Abstract
Schwannomas are benign, slow-growing peripheral nerve sheath tumors commonly occurring in the head, neck, and flexor regions of the extremities. Although most schwannomas are easily diagnosable, their variable morphology can occasionally create difficulty in diagnosis. Reporting pathologists should be aware that schwannomas can exhibit a broad spectrum of morphological patterns. Clinical and radiological examinations can show correlation and should be performed, in conjunction with ancillary tests, when appropriate. Furthermore, deferring a definitive diagnosis until excision may be necessary for small biopsy specimens and frozen sections. This report underscores these challenges through examination of two unique schwannoma cases, one predominantly cellular and the other myxoid, both of which posed significant challenges in histological interpretation.
Keywords: Neurilemmoma, Soft tissue neoplasms, Biopsy, Frozen sections, Immunohistochemistry, Pathologists, Extremities, Mitosis
Schwannomas are slow-growing, benign peripheral nerve sheath tumors that occur commonly in the head and neck region or flexor aspects of the extremities. Schwannomas also can occur, but are not limited to, the spinal or paraspinal region, cranial nerves, central nervous system, viscera, and bone. While more than 90% of cases are solitary and sporadic, multiple paraspinal schwannomas or involvement of the eight cranial nerves suggests neurofibromatosis 2 (NF2), and bilateral vestibular schwannoma is diagnostic of NF2. It is well known that tumorigenesis of schwannomas is due to the loss of merlin, a product of the NF2 gene, located at 22q2.2 [1].
A broad range of histological patterns can be observed in schwannomas, and the 5th edition of the World Health Organization Classification of soft tissue and bone tumors recognizes ancient, cellular, plexiform, epithelioid, and microcystic/reticular subtypes. Conventional schwannomas are composed almost entirely or entirely of Schwann cells with spindle morphology and typically exhibit Antoni A, Antoni B, and Verocay bodies. Although schwannomas are easily diagnosable on most occasions without ancillary testing, obtaining the correct diagnosis can be difficult due to their variable morphology.
Herein, we report two cases of schwannomas that exhibited histological features that were at opposite ends of the morphological spectrum, with differing cellularity and nuclear atypia, resulting in challenging biopsy and intraoperative frozen section interpretations.
CASE REPORT
Case 1
A 68-year-old male with no known medical or surgical history was referred to a tertiary hospital due to a left thigh mass that the patient had first noticed 3 years prior and that had recently increased in size. Additionally, two other masses were observed adjacent to the primary mass. The physical examination revealed no neurovascular compromise.
Magnetic resonance imaging (MRI) revealed a heterogeneously enhancing mass situated at the anterior aspect of the left thigh within the subcutaneous tissue plane. The largest of the masses exhibited central cystic changes and measured 7 cm at its maximum dimension, raising the possibility of a malignant soft tissue tumor (Fig. 1A).
Fig. 1.
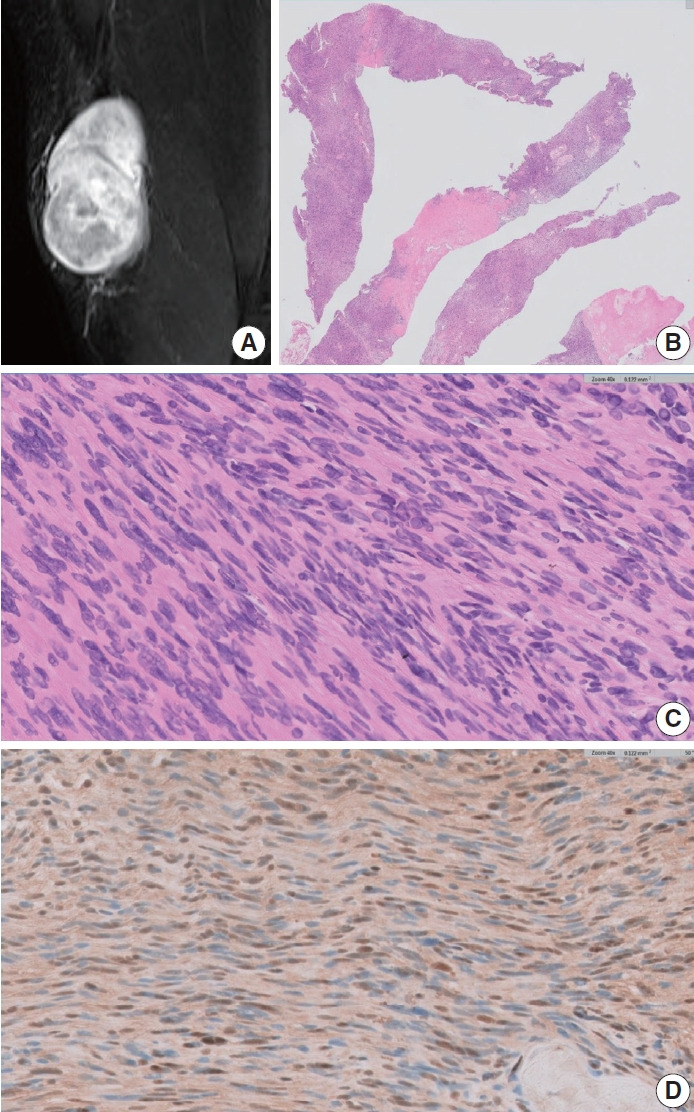
A cellular schwannoma. (A) Magnetic resonance imaging scan showing a heterogeneously enhancing mass within the subcutaneous tissue plane. (B) Core biopsies showing predominantly cellular areas, resulting in a “blue appearance” accompanied by pale degenerate areas. (C) Compact spindle cells showing hypercellularity and mild nuclear pleomorphism. (D) S-100 immunohistochemistry showing a focal weak staining pattern despite high cellularity.
A core needle biopsy was performed to rule out a malignant soft tissue neoplasm. The biopsy revealed densely packed short to long sweeping fascicles of spindle cells exhibiting marked hypercellularity and acellular degenerated areas (Fig. 1B). The tumor cells exhibited mild nuclear pleomorphism (Fig. 1C). S-100 immunohistochemistry (IHC) revealed positive staining in the tumor cells, with some areas exhibiting a weak staining pattern (Fig. 1D). The tumor cells also demonstrated diffuse strong positivity for SOX10, CD56, and CD99 while being negative for actin, desmin, pan-cytokeratin, epithelial membrane antigen, and CD34. Consequently, the biopsy results were not entirely conclusive, and a malignant peripheral nerve sheath tumor (MPNST) could not be definitively ruled out.
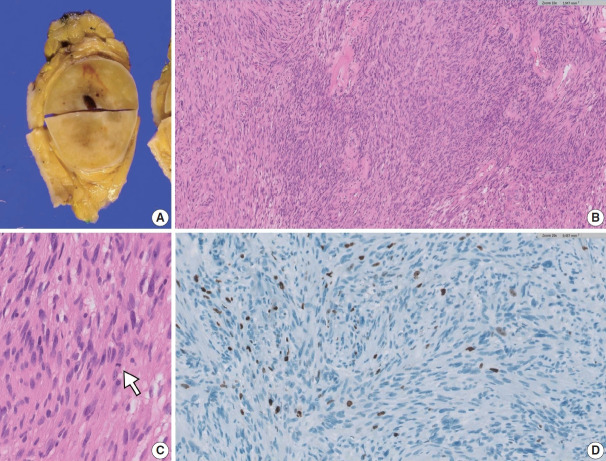
Upon gross examination of the excised specimen, the largest tumor was well surrounded by a thin fibrous capsule. The cut surface exhibited a solid yellow-to-tan appearance with cystic changes (Fig. 2A). The microscopic findings showed heterogeneous morphological patterns, including hypercellular regions lacking Verocay bodies (Fig. 2B) and areas characteristic of classic schwannoma. Rare mitotic figures were detected at 1 per mm2 (Fig. 2C) with a Ki-67 labeling index less than 5% (Fig. 2D). The final diagnosis of cellular schwannoma was made based on excision.
Fig. 2.
Excision specimen of a cellular schwannoma. (A) Solid yellow-to-tan lesion with a surrounding thin fibrous capsule. (B) Hypercellular region without well-formed Verocay bodies. (C) One of the rare mitotic figures detected (white arrow). (D) Ki-67 labelling index less than 5%.
Case 2
A 72-year-old female with a medical history of rheumatoid arthritis and no prior surgical interventions was referred to a tertiary care hospital due to a slow-growing mass in her left forearm that had been present for 4 years. The physical examination yielded an equivocal result on the Tinel test. MRI revealed a T2-bright mass demonstrating heterogeneous enhancement. The mass, measuring 12.3 cm at its maximum dimension, was suggestive of a benign peripheral nerve sheath tumor (Fig. 3A).
Fig. 3.
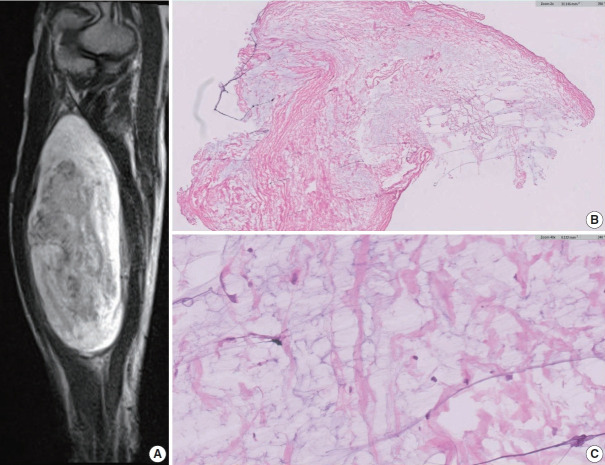
A schwannoma with myxoid change. (A) Magnetic resonance imaging showing a T2-bright mass displaying heterogeneous enhancement. (B, C) Low- and high-power views of the frozen section showing scattered cytologically benign spindle cells with surrounding loose myxoid stroma.
During surgery, a frozen section examination of three areas of the tumor exhibited an entirely myxoid, paucicellular pattern (Fig. 3B, C). The interim interpretation identified it as a low-grade myxoid tumor with a final determination deferred until the permanent sections could be evaluated.
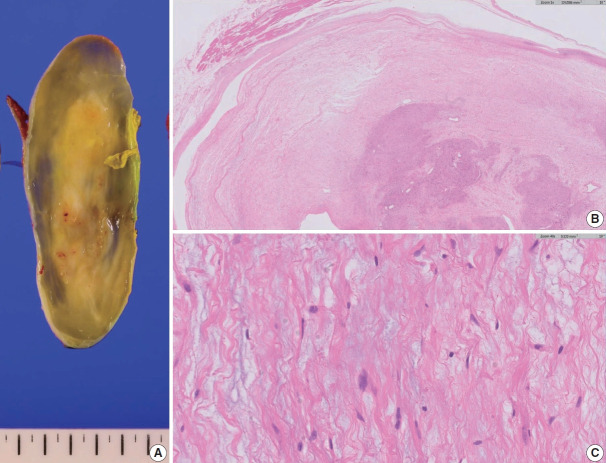
Upon gross examination of the excised specimen, the tumor was well-circumscribed, exhibiting a myxoid peripheral zone and a more solid, yellowish central area (Fig. 4A). Histopathological examination revealed a paucicellular area on the tumor’s superficial aspect, characterized by loose myxoid stroma interspersed with scattered spindle cells exhibiting wavy nuclei (Fig. 4B, C). These cells exhibited positive labeling with S-100 IHC. Deeper within the tumor, a region more characteristic of a conventional schwannoma was identified (Fig. 4B). The final diagnosis was schwannoma with myxoid change.
Fig. 4.
Excision specimen of a schwannoma with myxoid change. (A) Well-circumscribed, demonstrating a solid central area with a myxoid peripheral zone. (B) Interface between the loose myxoid area and more classic schwannoma-like area. Note that the classic area is observed at the deeper aspect. (C) High-power view exhibiting scattered spindle cells within the myxoid stroma.
DISCUSSION
Cellular schwannomas, as the name suggests, exhibit higher cellularity and increased mitotic figures compared to conventional schwannomas, although the mitotic count is typically less than 5 mitoses/mm2. These also typically reveal the absence of Verocay bodies and may exhibit hyperchromasia and microscopic foci of necrosis, further complicating the histological interpretation [2] and raising the possibility of malignant spindle cell neoplasms such as MPNST. Well-formed capsule and subcapsular lymphoid aggregates may support the diagnosis of schwannoma, but they are not always present. For this reason, additional ancillary tests are often required in this setting, and less diffuse reactivity with S-100 and loss of H3K27me3 by IHC are evidence against the diagnosis of schwannoma and favor MPNST [3].
In case 1, where a pre-operative diagnosis was obtained from a small biopsy, a retrospective analysis revealed characteristics suggestive of cellular schwannoma. However, this diagnosis was challenging due to the absence of hypocellular areas and Verocay bodies, and S-100 IHC did not demonstrate diffuse strong positivity. Although the mitotic count could have been informative in excluding MPNSTs [2], it remains an unreliable measure in biopsy specimens. Low-grade MPNSTs typically exhibit cytological atypia, hypercellularity, a mitotic count ranging from 1.5 to 4.5 mitoses/mm2 (equivalent to 3–9 mitotic figures per 10 high-power fields), and the absence of necrosis [4]. However, in the present case, the surgical specimen demonstrated only 1 mitosis per mm2, which falls below the diagnostic criteria for low-grade MPNST. Mitotic activity can be observed in cellular schwannomas but is typically less than 5 per 10 high-power fields [5]. Brisk mitotic activity may be present in rare instances, potentially exceeding 10 per 10 high-power fields [5]. However, if other diagnostic features of cellular schwannoma are present, this heightened proliferative activity remains aligned with a benign diagnosis. Cellular schwannomas are characterized by a specific pattern of Ki-67 labeling, primarily manifesting in the form of localized hotspots rather than a diffused increment [2]. Even in the presence of these hotspots, the Ki-67 index seldom exceeds 20%. The utilization of a Ki-67 stain can reveal variable labeling indices throughout a cellular schwannoma, with a significant concentration in hotspot regions [2]. By contrast, the Ki-67 stain typically exhibits a diffusely elevated labeling pattern in the context of an MPNST.
Although the identification of H3K27me3 loss can assist in confirming a diagnosis of high-grade MPNST, the presence of positive staining does not preclude the possibility of MPNST, as only 34% of cases demonstrated a loss of staining in a study conducted by Cleven et al. [3]. Moreover, H3K27me3 antibodies are not readily accessible in many settings. Consequently, it is often challenging to conclusively diagnose cellular schwannoma based solely on a small biopsy sample.
S-100 is a common, widely-used antibody for diagnostic use in histopathology as a neural marker, and it is useful in differentiating schwannomas and MPNSTs, as schwannomas typically exhibit diffuse and strong positivity, while MPNSTs demonstrate patchy or negative staining [5]. However, such a description is arbitrary, and there are no defined criteria on how diffuse and strong the staining has to be in a numerical or percentage form. There was focal weak staining with S-100 IHC in our cellular schwannoma case, which was problematic. Pekmezci et al. [2] used 90% as a cutoff value for diffuse positive staining and <5% for negative staining for a comparison between schwannomas and MPNSTs. No intensity of staining was documented. In Pekmezci et al. [2], 96% of cellular schwannomas stained diffusely, while only 14% of MPNSTs revealed diffuse positivity. With such findings, the authors believe the 90% cutoff value to be useful when used in conjunction with morphological and radiological evaluations.
Myxoid change is a well-described phenomenon in schwannomas and often causes diagnostic difficulties [6]. Case 2 revealed extensive myxoid change, particularly within the peripheral aspect of the tumor, and led to a diagnostic dilemma during the intraoperative frozen section. Chaurasia et al. [6] described a reticular schwannoma mimicking a low-grade fibromyxoid sarcoma, and this case also described extensive myxoid change. Many soft tissue tumors can exhibit myxoid change. For this reason, in small biopsy specimens of such cases, it would be prudent to report such cases as a low-grade myxoid neoplasm and recommend complete excision for a definitive assessment unless there are unequivocal histological features of schwannomas, including Antoni A, Antoni B, and Verocay bodies.
Interestingly, even after multiple incisional biopsies were performed for intraoperative frozen section of case 2, no diagnostic material for a schwannoma was obtained. The European Society for Medical Oncology (ESMO) guidelines [7] suggest that multiple core needles using 14 or greater gauge needles are used to sample different tumor locations and depths, correlating with radiological imaging. In hindsight, if the biopsies were performed from both superficial and deep aspects for this case, instead of multiple superficial biopsies only; these may have captured a more diagnostic area of the schwannoma.
Identifying extremely T2-bright and T1-hypointense enhancing lesions on MRIs involving the extremities or, less commonly, the trunk without visceral involvement suggests a myxoid neoplasm. However, these findings can also be observed in schwannomas [8]. The other radiological signs that can be seen in schwannomas include a fat split sign and a target sign [9]. For these reasons, correlation with radiological findings may be helpful in distinguishing between other myxoid neoplasms from a schwannoma with myxoid change.
A study by Pianta et al. [10] described 100% concordance between biopsy and excision in 41 cases of peripheral nerve sheath tumors, substantiating the accuracy of a core biopsy. Furthermore, a meta-analysis by Birgin et al. [11] revealed 88% sensitivity and 77% specificity for soft tissue sarcoma histotypes, further verifying the usefulness and high accuracy of core needle biopsies. However, histological heterogeneity is well known in soft tissue tumors [12], and obtaining a non-representative sample remains possible. Therefore, a definitive diagnosis is often deferred until after excision.
Reporting pathologists should be aware that a broad spectrum of morphological patterns in schwannomas can lead to a diagnostic challenge. Clinical and radiological exams and judicious use of ancillary tests is recommended when appropriate. Furthermore, deferring a definitive diagnosis to excision may be necessary for small biopsy specimens and frozen sections.
Footnotes
Ethics Statement
Formal written informed consent was not required with a waiver by the appropriate IRB (KC23ZASI0457).
Availability of Data and Material
Data sharing is not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Author contributions
Conceptualization: CKJ. Data curation: CK, YGC, CKJ. Formal analysis: CK, CKJ. Investigation: CK, YGC, CKJ. Supervision: CKJ. Writing—original draft: CK, CKJ. Writing—review & editing: CK, YGC, CKJ. Approval of final manuscript: all authors.
Conflicts of Interest
C.K.J., the editor-in-chief of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
This research was supported by a grant (HI21C0940) from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea.
References
- 1.WHO Classification of Tumours Editorial Board . WHO classification of tumours: soft tissue and bone tumours. 5th ed. Lyon: International Agency for Research on Cancer; 2020. [Google Scholar]
- 2.Pekmezci M, Reuss DE, Hirbe AC, et al. Morphologic and immunohistochemical features of malignant peripheral nerve sheath tumors and cellular schwannomas. Mod Pathol. 2015;28:187–200. doi: 10.1038/modpathol.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleven AH, Sannaa GA, Briaire-de Bruijn I, et al. Loss of H3K27 tri-methylation is a diagnostic marker for malignant peripheral nerve sheath tumors and an indicator for an inferior survival. Mod Pathol. 2016;29:582–90. doi: 10.1038/modpathol.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miettinen MM, Antonescu CR, Fletcher CD, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1: a consensus overview. Hum Pathol. 2017;67:1–10. doi: 10.1016/j.humpath.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez FJ, Folpe AL, Giannini C, Perry A. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295–319. doi: 10.1007/s00401-012-0954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaurasia JK, Afroz N, Sahoo B, Naim M. Reticular schwannoma mimicking myxoid sarcoma. BMJ Case Rep. 2014;2014:bcr2013202963. doi: 10.1136/bcr-2013-202963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casali PG, Abecassis N, Aro HT, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv51–67. doi: 10.1093/annonc/mdy096. [DOI] [PubMed] [Google Scholar]
- 8.Baheti AD, Tirumani SH, Rosenthal MH, et al. Myxoid soft-tissue neoplasms: comprehensive update of the taxonomy and MRI features. AJR Am J Roentgenol. 2015;204:374–85. doi: 10.2214/AJR.14.12888. [DOI] [PubMed] [Google Scholar]
- 9.Lee E, Lee GY, Cho WS, et al. Useful MRI features for distinguishing benign peripheral nerve sheath tumors and myxoid tumors in the musculoskeletal system. Investig Magn Reson Imaging. 2015;19:153–61. [Google Scholar]
- 10.Pianta M, Chock E, Schlicht S, McCombe D. Accuracy and complications of CT-guided core needle biopsy of peripheral nerve sheath tumours. Skeletal Radiol. 2015;44:1341–9. doi: 10.1007/s00256-015-2185-6. [DOI] [PubMed] [Google Scholar]
- 11.Birgin E, Yang C, Hetjens S, Reissfelder C, Hohenberger P, Rahbari NN. Core needle biopsy versus incisional biopsy for differentiation of soft-tissue sarcomas: a systematic review and meta-analysis. Cancer. 2020;126:1917–28. doi: 10.1002/cncr.32735. [DOI] [PubMed] [Google Scholar]
- 12.Mitsuyoshi G, Naito N, Kawai A, et al. Accurate diagnosis of musculoskeletal lesions by core needle biopsy. J Surg Oncol. 2006;94:21–7. doi: 10.1002/jso.20504. [DOI] [PubMed] [Google Scholar]