Abstract
Introduction:
Remote, internet-based methods for recruitment, screening, and longitudinally assessing older adults have the potential to facilitate Alzheimer’s disease (AD) clinical trials and observational studies.
Methods:
The Brain Health Registry (BHR) is an online registry that includes longitudinal assessments including self- and study partner-report questionnaires and neuropsychological tests. New initiatives aim to increase inclusion and engagement of commonly underincluded communities using digital, community-engaged research strategies. New features include multilingual support and biofluid collection capabilities.
Results:
BHR includes > 100,000 participants. BHR has made over 259,000 referrals resulting in 25,997 participants enrolled in 30 aging and AD studies. In addition, 28,278 participants are coenrolled in BHR and other studies with data linkage among studies. Data have been shared with 28 investigators. Recent efforts have facilitated the enrollment and engagement of underincluded ethnocultural communities.
Discussion:
The major advantages of the BHR approach are scalability and accessibility. Challenges include compliance, retention, cohort diversity, and generalizability.
Keywords: aging research, Alzheimer’s disease, Brain Health Registry, clinical trial recruitment, dementia, diversity, internet, internet registry, neuropsychological tests, neuroscience clinical research studies, online, remote assessment, remote biomarker collection
1 |. BACKGROUND
A major obstacle in the development of improved diagnostic methods and treatments for Alzheimer’s disease (AD) and other causes of cognitive decline and dementia is the recruitment of sufficient number of participants into clinical research.1–4 In addition, there is a pressing need for scalable instruments and tests that can be used to assess participants remotely, without the need for in-person visits to clinics.3 Finally, it has become increasingly obvious that many clinical research studies in the AD field, including clinical trials, predominantly enroll well-educated White individuals, with insufficient enrollment of Black, Latino, and other individuals from underrepresented groups, including those with lower educational attainment and socioeconomic status.5,6
A number of local and national US AD-related registries including the Alzheimer’s Prevention Registry7 and the Alzheimer Prevention Trial (APT) webstudy8,9 exist, and differ in format and purpose.10–14 Launched in 2014, the Brain Health Registry (BHR) is an online platform for the recruitment and assessment of participants for aging research.15–45 By amassing a large pool of prequalified participants, the BHR aims to make clinical trials and neuroscience research studies more efficient and innovative. Longitudinal monitoring of participants helps researchers obtain data to identify, assess, and monitor cognitive changes associated with aging and neurodegenerative disease progression. As the BHR has grown and evolved over the past 8 years, the BHR research team has implemented features to increase enrollment of underrepresented populations (URPs), integrate biomarker data, including genetic, plasma, as well as in-clinic data, and to enable the enrollment of study partners via the Caregiver and Study Partner Portal.22,29,36,39,41 These enhanced capabilities aim to generate more robust datasets and to increase the generalizability of research findings.
In 2018, we published a summary of BHR methods and activities.21 Since that time our cohort has grown substantially, and we have new projects, data, and publications. This report summarizes the current status of the BHR.
2 |. METHODS
2.1 |. BHR
The BHR study is approved by the University of California San Francisco (UCSF) Institutional Review Board. Any individual aged 18 or older who can provide online consent is eligible to participate in the BHR. The BHR includes a public-facing website, participant portal, investigator portal, and secure software platform for study management.
2.1.1 |. Software platform
The BHR’s software platform, Ebisu, is a web-based software designed by Derek Flenniken, BHR’s engineering director, to manage observational studies of human participants. Ebisu has multiple functionalities, including administration of tasks to participants, participant tracking and management, participant communications, study design, and data management. A detailed description of Ebisu’s development, maintenance, and capabilities was reported in our previous paper.21 In addition to the previously described capabilities, updated and new features in Ebisu include: multilingual support, biofluid collection capabilities, payment log, customizable registration page for coenrollments, new interface for datasets, new in-clinic data collection features, enhanced marketing features, and a redesigned user experience. Further details on these updated and new features can be found in Table 1.
TABLE 1.
Updated or new features in Ebisu.
| Updated or new feature | Description |
|---|---|
| Multilingual support | Studies can be configured to set which languages they support to allow participants to view content in their preferred language. |
| Biofluid collection capabilities | Software features facilitate collection of saliva and blood samples.29,36 Participants may provide a shipping address and receive a saliva collection kit or be provided with information to visit a local Quest Patient Service Center to have their blood drawn. A Vendor Portal allows fulfillment vendors to log in and download spreadsheets with participant information to create orders and send out saliva kits. The vendors can upload spreadsheets with additional information related to the kits that were sent out. Saliva and blood samples are returned to the BHR specimen bank and shipped out to labs for processing information related to the status of the received samples and can be uploaded into Ebisu. Ebisu captures/monitors data concerning shipment status, collection status, and analysis status. These results are automatically imported into the BHR database, where they are combined with online data collected in BHR. |
| Payment log | This facilitates disbursement of participant payments upon various events, including task completion, visit completion, and study completion. |
| Customizable registration page for coenrollments | The software tool for enrolling existing clinical cohorts from other studies into BHR with data linkage now contains a feature for a separate, customizable registration page to join BHR with a study-specific data-sharing consent form. This feature uses the existing BHR protocol and content, thereby reducing effort for new protocol development. |
| New interface for datasets | Anewinterfaceallows BHR staff to easily build customizable datasets, view the history of a dataset build, and specify data as “identifiable” (Protected Health Information).The interface also allows collaborators to securely download datasets directly from the investigator portal, instead of utilizing a third-party provider for data transfers. The “master data dictionary,” a consolidated list of all variables within a dataset, greatly facilitates data analysis. |
| New in-clinic data collection features | For projects with in-clinic components, Ebisu schedules in-clinic appointments and manages the collection of in-clinic data. Reports are provided for upcoming and overdue in-clinic appointments. In-clinic data are linked to any corresponding data collected online at home. In-clinic data collection may also include online tasks, to be conducted in a web browser at the clinic without requiring a participant to log in, but instead using a participant-specific appointment code to navigate to the online tasks. This system is used for a current study developing and validating electronic versions of the Clinical Dementia Rating and Financial Capacity Instrument-Short Form.21 |
| Enhanced marketing features | New features include the ability to gather and integrate data from Facebook and Google Adwords to analyze advertising efforts to recruit participants. |
| Redesigned user experience | To enhance the user experience and reduce subject burden, the following features were included: new color scheme, easier navigation and management of main study and substudies, participant-facing messaging with encouragements and guidance points, new task flow with balancing of activity types. |
2.1.2 |. Investigator Portal
Investigators and study coordinators use the Investigator Portal, an independent portal in Ebisu, to perform tasks as well as communicate with BHR staff about the enrollment and randomization of participants referred from BHR.
2.1.3 |. Dashboards
The BHR study team uses dashboards to visualize BHR data collected through Ebisu. The data, which are updated daily, pertain to participant and study partner enrollment, demographics including age, geographic location, gender, race, ethnicity, and education, self-reported mild cognitive impairment (MCI) and AD diagnoses, and task completion. Dashboards are customizable across different studies and help track results of recruitment, retention, and marketing efforts. Supplemental Figure S1 shows a screenshot of a dashboard.
2.2 |. Recruitment
Participants are recruited to join the BHR through news stories, word of mouth, paid digital ads, social media posts, other registries/research studies, email, and advocacy groups. The BHR’s recruitment projects are listed in Table 2. Supplemental Figure S2 shows the overall BHR participant flow starting from recruitment.
TABLE 2.
BHR projects.
| Project | Lead institution | Study type, design, population |
|---|---|---|
| Recruitment in BHR | ||
| Alzheimer’s Prevention Registry (APR) | BAI | Observational, registry recruitment, APR members |
| California Latino-Brain Health Registry (CAL-BHR) *43 | UCSF | Observational, community-engaged registry recruitment, Latino community |
| Study to expand registry participation of underrepresented populations (STEP-UP) * | BAI | Experimental, registry recruitment, Black, Hispanic, male |
| Referrals from BHR to other projects | ||
| ALLFTD mobile app study | UCSF | Observational, validation, mobile tests application validation, cognitively unimpaired controls |
| Alzheimer’s Disease Neuroimaging Initiative 3 (ADNI3)24 | NCIRE | Observational, in-clinic, older adults with or without MCI/AD |
| Alzheimer’s disease neuroimaging initiative-depression (ADNI-D) | UCSF | Observational, in-clinic, older adults with major depressive disorder |
| Alzheimer Prevention Trials (APT) Webstudy | USC | Observational, online registry recruitment, older adults without dementia |
| Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) | Eli Lilly and Company | Experimental, in-clinic phase 3 secondary AD prevention intervention trial, asymptomatic AD |
| β Amyloid Production and Effects on Cognition Study | Merck | Experimental, in-clinic phase 3 randomized placebo-controlled drug treatment intervention, older adults with prodromal AD |
| Butler Alzheimer’s Prevention Registry | Butler Hospital | Observational, recruitment to local Alzheimer’s prevention registry, older adults |
| Caregiver Attentional Awareness Pilot Study | UCSF | Experimental, in-clinic, and at-home iPad application intervention, older adult caregivers |
| Cleveland Clinic Lou Ruvo Center | Cleveland Clinic | Experimental, clinical trial for neurological disease, patients with Alzheimer’s, Parkinson’s, and Huntington’s diseases, multiple sclerosis, and frontotemporal dementia |
| EMERGE Study | Biogen | Experimental, in-clinic phase 3 randomized placebo-controlled drug treatment intervention, older adults with prodromal AD |
| Healthy Mind, Healthy You 93 | MGH | Experimental, online mindfulness intervention, all BHR participants |
| Hillblom Healthy Aging Study | UCSF | Observational, in-clinic observational aging/cognition study, cognitively unimpaired older adults |
| Hoarding behaviors study28,34,38,40,45 | UF | Observational, online registry assessment of hoarding, adults |
| Internet-Based Conversational Engagement Clinical Trial (I-CONECT) | OHSU | Experimental, community-based phase II behavioral intervention trial, socially isolated older adults |
| Phase II RCT of High-dose Vitamin D Supplements in Older Adults | UCD | Experimental, in-clinic, phase II RCT for vitamin D supplements, older adults |
| Repetitive Transcranial Magnetic Stimulation and Dementia Study | PAVAMC | Experimental, in-clinic repetitive transcranial magnetic stimulation veterans with dementia, MCI, or AD |
| Repetitive transcranial magnetic stimulation for mild cognitive impairment | PAVIR | Experimental, in-clinic, transcranial magnetic stimulation for mild cognitive impairment, older adults with noticeable decline in memory |
| Riluzole at Rockefeller | Rockefeller University | Experimental, in-clinic trial, older adults diagnosed with mild AD |
| Smell Study | Monell Chemical Senses Center | Observational, remote smell kit, adults |
| Study of Aging in Latinas/os for Understanding Dementias | Mount Sinai Medical Center | Observational, older Latino adults with and without HIV |
| UC Irvine Alzheimer’s Disease Research Center | UCI | Observational, in-clinic, neurological, cognitive, specimen collection, older adults |
| UPenn Healthy Brain Aging Study | UPenn | Experimental, randomized clinical trial, in-clinic, antidepressant medication effects on production of amyloid beta, healthy older adults |
| Direct-to-Site Referrals | ||
| Alzheimer’s Disease Neuroimaging Initiative 3 (ADNI3): ADNI3.org24 | NCIRE | Observational, recruitment to in-clinic study, older adults |
| Alzheimer’s Disease Neuroimaging Initiative 3 Diversity Task Force (ADNI3 DVT)*95 | NCIRE | Observational, culturally tailored recruitment to in-clinic study, older adults without or with MCI/AD from diverse ethnocultural communities (Black/African American, Latino) |
| Coenrollments | ||
| Alzheimer’s Disease Neuroimaging Initiative 3-Brain Health Registry (ADNI3-BHR)35 | NCIRE | Observational, online longitudinal monitoring, older adults who are cognitively unimpaired, have MCI or AD |
| Biomarker Prediction Study†36 | UCSF | Observational, remote blood collection, plasma biomarkers, older adults |
| Brain Health Registry-Affect: UCSF MAC Collaboration to Study Emotionalityt | UCSF | Survey, online affect-related questionnaires, all BHR participants |
| Brain Health Registry-GenePool Study*†29 | UCSF | Observational, remote DNA saliva collection, older adults |
| Brain Health Registry-Imaging Dementia — Evidence for Amyloid Scanning25,30,44 | UCSF | Observational, online registry participation, older Medicare beneficiaries with objectively confirmed cognitive impairment by a dementia |
| Buck Institute | Buck Institute | Recruitment effort with data-sharing consent for future research |
| Cognition After Surgery & Anesthesia-Brain Health Registry | UCSF | Observational, online longitudinal monitoring, postoperative decline, memory and thinking, MCI, dementia |
| Community Engaged Digital Alzheimer’s Research (CEDAR)* †49,50 | UCSF | Observational, community-engaged research registry engagement study, African American/Black BHR participants |
| Head Impact&Trauma Surveillance Study (HITSS)26 | BU | Observational, development of online head impact and trauma assessment, soccer and American football players |
| Healthy Brain Initiative-Brain Health Registry (HBI-BHR)/ageHAPPY (Healthy Ageing Project Population Youth-senior) | University of Melbourne | Observational, online registry for cognitive aging, adults in Australia |
| Memory assessment results initiative† | UCSF | Observational, pilot study providing unsupervised cognitive assessment results to online registry participants, all BHR participants |
| Striving Together to Prevent & Treat Alzheimer’s Disease | UTHSCSA | Observational, enrollment effort into registry, adults in an ADRC |
| Study to Evaluate Amyloid in Blood and Imaging Related to Dementia-Brain Health Registry | WashU | Observational, in-clinic, amyloid blood test, older adults |
| Veteran Brain Health Registry | NCIRE | Observational, online registry, veterans |
| Development and Validation of Online Assessments | ||
| Cambridge Cognition Paired Associates Learning | UCSF | Observational, remote validation of online unsupervised cognitive assessment, all BHR participants |
| Cogstate Brief Battery19,20,23,26,33 | UCSF | Observational, remote validation of online unsupervised cognitive assessment, all BHR participants |
| Electric Validation of Online Methods to Predict and Monitor Cognitive Declinet †‡27,39 | UCSF | Observational, in-clinic, and remote validation of online unsupervised diagnostic assessment, older adults |
| Lumos Labs NeuroCognitive Performance Tests23,26,37 | UCSF | Observational, remote validation of online unsupervised cognitive assessment, all BHR participants |
| MemTrax Memory Test23 | UCSF | Observational, remote validation of online unsupervised cognitive assessment, all BHR participants |
| Mobile Toolbox Study †‡ | UCSF | Observational, validation of a remote, smartphone-based cognitive assessment app validation, all BHR participants |
| Online neuropsychological test validation project with imaging pilot †‡ | UCSF | Observational, In-clinic and remote, validation of online unsupervised cognitive assessment, biomarkers, older adults |
| ReVeRe†‡31 | UCSF | Observational, in-clinic, and remote validation of online unsupervised cognitive assessment, biomarkers, older adults |
| Software as a Service (SaaS) | ||
| Alzheimer’s Disease Neuroimaging Initiative 4 (ADNI4) * ‡§ | NCIRE | Observational, recruitment to in-clinic, observational study, older adultswithout or with MCI/AD |
| Dutch Brain Health Registry32 | Amsterdam UMC | Observational, online registry for cognitive aging, adults |
| • * Focus on URP | ||
| • † Also a Referral from BHR toOther Projects | ||
| • ‡ Also a Coenrollment | ||
| • § Also a Direct-to-Site Referral | ||
| Lead Institutions | ||
| • BAI: Banner Alzheimer’s Institute | ||
| • UCSF: University of California, San Francisco | ||
| • NCIRE: Northern California Institute of Research and Education | ||
| • USC: University of Southern California | ||
| • MGH: Massachusetts General Hospital | ||
| • UF: University of Florida | ||
| • OHSU: Oregon Health and Science University | ||
| • UCD: University of California, Davis | ||
| • PAVAMC: Palo Alto Veterans Affairs Medical Center | ||
| • PAVIR: Palo Alto Veterans Institute for Research | ||
| • UCI: University of California, Irvine | ||
| • UPenn: University of Pennsylvania | ||
| • BU: Boston University | ||
| • UTHSCSA: University of Texas Health Science Center at San Antonio | ||
| • WashU:Washington University in St. Louis | ||
| • Amsterdam UMC: Amsterdam University Medical Center | ||
2.2.1 |. Self-reported recruitment source
Upon registering for the study, participants can select from a dropdown list of recruitment sources. Approximately 16% of participants do not report a recruitment source and about 18% report an unknown source, “Other.”
2.2.2 |. Trackable links
Trackable links that redirect to the BHR website are included in many digital communications, such as digital advertisements, email, online articles, and social media posts. These links can provide the sources through which participants enrolled.
2.2.3 |. URP recruitment
BHR’s failure to adequately recruit and enroll participants from diverse communities (eg, ethnocultural identity, socioeconomic background) is a critical limitation. Starting in 2019, BHR has made significant efforts to develop and evaluate culturally informed, internet-based, scalable methods using a community-engaged research (CER) approach to increase the recruitment and enrollment of individuals from communities that are commonly underincluded in medical research and often experience significant disparities in AD, dementia, MCI, prevalence, incidence, and outcomes.46–48 Initial efforts focused on recruitment and engagement of Latino participants in the California Latino-BHR (CAL-BHR) study.41 Our digital CER approach uses multicomponent strategies including (1) a collaboration with marketing professionals experienced in research recruitment in the Latino community; (2) formation of a community science partnership advisory board (CSPB), composed of BHR participants and other community stakeholders, who provide iterative feedback to guide the development and evaluation of all recruitment and enrollment of all strategies; (3) development and deployment of digital culturally and CSPB-informed digital recruitment strategies; and (4) development of multilingual Participant Portal and support to include Spanish.41 Digital efforts included multilingual and culturally informed messaging and imagery for (1) social media recruitment advertisements, (2) study recruitment landing pages, (3) and referral emails to other studies. In addition, efforts were also tailored to align with different age populations, for example, younger Latino individuals (more likely to be either U.S.-born or first, second, or third generation), middle-aged Latino individuals (more likely to be immigrants or first generation), and (3) older Latino individuals (more likely to be monolingual Spanish speaker). Another current effort, Community Engaged Digital Alzheimer’s Research (CEDAR),49 focuses on improving engagement and research participation of Black BHR participants using similar CER methods. The approach included establishing a Black CSPB, deploying a novel survey focused on facilitators and barriers to research participation, and developing and implementing novel digital engagement strategies (eg, monetary incentives, participant video testimonials, a culturally tailored email campaign). Efforts are under way evaluating the success of this approach, disseminating the findings professionally and in communities, as well as developing best practices, which can inform the continuous development of BHR and potentially be applied to other studies.
2.3 |. Participant Portal
After enrolling, participants are guided to the BHR Participant Portal, which consists of a structured list of study tasks, task descriptions, study dashboards with encouragements, a study navigator page for those enrolled in multiple studies, and a printable completion certificate (see examples in Supplemental Figure S3). A professional web design firm codesigned the Participant Portal to modernize and streamline our interface.
2.4 |. Consent forms
To register for BHR, individuals must enter their first and last name, email address, username, password, and month and year of birth on a registration form. All participants in BHR must sign an electronic informed consent form (see Supplemental Figure S3). The BHR platform supports administering multiple online consents for BHR and any related studies and agrees with any of our collaborators to allow the sharing of data between studies. Within their BHR profile, participants can view all the consents they have ever signed and view all the studies they have enrolled in through the platform. Participants have the option to withdraw from individual studies, which will in turn withdraw their associated consents.
2.5 |. Questionnaires
BHR participants are asked to complete a series of questionnaires during their baseline and longitudinal follow-up visits. These questionnaires are based on validated instruments that are used verbatim or that are adapted for an online setting.51–61 Questionnaires undergo periodic review to evaluate the utility and effectiveness of their deployment. Questionnaires are presented in a specified order, but participants may skip individual procedures or deviate from this order.
The initial questionnaire consists of basic demographic information, self-reported diagnoses of MCI, dementia, and AD, family history of AD, self-reported memory concerns, and questions about cognition and mental health. Subsequent questionnaires include measures of everyday cognition (ECog); mood (adapted from the Geriatric Depression Scale Short Form (GDS) and Patient Health Questionnaire (PHQ-8)); medical and depression history; head injuries (adapted from Quality of Life after Brain Injury Scale (QOLIBRI), Rivermead Post-Concussion Symptoms, Satisfaction with Life Scale (SWLS), and Ohio State Traumatic Brain Injury Form (OSU TBI)); family history of AD; hoarding and cluttering (adapted from the Hoarding Rating Scale, Activities of Daily Living for Hoarding Disorder, WHODAS, and Short Form Health Survey SF-12) and quality of life.54,57–60,62–71 In February 2021, we added a questionnaire asking Latino participants about nativity, immigration, bilingualism, and cross-border ties.41,53
2.6 |. Neuropsychological tests
The BHR platform currently administers the following online neuropsychological tests: (1) Cogstate Brief Battery72; (2) MemTrax Memory Test73,74; and (3) Cambridge Cognition Paired Associates Learning,75 which was added in the summer of 2021. The Lumos Labs NeuroCognitive Performance Tests76 was previously administered on the BHR platform for 5 years from 2014 until 2019.
2.7 |. Caregiver and Study Partner Portal
The BHR Caregiver and Study Partner Portal (CASPP) is a novel, scalable, web-based tool for remotely obtaining study partner data. Launched in 2016, this tool is a portal within the BHR that allows current participants to nominate a potential study partner by completing a My Study Partner task, which includes a description of the role of a study partner, questions about the potential study partner’s relationship to the participant, and a form requesting the name and email address of the potential study partner. Completion of the My Study Partner tasks triggers an automated invitation email to the potential study partner, with a description of the study partner role, instructions on how to enroll, and a link to a custom CASPP registration page within the BHR. From the registration page, the potential study partner creates an account and password, signs an online consent, and completes CASPP-specific tasks. CASPP tasks include study partner demographics, questions about the relationship between the participant and study partner (relationship type, how long they have known each other, whether they live together, how much time they spend together), and online adaptations of the Everyday Cognition Scale (ECog), Functional Activities Questionnaire (FAQ), Cognitive Functional Instrument (CFI), and Mild Behavioral Inventory Checklist.54,56,61 Study partners are invited to return to the CASPP at 6-month intervals to complete follow-up tasks. Supplemental Figure S4 shows the overall BHR study partner flow. An individual can serve as both a study partner and a participant in BHR. BHR participants can change their study partner at any time through their BHR account and request that a current study partner no longer serve in that role.
2.8 |. Participant support
Zendesk Support is a customer service software that uses a ticketing system to support and communicate with customers. Inquiries sent by participants, collaborators, or potential collaborators are forwarded to Zendesk via the info@brainhealthregistry.org email address. Each email is treated as a ticket, which is meant to be solved by designated BHR staff. BHR began using Zendesk Support on March 20, 2018. At any given time, one to three BHR staff members closely monitor and actively respond to participant questions using templated responses. To categorize and more efficiently respond to participant inquiries, tickets are sorted by type. BHR staff aim to respond to participants within 24 to 48 hours of when the participants sent their messages. Zendesk data are useful for tracking and addressing participant responses and attitudes and for identifying bugs or participant-facing issues with the BHR site.
2.9 |. Participant engagement
2.9.1 |. Email engagement
BHR uses a series of different emails to communicate with participants. Participants receive a welcome email after enrolling in BHR, reminder emails to complete procedures, and a thank you email after completing all procedures. Ahead of 6-month longitudinal follow-up visits, participants receive emails reminding them to return to the website to complete procedures.
Finally, in cases where someone begins enrollment but does not complete this three-step process (registration, account creation, online informed consent), emails are sent asking that they finish enrolling and participate in BHR.
2.9.2 |. BHR newsletters
Participants may opt in to receive electronic newsletters that cover developments in the AD research field and update participants on studies conducted by BHR researchers. Since BHR’s inception in 2014, newsletters have been in English but have been available in both English and Spanish since September 2021. All newsletters are archived on the BHR website at www.brainhealthregistry.org/newsletter/.
2.9.3 |. Social media
BHR uses Facebook and Twitter to share research findings in the greater Alzheimer’s field, as well as updates specific to BHR.
2.10 |. Types of BHR projects
There are five types of BHR projects: (1) referrals, (2) coenrollments, (3) data sharing, (4) development and validation of online assessments, and (5) software as a service. These BHR projects utilize Ebisu’s features to build both internal projects within the BHR team and external collaborative projects involving investigators outside of the BHR team, such as other investigators within UCSF or outside of UCSF, advocacy organizations, and private-sector entities involved in clinical neuroscience studies. All BHR projects are listed in Table 2. The BHR team carefully reviews requests for collaborations and decide which ones are within the capacities of the team based on several factors, including scientific merit, complexity, burden on BHR participants, value of data to the BHR, and cost.
2.10.1 |. Referrals
Referrals from BHR to other projects
Referrals originate from the pool of enrolled BHR participants who have completed self-report questionnaires and/or cognitive tests. This allows for screening prior to site referral, to identify likely candidates for the referral study. When participants who have expressed interest in participating in additional research and meets the eligibility criteria for a referral study, they will receive a series of referral emails telling them about the study. If participants are interested, they may sign a data-sharing consent form.
Direct to site referrals
Direct-to-site referrals consist of individuals referred directly to a research clinic site without the requirement to enroll in BHR and complete questionnaires and neuropsychological tests. These are done from a recruitment website, web-based form, or landing page managed by the BHR. Within the web-based form, interested individuals provide their contact information and any other applicable information (eg, birth year or zip codes), which can then be relayed to the site.
2.10.2 |. Coenrollments
Coenrollment means that participants are simultaneously enrolled in the BHR (signed the BHR consent) and another study. The goal of coenrollment is to link study data collected by both studies to create a more enriched dataset for analysis, papers, and presentations and to inform participants of future research projects.
2.10.3 |. Data sharing
BHR shares its data with interested qualifying researchers outside of the BHR research team, governed by a Data Use Agreement (DUA) and as part of most collaborations. The data-sharing infrastructure includes a secure dataset download portal and scalable, customizable data sets via Ebisu (Table 1). Interested collaborators work with the BHR team to determine the scope of the data shared and premises for potential collaboration in analysis. The BHR data-sharing guidelines are explained on the website at www.brainhealthregistry.org/for-investigators/de-identified-data-sharing/.
2.10.4 |. Development and validation of online assessments
Several studies are under way for developing and validating novel online assessments. These studies may include an examination of the feasibility, compliance, and usability of the assessment; investigation of the novel assessment to established in-clinic measures; and comparison of assessment performance in supervised settings versus performance in BHR.
2.10.5 |. Software as a service (SaaS)
BHR SaaS is a software platform created and maintained by the BHR team. BHR SaaS allows collaborators to build and manage their own cohorts through recruitment of participants into their online study, longitudinal data capture from participants, and participant communication management.
3 |. RESULTS
3.1 |. BHR participants
More than 100,000 participants are enrolled in the BHR. This number includes BHR study partners and participants coenrolled in other studies. Future reports will include detailed information on BHR study partners and those coenrolled in other studies. Here, we report data on the remaining 90,650 participants, of whom 67,395 (73.5%) are aged at least 55. Further, 21,938 (24.2%) of participants identify as male, 66,712 (73.6%) identify as female, and <1% as “other” or indicate “prefer not to say” on the gender questionnaire. On the ethnicity question, 12,002 (13.2%) participants marked “Latino,” 4191 (4.6%) identifed as Black or African American, 55,854 (61.6%) of participants reported a 4-year college degree or higher, and 42,520 (49.9%) of BHR participants had a self-reported memory concern. A total of 11,553 study partners are enrolled in the BHR.
The top three metropolitan areas in which BHR participants report residence are San Francisco-Oakland-Hayward, CA; Los Angeles-Long Beach-Anaheim, CA; and New York City-Newark-Jersey City, NY-NJPA. See Figure 1 for more details on BHR participants’ locations across the United States.
FIGURE 1.
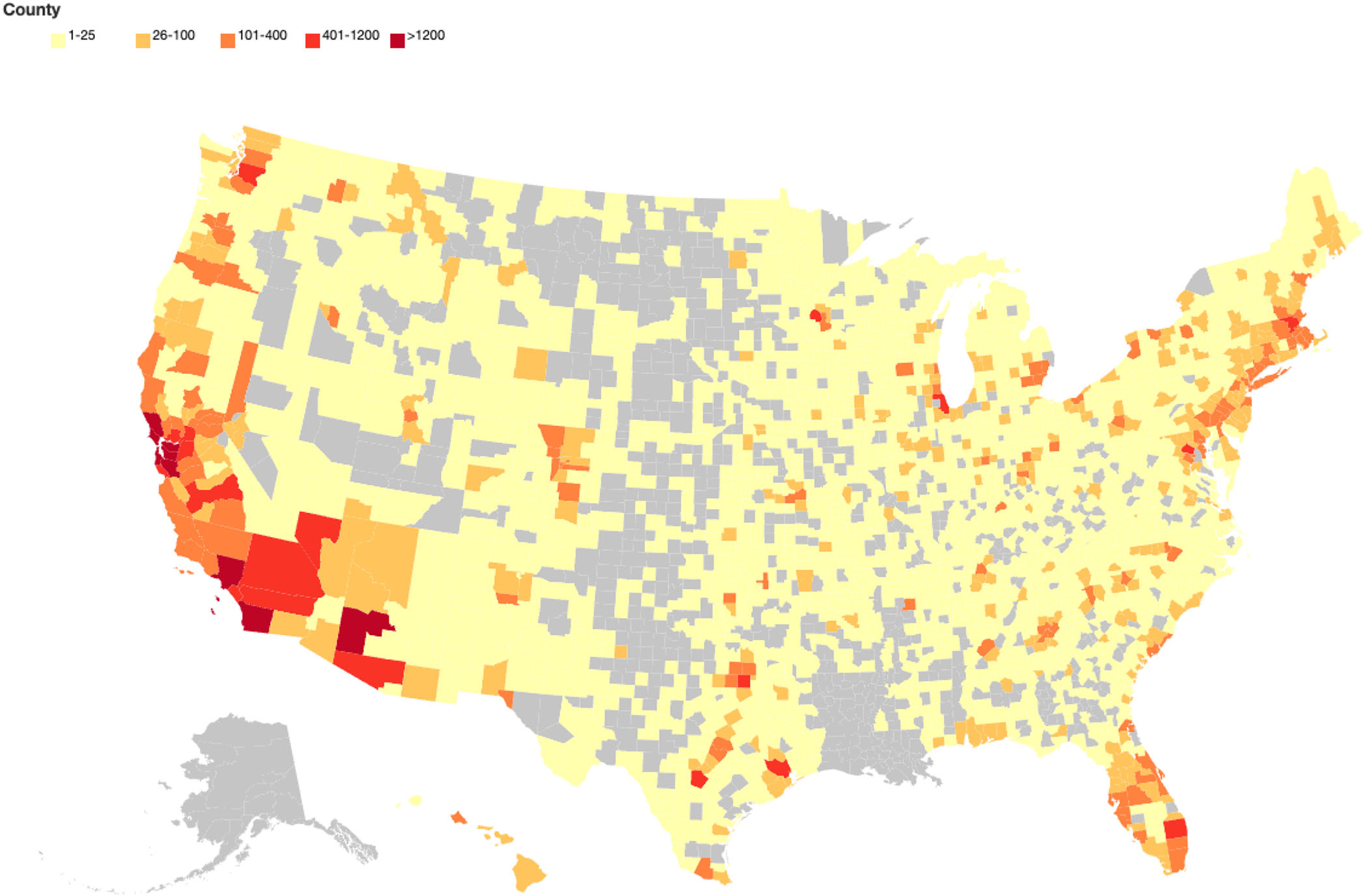
Heatmap of enrolled BHR participants from US counties.
See Table 3 for more detailed demographic information on the BHR cohort.
TABLE 3.
Demographics of BHRcohort.
| Demographics | N | % |
|---|---|---|
| Total | 90,650 | |
| Age, n = 89,315 | ||
| <50 | 15,165 | 17.0% |
| 50–59 | 16,632 | 18.6% |
| 60–69 | 27,827 | 31.2% |
| 70–79 | 22,185 | 24.8% |
| >80 | 7,506 | 8.4% |
| Gender, n = 90,650 | ||
| Female | 66,712 | 73.6% |
| Male | 21,938 | 24.2% |
| Other | 45 | <1% |
| Prefer not to say | 45 | <1% |
| Ethnicity, n = 90,650 | ||
| Latino | 12,002 | 13.2% |
| Not Latino | 71,376 | 78.7% |
| Prefer not to say | 2,372 | 2.6% |
| Unknown | 4,900 | 5.4% |
| Race, n = 90,650 | ||
| Asian | 3,138 | 3.5% |
| Black or African American | 4,191 | 4.6% |
| Native American | 2,697 | 3.0% |
| Pacific Islander | 429 | <1% |
| White | 71,716 | 79.1% |
| More than 1 race | 3,384 | 3.7% |
| Not collected | 1,658 | 1.8% |
| Other | 5,997 | 6.7% |
| Prefer not to say | 1,572 | 1.7% |
| Education, n = 90,650 | ||
| High school or less | 6,837 | 7.5% |
| Some college | 16,759 | 18.5% |
| 2-year college degree | 7,994 | 8.8% |
| 4-year college degree | 26,206 | 28.9% |
| Prefer not to say | 222 | <1% |
| Advanced degree | 29,648 | 32.7% |
| Memory concern and family history, n = 85,197; MCI, AD, dementia diagnosis, n = 63,103 | ||
| Memory concern | 42,520 | 49.9% |
| Family history of AD | 18,982 | 22.3% |
| Diagnosed with MCI | 3,658 | 5.8% |
| Diagnosed with AD | 518 | <1% |
| Diagnosed with dementia | 805 | 1.3% |
| Medical condition, n = 54,675 | ||
| Parkinson’s | 1,783 | 3.2% |
| Movement disorder | 2,499 | 4.6% |
| Motor neuron disease | 313 | <1% |
| Stroke | 1,807 | 3.3% |
| Schizophrenia | 102 | <1% |
| Heart disease | 3,185 | 5.8% |
| High blood pressure | 18,715 | 34.2% |
| Cholesterol | 22,025 | 40.3% |
| Diabetes | 5,028 | 9.2% |
| Cancer | 9,043 | 16.5% |
| Alcohol abuse | 6,664 | 12.1% |
| Drug abuse | 3,993 | 7.3% |
| Smoking | 20,867 | 38.2% |
3.2 |. Self-report questionnaire completion rates
Among BHR participants, 83,170 (94.2%) completed the initial questionnaire during their baseline time point, and 39,718 (45%) of participants have completed at least two initial questionnaires at any time point, including baseline and subsequent longitudinal time points.
3.3 |. Neuropsychological test completion rates
At baseline, 48,581 (55.7%) participants completed at least one neuropsychological test and 18,847 (21.6%) have completed at least two neuropsychological tests. Further, 56,788 (65.1%) have completed at least one neuropsychological test during at least two different time points. Finally, 32,096 (36.8%) of participants completed at least two neuropsychological tests during at least two different time points, including baseline and subsequent longitudinal time points.
3.4 |. Participant support
Since 2018, 20,537 tickets (requests for support) have been generated. The three most common ticket categories were General BHR Response, which contains advice for accessing and navigating the BHR portal; Technical Assistance Request, which provides troubleshooting advice if participants encounter issues accessing or completing study tasks; and Account Modification, which relates to account management, such as merging duplicate accounts or participant withdrawal.
Since BHR first began using Zendesk in 2018, 21,739 Zendesk tickets have been created for a total of 90,650 participants enrolled.
3.5 |. URP recruitment
The CAL-BHR project (aimed at increased enrollment of older Latino participants in BHR)41 and the CEDAR project49 (aimed at increasing engagement by Black BHR participants) represent the major BHR efforts to date. Efforts have resulted in the enrollment of 7013 individuals from underrepresented ethnocultural populations. In addition, a culturally tailored email campaign for recruitment and enrollment of Latino individuals into a remote genetics study increased the percentage of Latino participants from 2% to 21% in this study. More details have been reported in other publications.41
3.6 |. Referrals (Tables 2 and 4)
TABLE 4.
Summary of referral programs.
| Referral program | Referred | Responded | Response rate |
Enrolled | Enrollment rate |
|---|---|---|---|---|---|
| ALLFTD Mobile App Study | 298 | 148 | 50% | 40 | 13% |
| Alzheimer’s Disease Neuroimaging Initiative 3 (ADNI3) | 16153 | 3033 | 19% | 82 | 1% |
| Alzheimer’s Disease Neuroimaging Initiative-Depression | 1528 | 369 | 24% | 25 | 2% |
| (ADNI-D) | |||||
| Alzheimer Prevention Trials Webstudy (APT) | 31317 | 4739 | 15% | 1872 | 6% |
| Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) | 1212 | 297 | 25% | 11 | 1% |
| β Amyloid Production and Effects on Cognition Study | 139 | 48 | 35% | 0 | 0% |
| Biomarker Prediction Study | 7147 | 1308 | 18% | 864 | 12% |
| Brain Health Registry-Affect: UCSF MAC Collaboration to | 73795 | 11871 | 16% | 10233 | 14% |
| Study Emotionality | |||||
| Brain Health Registry-GenePool Study | 10675 | 2158 | 20% | 1690 | 16% |
| Butler Alzheimer’s Prevention Registry | 2384 | 484 | 20% | 23 | 1% |
| Caregiver Attentional Awareness Pilot Study | 14 | 4 | 29% | 0 | 0% |
| Community Engaged Digital Alzheimer’s Research | 3802 | 434 | 11% | 384 | 10% |
| (CEDAR) | |||||
| Cleveland Clinic Lou Ruvo Center | 497 | 84 | 17% | 0 | 0% |
| Electric Validation of Online Methods to Predict and | 3098 | 580 | 19% | 152 | 5% |
| Monitor Cognitive Decline | |||||
| EMERGE Study | 385 | 39 | 10% | 1 | 0% |
| Healthy Mind, Healthy You | 32200 | 4258 | 13% | 0 | 0% |
| Hillblom Healthy Aging Study | 1512 | 144 | 10% | 39 | 3% |
| Hoarding Behaviors Study | 13878 | 6290 | 45% | 1303 | 9% |
| Internet-Based Conversational Engagement Clinical Trial | 36 | 7 | 19% | 0 | 0% |
| (I-CONECT) | |||||
| Memory Assessment Results Initiative | 1304 | 415 | 32% | 380 | 29% |
| Mobile Toolbox Study | 48109 | 10099 | 21% | 7161 | 15% |
| Online neuropsychological test validation project with | 3220 | 942 | 29% | 550 | 17% |
| imaging pilot | |||||
| Phase II RCT of High-dose Vitamin D Supplements in Older | 1434 | 374 | 26% | 1 | 0% |
| Adults | |||||
| Repetitive Transcranial Magnetic Stimulation and | 290 | 62 | 21% | 2 | 1% |
| Dementia Study | |||||
| Repetitive Transcranial Magnetic Stimulation for Mild | 633 | 103 | 16% | 3 | 0% |
| Cognitive Impairment | |||||
| Riluzoleat Rockefeller | 21 | 6 | 29% | 0 | 0% |
| Smell Study | 3236 | 1841 | 57% | 1164 | 36% |
| Study of Aging in Latinas/osfor Understanding Dementias | 540 | 65 | 12% | 17 | 3% |
| UC Irvine Alzheimer’s Disease Research Center | 140 | 25 | 18% | 0 | 0% |
| UPenn Healthy Brain Aging Study | 284 | 70 | 25% | 0 | 0% |
| Total | 259142 | 50249 | 19% | 25997 | 10% |
A total of 30 studies have established referral programs with the BHR. Over 259,000 referrals to studies were sent out with 25,997 participants enrolled. Successful referral programs include (1) Monell Chemical Senses Center’s Smell Study, (2) Online Neuropsychological Test Validation Project with Imaging Pilot, and (3) BHR-GenePool Study.
3.7 |. Coenrollments (Tables 2 and 5)
TABLE 5.
Summary of coenrollments.
| Study | Enrolled |
|---|---|
| Alzheimer’s Disease Neuroimaging Initiative 3-Brain Health Registry (ADNI3-BHR) | 114 |
| Biomarker prediction study | 864 |
| Brain Health Registry-Affect: UCSF MAC Collaboration to Study Emotionality | 10233 |
| Brain Health Registry-GenePool Study | 1690 |
| Brain Health Registry-Imaging Dementia — Evidence for Amyloid Scanning | 981 |
| Buck Institute | 76 |
| Cognition After Surgery & Anesthesia-Brain Health Registry | 22 |
| Community Engaged Digital Alzheimer’s Research (CEDAR) | 384 |
| Electric validation of online methods to predict and monitor cognitive decline | 152 |
| Head Impact&Trauma Surveillance Study (HITSS) | 1000 |
| Healthy Brain Initiative-Brain Health Registry (HBI-BHR)/ageHAPPY (Healthy Ageing Project Population Youth-senior) | 4330 |
| Memory assessment results initiative | 380 |
| Mobile toolbox study | 7161 |
| Online neuropsychological test validation project with imaging pilot | 550 |
| Striving together to prevent & treat Alzheimer’s disease | 169 |
| Study to Evaluate Amyloid in Blood and Imaging Related to Dementia-Brain Health Registry | 165 |
| Veteran Brain Health Registry | 7 |
| Total | 28,278 |
A total of 28,278 BHR participants are coenrolled in 17 other studies. The Head Impact and Trauma Surveillance Study (HITSS), with 1000 enrolled participants, is an example of a successful coenrollment. The BHR platform facilitates the development of a large national cohort of participants who have had a history of head injury and/or are participating in sports or other activities where risk of head injury is high. Marketing activities direct interested participants to the HITSS website, which explains the project, and then the participants register for the BHR user experience.
3.8 |. Data sharing
The BHR shares data both as part of larger collaborations and with those interested in pursuing independent analysis questions. As of this writing, the BHR has shared data with 28 research groups.
3.9 |. Development and validation of online assessment tools
The BHR began by implementing Cogstate testing for all participants, leading to several publications showing the effects of advanced age and self-reported cognitive impairments,19 sleep,20 and depression26 on the Cogstate Brief Battery. Subsequently, MemTrax and the Cambridge Cognition Paired Associates Learning test were added. This experience with online assessments led to a National Institutes of Health (NIH)-funded project to develop and validate the electronic Clinical Dementia Rating,39 which is an online version of the widely used gold standard assessment known as the Clinical Dementia Rating. Finally, the BHR population and platform have been used to help validate the recently developed NIH-funded Mobile Toolbox.77
4 |. DISCUSSION
The major accomplishments of the BHR are as follows: enrollment of over 100,000 participants including over 11,000 study partners and coenrolled participants, over 259,000 referrals to 30 studies, coenrollment of 28,278 participants with 17 other studies, development of improved digital marketing methods for the enrollment of URPs, serving as a platform for the development of novel online assessment tools, and data sharing with collaborators and those requesting data. Major limitations have been high levels of participant dropout and persistent underrepresentation of historically excluded ethnocultural and education groups. Taken together, these accomplishments demonstrate that the BHR provides a unique adjunct for clinical neuroscience research.
4.1 |. Caregiver and Study Partner Portal
A unique feature of the BHR is the CASPP, a novel and scalable tool for obtaining dyadic (participant, study partner (SP) pair) data remotely. This approach has many advantages. Dyad report decline: (1) efficiently captures change within a single, cross-sectional assessment by asking about recent changes; (2) provides unique insight into complex activities of daily living that may begin to decline early in the AD continuum, are associated with AD biomarkers,78–81 and are difficult to assess using neuropsychological testing; and (3) offers good portability across cultures, languages, and educational levels. SP report of cognitive and functional decline has further advantages. The accuracy of self-reporting can be limited by overreporting decline due to being “worried well” or mood and personality traits82–85 or underreporting decline due to a lack of insight/awareness about one’s condition.86–88.
4.2 |. Referrals into other studies
The major goal of all cohort registries is to provide a reservoir of participants who can join more intensive in-clinic studies, especially randomized clinical trials. A major problem for the BHR and for other registries serving a similar function has been accurately tracking the success of these referrals. Ideally, the BHR would receive data back from clinics concerning the number of BHR participants who made contact with the site, how many were screened, and how many were finally enrolled by providing informed consent. This data linkage is particularly difficult, and without such linkage it’s not possible to quantify the success of the referral program. There are at least two causes of this problem. First, in many cases, especially for industry-sponsored randomized clinical trials, the protocol prevents release of any Protected Health Information to an outside source. Second, the clinic staff are overburdened and focus on recruitment and enrollment; tasking clinic staff with providing information back to the BHR adds a significant time burden. One solution to this problem is to include the BHR and other registries and referral sources in the main protocol of the study, and to require data linkage and accurate reporting of successful referrals by the clinic sites. Such data linkage has been accomplished between the BHR and Alzheimer’s Disease Neuroimaging Initiative (ADNI) (because Dr. Weiner is the primary investigator of both studies), demonstrating the feasibility of this approach. Once a successful data linkage approach is implemented, this allows complete tracking of the successes and failures of such an approach, facilitating optimization. Furthermore, data linkage would allow the in-clinic study to utilize information obtained by the referral source. This could be used to reduce screen fails and to provide other data useful to the study.
4.3 |. Coenrollments with other studies
A very unique feature of the BHR platform is the ability to conduct coenrollments with other studies. There are many NIH- and industry-funded studies where budget limitations prevent frequent in-clinic or telephone follow-up. Coenrollment with the BHR facilitates long-term follow-up at relatively low cost because most BHR functions are completely automated. Coenrollment also provides a unique opportunity to validate online assessment by examining the relationship between online measures and in-clinic measures.
4.4 |. Recruitment of underrepresented participants
Like other research studies, the BHR fails to adequately include underrepresented individuals, which is one of the most crucial limitations of research as it impacts the generalizability of research findings and perpetuates health-related inequities.21,3,5,6,89–91 To address this, the BHR has focused on developing culturally informed digital marketing efforts to include and engage Black and Latino individuals, with website landing pages designed to appeal to these populations. The success of the CAL-BHR and CEDAR projects, which focused on improving diversity in the BHR, has led to the use of similar digital marketing and racial/ethnic designed landing pages for Alzheimer’s Disease Neuroimaging Initiative 3 (ADNI3),92,93 and we are extending this approach for ADNI4. We believe that this digital marketing approach has proven to be a cost-effective adjunct to conventional “boots-on-the-ground” recruitment94 aimed at underrepresented groups. Although there is still a long way to go in assembling a representative cohort, diversity efforts to date have demonstrated the feasibility of our approach and begun to identify best practices in this area.
4.5 |. Development and validation of online assessment tools
The online cohort of the BHR has been a valuable participant pool for the development and validation of online assessments. The advantages of online unsupervised assessments include efficiency, scalability, reduced cost and resource use, frequent data collection, and ability to include those who cannot take part in in-clinic assessments due to location, competing demands, and other barriers that disproportionately affect URPs. The recent COVID pandemic further highlights the importance of remote assessments.
4.6 |. Data sharing with collaborators and those requesting data
As with ADNI, which makes all data available to requestors, the BHR has freely shared data with interested investigators. Our data sharing has led to a number of publications.15–44 The BHR data are extremely large and complex, especially due to various changes that are made to add or remove various features. For this reason, we recommend that investigators interested in using the BHR dataset to explore or test hypotheses collaborate with BHR scientists who are familiar with the dataset and its limitations.
4.7 |. Limitations
The limitations of the BHR include selection biases, issues of data integrity, lack of clinically confirmed data, missing data, and limited ability to engage and retain participants across longitudinal time points. All online research studies have selection biases for individuals with adequate digital devices and internet access and literacy. On the other hand, the online approach permits expanded access to research for individuals who may not be able to participate in in-person studies due to geographic constraints and time burdens. There are likely to be additional selection biases, both for enrollment and retention, related to the health, cognitive, and functional status of participants. In terms of data integrity, we have no way to validate or check the accuracy of the data provided by our participants, and it is possible that, for example, participants get help from others when taking the neuropsychological tests. Another major concern is the very high dropout rate. Furthermore, dropout increases with each successive visit. Nevertheless, many thousands of participants have returned to the BHR twice per year for many years, providing extensive longitudinal data. Related to dropout is the problem of completion. Only a small fraction of participants complete the entire experience. BHR frontloads the initial questionnaire to capture the information deemed most important. A number of methods to reduce dropout and increase completion rates are being considered and tested. Lack of completion and high dropout are clearly major limitations of the BHR and other online registries.
4.8 |. Summary
Despite the previously discussed limitations, when taken together, the accomplishments of the BHR (referrals, coenrollments, study partner data, recruitment of underrepresented participants, development/validation of online assessment methods, and data sharing) demonstrate the value of this unique approach to facilitate clinical neuroscience research.
Supplementary Material
Highlights.
Brain Health Registry (BHR) is an online, longitudinal platform of > 100,000 members.
BHR made > 259,000 referrals, which enrolled 25,997 participants in 32 studies.
New efforts increased enrollment and engagement of underincluded communities in BHR.
The major advantages of the BHR approach are scalability and accessibility.
BHR provides a unique adjunct for clinical neuroscience research.
RESEARCH IN CONTEXT.
1. Systematic Review:
A review of the literature was conducted using electronic databases and online search engines. A number of different Alzheimer’s disease-related local and national registries exist. In 2018, the first publication of the Brain Health Registry (BHR) provided a summary of the methods, activities, and results. Increased BHR participation and referral numbers, as well as new diversity and remote data collection efforts, warrant an update.
2. Interpretation:
This updated summary of the BHR highlights the registry’s success in enrolling participants, referring participants to other studies, coenrolling participants, sharing data, serving as a platform for novel online assessment tools, and increasing the inclusion of ethnoculturally diverse, historically excluded participants in the registry. These accomplishments demonstrate that BHR provides a unique adjunct for clinical neuroscience research.
3. Future Directions:
Much needed efforts are under way and planned to increase participant engagement and to further increase ethnocultural and socioeconomic diversity of the BHR cohort.
ACKNOWLEDGMENTS
We would like to thank all participants, study partners, Community Scientific Partnership Boards, and collaborators. Finally, we are grateful for the support of all our past and current BHR team members. This work was supported by the following present and past funding sources: National Institutes of Health (Grants R01 AG053798, R01 NS119651, R33 AG062867, RF1 AG059009, U2C AG060426, U19 AG024904, R01 AG063954, U24 AG057437), California Department of Public Health (Grants 16-10054, 18-10929, 19-10616), Department of Veterans Affairs, Alzheimer’s Association (Grant BHR-16-459161), Global Alzheimer’s Platform Foundation, Alzheimer’s Drug Discovery Foundation (Grant 20150802), Larry L. Hillblom Foundation (Grant 2015-A-011-NET), Monell Chemical Senses, Patient Centered Outcomes Research Institute (Grants PPRN-1501-26817, PPRN-1512-33786), VU University Medical Center, Australian Catholic University, Janssen Pharmaceutica, and Biogen, Inc. (Grant 174552), Genentech, Kevin and Connie Shanahan, Ray and Dagmar Dolby Family Fund, Rosenberg Alzheimer’s Project, and the Drew Foundation.
Footnotes
CONFLICTS OF INTEREST STATEMENT
Anna Aaronson, Joseph Eichenbaum, Winnie Kwang, Shilpa Gummadi, Jessica Santhakumar, Monica Camacho, Derek Flenniken, Juliet Fockler, Diana Truran-Sacrey, and Aaron Ulbricht report no potential conflicts of interest. Miriam Ashford receives support from the NIH’s National Institute on Aging, Grant F32AG072730-01 (made to institution), and declares no potential conflicts of interest. R. Scott Mackin declares to have received grants from the following entities in the past 36 months: National Institute of Mental Health and National Institute on Aging Grants R01MH117114, Ro1MH 098062, R01MH101472, and R01MH125928, with all grant payments made to the university. Rachel L. Nosheny has received support from the NIH (support to institution) for the present manuscript and grants from the NIH (grant to institution), California Department of Public Health (grant to institution) Genentech, Inc. Health Equity Innovations Fund (grant to institution), and Alzheimer’s Association (grant to institution). Michael W. Weiner receives support for his work from the following funding sources: NIH, grants from Department of Defense (DOD), grants from Patient-Centered Outcomes Research Institute (PCORI), grants from California Department of Public Health (CDPH), grants from University of Michigan, grants from Siemens, grants from Biogen, grants from Hillblom Foundation, grants from Alzheimer’s Association, grants from The State of California, grants from Johnson & Johnson, grants from Kevin and Connie Shanahan, grants from GE, grants from VU University Medical Center, grants from Australian Catholic University (HBI-BHR), grants from The Stroke Foundation, grants from Veterans Administration, personal fees from Acumen Pharmaceutical, personal fees from Cerecin, personal fees from Dolby Family Ventures, personal fees from Eli Lilly, personal fees from Merck Sharp & Dohme Corp., personal fees from National Institute on Aging (NIA), personal fees from Nestle/Nestec, personal fees from PCORI/PPRN, personal fees from Roche, personal fees from University of Southern California (USC), personal fees from NervGen, personal fees from Baird Equity Capital, personal fees from BioClinica, personal fees from Cytox, personal fees from Duke University, personal fees from Eisai, personal fees from FUJIFILM-Toyama Chemical (Japan), personal fees from Garfield Weston, personal fees from Genentech, personal fees from Guide-point Global, personal fees from Indiana University, personal fees from Japanese Organization for Medical Device Development, Inc., personal fees from Medscape, personal fees from Peerview Internal Medicine, personal fees from Roche, personal fees from T3D Therapeutics, personal fees from WebMD, personal fees from Vida Ventures, personal fees from the Buck Institute for Research on Aging, personal fees from China Association for Alzheimer’s Disease, personal fees from Japan Society for Dementia Research, personal fees from Korean Dementia Society, outside the submitted work; additionally, he holds stocks or options with Alzheon Inc., Alzeca, and Anven. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All participants in the BHR provided informed consent by signing an electronic consent form.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Grill JD, Galvin JE. Facilitating Alzheimer disease research recruitment. Alzheimer Dis Assoc Disord. 2014;28(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langbaum JB, Zissimopoulos J, Au R, et al. Recommendations to address key recruitment challenges of Alzheimer’s disease clinical trials. Alzheimers Dement. 2023;19(2):696–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fargo KN, Carrillo MC, Weiner MW, Potter WZ, Khachaturian Z. The crisis in recruitment for clinical trials in Alzheimer’s and dementia: an action plan for solutions. Alzheimers Dement. 2016;12(11):1113–1115. [DOI] [PubMed] [Google Scholar]
- 4.Elliott CL. Together we make the difference: national strategy for recruitment and participation in Alzheimer’s and related dementias clinical research. Ethn Dis. 2020;30(2):705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilmore-Bykovskyi AL, Jin Y, Gleason C, et al. Recruitment and retention of underrepresented populations in Alzheimer’s disease research: a systematic review. Alzheimers Dement (N Y). 2019;5:751–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin J, Doraiswamy PM. Underrepresentation of African-Americans in Alzheimer’s trials: a call for affirmative action. Front Aging Neurosci. 2016;8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langbaum JB, High N, Nichols J, Kettenhoven C, Reiman EM, Tariot PN. The Alzheimer’s Prevention registry: a large internet-based participant recruitment registry to accelerate referrals to Alzheimer’s-focused studies. J Prev Alzheimers Dis. 2020;7(4):242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter S, Clanton TB, Langford OG, et al. Recruitment into the Alzheimer prevention trials (APT) Webstudy for a trial-ready cohort for preclinical and prodromal Alzheimer’s disease (TRC-PAD). J Prev Alzheimers Dis. 2020;7(4):219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aisen PS, Sperling RA, Cummings J, et al. The trial-ready cohort for preclinical/prodromal Alzheimer’s disease (TRC-PAD) project: an overview. J Prev Alzheimers Dis. 2020;7(4):208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders KT, Langbaum JB, Holt CJ, et al. Arizona Alzheimer’s registry: strategy and outcomes of a statewide research recruitment registry. J Prev Alzheimers Dis. 2014;1(2):74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong K, Cummings J. Healthybrains.org: from registry to randomization. J Prev Alzheimers Dis. 2016;3(3):123–126. [DOI] [PubMed] [Google Scholar]
- 12.Langbaum JB, Gordon D, Walsh T, et al. P3–024: the Alzheimer’s prevention registry’s Genematch program: update on progress and lessons learned in helping to accelerate enrollment into Alzheimer’s prevention studies. Alzheimers Dement. 2018;14(20):P1073–P1073. 7S_Part. [Google Scholar]
- 13.Grill JD, Hoang D, Gillen DL, et al. Constructing a local potential participant registry to improve Alzheimer’s disease clinical research recruitment. J Alzheimers Dis. 2018;63(3):1055–1063. [DOI] [PubMed] [Google Scholar]
- 14.Johnson SC, Koscik RL, Jonaitis EM, et al. The Wisconsin registry for Alzheimer’s prevention: a review of findings and current directions. Alzheimers Dement (Amst). 2018;10:130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiner MW. The BrainHealthRegistry.org: using the Internet for identification, assessment, screening, recruitment, and longitudinal monitoring of subjects for neuroscience and Alzheimer’s disease studies. J Prev Alzheimers Dis. 2014;1(2):59–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geyer J, Insel P, Farzin F, et al. Evidence for age-associated cognitive decline from Internet game scores. Alzheimers Dement (Amst). 2015;1(2):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aisen P, Touchon J, Andrieu S, et al. Registries and cohorts to accelerate early phase Alzheimer’s trials. A report from the E.U./U.S. clinical trials in Alzheimer’s disease task force. J Prev Alzheimers Dis. 2016;3(2):68–74. [DOI] [PubMed] [Google Scholar]
- 18.Insel PS, Palmqvist S, Mackin RS, et al. Assessing risk for preclinical β-amyloid pathology with APOE, cognitive, and demographic information. Alzheimers Dement (Amst). 2016;4:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackin RS, Insel PS, Truran D, et al. Unsupervised online neuropsychological test performance for individuals with mild cognitive impairment and dementia: results from the Brain Health Registry. Alzheimers Dement (Amst). 2018;10:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohlenhoff BS, Insel PS, Mackin RS, et al. Total sleep time interacts with age to predict cognitive performance among adults. J Clin Sleep Med. 2018;14(9):1587–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiner MW, Nosheny R, Camacho M, et al. The Brain Health Registry: an internet-based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement. 2018;14(8):1063–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nosheny RL, Camacho MR, Insel PS, et al. Online study partner-reported cognitive decline in the Brain Health Registry. Alzheimers Dement (N Y). 2018;4:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cholerton B, Weiner MW, Nosheny RL, et al. Cognitive performance in Parkinson’s disease in the Brain Health Registry. J Alzheimers Dis. 2019;68(3):1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barger C, Fockler J, Kwang W, et al. Data-driven participant recruitment: findings from the Alzheimer’s disease neuroimaging initiative 3. J Prev Alzheimers Dis. 2020;7(2):122–127. [DOI] [PubMed] [Google Scholar]
- 25.Ashford MT, Neuhaus J, Jin C, et al. Predicting amyloid status using self-report information from an online research and recruitment registry: the Brain Health Registry. Alzheimer’s & Dementia: DADM. 2020;12(1):e12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alosco ML, Tripodis Y, Baucom ZH, et al. Late contributions of repetitive head impacts and TBI to depression symptoms and cognition. Neurology. 2020;95(7):e793–e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Xiong C, Aschenbrenner AJ, et al. Item response theory analysis of the clinical dementia rating. Alzheimers Dement. 2021;17(3):534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nutley SK, Camacho MR, Eichenbaum J, et al. Hoarding disorder is associated with self-reported cardiovascular/metabolic dysfunction, chronic pain, and sleep apnea. J Psychiatr Res. 2021;134:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fockler J, Kwang W, Ashford MT, et al. Brain health registry GenePool study: a novel approach to online genetics research. Alzheimers Dement (N Y). 2021;7(1):e12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albright J, Ashford MT, Jin C, et al. Machine learning approaches to predicting amyloid status using data from an online research and recruitment registry: the Brain Health Registry. Alzheimers Dement (Amst). 2021;13(1):e12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackin RS, Rhodes E, Insel PS, et al. Reliability and validity of a home-based self-administered computerized test of learning and memory using speech recognition. Aging Neuropsychol Cogn. 2021:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zwan MD, van der Flier WM, Cleutjens S, et al. Dutch Brain Research Registry for study participant recruitment: design and first results. Alzheimers Dement (N Y). 2021;7(1):e12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banh T, Jin C, Neuhaus J, et al. Unsupervised performance of the CogState Brief Battery in the Brain Health Registry: implications for detecting cognitive decline. J Prev Alzheimers Dis. 2022;9(2):262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nutley SK, Read M, Eichenbaum J, et al. Poor sleep quality and daytime fatigue are associated with subjective but not objective cognitive functioning in clinically relevant hoarding. BP:GOS. 2022;2(4):480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howell T, Neuhaus J, Glymour MM, Weiner MW, Nosheny RL. Alzheimer’s disease neuroimaging I. Validity of online versus in-clinic self-reported everyday cognition scale. J Prev Alzheimers Dis. 2022;9(2):269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fockler J, Ashford MT, Eichenbaum J, et al. Remote blood collection from older adults in the Brain Health Registry for plasma biomarker and genetic analysis. Alzheimers Dement. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kassam F, Chen H, Nosheny RL, et al. Cognitive profile of people with mild behavioral impairment in Brain Health Registry participants. Int Psychogeriatr. 2022:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sordo Vieira L, Nguyen B, Nutley SK, et al. Self-reporting of psychiatric illness in an online patient registry is a good indicator of the existence of psychiatric illness. J Psychiatr Res. 2022;151:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howell T, Gummadi S, Bui C, et al. Development and implementation of an electronic clinical dementia rating and financial capacity instrument-short form. Alzheimers Dement (Amst). 2022;14(1):e12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nutley SK, Read M, Eichenbaum J, et al. Health-related quality of life in hoarding: a comparison to chronic conditions with high disease burden. J Psychiatr Res. 2022;149:68–75. [DOI] [PubMed] [Google Scholar]
- 41.Ashford MT, Camacho MR, Jin C, et al. Digital culturally tailored marketing for enrolling Latino participants in a web-based registry: baseline metrics from the Brain Health Registry. Alzheimers Dement. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mindt MR, Okonkwo O, Weiner MW, et al. Improving generalizability and study design of Alzheimer’s disease cohort studies in the United States by including under-represented populations. Alzheimers Dement. n/a(n/a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nosheny RL, Camacho MR, Jin C, et al. Validation of online functional measures in cognitively impaired older adults. Alzheimers Dement. 2020;16(10):1426–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iaccarino L, La Joie R, Koeppe R, et al. rPOP: robust PET-only processing of community acquired heterogeneous amyloid-PET data. Neuroimage. 2022;246:118775. [DOI] [PubMed] [Google Scholar]
- 45.Nutley SK, Bertolace L, Vieira LS, et al. Internet-based hoarding assessment: The reliability and predictive validity of the internet-based Hoarding Rating Scale, Self-Report. Psychiatry research. 2020;294:113505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manly JJ, Jones RN, Langa KM, et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 health and retirement study harmonized cognitive assessment protocol project. JAMA Neurol. 2022;79(12):1242–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang M-X, Stern Y, Marder K, et al. The APOE-ϵ4 allele and the risk of Alzheimer disease among African Americans, Whites, and Hispanics. JAMA. 1998;279(10):751–755. [DOI] [PubMed] [Google Scholar]
- 48.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aaronson A, Ashford MT, Zhu D, et al. The community engaged digital alzheimer’s research (CEDAR) study: digital engagement strategies to increase ADRD research participation of black Americans. J Prev Alzheimers Dis. 2022;9.(15th Conference Clinical Trials Alzheimer’s Disease, November 29–December 2, 2022, San Francisco, CA, USA: Posters (Clinical Trial Alzheimer’s Disease)):51–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ashford MT, Zhu D, Bride J, et al. Understanding Online Registry Facilitators and Barriers Experienced by Black Brain Health Registry Participants: The Community Engaged Digital Alzheimer’s Research (CEDAR) Study. J Prev Alzheimers Dis. 2023:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy, White women. Health Psychol. 2000;19(6):586. [DOI] [PubMed] [Google Scholar]
- 52.Goodman E, Adler NE, Kawachi I, Frazier AL, Huang B, Colditz GA. Adolescents’ perceptions of social status: development and evaluation of a new indicator. Pediatrics. 2001;108(2):e31–e31. [DOI] [PubMed] [Google Scholar]
- 53.Torres JM, Lee A, González HM, Garcia L, Haan MN. A longitudinal analysis of cross-border ties and depression for Latino adults. Soc Sci Med. 2016;160:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farias ST, Mungas D, Reed BR, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22(4):531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amariglio RE, Donohue MC, Marshall GA, et al. Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer’s Disease Cooperative Study Cognitive Function Instrument. JAMA Neurol. 2015;72(4):446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfeffer RI, Kurosaki TT, Harrah CH Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–329. [DOI] [PubMed] [Google Scholar]
- 57.Corrigan JD, Bogner J. Initial reliability and validity of the Ohio State University TBI Identification Method. J Head Trauma Rehabil. 2007;22(6):318–329. [DOI] [PubMed] [Google Scholar]
- 58.von Steinbuechel N, Wilson L, Gibbons H, et al. QOLIBRI overall scale: a brief index of health-related quality of life after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2012;83(11):1041–1047. [DOI] [PubMed] [Google Scholar]
- 59.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 60.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. [DOI] [PubMed] [Google Scholar]
- 61.Ismail Z, Aguera-Ortiz L, Brodaty H, et al. The mild behavioral impairment checklist (MBI-C): a rating scale for neuropsychiatric symptoms in pre-dementia populations. J Alzheimers Dis. 2017;56(3):929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 63.King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587–592. [DOI] [PubMed] [Google Scholar]
- 64.Pietrzak RH, Maruff P, Snyder PJ. Convergent validity and effect of instruction modification on the groton maze learning test: a new measure of spatial working memory and error monitoring. Int J Neurosci. 2009;119(8):1137–1149. [DOI] [PubMed] [Google Scholar]
- 65.Mathewson KJ, Dywan J, Snyder PJ, Tays WJ, Segalowitz SJ. Aging and electrocortical response to error feedback during a spatial learning task. Psychophysiology. 2008;45(6):936–948. [DOI] [PubMed] [Google Scholar]
- 66.Chen KH, Chuah LY, Sim SK, Chee MW. Hippocampal region-specific contributions to memory performance in normal elderly. Brain Cogn. 2010;72(3):400–407. [DOI] [PubMed] [Google Scholar]
- 67.Lim YY, Ellis KA, Harrington K, et al. Use of the CogState Brief Battery in the assessment of Alzheimer’s disease related cognitive impairment in the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J Clin Exp Neuropsychol. 2012;34(4):345–358. [DOI] [PubMed] [Google Scholar]
- 68.Lim YY, Jaeger J, Harrington K, et al. Three-month stability of the CogState brief battery in healthy older adults, mild cognitive impairment, and Alzheimer’s disease: results from the Australian Imaging, Biomarkers, and Lifestyle-rate of change substudy (AIBL-ROCS). Arch Clin Neuropsychol. 2013;28(4):320–330. [DOI] [PubMed] [Google Scholar]
- 69.Fredrickson J, Maruff P, Woodward M, et al. Evaluation of the usability of a brief computerized cognitive screening test in older people for epidemiological studies. Neuroepidemiology. 2010;34(2):65–75. [DOI] [PubMed] [Google Scholar]
- 70.Sternberg DAH, Joseph L, Ben Katz, Kacey Ballard, Michael Scanlon. The Brain Performance Test: Preliminary Findings of transfer from cognitive training to a repeatable, dynamically generated assessment. Paper presented at: Society for Neuroscience2012. [Google Scholar]
- 71.Ashford JW, Gere E, Bayley PJ. Measuring memory in large group settings using a continuous recognition test. J Alzheimers Dis. 2011;27(4):885–895. [DOI] [PubMed] [Google Scholar]
- 72.Maruff P, Thomas E, Cysique L, et al. Validity of the CogState Brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24(2):165–178. [DOI] [PubMed] [Google Scholar]
- 73.Ashford J Memtrax computerized memory test, a one-minute dementia screen. Alzheimers Dement. 2005;1:S23–S23. [Google Scholar]
- 74.Ashford JW, Clifford JO, Anand S, Bergeron MF, Ashford CB, Bayley PJ. Correctness and response time distributions in the MemTrax continuous recognition task: analysis of strategies and a reverse-exponential model. FrontAging Neurosci. 2022:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barnett JH, Blackwell AD, Sahakian BJ, Robbins TW. The paired associates learning (PAL) test: 30 years of CANTAB translational neuroscience from laboratory to bedside in dementia research. Curr Top Behav Neurosci. 2016;28:449–474. [DOI] [PubMed] [Google Scholar]
- 76.Morrison GE, Simone CM, Ng NF, Hardy JL. Reliability and validity of the NeuroCognitive Performance Test, a web-based neuropsychological assessment. Front Psychol. 2015;6:1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gershon RC, Sliwinski MJ, Mangravite L, et al. The Mobile Toolbox for monitoring cognitive function. Lancet Neurol. 2022;21(7):589–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okonkwo OC, Alosco ML, Griffith HR, et al. Cerebrospinal fluid abnormalities and rate of decline in everyday function across the dementia spectrum: normal aging, mild cognitive impairment, and Alzheimer disease. Arch Neurol. 2010;67(6):688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marshall GA, Lorius N, Locascio JJ, et al. Regional cortical thinning and cerebrospinal biomarkers predict worsening daily functioning across the Alzheimer’s disease spectrum. J Alzheimers Dis. 2014;41(3):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lilamand M, Cesari M, del Campo N, et al. Brain amyloid deposition is associated with lower instrumental activities of daily living abilities in older adults. results from the MAPT study. J Gerontol A Biol Sci Med Sci. 2016;71(3):391–397. [DOI] [PubMed] [Google Scholar]
- 81.Ferreira D, Falahati F, Linden C, et al. A ‘Disease Severity Index’ to identify individuals with Subjective Memory Decline who will progress to mild cognitive impairment or dementia. Sci Rep. 2017;7:44368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoon JS, Charness N, Boot WR, Czaja SJ, Rogers WA. Depressive symptoms as a predictor of memory complaints in the PRISM sample. J Gerontol B Psychol Sci Soc Sci. 2017;00(00):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yates JA, Clare L, Woods RT, Matthews FE, Cognitive F. Ageing study W. subjective memory complaints are involved in the relationship between mood and mild cognitive impairment. J Alzheimers Dis. 2015;48(1):S115–123. Suppl. [DOI] [PubMed] [Google Scholar]
- 84.Jimenez-Huete A, Del Barrio A, Riva E, Campo P, Toledano R, Franch O. Subjective evaluation of mood and cognitive functions in a general neurology clinic: patients versus informants. J Clin Neurol. 2017;13(3):259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rattanabannakit C, Risacher SL, Gao S, et al. The cognitive change index as a measure of self and informant perception of cognitive decline: relation to neuropsychological tests. J Alzheimers Dis. 2016;51(4):1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scherling CS, Wilkins SE, Zakrezewski J, et al. Decreased self-appraisal accuracy on cognitive tests of executive functioning is a predictor of decline in mild cognitive impairment. Front Aging Neurosci. 2016;8:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry. 2005;20(9):827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rueda AD, Lau KM, Saito N, et al. Self-rated and informant-rated everyday function in comparison to objective markers of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1080–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Birkenbihl C, Salimi Y, Domingo-Fernándéz D, Lovestone S, Fröhlich H, Hofmann-Apitius M. Evaluating the Alzheimer’s disease data landscape. Alzheimers Dement (N Y). 2020;6(1):e12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Franzen S, Smith JE, van den Berg E, et al. Diversity in Alzheimer’s disease drug trials: the importance of eligibility criteria. Alzheimers Dement. 2022;18(4):810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ashford MT, Eichenbaum J, Williams T, et al. Effects of sex, race, ethnicity, and education on online aging research participation. Alzheimers Dement (N Y). 2020;6(1):e12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weiner MW, Veitch DP, Miller MJ, et al. Increasing participant diversity in AD research: plans for digital screening, blood testing, and a community-engaged approach in the Alzheimer’s Disease Neuroimaging Initiative 4. Alzheimers Dement. 2023;19(1):307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ashford MT, Raman R, Miller G, et al. Screening and enrollment of underrepresented ethnocultural and educational populations in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimers Dement. 2022;18(12):2603–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ashford MT, Okonkwo OC, Rivera Mindt M, et al. Efforts to improve recruitment and engagement of underrepresented populations in the Alzheimer’s disease neuroimaging initiative (ADNI) study. J Prev Alzheimers Dis. 2021;8(Suppl 1):S56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


