Abstract
The fungicidal activity of amphotericin B (AmB) was quantitated for several Candida species. Candida albicans and C. tropicalis were consistently susceptible to AmB, with less than 1% survivors after 6 h of exposure to AmB. C. parapsilosis and variants of C. lusitaniae and C. guilliermondii were the most resistant, demonstrating 50 to 90% survivors in this time period and as high as 1% survival after a 24-h exposure time. All Candida species were killed (<1% survivors) after 24 h of exposure to AmB. In contrast, overnight exposure to either fluconazole or itraconazole resulted in pronounced increases in resistance to subsequent exposures to AmB. Most dramatically, C. albicans was able to grow in AmB cultures after azole preexposure. Several other Candida species did not grow in AmB but showed little or no reduction in viability after up to 24 h in AmB. Depending on the growth conditions, Candida cells preexposed to azoles may retain AmB resistance for days after the azoles have been removed. If this in vitro antagonism applies to the clinical setting, treatment of patients with certain antifungal combinations may not be beneficial. The ability of some Candida isolates to survive transient exposures to AmB was not reflected in the in vitro susceptibility changes as measured by standard MIC assays. This finding should be considered in studies attempting to correlate patient outcome with in vitro susceptibilities of clinical fungal isolates. Patients who fail to respond to AmB may be infected with isolates that are classified as susceptible by standard in vitro assays but that may be resistant to transient antifungal exposures which may be more relevant in the clinical setting.
Consideration of the interactions between azoles and amphotericin B (AmB) has become clinically significant in recent years. Fluconazole and, to a lesser extent, itraconazole are widely used and largely effective but are not fungicidal. An additional limitation is that they are not effective against several Candida species, notably Candida krusei and C. glabrata (2, 4, 11, 17). AmB is a potent, fungicidal agent that is effective against most isolates of Candida but that has toxic side effects (1, 39). In addition, several Candida species, including C. lusitaniae, demonstrate intrinsic resistance to AmB (2, 6, 18, 19, 34, 35). Recent reports suggest that antifungal therapy may select for AmB-resistant variants of C. albicans and other susceptible species (5, 10, 14–16, 20, 21, 23, 35). However, mutants verified by in vitro testing to be resistant remain elusive (10). Inadvertent clinical selection for resistance to AmB may be more likely due to prolonged azole use than to AmB therapy. Some mutations in C. albicans that confer resistance to fluconazole act by altering the synthesis of ergosterol, the putative target of AmB action, and thereby confer cross-resistance (19).
We previously demonstrated that preexposing C. albicans in vitro to fluconazole or itraconazole conferred resistance to otherwise fungicidal concentrations of AmB (37). Depending on the conditions, up to 100% of the preexposed cells tolerated AmB at 2 μg/ml for up to 24 h. However, simultaneous exposure of C. albicans to azoles and AmB had much less effect, with only a small increase in the Candida population surviving relative to controls exposed to AmB alone. Moreover, several investigators have found synergistic interactions by simultaneous exposure of C. albicans to azoles and AmB (13, 30). One group, on the other hand, described antagonisms with preexposures of Candida to the more lipophilic azoles, such as itraconazole, but not to fluconazole (31, 32).
In this paper, we offer new observations describing the complex azole-AmB interactions. First, we compare the fungicidal effects of AmB on representative isolates of six species of Candida. We are able to show differences in AmB killing rates among some of these Candida isolates. Second, and most importantly, preexposure to azoles decreased the susceptibilities of all Candida species that were otherwise found to be susceptible to AmB by standardized in vitro susceptibility studies. C. albicans and, to a lesser extent, C. tropicalis demonstrated the greatest degree of antagonism. C. albicans was unique in that preexposure to azoles routinely allowed growth, not just survival, during subsequent incubations in AmB. Third, we show that fluconazole-mediated AmB tolerance is established by just a few hours of exposure to fluconazole. The protection endures for several days after azoles are removed, but only if the cells are maintained in a nongrowing state or if the exposure to AmB is continuous following azole incubation.
MATERIALS AND METHODS
Candida isolates.
The organisms tested included 123 clinical specimens recovered from individual patients at Harper Hospital, Detroit, Mich. The distribution of species included 93 C. albicans specimens, 25 C. tropicalis specimens, and 5 C. parapsilosis specimens. Representative isolates for each of six Candida species were chosen from the American Type Culture Collection (Table 1). Candida species were identified by germ tube and chlamydospore formation, morphology, Yeast API 20C method (bioMerieux, Hazelwood, Mo.) results, the phenotype on CHROMagar Candida plates (26), and the results of randomly amplified polymorphic DNA fingerprinting (33).
TABLE 1.
Representative Candida species
| Speciesa | Code | Isolate no. |
|---|---|---|
| C. albicans | Ca | B311b |
| C. krusei | Ckr | 7942 |
| C. lusitaniae | Cl | 7227 |
| C. parapsilosis | Cp | 90018b |
| C. tropicalis | Ct | 66029b |
| C. glabrata | Tg | 7650b |
Candida species as determined by conventional biochemical methodology.
American Type Culture Collection isolate (B311 corresponds to ATCC 32354; 7650 corresponds to ATCC 2001).
Drugs and reagents.
Antifungal agents approved for clinical use were used in the study, so that the results of in vitro studies would more closely approximate the potential results of in vivo studies. The following antifungal agents were used: AmB (as a lyophilized cake of AmB and sodium deoxycholate [Gensia Laboratories, Irvine, Calif.]); the AmB was suspended in sterile water at 1 mg/ml and stored frozen in light-protected vials), itraconazole (Janssen Pharmaceuticals, Titusville, N.J.), and fluconazole (Pfizer-Roerig, Inc., New York, N.Y.). Ergosterol was purchased from Sigma Chemicals, Inc. (St. Louis, Mo.), and dissolved at 5 μg/ml in chloroform. This was diluted into sterile yeast nitrogen base (YNB) medium supplemented with Tween 80, and the mixture was boiled with vigorous stirring to facilitate dispersement (12). For experiments that involved ergosterol, the control medium had the identical concentration of Tween 80.
Fungicidal activity assays.
The fungicidal effects of AmB were determined as previously described (37). Briefly, overnight cultures of each isolate were inoculated in YNB media with or without fluconazole (50 μg/ml). After an additional 14 h of incubation, cultures were diluted 50-fold into 1-ml cultures of YNB plus 2 μg of AmB per ml. The viabilities of these cultures were determined as indicated below. In some experiments, overnight cultures in fluconazole were incubated continuously in drug-free media, with or without daily subculturing.
Susceptibility studies.
The MICs for all of the representative ATCC isolates were determined in accordance with the National Committee for Clinical Laboratory Standards M27-A standards by a broth microdilution method (38).
RESULTS
Individual Candida isolates are killed by AmB at distinctive rates.
To measure the susceptibility of Candida cells to short-term exposures of AmB, freshly grown, overnight cultures were diluted 50-fold to 0.5 × 106 to 1 × 106 cells/ml and supplemented with AmB, typically at 2 μg/ml. At present time intervals, as well as at initial time points, yeast cultures were sampled, diluted, and plated on Sabouraud (SAB) agar to determine the number and percentage of surviving cells. Control cultures without AmB exposure always grew during the incubation interval.
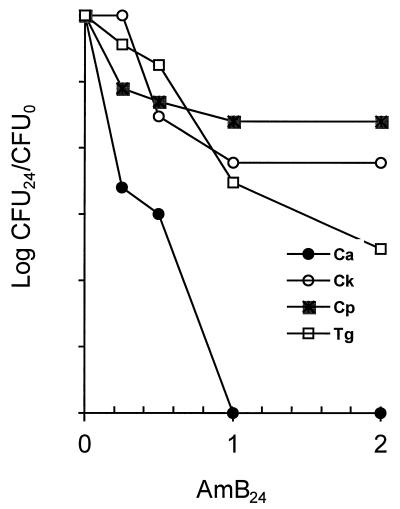
Figure 1 shows that individual isolates of four Candida species vary in susceptibility to AmB killing in 24-h exposures. The disparity among the different Candida species is most pronounced at 2 μg/ml. C. albicans is the most susceptible, with only about 1 in 106 cells surviving, while C. parapsilosis and C. krusei fare better, showing losses in viability of only 1 or 2 orders of magnitude. C. glabrata typically shows an intermediate level of survival. These relative survival levels among species were reproducible in three experiments.
FIG. 1.
Fungicidal effects of AmB on Candida species. Cultures were grown overnight and subcultured into YNB broth plus AmB at the indicated concentrations. After 24 h viability was determined by plating serial dilutions on SAB agar. The densities of control cultures incubated in the same way but without AmB increased more than 10-fold above the initial density, for all species. Ca, C. albicans; Ck, C. krusei; Cp, C. parapsilosis; Tg, C. glabrata.
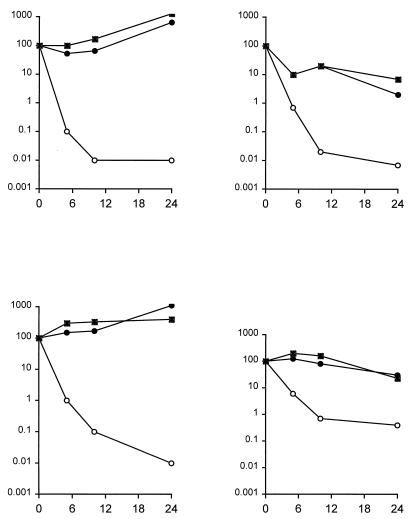
Figure 2 shows killing rates for four Candida species, each from an average of three to seven experiments. C. albicans, C. lusitaniae, and C. tropicalis are highly susceptible, with less than 0.1% survival after 8 h of exposure to AmB. The least susceptible of these four species was C. parapsilosis, with about a 1% survival rate even after 24 h in AmB. C. guilliermondii and C. glabrata were about as susceptible as C. tropicalis (data not shown). In most cases, viability reached its minimum after 8 h of AmB exposure.
FIG. 2.
Kill rates of Candida by AmB after azole exposure. Overnight cultures were exposed to 2 μg of AmB per ml for the indicated periods of time before serial dilutions were plated to determine viability (open circles). Parallel cultures were treated similarly after preexposure to either 50 μg of fluconazole per ml (solid circles) or 2 μg of itraconazole per ml (squares) for 16 h. Panels: upper left, C. albicans, upper right, C. lusitaniae; lower left, C. tropicalis; lower right, C. parapsilosis.
Preexposure to azoles confers a decreased AmB fungicidal effect.
Growth of all tested Candida species in 50 μg of fluconazole per ml before exposure to AmB resulted in dramatic decreases in AmB fungicidal activity. In the acidic YNB broth, this concentration of fluconazole is insufficient to inhibit growth to confluency, even though it is effective for most species in the same medium buffered with 100 mM sodium phosphate, pH 7.0 (37). Figure 2 shows that preexposed C. lusitaniae maintained at least 5% viability, that about half of preexposed C. parapsilosis cells remained viable, and that C. tropicalis and especially C. albicans grew in 2 μg of AmB per ml over a 24-h period. Cultures of C. albicans, uniquely among all tested species, became visibly turbid during this incubation. C. krusei and C. glabrata isolates were protected to the same extent, 0.1 to 0.01% of initially viable cells remaining viable, as C. tropicalis (data not shown).
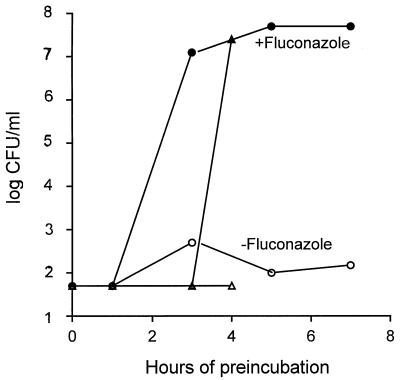
Presumably cells adapt to fluconazole and in so doing become resistant to subsequent AmB exposure. To determine the time required for this adaptation, overnight cultures of cells were diluted into replicate 1-ml cultures of YNB plus fluconazole as described above and at hourly intervals AmB was added to 2 μg/ml. Viable cells were assayed 24 h later (Fig. 3). In two independent experiments, cells were not protected after incubation for 1 to 2 h in fluconazole but were protected after 3 to 4 h of incubation (Fig. 3).
FIG. 3.
Time course of fluconazole protection. In independent tests (triangles and circles), C. albicans cells were diluted from overnight cultures and the mixture was supplemented with 50 μg of fluconazole per ml at time 0, while AmB at 2 μg/ml was added at the indicated intervals. Viability was determined as described for Fig. 1.
Is a 3- to 4-h fluconazole preincubation protective, or does it just allow sufficient time for growth of the yeast cells to a density at which AmB is less effective? To test this, yeast cells were incubated in the same concentration of AmB as previously used, but at initial densities of 2×, 5×, 10×, and 20× relative to the early cultures that were used in Fig. 3. No survivors were detected in the same time frame (data not shown), indicating that AmB killing is density independent. The protection is as effective at AmB concentrations of >6 μg/ml.
Azole-mediated resistance to AmB is maintained in nongrowing C. albicans for days after azole exposure.
How stable is the protective effect of azole exposure? This was addressed in two different experiments. In the first experiment, C. albicans cells were exposed to azoles as described for Fig. 2 and then incubated for 0, 1, 2, or 3 days in drug-free YNB broth at 30°C with shaking and without subculturing. After each day of drug-free incubation, cells were transferred to new cultures supplemented with AmB as before. Cell viability was assayed after 24 h of incubation. Figure 4 demonstrates that cells under these conditions remained resistant to AmB throughout the 3-day incubation. In contrast, controls that had no preexposure to fluconazole but that were otherwise treated identically were not protected, i.e., were highly susceptible to AmB.
FIG. 4.
Duration of azole-mediated protection from AmB killing. Cultures preexposed to azole were exposed to 2 μg of AmB per ml after 0, 1, 2, or 3 days of incubation in drug-free YNB broth. In one series, cultures were subcultured daily into fresh YNB broth (dashed lines). In a second series (solid lines), cultures were continuously incubated without subculturing until they were exposed to AmB. FLZ, fluconazole; ITZ, itraconazole.
In the second experiment, azole-exposed cells were subcultured daily in drug-free YNB media and grown overnight, thus allowing nearly continuous growth of cells. After each subculture and day of drug-free growth, they were diluted into YNB plus 2 μg of AmB per ml. After 24 h of incubation, cultures were assayed for viability. Such cells retained AmB resistance, i.e., retained a high level of viability, after 1 day of growth in drug-free media after azole exposure. However, after two or more days of this drug-free growth, these cells become as susceptible as cells that were never exposed to azoles (Fig. 4).
C. albicans was also exposed to fluconazole overnight, followed by daily serial passage in YNB plus AmB without fluconazole for 10 days. This culture continued to grow to stationary phase at approximately the same rate and to the same final turbidity as a parallel control culture with no AmB.
Intraspecies variability in AmB susceptibility.
Are isolates within a species of Candida equally susceptible to AmB killing? Among 93 random isolates of C. albicans, 91 were completely killed by a 24-h AmB incubation (Table 2). The five C. parapsilosis isolates demonstrated the highest percentage of survivors of any Candida isolates tested. Results for these species are consistent with those for their representative isolates (Fig. 1). In contrast, 12 of 25 C. tropicalis isolates showed a low but detectable percentage of survivors after a 24-h AmB incubation. This seems to indicate heterogeneity among isolates of this species.
TABLE 2.
Incidence of AmB survivors among clinical Candida isolates
| Candida species | Total no. of isolates | No. of AmB survivors |
|---|---|---|
| C. albicans (random set) | 93 | 2 |
| C. parapsilosis | 5 | 5 |
| C. tropicalis | 25 | 12 |
Survivors of 24-h exposures to AmB are not AmB-resistant variants.
Exposure of C. albicans and C. tropicalis to 2 μg of AmB per ml reduced viability by a factor of about 106, often below the level of detection of any survivors in 1-ml cultures. To determine whether rare survivors were stable, resistant variants, we exposed large populations of resistant variants from each species in 500-ml cultures at the same density (about 106/ml) to AmB. Approximately 1,000 cells survived and grew into colonies. These cells were pooled and reevaluated in the same way. There was no difference in survival noted. If even a single colony (conservatively, 106 cells) generated from the 1,000 survivors was persistently resistant, the second-generation selection would have produced at least 105 colonies, more if a putative resistant colony grew during the 24-h period of AmB exposure, instead of the observed 1,000 colonies. Therefore, none of the surviving colonies were stable AmB-resistant mutants.
Effects of ergosterol supplementation on AmB susceptibility.
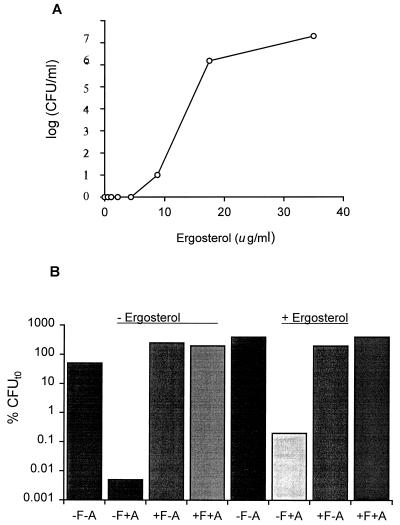
Does fluconazole act by depleting membranes of ergosterol, causing subsequent tolerance to AmB by removing its target? If so, adding ergosterol to the media during the fluconazole incubation may allow replacement in the membrane of ergosterol over less-favored lanosterol derivatives and thus restore the target for AmB and susceptibility to AmB.
Ergosterol is highly insoluble in YNB and must be solubilized with Tween 80. Under these conditions, the effect of exogenous ergosterol on AmB susceptibility is apparent (Fig. 5). As the concentration of ergosterol in the medium increases, the fungicidal effect of AmB decreases, when a fixed concentration of AmB is used on identical aliquots of C. albicans cells. This result shows that the ergosterol is solubilized freely into the medium where it antagonizes AmB activity, presumably by competing for AmB binding with the membrane-bound ergosterol.
FIG. 5.
Effects of exogenous ergosterol on AmB killing and azole-mediated protection from AmB killing. (A) Ergosterol at the indicated final concentrations was added to YNB–2 μg of AmB per ml. C. albicans viabilities were determined after 24 h. Control cultures at each concentration of ergosterol, without AmB, all grew to confluency in the same 24-h period. (B) Cultures were supplemented with 35 μg of ergosterol per ml during a 16-h fluconazole exposure before a 24-h incubation in 2 μg of AmB (+A). Control cultures received either no ergosterol, no azole (−F), or no AmB (−A).
The next experiment shows that cells exposed to fluconazole in medium containing the highest level of ergosterol, 35 μg/ml, were as resistant to subsequent AmB exposure as control cells in ergosterol-free medium (Fig. 5B). In both cases, fluconazole allowed growth of azole-pretreated cells during the 24-h exposure to AmB, whereas control cultures not pretreated with azole were effectively killed by AmB. Thus, a simple incorporation of ergosterol into the membrane either did not occur or was not effective in reversing fluconazole-mediated protection.
DISCUSSION
The results presented here demonstrate different kill rates of the individual Candida species by AmB. These differences may be important in choosing antifungal therapy, since AmB is rapidly absorbed or sequestered from the blood (7–9, 36). Hence, infecting cells may be transiently exposed to the highest fungicidal concentrations of AmB in serum.
Much more dramatic than species-to-species variation in AmB killing rates, however, were the levels of protection afforded by preincubation with fluconazole. C. albicans, the Candida species most susceptible to AmB, is able to grow in high concentrations of AmB after overnight exposure to azoles. In addition, the preincubation concentration does not need to inhibit growth. A concentration of 50 μg of fluconazole per ml is an effective protectant in YNB medium at pH 5.6 or 7.0, even though fluconazole is only inhibitory at the higher pH (37). Furthermore, fluconazole affords AmB protection even to those species such as C. krusei that are not susceptible to fluconazole.
A brief 3-h exposure to fluconazole suffices to establish protection from AmB killing. On the other hand, simultaneous exposure to both antifungals in vitro is lethal. This suggests that a process of adaptation to fluconazole may occur; this process then protects the cells during exposure to AmB. Given the short adaptation period, it seems unlikely that this effect is due to replacement of membrane ergosterol with a methylated sterol derivative that is not interactive with AmB, but this has not yet been investigated.
Incubation of C. albicans, C. krusei, C. parapsilosis, and C. tropicalis in fluconazole results in the depletion of ergosterol and the accumulation of lanosterol derivatives (28), the expected consequence of inhibiting lanosterol 14-α-demethylase. Ergosterol is normally synthesized de novo by yeasts even if it is available in the culture medium (22). However, under conditions which preclude ergosterol synthesis, notably anaerobiosis, it is imported efficiently (27). Under these conditions, other sterols such as cholesterol can substitute for ergosterol (24). If similar inhibitions promote ergosterol uptake in C. albicans, then one would expect ergosterol to reverse fluconazole-mediated protection from AmB. In our studies, however, it did not reverse the azole protection, suggesting that either exogenous ergosterol uptake did not occur or that ergosterol depletion is not the only mechanism involved in this interaction. Fluconazole does not block the ergosterol biosynthetic pathway but allows the formation of downstream C-14-α-methyl sterol derivatives (3, 19). A reasonable interpretation of the inability of ergosterol supplementation to reverse fluconazole-mediated protection from AmB, then, is that ergosterol uptake is not permitted in the presence of the C-14-α-methyl derivatives of lanosterol. More work is required to establish this and to determine if fluconazole mediates protection by another route. The latter is also suggested by the rapid rate at which protection is acquired.
A recent study shows that the MLC, the minimum lethal concentration, assayed at 2 days, was an effective predictor of microbiological failure in the patient. Furthermore, MICs were not effective predictors and were not correlated with MLCs (25). This is consistent with our observed lack of correlation among individual isolates between their MICs and their susceptibilities in our fungicidal activity assay. We did not assay this collection by using the E-test (38) or by doing MIC tests in antibiotic medium no. 3 (29). Since these are reported to be more sensitive in detecting AmB resistance, they may have shown more correlation with the fungicidal activity assay in this study. However, both of these tests depend on the growth of putative resistant isolates in the continuous presence of AmB. It is becoming clear that the most clinically useful assay for AmB susceptibility will ultimately be one that measures the extent to which an isolate survives exposure, not whether it grows in the presence of a given concentration.
Clinical implications of this study are apparent. If patients fail to respond to fluconazole, they are frequently switched to AmB. Our in vitro data suggests that these sequential treatments may be counterproductive. The protective effect of the azole lasts long after it is removed if the exposed cells are not actively growing or if they are maintained in AmB continuously. Cells that have adapted to AmB will, however, not appear resistant by any current standard assay in vitro after they have been subcultured in vitro in the absence of the drug; such isolates would have to be isolated from the patient directly under selective conditions. Furthermore, in an in vivo environment, even simultaneous exposure may have the same effect as sequential exposures in vitro, because of unknown and potentially variable pharmacokinetic differences of the two drugs in the patient. Finally, the degree to which this antagonism is clinically relevant depends on the Candida species and perhaps on the individual isolate, since some Candida species are not inhibited by AmB after azole exposure and have the ability to grow in its presence.
REFERENCES
- 1.Abadi J. Amphotericin B. Pediatr Rev. 1995;16:363–364. doi: 10.1542/pir.16-10-363-a. [DOI] [PubMed] [Google Scholar]
- 2.Arias A, Arevalo M P, Andreu A, Rodriguez C, Sierra A. Candida glabrata: in vitro susceptibility of 84 isolates to eight antifungal agents. Chemotherapy. 1996;42:107–111. doi: 10.1159/000239429. [DOI] [PubMed] [Google Scholar]
- 3.Bard M, Lees N D, Turi T, Craft D, Cofrin L, Barbuch R, Koegel C, Loper J C. Sterol synthesis and viability of erg11 (cytochrome P450 lanosterol demethylase) mutations in Saccharomyces cerevisiae and Candida albicans. Lipids. 1993;28:963–967. doi: 10.1007/BF02537115. [DOI] [PubMed] [Google Scholar]
- 4.Barry A L, Brown S D. In vitro studies of two triazole antifungal agents (voriconazole [UK-109,496] and fluconazole) against Candida species. Antimicrob Agents Chemother. 1996;40:1948–1949. doi: 10.1128/aac.40.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenguer J, Diaz-Guerra T M, Ruiz-Diez B, Bernaldo de Quiros J C, Rodriguez-Tudela J L, Martinez-Suarez J V. Genetic dissimilarity of two fluconazole-resistant Candida albicans strains causing meningitis and oral candidiasis in the same AIDS patient. J Clin Microbiol. 1996;34:1542–1545. doi: 10.1128/jcm.34.6.1542-1545.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumberg H M, Hendershot E F, Lott T J. Persistence of the same Candida albicans strain despite fluconazole therapy. Documentation by pulsed-field gel electrophoresis. Diagn Microbiol Infect Dis. 1992;15:545–547. doi: 10.1016/0732-8893(92)90106-4. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen K J, Bernard E M, Gold J W, Armstrong D. Distribution and activity of amphotericin B in humans. J Infect Dis. 1985;152:1037–1043. doi: 10.1093/infdis/152.5.1037. [DOI] [PubMed] [Google Scholar]
- 8.Collette N, van der Auwera P, Lopez A P, Heymans C, Meunier F. Tissue concentrations and bioactivity of amphotericin B in cancer patients treated with amphotericin B-deoxycholate. Antimicrob Agents Chemother. 1989;33:362–368. doi: 10.1128/aac.33.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collette N, van der Auwera P, Meunier F, Lambert C, Sculier J P, Coune A. Tissue distribution and bioactivity of amphotericin B administered in liposomes to cancer patients. J Antimicrob Chemother. 1991;27:535–548. doi: 10.1093/jac/27.4.535. [DOI] [PubMed] [Google Scholar]
- 10.Conly J, Rennie R, Johnson J, Farah S, Hellman L. Disseminated candidiasis due to amphotericin B-resistant Candida albicans. J Infect Dis. 1992;165:761–764. doi: 10.1093/infdis/165.4.761. . (Comment.) [DOI] [PubMed] [Google Scholar]
- 11.Cormican M G, Pfaller M A. Epidemiology of candidiasis. Compr Ther. 1995;21:653–657. [PubMed] [Google Scholar]
- 12.Geber A, Hitchcock C A, Swartz J E, Pullen F S, Marsden K E, Kwon-Chung K J, Bennett J E. Deletion of the Candida glabrata ERG3 and ERG11 genes: effect on cell viability, cell growth, sterol composition, and antifungal susceptibility. Antimicrob Agents Chemother. 1995;39:2708–2717. doi: 10.1128/aac.39.12.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghannoum M A, Fu Y, Ibrahim A S, Mortara L A, Shafiq M C, Edwards J E, Jr, Criddle R S. In vitro determination of optimal antifungal combinations against Cryptococcus neoformans and Candida albicans. Antimicrob Agents Chemother. 1995;39:2459–2465. doi: 10.1128/aac.39.11.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goetz M B. Relationship between fluconazole dosage regimens and the emergence of fluconazole-resistant Candida albicans. AIDS. 1996;10:335–336. doi: 10.1097/00002030-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Goff D A, Koletar S L, Buesching W J, Barnishan J, Fass R J. Isolation of fluconazole-resistant Candida albicans from human immunodeficiency virus-negative patients never treated with azoles. Clin Infect Dis. 1995;20:77–83. doi: 10.1093/clinids/20.1.77. [DOI] [PubMed] [Google Scholar]
- 16.Johnson E M, Warnock D W, Luker J, Porter S R, Scully C. Emergence of azole drug resistance in Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. J Antimicrob Chemother. 1995;35:103–114. doi: 10.1093/jac/35.1.103. [DOI] [PubMed] [Google Scholar]
- 17.Kan V L, Geber A, Bennett J E. Enhanced oxidative killing of azole-resistant Candida glabrata strains with ERG11 deletion. Antimicrob Agents Chemother. 1996;40:1717–1719. doi: 10.1128/aac.40.7.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly S L, Lamb D C, Kelly D E, Loeffler J, Einsele H. Resistance to fluconazole and amphotericin in Candida albicans from AIDS patients. Lancet. 1996;348:1523–1524. doi: 10.1016/S0140-6736(05)65949-1. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 19.Kelly S L, Lamb D C, Kelly D E, Manning N J, Loeffler J, Hebart H, Schumacher U, Einsele H. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol Δ5,6-desaturation. FEBS Lett. 1997;400:80–82. doi: 10.1016/s0014-5793(96)01360-9. [DOI] [PubMed] [Google Scholar]
- 20.Lacassin F, Damond F, Chochillon C, Longuet P, Lebras J, Vilde J L, Leport C. Response to fluconazole by 23 patients with human immunodeficiency virus infection and oral candidiasis: pharmacological and mycological factors. Antimicrob Agents Chemother. 1996;40:1961–1963. doi: 10.1128/aac.40.8.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Guennec R, Reynes J, Mallie M, Pujol C, Janbon F, Bastide J M. Fluconazole- and itraconazole-resistant Candida albicans strains from AIDS patients: multilocus enzyme electrophoresis analysis and antifungal susceptibilities. J Clin Microbiol. 1995;33:2732–2737. doi: 10.1128/jcm.33.10.2732-2737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis T L, Keesler G A, Fenner G P, Parks L W. Pleiotropic mutations in Saccharomyces cerevisiae affecting sterol uptake and metabolism. Yeast. 1988;4:93–106. doi: 10.1002/yea.320040203. [DOI] [PubMed] [Google Scholar]
- 23.McCullough M, Hume S. A longitudinal study of the change in resistance patterns and genetic relationship of oral Candida albicans from HIV-infected patients. J Med Vet Mycol. 1995;33:33–37. [PubMed] [Google Scholar]
- 24.Nes W, Sekula B, Nes W D, Adler J. The functional importance of structural features of ergosterol in yeast. J Biol Chem. 1978;253:6218. [PubMed] [Google Scholar]
- 25.Nguyen M H, Clancy C J, Yu V L, Yu Y C, Morris A J, Syndman D R, Sutton D A, Rinaldi M G. Do in vitro susceptibility data predict the microbiologic response to amphotericin B? Results of a prospective study of patients with Candida fungemia. J Infect Dis. 1998;177:425–430. doi: 10.1086/514193. [DOI] [PubMed] [Google Scholar]
- 26.Odds F C, Bernaerts R. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol. 1994;32:1923–1929. doi: 10.1128/jcm.32.8.1923-1929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parks L W, Casey W M. Physiological implications of sterol biosynthesis in yeast. Annu Rev Microbiol. 1995;49:95–116. doi: 10.1146/annurev.mi.49.100195.000523. [DOI] [PubMed] [Google Scholar]
- 28.Pfaller M, Riley J. Effects of fluconazole on the sterol and carbohydrate composition of four species of Candida. Eur J Clin Microbiol Infect Dis. 1992;11:152–156. doi: 10.1007/BF01967067. [DOI] [PubMed] [Google Scholar]
- 29.Rex J H, Cooper C R, Jr, Merz W G, Galgiani J N, Anaissie E J. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob Agents Chemother. 1995;39:906–909. doi: 10.1128/aac.39.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheven M, Scheven C. Quantitative screening for fluconazole-amphotericin B antagonism in several Candida albicans strains by a comparative agar diffusion assay. Mycoses. 1996;39:111–114. doi: 10.1111/j.1439-0507.1996.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 31.Scheven M, Scheven C, Hahn K, Senf A. Post-antibiotic effect and post-expositional polyene antagonism of azole antifungal agents in Candida albicans: dependence on substance lipophilia. Mycoses. 1995;38:435–442. doi: 10.1111/j.1439-0507.1995.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 32.Scheven M, Schwegler F. Antagonistic interactions between azoles and amphotericin B with yeasts depend on azole lipophilia for special test conditions in vitro. Antimicrob Agents Chemother. 1995;39:1779–1783. doi: 10.1128/aac.39.8.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steffan P, Vazquez J A, Boikov D, Xu C, Sobel J D, Akins R A. Identification of Candida species by randomly amplified polymorphic DNA fingerprinting of colony lysates. J Clin Microbiol. 1997;35:2031–2039. doi: 10.1128/jcm.35.8.2031-2039.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterling T R, Gasser R A, Jr, Ziegler A. Emergence of resistance to amphotericin B during therapy for Candida glabrata infection in an immunocompetent host. Clin Infect Dis. 1996;23:187–188. doi: 10.1093/clinids/23.1.187. [DOI] [PubMed] [Google Scholar]
- 35.Valentin A, Le Guennec R, Rodriguez E, Reynes J, Mallie M, Bastide J M. Comparative resistance of Candida albicans clinical isolates to fluconazole and itraconazole in vitro and in vivo in a murine model. Antimicrob Agents Chemother. 1996;40:1342–1345. doi: 10.1128/aac.40.6.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Etten E W, Otte-Lambillion M, van Vianen W, ten Kate M T, Bakker-Woudenberg A J. Biodistribution of liposomal amphotericin B (AmBisome) and amphotericin B-desoxycholate (Fungizone) in uninfected immunocompetent mice and leucopenic mice infected with Candida albicans. J Antimicrob Chemother. 1995;35:509–519. doi: 10.1093/jac/35.4.509. [DOI] [PubMed] [Google Scholar]
- 37.Vazquez J A, Arganoza M T, Vaishampayan J K, Akins R A. In vitro interaction between amphotericin B and azoles in Candida albicans. Antimicrob Agents Chemother. 1996;40:2511–2516. doi: 10.1128/aac.40.11.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wanger A, Mills K, Nelson P W, Rex J H. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob Agents Chemother. 1995;39:2520–2522. doi: 10.1128/aac.39.11.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wasan K M, Conklin J S. Evaluation of renal toxicity and antifungal activity of free and liposomal amphotericin B following a single intravenous dose to diabetic rats with systemic candidiasis. Antimicrob Agents Chemother. 1996;40:1806–1810. doi: 10.1128/aac.40.8.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]