Highlights
-
•
We investigated the impact of FDG-PET guided dose escalation in HNSCC.
-
•
Compared to a matched control group, local control rates were comparable.
-
•
Compared to a matched control group, late toxicity was increased.
-
•
Selection criteria for future, preferably, randomized trials need to be refined.
-
•
Only patients with high risk of LF &/or low risk of severe toxicity should be included.
Keywords: Dose escalation, HNSCC, FDG-PET, Late toxicity, Local control
Abstract
Purpose
To report on the late toxicity and local control (LC) of head and neck cancer patients treated with adaptive FDG-PET/CT response-guided radiotherapy (ADMIRE) with dose escalation (NCT03376386).
Materials and methods
Between December 2017 and April 2019, 20 patients with stage II-IV squamous cell carcinoma of the larynx, hypopharynx or oropharynx were treated within the ADMIRE study where FDG-PET/CT response-guided (Week 2&4) dose escalation was applied (total dose 70–78 Gy). Cisplatin or cetuximab was added to radiotherapy in case of T3-4 and/or N2c disease. To compare the LC and late toxicity of the study population, we used an external control group (n = 67) consisting of all eligible patients for the study (but not participated). These patients were treated in our institution during the same period with the current standard of 70 Gy radiotherapy. To reduce the effect of confounding, logistic regression analyses was done using stabilized inverse probability of treatment weighting (SIPTW).
Results
After median follow-up of 40 and 43 months for the ADMIRE and control groups, the 3-year LC-rates were 74% and 78%, respectively (adjusted HR after SIPTW 0.80, 95 %CI 0.25–2.52, p = 0.70). The incidences of any late G3 toxicity were 35% and 18%, respectively. The adjusted OR for any late G3 toxicity was 5.09 (95 %CI 1.64–15.8, p = 0.005), for any late G ≥ 2 toxicity was 3.67 (95 %CI 1.2–11.7, p = 0.02), for persistent laryngeal edema was 10.95 (95% CI 2.71–44.29, p = 0.001), for persistent mucosal ulcers was 4.67 (95% CI 1.23–17.7, p = 0.023), and for late G3 radionecrosis was 15.69 (95 %CI 2.43–101.39, p = 0.004).
Conclusion
Given the comparable LC rates with increased late toxicity in the ADMIRE group, selection criteria for future adaptive dose escalation trials (preferably randomized) need to be refined to include only patients at higher risk of local failure and/or lower risk of severe late toxicity.
Introduction
Despite the major improvements made in the detection of head and neck squamous cell carcinoma (HNSCC) and the introduction of different treatment strategies such as the use of systemic therapy and the altered fractionation schedules in the last few decades, the local control (LC) rates for certain groups of these patients are still disappointing [1], [2], [3], [4]. While there is a growing interest in treatment de-escalation in HPV-positive oropharyngeal carcinoma, patients with high-risk profiles (HPV-negative, heavy smoking history, locally-advanced disease) might still need treatment intensification to improve their oncologic outcomes. One way to intensify the treatment in those patients is by escalating the dose of radiation. However there are also concerns that this may lead to excessive toxicity. A potential solution is personalized dose escalation (DE), based on the individual response to treatment. In 2017, a prospective study was conducted in our institution (ADMIRE: Adaptive Dose-Escalated Multi-modality Image-guided RadiothErapy for head and neck cancer by twice re-imaging, re-delineation and re-planning during the course of radiotherapy) to investigate the feasibility and safety of escalating the dose of radiotherapy in 20 patients with stage II-IV non-metastatic HNSCC of the oropharynx, larynx and hypopharynx based on the tumor response, as monitored with serial FDG-PET/CT scans done at the end of the second and fourth weeks of radiotherapy. Gouw et al [5] concluded that FDG-PET/CT based DE to 74–78 Gy was considered feasible, as >80% of the adaptive plans were started within 2 fractions of the intended starting fraction and safe as no acute toxicity grade ≥ 4 was reported. The incidence of acute grade 3 dermatitis, mucositis and dysphagia were 15%, 15%, and 40%, respectively.
The aim of the current study is to report on late toxicity and LC of these patients and to compare these results with a control group matched to the inclusion criteria of the ADMIRE study.
Materials and methods
Study population and treatment
The ADMIRE study was approved by our institutional medical ethics committee (ClinicalTrials.gov identifier: NCT03376386). For the analysis of the control group, approval of our institutional review board was obtained (IRBd21-022). For a detailed description of the pre-treatment evaluation, inclusion criteria, treatment modality, indications for systemic treatment, radiation margins, imaging and image-guidance during treatment, the adaptive strategy, the response evaluation, and the follow-up scheme we refer the reader to the publication of Gouw et al. [5]. Briefly, 20 patients with stage II-IV HNSCC of oropharynx, larynx and hypopharynx treated were included. FDG-PET/CT scans were made at baseline and for re-delineation and re-planning at the end of week 2 and 4 of treatment. Depending on the metabolic response (MR), the remaining FDG-avid part of the primary tumor (CTVb) received a boost dose. In patients with complete MR (CMR) in week 2, the cumulative CTVb remained 70 Gy. In case of partial MR (PMR) in week 2 and CMR in week 4, the cumulative dose was 74 Gy. In patients with PMR in both weeks 2 and 4, the cumulative dose to the CTVb was escalated to 78 Gy. The control group consists of all the consecutive patients who were eligible for accrual but not included, either because they were not willing to participate (n = 15) or due to logistical limitations that allowed to treat only one patient a week because of the labor- and time-consuming logistical requirements of the study (n = 52). The control group was treated in our institution during the same period of time of accrual of the ADMIRE group between December 2017 and April 2019. Therefore, the whole treatment was identical to patients treated within ADMIRE study, with only two exceptions. Patients treated within the control group received the standard dose (SD) of radiation to the primary tumor (70 Gy; 35 fractions) and a single radiotherapy plan instead of the FDG PET/CT-guided DE with 2 planned adaptations.
End points
The primary endpoint was the feasibility and safety of FDG-PET/CT response-based DE protocol. The current study focusses on secondary endpoints; late toxicity (>90 days after treatment, using Common Terminology Criteria for Adverse Events, version 4.0) and LC as DE was only done to the primary tumor site. Other oncologic outcomes were also reported (loco-regional control; LRC, disease-free survival; DFS, and overall survival; OS).
Statistical analysis
LC and LRC were calculated from date of first radiotherapy fraction to local or loco-regional progression, respectively. Death as first event was censored as well as patients without any event at last follow-up. Patients with distant progression who received any salvage treatment were not censored for the LC/LRC analysis. Patients who were still alive and did not encounter any progression were censored at last follow-up. OS was calculated from date of first radiotherapy fraction to death from any cause, censoring patients who were still alive at last follow-up. LC, LRC, DFS, and OS were estimated with the Kaplan-Meier method and comparison between the cohorts was performed using the log-rank test. In addition, Hazard Ratios (HR) with the corresponding 95% Confidence Intervals (CI) were derived using Cox regression analyses. Median follow-up time was calculated as the median time to last follow-up or death and compared using a Kruskal-Wallis test and by reverse Kaplan-Meier method using log-rank test.
Late toxicity incidences were compared using Odds Ratios (OR) including 95% CI’s estimated by logistic regression analyses and categorical demographic variables were compared by means of Fisher’s exact test. For the comparison of the irradiated volumes and the doses at organs-at-risk (OAR), the Kruskal-Wallis test was used. P-values < 0.05 were considered significant.
To minimize the effect of confounding when estimating the difference between the ADMIRE and control group, stabilized inverse probability of treatment weighting (SIPTW) using propensity scores was applied when comparing the groups on LC and late toxicity. Propensity scores were estimated using logistic regression including important covariates for LC and late toxicity: T-stage, N-stage, tumor site, the addition of cisplatin or Cetuximab and PTV-total. For LC, Cox regression analysis was performed to estimate the adjusted HR after SIPTW and for late toxicity, logistic regression analyses were performed to estimate an adjusted OR after SIPTW. Computer code on how the SIPTW method was performed can be found in the supplementary material including results on covariate balance between the cohorts after weighting for assessing how well the SIPTW method worked.
For correlation of the site of local recurrence (LR) to the treated volumes the Center of mass (COM) approach was used: the CT scan with a LR was co-registered with the planning CT scan, using deformable registration. The original CTVb and PTVb were projected onto the recurrence CT scan. As the co-registration inaccuracy was 3 mm, a circle was created around the COM with a radius of 3 mm. The COM might be anywhere within that circle. A recurrence was considered in-field if the ratio of overlapping volume and recurrence volume was at least 95%, out-field if the ratio was<20%, and marginal if the ratio was between 20% and 95%.
Data preparation was performed using SAS software (version 9.4) and all statistical analyses were conducted in R (version 4.0.3).
Results
Twenty patients were treated within the ADMIRE group. One patient had a CMR in week 2 and received only 70 Gy without boost. Twelve patients received 78 Gy because of PMR at weeks 2 and 4 and 7 patients received 74 Gy, either because of CMR at week 4 (n = 3) or a missed FDG-PET/CT (n = 4).
Table 1 shows patients characteristics. No significant differences were seen between both groups, with exception of PTV-T in the control group, which was larger than in the ADMIRE group (median 451.2 cm3 vs. 540.2 cm3, respectively (p = 0.02).
Table 1.
Patients, tumor and treatment characteristics.
| ADMIRE (N = 20) | % | Control (N = 67) | % | p-value | |
|---|---|---|---|---|---|
| Patient's characteristics | |||||
| Follow-up; range (median) in months | 40.2 (7.8–54.2) | 43.4 (3.8–56.4) | 0.64 | ||
| Follow-up; range (median) in months (reverse KM method) | 48.6 (46–49.6) | 47.6 (46.6–49.2) | 0.37 | ||
| Age; range (median) in years | 64 (46–78) | 65 (42–83) | 0.39 | ||
| Gender; male | 11 | 55 | 45 | 67 | 0.43 |
| Site | 0.44 | ||||
| OPC | 10 | 50 | 37 | 55 | |
| LC | 9 | 45 | 21 | 31 | |
| HPC | 1 | 5 | 9 | 14 | |
| HPV-status in OPC | 5 | 50 | 15 | 40 | 0.72 |
| Smoking history | 0.76 | ||||
| never smoked | 2 | 10 | 6 | 9 | |
| <10 PY | 2 | 10 | 4 | 6 | |
| >10 PY | 16 | 80 | 57 | 85 | |
| Continued smoking during RT | 3 | 15 | 9 | 13 | 1.00 |
| T-stage | |||||
| T2 | 6 | 30 | 20 | 30 | 1.00 |
| T3 | 7 | 35 | 22 | 33 | |
| T4 | 7 | 35 | 25 | 37 | |
| N-stage | |||||
| N0 | 9 | 45 | 23 | 35 | 0.90 |
| N1 | 4 | 20 | 13 | 19 | |
| N2a | 0 | 0 | 1 | 2 | |
| N2b | 4 | 20 | 15 | 22 | |
| N2C | 3 | 15 | 15 | 22 | |
| AJCC stage | 1.00 | ||||
| stage 2 | 3 | 15 | 9 | 14 | |
| stage 3 | 3 | 15 | 13 | 19 | |
| stage 4 | 14 | 70 | 45 | 67 | |
| Concomitant cisplatin or cetuximab | 11 | 55 | 36 | 54 | 1.00 |
| Accelerated RT | 4 | 20 | 30 | 45 | 0.06 |
| DVH parameters | |||||
| GTV-P, median (range); cm3 | 16.14 (1.52–55.13) | 14.11 (2.2–98.4) | 0.64 | ||
| CTV-P, median (range); cm3 | 46.28 (7.32–111.2) | 36.5 (7.32–180.79) | 0.99 | ||
| PTV-P, median (range); cm3 | 81.25(15.06–156.57) | 59.1 (14.55–240.92) | 0.95 | ||
| PTV-T, median (range); cm3 | 451.26 (107.52–668.62) | 540.21 (248.41–1277.28) | 0.02 | ||
| Dmean ispilateral PG; median (range); Gy | 22.95 (11.5–42) | 27.13 (11.4–62.5) | 0.24 | ||
| Dmean contralateral PG; median (range); Gy | 18.15 (9.8–29.2) | 19.46 (0.62–61.54) | 0.2 | ||
| Dmean ispilateral SMG; median (range); Gy | 64.55 (38.9–71.1) | 66.31 (15.49–71.1) | 0.43 | ||
| Dmean contralateral SMG; median (range); Gy | 45.05 (32.3–67.6) | 45.35 (6.48–69.94) | 0.55 | ||
| Dmean constrictor muscle; median (range); Gy | 52 (36.7–58.7) | 49.55 (13.8–67.5) | 0.42 | ||
| Dmean oral cavity; median (range); Gy | 26.2 (12.5–64.4) | 28.6 (1.93–66.17) | 0.95 | ||
| Dmean larynx; median (range); Gy | 62.45 (36–74.8) | 60.49 (21.06–73.2) | 0.45 | ||
| Dmax mandible; median (range); Gy | 72.1 (32–74.6) | 66.04 (17.55–76.3) | 0.09 | ||
Abbreviations: KM: Kaplan-Meier; OPC: oropharygeal cancer; LC: larynxgeal cancer; HPC: hypopharyngeal cancer; HPV: human papilloma virus; PY: pack years; RT: radiotherapy; AJCC: American Joint Committee on Cancer; DVH: dose volume histogram; GTV-P: gross tumor volume primary; CTV-P: clinical target volume primary; PTV-P: planning target volume primary; PTV-T planning target volume total; Gy: Gray. PG: parotid gland; SMG: submandibular gland.
Table 2 shows the differences between both groups with regard to the incidence of late toxicity and the logistic regression analyses with and without SIPTW. No grade 4 or 5 toxicity was reported. Overall the incidence of late toxicities was higher in the ADMIRE group, compared to the control group, but the differences were not statistically significant, with the exception of persistent laryngeal edema (p < 0.05), but considering the number of tests this result is negligible. When comparing the two groups on late toxicity using SIPTW, the ADMIRE group has significantly higher odds of suffering from late toxicity. The adjusted OR for any late G3 toxicity was 5.09 (95 %CI 1.64–15.8, p = 0.005), for any late G ≥ 2 toxicity was 3.67 (95 %CI 1.2–11.7, p = 0.022), for persistent laryngeal edema was 10.95 (95% CI 2.71–44.29, p = 0.001), for persistent mucosal ulcers was 4.67 (95% CI 1.23–17.7, p = 0.023), and for late G3 radionecrosis was 15.69 (95 %CI 2.43–101.39, p = 0.004). However, the widths of the CIs show these OR’s are not very precise and these should therefore be interpreted with caution.
Table 2.
Logistic regression analysis for late radiation-related toxicity of both groups, with and without SIPTW.
| Incidence late toxicity |
Without SIPTW |
After SIPTW |
||||
|---|---|---|---|---|---|---|
| ADMIRE | Control | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Any late Grade 3 toxicity | 7 (35%) | 12 (18%) | 2.47 (0.81–7.5) | 0.11 | 5.09 (1.64–15.8) | 0.005 |
| Any late Grade ≥ 2 toxicity | 12 (60%) | 28 (42%) | 2.09 (0.76–5.78) | 0.16 | 3.67 (1.2–11.17) | 0.02 |
| Overall late Grade 3 dysphagia | 5 (25%) | 9 (13%) | 2.15 (0.63–7.36) | 0.22 | 3.42 (1–11.72) | 0.05 |
| Grade 3 dysphagia > 3 months | 4 (20%) | 9 (13%) | 1.61 (0.44–5.92) | 0.47 | 2.11 (0.57–7.81) | 0.26 |
| Grade 3 dysphagia > 6 months | 2 (10%) | 6 (9%) | 1.13 (0.21–6.09) | 0.89 | 2.02 (0.43–9.49) | 0.37 |
| Grade 3 dysphagia > 9 months | 1 (5%) | 3 (5%) | 1.12 (0.11–11.43) | 0.92 | 2.89 (0.45–18.62) | 0.26 |
| Grade 3 dysphagia > 12 months | 1 (5%) | 1 (2%) | 3.47 (0.21.58.18) | 0.39 | 8.16 (0.73–90.88) | 0.09 |
| Late Grade ≥ 2 dysphagia | 6 (30%) | 16 (24%) | 1.37 (0.45–4.14) | 0.58 | 1.86 (0.6–5.75) | 0.28 |
| Late Grade ≥ 2 dry mouth | 4 (20%) | 12 (18%) | 1.15 (0.32–4.04) | 0.83 | 1.27 (0.36--4.52) | 0.71 |
| Persistent laryngeal edema > 6 months | 5 (25%) | 4 (6%) | 5.25 (1.26–21.94) | 0.02 | 10.95 (2.71–44.29) | 0.001 |
| persistent mucosal ulcers > 6 months | 5 (25%) | 6 (9%) | 3.39 (0.91–12.62) | 0.07 | 4.67 (1.23–17.7) | 0.02 |
| Late Grade 3 radionecrosis | 3 (15%) | 2 (3%) | 5.74 (0.89–37.11) | 0.07 | 15.69 (2.43–101.39) | 0.004 |
Abbreviations: OR: odds ratio; CI: confidence interval; SIPTW: stabilized inverse probability of treatment weighting.
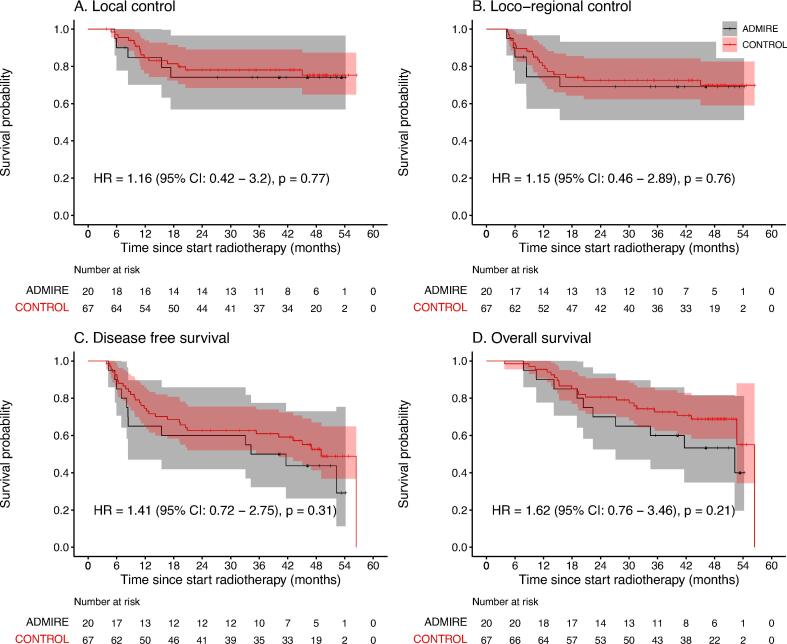
The 3-year LC rates for the ADMIRE and control groups were 74% and 78%, respectively (HR 1.16, 95 %CI 0.42–3.2; p = 0.77) (Fig. 1). The adjusted HR after SIPTW for LC has changed from 1.16 to 0.8. However, difference between both groups is still statistically not significant (HR 0.80, 95 %CI 0.25–2.52, p = 0.70).
Fig. 1.
The Kaplan-Meier curves of local control, loco-regional control, disease-free survival and overall survival. + censored.
The 3-years LRC rates for the ADMIRE and control groups were 69% and 73% (p = 0.76). The rates for DFS were 50% and 61%, respectively (p = 0.31) and for OS were 60% and 72% (p = 0.21).
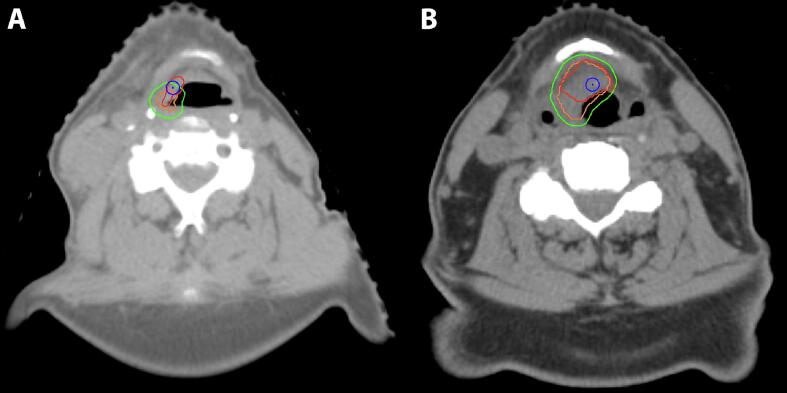
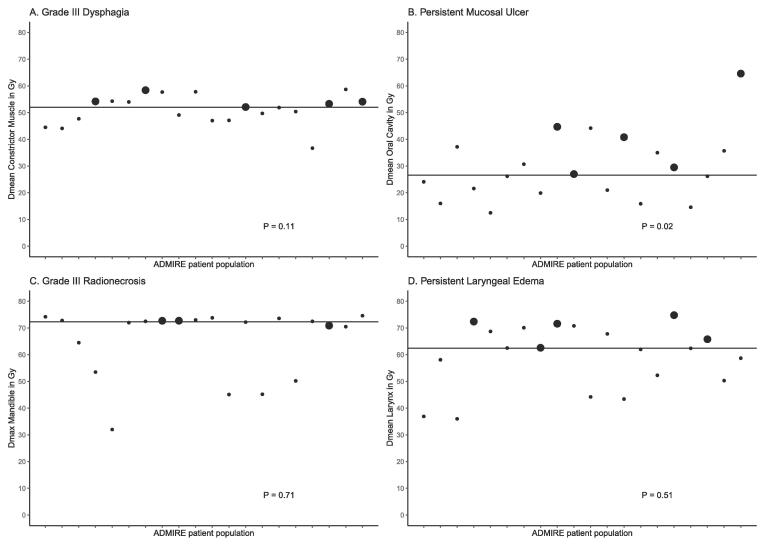
In the ADMIRE group, 5 patient developed local failure (LF) (Table3). The median time from end of radiation to LF was 7 months (range, 4–15.5). LF was seen in 2 of 3 patients who continued smoking during treatment (66%), compared to 3 of 17 patients (18%) who stopped smoking before radiotherapy. However, in a Cox regression analysis, the effect of continued smoking on LF was not significant (HR 4.86, 95% CI 0.79–29.78, p = 0.09). Patients with and without LF differed on PET variables, as the SUVpeak in patients without LF was reduced by a median of 50%, compared to a reduction of 26% in those who developed LF. Total lesion glycolysis (TLG) was decreased by a median of 54% in patients without LF, compared to increase of TLG by a median of 17% in patients who developed LF. However, the PET variables (changes in SUVpeak and in TLG) also had no significant effect on occurrence of a LF in the Cox regression analysis (HR 1.13, 95% CI 0.88–1.44, p = 0.34 for SUVpeak and HR 1.02, 95 %CI 0.99–1.04, p = 0.26 for TLG). Fig. 2 illustrates the correlation of local recurrence to the treated volumes, using the COM approach. Three of 5 LF were in-field LF within the PTV-boost and 2 were marginal Fig. 3 illustrates the correlation of the occurrence of 4 late toxicity items with the dose in the corresponding OAR in patients treated within the ADMIRE study. In all these items the dose in the corresponding OAR in patients with any of these toxicities was higher than those without toxicity and higher than the median dose of the whole group. The median Dmean of oral cavity in patients with, compared to those without, mucosal ulceration were 40.8 Gy and 24.1 Gy, respectively (p = 0.02).
Table 3.
Different characteristics of patients with lf (n = 5).
| LF1 | LF2 | LF3 | LF4 | LF5 | |
|---|---|---|---|---|---|
| Age | 64 | 61 | 61 | 75 | 77 |
| Site primary tumor | larynx | oropharynx | larynx | larynx | larynx |
| T-stage | 3 | 4 | 4 | 3 | 4 |
| AJCC stage | 4 | 4 | 4 | 3 | 4 |
| Continued smoking during RT | 0 | 1 | 1 | 0 | 0 |
| Dose of RT | 78 | 74 | 74 | 78 | 78 |
| Time to LF from end RT in months | 4 | 14 | 4,5 | 7 | 15,5 |
| Site of LF in relation to CTVb | marginal | marginal | in-field | in-field | out-field |
| Site of LF in relation to PTVb | in-field | marginal | in-field | in-field | marginal |
Abbreviations: LF: local failure; RT; radiotherapy; AJCC: American Joint Committee on Cancer; CTVb: clinical target volume boost; PTVb: planning target volume boost.
Fig. 2.
Center of mass (COM) approach for correlation of the site of local recurrence (LR) to the original treated volumes: CT scans with a LR (red colour). After co-registration, the original CTVb (orange) and original PTVb (green) were projected onto the recurrence CT scan. As the co-registration inaccuracy was 3 mm, a circle (blue) was created around the COM (blue dot) with a radius of 3 mm. The COM might be anywhere within the blue circle. Left panel A: the recurrence in this patient was considered as marginal CTVb and in-field PTVb. Right panel B: the recurrence in this patients was considered in-field for both CTVb and PTVb.
Fig. 3.
The correlation of the occurrence of 4 late toxicity items with the dose in the corresponding OAR in patients treated within the ADMIRE study. The dose in the corresponding OAR in patients with any of these toxicity (large dots) was higher than those without toxicity (small dots) and higher than the median dose of the whole group (the horizontal line).
Discussion
This prospective study aims to report on late toxicity and LC rate of patients treated within the ADMIRE study of adaptive FDG-PET/CT response-guided radiotherapy with DE and to compare these results with a control group of patients treated in our institution. Comparing the ADMIRE group with the control group using SIPTW shows that adjusted HR for LC was 0.8 (p = 0.70) and adjusted OR for any late grade 3 toxicity was 5.09 (p = 0.005), which might mean that FDG PET/CT-guided DE to the primary tumor results in comparable LC rates but at the cost of higher odds of suffering from late toxicity in the ADMIRE group. However, these conclusions should be interpreted with caution, given the widths of the CIs and the small number of LFs and toxicity events in the ADMIRE group and reinforce the need for larger, preferably randomized, trials to investigate the benefit of DE in HNSCC patients with high-risk profiles at need of treatment intensification.
Three other prospective PET/CT-based DE studies were published. The strategy in the RCT of the Welz et al. [6] was FMISO-based, where non-hypoxic tumors (n = 14) received 70 Gy and hypoxic tumors were randomized to 70 Gy (n = 20) or escalated dose (ED) (77 Gy/35 fractions) (n = 19). The 5-year LC-rates for non-hypoxic and hypoxic tumor were 100% and 74%, respectively (p = 0.03). The LC-rates for hypoxic tumor treated with ED and SD were 84% and 59%, respectively (p = 0.15). No significant differences in toxicity were seen between groups. They concluded that 10% DE is feasible without substantial risk of increased toxicity. Because of the premature closure of the study and the small sample size the statistically significant improvements in LC could not be reached. The Ghent group conducted a prospective study in 71 patients treated with FDG-PET-based DE (70.2–85.9 Gy/30–32 fractions) [7], [8], [9]. The 5-year LC-rate was 82%, compared to 73.6% in the control group treated with 70 Gy. The study showed a significant increase in acute and late dysphagia (p = 0.004 and 0.005, respectively) and late G4 mucosal ulceration was higher than in the control group (12.5% vs. 4.1%, p = 0.11). The Danish group [10] reported on FDG-PET-based DE trial where the GTV-PET was boosted to 79.7 Gy in 34 fractions in 15 patients. After 18 months of follow-up, one LF was observed. Mucosal ulcers were reported in 4 patients (27%), two of which were severe and persistent. Because of these 2 severe late toxicity events, the group decided to refrain from further DE in future studies.
Given the differences in inclusion criteria, the ART strategy and the radiation dose scheme used, the toxicity score reporting and all other differences regarding the radiotherapy workflow of these different institutions, it is quite difficult to compare the results of these 4 prospective DE trials (Supplementary Table 1). Nevertheless, some common concerns and suggestions for improvements can be noticed. First, in all of these studies concerns were raised about late toxicities like persistent mucosal ulceration, persistent need for tracheostomy or tube feeding or possible increase in the incidence of esophageal necrosis and/or osteoradionecrosis. Because of these concerns the selection of patients for future DE trials will need to be refined to include patients with relatively high risk of LF and low risk of severe late toxicity such as patients with HPV-negative locally-advanced HNSCC who are not suitable for concomitant cisplatin and are willing to stop smoking before treatment. Because it is quite difficult to identify patients at high risk of severe late toxicity, upfront treatment planning comparison could be made for standard and escalated dose to identify patients at increasing risk of toxicity using NTCP models for different toxicity items and to subsequently exclude these patients from DE trials [11], [12], [13]. A similar validated selection tool is used in the Netherlands for selecting HNSCC patients for proton therapy, the model-based selection tool [14]. Because of the frequently raised concerns about the duration and severity of mucosal ulceration, DE might preferably not be offered to patients with Dmean oral cavity > 26 Gy, as these patients might have a higher chance of developing persistent mucosal ulceration, or at least thoroughly discuss the risk of toxicity with these patients before accrual (Fig. 3). On the other hand, Bordin et al. found that Dmean oral cavity < 41.8 Gy was associated with lower risk of, especially, acute oral mucositis, compared to Dmean > 58.8 Gy [15].
The hypothesis that DE of 10–25% to the hypoxic (sub)volumes of the tumor would result in 10–20% better LC-rates could not be confirmed in different prospective studies, most probably because of the small sample size in these studies [6], [7], [8], [9], [10]. Welz et al. showed that there is a 25% improvement of LC-rate by escalating the dose with 10% to hypoxic sub-volumes, supporting the concept of FMISO-PET-based DE strategy [6], [16]. Berwouts et al. showed that DE up to 24% by DPBN resulted in an absolute benefit in LC of 8.7% at 5-years [8]. The results of two randomized trials from Belgium and the Netherlands (NCT01341535 and NCT01504815) need to be awaited. In these studies FDG-PET-based DE and dose-redistribution was compared to conventional non-adaptive IMRT [17], [18].
There is growing evidence that different PET parameters might be useful imaging biomarkers for hypoxia and for response prediction during the treatment of patients with HNSCC [19], [20]. Recent studies have shown that changes in different FDG-PET parameters might discriminate poor from good responders. Vos et al [21] showed that all HNSCC patients who were treated with neo-adjuvant immune therapy and subsequently developed pCR at the time of resection had at least 12.5% reduction of TLG and SUVpeak 4 weeks after treatment. None of the patients with ΔTLG of at least −12.5% developed LF after resection. This cut-off point might identify good responders with 95% accuracy. Whether these results could be directly extrapolated to HNSCC treated by radiotherapy is unknown. Nevertheless, in our study the same trend was seen, as the ΔSUV peak in patients without and with LF was reduced by a median of −50% and −26%, respectively and ΔTLG was decreased by a median of −54% in patients without LF, compared to increase of ΔTLG by a median of + 17% in patients who developed LF. Allen et al. [22] showed that a reduction of mid-treatment MTV by ≥ 50% from baseline is a possible cut-off point to de-escalate the radiation dose to 54 Gy in HPV-positive patients. In these patients acute toxicity was significantly reduced, compared to 70 Gy. However, the results of oncologic outcomes need to be awaited before adopting these parameters as biomarkers for the response evaluation in HNSCC.
The current study has a couple of limitations. The impact of this adaptive FDG-PET/CT response-guided radiotherapy on treatment efficacy cannot be efficiently evaluated because of the small sample size and the limited number of events in the study population. Furthermore, the SIPTW analyses performed to minimize the effect of confounding could not eliminate all biases related to matched case-control comparison. Future randomized trials with sample size powered for differences in LC-rates will overcome these limitations.
In conclusion, radiotherapy for HNSCC with FDG-PET/CT-based dose escalation to the primary tumor leads to comparable local control rates but at the cost of increased late toxicity. For future PET-based DE trials, selection criteria will need to be refined to include only patients at higher risk of local failure and/or lower risk of severe late toxicity.
Authors contribution
Abrahim Al-Mamgani : study set-up, check-up data control group, analysis site of recurrences, manuscript writing.
Rob Kessels : statistical analysis, review manuscript.
Zeno A.R. Gouw : study set-up, analysis site of recurrences, manuscript writing, review manuscript.
Arash Navran : check-up data control group, analysis site of recurrences, review manuscript.
Vineet Mohan : analysis site of recurrences, review manuscript.
Jeroen B. van de Kamer : analysis of PET outcomes, review manuscript.
Jan-Jakob Sonke : study set-up, review manuscript.
Wouter V. Vogel : study set-up, analysis of PET outcomes, review manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2023.100676.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Haddad R.I., Shin D.M. Recent advances in head and neck cancer. N Eng J Med. 2008;359:1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 2.Pignon J.P., le Maître A., Maillard E., Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Bonner J.A., Harari P.M., Giralt J., Cohen R.B., Jones C.U., Sur R.K., et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 4.Lacas B., Bourhis J., Overgaard J., Zhang Q., Grégoire V., Nankivell M., et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol. 2017;18(9):1221–1237. doi: 10.1016/S1470-2045(17)30458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gouw Z.A.R., La Fontaine M.D., Vogel W.V., van de Kamer J.B., Sonke J.J., Al-Mamgani A. Single-Center Prospective Trial Investigating the Feasibility of Serial FDG-PET Guided Adaptive Radiation Therapy for Head and Neck Cancer. Int J Radiat Oncol Biol Phys. 2020 Nov 15;108(4):960–968. doi: 10.1016/j.ijrobp.2020.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Welz S., Paulsen F., Pfannenberg C., Reimold M., Reischl G., Nikolaou K., et al. Dose escalation to hypoxic subvolumes in head and neck cancer: A randomized phase II study using dynamic [18F]FMISO PET/CT. Radiother Oncol. 2022;171:30–36. doi: 10.1016/j.radonc.2022.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Duprez F., De Neve W., De Gersem W., Coghe M., Madani I. Adaptive Dose Painting by Numbers for Head-and-Neck Cancer. Int J Radiat Oncol Biol Phys. 2011;80(4):1045–1055. doi: 10.1016/j.ijrobp.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Berwouts D., Madani I., Duprez F., Olteanu A.L., Vercauteren T., Boterberg T., et al. Long-term outcome of 18 F-fluorodeoxyglucose-positron emission tomography-guided dose painting for head and neck cancer: Matched case-control study. Head Neck. 2017 Nov;39(11):2264–2275. doi: 10.1002/hed.24892. [DOI] [PubMed] [Google Scholar]
- 9.Berwouts D., Olteanu L.A.M., Duprez F., Vercauteren T., De Gersem W., De Neve W., et al. Three-phase adaptive dose-painting-by-numbers for head-and-neck cancer: initial results of the phase I clinical trial. Radiother Oncol. 2013;107(3):310–316. doi: 10.1016/j.radonc.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen J.H., Håkansson K., Vogelius I.R., Aznar M.C., Fischer B.M., Friborg J., et al. Phase I trial of 18F-Fludeoxyglucose based radiation dose painting with concomitant cisplatin in head and neck cancer. Radiother Oncol. 2016;120(1):76–80. doi: 10.1016/j.radonc.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Beetz I., Schilstra C., van der Schaaf A., van den Heuvel E.R., Doornaert P., van Luijk P., et al. NTCP models for patient-rated xerostomia and sticky saliva after treatment with intensity modulated radiotherapy for head and neck cancer: The role of dosimetric and clinical factors. Radiother Oncol. 2012;105(1):101–106. doi: 10.1016/j.radonc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Christianen M.E.M.C., Schilstra C., Beetz I., Muijs C.T., Chouvalova O., Burlage F.R., et al. Predictive modelling for swallowing dysfunction after primary (chemo) radiation: Results of a prospective observational study. Radiother Oncol. 2012;105(1):107–114. doi: 10.1016/j.radonc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Rancati T., Fiorino C., Sanguineti G. NTCP modeling of subacute/late laryngeal edema scored by fiberoptic examination. Int J Radiat Oncol Biol Phys. 2009;75:915–923. doi: 10.1016/j.ijrobp.2009.04.087. [DOI] [PubMed] [Google Scholar]
- 14.Langendijk J.A., Lambin P., De Ruysscher D., Widder J., Bos M., Verheij M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: The model-based approach. Radiother Oncol. 2013;107:267–273. doi: 10.1016/j.radonc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Brodin N.P., Tomé W.A. Revisiting the dose constraints for head and neck OARs in the current era of IMRT. Oral Oncol. 2018 Nov;86:8–18. doi: 10.1016/j.oraloncology.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zips D., Zöphel K., Abolmaali N., Perrin R., Abramyuk A., Haase R., et al. Exploratory prospective trial of hypoxia-specific PET imaging during radiochemotherapy in patients with locally advanced head-and-neck cancer. Radiother Oncol. 2012;105(1):21–28. doi: 10.1016/j.radonc.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Heukelom J., Hamming O., Bartelink H., Hoebers F., Giralt J., Herlestam T., et al. Adaptive and innovative Radiation Treatment FOR improving Cancer treatment outcomE (ARTFORCE); a randomized controlled phase II trial for individualized treatment of head and neck cancer. BMC Cancer. 2013;13(1) doi: 10.1186/1471-2407-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.clinicaltrials.gov identifier NCT01341535.
- 19.Löck S., Perrin R., Seidlitz A., Bandurska-Luque A., Zschaeck S., Zöphel K., et al. Residual tumour hypoxia in head-and-neck cancer patients undergoing primary radiochemotherapy, final results of a prospective trial on repeat FMISO-PET imaging. Radiother Oncol. 2017;124(3):533–540. doi: 10.1016/j.radonc.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Riaz N., Sherman E., Pei X., Schöder H., Grkovski M., Paudyal R., et al. Precision Radiotherapy: Reduction in Radiation for Oropharyngeal Cancer in the 30 ROC Trial. J Natl Cancer Inst. 2021;113(6):742–751. doi: 10.1093/jnci/djaa184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vos J.L., Zuur C.L., Smit L.A., de Boer J.P., Al-Mamgani A., van den Brekel M.W.M., et al. [18F]FDG-PET accurately identifies pathological response early upon neoadjuvant immune checkpoint blockade in head and neck squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2022;49(6):2010–2022. doi: 10.1007/s00259-021-05610-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen S.G., Rosen B.S., Aryal M., Cao Y., Schipper M.J., Wong K.K., et al. Initial Feasibility and Acute Toxicity Outcomes from a Phase II trial of FDG-PET response-based de-escalated definitive chemoradiotherapy for p16+ oropharynx cancer, a Planned Interim Analysis. Int J Radiat Oncol Biol Phys. 2023 Mar 15;S0360–3016(23):00284–00285. doi: 10.1016/j.ijrobp.2023.03.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.