η2 = .039]. The interactive effect of negative affectivity and trial type on total looking time was also not significant [F(2,138) = 1.29, p = .278, partial η2 = .018]. Results are represented visually in Figure 3 and descriptive information on total looking time to faces across trial types is presented in Table 2 (descriptive information on total looking time to emotion faces and total looking time to neutral faces are presented in supplementary Table S4). Findings suggest that negative affectivity does not significantly relate to how much time infants spend attending to faces in general.
Figure 3. Negative Affectivity and Total Looking Time to Faces by Trial Type.
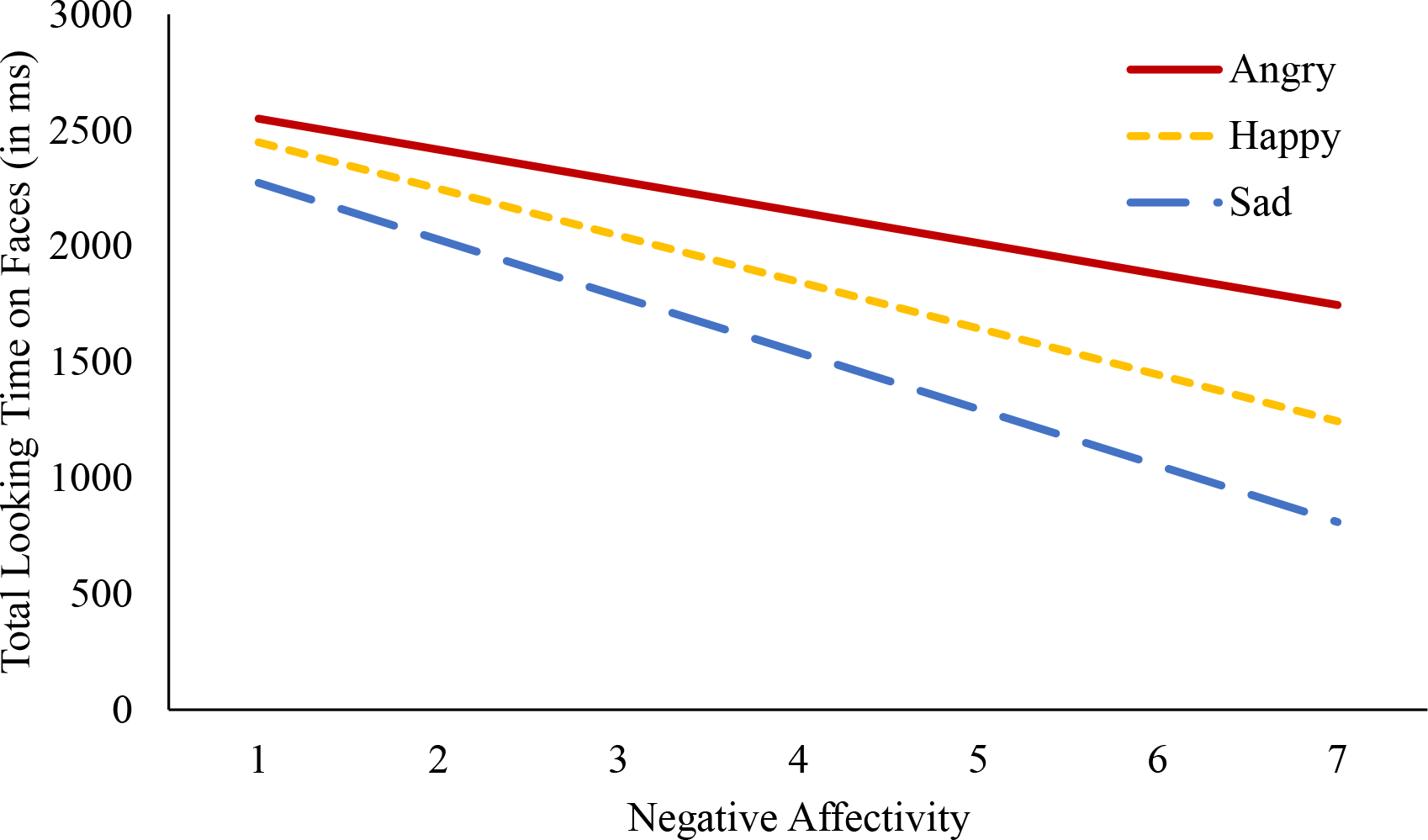
Note. Negative affectivity was negatively associated with total looking time to faces during angry (βAngry = −133.96, SE = 114.30, t = −1.17, p = .240, partial η2 = .020), happy (βHappy = −200.55, SE = 125.13, t = −1.60, p = .114, partial η2 = .036), and sad trials (βSad = −243.81, SE = 124.08, t = −1.97, p = .053, partial η2 = .053).
Testing Study Aims
Latency Difference Score.
Results from one-sample t-tests indicated that the latency difference score, our primary measure of attention orienting, did not significantly differ from a value of 0 for angry, happy, or sad trials (all p’s > .250), suggesting that on average infants took the same amount of time to initially orient to the emotional face as the neutral face it was paired within each trial type. In our general linear multivariate model, the within-subjects effect of the latency difference score was not significant [F(2,138) = 0.23, p = .765, partial η2 = .004], suggesting that on average the latency difference score did not differ across trial type (see Table 2 for additional descriptive information on the proportion of total looking time by trial type). The between-subjects effect of negative affectivity [F(1,69) = 0.16, p = .692, partial η2 = .002] as well as the negative affectivity by trial type within-subjects interaction [F(2,138) = 0.68, p = .511, partial η2 = .010] were also not significant. Results from this model are represented visually in Figure 4. In secondary analyses we also found that negative affectivity also does not relate to the proportion of trials in which the infant looked at the emotion face before looking at the neutral face (see Supplementary Table S5 and S6 for additional details). Taken together, results suggest infant negative affectivity does not relate to biases in attention orienting.
Figure 4. Negative Affectivity and Latency Difference Score by Trial Type.
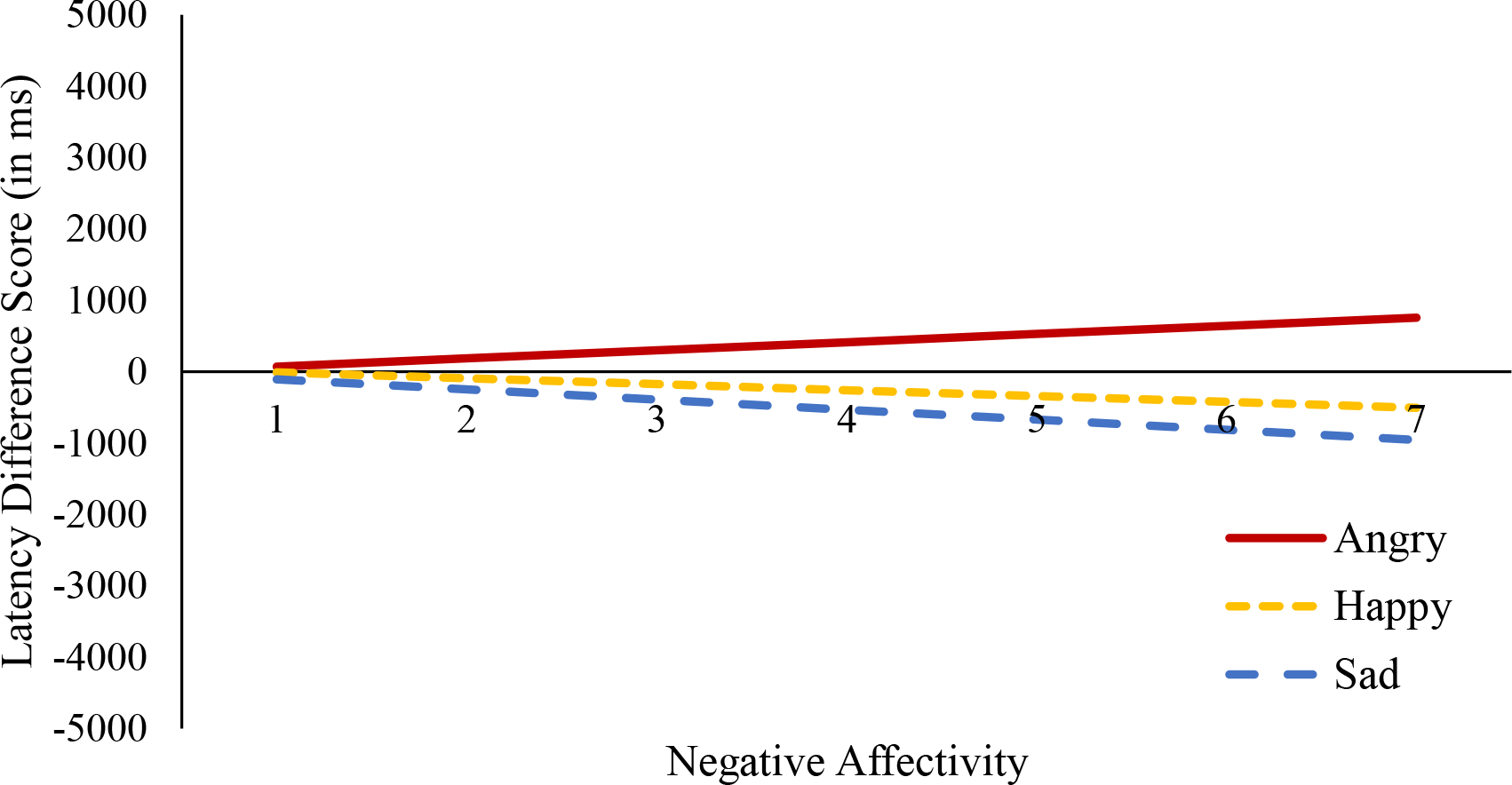
Note. Negative affectivity was not significantly associated with the latency difference score for angry (βAngry = 114.37, SE = 143.92, t = 0.80, p = .430, partial η2 = .009), happy (βHappy = −82.85, SE = 168.83, t = −0.49, p = .625, partial η2 = .003), or sad trials (βSad = −143.53, SE = 171.57, t = −0.83, p = .412, partial η2 = .010).
Proportion of Total Looking Time.
Results from one-sample t-tests indicated that the proportion of total looking time, our primary measure of attention holding, did not significantly differ from a value of 0.5 for angry, happy, or sad faces (all p’s > .250), suggesting that on average infants spent an equal proportion of time fixating on the emotional face as they did the neutral face it was paired with. Results from the general linear multivariate model indicate no significant within-subjects effect of trial type [F(2,138) = 0.89, p = .413, partial η2 = .013] on the proportion of total looking time, suggesting that overall, the proportion of total looking time did not vary based on the emotion of the stimuli (see Table 2 for additional descriptive information on the proportion of total looking time by trial type). There was also no significant between-subjects effect of negative affectivity [F(1,69) = 2.02, p = .159, partial η2 = .028]. However, the interactive within-subjects effect of trial type and negative affectivity was significant [F(2,138) = 5.71, p = .004, partial η2 = .076]. As illustrated in Figure 5, negative affectivity was associated with a lower proportion of total looking time to angry faces relative to neutral faces (β = −0.03, SE = 0.02, t = −2.13, p = .036, partial η2 = .062). In contrast, negative affectivity was associated with a higher proportion of total looking time to happy faces relative to neutral faces (β = 0.04, SE = 0.02, t = 2.48, p = .016, partial η2 = .081). Negative affectivity was associated with a higher proportion of total looking time to sad faces at the level of a trend (β = 0.03, SE = 0.02, t = 1.89, p = .064, partial η2 = .049). These findings suggest that infants high in negative affectivity spent less time attending to angry faces and more time attending to happy faces in comparison to their respective neutral pairs over the course of the trial.
Figure 5. Negative Affectivity and Proportion of Total Looking Time by Trial Type.
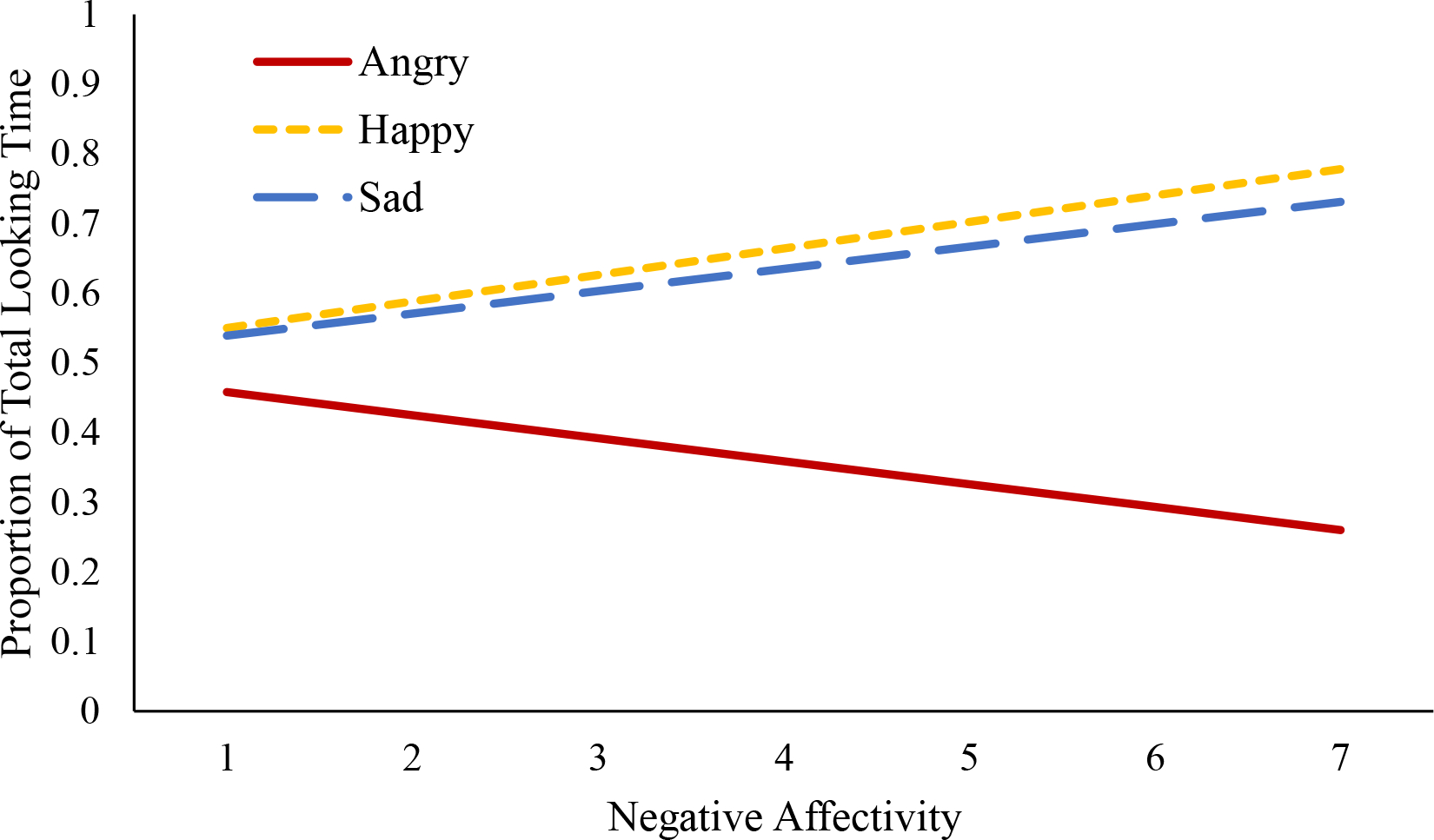
Note. Negative affectivity was associated with a lower proportion of total looking time to angry faces relative to neutral faces (βAngry = −0.03, SE = 0.02, t = −2.13, p = .036, partial η2 = .062) and was associated with a higher proportion of total looking time to happy faces relative to neutral faces (βHappy = 0.04, SE = 0.02, t = 2.47, p = .016, partial η2 = .081). Negative affectivity associated with a higher proportion of total looking time to sad faces relative to neutral faces at the level of a trend (βSad = 0.03, SE = 0.02, t = 1.88, p = .064, partial η2 = .049).
Discussion
The ability to preferentially attend to and visually process emotional faces is closely interwoven with the trajectory of socioemotional development across the lifespan. The current study investigated the relation between affect-biased attention and infant negative affectivity, a key predictor of poor mental health outcomes (Muris & Ollendick, 2005; Nigg, 2006). Affect-biased attention was assessed by jointly evaluating both attention orienting and holding on emotion faces in a free viewing task. Utilizing this approach, we found that overall, infant attention did not vary across trial type on our primary metrics of initial orienting (i.e., latency difference score) and attention holding (i.e., proportion of total looking time). When exploring the associations among negative affectivity and affect-biased attention, we found that infant negative affectivity relates to patterns of attention holding but not initial orienting towards emotional faces. Specifically, we found that after detecting both the emotion face and its neutral face pair, infants high in negative affectivity spent a lower proportion of time attending to angry faces and a greater proportion of time attending to happy faces in comparison to neutral face pairs. Findings provide evidence of an association between increased negative affectivity and biases in attention holding towards positive, happy faces and away from threat-cueing, angry faces in contrast to neutral faces.
We did not find any overall patterns of bias in attention orienting, as latencies to detect faces did not significantly vary across happy, angry, and sad trials, nor did they differ when assessing orienting to the emotion face in contrast to the neutral face pair. Prior studies suggest that a normative bias towards threat-cuing stimuli emerges between 5 and 7 months of age (Fu & Pérez-Edgar, 2019; LoBue & DeLoache, 2010; Morales et al., 2017; Nelson & Dolgin, 1985; Peltola et al., 2009). However, notably much of this work has been based on quicker detection on fear faces whereas less research has explored latency to angry faces in infants (Fu & Pérez-Edgar, 2019). It is therefore possible that attention orienting biases to angry faces may develop more slowly in comparison to fearful faces, or that such biases may not have been elicited due to factors related to study design (e.g., in this free viewing paradigm, attention to emotion faces is always assessed within the context of simultaneously presented neutral faces).
When assessing the main effect of emotion on total looking time to faces, we found that infants spent less time holding attention on faces during sad trials in comparison to happy and angry trials. This finding underscores the importance of including sad faces in infant eye tracking studies, as very limited work has assessed how infants visually explore sad faces. There is some evidence from past work that by 7 months of age, infants hold attention more on faces displaying salient threat cuing emotions, such as fear (Nelson & Dolgin, 1985) and are less likely to disengage from these faces in comparison to happy or neutral faces (Peltola et al., 2013). However, in the current sample of 6-month-old infants we saw no overall differences in attention holding between angry and happy trials. Further, when total looking time to emotion faces was assessed in comparison to the neutral face pair with the proportion of total looking time metric, we saw no overall differences in attention holding across any of the emotion categories. It is therefore possible that additional biases in attention holding, such as biases toward threat-cuing faces, may emerge in the presence of specific risk factors, such as negative affectivity. Null findings could also be explained by the inclusion of angry faces instead of fear faces or could be attributed to the specific study design (i.e., free viewing paradigm) implemented in the current investigation.
Findings linking negative affectivity towards biases in attention holding but not orienting align with past observations in the extent literature. Similar to Fu and colleagues (2020) and Vallorani and colleagues (2021), we did not find evidence of a main effect of negative affectivity on attention orienting to emotion faces. However, we did find that after having initially fixated on both faces in an emotional-neutral face pair, infants high in negative affectivity demonstrated less attention holding on angry faces in comparison to neutral face pairs. This finding suggests that infants high in negative affectivity are less likely to exhibit preferential holding of attention on threat-cuing stimuli when in the presence of a competing neutral stimuli. It is possible that this reduction in sustained attention on angry faces could reflect a reduced tolerance and avoidance of threat-cuing stimuli. Notably, this pattern of less attention holding on threat-cuing faces in comparison to neutral faces has also been observed in children and adolescents with elevated anxiety symptoms (Lisk et al., 2019). Similarly in adults, anxious individuals also tend to exhibit sustained avoidance of threat in the more voluntary stage of attention holding, underscoring possible implications for psychopathological risk if negative emotionality and attentional biases are maintained over time (Armstrong & Olatunji, 2012). We also found that infants higher in negative affectivity also looked longer at happy faces in comparison to the neutral faces. Similar findings have been reported in eye tracking studies of children experiencing depression, which linked depression to a greater proportion of total looking time to happy faces in comparison to other emotion faces in a visual search paradigm (Harrison & Gibb, 2014). It is also plausible that attentional biases towards happy faces and away from angry faces could be a compensatory strategy for infants high in emotionality to avoid over-arousal, highlighting the importance of continuing to explore how these biases relate to negative emotionality and other facets of psychopathological risk over time (Martinos et al., 2012; Morales et al., 2016; Peltola et al., 2015). Taken together, findings of the current study suggest that 6-month-old infants who are higher in negative affectivity are no quicker to detect emotion faces but exhibit attentional biases in how they subsequently process those faces in the more voluntary stage of attention holding. This pattern of attentional behavior suggests that infants who are high in negative affectivity and at elevated risk for later psychopathology may differ in how they deploy attention to explore emotionally salient stimuli and faces in their environments, and more work is needed to explore the function these attentional behaviors may serve.
Strengths, Limitations, and Future Directions
The current study has several key strengths. First, the study task involved the free viewing of emotional faces. This approach allowed us to explore how infants viewed faces for extended periods of time with minimal constraints, rather than brief and rapid exposures. Through this study design, we were also able to explore how negative affectivity relates to each subcomponent of attention within a singular task. Second, as previously stated, sad faces in addition to happy and angry faces were included in the study design. Offering a more complete range of emotion faces allowed us to distinguish how looking behaviors differ across negative emotional faces and underscores the importance of including a variety of emotion types in the study of affect-biased attention. Finally, infants in the study sample represent children from a range of racial, ethnic, and socioeconomic backgrounds, many of which are underrepresented in infant eye tracking research. This diversity of the study sample is important to support generalizability of study findings.
Despite these strengths, there are also several limitations and key areas for future research. First, negative affectivity was assessed via maternal report. Future studies should utilize multi-rater measures and may assess whether these findings align with laboratory assessments of infant negative affectivity. Second, affect-biased attention and negative affectivity were assessed at one time point. A growing body of research has suggested that the interplay between attention and socioemotional functioning is dynamic across the first few years of life. Developmental theories such as that of Field and Lester (2010), suggest that innate biases in attention towards faces are moderated across development by factors both intrinsic and extrinsic to the child in a bidirectional relation. Future studies should therefore continue to explore how the observed attentional biases related to negative affectivity at 6 months of age may be perpetuated or modified across infancy, and even into childhood and adolescence when diagnosable psychopathology begins to emerge. This line of research may also be further supported by studies that integrate both eye tracking as well as neuroimaging measures, such as fMRI and ERP. This is an important direction for future research as recent work suggests that attention orienting and holding may be supported by differing neural subnetworks that are integrated in the dorsal front-parietal and midcingulo-insular network (Markett et al., 2022). Incorporating neural measures into a multimodal approach would provide an opportunity to see if patterns of differential attending and processing of emotional faces in the current study map onto differences in brain networks. Additionally, all infants were presented with the same images in a fixed order to reduce task related variability in the measurement of individual differences. These images included in the current study design include only the faces of White, female actors. Although race-based biases in attention orienting and attention holding (e.g., preferential looking towards other race faces) are not reliably demonstrated by 6 months of age (Hunter & Markant, 2021; Kelly et al., 2007; Kelly et al., 2009; Liu et al., 2013), it is important that future studies include images that are representative of caregivers, especially across later development as by 9 months infants demonstrate greater difficulty discriminating other-race faces (Kelly et al., 2007; Kelly et al., 2009; Markant et al., 2016). Future studies should continue to assess how the race, ethnicity, and gender of actors and infant experiences shape visual exploration of emotion faces and investigate whether these factors moderate any observed associations. Lastly, although we observe differences in infant gaze, we do not know the cognitive mechanisms underlying these observed behaviors (e.g., whether infants indeed perceive angry faces to be threatening or whether they are responding to other visual factors such as novelty or visual distinctiveness).
Clinical Implications
Current study findings have important implications for understanding the role of affect-biased attention in the development of psychopathology. Infants rely heavily on the ability to detect and process faces around them, particularly those of their caregivers. This ability develops along with the capacity to use attention as a way to regulate emotional experiences. As infants become children and then adults, sustained patterns of exaggerated attentional biases may relate to difficulty regulating emotional states and could support the development of co-occurring mental health challenges. Notably, biases in attention holding away from threat-cuing faces and towards positive, happy faces have also been observed in children and adolescents with anxiety and depression respectively, further underscoring the importance of exploring how these attentional patterns relate to socioemotional development over time. Continuing to explore the relation between negative affectivity and affect-biased attention in early life is important as it may further our understanding of the etiology of psychiatric disorders. This work may inform the development and implementation of more targeted early interventions that support the use more adaptive attention strategies and provide supportive caregiving behaviors for infants who are more emotionally reactive and thus at elevated risk.
Supplementary Material
Acknowledgements:
The authors wish to thank the families who participated in this project. The assistance of the Care Project research team, who made this work possible, is gratefully acknowledged. We would also like to thank Dr. Koraly Pérez-Edgar for her consultation on the processing of eye tracking data. This work was funded by the National Institute of Mental Health (NIMH R01 MH109662). Author D.A.S. is supported by the National Institute of Mental Health (NIMH T32 MH093315).
Footnotes
Conflicts of Interest: None
Ethics Approval Statement: This study was approved by the Colorado Multiple Institutional Review Board (COMIRB) (approval no. 16–2040) and the University of Denver Institutional Review Board (approval no. 956810).
Informed Consent Statement: All participants provided written informed consent prior to enrollment in the study.
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author, D. A. S., upon reasonable request.
References
- Armstrong T, & Olatunji BO (2012). Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clinical Psychology Review, 32(8), 704–723. 10.1016/j.cpr.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, & Van Ijzendoorn MH (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin, 133(1), 1–24. 10.1037/0033-2909.133.1.1 [DOI] [PubMed] [Google Scholar]
- Barrett LF, Adolphs R, Marsella S, Martinez AM, & Pollak SD (2019). Emotional expressions reconsidered: Challenges to inferring emotion from human facial movements. Psychological Science in the Public Interest, 20(1), 1–68. 10.1177/1529100619832930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, & Arterberry ME (2003). Recognition, discrimination and categorization of smiling by 5-month-old infants. Developmental Science, 6(5), 585–599. 10.1111/1467-7687.00314 [DOI] [Google Scholar]
- Burris JL, Barry-Anwar RA, & Rivera SM (2017). An eye tracking investigation of attentional biases towards affect in young children. Developmental Psychology, 53(8), 1418–1427. 10.1037/dev0000345 [DOI] [PubMed] [Google Scholar]
- Burris JL, Buss K, LoBue V, Pérez-Edgar K, & Field AP (2019). Biased attention to threat and anxiety: On taking a developmental approach. Journal of Experimental Psychopathology, 10(3), 1–21. 10.1177/2043808719860717 [DOI] [Google Scholar]
- Caron AJ, Caron RF, & MacLean DJ (1988). Infant discrimination of naturalistic emotional expressions: The role of face and voice. Child Development, 59(3), 604–616. 10.2307/1130560 [DOI] [PubMed] [Google Scholar]
- Cohen LB (1972). Attention-getting and attention-holding processes of infant visual preferences. Child Development, 43, 869–879. 10.2307/1127638 [DOI] [PubMed] [Google Scholar]
- Conejero Á, & Rueda MR (2018). Infant temperament and family socio-economic status in relation to the emergence of attention regulation. Scientific Reports, 8(1), 1–11. 10.1038/s41598-018-28831-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courage ML, Reynolds GD, & Richards JE (2006). Infants’ visual attention to patterned stimuli: Developmental change from 3-to 12-months of age. Child Development, 77, 680–695. 10.1111/j.1467-8624.2006.00897.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Hankin BL, Swales DA, & Hoffman MC (2018). An experimental test of the fetal programming hypothesis: Can we reduce child ontogenetic vulnerability to psychopathology by decreasing maternal depression? Development and Psychopathology, 30(3), 787–806. 10.1017/S0954579418000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan M, Belsky J, Reid V, Volein A, & Johnson MH (2004). Maternal personality and infants’ neural and visual responsivity to facial expressions of emotion. Journal of Child Psychology and Psychiatry, 45(7), 1209–1218. 10.1111/j.1469-7610.2004.00320.x [DOI] [PubMed] [Google Scholar]
- De Haan M, Johnson MH, & Halit H (2003). Development of face-sensitive event-related potentials during infancy: A review. International Journal of Psychophysiology, 51(1), 45–58. 10.1016/s0167-8760(03)00152-1 [DOI] [PubMed] [Google Scholar]
- Field AP, & Lester KJ (2010). Is there room for ‘development’ in developmental models of information processing biases to threat in children and adolescents? Clinical Child and Family Psychology Review, 13(4), 315–332. 10.1007/s10567-010-0078-8 [DOI] [PubMed] [Google Scholar]
- Frank MC, Vul E, & Johnson SP (2009). Development of infants’ attention to faces during the first year. Cognition, 110(2), 160–170. 10.1016/j.cognition.2008.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Morales S, LoBue V, Buss KA, & Pérez-Edgar K (2020). Temperament moderates developmental changes in vigilance to emotional faces in infants: Evidence from an eye-tracking study. Developmental Psychobiology, 62(3), 339–352. 10.1002/dev.21920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, & Pérez-Edgar K (2019). Threat-related attention bias in socioemotional development: A critical review and methodological considerations. Developmental Review, 51, 31–57. 10.1016/j.dr.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger LD, Danziger S, & Schoeni RF (2009). Accurately measuring the trend in poverty in the United States using the Panel Study of Income Dynamics. Journal of Economic and Social Measurement, 34(2–3), 105–117. 10.3233/JEM-2009-0313 [DOI] [Google Scholar]
- Haith MM, Bergman T, & Moore MJ (1977). Eye contact and face scanning in early infancy. Science, 198(4319), 853–855. 10.1126/science.918670 [DOI] [PubMed] [Google Scholar]
- Harrison AJ, & Gibb BE (2015). Attentional biases in currently depressed children: An eye-tracking study of biases in sustained attention to emotional stimuli. Journal of Clinical Child & Adolescent Psychology, 44(6), 1008–1014. 10.1080/15374416.2014.930688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnius S, & Geuze RH (2004). Developmental changes in visual scanning of dynamic faces and abstract stimuli in infants: A longitudinal study. Infancy, 6(2), 231–255. 10.1207/s15327078in0602_5 [DOI] [PubMed] [Google Scholar]
- Hunnius S, & Geuze RH (2004). Gaze shifting in infancy: A longitudinal study using dynamic faces and abstract stimuli. Infant Behavior and Development, 27(3), 397–416. 10.1016/j.infbeh.2004.02.003 [DOI] [PubMed] [Google Scholar]
- Hunter BK, & Markant J (2021). Differential sensitivity to species-and race-based information in the development of attention orienting and attention holding face biases in infancy. Developmental Psychobiology, 63(3), 461–469. 10.1002/dev.22027 [DOI] [PubMed] [Google Scholar]
- Joormann J, Talbot L, & Gotlib IH (2007). Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology, 116(1), 135–143. 10.1037/0021-843x.116.1.135 [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Ge L, & Pascalis O (2007). The other-race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science, 18(12), 1084–1089. 10.1111/j.1467-9280.2007.02029.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Liu S, Lee K, Quinn PC, Pascalis O, Slater AM, & Ge L (2009). Development of the other-race effect during infancy: Evidence toward universality?. Journal of Experimental Child Psychology, 104(1), 105–114. 10.1016/j.jecp.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyrka-Allchorne K, Wass SV, & Sonuga-Barke EJ (2020). Research review: Do parent ratings of infant negative emotionality and self-regulation predict psychopathology in childhood and adolescence? A systematic review and meta-analysis of prospective longitudinal studies. Journal of Child Psychology and Psychiatry, 61(4), 401–416. 10.1111/jcpp.13144 [DOI] [PubMed] [Google Scholar]
- Leppänen JM (2016). Using eye tracking to understand infants’ attentional bias for faces. Child Development Perspectives, 10(3), 161–165. 10.1111/cdep.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM, Moulson MC, Vogel-Farley VK, & Nelson CA (2007). An ERP study of emotional face processing in the adult and infant brain. Child Development, 78(1), 232–245. 10.1111/j.1467-8624.2007.00994.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM, & Nelson CA (2006). The development and neural bases of facial emotion recognition. Advances in Child Development and Behavior, 34, 207–246. 10.1016/s0065-2407(06)80008-x [DOI] [PubMed] [Google Scholar]
- Leppänen JM, & Nelson CA (2009). Tuning the developing brain to social signals of emotions. Nature Reviews Neuroscience, 10(1), 37–47. 10.1038/nrn2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisk S, Vaswani A, Linetzky M, Bar-Haim Y, & Lau JY (2020). Systematic review and meta-analysis: Eye-tracking of attention to threat in child and adolescent anxiety. Journal of the American Academy of Child & Adolescent Psychiatry, 59(1), 88–99. 10.1016/j.jaac.2019.06.006 [DOI] [PubMed] [Google Scholar]
- Liu S, Xiao WS, Xiao NG, Quinn PC, Zhang Y, Chen H, . . . Lee K (2015). Development of visual preference for own-versus other-race faces in infancy. Developmental Psychology, 51(4), 500–511. 10.1037/a0038835 [DOI] [PubMed] [Google Scholar]
- LoBue V, & DeLoache JS (2010). Superior detection of threat-relevant stimuli in infancy. Developmental Science, 13(1), 221–228. 10.1111/j.1467-7687.2009.00872.x [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, Phillips BM, & Hooe ES (2003). Relations of positive and negative affectivity to anxiety and depression in children: evidence from a latent variable longitudinal study. Journal of Consulting and Clinical Psychology, 71(3), 465–481. 10.1037/0022-006x.71.3.465 [DOI] [PubMed] [Google Scholar]
- Markant J, Oakes LM, & Amso D (2016). Visual selective attention biases contribute to the other-race effect among 9-month-old infants. Developmental Psychobiology, 58(3), 355–365. 10.1002/dev.21375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markett S, Nothdurfter D, Focsa A, Reuter M, & Jawinski P (2022). Attention networks and the intrinsic network structure of the human brain. Human Brain Mapping, 43(4), 1431–1448. 10.1002/hbm.25734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinos M, Matheson A, & de Haan M (2012). Links between infant temperament and neurophysiological measures of attention to happy and fearful faces. Journal of Child Psychology and Psychiatry, 53(11), 1118–1127. 10.1111/j.1469-7610.2012.02599.x [DOI] [PubMed] [Google Scholar]
- Morales S, Brown KM, Taber-Thomas BC, LoBue V, Buss KA, & Pérez-Edgar KE (2017). Maternal anxiety predicts attentional bias towards threat in infancy. Emotion, 17(5), 874–883. 10.1037/emo0000275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales S, Fu X, & Pérez-Edgar KE (2016). A developmental neuroscience perspective on affect-biased attention. Developmental Cognitive Neuroscience, 21, 26–41. 10.1016/j.dcn.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, & Ollendick TH (2005). The role of temperament in the etiology of child psychopathology. Clinical Child and Family Psychology Review, 8(4), 271–289. 10.1007/s10567-005-8809-y [DOI] [PubMed] [Google Scholar]
- Nakagawa A, & Sukigara M (2019). Attentional bias assessed by a facial expression cuing paradigm in infants. Scientific Reports, 9(1), 1–7. 10.1038/s41598-018-36806-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA (1987). The recognition of facial expressions in the first two years of life: Mechanisms of development. Child Development, 58(4), 889–909. 10.2307/1130530 [DOI] [PubMed] [Google Scholar]
- Nelson CA, & De Haan M (1996). Neural correlates of infants’ visual responsiveness to facial expressions of emotion. Developmental Psychobiology, 29(7), 577–595. [DOI] [PubMed] [Google Scholar]
- Nelson CA, & Dolgin KG (1985). The generalized discrimination of facial expressions by seven-month-old infants. Child Development, 56(1), 58–61. 10.2307/1130173 [DOI] [PubMed] [Google Scholar]
- Nigg JT (2006). Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry, 47(3–4), 395–422. 10.1111/j.1469-7610.2006.01612.x [DOI] [PubMed] [Google Scholar]
- Peckham AD, McHugh RK, & Otto MW (2010). A meta-analysis of the magnitude of biased attention in depression. Depression and Anxiety, 27(12), 1135–1142. 10.1002/da.20755 [DOI] [PubMed] [Google Scholar]
- Peltola MJ, Forssman L, Puura K, van IJzendoorn MH, & Leppänen JM (2015). Attention to faces expressing negative emotion at 7 months predicts attachment security at 14 months. Child Development, 86(5), 1321–1332. 10.1111/cdev.12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola MJ, Hietanen JK, Forssman L, & Leppänen JM (2013). The emergence and stability of the attentional bias to fearful faces in infancy. Infancy, 18, 905–926. doi: 10.1111/infa.12013 [DOI] [Google Scholar]
- Peltola MJ, Leppänen JM, Mäki S, & Hietanen JK (2009). Emergence of enhanced attention to fearful faces between 5 and 7 months of age. Social Cognitive and Affective Neuroscience, 4(2), 134–142. 10.1093/scan/nsn046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Morales S, LoBue V, Taber-Thomas BC, Allen EK, Brown KM, & Buss KA (2017). The impact of negative affect on attention patterns to threat across the first 2 years of life. Developmental Psychology, 53(12), 2219–2232. 10.1037/dev0000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Taber-Thomas B, Auday E, & Morales S (2014). Temperament and attention as core mechanisms in the early emergence of anxiety. Contributions to Human Development, 26, 42–56. 10.1159/000354350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, & Posner MI (2012). The attention system of the human brain: 20 years after. Annual Review of Neuroscience, 35, 73–89. 10.1146/annurev-neuro-062111-150525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam SP, Rothbart MK, & Gartstein MA (2008). Homotypic and heterotypic continuity of fine-grained temperament during infancy, toddlerhood, and early childhood. Infant and Child Development: An International Journal of Research and Practice, 17(4), 387–405. 10.1002/icd.582 [DOI] [Google Scholar]
- Posner MI, & Petersen SE (1990). The attention system of the human brain. Annual Review of Neuroscience, 13(1), 25–42. 10.1146/annurev.ne.13.030190.000325 [DOI] [PubMed] [Google Scholar]
- Quinn PC, Yahr J, Kuhn A, Slater AM, & Pascalis O (2002). Representation of the gender of human faces by infants: A preference for female. Perception, 31(9), 1109–1121. 10.1068/p3331 [DOI] [PubMed] [Google Scholar]
- Righi G, Westerlund A, Congdon EL, Troller-Renfree S, & Nelson CA (2014). Infants’ experience-dependent processing of male and female faces: Insights from eye tracking and event-related potentials. Developmental Cognitive Neuroscience, 8, 144–152. 10.1016/j.dcn.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK (1981). Measurement of temperament in infancy. Child Development, 52, 569–578. 10.2307/1129176 [DOI] [Google Scholar]
- Rothbart MK, & Bates JE (2006). Temperament. In Eisenberg N, Damon W, & Lerner RM (Eds.), Handbook of child psychology: Social, emotional, and personality development (pp. 99–166). John Wiley & Sons, Inc. [Google Scholar]
- Segal SC, Marquis AR, & Moulson MC (2021). Are our samples representative? Understanding whether temperament influences infant dropout rates at 3 and 7 months. Infant Behavior and Development, 65, 101630. 10.1016/j.infbeh.2021.101630 [DOI] [PubMed] [Google Scholar]
- Serrano JM, Iglesias J, & Loeches A (1992). Visual discrimination and recognition of facial expressions of anger, fear, and surprise in 4-to 6-month-old infants. Developmental Psychobiology, 25(6), 411–425. 10.1002/dev.420250603 [DOI] [PubMed] [Google Scholar]
- Simion F, Macchi Cassia V, Turati C, & Valenza E (2001). The origins of face perception: specific versus non-specific mechanisms. Infant and Child Development, 10(1–2), 59–65. 10.1002/icd.247 [DOI] [Google Scholar]
- Tobii Pro AB (2014). Tobii Pro Lab (Version 1.162). Danderyd, Sweeden: Tobii Pro AB. [Google Scholar]
- Todd RM, Cunningham WA, Anderson AK, & Thompson E (2012). Affect-biased attention as emotion regulation. Trends in Cognitive Sciences, 16(7), 365–372. 10.1016/j.tics.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Tottenham N, Borscheid A, Ellertsen K, Marcus D, & Nelson C (2002). The NimStim face set. Retrieved from http://www.macbrain.org/faces/index.htm.
- United States Census Bureau (2020). How the Census Bureau measures poverty. Retrieved February 2021 from
- Vallorani A, Fu X, Morales S, LoBue V, Buss KA, & Pérez-Edgar K (2021). Variable-and person-centered approaches to affect-biased attention in infancy reveal unique relations with infant negative affect and maternal anxiety. Scientific Reports, 11(1), 1–14. 10.1038/s41598-021-81119-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele B, Verschuere B, Tibboel H, De Houwer J, Crombez G, & Koster EH (2014). A review of current evidence for the causal impact of attentional bias on fear and anxiety. Psychological Bulletin, 140(3), 682–721. 10.1037/a0034834 [DOI] [PubMed] [Google Scholar]
- Viola Macchi C, Turati C, & Simion F (2004). Can a nonspecific bias toward top-heavy patterns explain newborns’ face preference? Psychological Science, 15(6), 379–383. 10.1111/j.0956-7976.2004.00688.x [DOI] [PubMed] [Google Scholar]
- Wieser MJ, Pauli P, Weyers P, Alpers GW, & Mühlberger A (2009). Fear of negative evaluation and the hypervigilance-avoidance hypothesis: An eye-tracking study. Journal of Neural Transmission, 116(6), 717–723. 10.1007/s00702-008-0101-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, D. A. S., upon reasonable request.


