Abstract
Background
T cell-mediated acute rejection(AR) after heart transplantation(HT) ultimately results in graft failure and is a common indication for secondary transplantation. It’s a serious threat to heart transplant recipients. This study aimed to explore the novel lncRNA-miRNA-mRNA networks that contributed to AR in a mouse heart transplantation model.
Methods
The donor heart from Babl/C mice was transplanted to C57BL/6 mice with heterotopic implantation to the abdominal cavity. The control group was syngeneic heart transplantation with the same kind of mice donor. The whole-transcriptome sequencing was performed to obtain differentially expressed mRNAs (DEmRNAs), miRNAs (DEmiRNAs) and lncRNAs (DElncRNAs) in mouse heart allograft. The biological functions of ceRNA networks was analyzed by GO and KEGG enrichment. Differentially expressed ceRNA involved in programmed cell death were further verified with qRT-PCR testing.
Results
Lots of DEmRNAs, DEmiRNAs and DElncRNAs were identified in acute rejection and control after heart transplantation, including up-regulated 4754 DEmRNAs, 1634 DElncRNAs, 182 DEmiRNAs, and down-regulated 4365 DEmRNAs, 1761 DElncRNAs, 132 DEmiRNAs. Based on the ceRNA theory, lncRNA-miRNA-mRNA regulatory networks were constructed in allograft acute rejection response. The functional enrichment analysis indicate that the down-regulated mRNAs are mainly involved in cardiac muscle cell contraction, potassium channel activity, etc. and the up-regulated mRNAs are mainly involved in T cell differentiation and mononuclear cell migration, etc. The KEGG pathway enrichment analysis showed that the down-regulated DEmRNAs were mainly enriched in adrenergic signaling, axon guidance, calcium signaling pathway, etc. The up-regulated DEmRNAs were enriched in the adhesion function, chemokine signaling pathway, apoptosis, etc. Four lncRNA-mediated ceRNA regulatory pathways, Pvt1/miR-30c-5p/Pdgfc, 1700071M16Rik/miR-145a-3p/Pdgfc, 1700071M16Rik/miR-145a-3p/Tox, 1700071M16Rik/miR-145a-3p/Themis2, were finally validated. In addition, increased expression of PVT1, 1700071M16Rik, Tox and Themis2 may be considered as potential diagnostic gene biomarkers in AR.
Conclusion
We speculated that Pvt1/miR-30c-5p/Pdgfc, 1700071M16Rik/miR-145a-3p/Pdgfc, 1700071M16Rik/miR-145a-3p/Tox and 1700071M16Rik/miR-145a-3p/Themis2 interaction pairs may serve as potential biomarkers in AR after HT.
Keywords: ceRNA, heart transplant, acute rejection (AR), lncRNA, microRNA, mRNA
Background
Heart transplantation is an effective method of treating patients with end-stage heart failure. However, patients are at risk for several complications after transplantation, the most common of which is acute rejection. Recipients mortality due to acute rejection has been reported up to 11% within 3 years after heart transplantation (1).
Over the past decade, with the appearance of numerous high-throughput genomic platforms and bioinformatics, it has been found that more than 90% of genes in the genome belong to non-coding RNA (non-coding RNA, ncRNA), and less than 2% of genes belong to proteins-encoding RNA (2–4). long non-coding RNA(lncRNA), which comprises a variety of RNA species longer than 200nt, is the majority of non-coding RNA in human body (5). MiRNA is a single-stranded RNA of 21-25 nt in length that binds to other RNAs through complementary nucleotide sequences to influence the function and translation of other RNAs and then regulate gene expression.
In heart transplantation, ncRNAs are involved in the process of heart transplant rejection mainly by regulating immune and inflammatory responses. LncRNAs can modulate the immune response and affect graft survival. Studies have reported that lncRNAs alter the recipient immune environment by regulating Treg, Th1 or DC cell ratios or phenotypic alterations, affecting immune rejection and regulating graft survival (6–8). MiRNAs are also closely related to transplantation immune rejection. miRNAs regulate the immune and inflammatory microenvironment in the recipient, and induce immune tolerance or rejection in the graft (9–13). Therefore, differential expression of miRNAs can be used to predict rejection after heart transplantation (14–19). It is worth mentioning that although miRNA has been shown to predict heart transplant rejection, some studies have demonstrated that the predictive power of miRNA is still far from adequate compared to troponin T (20). Besides, circle RNA can also be involved in immune rejection of heart transplantation (21).
In this study, we used whole transcriptome sequencing technology to preliminarily explore and validate the ceRNA regulatory network affecting acute rejection and to elucidate the possible biomarkers of acute rejection. Here, we tentatively propose RNAs critical for acute rejection of heart transplantation that may play a role in various immune responses after heart transplantation.
Methods
Animals
Babl/C and C57/B6 mice (6-8 weeks) were purchased from Guangdong Medical Laboratory Animal Center. The mice were housed in the animal facility of Sun Yat-sen University, where they were kept in a specific pathogen-free environment with a 12:12-hour light-dark cycle, on sterile chow and sterilized water. All animal experiments were approved by the ethics committee of the First Affiliated Hospital, Sun Yat-sen University.
Heart transplantation and histology
We construct the mice heart transplantation acute rejection model by transplanting the heart of Babl/c or C57BL/6 donor mice into the abdominal cavity of C57/B6 mice, as previously reported (22). Allogeneic heart transplantation was defined as the acute rejection(AR) group, and homogeneous heart transplantation was the control(CON) group. After transplantation, the rejection of the transplanted heart was determined by observing the abdominal pulsation. Rejection was considered to occur if the heartbeat stopped. Specimens of transplanted hearts were embedded and sections, stained with hematoxylin/eosin for microscopic evaluation.
Screening strategy for DEmRNAs, DElncRNAs and DEmiRNAs
The differential expression of mRNAs, lncRNAs and miRNAs between the AR and CON groups was analyzed using the DESeq2 method. The screening thresholds for significant differences in gene expression were adjusted to p<0.05 and |log2FC(fold change)|>1.5. Heat maps and volcano maps were generated for visual analysis.
lncRNA-miRNA-mRNA regulatory network
The DElncRNA-DEmiRNA-DEmRNA network was constructed based on the ceRNA hypothesis: (1) the interaction information of miRNA-mRNAs in miRmap, Miranda, miRDB, TargetScan and MitarBase and miRNA-lncRNAs in Starbase was extracted; (2) If both lncRNAs and mRNAs were targeted and negatively expressed with a common miRNA, the lncRNA-miRNA-mRNA set was identified as a co-expression competition triad and the corresponding ceRNA regulatory network was constructed. ceRNA regulatory network was visualized with Cytoscape 3.7.1
KEGG and GO function enrichment analysis
To predict the potential biological functions of genes in the ceRNA network, the GO pathway (e.g., biological process, BP; cellular component, CC and molecular function, MF) and the KEGG pathway were implemented using the ClueGO app. p<0.05 was considered statistically significant and the results were visualized with bubble plots.
PCR
Total RNA of the heart was extracted using RNA Extraction Kit (Accurate Biology, Hunan, China) following the manufacturer’s instructions. The Evo M-MLV RT Premix for qPCR (Accurate Biology, Hunan, China) and a polymerase chain reaction (PCR) System generated cDNA. The PCR was performed with the SYBR Green Premix Pro Taq HS qPCR KIT (Accurate Biology, Hunan, China). The results were analyzed by the 2-ΔΔCT method. Gene expression data were shown as relative to the control group, which was set as 100%.
Statistical analysis
In this study, GraphPad Prism 8 (GraphPad Software, San Diego, California, USA) was used for data statistics and analysis. Differences in the expression of mRNAs, lncRNAs and miRNAs between the AR group and the CON group were analyzed using t-test. p<0.05 was considered a significant difference.
Results
Construction of acute rejection model of heart transplantation in mice
The mice acute rejection model was constructed by allogeneic heart transplantation, and cardiac allograft rejection or survival was confirmed by visualization and unpalpation. We constructed Allogeneic and homogeneous heart transplantation for comparative analysis. The survival of graft was shown in Figure 1A , the AR group (n=6) started to show rejection on day 1 after transplantation, and all grafts stopped beating by day 4. While the CON group (n=6) began to show rejection on day 6 and by the end of the observation period three grafts stopped beating, which was a significant difference. The mice transplanted hearts were subsequently subjected to pathological analysis. The transplanted hearts in the AR group showed a series of acute rejection manifestations such as myocardial vacuolar lesions, disorganized cell morphology, and loss of nuclei, while the hearts from the CON group showed normal cardiomyocyte manifestations, as shown in Figure 1B .
Figure 1.
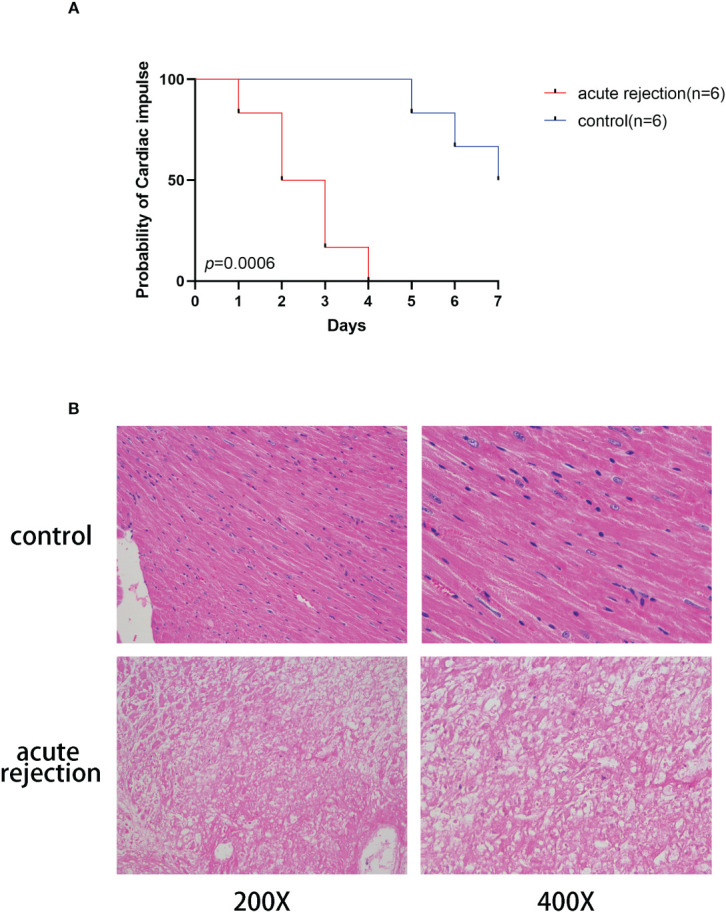
Construction of acute rejection model of heart transplantation in mice. (A)The cardiac arrest curve in mice within 7 days after transplantation. Red represents the acute rejection group(n=6) and blue indicates the control group(n=6). (B) Myocardial pathology in control and acute rejection group.
Screening of DEmRNAs, DElncRNAs and DEmiRNAs
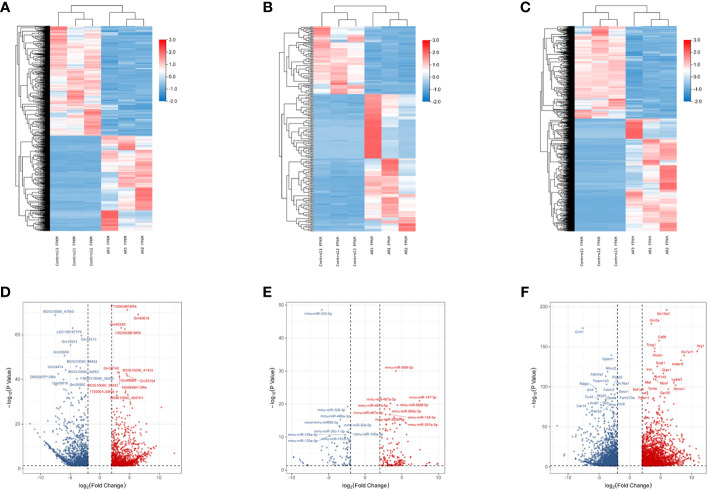
The whole-transcriptome sequencing results demonstrated 12925 DE-RNAs significantly associated with acute rejection. There were 9119 DE-mRNAs in the transcriptome, including 4754 up-regulated DEmRNAs and 4365 down-regulated DEmRNAs. Likewise, there were 1634 up-regulated DE-lncRNAs, 1761 down-regulated DE-lncRNAs, 182 up-regulated DE-miRNAs and 132 down-regulated DE-miRNAs. The heat map and volcano plot of DEmRNAs, DElncRNAs and DEmiRNAs were shown in Figure 2 .
Figure 2.
Heatmap plots and volcano plots of DelncRNAs, DemiRNAs, and DemRNAs during rejection and control group in an acute rejection heart transplant model. A comparative analysis for expression profiles of lncRNAs, miRNAs and mRNAs between allogeneic transplantation group and syngenic transplantation controls was performed with P < 0.05 and |log2 fold change [FC]|>1.5 as threshold. (A) Heatmap of differentially expressed lncRNAs; (B) Heatmap of differentially expressed miRNAs; (C) Heatmap of differentially expressed mRNAs; (D) Volcano plot of 2175 differentially expressed lncRNAs; (E) Volcano plot of 314 differentially expressed miRNAs; (F) Volcano plot of 3958 differentially expressed mRNAs. Red represents upregulated genes and blue indicates downregulated genes. DE, differentially expressed; FC, fold change.
Differential gene interaction network construction
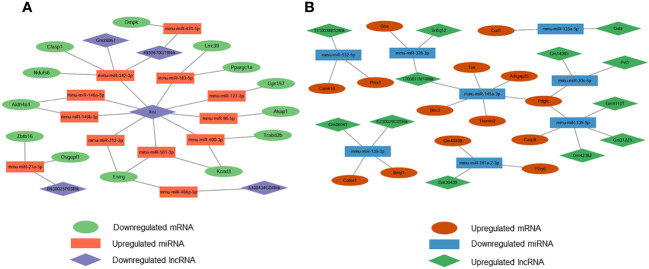
We constructed DE-miRNA-mediated ceRNA regulatory networks based on base sequences and expression levels. Based on the interaction elements, 14 miRNA-lncRNA pairs and 16 miRNA-mRNA pairs were identified in the upregulated miRNA ceRNA network, and 13 miRNA-lncRNA pairs and 16 miRNA-mRNA pairs in the downregulated miRNA ceRNA network. Based on this, the down-regulated ceRNA network including 5 lncRNA nodes, 12 miRNA nodes and 13 mRNA nodes, and the up-regulated ceRNA network including 12 lncRNA nodes, 8 miRNA nodes and 14 mRNA nodes were constructed. The downregulated and upregulated ceRNA networks were shown in Figure 3 separately.
Figure 3.
Construction of lncRNA-mediated ceRNA regulatory network. (A) The lncRNA-mediated downregulated ceRNA network; (B) The lncRNA-mediated upregulated ceRNA network. Green means downregulated LncRNA, red means upregulated LncRNA, blue indicates upregulated miRNAs and mRNAs, yellow represents downregulated miRNAs, orange represents downregulated mRNAs.
KEGG and GO functional enrichment analysis
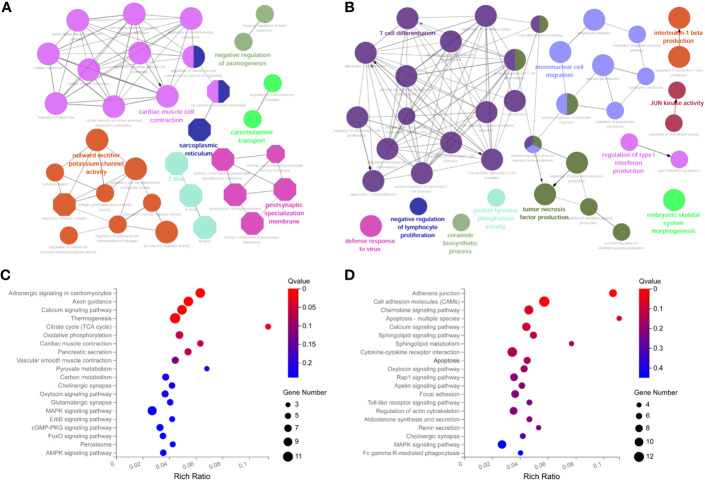
To further explore the potential functions of ceRNA network-related RNAs, we performed functional enrichment analysis using the ClueGO app. The results indicate that the down-regulated mRNAs are mainly involved in cardiac muscle cell contraction, potassium channel activity, etc ( Figure 4A ). the up-regulated mRNAs are mainly involved in T cell differentiation and mononuclear cell migration, etc ( Figure 4B ). The KEGG pathway enrichment analysis showed that the down-regulated DEmRNAs were mainly enriched in adrenergic signaling, axon guidance, calcium signaling pathway, etc ( Figure 4C ). The up-regulated DEmRNAs were enriched in the adhesion function, chemokine signaling pathway, apoptosis, etc ( Figure 4D ).
Figure 4.
Functional enrichment analysis of DemRNAs in ceRNA networks with ClueGO app. (A) GO biological functional analyses of downregulated mRNA; (B) GO biological function analyses of upregulated mRNA; (C) KEGG pathway analyses of downregulated mRNA; (D) KEGG pathway analyses of upregulated mRNA. BP, biological process; CC, cellular component; MF, molecular function; GO, Gene Ontology.
qRT-PCR validation
To find the key genes in the ceRNA network, we validated the DERNAs found by sequencing in vivo (n=3) by qRT-PCR, and the results were shown in Figure 5A . We identified genes that play a key role in acute rejection, including PDGFC(t=11.200, p<0.000), Birc3(t=3.829,p<0.001), TOX(t=13.770, p<0.000), Themis2(t=23.770, p<0.000), PVT1(t=11.420, p<0.000), 1700071M16Rik(t=8.672, p<0.000), miR-145a-3p(t=10.530,p<0.000), miR-30c-5p(t=7.901, p<0.000). The interaction network was shown in Figure 5B .
Figure 5.
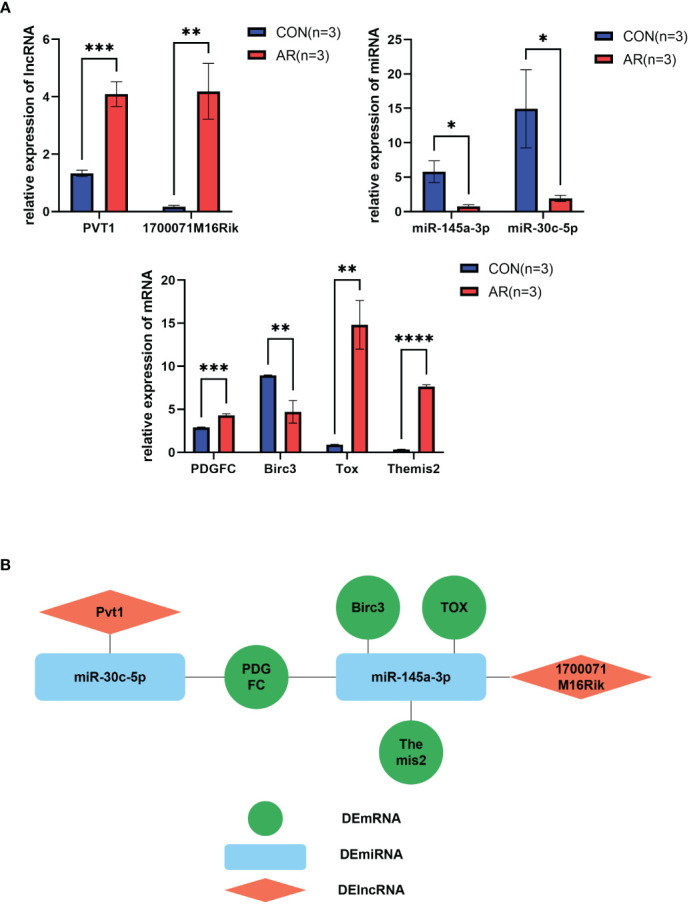
Validation of key RNAs in ceRNA networks. (A) Significance qPCR validation results of DEmiRNA, DElncRNA and DEmRNA within ceRNA networks(n=3). (B) Diagram of key RNA regulatory networks. Diamond shape represents DElncRNA, rounded rectangle represents DEmiRNA, circle represents DEmRNA. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Identification of programmed cell death-associated genes in ceRNA network
We compared 75 differentially expressed mRNAs in the ceRNA network with programmed cell death-related genes in GeneCard and obtained 62 common genes. Comparing these common genes with the key genes validated above, we found that among them pyroptosis gene Birc3, and apoptosis genes PDGFC, TOX and THEMIS2 were common, and these genes are likely to be involved in cell death caused by acute rejection.
Conclusion
In our study, we confirmed the existence of lncRNA-miRNA-mRNA interactions network in the heart transplantation acute rejection model. The regulatory network between mRNA, lncRNA and miRNA was constructed based on the ceRNA network theory by comparing the whole transcriptome of acute rejection group and control group. KEGG and GO analyses demonstrated that genes within this network are mainly involved in inflammation and immune-related functions. Finally, qRT-PCR validated the key genes in this network, including Pvt1/miR-30c-5p/Pdgfc, 1700071M16RIK/miR-145a-3p/Pdgfc, 1700071M16RIK/miR-145a-3p/TOX, 1700071M16RIK/miR-145a-3p/THEMIS2.
To our knowledge, PDGFC and PVT1 have been shown to be associated with acute rejection of transplanted hearts. PDGFC, a member of platelet-derived growth factor (PDGF), has been reported to be associated with chronic rejection of cardiac transplantation, upregulating TGF-β1 and promoting myocardial fibrosis and atherosclerosis (23). Overexpression of PVT1 upregulates TNF receptor-associated factor (TRAF) 6 expression through targeting miRNAs to alter Treg autophagy and inhibit cardiac transplant rejection (6).
Themis2 is associated with B-cell development and macrophage immune responses (24, 25).TOX regulates transcriptional processes by binding to DNA in a structure-dependent way. TOX is involved in T cell development and autoimmune regulation, and plays a critical role in T cell depletion (26). BIRC3 is one of the eight members of the human inhibitors of apoptosis proteins family. The literature reports that Birc3 expression is associated with tumor prognosis, inflammatory response and immune disorders (27–29). Birc3 inhibits cellular pyroptosis by inactivating NLRC4 inflammatory vesicles (30).
miR-30c-5p has been shown to be associated with cardiac IRI and ameliorates myocardial injury by suppressing apoptosis-related genes such as BCL2, Bach1 (31, 32). Chen J et al. reported that miR-30c-5p can activate NF-κB pathway to promote myocardial IRI (33). miR-145a-3p is associated with lipid metabolism, but the relationship with rejection is unclear (34). Besides, miR-30c-5p may be associated with immunosuppression/graft tolerance induction in liver transplant recipients after transplantation. Morsiani C et al. found that miR-30c-5p decreased in liver transplant patients in early to mid-term follow-up, but returned to normal levels 19 months after liver transplantation (35).
Antibody-mediated rejection is a major cause of heart transplant failure, and previous studies have reported that C4d deposition is associated with heart transplants acute rejection (36, 37). Using C4d-targeted microbubbles loaded with nitric oxide could improve the therapeutic efficacy of heart transplant rejection (38). We found that the positivity of C4d was higher in the AR group compared to the CON group (p=0.0159), the results are shown in Supplementary Figure 1 . The relationship between C4d deposition and the above RNAs has not yet been reported, and it remains to be investigated which kind of RNA could monitor the C4d deposition.
In this study, we propose the ceRNA regulatory network in heart transplantation acute rejection and elucidate the possible mechanisms underlying the influence of this regulatory network on the survival of transplanted hearts. However, this study only used a mice heart transplantation model and was not validated in vitro or patients, so the ceRNA network may not apply to humans. In the future, we will continue to conduct in vitro experiments and collect serum from patients for further analysis. In addition, this study has not verified the protein expression alteration of differential mRNAs, and since the function of mRNAs needs to be further exerted at the protein level, the regulatory network may also be altered due to translation modifications.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA943392.
Ethics statement
The animal study was approved by Medical Ethics Committee, The First Affiliated Hospital of Sun Yat-sen University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YG, MC and YZ contributed to conception and design of the study. YD and ZC established the animal model. XtH, MZ and ZX performed the statistical analysis. SW and YL performed the bioinformatic analysis. WJ and XsH wrote the first draft of the manuscript. CZ, YG and YZ wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding Statement
Supported by grants as follows: National Natural Science Foundation of China (81401324 and 81770410), the Guangdong Provincial Key Laboratory Construction Projection on Organ Donation and Transplant Immunology (2013A061401007, 2017B030314018 and 2020B1212060026).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1184409/full#supplementary-material
References
- 1. Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report–2014; focus theme: retransplantation. J Heart Lung Transplant (2014) 33(10):996–1008. doi: 10.1016/j.healun.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 2. Wilusz JESH, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev (2009) 23:1494–504. doi: 10.1101/gad.1800909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Djebali SDC, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, et al. Landscape of transcription in human cells. Nature (2012) 6(7414):101–8. doi: 10.1038/nature11233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Derrien TJR, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res (2012) 22:1775–89. doi: 10.1101/gr.132159.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cui MZM, Sun B, Wang Y, Ye L, Zhang X. A long noncoding RNA perturbs the circadian rhythm of hepatoma cells to facilitate hepatocarcinogenesis. Neoplasia (2015) 17:79–88. doi: 10.1016/j.neo.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu J, Wang X, Zhang B, Li P, Du X, Qi F. The lncRNA PVT1 regulates autophagy in regulatory T cells to suppress heart transplant rejection in mice by targeting miR-146a. Cell Immunol (2021) 367:104400. doi: 10.1016/j.cellimm.2021.104400 [DOI] [PubMed] [Google Scholar]
- 7. Gu G, Huang Y, Wu C, Guo Z, Ma Y, Xia Q, et al. Differential expression of long noncoding RNAs during cardiac allograft rejection. Transplantation (2017) 101(1):83–91. doi: 10.1097/TP.0000000000001463 [DOI] [PubMed] [Google Scholar]
- 8. Wu J, Zhang H, Zheng Y, Jin X, Liu M, Li S, et al. The Long Noncoding RNA MALAT1 Induces Tolerogenic Dendritic Cells and Regulatory T Cells via miR155/Dendritic Cell-Specific Intercellular Adhesion Molecule-3 Grabbing Nonintegrin/IL10 Axis. Front Immunol (2018) 9:1847. doi: 10.3389/fimmu.2018.01847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tuo L, Song H, Jiang D, Bai X, Song G. Mesenchymal stem cells transfected with anti-miRNA-204-3p inhibit acute rejection after heart transplantation by targeting C-X-C motif chemokine receptor 4 (CXCR4) in vitro . J Thorac Dis (2021) 13(8):5077–92. doi: 10.21037/jtd-21-1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fujino M, Zhu P, Cai S, Nishio Y, Zhuang J, Li XK. MicroRNAs involved in acute rejection and tolerance in murine cardiac allografts. Exp Clin Transplant (2016) 14(4):424–30. doi: 10.6002/ect.2015.0251 [DOI] [PubMed] [Google Scholar]
- 11. Li C, Wang X, Yuan F, Zhao Z, Zhang B, Zhang J, et al. MiR-669b-3p regulates CD4(+) T cell function by down-regulating indoleamine-2, 3-dioxygenase. Transpl Immunol (2020) 62:101320. doi: 10.1016/j.trim.2020.101320 [DOI] [PubMed] [Google Scholar]
- 12. Chen J, Li S, Wang X, Fang Z, Cheng C. MiR155 relieves acute heart transplantation in mice by modulating th1/th17 immune response. Cell Mol Biol (Noisy-le-grand) (2022) 68(1):35–41. doi: 10.14715/cmb/2022.68.1.6 [DOI] [PubMed] [Google Scholar]
- 13. Wang X, Cao J, Yu Y, Ma B, Gao C, Lu J, et al. Role of microRNA 146a in regulating regulatory T cell function to ameliorate acute cardiac rejection in mice. Transplant Proc (2019) 51(3):901–12. doi: 10.1016/j.transproceed.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 14. Guo S, Guo X, Wang S, Nie Q, Ni G, Wang C. Role of miR-29 as marker of risk of acute rejection after heart transplant. Br J BioMed Sci (2017) 74(4):187–92. doi: 10.1080/09674845.2017.1333265 [DOI] [PubMed] [Google Scholar]
- 15. Duong Van Huyen JP, Tible M, Gay A, Guillemain R, Aubert O, Varnous S, et al. MicroRNAs as non-invasive biomarkers of heart transplant rejection. Eur Heart J (2014) 35(45):3194–202. doi: 10.1093/eurheartj/ehu346 [DOI] [PubMed] [Google Scholar]
- 16. Sukma Dewi I, Hollander Z, Lam KK, McManus JW, Tebbutt SJ, Ng RT, et al. Association of serum miR-142-3p and miR-101-3p levels with acute cellular rejection after heart transplantation. PloS One (2017) 12(1):e0170842. doi: 10.1371/journal.pone.0170842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pérez-Carrillo L, Sánchez-Lázaro I, Triviño JC, Feijóo-Bandín S, Lago F, González-Juanatey JR, et al. Diagnostic value of serum miR-144-3p for the detection of acute cellular rejection in heart transplant patients. J Heart Lung Transplant (2022) 41(2):137–47. doi: 10.1016/j.healun.2021.10.004 [DOI] [PubMed] [Google Scholar]
- 18. Kennel PJ, Yahi A, Naka Y, Mancini DM, Marboe CC, Max K, et al. Longitudinal profiling of circulating miRNA during cardiac allograft rejection: a proof-of-concept study. ESC Heart Fail (2021) 8(3):1840–9. doi: 10.1002/ehf2.13238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nováková T, Macháčková T, Novák J, Hude P, Godava J, Žampachová V. Oppelt J, Zlámal F, Němec P, Bedáňová H, Slabý O, Bienertová-Vašků J, Špinarová L, Krejčí J. Identification of a Diagnostic Set of Endomyocardial Biopsy microRNAs for Acute Cellular Rejection Diagnostics in Patients after Heart Transplantation Using Next-Generation Sequencing. Cells (2019) 8(11):1400. doi: 10.3390/cells8111400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Esmaeili-Bandboni A, Bagheri J, Bakhshandeh AR, Mohammadnejad J, Sadroddiny E. Serum Levels of miR-155, miR-326, and miR-133b as Early Diagnostic Biomarkers for the Detection of Human Acute Heart Allograft Rejection in Comparison with Serum Cardiac Troponin T. Heart Surg Forum (2018) 21(2):E101–e107. doi: 10.1532/hsf.1887 [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y, Zhang G, Liu Y, Chen R, Zhao D, McAlister V, et al. GDF15 regulates Malat-1 circular RNA and inactivates NFκB signaling leading to immune tolerogenic DCs for preventing alloimmune rejection in heart transplantation. Front Immunol (2018) 9:2407. doi: 10.3389/fimmu.2018.02407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyahara Y, Khattar M, Schroder PM, Mierzejewska B, Deng R, Han R, et al. Anti-TCRβ mAb induces long-term allograft survival by reducing antigen-reactive T cells and sparing regulatory T cells. Am J Transplant (2012) 12(6):1409–18. doi: 10.1111/j.1600-6143.2012.04006.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tuuminen R, Nykänen AI, Krebs R, Soronen J, Pajusola K, Keränen MA, et al. PDGF-A, -C, and -D but not PDGF-B increase TGF-beta1 and chronic rejection in rat cardiac allografts. Arterioscler Thromb Vasc Biol (2009) 29(5):691–8. doi: 10.1161/ATVBAHA.108.178558 [DOI] [PubMed] [Google Scholar]
- 24. Peirce MJ, Brook M, Morrice N, Snelgrove R, Begum S, Lanfrancotti A, et al. Themis2/ICB1 is a signaling scaffold that selectively regulates macrophage Toll-like receptor signaling and cytokine production. PloS One (2010) 5(7):e11465. doi: 10.1371/journal.pone.0011465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng D, Deobagkar-Lele M, Zvezdova E, Choi S, Uehara S, Baup D, et al. Themis2 lowers the threshold for B cell activation during positive selection. Nat Immunol (2017) 18(2):205–13. doi: 10.1038/ni.3642 [DOI] [PubMed] [Google Scholar]
- 26. Cheng Y, Shao Z, Chen L, Zheng Q, Zhang Q, Ding W, et al. Role, function and regulation of the thymocyte selection-associated high mobility group box protein in CD8(+) T cell exhaustion. Immunol Lett (2021) 229:1–7. doi: 10.1016/j.imlet.2020.11.004 [DOI] [PubMed] [Google Scholar]
- 27. Frazzi R. BIRC3 and BIRC5: multi-faceted inhibitors in cancer. Cell Biosci (2021) 11(1):8. doi: 10.1186/s13578-020-00521-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vinogradova EV, Zhang X, Remillard D, Lazar DC, Suciu RM, Wang Y, et al. An activity-guided map of electrophile-cysteine interactions in primary human T cells. Cell (2020) 182(4):1009–1026.e1029. doi: 10.1016/j.cell.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pradhan M, Baumgarten SC, Bembinster LA, Frasor J. CBP mediates NF-κB-dependent histone acetylation and estrogen receptor recruitment to an estrogen response element in the BIRC3 promoter. Mol Cell Biol (2012) 32(2):569–75. doi: 10.1128/MCB.05869-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu M, Shao Z. Lactobacillus pentosus Alleviates Lipopolysaccharide-Induced Neuronal Pyroptosis via Promoting BIRC3-Mediated Inactivation of NLRC4. Evid Based Complement Alternat Med (2022) 2022:2124876. doi: 10.1155/2022/2124876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meng S, Hu Y, Zhu J, Feng T, Quan X. miR-30c-5p acts as a therapeutic target for ameliorating myocardial ischemia-reperfusion injury. Am J Transl Res (2021) 13(4):2198–212. [PMC free article] [PubMed] [Google Scholar]
- 32. Sun M, Guo M, Ma G, Zhang N, Pan F, Fan X, et al. MicroRNA-30c-5p protects against myocardial ischemia/reperfusion injury via regulation of Bach1/Nrf2. Toxicol Appl Pharmacol (2021) 426:115637. doi: 10.1016/j.taap.2021.115637 [DOI] [PubMed] [Google Scholar]
- 33. Chen J, Zhang M, Zhang S, Wu J, Xue S. Rno-microRNA-30c-5p promotes myocardial ischemia reperfusion injury in rats through activating NF-κB pathway and targeting SIRT1. BMC Cardiovasc Disord (2020) 20(1):240. doi: 10.1186/s12872-020-01520-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sud N, Zhang H, Pan K, Cheng X, Cui J, Su Q. Aberrant expression of microRNA induced by high-fructose diet: implications in the pathogenesis of hyperlipidemia and hepatic insulin resistance. J Nutr Biochem (2017) 43:125–31. doi: 10.1016/j.jnutbio.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morsiani C, Collura S, Sevini F, Ciurca E, Bertuzzo VR, Franceschi C, et al. Circulating miR-122-5p, miR-92a-3p, and miR-18a-5p as Potential Biomarkers in Human Liver Transplantation Follow-Up. Int J Mol Sci (2023) 24(4):3457. doi: 10.3390/ijms24043457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mohamedali B, Pyle J, Bhat G. Acute cellular rejection and C4d positivity in heart transplantation: A manifestation of asymptomatic antibody-mediated rejection?. Am J Clin Pathol (2016) 145(2):238–43. doi: 10.1093/ajcp/aqv026 [DOI] [PubMed] [Google Scholar]
- 37. Alsughayyir J, Chhabra M, Qureshi MS, Mallik M, Ali JM, Gamper I, et al. Relative frequencies of alloantigen-specific helper CD4 T cells and B cells determine mode of antibody-mediated allograft rejection. Front Immunol (2018) 9:3039. doi: 10.3389/fimmu.2018.03039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liao T, Li Q, Zhang Y, Yang Z, Huang Z, Han F, et al. Precise treatment of acute antibody-mediated cardiac allograft rejection in rats using C4d-targeted microbubbles loaded with nitric oxide. J Heart Lung Transplant (2020) 39(5):481–90. doi: 10.1016/j.healun.2020.02.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA943392.