Abstract
Introduction
In this study, we evaluate the versatility of smartphone thermal imaging technology as a valuable intraoperative modality in different stages of perforator flap surgery aiming to minimize the complications and achieve the best postoperative outcome.
Patients and methods
Thermography was performed in 20 perforator flaps in 20 patients at different surgical stages in three different ways to identify the most dominant perforator: first, by measuring the surface temperature of the skin; second, by using the dynamic infrared thermography technique; and third, by assessing the perfusion pattern when the flap was supplied by each perforator separately. Thermography was used to help in discarding the least perfused area of the flap. After microvascular anastomosis, the flap reheating pattern was evaluated.
Results
Seventeen free and three pedicled perforator flaps were included. Intraoperatively, each of the selected perforators had a corresponding hotspot. The perforator with the hottest hotpot, best rewarming, and provision of best flap perfusion on thermography was found clinically dominant. After microvascular anastomosis in free flaps, rapid rewarming was recorded in 15 cases. In two deep inferior epigastric perforator flaps, no rapid rewarming was observed. The pedicle was kinked in one case and there was a venous insufficiency in another case that required a cephalic turndown. All flaps showed good perfusion on thermography after inset.
Conclusion
Smartphone thermography has proven to be a valuable, cheap, rapidly employed, and objective tool not only for the design of perforator flaps, but also for the decision making intraoperatively to achieve the best surgical outcome.
Key words: Thermography, perforator flaps, microsurgery, DIEP flaps
Introduction
Perforator flaps are commonly used, in the form of pedicled or free tissue transfer, for the reconstruction of soft tissue defects. They gained popularity in the recent years in the field of reconstructive microsurgery because of their ability to reduce the donor site morbidity.1 Accurate identification of the exact location of the perforators is critical component of successful reconstructive surgical procedures. However, skin perforators do not have a fixed anatomical location, which makes their intraoperative identification challenging.2,3
Previously, locating the most ideal perforators depended on specific anatomical landmarks that were mainly guided by cadaveric dissection studies. In 1987, Taylor and Palmer4 explained the angiosome concept using lead oxide injection and defined it as composite tissue blocks perfused by a single source artery. Surface topographic landmarks are generally useful as a starting point in perforator flap design, but they can vary considerably among patients, hence not entirely reliable.5,6
Recently, technological advancements within imaging modalities have proved effective in preoperative mapping and intraoperative assessment. Computed tomographic angiography (CTA), although remains the gold standard for preoperative mapping of perforators, is expensive, exposes the patient to the risk of intravenous contrast and radiation, requires a radiologist for interpretation and, more importantly, cannot be used intraoperatively.7 Color ultrasound Doppler has been used for assessment of vessel caliber, but it is highly operator dependent.8
These imaging tools assess perforators during the preoperative planning phase; however, most of the surgeons still rely on the clinical assessment of the vessel location and size intraoperatively as the final determining factor.9 Lindsey10 highlighted the importance of intraoperative evaluation for perforator selection in deep inferior epigastric perforator (DIEP) and muscle sparing free transverse rectus abdominis myocutaneous flaps. Intraoperative assessment enables the surgeon to evaluate the perforator after releasing fascial collar and attachments that cannot be achieved using preoperative imaging, helping the surgeon to find out the dominant perforator intraoperatively.
Indocyanine green fluorescence (ICG) imaging has recently been utilized intraoperatively in perforator flap surgery to assess flap perfusion, but not without its own disadvantages. It is invasive, contraindicated in patients with hepatic and renal diseases, and can cause allergic reactions.11 It also requires devices that are costly, either inbuilt into the microscope or a large standalone device.12
However, thermal imaging technology detects infrared radiation from the skin, which is well correlated with the local skin temperature changes, and the cutaneous perfusion.13,14 This process was first described by Arai and Fukuda.15 They introduced “hotspots” that have been used as surface landmarks for perforator mapping.16 Later on, dynamic infrared thermography (DIRT) was described, in which the skin surface is exposed to a cold challenge and then the pattern of skin rewarming is analyzed.17 Thermography has been proposed by many authors being a noninvasive low-cost technique, easily used and compatible with hand-held smartphone devices;18, 19, 20 however, the versatility and applicability of using smartphone thermal imaging technology intraoperatively in different kinds of perforator flaps is unclear.
In this study, we evaluate the uses of smartphone thermal imaging technology as a valuable intraoperative modality in perforator flap surgery. Although CTA is the gold standard in terms of preoperative decision making, we believe it is crucial to have a reliable intraoperative imaging modality where the CTA cannot be used. This low-cost imaging tool is easy to use and may be advantageous in resource poor settings. We aim to evaluate the reliability of the smartphone thermography to aid intraoperative decision making and its versatility to be used in complex reconstructive procedures, especially in places with limited resources.
Patients and methods
Inclusion and exclusion criteria
Twenty perforator flaps in 20 patients were included in this prospective study; 13 DIEP flaps, 4 anterior lateral thigh (ALT) flaps, 2 posterior tibial artery perforator (PTAP) flaps, and 1 thoracodorsal artery perforator (TDAP) flap (Table 1). Exclusion criteria were active smokers, body mass index (BMI) over 30 kg/m2, patients not fit for surgery (American Society of Anesthesiologists classification > 4), and patients with medical conditions that could alter tissue temperature such as acute inflammation, osteomyelitis, peripheral vascular disease or vasculitis, chronic kidney or liver disease, cardiac dysfunction, or the intake of vasoactive medication (beta-blockers, calcium channel blockers, and nitroglycerine). Informed consents were signed by the patients who participated in the study.
Table 1.
Patient characteristics.
| Study population | Patients (n=20) |
|---|---|
| Patients characteristics Age: mean (min-max) |
43.8 (10-67) |
| BMI (kg/m2): mean (min-max) | 26.4 (18-30) |
| Free flap | 17 |
| - DIEP | 13 |
| - ALT | 3 |
| - TDAP | 1 |
| Pedicled flap | 3 |
| - PTAP | 2 |
| - ALT | 1 |
| Immediate | 11 |
| Delayed | 9 |
| Reconstructed region | |
| - Breast | 13 |
| - Head and neck (right side of neck) | 1 |
| - Lower limb | 6 |
| • Groin | 1 |
| • Knee | 1 |
| • Leg | 1 |
| • Ankle | 1 |
| • Foot | 2 |
| Indications for reconstruction | |
| - Breast cancer | 13 |
| - Sarcoma | 1 |
| - Post-traumatic skin loss with bone/tendon exposure | 3 |
| - Chronic ulcer | 1 |
| - Scar contracture | 2 |
Preoperative mapping
Preoperative perforator mapping was performed using a 64-section multidetector CT system (Philips Medical Systems, Cleveland, OH, USA). After induction of general anesthesia and before surgery, several perforators were selected based on preoperative imaging and marked on the skin. This was then confirmed using the hand-held Doppler ultrasound (8 MHz, Multi Dopplex II, Huntleigh Healthcare, Cardiff, UK) and thermal hotspots using smartphone thermal imaging camera (FLIR ONE Pro, FLIR Systems, Inc., Wilsonville, Ore.) (Figure 1).
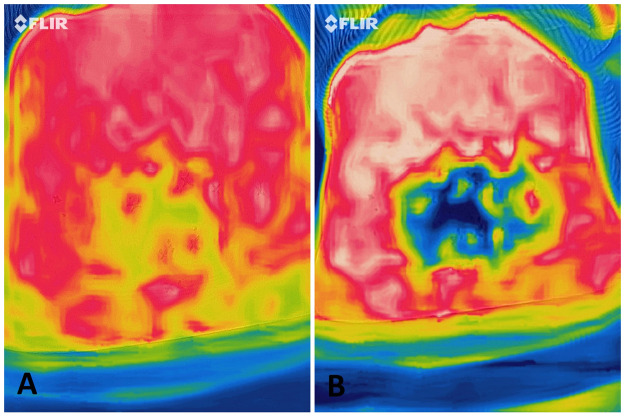
Figure 1.
A: Perforator mapping of the abdomen before the cold challenge. B: After the cold challenge test.
Intraoperative
Dominant perforator selection
Thermal imaging was used during different stages of the surgical procedure in all patients (Table 2). The operating room temperature was kept between 23 and 28°C. Before capturing any thermal images, no pressure was applied to the skin for at least 3 minutes. The optimal camera temperature range focus was set to 28 to 32°C, and the distance between the camera and the skin of the patients was kept between 40 and 70 cm.
Table 2.
Different intraoperative uses of thermography.
| The surgical step | Expected findings | Importance |
|---|---|---|
| 1- Before skin incision | Location of different perforators | Identification of exact location of perforators that helps to accelerate the flap harvest procedure |
| 2- After suprafascial dissection of selected perforators | One perforator is hotter than the others. Different ways to determine that:
|
Identification of the dominant perforator |
| 3- Assessment of the flap perfusion when based on the dominant perforator | Areas of good perfusion, poor perfusion, and no perfusion | Multiple surgical decisions can be made:
|
| 4- Assessment of flap perfusion after anastomosis and flap inset | Pattern of flap reperfusion | Identification of early vascular compromise of the pedicle/pedicle kinking or excessive compression |
The selected perforators were dissected in the supramuscular fascia plane. Intraoperative assessment of perforators was performed first by taking thermal images of the flap and ensuring that the hotspots were corresponding to the underlying perforators, following which the surface temperature of each hotspot was measured using the device and compared with each other. The hottest hotspot should correspond to the most dominant perforator. If temperature difference was inconclusive (two hotspots had the same surface temperature), then evaluation of perforators was performed with the aid of DIRT technique. The cold challenge was performed using a cold reusable gel pad. The gel pad was kept in a refrigerator (4°C) for 20 minutes. Then, it was coated with a thin sterile plastic bag and applied to the region of interest for 15 seconds and then removed. Care was taken to eliminate water condensate and wrinkles on the outside of the plastic cover to reach an equal cooling of the examined skin. During the recovery session, the first appearing hotspot was selected as the dominant perforator (Figure 2).
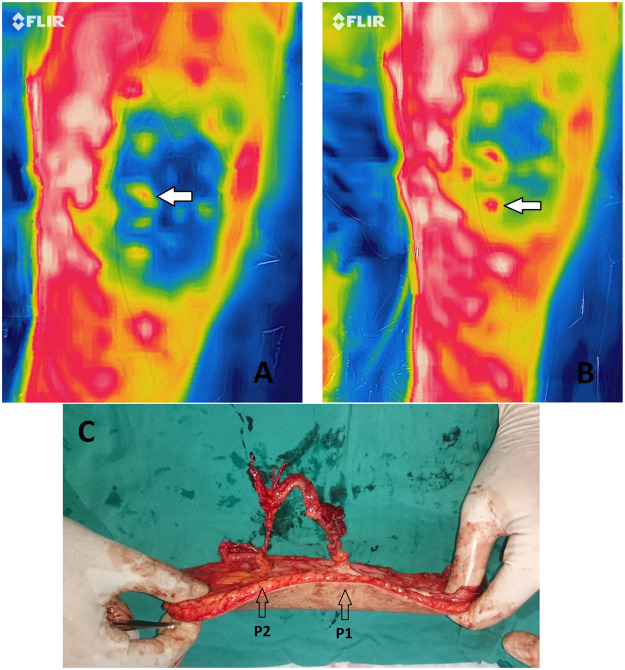
Figure 2.
A: Thermal imaging of the left thigh showing the anterior lateral thigh ALT flap perforators. Note the white arrow pointing to the first rewarming perforator P1 after the cold challenge test. B: The white arrow pointing to the second rewarming perforator P2. C: Intraoperative picture of the raised ALT free flap. Intraoperatively, both P1 and P2 were found to be corresponding to the best two perforators (in terms of size and pulsatility); hence, both of them were harvested and dissected to their common origin.
To assess flap perfusion when based on each single perforator, microvascular clamps were used to clamp the perforators until the flap showed no heated/perfused areas, then one perforator was unclamped each time and the pattern of perfusion was assessed both clinically and thermographically, giving an idea about the effect of each perforator on flap perfusion (Figure 3). The correspondence between the thermal and clinical findings was recorded, and the dominant perforator was selected.
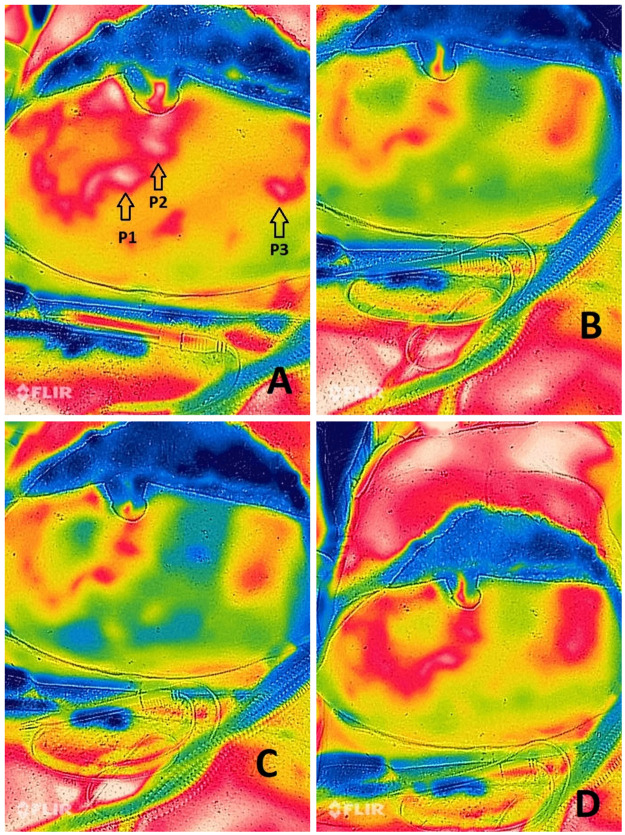
Figure 3.
Thermal imaging of a DIEP flap raised in the supra-muscular fascia plane with only 3 perforators left attached. Microvascular clamps were used to clamp the perforators until the flap showed no heated/perfused areas, then one perforator was unclamped each time and the pattern of perfusion was recorded giving an idea about the effect of each perforator on the flap perfusion. A: The three arrows pointing to the location of the perforators before clamping. B: P1 perforator was unclamped and the pattern of perfusion was captured. C: P1 perforator was re-clamped and P2 perforator was unclamped. The pattern of flap perfusion was then captured again. D: P1 and P2 were clamped and P3 unclamped. Notice the flap perfusion on thermal imaging is best when based on P3. This was corresponding to the clinical findings which meant that P3 was the dominant perforator and the decision was made to raise the flap based on it.
Perforator dominancy was checked clinically, defined by the ability of the perforator to provide blood supply to the largest area of the flap, and then compared with the thermal findings. The perforator with the hottest hotpot, best progressive rewarming, and provision of best flap perfusion on thermography was considered thermographically dominant.
Assessment of perfusion before flap transfer
After completion of perforator dissection, the least perfused part of the flap was discarded based on the clinical assessment after comparing that with the thermography findings (Figure 4).
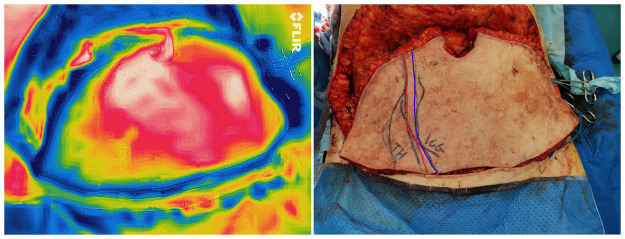
Figure 4.
DIEP flap was raised and thermal imaging was used just before division of the pedicle to mark and discard the least perfused part of the flap. Note the least perfused area of the flap in thermal imaging was most of Zone IV and was marked on the skin (red line). In this case, ICG imaging was also used to assess the flap perfusion before division and the findings/markings (blue line) were noticeably corresponding to thermography findings.
Assessment of perfusion after flap transfer/microvascular anastomosis
After completion of the microvascular anastomosis, in cases of free flap transfer, the pattern of flap reheating/reperfusion was evaluated. The final intraoperative measurement was performed after flap inset to assess the patency of the pedicle after manipulation (Figure 5).
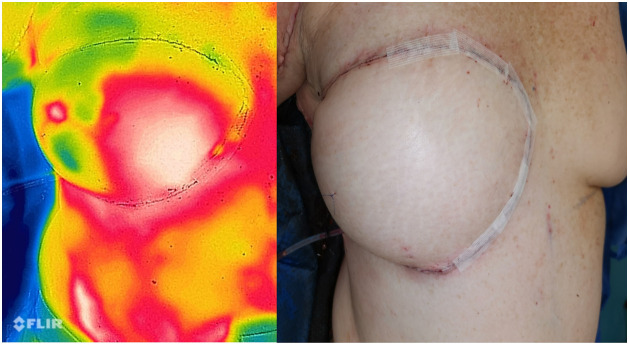
Figure 5.
The final intraoperative measurement was performed after flap inset to assess the patency of the pedicle. In this case, DIEP flap was used for delayed breast reconstruction after a previous mastectomy. Note the good flap perfusion assessed by thermography and clinically.
Results
Twenty perforator flaps were performed in 20 patients with a mean age of 43.8 years (range, 10-67 years) and mean BMI of 26.4 kg/m2 (range, 18-30 kg/m2). Seventeen free perforator flaps (13 free DIEP, 3 free ALT, and 1 free TDAP), and 3 pedicled perforator flaps (1 pedicled ALT and 2 pedicled PTAP) were included. Eleven patients had immediate reconstruction and 9 had delayed reconstruction.
The number of perforators selected for dissection was 2 in 16 cases and 3 in 4 cases. Perforators with the largest diameter in the preoperative CTA were marked on the skin, and then tested with thermal imaging and Doppler US. They were all found to have the hottest hotspots on thermography, confirmed with DIRT, and good Doppler signals.
Intraoperatively, after dissection of 44 perforators in the plane of the supramuscular fascia in all patients, each of them had a corresponding hotspot. All the selected clinically dominant perforators on which the flaps were raised on were found thermographically dominant.
In all DIEP flaps, the clinically least perfused parts were found corresponding to the thermography findings. In the rest of cases, no hypoperfused parts were detected clinically or thermographically.
In free flap cases, after microanastomosis, rapid rewarming was recorded in 15 free flaps. In one DIEP flap, no rapid rewarming was observed, and the flap was pale with no dermal bleeding noted. The absence of rewarming was recorded immediately after completing the anastomosis before clinical signs became apparent. Pedicle exploration showed kinking and arterial spasm. The pedicle was untwisted and topical verapamil was applied, which helped to improve the flap perfusion (Supplementary Material; Figure 6). In another DIEP flap, rewarming occurred only in the perforator/hotspot zone with significant reduced perfusion in the rest of the flap. Clinically, the flap was congested and dermal bleeding showed a venous pattern. The superficial inferior epigastric vein (SIEV) of the flap was also engorged with blood suggesting insufficient venous drainage of the flap. Cephalic turndown to SIEV was performed to improve the venous drainage, after which the flap perfusion on thermography improved dramatically (Supplementary Material; Figure 7) (Video 1). All flaps showed good perfusion on thermography after inset.
Table 3 summarizes the overall postoperative complications. Postoperatively, there was no total flap loss in any of the cases. However, distal flap necrosis occurred in one PTAP flap. The flap was used for posttraumatic reconstruction of a skin defect with exposed talus and medial malleolus. Fortunately, the part of the flap that was covering the exposed bones was viable, and it was only the distal tip that was affected with superficial necrosis. This part was debrided and negative pressure wound therapy was applied, and reconstructed later on with a skin graft.
Table 3.
Clinical outcomes/postoperative complications.
| Complications | Number of cases | Flap type | Further procedure(s) |
|---|---|---|---|
| Wound dehiscence | 2 | 1 DIEP 1 Free ALT |
Healed by secondary intention |
| Fat necrosis | 2 | DIEP | None |
| Significant breast asymmetry | 1 | DIEP | Contralateral symmetrizing mastopexy |
| Bilateral abdominal dog ears | 1 | DIEP | Revision of dog ears |
| Distal flap necrosis | 1 | PTAP | Split thickness skin graft |
Discussion
This study showed the versatility and applicability of using the smartphone thermal imaging technology intraoperatively in different kinds of perforator flaps, and in each single operative step. It also showed the correlation between thermal and clinical mapping of perforators and the reliability of this technology in reducing the risk of total/partial flap failure. We highlight the importance of intraoperative assessment in perforator flap surgery, and the surgeon's need for an easy, low-cost, and portable imaging tool that can help to make quick crucial operative decisions. Thermal imaging proved to be perfectly suitable for that purpose, and additionally, it requires minimal training, no contrast or ionizing radiation, and with no adverse effects.21
Intraoperative monitoring of perforator flaps is essential to reduce the flap failure risk.22 Routinely, flap viability is evaluated intraoperatively by assessing the skin color, turgor, capillary refill, and dermal bleeding. However, these methods are subjective, have a learning curve, and could be difficult to assess in dark-skinned patients. Very few objective techniques are available to use in theater such as ICG angiography.23,24 Thermography provides reliable alternative intraoperative objective method for flap design and perfusion assessment by monitoring the thermal hot spots that correspond to the high-vascularization areas.21,25 These spots can be captured statically or through DIRT.14 In one DIEP case, we compared thermography with ICG in terms of flap perfusion assessment and findings were very similar (Figure 4, Video 1). Weum et al.26 concluded that 95.6% of hotspots detected by DIRT corresponded to perforators of the DIEP flap quadrant on CTA. Pereira et al.27 reported very high concordance in perforator detection between thermal images taken with a smartphone device and CTA. They stated that the high sensitivity and specificity obtained using these smartphone-compatible cameras were comparable with those of the gold standard.
Intraoperative uses of thermography can be summarized into four main steps:
-
1-
Identification of perforators location.
-
2-
Identification of the dominant perforator.
-
3-
Assessment of flap perfusion when based on the dominant perforator and helping to determine the appropriate flap size and discard the least perfused parts. This step can also help the surgeon to determine if a bipedicled flap is needed, or the perfusion provided through a single pedicle is sufficient.
-
4-
Assessment of flap perfusion after anastomosis and flap inset. This can help to early identify vascular compromise.
After dissection of the chosen perforators, thermography can help the surgeon to decide which perforator is the best to harvest the flap on. Cooling the flap skin surface helps to distinguish between perforators in terms of blood flow. In all patients, rapid rewarming occurred and the first appearing hot spot corresponded to the dominant perforator clinically. In one free ALT flap, the cold challenge showed two perforators to have two good hot spots with the same rewarming interval. The two perforators were close to each other and clinical examination showed good size for each of them, so decision was made to dissect both perforators till their common origin from the descending branch of the lateral circumflex femoral artery and harvest the flap on both perforators (Figure 2). In flaps where multiple perforators are dissected and selection of the most dominant one cannot be determined by one of these methods, the impact of each perforator on perfusion can be assessed by alternate clamping of the perforators, and the perforator with the largest perfused area is selected.17
After the dominant perforator was selected, all other perforators were ligated and the main perforator was dissected to its origin; we found thermal imaging very useful to provide information about the least perfused part of the flap to be discarded, helping to reduce the incidence of partial flap ischemia and fat necrosis. The next measurement was obtained after the flap pedicle was successfully microanastomosed to the recipient vessel. During the ischemia time between flap separation and revascularization, the nonperfused flap cools down which means that a cold challenge is not needed. The easy use of this technique allows for frequent monitoring of the flap perfusion intraoperatively that helps with early detection of vascular compromise.
The final thermal measurement was performed after flap shaping. During inset, the flap pedicle can be potentially kinked or compressed. Thermography can diagnose perfusion problems before becoming clinically detectable. In a recent study conducted by Cruz-Segura et al.28 on early detection of vascular obstruction using thermography, they concluded that this tool allows us to identify the obstruction between 2 and 12 hours earlier compared with the usual clinical method. This would enhance the probability of saving the flap by providing more time to intervene.
Thermography is not only useful in assessment of free perforator flaps, but in pedicled perforator flaps as well. One of the difficult intraoperative decisions to make in propeller flaps is whether to twist the flap in a clockwise or counterclockwise direction. Thermal imaging can help to determine which direction of rotation will least reduce flap perfusion.29 Pereira and Hallock30 referred to the use of thermal imaging in local perforator flaps from a different perspective. They reported not only the hotspots that are of good use but also the cold zones. In keystone flaps, if thermography detects multiple hotspots, subfascial elevation of a cold portion of the flap can be safely done to achieve tension free flap advancement.
In breast reconstruction, mastectomy flaps perfusion can also be evaluated to avoid postoperative mastectomy flap ischemia and wound dehiscence. In one DIEP patient, the flap was monitored immediately after inset and showed good perfusion; however, the central part of the lower mastectomy flap was not well perfused. Clinical assessment of this part of the mastectomy flap was satisfactory and a decision was made to manage it conservatively. However, in the postoperative follow-up days, this part turned ischemic (Supplementary Material; Figure 8).
Although the professional thermal camera identifies slightly more hotspots, verification data are very similar to the smartphone-compatible thermal camera, and for clinical purposes, these differences are negligible.31 There is no learning curve to acquire the skills to use the smartphone-compatible device, which should now enable any plastic surgeon to raise any type of local perforator flap as they find suitable.32
Patel and Keller33 reported that the largest caliber vessel is the most suitable to perfuse the flap, consistent with the current general consensus of 1.5 mm diameter. With multiple perforators meeting such criteria, the next step is to detect which will best perfuse the flap.3 CTA, although is the gold standard, cannot distinguish between perforators in terms of perfusion if they have the same diameter. This also highlights the importance of the thermal imaging use intraoperatively to guide the surgeon through each surgical step.
Thermal imaging technology is becoming more universally useful around the world in both the developing and developed countries. Taking into account the current global financial crisis that worsened the long-lasting problem of lack of facilities especially in the developing countries, we believe that it is time to expand the use of this practical tool that can be affordable in all healthcare facilities, helping the reconstructive surgeons to get the most important findings that they need to make decisions and operate safely in such critical surgical specialty without the need for complex expensive imaging tools, which may not be available in many occasions and in the majority of places in these countries.
Conclusion
Smartphone thermography has proven to be a valuable, cheap, rapidly employed, and objective tool not only for the design of perforator flaps, but also for management of the flap intraoperatively in each surgical step. This noninvasive technique provides the surgeon with real-time visualization of the most suitable perforator and allows continuous monitoring of the flap perfusion in different stages before and after flap harvest, helping to achieve the best possible outcome.
Video
Flap perfusion assessment in a DIEP flap, after completion of microvascular anastomosis (and a cephalic turndown in this case) and before flap inset, using both thermography and ICG imaging. There was a high correlation between both imaging techniques in terms of flap perfusion. The white arrow is pointing to the nonperfused part of the flap in thermography that was also black/nonperfused in ICG.
Conflicts of interest and financial declaration statement
There are no conflicts of interest to declare, and the authors have no financial interest concerning this article.
Acknowledgments
Ethical approval
This prospective study was approved by the Research Ethics Committee REC, Health Research Authority HRA, Health and Care Research Wales HCRW (Integrated Research Application System IRAS project ID: 279874, REC reference: 21/NS/0096), and Institutional Research Board IRB, Faculty of Medicine, Mansoura University, Egypt (Proposal Code: MD.18.05.45).
Funding
None
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jpra.2023.08.004.
Appendix. Supplementary materials
A: Thermal imaging of DIEP flap (outlined with black lines) after completion of microanastomosis showing no flap re-warming (flap is blue; cold). B: Significant improvement in flap perfusion after pedicle de-kinking and application of a vasodilator.
A: After microanastomosis, thermography showing perfusion only at the perforator zone. B: Intraoperative picture of the flap on the chest. The flap was clinically congested. C: A cephalic turndown was performed, after which the flap perfusion on thermography improved.
A and B: A thermal image and a clinical photograph of a reconstructed breast using DIEP flap captured immediately postoperatively after flap inset. The flap skin paddle was hot/well perfused; however, the central part of the lower mastectomy flap was cold/nonperfused on thermography. C: Although looked clinically well on the operating table, the central part of the lower mastectomy flap turned ischemic postoperatively. This picture was taken on postoperative day (POD) 3. Note the correlation between the nonperfused area in thermography in the immediate postoperative phase, and the ischemic area of the mastectomy flap in POD 3. The white arrow in A and C is showing the upward extension of the ischemia of the mastectomy flap edge that can be seen matching in both pictures.
References
- 1.Geddes CR, Morris SF, Neligan PC. Perforator flaps: evolution, classification, and applications. Annals of plastic surgery. 2003;50(1):90–99. doi: 10.1097/00000637-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Saint-Cyr M, Schaverien MV, Rohrich RJ. Perforator flaps: history, controversies, physiology, anatomy, and use in reconstruction. Plastic and reconstructive surgery. 2009;123(4):132e–145e. doi: 10.1097/PRS.0b013e31819f2c6a. [DOI] [PubMed] [Google Scholar]
- 3.Rammos CK, Jones GE, Taege SM, Lemaster CM. The use of multispectral imaging in DIEP free flap perforator selection: a case study. Plastic and reconstructive surgery. Global open. 2020;8(11):e3245. doi: 10.1097/GOX.0000000000003245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. British journal of plastic surgery. 1987;40(2):113–141. doi: 10.1016/0007-1226(87)90185-8. [DOI] [PubMed] [Google Scholar]
- 5.Smith RK, Wykes J, Martin DT, Niles N. Perforator variability in the anterolateral thigh free flap: a systematic review. Surgical and radiologic anatomy. 2017;39(7):779–789. doi: 10.1007/s00276-016-1802-y. [DOI] [PubMed] [Google Scholar]
- 6.Holm C, Mayr M, Höfter E, Raab N, Ninkovic M. Interindividual variability of the SIEA angiosome: effects on operative strategies in breast reconstruction. Plastic and reconstructive surgery. 2008;122(6):1612–1620. doi: 10.1097/PRS.0b013e31818a9a3f. [DOI] [PubMed] [Google Scholar]
- 7.Aubry S, Pauchot J, Kastler A, Laurent O, Tropet Y, Runge M. Preoperative imaging in the planning of deep inferior epigastric artery perforator flap surgery. Skeletal radiology. 2013;42(3):319–327. doi: 10.1007/s00256-012-1461-y. [DOI] [PubMed] [Google Scholar]
- 8.Cina A, Salgarello M, Barone-Adesi L, Rinaldi P, Bonomo L. Planning breast reconstruction with deep inferior epigastric artery perforating vessels: multidetector CT angiography versus color Doppler US. Radiology. 2010;255(3):979–987. doi: 10.1148/radiol.10091166. [DOI] [PubMed] [Google Scholar]
- 9.Matsui A, Lee BT, Winer JH, Kianzad V, Frangioni JV. Image-guided perforator flap design using invisible near-infrared light and validation with x-ray angiography. Annals of plastic surgery. 2009;63(3):327–330. doi: 10.1097/SAP.0b013e318193493d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindsey JT. Integrating the DIEP and muscle-sparing (MS-2) free TRAM techniques optimizes surgical outcomes: presentation of an algorithm for microsurgical breast reconstruction based on perforator anatomy. Plastic and reconstructive surgery. 2007;119(1):18–27. doi: 10.1097/01.prs.0000244743.90178.89. [DOI] [PubMed] [Google Scholar]
- 11.Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plastic and reconstructive surgery. 2012;129(5):778e–788e. doi: 10.1097/PRS.0b013e31824a2ae8. [DOI] [PubMed] [Google Scholar]
- 12.Faderani R, Yassin AM, Brady C, Caine P, Nikkhah D. Versatility of indocyanine green (ICG) dye in microsurgical flap reconstruction. Journal of plastic, reconstructive and aesthetic surgery. 2023;76:118–120. doi: 10.1016/j.bjps.2022.11.025. [DOI] [PubMed] [Google Scholar]
- 13.Smit JM, Negenborn VL, Jansen SM, et al. Intraoperative evaluation of perfusion in free flap surgery: a systematic review and meta-analysis. Microsurgery. 2018;38(7):804–818. doi: 10.1002/micr.30320. [DOI] [PubMed] [Google Scholar]
- 14.de Weerd L, Mercer JB, Weum S. Dynamic infrared thermography. Clinics in plastic surgery. 2011;38(2):277–292. doi: 10.1016/j.cps.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Arai K, Fukuda O. [Clinical application of thermometers and thermography in plastic surgery] Keisei geka. Plastic and reconstructive surgery. 1968;11(3):239–250. [PubMed] [Google Scholar]
- 16.Sheena Y, Jennison T, Hardwicke JT, Titley OG. Detection of perforators using thermal imaging. Plastic and reconstructive surgery. 2013;132(6):1603–1610. doi: 10.1097/PRS.0b013e3182a80740. [DOI] [PubMed] [Google Scholar]
- 17.Thiessen FEF, Vermeersch N, Tondu T, et al. Dynamic infrared thermography (DIRT) in DIEP flap breast reconstruction: a clinical study with a standardized measurement setup. European journal of obstetrics, gynecology, and reproductive biology. 2020;252:166–173. doi: 10.1016/j.ejogrb.2020.05.038. [DOI] [PubMed] [Google Scholar]
- 18.Hardwicke JT, Osmani O, Skillman JM. Detection of perforators using smartphone thermal imaging. Plastic and reconstructive surgery. 2016;137(1):39–41. doi: 10.1097/PRS.0000000000001849. [DOI] [PubMed] [Google Scholar]
- 19.Sheikh R, Memarzadeh K, Torbrand C, Blohmé J, Lindstedt S, Malmsjö M. Blood perfusion in a full-thickness eyelid flap, investigated by laser Doppler velocimetry, laser speckle contrast imaging, and thermography. EPlasty. 2018;18:e9. [PMC free article] [PubMed] [Google Scholar]
- 20.Paul SP. Using a thermal imaging camera to locate perforators on the lower limb. Archives of plastic surgery. 2017;44(3):243–247. doi: 10.5999/aps.2017.44.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chubb D, Rozen WM, Whitaker IS, Ashton MW. Images in plastic surgery: digital thermographic photography (‘thermal imaging’) for preoperative perforator mapping. Annals of plastic surgery. 2011;66(4):324–325. doi: 10.1097/SAP.0b013e31820bcc5e. [DOI] [PubMed] [Google Scholar]
- 22.Khouri RK. Avoiding free flap failure. Clinics in plastic surgery. 1992;19(4):773–781. [PubMed] [Google Scholar]
- 23.Li K, Zhang Z, Nicoli F, et al. Application of indocyanine green in flap surgery: a systematic review. Journal of reconstructive microsurgery. 2018;34(2):77–86. doi: 10.1055/s-0037-1606536. [DOI] [PubMed] [Google Scholar]
- 24.Ludolph I, Arkudas A, Schmitz M, et al. Cracking the perfusion code?: laser-assisted indocyanine green angiography and combined laser Doppler spectrophotometry for intraoperative evaluation of tissue perfusion in autologous breast reconstruction with DIEP or ms-TRAM flaps. Journal of plastic, reconstructive and aesthetic surgery. 2016;69(10):1382–1388. doi: 10.1016/j.bjps.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 25.de Weerd L, Mercer JB, Setså LB. Intraoperative dynamic infrared thermography and free-flap surgery. Annals of plastic surgery. 2006;57(3):279–284. doi: 10.1097/01.sap.0000218579.17185.c9. [DOI] [PubMed] [Google Scholar]
- 26.Weum S, Mercer JB, de Weerd L. Evaluation of dynamic infrared thermography as an alternative to CT angiography for perforator mapping in breast reconstruction: a clinical study. BMC medical imaging. 2016;16(1):43. doi: 10.1186/s12880-016-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira N, Valenzuela D, Mangelsdorff G, Kufeke M, Roa R. Detection of perforators for free flap planning using smartphone thermal imaging: a concordance study with computed tomographic angiography in 120 perforators. Plastic and reconstructive surgery. 2018;141(3):787–792. doi: 10.1097/PRS.0000000000004126. [DOI] [PubMed] [Google Scholar]
- 28.Cruz-Segura A, Cruz-Domínguez MP, Jara LJ, et al. Early detection of vascular obstruction in microvascular flaps using a thermographic camera. Journal of reconstructive microsurgery. 2019;35(7):541–548. doi: 10.1055/s-0039-1688749. [DOI] [PubMed] [Google Scholar]
- 29.Hallock GG. Smartphone thermal imaging can enable the safer use of propeller flaps. Seminars in plastic surgery. 2020;34(3):161–164. doi: 10.1055/s-0040-1714291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira N, Hallock GG. Smartphone thermography for lower extremity local flap perforator mapping. Journal of reconstructive microsurgery. 2021;37(1):59–66. doi: 10.1055/s-0039-3402032. [DOI] [PubMed] [Google Scholar]
- 31.Obinah MPB, Nielsen M, Hölmich LR. High-end versus low-end thermal imaging for detection of arterial perforators. Plastic and reconstructive surgery. Global open. 2020;8(10):e3175. doi: 10.1097/GOX.0000000000003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallock GG. The use of smartphone thermography to more safely unmask and preserve circulation to keystone advancement flaps in the lower extremity. Injury. 2020;51(Suppl 4):S121–S125. doi: 10.1016/j.injury.2020.05.029. [DOI] [PubMed] [Google Scholar]
- 33.Patel SA, Keller A. A theoretical model describing arterial flow in the DIEP flap related to number and size of perforator vessels. Journal of plastic, reconstructive and aesthetic surgery. 2008;61(11):1316–1320. doi: 10.1016/j.bjps.2007.08.020. discussion 1320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Thermal imaging of DIEP flap (outlined with black lines) after completion of microanastomosis showing no flap re-warming (flap is blue; cold). B: Significant improvement in flap perfusion after pedicle de-kinking and application of a vasodilator.
A: After microanastomosis, thermography showing perfusion only at the perforator zone. B: Intraoperative picture of the flap on the chest. The flap was clinically congested. C: A cephalic turndown was performed, after which the flap perfusion on thermography improved.
A and B: A thermal image and a clinical photograph of a reconstructed breast using DIEP flap captured immediately postoperatively after flap inset. The flap skin paddle was hot/well perfused; however, the central part of the lower mastectomy flap was cold/nonperfused on thermography. C: Although looked clinically well on the operating table, the central part of the lower mastectomy flap turned ischemic postoperatively. This picture was taken on postoperative day (POD) 3. Note the correlation between the nonperfused area in thermography in the immediate postoperative phase, and the ischemic area of the mastectomy flap in POD 3. The white arrow in A and C is showing the upward extension of the ischemia of the mastectomy flap edge that can be seen matching in both pictures.