Summary
Background
Older adults are at increased risk of SARS-CoV-2 Omicron infection and severe disease, especially those in congregate living settings, despite high SARS-CoV-2 vaccine coverage. It is unclear whether hybrid immunity (combined vaccination and infection) after one Omicron infection provides increased protection against subsequent Omicron reinfection in older adults.
Methods
Incidence of SARS-CoV-2 Omicron infection was examined in 750 vaccinated residents of long-term care and retirement homes in the observational cohort COVID in Long-Term Care Study in Ontario, Canada, within a 75-day period (July to September 2022). Risk of infection was assessed by Cox proportional hazards regression. Serum anti-spike and anti-RBD SARS-CoV-2 IgG and IgA antibodies, microneutralization titres, and spike-specific T cell memory responses, were examined in a subset of 318 residents within the preceding three months.
Findings
133 of 750 participants (17.7%) had a PCR-confirmed Omicron infection during the observation period. Increased infection risk was associated with prior Omicron infection (at 9–29 days: 47.67 [23.73–95.76]), and this was not attributed to days since fourth vaccination (1.00 [1.00–1.01]) or residence outbreaks (>6 compared to ≤6: 0.95 [0.37–2.41]). Instead, reinfected participants had lower serum neutralizing antibodies to ancestral and Omicron BA.1 SARS-CoV-2, and lower anti-RBD IgG and IgA antibodies, after their initial Omicron infection.
Interpretation
Counterintuitively, SARS-CoV-2 Omicron infection was associated with increased risk of Omicron reinfection in residents of long-term care and retirement homes. Less robust humoral hybrid immune responses in older adults may contribute to risk of Omicron reinfection.
Funding
COVID-19 Immunity Task Force of the Public Health Agency of Canada.
Keywords: SARS-CoV-2, COVID-19, Omicron, Older adults, Hybrid immunity
Research in context.
Evidence before this study
Older adults, particularly those in long-term care facilities, remain the most vulnerable to breakthrough SARS-CoV-2 infections and severe COVID-19 despite high vaccination rates, so understanding hybrid immunity and its duration after infection is essential to optimize vaccination strategies. We previously reported that early Omicron (BA.1 and BA.2) infection risk was decreased in long-term care and retirement home residents with recent vaccination and a pre-Omicron infection, and several studies have found that Omicron BA.1/BA.2 SARS-CoV-2 infection has protective effects against reinfection with BA.5 in community-dwelling adults. However, data on hybrid immunity in older adults in congregate care facilities remains scarce, and no studies have investigated Omicron-associated hybrid immunity against Omicron reinfection in this population.
Added value of this study
To our knowledge, this is the first study to examine risk factors that may contribute to Omicron reinfection in older adults in congregate living facilities. In this observational study of data from a large Canadian cohort of long-term care and retirement home residents with four monovalent mRNA vaccinations, we counterintuitively found that one early Omicron infection (BA.1/BA.2) was associated with increased risk of subsequent Omicron infection (BA.5), whereas resident age, sex, frailty, time since vaccination, and number of prior residence outbreaks did not significantly affect risk, implying that there may be an immunological contribution. Our further exploration of humoral and T cell immunity after initial Omicron infection suggested that while many older adults did experience an increase in antibody levels and neutralizing antibodies after one Omicron infection, individuals with reinfections had weak humoral hybrid immune responses.
Implications of all the available evidence
Our results suggest that the generation of protective humoral hybrid immunity is influenced by the heterogeneity of immune aging as well as the specific SARS-CoV-2 variant of infection. Many residents of long-term care and retirement homes do not experience a period of enhanced post-infection protection against subsequent infection, which highlights the importance of maintaining high coverage of booster vaccinations in older adults in both congregate care facilities and in the community.
Introduction
Infection with severe acute respiratory virus syndrome coronavirus 2 (SARS-CoV-2) has lasting and broad effects on cellular and humoral immunity.1 Hybrid immunity (i.e., immunity resulting from both vaccination and natural infection) generally provides transient protection against reinfection and longer-lasting protection from severe COVID-19.2, 3, 4 For older adults, their complex healthcare needs, multiple comorbidities, as well as age-associated changes to the immune system, may contribute to heterogenous and less durable hybrid immunity.
The emergence of the highly transmissible and immune evasive SARS-CoV-2 Omicron (B1.1.529) variant caused significant breakthrough infections globally in 2022.5,6 Older adults in congregate living facilities are disproportionately vulnerable to COVID-19 when community transmission is high.7 Accordingly, there were initial province-wide peaks in SARS-CoV-2 infection numbers in Ontario, Canada, in mid-January (BA.1) and mid-April (BA.1/BA.2), with concurrent outbreaks in many long-term care and retirement homes.8 Due to concerns about waning immunity, fourth monovalent mRNA vaccine doses were offered to those populations.9 We and others have found that recent monovalent vaccination was protective against symptomatic early Omicron BA.1/BA.2 infection.10, 11, 12 In addition, we found that infection with a pre-Omicron variant provided three months of protection against infection with Omicron BA.1/BA.2.10 Thus, recent monovalent vaccination and hybrid immunity protected some older adults against early Omicron infection.
As of July 2022, genomic surveillance showed that over half of COVID-19 cases in Ontario were caused by the Omicron subvariant BA.5 (B.1.1.529.5).13 Despite the high number of early Omicron infections, and presumed hybrid immune protection, there was a province-wide wave of Omicron BA.5 infections and outbreaks in long-term care homes.8 It was unclear whether less robust or waning protection from vaccination or hybrid immunity contributed to infection risk in older adults.
At the start of this BA.5-dominanted wave, within our COVID in Long-Term Care Study cohort of over 1000 residents of 27 long-term care and retirement homes, ∼90% of participants had received a fourth SARS-CoV-2 monovalent vaccination, and ∼35% had a prior SARS-CoV-2 infection. We thus retrospectively investigated the degree to which recent monovalent vaccination and/or infection provided protection against Omicron BA.5 infection. We found counterintuitively that recent Omicron BA.1/2 infection was associated with increased risk of Omicron BA.5 infection. Individuals with lower humoral hybrid immune responses after their initial Omicron infection had Omicron reinfections.
Methods
Ethics
All protocols were approved by the Hamilton Integrated Research Ethics Board (HIREB #13059) and other site-specific research ethics boards, and informed consent was obtained.
Study cohort characteristics, determination of infection, and blood collection
Study data are from the COVID in Long-Term Care Study (https://covidinltc.ca). Participants were recruited from 27 long-term care and retirement homes in Ontario, Canada, beginning in March 2021. Participant demographic data were collected at enrollment. Data on chronological age, sex assigned at birth, vaccinations, medications and comorbidities, and for determination of the Clinical Frailty Scale (CFS; a 9-point scale from 1 ‘very fit’ to 9 ‘terminally ill’),14 were collected by study site coordinators from medical records and/or direct participant communication. SARS-CoV-2 infection history was determined from documentation of a positive nasopharyngeal PCR test. The number of facility outbreaks was identified from the Ontario Ministry of Long-Term Care COVID-19 datasets for ‘Active outbreaks’ and ‘Resolved outbreaks’.15
As per Province of Ontario guidelines, most participants received two doses of Moderna Spikevax 100 μg (mRNA-1273) or Pfizer Cominarty 30 μg (BNT162b2) following manufacturer-recommended schedules, and a third monovalent mRNA vaccination in Fall 2021 at least 5 months from their second dose.16 Fourth monovalent mRNA vaccinations began in early 2022.9 750 actively enrolled participants with four monovalent mRNA vaccine doses as of July 1, 2022, without SARS-CoV-2 infection within seven days of their fourth vaccination, were included in this Omicron infection risk study cohort. Infections from December 15, 2021, onward (the initial Omicron wave as defined by Public Health Ontario) were assumed to be Omicron infections, as genetic sequencing was unavailable. The Ontario COVID-19 Genomic Network reported that in Ontario, Canada, the Omicron lineage accounted for 67.1% of cases December 12–18, 2021, 88.8% of cases December 19–25, and 97.7% of cases December 27, 2021 through January 1, 2022.17,18 Omicron BA.5 accounted for 60.3% of COVID-19 cases in Ontario as of July 1, 2022 at the beginning of the observation period,19 and 89.7% of cases as of September 13, 2022 at the end of the observation period.20
The primary goal of the COVID in LTC study is to assess SARS-CoV-2 vaccine immunogenicity. Approximately every 3 months after vaccinations (i.e., 3 months, 6 months, 9 months etc …), dependent on the absence of facility outbreaks and participant availability, venous blood is collected following standard protocols.21 Serum was isolated from anti-coagulant-free vacutainers for antibody assays, and whole blood was collected in heparin-coated vacutainers for T cell assays. Of the 750 participants in this study cohort, humoral and cellular measures were examined in a random subset of 318 participants with blood draws in the three months preceding the start of the observation window (i.e., April 1 to June 30, 2022), at least 7 days from fourth dose vaccinations and/or documented infections.
Measurements of serum anti-SARS-CoV-2 antibodies and neutralization capacity
Serum IgG and IgA anti-SARS-CoV-2 spike and receptor binding domain (RBD) antibodies were measured by validated ELISA, with assay cutoff defined from a pre-COVID-19 population, as previously described.22 Purified ancestral Wuhan Hu-1 SARS-CoV-2 spike antigen was obtained from R&D Systems (cat# 11058-CV-100; YP_009724390.1). The following reagent was produced under HHSN272201400008C and obtained through BEI Resources, NIAID, NIH: Vector pCAGGS Containing the SARS-Related Coronavirus 2, Wuhan-Hu-1 Spike Glycoprotein Receptor Binding Domain (RBD), NR-52309. Serum antibody neutralization was assessed by cell culture assays with live ancestral (SB3) and Omicron BA.1 SARS-CoV-2, and Vero E6 (ATCC CRL-1586) cells, following established protocols.22 Data were reported as geometric microneutralization titers at 50% (MNT50), from below detection (MNT50 = 5; 1:10 dilution) to MNT50 = 1280.22
Activation-Induced Marker (AIM) assays of T cell memory responses
T cell recall responses were assessed as published.1,21 Whole blood was stimulated with overlapping SARS-CoV-2 peptide pools (1 μg/mL) of the complete ancestral spike protein (#130-127-953; Miltenyi Biotec), the immunodominant regions of the spike protein (#130-126-701; Miltenyi Biotec), or the complete Omicron B1.1.529 (BA.1) spike protein (PM-SARS2-SMUT08-1, JPT), with inclusion of a negative media control (unstimulated) and a positive polyclonal stimulation control (CytoStim™; #130-092-173; Miltenyi Biotec). Samples were stained with fluorophore-conjugated monoclonal antibodies, captured with a CytoFLEX LX (4 laser, Beckman Coulter, Brea, CA, USA), and analyzed with FlowJo v10.8.1 Software (BD Life Sciences) following established protocols.1,21 CD4+AIM+ and CD8+AIM+ T cells were identified by co-expression of CD25 and CD134 (OX40) on CD4+ T cells, and co-expression of CD69 and CD137 (4-1BB) on CD8+ T cells, with subtraction of the unstimulated sample (i.e., negative control).1,21
Statistics
Participant characteristics and antibody and cellular data were assessed by Student's t-test (mean; parametric) or Mann–Whitney U test (median; nonparametric) for continuous variables according to data normality as assessed by Shapiro–Wilk test, or by Chi-square test of independence (proportions) for categorical variables. Cumulative incidence of Omicron infection within the observation period of July 1 to September 13, 2022 was plotted by Kaplan–Meier curves, in individuals with no prior PCR-confirmed SARS-CoV-2 infections (i.e., no infections between March 2020 and June 30, 2022), one prior Omicron infection (i.e., between December 15, 2021 and June 30, 2022), one pre-Omicron infection (i.e., prior to December 15, 2021),23 and multiple prior infections (i.e., prior to and/or between December 15, 2021 and July 1, 2022), with statistical significance between the curves determined by log-rank test. Right censoring occurred when participants died, declined to continue in the study, or did not develop the outcome of a documented PCR-positive SARS-CoV-2 infection at the end of the follow-up period. Estimation of Omicron infection hazard ratios, within the observation period of July 1 to September 13, 2022, was performed by the Cox proportional-hazards regression model, with the baseline hazard on July 1, 2022. Variables reflect characteristics at baseline: age (years), sex assigned at birth (female, male), hybrid immunity status (one Omicron infection (0–8 days), one Omicron infection (9–29 days), one Omicron infection (30–75 days), one pre-Omicron infection, multiple infections, never infected), four-dose vaccine combination (mRNA-1273 × 4, BNT162b2 × 4, any mRNA combination × 4), place of residence (retirement home, long-term care home), number of facility outbreaks prior to July 1, 2022 (>6, ≤6), Clinical Frailty Scale (<6 (very fit to living with mild frailty), 6 (living with moderate frailty), >6 (living with severe frailty to terminally ill), and/or missing), and time since vaccination (days). The proportional hazard (PH) assumption was assessed with the Schoenfeld residual test. One subgroup level of the hybrid immunity status variable (i.e., one prior Omicron infection) violated the PH assumption. Interaction terms between that subgroup level and time were categorized into intervals of 0–8 days, 9–29 days, and 30–75 days in the model and hazard ratios were estimated within each interval. These time intervals were selected from the Kaplan–Meier curve case distribution, which was low between 0 and 8 days, steeply increased between 9 and 29 days, and then flattened. This is summarized by the following formula: H(t) = h0(t) exp [B1(Age) + B2(Sex) + B3(Prior Omicron Infection∗0–8 days) + B4(Prior Omicron Infection∗9–29 days) + B5(Prior Omicron Infection∗30–75 days) + B6(Prior Pre-Omicron Infection) + B7(Multiple Prior Infections) + B8(mRNA-1273 × 4) + B9(Other mRNA vaccine combination × 4) + B10(Place of Residence) + B11(Number of Facility Outbreaks), B12(Clinical Frailty Scale) + B13(Time Since Vaccination). Hazard ratios are presented with 95% confidence intervals based on robust standard errors, accounting for the clustering of participants by facility. Kaplan–Meier curves were plotted with R v4.2.0 (R Core Team). Regression analysis was performed using SAS v9.4 (SAS Institute Inc). GraphPad Prism v9.4.0 was used to plot and analyze antibody and cellular data. A P value of <0.05 was considered statistically significant.
Role of funding source
This work was funded by a grant from the Canadian COVID-19 Immunity Task Force of the Public Health Agency of Canada (021-HQ-000138) awarded to APC and DMEB. DMEB is the Canada Research Chair in Aging & Immunity. APC is the Schlegel Chair in Clinical Epidemiology and Aging. MSM holds the Canada Research Chair in Viral Pandemics. JAB was supported by a McMaster Institute for Research on Aging Postdoctoral Fellowship. The study funders had no input in the study design, data collection, analyses, and interpretation, manuscript writing, and the decision to submit for publication. Statements in this article do not necessarily reflect the position or policies of the funding bodies.
Results
Participant characteristics and Omicron infections
Incidence of Omicron infection during the initial Omicron BA.5 wave was retrospectively examined in 750 residents of retirement and long-term care homes between July 1 and September 13, 2022. At baseline (i.e., July 1, 2022), median participant age was 87.0 years, 64.4% of participants were female (n = 483), and 57.1% were in a long-term care residence (n = 428) (Table 1). All participants had received four doses of monovalent mRNA vaccines and had not yet received a bivalent vaccine. Most participants received a combination of mRNA-1273 and BNT162b2 vaccines (46.1%, n = 346).
Table 1.
Cohort demographics at baseline by observation period infection outcome.a
| N | No Omicron Infection |
Omicron Infection |
Total |
Pb |
|---|---|---|---|---|
| 617 | 133 | 750 | ||
| Age (years) | ||||
| Mean (SD) | 84.9 (9.7) | 83.4 (11.5) | 84.7 (10.1) | – |
| Median (IQR) | 87.0 (80.0–92.0) | 86.0 (78.0–92.0) | 87.0 (80.0–92.0) | 0.36 |
| Sex—N (%) | ||||
| Female | 402 (65.2%) | 81 (60.9%) | 483 (64.4%) | 0.35 |
| Male | 215 (34.9%) | 52 (39.1%) | 267 (35.6%) | |
| Hybrid immunity status—N (%) | ||||
| Never infected | 438 (71.0%) | 44 (33.1%) | 482 (64.3%) | <0.0001 |
| Multiple infections | 58 (9.4%) | 3 (2.3%) | 61 (8.1%) | |
| One pre-Omicron infection | 66 (10.7%) | 10 (7.5%) | 76 (10.1%) | |
| One prior Omicron infection | 55 (8.9%) | 76 (57.1%) | 131 (17.5%) | |
| mRNA four-dose vaccine combination—N (%) | ||||
| mRNA1273 × 4 | 219 (35.5%) | 47 (35.3%) | 266 (35.5%) | <0.0001 |
| BNT162b2 × 4 | 89 (14.4%) | 49 (36.8%) | 138 (18.4%) | |
| Other mRNA combination × 4 | 309 (50.1%) | 37 (27.8%) | 346 (46.1%) | |
| Time since fourth vaccination to baseline (days)c | ||||
| Mean (SD) | 141 (33) | 125 (46) | 138 (37) | – |
| Median (IQR) | 155 (135–185) | 147 (65–176) | 154 (130–185) | 0.0041 |
| Residence type–N (%) | ||||
| Long-term care residence | 319 (51.7%) | 109 (82.0%) | 428 (57.1%) | <0.0001 |
| Retirement residence | 298 (48.3%) | 24 (18.0%) | 322 (42.9%) | |
| Residence outbreaks—N | ||||
| Mean (SD) | 6.8 (1.8) | 6.2 (1.6) | 6.7 (1.8) | – |
| Median (IQR) | 6.0 (6.0–7.0) | 6.0 (5.0–7.0) | 6.0 (6.0–7.0) | <0.0001 |
| Participants in residences with outbreaks–N (%) | ||||
| ≤6 outbreaks | 351 (56.9%) | 97 (72.9%) | 448 (59.7%) | 0.0006 |
| >6 outbreaks | 266 (43.1%) | 36 (27.1%) | 302 (40.3%) | |
| Clinical Frailty Scale—(1 to 9 ranking)d | ||||
| Missing (N) | 120 | 12 | 132 | – |
| Mean (SD) | 6.5 (1.1) | 6.7 (0.8) | 6.6 (1.1) | – |
| Median (IQR) | 7.0 (6.0–7.0) | 7.0 (7.0–7.0) | 7.0 (6.0–7.0) | 0.036 |
| Clinical Frailty Scale—number of participants—N (%)d | ||||
| CFS <6 | 66 (10.7%) | 6 (4.5%) | 72 (9.6%) | 0.016 |
| CFS 6 | 111 (18.0%) | 23 (17.3%) | 134 (17.9%) | |
| CFS >6 | 320 (51.9%) | 92 (69.2%) | 412 (54.9%) | |
| Comorbidities–Ne | ||||
| Missing (N) | 59 | 4 | 63 | – |
| Mean (SD) | 4.6 (2.0) | 4.7 (2.1) | 4.7 (2.1) | – |
| Median (IQR) | 4.0 (3.0–6.0) | 4.0 (3.0–6.0) | 4.0 (3.0–6.0) | 0.90 |
| Immunosuppressive medications—number of participants—Nf | ||||
| Missing | 118 | 8 | 126 | – |
| No | 472 (94.6%) | 124 (99.2%) | 596 (95.5%) | 0.026 |
| Yes | 27 (5.4%) | 1 (0.8%) | 28 (4.5%) | |
Data as of baseline on July 1, 2022. Residence outbreaks and Clinical Frailty Scale are reported individually and by grouping participants.
Data were assessed by non-parametric Mann–Whitney U-test for two-group comparisons of continuous variables including CFS and by Chi-square test for categorical variables.
All participants had four mRNA vaccine vaccinations at the start of the observation window on July 1, 2022.
Clinical Frailty Scale is a 9-point scale from 1 (very fit) to 9 (terminally ill). Data are reported as the CFS and by grouping participants with a CFS <6 (very fit to living with mild frailty), 6 (living with moderate frailty), and >6 (living with severe frailty to terminally ill).
Incidence of comorbidities also summarized in Supplementary Table S1. List of comorbidities: emphysema, hypertension, diabetes, heart disease, angina, cancer, memory problems, Alzheimer's disease/dementia, osteoarthritis, rheumatoid arthritis, peripheral vascular disease, stroke, transient ischemic attack, Parkinson's disease, stomach ulcers, bowel disorder, cataracts, glaucoma, macular degeneration, osteoporosis, back problems, thyroidism, and kidney disease.
Number of participants prescribed immunosuppressive medications; a list of medications is provided in Supplementary Table S2 and a summary of medication use within the cohort is provided in Supplementary Table S3.
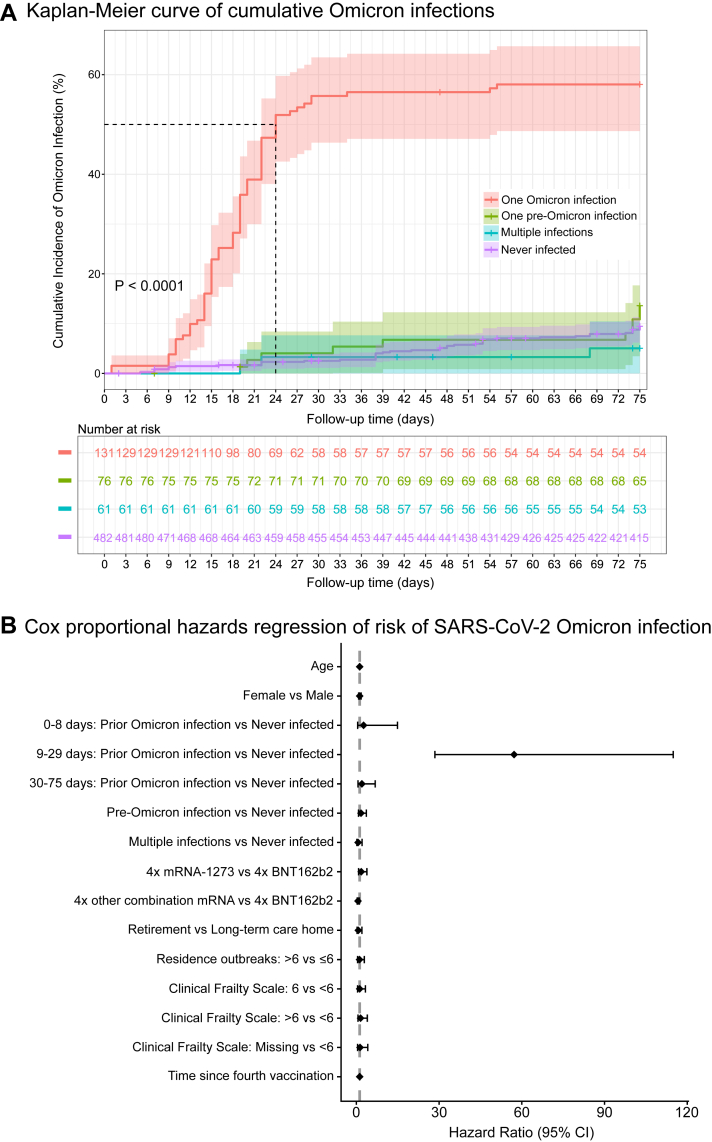
Prior to the observation period, 35.7% (n = 268/750) of participants had at least one prior SARS-CoV-2 infection, 17.5% (n = 131/750) of whom had one Omicron infection. During the observation period, 17.7% (n = 133/750) of participants had an Omicron infection. Age and sex were similar for participants with and without Omicron infection during the observation period. Participants with an Omicron infection more often received four BNT162b2 vaccines (Omicron Infection: 36.8%, n = 49/133; No Omicron Infection: 14.4%, n = 89/617) and resided in long-term care homes (Omicron Infection: 82.0%, n = 109/133; Infection: 51.7%, n = 319/617), though the incidence of residence outbreaks was lower (≤6 outbreaks—Omicron Infection: 72.9%, n = 97/133; No Omicron Infection: 56.9%, n = 351/617). Participants with an Omicron infection in the observation period were also more likely to have a Clinical Frailty Score greater than 6 (Omicron Infection: 69.2%, n = 92/133; No Infection: 51.9%, n = 320/617), though the number of comorbidities was similar (Omicron Infection median [IQR]: 4.0 (3.0–6.0); No Omicron Infection: 4.0 (3.0–6.0) (also see Supplementary Table S1), and less than 5% of participants were prescribed immunosuppressive medications (also see Supplementary Tables S2 and S3). During the observation period, 57.1% (n = 76/133) of participants with an Omicron infection outcome had a prior Omicron infection, whereas for participants with an outcome of no Omicron infection, 71.0% (n = 438/617) had no prior SARS-CoV-2 infections. This observation is also apparent by Kaplan–Meier plot (Fig. 1 Panel A), as cumulative probability of Omicron infection was highest in individuals with one Omicron infection (and no pre-Omicron SARS-CoV-2 infections) prior to the observation period (P < 0.0001).
Fig. 1.
SARS-CoV-2 Omicron infection incidence and risk. In Panel A, time to SARS-CoV-2 Omicron infection from July 1 through September 13, 2022, was estimated in individuals with one prior Omicron infection (i.e., between December 15, 2021 and June 30, 2022, with no pre-Omicron infections), one pre-Omicron infection (i.e., infection prior to December 15, 2021), multiple prior infections (i.e., prior to and/or between December 15, 2021 and July 1, 2022), and no prior infections (i.e., no infections between March 2020 and June 30, 2022), by means of the Kaplan–Meier method. Statistical significance between the curves was determined by log-rank test. Shading around the mean line indicates the 95% confidence interval. The dotted horizontal-vertical line indicates 50% cumulative incidence. In Panel B, the Cox proportional-hazards regression model was used to estimate hazard ratios of Omicron infection between July 1 and September 13, 2022, with the baseline hazard on July 1, 2022. Variables (age, sex, previous infection, mRNA vaccine combination, residence type, number of outbreaks, clinical frailty scale, time since fourth vaccination) reflect characteristics at baseline, with adjustments for study site. The hazard ratios are presented with 95% confidence intervals based on robust standard errors.
Risk factors for Omicron BA.5 infection
We next assessed factors that contributed to risk of Omicron infection using a Cox proportional hazards regression model (Fig. 1 Panel B; Supplementary Figure S1; Supplementary Tables S4 and S5). Risk of Omicron infection was not associated with chronological age, sex assigned at birth, residence type (retirement home compared to long-term care home), or number of previous residence outbreaks. Comparison of four mRNA-1273 vaccines to four BNT162b2 vaccines revealed no significant changes in Omicron infection risk, though there was an associated decreased risk of infection in participants with four-dose combinations of both mRNA-1273 and BNT162b2 vaccines compared to four BNT162b2 vaccines (P = 0.023; HR [95% CI]: 0.49 [0.26–0.90]). Frailty and time since fourth vaccination were also not associated with Omicron infection risk. Individuals with a prior Omicron infection had the highest associated risk of an Omicron infection during the observation period, in particular between days 9 and 29, with a large confidence interval (P < 0.0001; 47.67 [23.73–95.76]). Most Omicron reinfections occurred at ∼5 months after the initial infection (days to reinfection: mean ± SD 156.2 ± 41.4 days; median [IQR] 173.0 [118.5–181.0] days). We hypothesized that there may be individual immune-associated factors that are modifiers of Omicron reinfection risk after one Omicron infection.
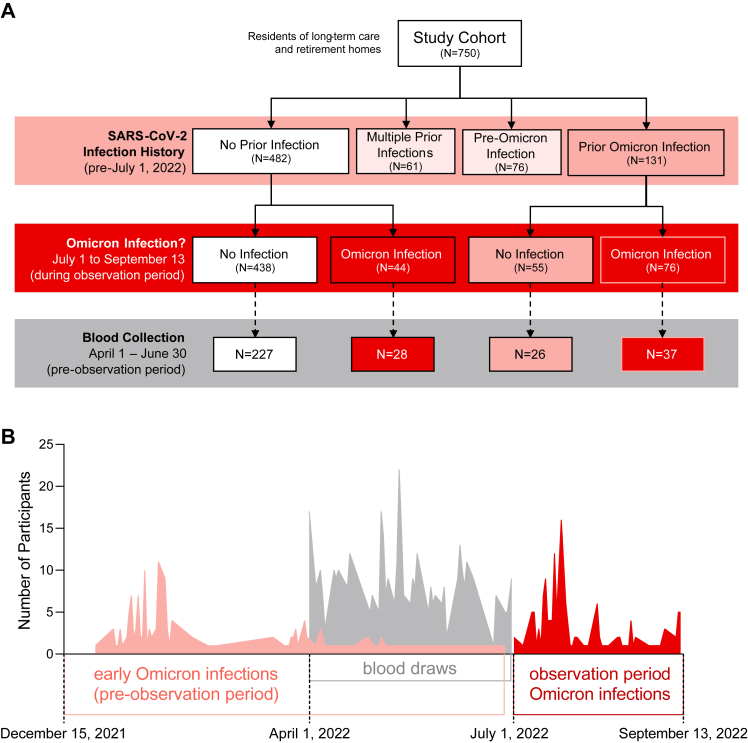
Description of immune analysis cohort
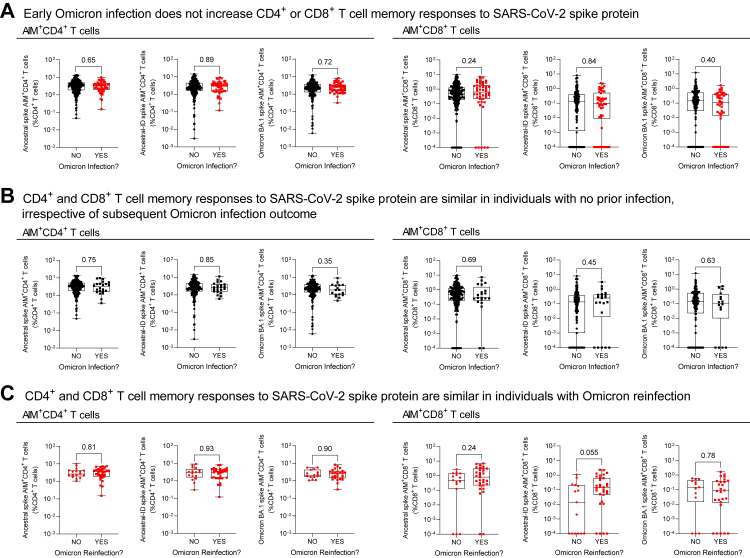
We measured immunological parameters in a random subset of participants with blood collections (n = 318), as part of routine vaccine immunogenicity surveillance, within three months of the start of the observation period (i.e., April 1 to June 30, 2022). We considered participant early Omicron infection history (i.e., presumptive Omicron BA.1/2 infection after December 15, 2021) prior to their blood collection and July 1, 2022 (i.e., the start of the observation period), as well as Omicron infection outcome (i.e., presumptive Omicron BA.5 infection) post-blood collection between July 1 and September 13, 2022 (i.e., the observation period) in context of their early Omicron BA.1/2 infection history (Fig. 2). Immune analysis cohort data by pre-observation period Omicron BA.1/2 infection history are summarized in Supplementary Tables S6–S9, and immune analysis cohort data by pre- and post-observation period Omicron BA.5 infection history are summarized in Supplementary Tables S7–S11. The time intervals between blood collections and the start of the observation period were similar between all comparator groups. Serum anti-spike and anti-RBD IgG and IgA antibodies (Fig. 3), serum antibody neutralization of ancestral and Omicron BA.1 subvariant SARS-CoV-2 (Fig. 4), and whole blood CD4+ and CD8+ T cell memory responses to ancestral and Omicron BA.1 spike protein (Fig. 5), were assessed.
Fig. 2.
Overview of the study cohort. An overview of the study cohort by participant infection history and blood collections is shown in Panel A by flow chart and Panel B as a timeline.
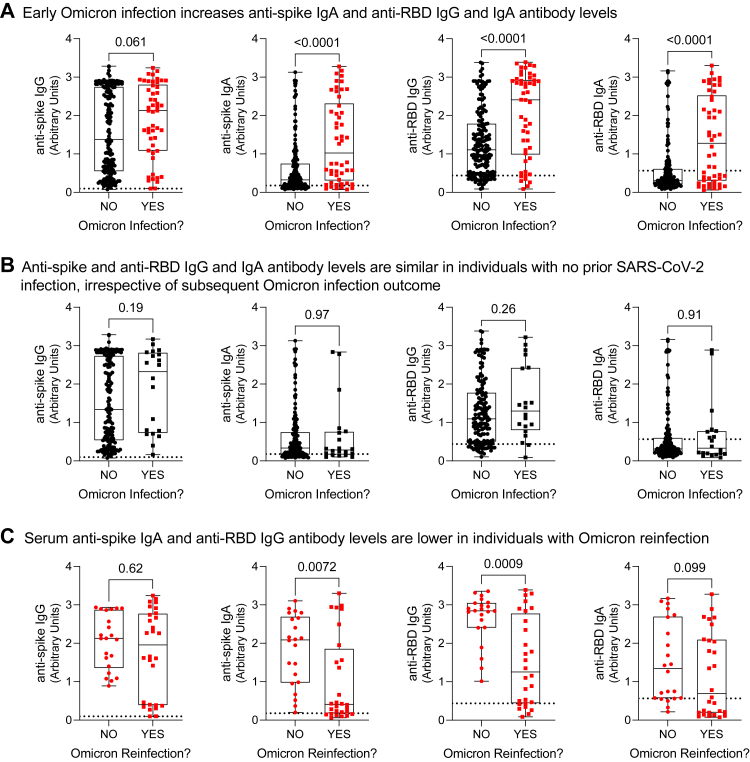
Fig. 3.
Serum antibody levels in residents of long-term care and retirement facilities in context of hybrid immunity. Ancestral anti-spike and anti-RBD (receptor binding domain) IgG and IgA antibody levels were measured by ELISA in serum samples collected within three months prior to the start of the observation period (i.e., collected between April 1 and June 30 before the July 1 to September 13, 2022 observation period). Panel A shows data according to Omicron infection history prior to July 1, 2022. Panel B stratifies data by Omicron infection outcome between July 1 and September 13 in individuals with no prior Omicron infection from panel A. Panel C stratifies data by Omicron reinfection outcome between July 1 and September 13 in individuals with prior Omicron infection from panel A. Each data point indicates an individual participant. Data are presented as box and whisker plots, minimum to maximum, with the center line at the median. Dotted lines indicate cutoff thresholds. Statistical significance was assessed by Mann–Whitney U test. All P-values are shown.
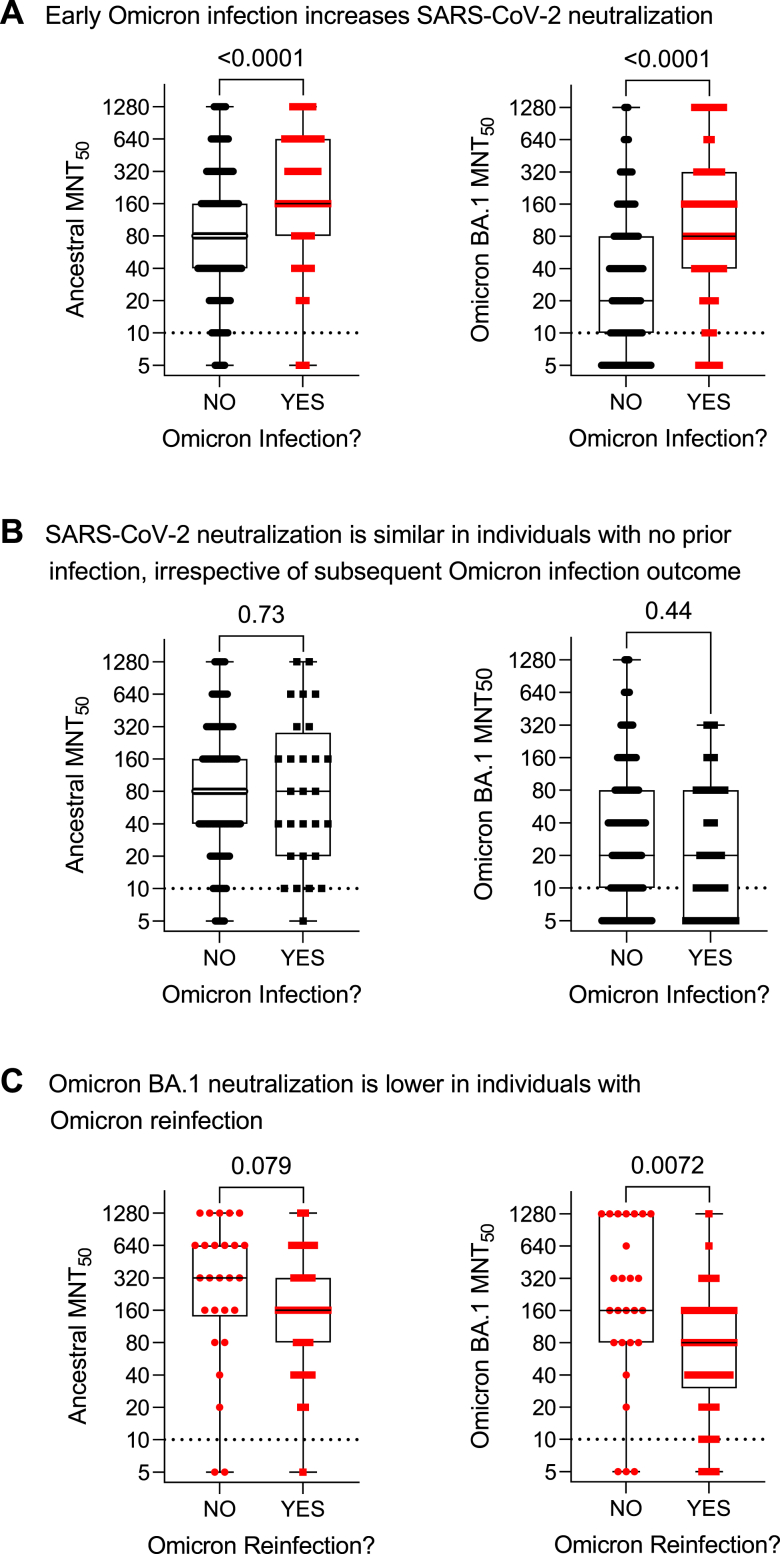
Fig. 4.
Serum anti-SARS-CoV-2 microneutralization titres in residents of long-term care and retirement facilities in context of hybrid immunity. Microneutralization titres (MNT50) of ancestral and Omicron BA.1 anti-SARS-CoV-2 antibodies in serum samples collected within three months prior to the start of the observation period (i.e., collected between April 1 and June 30 before the July 1 to September 13, 2022 observation period). Panel A shows data according to Omicron infection history prior to July 1, 2022. Panel B stratifies data by Omicron infection outcome between July 1 and September 13 in individuals with no prior Omicron infection from panel A. Panel C stratifies data by Omicron reinfection outcome between July 1 and September 13 in individuals with prior Omicron infection from panel A. Dotted lines indicate cutoff thresholds. Each data point indicates an individual participant. Data are presented on a log2 scale as box and whisker plots, minimum to maximum, with the center line at the median. Dotted lines indicate cutoff thresholds. Statistical significance was assessed by Mann–Whitney U test. All P-values are shown.
Fig. 5.
Memory T cell recall responses to SARS-CoV-2 spike protein in residents of long-term care and retirement facilities in context of hybrid immunity. Memory T cell recall responses to SARS-CoV-2 complete ancestral spike protein, immunodominant regions of ancestral spike protein (ancestral-ID), and complete Omicron BA.1 spike protein in whole blood were assessed by an Activation-Induced Marker (AIM) flow cytometry assay within three months prior to the observation period (i.e., collected between April 1 and June 30 before the July 1 to September 13, 2022 observation period). Panel A shows data according to Omicron infection history prior to July 1, 2022. Panel B stratifies data by Omicron infection outcome between July 1 and September 13 in individuals with no prior Omicron infection from panel A. Panel C stratifies data by Omicron reinfection outcome between July 1 and September 13 in individuals with prior Omicron infection from panel A. Data are plotted on a log10 scale. Each data point indicates an individual participant. Data are presented as box and whisker plots, minimum to maximum, with the center line at the median. Statistical significance was assessed by Mann–Whitney U test. All P-values are shown.
Humoral and cellular immunity after early Omicron BA.1/2 infection
We first considered all participants with immunogenicity data in context of their early Omicron infection history, comparing humoral and cellular data collected from participants with no infections prior to the observation period (i.e., prior to July 1, 2022) to data collected from participants after an early Omicron infection before the observation period (i.e., between December 15, 2021 and June 30, 2022). In the three months before the observation period, relative to participants with no Omicron infection, individuals with a prior Omicron infection (days since Omicron infection median [IQR]: 101.0 [55.0–127.0]) had increased serum antibodies, in particular anti-spike IgA and anti-RBD IgG and IgA (Fig. 2, panel A; Supplementary Table S9), as well as increased neutralization capacity against ancestral and Omicron BA.1 subvariant SARS-CoV-2 (Fig. 3, panel A). Notably, serum antibody quantities and their neutralizing capacity showed considerable heterogeneity (Supplementary Figure S2). While on a population-level there is a positive correlation between microneutralization titres and IgG and IgA antibodies, these measurements do not necessarily correlate on an individual basis. T cell memory responses to the SARS-CoV-2 spike protein were not different (Fig. 4, panel A). In summary, humoral immunity was enhanced in participants with hybrid immunity.
Humoral and cellular immunity with no prior SARS-CoV-2 infection in context of Omicron BA.5 infection outcome
We next compared humoral and cellular data collected from participants with no infections prior to the observation period (i.e., participants with no infections from Panel A in Fig. 3, Fig. 4, Fig. 5) in context of their Omicron infection outcome between July 1 and September 13, 2022 (i.e., no Omicron infection or Omicron infection). There were no distinct differences in humoral and cellular immunogenicity in this population by infection outcome. In the three months preceding the observation period, individuals with no prior Omicron infection had similar antibody levels (Fig. 3, panel B; Supplementary Table S10), microneutralization titres (Fig. 4, panel B), and T cell memory responses (Fig. 5, panel B), irrespective of Omicron infection outcome during the observation period.
Humoral and cellular immunity after Omicron BA.1/2 infection in context of Omicron BA.5 infection outcome
Finally, we compared humoral and cellular data collected after an early Omicron infection (i.e., between December 15, 2021 and June 30, 2022) in participants with prior Omicron infections from Panel A in Fig. 3, Fig. 4, Fig. 5, according to their Omicron infection outcome between July 1 and September 13, 2022 (i.e., no Omicron infection or Omicron reinfection). The time interval between prior Omicron infection to the date of blood draws was comparable (P = 0.34) between individuals not reinfected (median [IQR]: 105 [49–139] days) and reinfected (101 [65–108] days). Omicron reinfections occurred (median [IQR]) 72 [48–79] days after the blood collection. This assessment showed that although prior Omicron infection overall promoted higher serum antibody levels and neutralization in the three months before the observation period (Fig. 2, Fig. 3, Fig. 4 panel A), this effect was not consistent. Rather, there were distinct immunological differences according to whether individuals were reinfected with Omicron (Fig. 2, Fig. 3, Fig. 4, panel C; Supplementary Table S11). Individuals with a documented Omicron infection preceding the observation period, if they were reinfected during the observation period, had significantly lower post-initial Omicron infection serum anti-RBD IgG and anti-spike IgA antibodies, and a tendency toward lower anti-spike IgG antibodies, as well as lower neutralization of the Omicron BA.1 variant. Memory T cell responses were similar irrespective of infection history or outcome. Therefore, there were lower humoral but not cellular immune responses after initial Omicron infection in individuals with an Omicron reinfection.
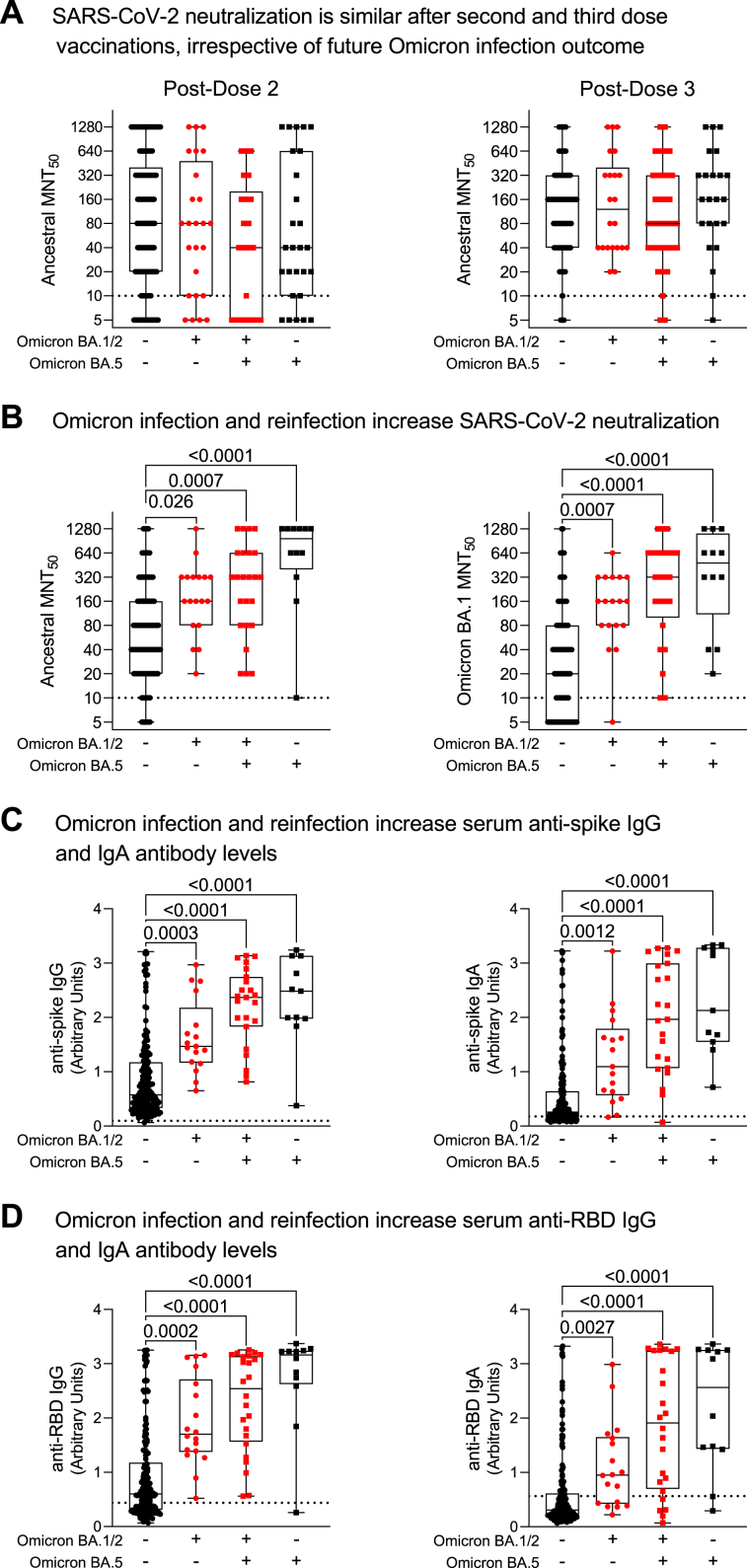
Humoral immunity before and after Omicron reinfection
To determine if individuals with Omicron reinfections had normal immune responses prior to their Omicron infections, we measured their vaccine responses post-second and third doses (Fig. 6, Panel A). Prior to initial Omicron infections, there were no statistically significant differences in vaccine responses (as measured by neutralizing antibodies) between individuals who were subsequently never infected, had a single Omicron infection, or those with Omicron reinfection. These data indicate that in individuals with subsequent Omicron reinfection, there were no immune abnormalities impeding the generation of hybrid humoral immunity against SARS-CoV-2 after their initial presumptive Omicron BA.1/2 infection, despite significant differences in humoral immunity after their initial Omicron BA.1/2 infection (Fig. 3, Fig. 4, Fig. 5). When immune responses after presumptive Omicron BA.5 infections were investigated, individuals who had two Omicron infections were able to mount strong hybrid immune responses after Omicron BA.5 infection (i.e., elevated IgG, IgA and neutralizing antibodies; Supplementary Figure S3). This humoral hybrid immune response after Omicron reinfection was similar to that of other individuals after a single Omicron infection, whether BA.1/2 or BA.5 (Fig. 6, Panels B to D). Collectively, these data support the hypothesis that the increased risk of Omicron reinfection after an initial Omicron infection was a result of unique differences in biological responses to the initial infection, not an underlying immune defect or immunosuppression.
Fig. 6.
Humoral responses to SARS-CoV-2 vaccination and Omicron infections. In Panel A, post-vaccination neutralizing antibodies against ancestral SARS-CoV-2 were measured after two and three mRNA vaccine doses and prior to future Omicron BA.1/2 and BA.5 infection outcomes. Humoral responses were examined in Panels B to D in serum samples collected after the observation period baseline and Omicron BA.5 infections. Ancestral anti-spike and anti-RBD (receptor binding domain) IgG and IgA antibody levels were measured by ELISA. Neutralizing antibodies against ancestral and Omicron BA.1 anti-SARS-CoV-2 were assessed by microneutralization assays (MNT50). Each data point indicates an individual participant. Data are presented as box and whisker plots, minimum to maximum, with the center line at the median. Data in Panels A and B are presented on a log2 scale. Dotted lines indicate cutoff thresholds. Statistical significance was assessed by Kruskal–Wallis test with Dunn's multiple comparisons test. P values are only shown for post-hoc comparisons of statistical significance.
Discussion
In this study, we found that residents of long-term care and retirement homes who received monovalent vaccinations and were infected with the SARS-CoV-2 Omicron BA.1/2 variants in early 2022 had an associated increased risk of subsequent Omicron BA.5 infection, compared to individuals who never had an Omicron infection. As all study participants with an early Omicron infection had received four mRNA vaccines before the observation period, and time since vaccination did not significantly contribute to Omicron infection risk, these findings challenged the concept that hybrid immunity increases protection against subsequent SARS-CoV-2 infection with Omicron subvariants.
Further investigation of those individuals with an early presumptive Omicron BA.1/2 infection revealed that many individuals with hybrid immunity (i.e., vaccination with natural infection) had elevated post-infection humoral immune responses. Despite similar generation of neutralizing antibodies after second and third vaccinations prior to initial Omicron infections, individuals with an Omicron reinfection during the observation period generally had reduced Omicron neutralization and lower levels of serum antibodies after their initial Omicron infection compared to individuals without Omicron reinfection. Yet, after a presumptive Omicron BA.5 infection, individuals who had both BA.1/2 and BA.5 infections were able to mount hybrid humoral immune responses that were comparable to responses of individuals with a single Omicron infection, whether Omicron BA.1/2 or Omicron BA.5. Moreover, our data showed that older adults have considerable heterogeneity in humoral responses post-vaccination and with hybrid immunity, even with similar vaccination and infection histories. Quantities of serum IgG and IgA antibodies were not always indicative of SARS-CoV-2 neutralizing capacity, or vice versa. This heterogeneity of humoral responses is likely reflected in the large confidence interval observed for the association between early Omicron infection and reinfection risk.
Despite their established importance in limiting progression of SARS-CoV-2 infection,24 there were no differences in ancestral and Omicron BA.1 SARS-CoV-2 spike-specific CD4+ and CD8+ T cell memory responses in individuals with an early Omicron BA.1/2 infection, irrespective of whether they had a subsequent Omicron BA.5 infection. However, this does not preclude the possibility that there may be differences in T cell polyfunctional cytokine responses or the roles of CD4+ T cells in supporting antibody production. Future studies may provide insight into whether the generation of memory T cells against other components of the SARS-CoV-2 virus after an initial Omicron infection may also differ in individuals with Omicron reinfections.
Older adults in congregate living in Canada have been a priority group for SARS-CoV-2 vaccinations.25 This is likely a contributing factor to their lower rates of Omicron infections when compared to community-dwelling older adults, though they still have more severe illness and higher hospitalization rates.7 Many countries, including Canada, have adopted vaccination guidelines stating that after a confirmed SARS-CoV-2 infection, the ideal time for subsequent vaccination is 6 months, though a shortened interval of 3 months may be considered in the context of increased risk of infection or severe outcomes.26 The study cohort included participants from public and private facilities of a range of sizes, both non-profit and for-profit, which follow provincially-mandated directives concerning infection prevention and control practices, and are representative of typical long-term care and retirement homes in Ontario, Canada. As most individuals in our study cohort had an Omicron reinfection at less than 6 months from their prior Omicron infection, a shortened vaccination interval may be beneficial in maintaining immune protection within this population. Alternatively, bivalent vaccines, with ancestral and Omicron variant mRNA, may provide superior longevity of immune protection. Irrespective, when considering older adults, a more nuanced perspective of protection provided by hybrid immunity may be necessary, which considers individualized risk assessments to optimize vaccination strategies. This risk assessment may need to incorporate measurements of immunity rather than more traditional assessments including frailty, as we did not observe an association between Omicron infection risk and the Clinical Frailty Scale. As noted above, we found that individuals who were not reinfected had higher serum antibody levels and neutralization capacity, compared to individuals with Omicron reinfection, suggesting that there were differences in either their initial hybrid immune response, or the durability of their immunity. While correlates of protection against Omicron variant reinfection remain poorly defined, these observations imply that maintenance of antibody levels and neutralization in older adults by vaccination, irrespective of infection history, is critically important for longevity of immune protection. Our findings likely extend to community-dwelling older adults, who may not have the same immune histories as those in retirement or long-term care homes, but who also experience detrimental immunosenescence and inflammaging.27
Individual histories of SARS-CoV-2 vaccination, variant exposure, and infection may have an imprinting effect on immunity and modify protection against current and emerging variants of concern.28,29 Pre-Omicron infections were reported to provide lasting protection against reinfection with pre-Omicron variants in older adults in care homes.30 As mentioned, we in addition found in older adults that SARS-CoV-2 infection in fall 2022 (i.e., presumptive Delta variant infection) was associated with reduced risk of early Omicron BA.1/2 infection.10 Early Omicron infections in healthy younger adults were initially implicated in reducing risk of subsequent Omicron infection, whether in absence of vaccination, or in context of hybrid immunity.31,32 However, the extent and interval of protection after early Omicron variant infection may be shorter than after infection with pre-Omicron variants of concern.33,34 Neutralizing antibodies produced after Omicron BA.1 infection were reported to be less efficacious against Omicron BA.5 compared to early Omicron subvariants and pre-Omicron variants, whether after early Omicron infection or within infection-naïve vaccinated individuals.29,35,36 After emergence of the more transmissible Omicron subvariant BA.5, for example, it was reported that both mRNA1273 and BNT162b2 vaccines, after three or four doses, may only offer short-term protection from BA.5 infection.37 These observations may explain why Omicron BA.5 replaced BA.1/BA.2 as the dominant circulating virus, and why individuals in our cohort who had lower humoral hybrid immunity, including reduced neutralization of the Omicron BA.1 virus, had an Omicron reinfection (most likely a BA.5 infection after initial BA.1/BA.2 infection, based on infection dates and provincial genomic surveillance data13). Therefore, consideration of SARS-CoV-2 variant-specific infection history, as well as circulating Omicron sub-lineage, are likely important when exploring associations of Omicron-associated hybrid immunity and vaccine efficacy. Further investigations of vaccine immunogenicity and hybrid immunity in context of other Omicron subvariants may provide additional insights to explain our observations of disparate post-early Omicron infection humoral responses, and may facilitate identification of risk factors that contribute to poor hybrid immunity in older adults.
This study has several limitations. As this was a retrospective observational study, individuals were not matched by age, sex, or other factors, asymptomatic infections may have been overlooked, and data should not be interpreted to confer causality. Although we were able to rule out residence type or outbreak history as contributing factors, other environmental and behavioural factors may have influenced individual risk of exposure. In addition, while frailty was not associated with increased infection risk, differences in individual requirements for daily care may require increased interactions, potentially increasing risk of exposure and infection. Genomic sequencing was unavailable to confirm infection by a specific Omicron variant. Based on public health surveillance data, it was assumed that infections were caused by an Omicron BA.1/BA.2 variant prior to the observation period, or by Omicron BA.5 during the observation period. It was outside the scope of this study to consider how facility-associated and individual health-associated factors influenced humoral and cellular assessments. As infection symptoms, severity and outcomes were unknown, risk analyses and immunological measurements were not stratified by those data.
Overall, our observations caution that immunological features of hybrid immunity are not the same in all older adults, and hybrid immunity should not be considered a panacea against future SARS-CoV-2 infection, whether from cross-subvariant Omicron infections, or future variants of concern. Continued public health surveillance and research are necessary to assess longevity of vaccine responses and hybrid immunity as new variants of concern continue to emerge. More extensive characterization of humoral and cellular immunity after SARS-CoV-2 infection may aid in developing interventions to prevent exacerbation of age-associated immune dysfunction and reinfection risk.
Contributors
JAB—investigation, data curation, formal analysis, validation, visualization, writing—original draft, writing—review and editing. AR—investigation, data curation, formal analysis, validation, visualization, writing—review and editing. HB, AZ, JA, HDS, LL, AK—investigation, methodology, validation, data curation. MH—methodology, validation, data curation, project administration. TK—data curation, project administration. RC—investigation, methodology, validation, data curation, writing—review and editing. JLB, IN, MSM—investigation, methodology, resources, supervision, writing—review and editing. APC, DMEB—conceptualization, funding acquisition, resources, supervision, validation, writing—review and editing. All authors had access to the data, accepted responsibility to submit for publication, and read and approved the final version of the manuscript. JAB, AR, APC and DMEB verified the underlying data. APC and DMEB accepted full responsibility for the work and controlled the decision to publish.
Data sharing statement
Deidentified participant data from this study are available upon reasonable request from the corresponding author.
Declaration of interests
TK has received funding from the COVID-19 Immunity Task Force of the Public Health Agency of Canada to attend a conference of the COVID-19 Immunity Task Force. MSM has received research grants from Providence Therapeutics, Medicago, Bay Area Health Trust, and Lactiga, and honoraria for speaking at Boehringer Ingelheim and development of COVOICES Educational Initiatives at Sanofi, as well as consulting fees from Seqirus, Sanofi, and Grifols, has patents in unrelated technology, holds stock with Aeroimmune Biotherapeutics and stock options with Kapoose Creek Wellness, and is a member of the COVID-19 working group of the National Advisory Committee on Immunization (NACI). APC has received other research funding from the COVID-19 Immunity Task Force of the Public Health Agency of Canada. DMEB has received honoraria from AstraZeneca Mexico for a lecture, consulting fees from Pfizer and AstraZeneca, and holds a volunteer position on the Board of Directors of the Ontario Lung Health Foundation. All other authors declare no competing interests.
Acknowledgements
Data in this study were collected by the COVID in LTC Study Group. Other members of the COVID in LTC Study investigator group include Kevin Brown, David C. Bulir, Judah Denburg, George A. Heckman, John P. Hirdes, Aaron Jones, Mark Loeb, Janet E. McElhaney (posthumous), Nathan M. Stall, Marek Smieja, Ahmad Von Schlegell, Kevin Stinson, Arthur Sweetman, Chris Verschoor, and Gerry Wright. We acknowledge administrative and technical assistance from other members of the COVID in LTC Study team including Lucas Bilaver, Timothy Boniface, Jessa Calderon, Braeden Cowbrough, Waverley Fung, Sussan Kianpour, Zain Pasat, Maya Potter, Javier Ruiz Ramírez, Lindsay Scherer, Leslie Tan, Jodi C. Turner, and Ying Wu. We thank our participants, their families, as well as staff, for their support of this study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102148.
Appendix A. Supplementary data
References
- 1.Kennedy A.E., Cook L., Breznik J.A., et al. Lasting changes to circulating leukocytes in people with mild SARS-CoV-2 infections. Viruses. 2021;13(11):2239. doi: 10.3390/v13112239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forecasting Team C-aL, SS Past SARS-CoV-2 infection protection against reinfection: a systematic review and meta-analysis. Lancet. 2022;401(10379):833–842. doi: 10.1016/S0140-6736(22)02465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobrovitz N., Ware H., Ma X., et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023;23(5):556–567. doi: 10.1016/S0140-6736(22)02465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng L., Li P., Zhang X., et al. Risk of SARS-CoV-2 reinfection: a systematic review and meta-analysis. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-24220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung C., Kmiec D., Koepke L., et al. Omicron: what makes the latest SARS-CoV-2 variant of concern so concerning? J Virol. 2022;96(6) doi: 10.1128/jvi.02077-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . 2022. COVID-19 weekly epidemiological update.https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---18-may-2022 [Google Scholar]
- 7.Rochon P.A., Li J.M., Johnstone J., et al. 2022. Born on behalf of the congregate care setting working group and the Ontario COVID-19 science advisory table. The COVID-19 pandemic's impact on long-term care homes: five lessons learned: Ontario COVID-19 science advisory table. [Google Scholar]
- 8.Public Health Ontario . 2023. Ontario COVID-19 data tool.https://www.publichealthontario.ca/en/data-and-analysis/infectious-disease/covid-19-data-surveillance/covid-19-data-tool [Google Scholar]
- 9.Ontario Agency for Health Protection and Promotion (Public Health Ontario) Ontario Immunization Advisory Committee Recommendations: fourth COVID-19 vaccine dose long-term care home residents and older adults in other congregate settings. https://www.publichealthontario.ca/-/media/documents/ncov/vaccines/2022/01/covid-19-oiac-4th-dose-recommendations-older-adults-ltc.pdf?sc_lang=en Toronto, Ontario.
- 10.Breznik J.A., Rahim A., Kajaks T., et al. Protection from omicron infection in residents of nursing and retirement homes in Ontario, Canada. J Am Med Dir Assoc. 2023;24(5):753–758. doi: 10.1016/j.jamda.2023.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsang N.N.Y., So H.C., Cowling B.J., Leung G.M., Ip D.K.M. Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and symptomatic infection of SARS-CoV-2 omicron BA.2 in Hong Kong: a prospective cohort study. Lancet Infect Dis. 2023;23(4):421–434. doi: 10.1016/S1473-3099(22)00732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fong C.H., Zhang X., Chen L.L., et al. Effect of vaccine booster, vaccine type, and hybrid immunity on humoral and cellular immunity against SARS-CoV-2 ancestral strain and Omicron variant sublineages BA.2 and BA.5 among older adults with comorbidities: a cross sectional study. eBioMedicine. 2023;88 doi: 10.1016/j.ebiom.2023.104446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ontario Agency for Health Protection and Promotion (Public Health Ontario) 2022. Epidemiologic summary: SARS-CoV-2 whole genomic sequencing in Ontario.https://www.publichealthontario.ca/-/media/Documents/nCoV/Archives/Genome/2022/07/sars-cov-2-genomic-surveillance-report-2022-07-19.pdf?rev=da4351fd7fab421ca5cdfa0b695c477f&sc_lang=en Torongto, ON. [Google Scholar]
- 14.Theou O., Pérez-Zepeda M.U., van der Valk A.M., Searle S.D., Howlett S.E., Rockwood K. A classification tree to assist with routine scoring of the Clinical Frailty Scale. Age Ageing. 2021;50(4):1406–1411. doi: 10.1093/ageing/afab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Care OMoL-T, editor. Long-term care home COVID-19 data. 2023. Long-term care home COVID-19 data. Ontario Data Catalogue. [Google Scholar]
- 16.National Advisory Committee on Immunization . Public Health Agency of Canada; 2021. Guidance on booster COVID-19 vaccine doses in Canada - update December 3, 2021. [Google Scholar]
- 17.Ontario Agency for Health Protection and Promotion (Public Health Ontario) 2022. Epidemiologic summary: SARS-CoV-2 whole genome sequencing in Ontario, January 11, 2022. Toronto, ON. [Google Scholar]
- 18.Ontario Agency for Health Protection and Promotion (Public Health Ontario) 2022. Epidemiologic summary: SARS-CoV-2 whole genome sequencing in Ontario, January 25, 2022. Toronto, ON. [Google Scholar]
- 19.Ontario Agency for Health Protection and Promotion (Public Health Ontario) 2022. Epidemiologic summary: SARS-CoV-2 whole genome sequencing in Ontario, July 22, 2022. Toronto, ON. [Google Scholar]
- 20.Ontario Agency for Health Protection and Promotion (Public Health Ontario) 2022. Epidemiologic summary: SARS-CoV-2 whole genomic sequencing in Ontario, October 7, 2022. Toronto, Ontario. [Google Scholar]
- 21.Breznik J.A., Huynh A., Zhang A., et al. Cytomegalovirus seropositivity in older adults changes the T cell repertoire but does not prevent antibody or cellular responses to SARS-CoV-2 vaccination. J Immunol. 2022;209(10):1892–1905. doi: 10.4049/jimmunol.2200369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huynh A., Arnold D.M., Smith J.W., et al. Characteristics of anti-SARS-CoV-2 antibodies in recovered COVID-19 subjects. Viruses. 2021;13(4):697. doi: 10.3390/v13040697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ontario Agency for Health Protection and Promotion (Public Health Ontario) 2022. COVID-19 in long-term care homes: focus on April 24, 2022 to May 7, 2022. Toronto, ON. [Google Scholar]
- 24.Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 25.Zhao L., Ismail S.J., Tunis M.C. Ranking the relative importance of COVID-19 vaccination strategies in Canada: a priority-setting exercise. CMAJ Open. 2021;9(3):E848–E854. doi: 10.9778/cmajo.20200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Advisory Committee on Immunization (NACI) An advisory committee statement (ACS) national advisory committee on immunization (NACI) guidance on COVID-19 vaccine booster doses: initial considerations for 2023. https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/guidance-covid-19-vaccine-booster-doses-initial-considerations-2023/guidance-covid-19-vaccine-booster-doses-initial-considerations-2023.pdf
- 27.Fulop T., Larbi A., Dupuis G., et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2018;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall V., Foulkes S., Insalata F., et al. Protection against SARS-CoV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207–1220. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds C.J., Pade C., Gibbons J.M., et al. Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science. 2022;377(6603) doi: 10.1126/science.abq1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeffery-Smith A., Rowland T.A.J., Patel M., et al. Reinfection with new variants of SARS-CoV-2 after natural infection: a prospective observational cohort in 13 care homes in England. Lancet Healthy Longev. 2021;2(12):e811–e819. doi: 10.1016/S2666-7568(21)00253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carazo S., Skowronski D.M., Brisson M., et al. Protection against omicron (B.1.1.529) BA.2 reinfection conferred by primary omicron BA.1 or pre-omicron SARS-CoV-2 infection among health-care workers with and without mRNA vaccination: a test-negative case-control study. Lancet Infect Dis. 2023;23(1):45–55. doi: 10.1016/S1473-3099(22)00578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andeweg S.P., de Gier B., Eggink D., et al. Protection of COVID-19 vaccination and previous infection against Omicron BA.1, BA.2 and Delta SARS-CoV-2 infections. Nat Commun. 2022;13(1):4738. doi: 10.1038/s41467-022-31838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmer H.K., Mackey K., Fiordalisi C.V., Helfand M. Major update 2: antibody response and risk for reinfection after SARS-CoV-2 infection-final update of a living, rapid review. Ann Intern Med. 2023;176(1):85–91. doi: 10.7326/M22-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altarawneh H.N., Chemaitelly H., Hasan M.R., et al. Protection against the omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 2022;386(13):1288–1290. doi: 10.1056/NEJMc2200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao Y., Yisimayi A., Jian F., et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608(7923):593–602. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan K., Karim F., Ganga Y., et al. Omicron BA.4/BA.5 escape neutralizing immunity elicited by BA.1 infection. Nat Commun. 2022;13(1):4686. doi: 10.1038/s41467-022-32396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tartof S.Y., Slezak J.M., Puzniak L., et al. BNT162b2 vaccine effectiveness against SARS-CoV-2 omicron BA.4 and BA.5. Lancet Infect Dis. 2022;22(12):1663–1665. doi: 10.1016/S1473-3099(22)00692-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.