Summary
Background
Molnupiravir and nirmatrelvir-ritonavir have emerged as promising options for COVID-19 treatment, but direct comparisons of their effectiveness have been limited. This study aimed to compare the effectiveness of these two oral antiviral drugs in non-hospitalised and hospitalised patients with COVID-19.
Methods
In this target trial emulation study, we used data from a territory-wide electronic health records database on eligible patients aged ≥18 years infected with COVID-19 who were prescribed either molnupiravir or nirmatrelvir-ritonavir within five days of infection between 16 March 2022 and 31 December 2022 in the non-hospitalised and hospitalised settings in Hong Kong. A sequence trial approach and 1:1 propensity score matching was applied based on age, sex, number of COVID-19 vaccine doses received, Charlson comorbidity index, comorbidities, and drug use within past 90 days. Cox regression adjusted with patients’ characteristics was used to compare the risk of effectiveness outcomes (all-cause mortality, intensive care unit (ICU) admission or ventilatory support and hospitalisation) between groups. Subgroup analyses included age (<70; ≥70 years); sex, Charlson comorbidity index (<4; ≥4), and number of COVID-19 vaccine doses received (0–1; ≥2 doses).
Findings
A total of 63,522 non-hospitalised (nirmatrelvir-ritonavir: 31,761; molnupiravir: 31,761) and 11,784 hospitalised (nirmatrelvir-ritonavir: 5892; molnupiravir: 5892) patients were included. In non-hospitalised setting, 336 events of all-cause mortality (nirmatrelvir-ritonavir: 71, 0.22%; molnupiravir: 265, 0.83%), 162 events of ICU admission or ventilatory support (nirmatrelvir-ritonavir: 71, 0.22%; molnupiravir: 91, 0.29%), and 4890 events of hospitalisation (nirmatrelvir-ritonavir: 1853, 5.83%; molnupiravir: 3037, 9.56%) were observed. Lower risks of all-cause mortality (absolute risk reduction (ARR) at 28 days: 0.61%, 95% CI: 0.50–0.72; HR: 0.43, 95% CI: 0.33–0.56) and hospital admission (ARR at 28 days: 3.73%, 95% CI: 3.31–4.14; HR: 0.72, 95% CI: 0.67–0.76) were observed in nirmatrelvir-ritonavir users compared to molnupiravir users. In hospitalised setting, 509 events of all-cause mortality (nirmatrelvir-ritonavir: 176, 2.99%; molnupiravir: 333, 5.65%), and 50 events of ICU admission or ventilatory support (nirmatrelvir-ritonavir: 26, 0.44%; molnupiravir: 24, 0.41%) were observed. Risk of all-cause mortality was lower for nirmatrelvir-ritonavir users than for molnupiravir users (ARR at 28 days: 2.66%, 95% CI: 1.93–3.40; HR: 0.59, 95% CI: 0.49–0.71). In both settings, there was no difference in the risk of intensive care unit admission or ventilatory support between groups. The findings were consistent across all subgroup’s analyses.
Interpretation
Our analyses suggest that nirmatrelvir-ritonavir was more effective than molnupiravir in reducing the risk of all-cause mortality in both non-hospitalised and hospitalised patients. When neither drug is contraindicated, nirmatrelvir-ritonavir may be considered the more effective option.
Funding
HMRF Research on COVID-19, The Hong Kong Special Administrative Region (HKSAR) Government; Collaborative Research Fund, University Grants Committee, the HKSAR Government; and Research Grant from the Food and Health Bureau, the HKSAR Government; the Laboratory of Data Discovery for Health (D24H) funded by the AIR@InnoHK administered by Innovation and Technology Commission.
Keywords: SARS-CoV-2, Viral disease, Molnupiravir, Nirmatrelvir-ritonavir
Research in context.
Evidence before this study
Two oral antivirals, nirmatrelvir-ritonavir and molnupiravir, have been applied as the treatments for patients with COVID-19 to reduce the risks of hospitalisation and death. We searched Scopus and PubMed for studies published before 12, June 2023, with search terms (“SARS-CoV-2” OR “COVID-19”) AND ((“molnupiravir” OR “Lagevrio” OR “EIDD-2801”) OR (“nirmatrelvir” OR “Paxlovid” OR “PF-07321332”)). The majority of current evidence including the randomised control trials and observational studies reported the effectiveness of nirmatrelvir-ritonavir and molnupiravir treatments, respectively, compared to non-users. Additional studies directly comparing the efficacy of nirmatrelvir-ritonavir and molnupiravir in both non-hospitalised and hospitalised patients with COVID-19 are needed to inform clinical use of these oral antivirals.
Added value of this study
This is one of the first real-world studies conducting direct comparison on the effectiveness of nirmatrelvir-ritonavir and molnupiravir in both non-hospitalised and hospitalised patients with COVID-19. Nirmatrelvir-ritonavir was associated with significant reduced risk of all-cause mortality at 28 days among both non-hospitalised and hospitalised patients. For non-hospitalised patients, nirmatrelvir-ritonavir also associated significantly with decreased risk of hospitalisation.
Implications of all the available evidence
Several clinical guidelines prioritise the use of nirmatrelvir-ritonavir over molnupiravir for patients without contraindications to either treatment. Our study, which utilised a large real-world population, directly compared the clinical outcomes of nirmatrelvir-ritonavir and molnupiravir in both non-hospitalised and hospitalised patients, and revealed a greater clinical benefit with nirmatrelvir-ritonavir treatment. However, further research is needed to confirm the effectiveness of nirmatrelvir-ritonavir relative to molnupiravir.
Introduction
The ongoing spread of COVID-19 has highlighted the need for effective antiviral treatments. Two oral antiviral drugs, molnupiravir (Lagevrio) and nirmatrelvir-ritonavir (Paxlovid) have shown promise as novel treatment options for adults with COVID-19 from phase III randomised controlled trials, namely the Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial and the Molnupiravir for Oral Treatment of COVID-19 in an Outpatient Setting (MOVe-OUT) trial.1,2 There is limited evidence directly comparing the effectiveness of these two antiviral treatments. Nevertheless, several clinical guidelines, including National Institutes of Health from US and National Health and Medical Research Council in Australia, recommend nirmatrelvir–ritonavir over molnupiravir, provided that patients do not have any contraindications to either drugs.3,4 Consequently, understanding their relative effectiveness profiles is crucial for informing public health policy and guiding clinical practice.
The evidence on direct comparison of the efficacy of these two antiviral treatments are limited. The results from EPIC-HR trial and MOVe-OUT trial revealed 89% and 30% relative risk reduction in hospitalisation and mortality for nirmatrelvir-ritonavir and molnupiravir treatments, respectively, compared to non-users among unvaccinated patients.1,2 This indirect comparison has limitations due to differences in study design and patient populations, which may affect the validity of the conclusion. Meanwhile, only one recent observational study conducted in US Veterans population concluded no difference in hospitalisation and mortality between 1750 patients in each nirmatrelvir-ritonavir and molnupiravir treatment group.5 However, their population has a much higher proportion of males and prevalence of mental and physical health conditions compared to the general population, which may not be fully representative. Moreover, the study population consisted of a relatively small sample size of non-hospitalised patients with COVID-19 and, as a result, a limited number of events. This may contribute to insufficient statistical power for identifying differences between the groups. A head-to-head comparison of the effectiveness of these two antiviral treatments in the inpatient setting is currently lacking. Given that antivirals are typically prioritised for high-risk patients and previous studies, including our own, have demonstrated potential advantages in using nirmatrelvir-ritonavir and molnupiravir for hospitalised patients with COVID-19,6,7 it is of paramount importance to validate the existing evidence and assess the comparative benefits in hospital setting using a larger sample size.
In Hong Kong, the Department of Health (DH) has approved the use of nirmatrelvir-ritonavir and molnupiravir in patients with COVID-19.8,9 Since February 2022, amidst a large local Omicron-dominant outbreak,10 the government has been distributing these two drugs free of charge to individual COVID patients aged over 60 or at high risk of medical illness in both non-hospitalised and hospitalised settings. Using a target trial emulation design, the aim of this study was to compare the effectiveness of molnupiravir and nirmatrelvir-ritonavir in both non-hospitalised and hospitalised patients with COVID-19 based on large real-world population.
Methods
Data sources
We acquired clinical data from the Hospital Authority's (HA) routine electronic health records database, vaccination records and confirmed COVID-19 case records from the Department of Health (DH) of the Government of the Hong Kong Special Administrative Region (HKSAR). To integrate these databases, we used anonymised unique patient identifiers. The HA, a statutory administrative organisation in Hong Kong, manages all public inpatient services and most public outpatient services. The HA's electronic health records database contains information on patient demographics, diagnoses, prescriptions, and laboratory tests, providing real-time data to support clinical management across all clinics and hospitals within the HA. The DH maintains a comprehensive database of vaccination records for all individuals in Hong Kong, and keeps a database documenting every confirmed COVID-19 case, based on both mandatory and voluntary reporting of positive Polymerase Chain Reaction (PCR) and Rapid Antigen Test (RAT) results. Death records were extracted from the Hong Kong Deaths Registry, a government agency under the HKSAR government responsible for maintaining records of all registered deaths for all residents in Hong Kong. These population-based databases have been widely utilised in previous studies evaluating the effectiveness of COVID-19 drugs and vaccinations.11, 12, 13, 14, 15, 16, 17, 18, 19 This study was approved by the Central Institutional Review Board of the Hospital Authority of Hong Kong (CIRB-2021-005-4) and the DH Ethics Committee (LM171/2021). Anonymous data were extracted, and written informed consent has been waived by the ethics committee for de-identified electronic health records.
Study design and eligibility criteria
This study was a target trial emulation conducted using territory-wide electronic health records databases in Hong Kong. The use of target trial emulation helps mitigate some of the typical challenges in observational study designs such as immortal time and selection biases.20, 21, 22 The specification and emulation of the target trial is detailed in Supplementary Table S1. The inclusion period commenced from 16 March 2022 (when both molnupiravir and nirmatrelvir-ritonavir became available in Hong Kong) to 31 December 2022 (to allow 28 days of follow-up). Patients aged ≥18 years who had a COVID-19 infection (documented as a PCR/RAT positive result confirmed by DH) and received COVID-19 oral antivirals (molnupiravir or nirmatrelvir-ritonavir) within five days were eligible. The index date was defined as the date of molnupiravir or nirmatrelvir-ritonavir prescription. Exclusion criteria include: (i) patients who had a history of COVID-19 infection before index date and (ii) patients with contraindications to nirmatrelvir-ritonavir or molnupiravir,23,24 including severe liver impairment (cirrhosis, hepatocellular carcinoma, or liver transplant), chronic kidney disease, and use of interacting drugs (i.e., amiodarone, apalutamide, rifampicin, rifapentine, carbamazepine, primidone, phenobarbital, or phenytoin, direct oral anticoagulants) within 90 days before index date. For the analysis of each of each outcome, patients who had a history of the outcome before index date were also excluded.
Sequence trial emulation
Two emulated target trials of the same design were conducted separately for non-hospitalised and hospitalised patients with COVID-19 respectively, where non-hospitalised patients referred to those not hospitalised on or before index date, and hospitalised patients were defined as those admitted to hospital within five days before or on the index date. A sequence trial approach was adopted to compare the risk of outcomes between patients who received nirmatrelvir-ritonavir and patients who received molnupiravir.25,26 All eligible non-hospitalised patients newly prescribed molnupiravir were matched 1:1 to eligible non-hospitalised patients newly prescribed nirmatrelvir-ritonavir on each day during the inclusion period. For eligible hospitalised patients, matching was performed between molnupiravir and nirmatrelvir-ritonavir users on each week during the inclusion period. Propensity score matching was used with caliper of 0.2 to emulate randomisation of treatment assignment. Propensity scores were estimated using logistic regression to predict the probability of treatment assignment given the following baseline covariates: age, sex, number of COVID-19 vaccine doses received, Charlson comorbidity index (CCI), comorbidities (cancer, respiratory disease, diabetes, myocardial infarction, cerebrovascular disease, hypertension), and drug use (renin-angiotensin-system agents, beta blockers, calcium channel blockers, diuretics, nitrates, lipid lowering agents, insulin, oral antidiabetic drugs, antiplatelets, immuno-suppressants, corticosteroids, proton pump inhibitors, histamine H2 receptor antagonists, tocilizumab, baricitinib, remdesivir and interferon beta-1b) within past 90 days. These covariates were selected since they were potential confounders of COVID-19 oral antiviral treatments and mortality. Individuals were followed up from the index date till the earliest outcome occurrence, death, 28 days after index date or the end of data availability (31 January 2023).
Outcomes
Effectiveness outcomes included (i) 28-day all-cause mortality; (ii) intensive care unit (ICU) admission or ventilatory support within 28 days; and (iii) hospitalisation within 28 days (for community setting only). Use of ventilatory support was identified using ICD-9 procedure codes (39.65, 89.18, 93.90, 93.95, 93.96, 96.7, 96.04).
Statistical analysis
Covariate balance in the matched cohort was assessed where a standardised mean difference (SMD) between groups of 0.1 or less for all covariates was considered acceptable.27 Incidence rates were reported with 95% confidence intervals estimated based on Poisson distribution. Cox proportional hazards regression adjusted with baseline covariates, which were the same as those used in logistic regression in propensity score matching, were used to compare the risk of outcomes between molnupiravir recipients and nirmatrelvir-ritonavir recipients. Hazard ratios with 95% confidence intervals were reported. Absolute risk reduction (ARR) was reported as the difference in rate of events for nirmatrelvir-ritonavir recipients as compared with molnupiravir recipients.
Pre-specified subgroup analyses stratified by vaccination status (0–1, ≥2 vaccine doses received), age (<70, ≥70 years), sex (male, female), and CCI (0–3, ≥4) were carried out. Interaction effects between treatment and vaccination status, age (continuous variable), sex and CCI (continuous variable) were also tested and the P-values for interaction were reported.
Three sensitivity analyses were performed to evaluate the robustness of the findings from the main analysis. First, risk of outcomes among patients who received COVID-19 drug treatments within three days instead of five days were performed. Second, due to the slight imbalance in baseline history of myocardial infection between groups among hospitalised patients (SMD 0.130), the patients with baseline history of myocardial infection were excluded. Third, E-value was computed to assess the robustness of conclusions to potential unmeasured confounding. E-value is defined as RR + , in which RR refers to risk ratio between treatment and outcome (reciprocal of RR will be taken for RR <1, and RR is interchangeable with HR for rate of event <15% by the end of follow-up). It is a measurement of the minimal strength required for a confounder to be associated with both treatment and outcome to fully explain away the observed association between treatment and outcome.28
All statistical tests were two-sided, with P values below 0.05 deemed statistically significant. Statistical analyses were performed using R version 4.0.3 (www.R-project.org). For quality assurance purposes, two researchers (VY, ZW) carried out the statistical analyses independently. To ensure transparent reporting of the cohort study, the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement checklist was followed.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and took final responsibility for the decision to submit for publication.
Results
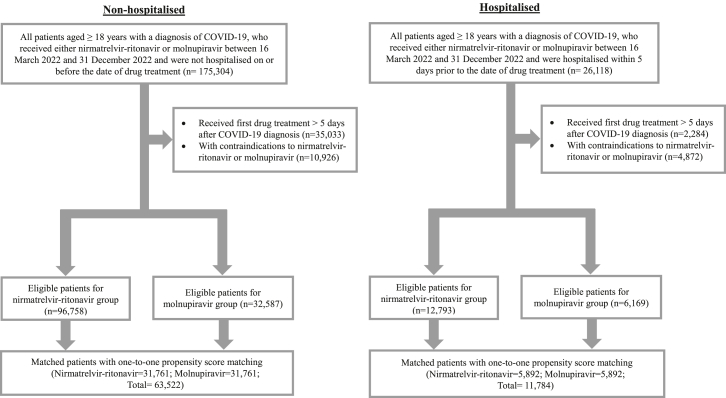
After applying the eligibility criteria and matching, 63,522 non-hospitalised (nirmatrelvir-ritonavir: 31,761; molnupiravir: 31,761) and 11,784 hospitalised (nirmatrelvir-ritonavir: 5892; molnupiravir: 5892) patients were included (Fig. 1). Among the non-hospitalised patients, the mean (SD) age and proportion of male were 69.77 (12.8) years and 48.0% for nirmatrelvir-ritonavir recipients, and 70.87 (14.1) years and 47.9% for molnupiravir recipients (Table 1). The majority of both treatment groups were previously vaccinated with BNT161b2 or CoronaVac [≥3 doses: 79.0% (nirmatrelvir-ritonavir) vs 77.4% (molnupiravir); ≥2 doses: 91.3% (nirmatrelvir-ritonavir) vs 89.4% (molnupiravir)]. All baseline characteristics were well-balanced between the two treatment groups with SMD <0.1 (Table 1). Among hospitalised patients, the mean (SD) age and proportion of male were 74.92 (14.6) years and 50.0% for nirmatrelvir-ritonavir recipients, and 75.77 (15.8) years and 50.1% for molnupiravir recipients (Table 1). Most patients from both treatment groups were vaccinated [≥3 doses: 63.8% (nirmatrelvir-ritonavir) vs 63.1% (molnupiravir); ≥2 doses: 80.5% (nirmatrelvir-ritonavir) vs 79.1% (molnupiravir)]. All baseline characteristics were also well-balanced between the two treatment groups with SMD <0.1, except for pre-existing myocardial infarction (SMD 0.130) (Table 1). The baseline characteristics of eligible patients before matching was also displayed in Supplementary Table S2.
Fig. 1.
Study flow diagram. Number of individuals included and excluded from the study based on the inclusion and exclusion criteria, and performance of 1:1 propensity score matching was illustrated for non-hospitalised and hospitalised groups in the study flow diagram. Notes: The patients were matched by gender, age, Charlson Comorbidity Index, vaccination status, pre-existing comorbidities and medication use within 90 days at baseline.
Table 1.
Baseline characteristics of COVID-19 patients after one-to-one propensity score matching.
| Characteristics | Non-hospitalised (N = 63,522) |
Hospitalised (N = 11,784) |
||||
|---|---|---|---|---|---|---|
| Nirmatrelvir-ritonavir (N = 31,761) | Molnupiravir (N = 31,761) | SMDa | Nirmatrelvir-ritonavir (N = 5892) | Molnupiravir (N = 5892) | SMDa | |
| Age, year–mean (SD) | 69.77 (12.8) | 70.87 (14.1) | 0.081 | 74.92 (14.6) | 75.77 (15.8) | 0.056 |
| Sex, Male (%) | 15,250 (48.0) | 15,216 (47.9) | 0.002 | 2948 (50.0) | 2951 (50.1) | 0.001 |
| Charlson Comorbidity Index–mean (SD) | 3.19 (1.7) | 3.36 (1.8) | 0.099 | 4.08 (2.1) | 4.24 (2.2) | 0.071 |
| COVID-19 vaccination (%) | 0.072 | 0.071 | ||||
| Unvaccinated | 1885 (5.9) | 2176 (6.9) | 867 (14.7) | 856 (14.5) | ||
| 1 dose | 859 (2.7) | 1191 (3.7) | 280 (4.8) | 374 (6.3) | ||
| 2 doses | 3916 (12.3) | 3801 (12.0) | 984 (16.7) | 942 (16.0) | ||
| ≥3 doses | 25,101 (79.0) | 24,593 (77.4) | 3761 (63.8) | 3720 (63.1) | ||
| Pre-existing comorbidities (%) | ||||||
| Cancer | 2074 (6.5) | 2171 (6.8) | 0.012 | 591 (10.0) | 567 (9.6) | 0.014 |
| Respiratory disease | 1304 (4.1) | 1582 (5.0) | 0.042 | 506 (8.6) | 512 (8.7) | 0.004 |
| Diabetes | 9038 (28.5) | 9127 (28.7) | 0.006 | 1854 (31.5) | 1929 (32.7) | 0.027 |
| Myocardial infarction | 410 (1.3) | 755 (2.4) | 0.081 | 225 (3.8) | 377 (6.4) | 0.117 |
| Cerebrovascular disease | 2931 (9.2) | 3500 (11.0) | 0.059 | 1067 (18.1) | 1208 (20.5) | 0.061 |
| Hypertension | 16,133 (50.8) | 16,273 (51.2) | 0.009 | 3192 (54.2) | 3241 (55.0) | 0.017 |
| Medication use within 90 days (%) | ||||||
| Renin-angiotensin-system agents | 10,194 (32.1) | 10,679 (33.6) | 0.033 | 1952 (33.1) | 2062 (35.0) | 0.039 |
| Beta blockers | 6695 (21.1) | 7224 (22.7) | 0.040 | 1371 (23.3) | 1511 (25.6) | 0.055 |
| Calcium channel blockers | 13,552 (42.7) | 14,063 (44.3) | 0.032 | 2614 (44.4) | 2711 (46.0) | 0.033 |
| Diuretics | 1996 (6.3) | 2630 (8.3) | 0.077 | 728 (12.4) | 883 (15.0) | 0.077 |
| Nitrates | 2129 (6.7) | 2727 (8.6) | 0.071 | 605 (10.3) | 716 (12.2) | 0.060 |
| Lipid lowering agents | 16,212 (51.0) | 16,733 (52.7) | 0.033 | 2855 (48.5) | 3034 (51.5) | 0.061 |
| Insulins | 1120 (3.5) | 1434 (4.5) | 0.050 | 446 (7.6) | 534 (9.1) | 0.054 |
| Antidiabetic drugs | 7710 (24.3) | 7862 (24.8) | 0.011 | 1527 (25.9) | 1589 (27.0) | 0.024 |
| Antiplatelets | 7928 (25.0) | 9091 (28.6) | 0.083 | 2030 (34.5) | 2291 (38.9) | 0.092 |
| Immuno-suppressants | 326 (1.0) | 650 (2.0) | 0.083 | 99 (1.7) | 174 (3.0) | 0.085 |
| Corticosteroids | 643 (2.0) | 1117 (3.5) | 0.091 | 379 (6.4) | 430 (7.3) | 0.034 |
| Proton pump inhibitors | 7249 (22.8) | 8561 (27.0) | 0.096 | 2124 (36.0) | 2347 (39.8) | 0.078 |
| Histamine H2 receptor antagonists | 7306 (23.0) | 7463 (23.5) | 0.012 | 1452 (24.6) | 1493 (25.3) | 0.016 |
| Tocilizumab | 0 (0.0) | 0 (0.0) | <0.001 | 0 (0.0) | 1 (0.0) | 0.018 |
| Baricitinib | 0 (0.0) | 0 (0.0) | <0.001 | 3 (0.1) | 1 (0.0) | 0.018 |
| Remdesivir | 0 (0.0) | 0 (0.0) | <0.001 | 34 (0.6) | 35 (0.6) | 0.002 |
| Interferon beta-1b | 0 (0.0) | 0 (0.0) | <0.001 | 2 (0.0) | 2 (0.0) | <0.001 |
SMD = standardised mean difference; SD = standard deviation.
SMD <0.1 indicates balance between groups.
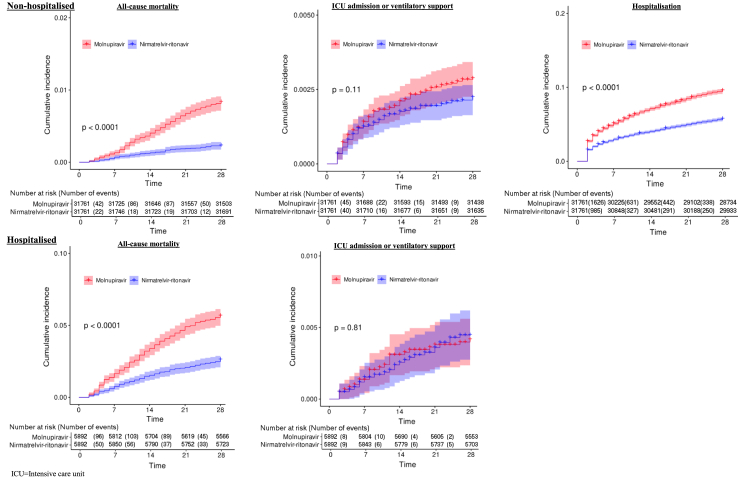
The 28-day cumulative incidence of outcomes between groups were shown in Fig. 2. Both non-hospitalised and hospitalised groups have been followed up for a median of 28 days (Interquartile range (IQR) 0 day). In non-hospitalised setting, 336 events of all-cause mortality (nirmatrelvir-ritonavir: 71, 0.22%; molnupiravir: 265, 0.83%), 162 events of ICU admission or ventilatory support (nirmatrelvir-ritonavir: 71, 0.22%; molnupiravir: 91, 0.29%), and 4890 events of hospitalisation (nirmatrelvir-ritonavir: 1853, 5.83%; molnupiravir: 3037, 9.56%) were observed. Among these non-hospitalised individuals, nirmatrelvir-ritonavir users had lower rates of mortality (Absolute risk reduction (ARR) at 28 days 0.61%, 95% CI: 0.50–0.72) and hospitalisation (ARR at 28 days 3.73%, 95% CI: 3.31–4.14), and had similar rate of ICU admission or ventilatory support (ARR at 28 days 0.07%, 95% CI: −0.02 to 0.14) compared to molnupiravir users (Table 2). In hospitalised setting, 509 events of all-cause mortality (nirmatrelvir-ritonavir: 176, 2.99%; molnupiravir: 333, 5.65%), and 50 events of ICU admission or ventilatory support (nirmatrelvir-ritonavir: 26, 0.44%; molnupiravir: 24, 0.41%) were observed. Among the hospitalised individuals, the nirmatrelvir-ritonavir group demonstrated a lower mortality rate compared to the molnupiravir group (ARR at 28 days 2.66%, 95% CI: 1.93–3.40), and the rates of ICU admission or ventilatory support were similar between the two groups (ARR at 28 days −0.03%, 95% CI: −0.27 to 0.20) (Table 2). Consistent results were observed in relative risk adjusted with patient characteristics. Nirmatrelvir-ritonavir was associated with significantly reduced risk of all-cause mortality among both non-hospitalised (HR: 0.43, 95% CI: 0.33–0.56) and hospitalised patients (HR: 0.59, 95% CI: 0.49–0.71). For non-hospitalised patients, nirmatrelvir-ritonavir was also associated with a decreased risk of hospitalisation (HR: 0.72, 95% CI: 0.67–0.76) (Table 2).
Fig. 2.
28-day cumulative incidence of outcomes. The 28-day cumulative incidence of outcomes between molnupiravir and nirmatrelvir-ritonavir users in non-hospitalised and hospitalised setting was displayed. ICU = intensive care unit.
Table 2.
Risk of outcomes for COVID-19 patients receiving nirmatrelvir-ritonavir compared with molnupiravir
| Outcomes | Non-hospitalised (N = 63,522) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nirmatrelvir-ritonavir (N = 31,761) |
Molnupiravir (N = 31,761) |
ARR (95% CI) (%) | Adjusted HR (95% CI)a | |||||||
| Events | Rate (%) | Follow-up (days) | Incidence rate (per 10,000 person days) | Events | Rate (%) | Follow-up (days) | Incidence rate (per 10,000 person days) | |||
| Effectiveness outcome | ||||||||||
| All-cause mortality | 71 | 0.22 | 888,257 | 0.80 (0.62, 1.01) | 265 | 0.83 | 885,787 | 2.99 (2.64, 3.37) | 0.61 (0.50, 0.72) | 0.43 (0.33, 0.56) |
| ICU admission or ventilatory support | 71 | 0.22 | 887,099 | 0.80 (0.63, 1.01) | 91 | 0.29 | 884,419 | 1.03 (0.83, 1.26) | 0.07 (−0.02, 0.14) | 0.98 (0.72, 1.35) |
| Hospitalisation | 1853 | 5.83 | 855,325 | 21.66 (20.69, 22.67) | 3037 | 9.56 | 831,893 | 36.51 (35.22, 37.83) | 3.73 (3.31, 4.14) | 0.72 (0.67, 0.76) |
| Hospitalised (N = 11,784) | ||||||||||
| Nirmatrelvir-ritonavir (N = 5892) | Molnupiravir (N = 5892) | ARR (95% CI) (%) | Adjusted HR (95% CI)a | |||||||
| Events | Rate (%) | Follow-up (days) | Incidence rate (per 10,000 person days) | Events | Rate (%) | Follow-up (days) | Incidence rate (per 10,000 person days) | |||
| Effectiveness outcome | ||||||||||
| All-cause mortality | 176 | 2.99 | 162,361 | 10.84 (9.30, 12.57) | 333 | 5.65 | 159,934 | 20.82 (18.64, 23.18) | 2.66 (1.93, 3.40) | 0.59 (0.49, 0.71) |
| ICU admission or ventilatory support | 26 | 0.44 | 162,046 | 1.60 (1.05, 2.35) | 24 | 0.41 | 159,628 | 1.50 (0.96, 2.24) | −0.03 (−0.27, 0.20) | 1.09 (0.63, 1.91) |
ARR = absolute risk reduction; CI = confidence interval; HR = hazard ratio; ICU = intensive care unit.
Hazard ratios were obtained from Cox proportional hazard regression adjusted by sex, age, Charlson Comorbidity Index and vaccination status, pre-existing comorbidities, and medication use within 90 days at baseline.
The results were consistent for all-cause mortality among both non-hospitalised and hospitalised individuals across all subgroups (Table 3). The findings on ICU admission or ventilatory support and hospitalisation were also similar across all subgroups for both non-hospitalised and hospitalised patients (Supplementary Table S3) Two sensitivity analyses, (i) restricting patients received COVID-19 drug treatments within three days from diagnosis, or (ii) excluding patients with pre-existing myocardial infarction, showed similar results as the main analysis (Supplementary Tables S4 and S5). The E-value for all-cause mortality using estimated of HR in non-hospitalised and hospitalised groups were 4.08 and 2.78, respectively (Supplementary Table S6). These findings suggest that unobserved confounding variable with at least a 4.08 and 2.78-fold stronger association with mortality would be needed to explain away these significant HR, thus our conclusions are likely robust to potential unmeasured confounding.
Table 3.
Subgroup analysis for all-cause mortality.
| Subgroups | Non-hospitalised (N = 63,542) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nirmatrelvir-ritonavir (N = 31,771) |
Molnupiravir (N = 31,771) |
ARR (95% CI) (%) | Adjusted HR (95% CI)a | P-value for interaction | |||||||
| Events | Rate (%) | Follow-up (days) | Incidence rate (per 10,000 person days) | Events | Rate (%) | Follow-up (days) | Incidence rate (per 10,000 person days) | ||||
| All-cause mortality | |||||||||||
| Age, years | 0.69 | ||||||||||
| <70 | 5 | 0.03 | 418,569 | 0.12 (0.04, 0.28) | 11 | 0.08 | 393,311 | 0.28 (0.14, 0.50) | 0.05 (−0.01, 0.10) | 0.48 (0.16, 1.44) | |
| ≥70 | 66 | 0.39 | 469,688 | 1.41 (1.09, 1.79) | 254 | 1.43 | 492,476 | 5.16 (4.54, 5.83) | 1.04 (0.84, 1.24) | 0.42 (0.32, 0.56) | |
| Sex | 0.36 | ||||||||||
| Male | 41 | 0.27 | 426,346 | 0.96 (0.69, 1.30) | 123 | 0.81 | 424,446 | 2.90 (2.41, 3.46) | 0.54 (0.38, 0.70) | 0.46 (0.32, 0.66) | |
| Female | 30 | 0.18 | 461,911 | 0.65 (0.44, 0.93) | 142 | 0.86 | 461,341 | 3.08 (2.59, 3.63) | 0.68 (0.52, 0.83) | 0.38 (0.25, 0.56) | |
| CCI | 0.13 | ||||||||||
| 0–3 | 4 | 0.02 | 534,382 | 0.07 (0.02, 0.19) | 19 | 0.11 | 487,031 | 0.39 (0.23, 0.61) | 0.09 (0.04, 0.14) | 0.20 (0.07, 0.58) | |
| ≥4 | 67 | 0.53 | 353,875 | 1.89 (1.47, 2.40) | 246 | 1.71 | 398,756 | 6.17 (5.42, 6.99) | 1.18 (0.94, 1.43) | 0.45 (0.34, 0.59) | |
| COVID-19 vaccination | 0.66 | ||||||||||
| 0–1 dose | 14 | 0.74 | 52,553 | 2.66 (1.46, 4.47) | 73 | 3.35 | 59,969 | 12.17 (9.54, 15.31) | 2.61 (1.76, 3.46) | 0.36 (0.20, 0.64) | |
| ≥2 doses | 57 | 0.19 | 835,704 | 0.68 (0.52, 0.88) | 192 | 0.65 | 825,818 | 2.32 (2.01, 2.68) | 0.46 (0.35, 0.56) | 0.44 (0.33, 0.60) | |
| Hospitalised (N = 11,784) | |||||||||||
| Nirmatrelvir-ritonavir (N = 5892) | Molnupiravir (N = 5892) | ARR (95% CI) (%) | Adjusted HR (95% CI)a | P-value for interaction | |||||||
| Events | Rate (%) | Follow-up (days) | Incidence rate (per 10,000 person days) | Events | Rate (%) | Follow-up (days) | Incidence rate (per 10,000 person days) | ||||
| All-cause mortality | |||||||||||
| Age, years | 0.87 | ||||||||||
| <70 | 21 | 1.27 | 45,939 | 4.57 (2.83, 6.99) | 40 | 2.42 | 45,577 | 8.78 (6.27, 11.95) | 1.15 (0.23, 2.07) | 0.44 (0.25, 0.76) | |
| ≥70 | 155 | 3.66 | 116,422 | 13.31 (11.30, 15.58) | 293 | 6.91 | 114,357 | 25.62 (22.77, 28.73) | 3.25 (2.31, 4.20) | 0.63 (0.51, 0.76) | |
| Sex | 0.14 | ||||||||||
| Male | 102 | 3.46 | 80,997 | 12.59 (10.27, 15.29) | 158 | 5.35 | 80,298 | 19.68 (16.73, 23.00) | 1.89 (0.85, 2.94) | 0.67 (0.52, 0.86) | |
| Female | 74 | 2.51 | 81,364 | 9.09 (7.14, 11.42) | 175 | 5.95 | 79,636 | 21.97 (18.84, 25.48) | 3.44 (2.41, 4.46) | 0.52 (0.39, 0.69) | |
| CCI | 0.51 | ||||||||||
| 0–3 | 16 | 0.78 | 57,398 | 2.79 (1.59, 4.53) | 26 | 1.40 | 51,649 | 5.03 (3.29, 7.38) | 0.62 (−0.03, 1.28) | 0.47 (0.25, 0.90) | |
| ≥4 | 160 | 4.17 | 104,963 | 15.24 (12.97, 17.80) | 307 | 7.61 | 108,285 | 28.35 (25.27, 31.71) | 3.44 (2.40, 4.47) | 0.60 (0.50, 0.73) | |
| COVID-19 vaccination | 0.050 | ||||||||||
| 0–1 dose | 45 | 5.19 | 23,594 | 19.07 (13.91, 25.52) | 83 | 9.70 | 22,794 | 36.41 (29.00, 45.14) | 4.51 (2.03, 6.98) | 0.53 (0.36, 0.77) | |
| ≥2 doses | 131 | 2.61 | 138,767 | 9.44 (7.89, 11.20) | 250 | 4.96 | 137,140 | 18.23 (16.04, 20.63) | 2.35 (1.61, 3.10) | 0.60 (0.48, 0.74) | |
ARR = absolute risk reduction; CI = confidence interval; HR = hazard ratio; CCI = Charlson Comorbidity Index.
Hazard ratios were obtained from Cox proportional hazard regression adjusted by sex, age, Charlson Comorbidity Index and vaccination status, pre-existing comorbidities, and medication use within 90 days at baseline.
Discussion
There was a greater reduction in the risk of all-cause mortality when comparing nirmatrelvir-ritonavir treatment to molnupiravir in both non-hospitalised and hospitalised patients. When neither drug is contraindicated, nirmatrelvir-ritonavir may be considered as a more effective option.
Our study findings differ from a previous study conducted on the US Veterans population, which found no observable difference between nirmatrelvir-ritonavir and molnupiravir treatments.6 The discrepancy in hospitalisation risk between the two studies may be attributed to various factors, including differences in health seeking behaviours and hospitalisation criteria between Hong Kong and the US. However, the point estimator of risk of mortality may be similar between their (risk ratio: 0.50, 95% CI: 0.09–2.73) and our study (hazard ratio: 0.43, 95% CI: 0.33–0.56). Compared to their study, our study had a higher sample size and number of events, providing sufficient statistical power to detect differences. Indeed, our conclusion is also supported by indirect evidence from the EPIC-HR and MOVe-OUT trials.1,2 Further, a UK-based Platform Adaptive trial of NOvel antiviRals for eArly treatMent of Covid-19 In the Community (PANORAMIC) trial in the United Kingdom even found no reduction of hospitalisations or mortality among molnupiravir group compared to control group among high-risk vaccinated non-hospitalised patients.29 Two US-based observational studies reported a reduction in hospitalisation and mortality among nirmatrelvir-ritonavir users compared to non-users in populations with high COVID-19 vaccine uptake (HR of hospitalisation or mortality: 0.20, 95% CI: 0.02–0.5030; Odd ratio (OR) of hospitalisation: 0.45, 95% CI: 0.33–0.6231; OR of mortality: 0.15, 95% CI: 0.03–0.5031). Another US-based observational study demonstrated that molnupiravir was associated with a decrease in hospital admissions or mortality (relative risk 0.72, 95% CI: 0.64–0.7932). In the inpatient setting, two Hong Kong-based studies showed reductions in hospitalisation and mortality among nirmatrelvir-ritonavir and molnupiravir users compared to non-users (HR in nirmatrelvir-ritonavir: 0.77, 95% CI: 0.66–0.906; HR in molnupiravir: 0.87, 95% CI: 0.81–0.936; HR in nirmatrelvir-ritonavir: 0.34, 95% CI: 0.23–0.507; HR in molnupiravir: 0.48, 95% CI: 0.40–0.597). Notably, the molnupiravir for Oral Treatment of COVID-19 in an outpatient setting (MOVe-In) showed no reduction in mortality among the majority of patients with severe illness who required oxygen therapy or remdesivir.33 These results from indirect comparison indicated that the risk of hospitalisation and mortality among nirmatrelvir-ritonavir or molnupiravir users was lower compared to non-users, and the magnitude of risk reduction was reported to be lower among nirmatrelvir-ritonavir users than molnupiravir users relative to non-users. On the top of current evidence, our findings provided a direct comparison between nirmatrelvir-ritonavir and molnupiravir in both non-hospitalised and hospitalised patients and highlighted a greater clinical benefit in nirmatrelvir-ritonavir treatment.
Nirmatrelvir-ritonavir and molnupiravir have distinct mechanisms of action, which may contribute to differences in their effectiveness. Nirmatrelvir-ritonavir targets the main protease of the SARS-CoV-2 virus,34 inhibiting its replication, while molnupiravir interferes with the viral RNA replication process by causing errors during replication.35 In addition, ritonavir, serves as a pharmacokinetic enhancer for nirmatrelvir, increasing its blood concentration and resulting in greater antiviral activity against the virus.23 These differences in mechanisms of action and pharmacokinetics may play a role in the variations observed in the effectiveness of these two antiviral drugs. Further research is warranted to fully elucidate how their mechanisms of action and pharmacokinetics could impact antiviral effectiveness.
Our current findings showed that nirmatrelvir-ritonavir users were associated with a lower risk of mortality but not ICU admission or the need for ventilatory support compared to molnupiravir users. It is important to note that the present study was conducted amidst the peak of the fifth wave of COVID-19 in Hong Kong. Due to overwhelmed public hospitals and limited ICU bed space, some patients may have unfortunately died while waiting for ICU admission. Additionally, the diagnosis coding used to determine ventilator use in the electronic database could have led to underdiagnosis. Hence, the risk of ICU admission or the need for ventilatory support may have been underestimated in our study. Despite these limitations, our findings suggest that nirmatrelvir-ritonavir is an effective choice of oral antivirals in reducing all-cause mortality for both non-hospitalised and hospitalised patients. Further studies are needed to evaluate the effectiveness of both drugs in reducing COVID-19 complications using other outcome measures.
While our study revealed higher treatment effectiveness with nirmatrelvir-ritonavir compared to molnupiravir, this finding should be interpreted with caution. Ritonavir is a potent inhibitor of the CYP3A4 enzyme, which plays a critical role in the metabolism of numerous drugs. The concurrent use of ritonavir with drugs like anticoagulants and antiarrhythmic drugs, which are metabolised by CYP3A4, may result in elevated blood levels of the concomitant drugs, potentially leading to significant drug interactions and subsequent adverse effects or toxicity.36 Furthermore, nirmatrelvir-ritonavir is not recommended for patients with severe liver or renal impairment.36 In contrast, molnupiravir is contraindicated for pregnant or breastfeeding patients, but no known drug-drug interactions have been reported.24 Consequently, nirmatrelvir-ritonavir has several limitations and is not suitable for all patients. Given the observed clinical improvement in molnupiravir users compared to non-users, molnupiravir remains a valuable treatment choice for patients with COVID-19 when nirmatrelvir-ritonavir is less clinically appropriate.
This study has several strengths and limitations. Among the strengths, this is the first territory-wide real-world study comparing molnupiravir and nirmatrelvir-ritonavir in both non-hospitalised and hospitalised settings. Our findings supplement RCT data with direct evidence of how the two oral antiviral drugs compare to each other in terms of the effectiveness. The use of comprehensive data from territory-wide electronic health records database in Hong Kong confers high population representativeness, and the adoption of a target trial emulation approach helped mitigate many of the typical challenges and biases in observational data analyses.
This study had several limitations. Firstly, information on COVID-19 symptom onset was not available and was proxied with the date of first positive PCR/RAT result. Secondly, data on body mass index was not available and could not be accounted for despite a higher BMI being an independent indicator of risk of long COVID.37 However, common sequalae of obesity including diabetes, use of lipid-lowering drugs and hypertension were accounted for in our analyses. Moreover, these two limitations are applied in both treatment groups, and thus should not affect the interpretation of the results. Thirdly, information on adherence to treatments and reasons for drug discontinuation were not available. Fourth, information on mortality caused by COVID-19 was not available. Fifth, since molnupiravir and nirmatrelvir-ritonavir were distributed free of charge to COVID patients aged over 60 in both non-hospitalised and hospitalised settings by the Hong Kong government started from February 2022, the mean age of the study population was relatively high. Lastly, as with all observational studies, there remains a possibility of residual confounding by indication, even though patients with potential contraindications to either oral antiviral had been excluded from the analyses.
To conclude, in this population-based target trial emulation, our findings suggest that nirmatrelvir-ritonavir treatment is more effective than molnupiravir in reducing the risk of all-cause mortality in both hospitalised and non-hospitalised patients. In clinical settings where there are no contraindications to the use of either antiviral drugs, nirmatrelvir-ritonavir may be superior based on drug effectiveness. Our study highlights the importance of considering the relative effectiveness of different treatment options when making clinical decisions.
Contributors
Concept and design: EYFW, VKCY, ICKW, EWYC.
Acquisition of data: ICKW.
Access, and verification of data: EYFW, VKCY, ZCTW, ICKW, EWYC.
Analysis, or interpretation of data: All authors.
Drafting of the manuscript: EYFW, VKCY, ZCTW.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: EYFW, VKCY, ZCTW.
Administrative, technical, or material support: ICKW, EWYC.
Supervision: ICKW, EWYC.
Data sharing statement
Data are not available, as the data custodians (the Hospital Authority and the Department of Health of Hong Kong SAR) have not given permission for sharing due to patient confidentiality and privacy concerns. Local academic institutions, government departments, or non-governmental organizations may apply for the access to data through the Hospital Authority’s data sharing portal (https://www3.ha.org.hk/data).
Declaration of interests
EYFW has received research grants from the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, the Hong Kong Research Grants Council of the Government of the Hong Kong SAR, Narcotics Division, Security Bureau of the Government of the Hong Kong SAR, and National Natural Science Foundation of China, outside the submitted work. FTTL has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council and has received research grants from the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, outside the submitted work. CSLC has received grants from the Food and Health Bureau of the Hong Kong Government, the Hong Kong Research Grants Council of the Government of the Hong Kong SAR, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, MSD, and Amgen; and personal fees from PrimeVigilance; outside the submitted work. XL has received research grants from the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region; research grants from the Hong Kong Research Grants Council (Early Career Scheme, and Research Impact Fund) of the Government of the Hong Kong SAR; research and educational grants from Janssen and Pfizer; internal funding from the University of Hong Kong; and consultancy fees from Pfizer, and Merck Sharp & Dohme; Dohme, unrelated to this work. CKHW has received research grants from the Hong Kong Research Grants Council of the Government of the Hong Kong SAR, the EuroQol Group Research Foundation, AstraZeneca, and Boehringer Ingelheim, unrelated to this work. IFNH received payments as member of Advisory Board for Pfizer, MSD, Moderna, GSK, and Gilead. ICKW reports research funding from Amgen, Bristol Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong Research Grants Council of the Government of the Hong Kong SAR, the Hong Kong Health and Medical Research Fund, the National Institute for Health Research in England, the European Commission, and the National Health and Medical Research Council in Australia, consulting fees from IQVIA and World Health Organization, payment for expert testimony for Appeal Court of Hong Kong and is a non-executive director of Jacobson Medical in Hong Kong and Therakind in England, outside of the submitted work. ICKW reports role as member of Pharmacy and Poisons Board in Hong Kong, the Expert Committee on Clinical Events Assessment Following COVID-19 Immunization, and the Advisory Panel on COVID-19 Vaccines of the Hong Kong Government. EWYC reports grants from the Hong Kong Research Grants Council of the Government of the Hong Kong SAR, Research Fund Secretariat of the Food and Health Bureau, National Natural Science Fund of China, Wellcome Trust, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, RGA Reinsurance Company, AstraZeneca, Narcotics Division of the Security Bureau of the Hong Kong Special Administrative Region, Innovation and Technology Commission of the Government of the Hong Kong Special Administrative Region, Novartis, National Health and Medical Research Council Australia; honorarium from Hospital Authority; outside the submitted work. EWYC reports unpaid role of president of International Society for Pharmacoeconomics and Outcomes Research (ISPOR), Hong Kong Regional Chapter. All other authors declare no competing interests.
Acknowledgments
This study was funded by HMRF Research on COVID-19, The Hong Kong Special Administrative Region (HKSAR) Government (Principal Investigator (WP2): EWYC; Ref No. COVID1903011); Collaborative Research Fund, University Grants Committee, the HKSAR Government (Principal Investigator: ICKW; Ref. No. C7154-20GF); and Research Grant from the Food and Health Bureau, the HKSAR Government (Principal Investigator: ICKW; Ref. No. COVID19F01). ICKW and FTTL are partially supported by the Laboratory of Data Discovery for Health (D24H) funded by the by AIR@InnoHK administered by Innovation and Technology Commission. We gratefully acknowledge the Centre for Health Protection, the Department of Health and the Hospital Authority for facilitating data access. We would like to thank Stacy Yan Yu Pan for providing support on analysis of data.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102225.
Contributor Information
Ian Chi Kei Wong, Email: wongick@hku.hk.
Esther Wai Yin Chan, Email: ewchan@hku.hk.
Appendix A. Supplementary data
References
- 1.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammond J., Leister-Tebbe H., Gardner A., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institutes of Health . 2023. Therapeutic management of nonhospitalized adults with COVID-19.https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults--therapeutic-management/ [Google Scholar]
- 4.National COVID-19 Clinical Evidence Taskforce Australian guidelines for the clinical care of people with COVID-19. https://files.magicapp.org/guideline/0b4c8c7d-f57c-4524-b60d-e690ff0b4452/published_guideline_7252-74_1.pdf
- 5.Bajema K.L., Berry K., Streja E., et al. Effectiveness of COVID-19 treatment with nirmatrelvir–ritonavir or molnupiravir among U.S. veterans: target trial emulation studies with one-month and six-month outcomes. Ann Intern Med. 2023;176(6):807–816. doi: 10.7326/M22-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan E.Y.F., Yan V.K.C., Mok A.H.Y., et al. Effectiveness of molnupiravir and nirmatrelvir–ritonavir in hospitalized patients with COVID-19: a target trial emulation study. Ann Intern Med. 2023;176(4):505–514. doi: 10.7326/M22-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong C.K., Au I.C., Lau K.T., Lau E.H., Cowling B.J., Leung G.M. Real-world effectiveness of early molnupiravir or nirmatrelvir–ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA. 2 wave: a retrospective cohort study. Lancet Infect Dis. 2022;22(12):1681–1693. doi: 10.1016/S1473-3099(22)00507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.HKSAR Government . 2022. Free COVID-19 oral drugs plan set. [Google Scholar]
- 9.Pfizer . 2022. PAXLOVID™ (nirmatrelvir tab; ritonavir tab) [Google Scholar]
- 10.Ho K. Hong Kong Free Press; 2022. Hong Kong’s Hospital Authority expands use of Covid-19 oral drugs.https://hongkongfp.com/2022/03/22/hong-kongs-hospital-authority-expands-use-of-covid-19-oral-drugs/ [Google Scholar]
- 11.Wan E.Y.F., Chui C.S.L., Lai F.T.T., et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2021;21:451–455. doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua G.T., Kwan M.Y.W., Chui C.S.L., et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following comirnaty vaccination. Clin Infect Dis. 2021;75(4):673–681. doi: 10.1093/cid/ciab989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X., Tong X., Yeung W.W.Y., et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2021;81(4):564–568. doi: 10.1136/annrheumdis-2021-221571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai F.T.T., Huang L., Chui C.S.L., et al. Multimorbidity and adverse events of special interest associated with Covid-19 vaccines in Hong Kong. Nat Commun. 2022;13(1):411. doi: 10.1038/s41467-022-28068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai F.T.T., Li X., Peng K., et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine: a case–control study. Ann Intern Med. 2022;175(3):362–370. doi: 10.7326/M21-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai F.T.T., Huang L., Peng K., et al. Post-Covid-19-vaccination adverse events and healthcare utilization among individuals with or without previous SARS-CoV-2 infection. J Intern Med. 2022;291(6):864–869. doi: 10.1111/joim.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., Tong X., Wong I.C.K., et al. Lack of inflammatory bowel disease flare-up following two-dose BNT162b2 vaccine: a population-based cohort study. Gut. 2022;71:2608–2611. doi: 10.1136/gutjnl-2021-326860. [DOI] [PubMed] [Google Scholar]
- 18.Wan E.Y.F., Chui C.S.L., Wang Y., et al. Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: a self-controlled case series and nested case-control study. Lancet Reg Health West Pac. 2022;21 doi: 10.1016/j.lanwpc.2022.100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong X., Wong C.K.H., Au I.C.H., et al. Safety of inactivated and mRNA COVID-19 vaccination among patients treated for hypothyroidism: a population-based cohort study. Thyroid. 2022;32(5):505–514. doi: 10.1089/thy.2021.0684. [DOI] [PubMed] [Google Scholar]
- 20.Hernán M.A. Methods of public health research—strengthening causal inference from observational data. N Engl J Med. 2021;385(15):1345–1348. doi: 10.1056/NEJMp2113319. [DOI] [PubMed] [Google Scholar]
- 21.Hernán M.A., Alonso A., Logan R., et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernán M.A., Robins J.M. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758–764. doi: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Food & Drug Administration . 2023. Fact sheet for healthcare providers: emergency use authorization for Paxlovid.https://www.fda.gov/media/155050/download [Google Scholar]
- 24.U.S. Food & Drug Administration . 2023. Fact sheet for healthcare providers: emergency use authorization for lagevrio (molnupiravir) capsules.https://www.fda.gov/media/155054/download [Google Scholar]
- 25.Danaei G., Rodríguez L.A., Cantero O.F., Logan R., Hernán M.A. Observational data for comparative effectiveness research: an emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat Methods Med Res. 2013;22(1):70–96. doi: 10.1177/0962280211403603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung K.S., Leung W.K., Seto W.K. Application of big data analysis in gastrointestinal research. World J Gastroenterol. 2019;25(24):2990–3008. doi: 10.3748/wjg.v25.i24.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VanderWeele T.J., Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 29.Butler C.C., Hobbs F.R., Gbinigie O.A., et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet. 2023;401(10373):281–293. doi: 10.1016/S0140-6736(22)02597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewnard J.A., McLaughlin J.M., Malden D., et al. Effectiveness of nirmatrelvir–ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system. Lancet Infect Dis. 2023;23(7):806–815. doi: 10.1016/S1473-3099(23)00118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aggarwal N.R., Molina K.C., Beaty L.E., et al. Real-world use of nirmatrelvir–ritonavir in outpatients with COVID-19 during the era of omicron variants including BA. 4 and BA. 5 in Colorado, USA: a retrospective cohort study. Lancet Infect Dis. 2023;23(6):696–705. doi: 10.1016/S1473-3099(23)00011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Y., Bowe B., Al-Aly Z. Molnupiravir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ. 2023;380 doi: 10.1136/bmj-2022-072705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arribas J.R., Bhagani S., Lobo S.M., et al. Randomized trial of molnupiravir or placebo in patients hospitalized with Covid-19. NEJM Evid. 2022;1(2) doi: 10.1056/EVIDoa2100044. [DOI] [PubMed] [Google Scholar]
- 34.Extance A. Covid-19: what is the evidence for the antiviral Paxlovid? BMJ. 2022;377 doi: 10.1136/bmj.o1037. [DOI] [PubMed] [Google Scholar]
- 35.Extance A. Covid-19: what is the evidence for the antiviral molnupiravir? BMJ. 2022;377 doi: 10.1136/bmj.o926. [DOI] [PubMed] [Google Scholar]
- 36.Pfizer Inc . 2023. Fact sheet for healthcare providers: emergency use authorization for Paxlovid. [Google Scholar]
- 37.Chudzik M., Lewek J., Kapusta J., Banach M., Jankowski P., Bielecka-Dabrowa A. Predictors of long COVID in patients without comorbidities: data from the polish long-COVID cardiovascular (PoLoCOV-CVD) study. J Clin Med. 2022;11(17):4980. doi: 10.3390/jcm11174980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.