Abstract
As an essential micronutrient for humans, the metabolism of copper is fine-tuned by evolutionarily conserved homeostatic mechanisms. Copper toxicity occurs when its concentration exceeds a certain threshold, which has been exploited in the development of copper ionophores, such as elesclomol, for anticancer treatment. Elesclomol has garnered recognition as a potent anticancer drug and has been evaluated in numerous clinical trials. However, the mechanisms underlying elesclomol-induced cell death remain obscure. The discovery of cuproptosis, a novel form of cell death triggered by the targeted accumulation of copper in mitochondria, redefines the significance of elesclomol in cancer therapy. Here, we provide an overview of copper homeostasis and its associated pathological disorders, especially copper metabolism in carcinogenesis. We summarize our current knowledge of the tumor suppressive mechanisms of elesclomol, with emphasis on cuproptosis. Finally, we discuss the strategies that may contribute to better application of elesclomol in cancer therapy.
Keywords: Elesclomol, Cuproptosis, Oxidative stress, Copper metabolism, Cancer therapy
1. Introduction
Redox active copper functions as a structural and catalytic cofactor for a diverse array of biological processes, including mitochondrial respiration, antioxidant defense, DNA damage response, and lipid metabolism [1]. Although copper is a physiological necessity, excess amounts of copper can lead to cytotoxicity by generating reactive oxygen species (ROS) through Fenton or Fenton-like reactions, a process of Cu + catalyzing H2O2 to produce highly oxidized HO● (Cu+ +H2O2 →Cu2+ +HO● +HO−). Thus, intricate homeostatic systems have evolved in both prokaryotic and eukaryotic organisms to maintain intracellular copper levels [2]. The connection between copper and cancer has been acknowledged for over a century, with numerous observations indicating a higher demand for copper in tumors compared to healthy tissue [3]. Studies have reported elevated copper concentrations in tumors or serum of patients with different types of cancer [4]. Copper could fuel tumorigenesis by facilitating angiogenesis, proliferation, and metastasis [[5], [6], [7], [8], [9]]. However, the finely tuned homeostatic system would be overwhelmed by disease or copper ionophores-induced copper overload, leading to cell death.
Elesclomol is a copper ionophore, originally developed as a chemotherapeutic adjuvant by Synta Pharmaceuticals [10]. It is a novel, first-in-class investigational drug that exerts anticancer activities primarily by generating deleterious ROS and triggering mitochondrial apoptosis in a copper-dependent manner [11]. Over the past decade, elesclomol has been evaluated in various clinical trials engaging patients with melanoma, acute myeloid leukemia or some solid tumors, either alone or in combination with paclitaxel [[11], [12], [13], [14], [15]]. However, the underlying mechanisms responsible for the cytotoxic effects of elesclomol have not yet been fully elucidated, which contributes to its suboptimal anticancer efficacy in clinical studies. Given that tumor cells with a higher reliance on mitochondrial metabolism are more susceptible to elesclomol, the identification of the most relevant cancer settings and screening patient populations using biomarkers, such as indicators of tumor hypoxia, may help achieve favorable clinical trial results. In addition, combinations of elesclomol with other drugs, such as chemotherapeutic agents, proteasome inhibitors, and glycolysis inhibitors, may synergistically enhance the anticancer efficacy. However, the rationale for this combinatory regimen heavily relies on a comprehensive understanding of the cytotoxic mechanisms of elesclomol. Encouragingly, the characterization of cuproptosis as a copper-triggered modality of mitochondrial cell death opens a door to exploring the reapplication of elesclomol in clinical settings. Despite a high safety profile demonstrated by clinical data, the challenging pharmacokinetics of elesclomol poses a barrier to its utilization in anticancer treatment. Recently, the application of nanomedicine in elesclomol-induced cuproptosis for cancer therapy invigorates studies on developing advanced nanotechnology to modulate the pharmacological properties of elesclomol. Here, we review the tumor suppressive mechanisms and the clinical therapeutic applications of elesclomol. We then discuss the rationale behind strategies aimed at achieving tangible clinical benefits through elesclomol-based therapy.
2. Copper homeostasis is fine-tuned at multiple levels
Copper is an essential micronutrient that plays a crucial role in a wide range of physiological processes, including mitochondrial respiration, antioxidant defense, energy metabolism and bio-compound synthesis [16]. It is a double-edged sword as even modest increases can cause cytotoxicity, leading to cell death [17]. Thus, copper homeostasis is tightly regulated by a series of copper transporters, chaperones, and metallochaperones. Genetic variation that causes copper accumulation results in life-threatening pathological conditions [18,19].
2.1. Systemic copper homeostasis
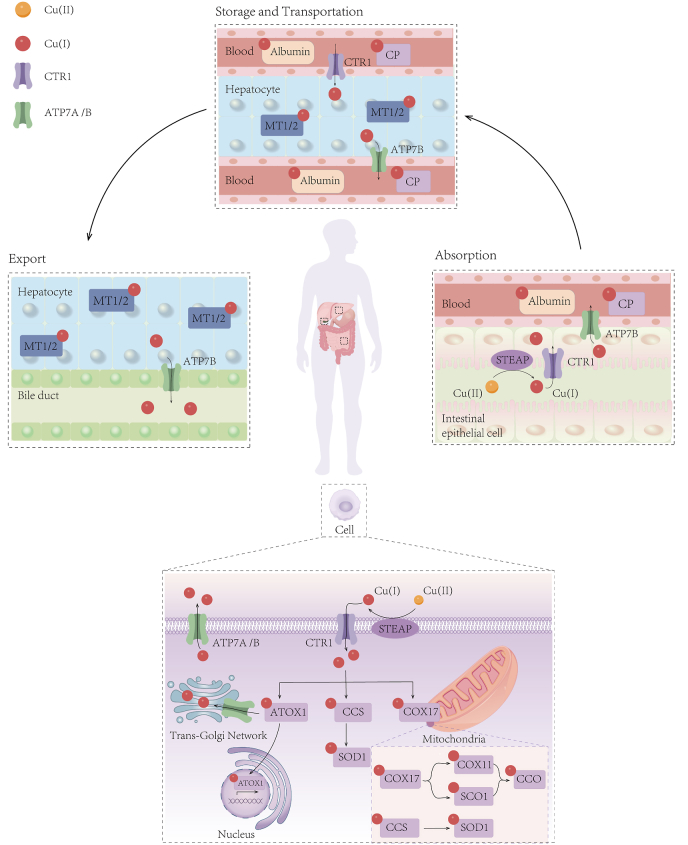
In the body, the absorption of dietary copper occurs mainly at the duodenum and small intestine. Subsequently, copper is transported to the liver by serum carriers including albumin, alpha2 microglobulins, histidines, and transcuprein [16,20]. About 75% of portal copper is stored primarily in the liver, which serves to distribute copper to peripheral organs via blood or to excrete from the body through the bile. Copper ions are transported by binding to proteins rather than being free and ceruloplasmin (CP) functions as the primary carrier for exchangeable copper in the blood. Due to the rapid degradation of CP when it is metal-free, the abundance of CP in plasma serves as a biomarker for systemic copper deficiency. This biomarker has been widely used in clinical trials investigating therapeutic copper depletion in cancer patients [21]. Excess copper is stored within liver cells by metallothionein 1 (MT1) and MT2 or utilized for the synthesis of copper-dependent enzymes [22,23] (Fig. 1). Other pathways for copper elimination such as urine, sweat, and menses play a relatively minor role in copper loss [16].
Fig. 1.
Overview of systemic and cellular copper homeostasis.
The human body primarily absorbs copper through the small intestine, which is then transported to the liver via the bloodstream and excreted into bile. CP serves as the main protein carrier for exchangeable copper in the blood plasma, while excess copper is stored in hepatocytes by MT1 and MT2. At the cellular level, the metal reductase STEAP and the copper transporter CTR1 enable high-affinity copper uptake. Copper is transported to different subcellular organelles through various copper-binding proteins, such as COX17, CCS, and ATOX1. These proteins play a crucial role in ensuring the availability of copper within the cell. Ultimately, ATP7A and ATP7B transfer copper from the cytosol to the TGN lumen. When intracellular copper levels are high, ATP7A and ATP7B exit the TGN, facilitating the efflux of copper from the cell. CP, ceruloplasmin; MT1, metallothionein 1; STEAP, six-transmembrane epithelial antigen of the prostate; CTR1, copper transporter 1; COX17, cytochrome C oxidase copper chaperone 17; CCS, copper chaperone for superoxide dismutase; ATOX1, antioxidant-1; SOD1, superoxide dismutase 1; SCO1, synthesis of cytochrome c oxidase 1; COX11, cytochrome C oxidase copper chaperone 11; CCO, cytochrome C oxidase; ATP7A, ATPase Copper Transporting Alpha; TGN, trans-Golgi network.
At the cellular level, a sophisticated network of copper-dependent proteins works together to coordinate the intake, release, and intracellular utilization of copper. This ensures the levels of copper maintain within a safe and optimal range, preventing harmful effects. Extracellular Cu(II) is reduced by the metalloreductases such as STEAP (six-transmembrane epithelial antigen of the prostate) to Cu(I), which is then transported into the cell mainly through copper transporter 1 (CTR1) [24]. CTR1 encoded by the SLC31A1 gene is structurally and functionally conserved from yeast to humans, and is responsible for the majority of copper uptake into cells [25]. Moreover, both in vitro and in vivo studies reported a negative-feedback regulatory loop of CTR1 expression by copper [26,27]. Another copper transporter, called CTR2 (encoded by SLC31A2), functions as a low-affinity copper importer and also plays a role in maintaining intracellular copper homeostasis [28,29]. Besides, several studies demonstrated that CTR1 and divalent metal transporter 1 (DMT1) would compensate for each other for copper uptake in mammalian cells, although different types of cells may use either one as a predominant copper importer under certain physiological circumstances [28,30]. More receptors involved in copper uptake need to be identified.
2.2. Copper balance is crucial for cellular maintenance and metabolism
Within the cytoplasm, copper trafficking is precisely orchestrated by a meticulously organized network of high-affinity copper chaperons: (1) copper chaperone for superoxide dismutase (CCS) and superoxide dismutase 1 (SOD1) are localized in both the cytoplasm and mitochondrial intermembrane space (IMS), where they detoxify mitochondria-derived ROS [31,32]. CCS delivers copper to SOD1 for the detoxification of ROS and maintain copper homeostasis; (2) cytochrome C oxidase copper chaperone 17 (COX17) located in the IMS, plays a key role in delivering copper to either synthesis of cytochrome c oxidase 1 (SCO1) or cytochrome C oxidase copper chaperone 11 (COX11). These two chaperones then transfer copper to the cytochrome C oxidase (CCO) subunit, thereby activating the enzyme activity within the respiratory chain [33,34]; (3) Antioxidant-1 (ATOX1) transports copper to the nucleus, where copper binds to transcription factors and drives gene expression [35]. Additionally, ATOX1 is responsible for transferring copper to the copper transporting ATPase1 (ATP7A and ATP7B) in the trans-Golgi network (TGN) and facilitating the synthesis of cuproenzymes such as ceruloplasmin [[35], [36], [37]]. Mice lacking the Atox1 gene were found to be perinatal lethal due to disrupted copper balance, highlighting the central role of this chaperone plays in copper homeostasis [38]. Finally, ATP7A and ATP7B transfer copper from the cytosol to the TGN lumen. When cytosolic copper levels increase, these efflux proteins exit the TGN to export copper by fusing with the plasma membrane [39] (Fig. 1). Importantly, previous studies have documented that mutations in ATP7A and ATP7B cause inherited disorders of copper metabolism, which are recognized as Menkes disease (MD) and Wilson disease (WD), respectively [40].
3. Copper metabolism involves in carcinogenesis
The inability to maintain a proper balance of copper at both systemic and subcellular levels has been linked to various pathologic conditions. For example, malfunctions of ATP7A cause Menkes disease, which is an X-linked recessive disorder characterized by impaired copper absorption across the intestinal epithelium and severe systemic copper deficiency [[41], [42], [43]]. In contrast, mutations in ATP7B, primarily expressed in the liver, give rise to Wilson disease in which defective biliary copper excretion causes excess copper deposit in the brain, liver, and other tissues, ultimately leading to systemic poisoning [44,45]. In addition, defects in the mitochondrial and cellular distributions and homeostasis of copper lead to severe neurodegenerative conditions (such as Alzheimer's disease, Parkinson's disease, and Huntington's disease), mitochondrial myopathies, and metabolic diseases [46]. Notably, copper exhibits a dual nature in carcinogenesis, acting as both a promoter of tumor growth and an inducer of redox stress in cancer.
3.1. Protumor activities of copper and corresponding anticancer strategies
The notion that tumor cells have a higher demand for copper than normal tissues and non-dividing cells has been increasingly acknowledged [3]. Increased copper levels have been reported in tumor tissues and serum obtained from patients with various types of cancer [4]. Further, elevated levels of serum copper (ceruloplasmin-bound copper) in patients have been positively correlated with tumor stage and disease progression of breast, colorectal, and lung cancers [[47], [48], [49]]. The tumor-promoting effects of copper have been revealed in preclinical models for breast, pancreatic and lung cancers involved in regulating protein kinase cascade, such as EGFR/ERK/c-Fos, MEK-ERK1/2 [[50], [51], [52]]. Copper directly binds or activates EGFR, PDK1 or PI3K to promotes tumorigenesis. Copper also affects MAPK and autophagic pathways or indirectly changes c-Myc stability to influence tumor growth [[53], [54], [55]]. The pro-angiogenic properties of copper were initially raised with the observation that copper could induce endothelial cell migration which was further supported by the finding that copper induced corneal neovascularization in rabbits [5,6]. Copper primarily promotes angiogenesis by regulating angiogenic molecules, such as IL-1α, vascular endothelial growth factor (VEGF), and hypoxia-inducible factor 1 (HIF-1) [[7], [8], [9]]. Additionally, copper chaperons like SOD1 and ATOX1 have been found to facilitate tumor angiogenesis [56]. Copper enhances epithelial-mesenchymal transition (EMT) by activating metastasis-related enzymes and signaling cascades. For instance, a single-cell tracking analysis suggested that ATOX1 mediates breast cancer migration via coordinated copper transport in the ATP7A-LOX (copper-dependent protein lysyl oxidase) axis [57]. The secreted copper-binding glycoprotein SPARC (secreted protein acidic and cysteine-rich, or osteonectin) has been shown to modulate cell-matrix interactions and promote tumor invasion and metastasis [58]. It worth mentioning that independent of their canonical function in copper transport, endothelial CTR1 and ATP7A have been shown to promote angiogenesis through vascular endothelial growth factor receptor type 2 (VGEFR2) signaling pathway [59,60]. Intriguingly, copper also plays a role in modulating the expression of immune checkpoint protein programmed death-ligand1(PD-L1). Specifically, copper depletion expedited the degradation of PD-L1, thereby reducing tumor growth and improving survival in a neuroblastoma xenograft mouse model [61].
The fact that cancer cells typically exhibit a higher demand for copper compared to quiescent healthy cells can be leveraged by utilizing copper chelators to hinder tumor progression and manage copper overload in Wilson disease. Indeed, chelation therapy is well tolerated for long-term use in chronic genetic diseases of copper misregulation [62]. Therefore, several copper-chelating agents (e.g., tetrathiomolybdate, trientine, and d-penicillamine) have been developed and evaluated for their antitumor effectivity in preclinical animal models and clinical trials (Table 1). The utilization of chelators to deplete copper has demonstrated the ability to delay cancer metastasis by impeding lesion vascularization in various animal models for different types of cancer [[63], [64], [65]]. In line with the molecular mechanisms of copper-promoted angiogenesis, copper chelators inhibit angiogenesis by targeting key angiogenic factors, including VEGF, HIF-1α, CD31, IL-1α and IL-18. Among the various copper chelators, tetrathiomolybdate (TTM) has been extensively studied and assessed in different phases of clinical trials as an anti-angiogenic drug [66,67].
Table 1.
Clinical trials of copper chelators in cancer therapy.
| Drugs | Status | Conditions | Phases | Identifier |
|---|---|---|---|---|
| Tetrathiomolybdate | Active, not recruiting | Breast Cancer | Phase 2 | NCT00195091 |
| Completed | Prostate Cancer | Phase 2 | NCT00150995 | |
| Completed | Esophageal Carcinoma | Phase 2 | NCT00176800 | |
| Completed | Colorectal Carcinoma | Phase 2 | NCT00176774 | |
| Completed | Hepatocellular carcinoma | Phase 2 | NCT00006332 | |
| Completed | Non-Small Cell Lung Cancer | Phase 1 | NCT01837329 | |
| Withdrawn | Non-Small Cell Lung Cancer | Phase 1 | NCT00560495 | |
| Choline tetrathiomolybdate (ATN-224) | Terminated | Breast Cancer | Phase 2 | NCT00674557 |
| Unknown | Prostate Cancer | Phase 2 | NCT00405574 | |
| Unknown | Melanoma | Phase 2 | NCT00383851 | |
| Terminated | Multiple Myeloma | Phase 1, Phase 2 | NCT00352742 | |
| Trientine | Completed | Epithelial Ovarian Cancer | Phase 1, Phase 2 | NCT03480750 |
| Tubal Cancer | ||||
| Primary Peritoneal Cancer | ||||
| Completed | Advanced Cancers | Phase 1 | NCT01178112 | |
| Withdrawn | Melanoma | Phase 1 | NCT02068079 | |
| Penicillamine | Completed | Glioblastoma | Phase 2 | NCT00003751 |
| Clioquinol | Terminated | Relapsed or Refractory Hematological Malignancy | Phase 1 | NCT00963495 |
3.2. Harnessing copper-induced cytotoxicity for cancer treatment
While copper is essential for cell survival and constitutes an exploitable dependency in cancer, excess copper levels may cause cytotoxicity, although a clear picture of the underlying mechanisms has not yet emerged. Over the past 30 decades, numerous studies have revealed that copper ionophores such as disulfiram (DSF) and elesclomol, exhibit anticancer properties by elevating intracellular copper levels.
DSF, an irreversible inhibitor of aldehyde dehydrogenase (ALDH) that was approved by the Food and Drug Administration (FDA) in 1951 for the treatment of alcoholism, has been demonstrated to potentiate the efficacy of certain anticancer drugs [68]. In acidic environments like the stomach, DSF is reduced to diethyldithiocarbamate (DDTC), which is a strong chelator of divalent transition metal, including Cu(II) [69]. The DDTC–Cu (II) complex preferentially displayed cytotoxicity to ALDH+ cancer stem cells by induction of ROS and abolishing ALDH activity [70,71]. It can activate PI3K-AKT signaling in breast cancer by suppressing PTEN expression [72], or induce apoptosis through inhibition of the proteasomal activity in xenograft breast cancer model [73]. In addition, DDTC–Cu (II) complex functions as a potent proteasome inhibitor, impairing DNA repair pathways and augmenting the therapeutic effects of DNA-damaging agents (temozolomide and radiation) for the treatment of glioblastoma [74]. Due to its ability to penetrate the blood-brain barrier, extensive clinical trials assessing the effectiveness of DSF in treating glioblastomas have been conducted. A phase I clinical trial (NCT01907165) demonstrated that the combination of DSF and temozolomide resulted in improved progression-free survival (PFS) for patients with glioblastoma [75]. And a phase II study (NCT03034135) suggested that a combination of DSF-copper to temozolomide for treating glioblastoma patients resistant to other therapies was well tolerated (Table 2). However, further research is needed to determine the patient populations that would respond better to this treatment regimen [76]. Overall, DSF has shown strong anticancer and chemosensitization activities through multiple mechanisms.
Table 2.
Clinical trials of disulfiram (DSF) in cancer therapy.
| NCT number | Conditions | Drugs | Phases | Results |
|---|---|---|---|---|
| NCT00312819 | Non-small Cell Lung Cancer | Chemotherapy, Disulfiram | Phase 2, Phase 3 | Disulfiram was well tolerated and had potential to prolong survival in patients with newly diagnosed non-small cell lung cancer when added to cisplatin and vinorelbine combination regimen. |
| NCT03034135 | Recurrent Glioblastoma | Disulfiram/Copper, Temozolomide | Phase 2 | DSF/Cu in combination with TMZ for TMZ-resistant IDH-wild type GBM seemed to be well tolerated, but had little activity in the unselected group. |
| NCT02101008 | Melanoma | Disulfiram, chelated zinc | Phase 2 | A serious adverse event (1/12) occurred during the trial, that the patient developed psychiatric disorders. |
| NCT01118741 | Prostate Cancer | Disulfiram | Phase 1 | Some patients had transient global PBMC demethylation changes, which limited the efficacy of disulfiram. |
| NCT02678975 | Glioma | Disulfiram, Copper, Alkylating Agents | Phase 2, Phase 3 | Unpublished |
| Glioblastoma | ||||
| NCT03714555 | Metastatic Pancreatic Cancer | Disulfiram, Copper Gluconate, Chemotherapy | Phase 2 | Unpublished |
| NCT00256230 | Stage IV Melanoma | Disulfiram | Phase 1, Phase 2 | Unpublished |
| NCT00742911 | Hepatic metastases from solid tumors | Disulfiram, Copper Gluconate | Phase 1 | Unpublished |
| NCT01907165 | Glioblastoma | Temozolomide, Disulfiram, Copper Gluconate | Early Phase 1 | Unpublished |
Elesclomol [N-malonyl-bis (N′-methyl-N′-thiobenzoyl hydrazide)] is another copper ionophore originally identified through a cell-based phenotypic screen for small molecules with potent pro-apoptotic activity. Due to its demonstrated antitumor activity against a wide range of cancer cell types and substantial enhancement of chemotherapeutic agents' efficacy, such as paclitaxel, it was further developed into a chemotherapeutic adjuvant by Synta Pharmaceuticals [10,77]. It is known that heightened oxidative stress beyond the threshold in cancer cells can induce apoptosis [78]. The anticancer mechanism of elesclomol has long been interpreted to involve the generation of intracellular ROS in cancer cells, and this process could be abrogated by the antioxidant N-acetylcysteine (NAC) [79]. However, the exact anticancer mechanisms of elesclomol are not yet fully elucidated.
4. The anticancer mechanisms of elesclomol
4.1. The toxicity of elesclomol to cancer cells is reliant on copper
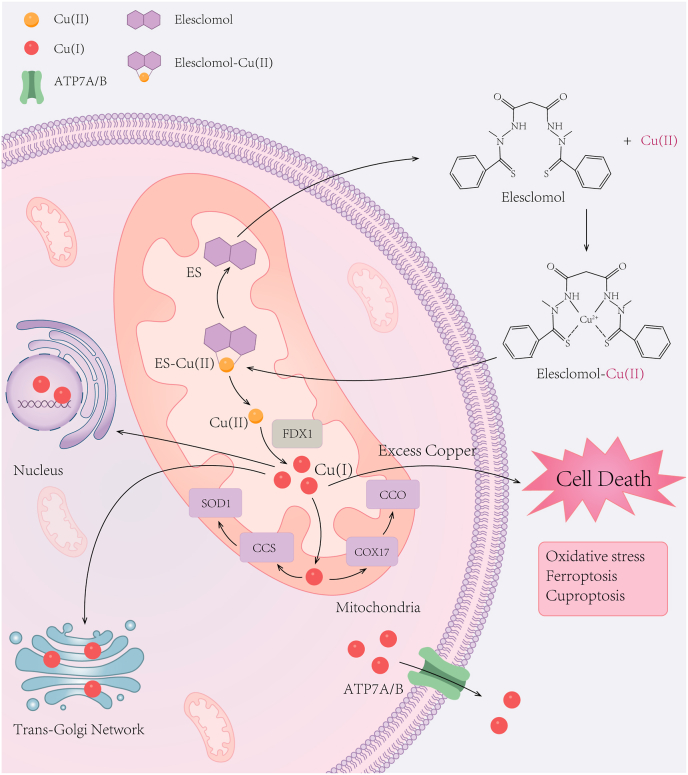
Cu(I) is regarded as the predominant form in the reducing environment of the cell cytosol. As a highly lipophilic Cu(II)-binding molecule, elesclomol forms a 1:1 permeable complex with Cu(II) extracellularly which rapidly transports copper to mitochondria and releases copper after it is reduced to Cu(I) by ferredoxin 1(FDX1) [80,81] (Fig. 2). Cu(I) in the mitochondria can generate ROS and cause unmitigated oxidative stress once ROS is excess. Afterward, elesclomol is effluxed from cells and continues shuttling elesclomol-Cu(II) complexes into the intracellular compartments. The mitochondrial selectivity for copper enrichment exhibited by elesclomol is distinct from other ionophores, such as DSF [80]. The reduced viability of MDA-MB435 melanoma cells upon elesclomol treatment was restored by addition of copper, while iron, manganese, and zinc did not have similar rescuing effects [80]. It was reported that when elesclomol paired with redox inert metals including Cu(II), Ni(II), and Pt(II), the killing effect of elesclomol-Ni(II)and elesclomol-Pt(II) complexes in human leukemia K562 cells is 34-times and 1040-times lower, respectively, compared to the elesclomol-Cu(II) complex [53], highlighting that elesclomol exerts its cytotoxicity mainly through preferential sequestration of Cu(II).
Fig. 2.
The structure and cellular entry mechanism of elesclomol.
Elesclomol forms a 1:1 complex with Cu(II) extracellularly, which rapidly transports copper to mitochondria and releases copper upon reduction to Cu(I) by FDX1. Subsequently, elesclomol-Cu(II) complexes lead to cell death through various mechanisms, including the generation of oxidative stress, targeting metabolically active mitochondria, disruption of iron homeostasis, promoting ferroptosis and inducing cuproptosis. FDX1, ferredoxin 1.
4.2. The cytotoxicity of elesclomol is primarily attributed to mitochondrial oxidative stress
The induction of mitochondrial oxidative stress and subsequent apoptosis through the Cu II-I redox cycling process is a well-established anticancer mechanism of elesclomol [79]. Compared to normal cells, many types of cancer cells have inherently elevated levels of oxidative stress, which would be further induced by elesclomol and exceeded a critical threshold in tumors [14]. For example, BRAFV600E inhibitor(vemurafenib)-resistant melanoma cells displayed high levels of basal mitochondrial respiration and ROS production which rendered them more vulnerable to elesclomol [82]. Likewise, elesclomol selectively killed cisplatin-resistance lung cancer cells through increased mitochondria respiration [83]. It has also been reported to exhibit cytotoxic effects on cisplatin-resistance lung cancer cells by impeding the clearance of ROS. These effects are mediated by downregulation of thioredoxin-1 (TRX1), a crucial antioxidant molecule responsible for maintaining the intracellular reducing response, and glutathione (GSH), which effectively scavenge intracellular ROS [84]. However, enhanced mitochondrial metabolism is observed only in certain subtypes of tumors, such as melanoma, breast cancer and hepatocellular carcinoma. Whereas high-grade serous ovarian cancer and diffuse large B-cell lymphoma show metabolic heterogeneity [85,86].
Cancer stem cells (CSC) represent a distinct and elusive subset of cancer cells characterized by their exceptional capacity for self-renewal, disease initiation and metastasis. They are heavily reliant on mitochondrial respiration and OXPHOS activity to fuel tumorigenesis [87]. With a high-throughput drug screening targeted the stem-like tumor-initiating cell (TIC) population in ovarian cancer (OC), pro-oxidants including elesclomol and DSF were identified. These two drugs reduced the phenotypic features and sphere-formation ability of TICs, implying that the heightened mitochondrial respiration of CSC may contribute to their elevated sensitivity to elescolmol [88]. In addition, glioblastoma stem cells (GSCs) also highly rely on OXPHOS and resist current glioblastoma (GBM) therapies [89]. GSCs have been demonstrated to contribute to GBM-associated neovascularization process through transdifferentiation into GSC-derived endothelial cells (GdECs) [90]. Elesclomol showcased remarkable efficacy in killing both GSCs and GdECs at submicromolar concentrations via elevated levels of ROS, and led to a non-apoptotic copper-dependent cell death [91].
Therefore, elevated levels of oxidative stress would create a therapeutic vulnerability to oxidative stress-inducing drugs. For instance, aberrant activation of oncogene SOX6 in ewing sarcoma (EwS), an aggressive childhood cancer, promoted malignancy but conferred sensitivity to elesclomol by increased mitochondrial ROS. Mechanistically, SOX6 upregulated the expression of thioredoxin binding protein (TXNIP), which induces oxidative stress by inhibiting the antioxidative function of thioredoxin (TXN) [92].
4.3. Cancer cells with low glycolytic activity are more sensitive to elesclomol
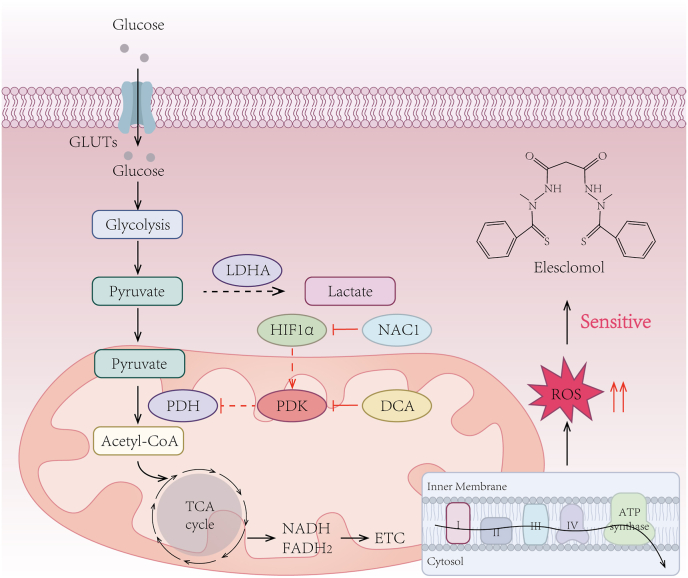
The anticancer effects of elesclomol rely on the presence of oxygen, which primarily drives energy metabolism through OXPHOS. However, many solid tumors face the challenge of insufficient oxygen supply, termed hypoxia [93]. Under hypoxic conditions, tumors undergo an energy metabolic shift to glycolysis in the cytoplasm instead of mitochondrial respiration [94], the process of which is governed largely by a family of transcription factors known as hypoxia-inducible factors (HIFs) [95]. It was reported that blocking HIF-1α signaling pathway restored mitochondrial respiration via downregulating the expression of pyruvate dehydrogenase kinase-3 (PDK3) [96,97]. Consequently, inhibiting PDK3 activity by dichloroacetate (DCA) increased the onset of oxidative stress and potentiated the antitumor effects of elesclomol in melanoma [96]. Moreover, nucleus accumbens-1 (NAC1) has been revealed to facilitate oxidative stress and resistance against elesclomol as a crucial upstream regulator of HIF-1α/PDK3 axis [98] (Fig. 3). These studies underscore the potential significance of targeting glycolysis as an adjunctive strategy to enhance the antitumor efficacy of elesclomol.
Fig. 3.
Cancer cells exhibiting elevated levels of mitochondrial oxidative stress are more sensitive to elesclomol.
The effectiveness of elesclomol as an anticancer agent relies on the presence of oxygen, which predominantly fuels cellular energy metabolism through OXPHOS rather than glycolysis. Consequently, targeting glycolysis to enhance mitochondrial respiration may enhance the antitumor efficacy of elesclomol. Blocking the HIF-1α/PDK3 axis by inhibiting the upstream factor NAC1 or utilizing a PDK inhibitor such as DCA can promote oxidative stress and enhance the antitumor efficacy of elesclomol. OXPHOS, oxidative phosphorylation; GLUTs, glucose transporters; LDHA, lactate dehydrogenase A; PDH, pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinases; DCA, dichloroacetate; HIF1, hypoxia-inducible factor 1; NAC1, nucleus accumbens-1; TCA, tricarboxylic acid; ETC, electron transport chain; ROS, reactive oxygen species.
Hypoxia contributes to tumor progression and induces glycolytic metabolism, wherein pyruvate is converted into lactate as the end product of glycolysis by the enzyme lactate dehydrogenase (LDH). Elevated serum LDH levels not only correlate with poor prognosis in numerous types of cancer but also serve as a biomarker of tumor hypoxia [99,100]. Based on the understanding that elevated levels of glycolysis impair tumor sensitivity to elesclomol, it can be inferred that patients with high LDH levels may exhibit increased resistance to elesclomol. This inference was validated by a randomized, double-blind phase III clinical trial, aiming to evaluate the efficacy of elesclomol in combination with paclitaxel in patients with advanced melanoma [11]. The study revealed that the addition of elesclomol had no effect on patients with high LDH levels. However, it was observed to be effective in patients with lower levels of serum LDH, resulting in improved PFS. Therefore, serum LDH levels may serve as an indicator of patients’ sensitivity to elesclomol. Of note, the identification of novel tumor hypoxia biomarkers could potentially customize the administration of elesclomol.
4.4. Ferroptosis accounts for the anticancer activity of elesclomol
Copper (Cu) and iron (Fe) are essential trace minerals with two oxidative states. They serve as cofactors for numerous essential cellular enzymes due to their high reactivity in redox reactions [101]. Since the excess level of intracellular copper and iron are cytotoxic, the intracellular level of copper and iron is fine-tuned by an active homeostatic metabolism network. The disruption of iron and copper metabolism, as well as their interaction, is of significant importance in the pathogenesis of various diseases [102].
While previous studies have established iron as the exclusive metal ion responsible for triggering ferroptosis [103], emerging evidence suggests that intracellular copper accumulation could also facilitate ferroptosis [104]. For example, Cu(II) has been reported to promote tax1 binding protein 1 (TAX1BP1)-dependent autophagic degradation of glutathione peroxidase 4 (GPX4), a well-documented negative regulator of ferroptosis, leading to ferroptosis-mediated tumor suppressing in a preclinical model of pancreatic cancer. As a result, the use of copper chelators demonstrated an attenuated experimental acute pancreatitis associated with ferroptosis [104]. By co-delivering Cu(II), glucose oxidase (GOx) and cinnamaldehyde (Cin) with copper-alanine nanoparticles (CACG) into the tumor microenvironment, GSH was depleted partially through the reduction of Cu(II) into Cu(I) and the cascade of Cu(I)-catalyzed Fenton reactions. This efficient GSH depletion resulted in ROS production and enhanced ferroptosis, consequently leading to the retardation of tumor growth in 4T1 tumor-bearing mouse model [105].
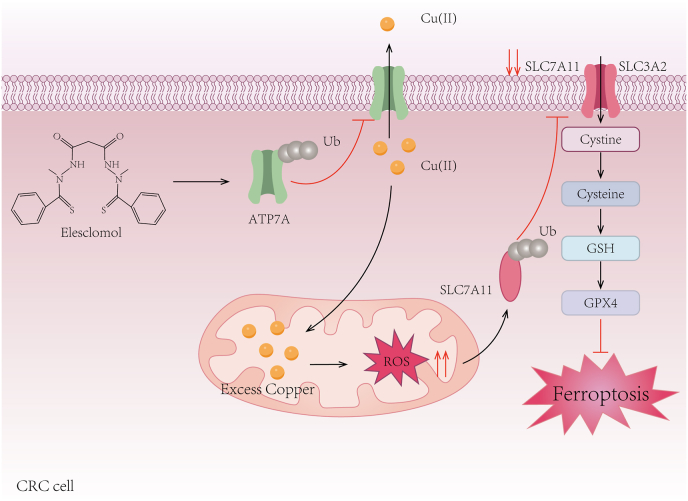
As a copper ionophore, in vitro studies have shown that elesclomol could directly bind Fe2+ [106]. In vivo, elesclomol treatment not only increased cellular copper content but also indirectly elevated mitochondrial iron levels by stimulating the copper-dependent iron import machinery [107]. At the molecular level, Cu(II) derived from elesclomol-Cu(II) was pumped into the Golgi lumen, where it was utilized for the metalation of the iron transport multicopper oxidase Fet3. The metallated Fet3 then oxidized Fe2+ to Fe3+, which enabled mitochondria enrichment of iron via the iron importer transferrin receptor 1 (Ftr1), resulting in dysregulation of iron homeostasis [107]. In addition, elesclomol has been reported to promote the degradation of copper-transporting ATPase 1 (ATP7A), which plays a vital role in maintaining intracellular copper homeostasis by actively exporting excess copper out of the cells [108,109]. The combined treatment of elesclomol and copper worsened the accumulation of ROS due to the loss of ATP7A, which subsequently promoted the degradation of SLC7A11, ultimately resulting in ferroptosis in colorectal cancer cells [108] (Fig. 4). In summary, elesclomol has the potential to modulate ferroptosis either through copper-ion interactions or by inducing oxidative stress independent of iron metabolism. However, the mechanisms by which elesclomol inhibits tumor growth by inducing ferroptosis and whether this inhibition relies on the type of cancer warrant more exploration.
Fig. 4.
Ferroptosis accounts for the anticancer activity of elesclomol.
Elesclomol increases Cu(II) levels within the mitochondria and reduces ATP7A expression, resulting in intracellular Cu(II) retention and ROS accumulation. These effects contribute to the degradation of SLC7A11, further amplifying oxidative stress and ultimately triggering ferroptosis in CRC cells. Ub, ubiquitin; SLC7A11, solute carrier family 7 member 11; SLC3A2, solute carrier family 3 member 2; GSH, glutathione; GPX4, glutathione peroxidase 4; CRC: colorectal cancer.
4.5. Elesclomol exerts anticancer effects through cuproptosis
A comprehensive understanding of the mechanisms underlying the cytotoxicity induced by copper or copper ionophore has not yet been established. Besides apoptosis [79,110] and ROS-induced cell death [80,111], copper has been revealed as an inducer of endoplasmic reticulum (ER) stress, leading to a caspase-independent paraptotic death [112]. Moreover, it has been found to target the ubiquitin-proteasome system [73,113]. Whether copper overload induces a distinct type of programmed cell death has long been debated until the mechanism of cuproptosis was unveiled in 2022.
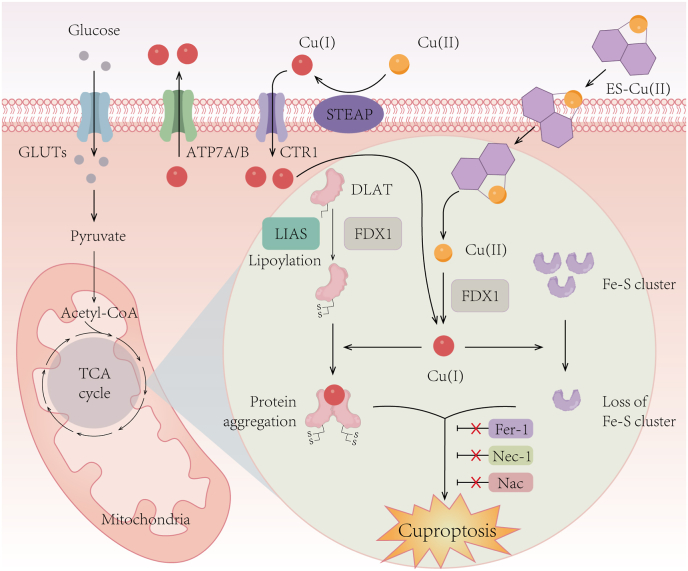
Cuproptosis is a copper-dependent and unique kind of programmed cell death that is different from existing other forms of cell death, including apoptosis, ferroptosis, pyroptosis and necroptosis. Elesclomol has been shown to induce cuproptosis dependent on mitochondrial respiration, as cells more reliant on mitochondrial respiration were nearly 1000-fold more sensitive to copper ionophores than cells undergoing glycolysis [114]. Mechanistically, copper directly binds to lipoylated components of the tricarboxylic acid (TCA) cycle, leading to the aggregation of lipoylated protein and subsequent destabilization of the iron-sulfur (Fe–S) cluster proteins. This process results in proteotoxic stress and ultimately cell death [114]. Elesclomol-induced cuproptosis relies on its direct target FDX1, which reduces Cu(II) to Cu(I), facilitating the lipoylation and aggregation of enzymes (especially dihydrolipoamide S-acetyltransferase, (DLAT)) involved in mitochondrial TCA cycle, and causes the loss of Fe–S cluster proteins [114] (Fig. 5). The identification and definition of cuproptosis not only advances research on targeting copper metabolism in cancer, but also challenges the conventional view that oxidative stress is a fundamental molecular mechanism of meta-induced toxicity. Moreover, the prognostic model constructed with cuproptosis-related genes has shown excellent predictive capability of patient outcomes in various cancers, and the expression levels of cuproptosis-related genes may influence the sensitivity of patients to immunotherapy, radiotherapy, and chemotherapy [[115], [116], [117]].
Fig. 5.
Schematic diagram of the mechanism of cuproptosis.
Elesclomol-induced cuproptosis primarily relies on its direct target FDX1, which catalyzes the reduction of Cu(II) to Cu(I). This reduction event facilitates the lipoylation and aggregation of enzymes, particularly DLAT, involving in the mitochondrial TCA cycle. Additionally, it triggers the loss of Fe–S cluster proteins. These aberrant processes collectively lead to proteotoxic stress and eventual cell death. Importantly, inhibitors targeting ferroptosis (Fer-1), necroptosis (Nec-1), and oxidative stress (NAC) do not affect the occurrence of cuproptosis. DLAT, dihydrolipoamide S-acetyltransferase; LIAS, lipoic acid synthetase; Fer-1, ferrostatin-1; Nec-1, necrostatin-1; NAC, N-acetylcysteine.
Recently, a ROS-responsive nanomedicine loaded with elesclomol and copper (NP@ESCu) has been demonstrated to not only kill tumor cells through cuproptosis, but also reprogram the tumor microenvironment via upregulating the expression of PD-L1 in a mice model with subcutaneous bladder cancer. This synergistic effect has shown an improvement in the responsiveness of tumors to immunotherapy [118]. Interestingly, ferroptosis inducers (FINs, sorafenib and erastin) has found to intensify elesclomol-induced cuproptosis in liver cancer cells through upregulated protein lipoylation via suppressing FDX1 protein degradation and suppressing intracellular GSH synthesis. The combination of FINs and elesclomol significantly inhibited liver cancer growth in vivo, suggesting that co-targeting ferroptosis and cuproptosis may provide novel therapeutic opportunities for liver cancer or other elesclomol-sensitive cancers [119]. There are still many unanswered questions in elesclomol-induced cuproptosis, such as (1) the cellular morphology and molecular manifestations of copper-induced cell death; (2) the downstream cascades that are activated by DLAT oligomers; (3) the molecular interactions that contribute to cellular proteotoxic stress; (4) the specific role of FDX1 and how its regulatory network contributes to cuproptosis; (5) how do disorders associated with elevated intracellular copper avoid cuproptosis. Furthermore, the involvement of copper-induced oxidative stress in the progression of cuproptosis and potential therapeutic targets for the prevention and treatment of cuproptosis remain largely unknown.
5. Therapeutic application potential of elesclomol in human diseases
5.1. Elesclomol targets mitochondrial metabolism in cancer therapy
Mitochondrial metabolism plays a crucial role in tumorigenesis, metastasis, and drug resistance [87]. Many types of cancer cells, including cancer stem cells, metastatic tumor cells, therapy-resistant tumor cells, and tumors with inhibited glycolysis, depend on mitochondria respiration through enhanced OXPHOS activity to fuel tumorigenesis. Therefore, elesclomol exhibited tremendous toxicity to these kinds of cancers.
The stem-like tumor-initiating cells (TICs) in OC are associated with disease recurrence following chemotherapy, resulting in poor prognosis for OC patients [120]. OC TICs prefer OXPHOS rather than glycolysis to maintain stemness. Under oxidative stress, enhanced antioxidant and drug metabolism responses provide a survival advantage for TICs, but also point to a potential vulnerability [121]. Indeed, both DSF and elesclomol were identified from a high-throughput drug screening that targeted TICs. These two compounds were found to diminish the stemness properties of TICs and enhance the cytotoxic effects of carboplatin [88]. Elesclomol was also identified as the most effective agent in killing glioblastoma stem cells (GSCs) through another drug screen specifically designed to target GSCs. Moreover, the combination of elesclomol with the alkylating agent temozolomide (TMZ) enhanced the cytotoxicity compared to TMZ alone [91].
A subset of slow-cycling melanoma cells which are characterized by the high expression of Jumonji AT-rich interactive domain 1B (JARID1B), manifest inherent resistance to multiple drugs such as vemurafenib or cisplatin. As these cells possess elevated mitochondrial metabolism properties, elesclomol effectively targeted them and reversed their enrichment caused by cisplatin monotherapy [122]. The mitochondrial oxidative signature in BRAFV600E melanoma cells also presented an opportunity for elesclomol to surpass tumor resistance to the BRAF inhibitor vemurafenib [82]. Elesclomol has been reported to selectively display toxicity to cisplatin-resistant lung cancer cells that possessed high basal levels of ROS, while sparing normal cells and the parental counterparts [83]. A comprehensive functional genomic analysis revealed a correlation between mitochondrial metabolism and the sensitivity of tumors to proteasome inhibitors. When cells were compelled to rely on OXPHOS rather than glycolysis, they developed resistance to proteasome inhibitors [81]. This mitochondrial state gave rise to a distinct vulnerability of breast cancer cells to elesclomol [81]. Chemotherapeutic drugs trigger a metabolic shift in cancer cells, making them more dependent on OXPHOS. This increased reliance renders tumors more susceptible to elesclomol, positioning it as a promising adjuvant in chemotherapy. Notably, inhibition of glucose metabolism would not only enfeeble the malignant potential of tumor cells, but also sensitize them to copper ionophores. Therefore, the combination of glycolysis inhibitors and elesclomol may have a synergistic inhibitory effect on tumorigenesis [96].
5.2. Elesclomol-related anticancer clinical trials
Elesclomol, as a first-in-class investigational drug, has demonstrated its ability to enhance the therapeutic effectiveness of paclitaxel in patients with refractory solid tumors and those with stage IV metastatic melanoma (Table 3). During this phase I clinical trial, the combination of elesclomol and paclitaxel was well tolerated with a toxicity profile similar to that of single-agent paclitaxel. Partial responses were achieved in one patient with Kaposi's sarcoma and another with ovarian cancer that progressed during prior treatment with paclitaxel [12]. In a small phase II randomized, double-blinded, and multi-center trial enrolling 81 patients with stage IV metastatic melanoma, the combination of elesclomol and paclitaxel showed a significant improvement in median PFS, doubling the duration compared to the use of paclitaxel alone. The combination therapy demonstrated a remarkable 41.7% reduction in the risk of disease progression, with increased overall survival (OS) rates [15]. Unfortunately, except for the above clinical trials, despite elesclomol exhibiting a favorable clinical safety profile alone or in combination with paclitaxel, it did not yield the desired clinical responses in patients with relapse and refractory acute myeloid leukemia [13] and patients with recurrent of persistent platinum-resistant ovarian, fallopian tube or primary peritoneal cancers [14]. While a subsequent phase III trial involving chemotherapy-naïve patients with advanced melanoma demonstrated that elesclomol combined with paclitaxel showed a lack of efficacy in the unselected population. A prospectively defined subgroup analysis revealed that patients with normal baseline levels of LDH were more sensitive to elesclomol [11]. As increased LDH activity is associated with tumor hypoxia, this finding is consistent with the requirement of active mitochondrial respiration for the action of elesclomol.
Table 3.
Elesclomol-related clinical trials in cancer therapy.
| NCT number | Status | Phases | Conditions | Enrollment | Drugs | Results | Reference |
|---|---|---|---|---|---|---|---|
| NCT00088114 | Completed | Phase 1 | Neoplasms | 50 | Elesclomol sodium, Paclitaxel | Patients tolerated the combination of elesclomol and paclitaxel well, and the toxicity profile of elesclomol was comparable to that of single-agent paclitaxel. | 17255281 |
| NCT01280786 | Unknown | Phase 1 | Acute Myeloid Leukemia | 36 | Elesclomol sodium | Elesclomol exhibited a favorable clinical safety profile; however, no clinical responses were observed in the patients treated with elesclomol. | 26732437 |
| NCT00084214 | Completed | Phase 1, Phase 2 | Melanoma | 103 | Elesclomol sodium, Paclitaxel | The combination of elesclomol and paclitaxel led to a significant doubling of median PFS, demonstrating an acceptable toxicity profile and promising OS. | 19826135 |
| NCT00888615 | Completed | Phase 2 | Recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal carcinoma | 58 | Elesclomol sodium, Paclitaxel | Elesclomol combined with paclitaxel was well tolerated, but the proportion responding was low. | 30309721 |
| NCT00522834 | Terminated | Phase 3 | Melanoma | 630 | Elesclomol sodium, Paclitaxel | Elesclomol combined with paclitaxel did not significantly improve PFS in advanced melanoma patients; and patients with normal baseline LDH showed a statistically significant increase in median PFS with the combination therapy. | 23401447 |
| NCT00087997 | Completed | Phase 2 | Soft Tissue Sarcoma | 80 | Elesclomol sodium | Elesclomol through the role of HSP70 enhanced the efficacy of taxane. | 16784029 |
| NCT00088088 | Completed | Phase 1, Phase 2 | Stage IIIB/IV Non-Small Cell Lung Cancer | 86 | Paclitaxel, Carboplatin, Elesclomol sodium | Unpublished | – |
| NCT00808418 | Completed | Phase 1 | Prostate Cancer | 34 | Elesclomol sodium, Docetaxel | Unpublished | – |
| NCT00827203 | Suspended | Phase 1 | Metastatic Solid Tumors | 30 | Elesclomol sodium | Unpublished | – |
Elesclomol in combination with paclitaxel has been extensively studied in clinical trials mostly focusing on advanced-stage melanomas. While published results indicate that the clinical anticancer efficacy of elesclomol is suboptimal, it does demonstrate an acceptable safety profile. Furthermore, several clinical trials related to elesclomol treatment are currently underway in different types of cancers, including prostate cancer, stage IIIB/IV non-small cell lung cancer, and soft tissue sarcoma [123]. Given that tumors reliant on mitochondrial metabolism are particularly susceptible to elesclomol, this compound may hold great promise as a therapeutic agent for a specific subset of cancers. Future research should be conducted using available biomarkers to identify the appropriate patient population. Furthermore, a comprehensive understanding of the drug's mechanism of action is crucial to guide clinical studies.
5.3. Elesclomol holds promise for the treatment of hereditary copper deficiency disorders
Unlike cancer cells, elesclomol exhibits minimal toxicity in healthy cells, indicating that the endogenous antioxidant capacity of healthy cells may overcome potential toxicity issues [80]. Elesclomol was identified as the most efficient copper delivery agent that can restore mitochondrial function in the context of defective copper transport in yeast, zebrafish, and mammalian cell lines [124]. Taking advantage of its membrane permeating properties and mitochondria-directed release mechanism, elesclomol was reported to substantially ameliorate pathology and lethality in a mouse model of Menkes disease. It corrected defective CTR1 and ATP7A membrane-copper transport with targeted improvement of mitochondrial CcO metalation [125]. This promising preclinical study paves the way for a new approach in the treatment of Menkes and associated disorders. Furthermore, using genetic models of copper-deficient C. elegans and mice, dietary elesclomol supplementation fully rescued copper deficiency phenotypes. Remarkably, oral gavage with elesclomol rescued intestine specific Ctr1 knockout mice from postnatal mortality without additional copper supplementation. These findings suggest that elesclomol facilitates copper delivery from dietary sources independent of the intestinal copper transporting system [126]. However, before repurposing elesclomol for the treatment of copper deficiency disorders in human patients, several crucial issues must be resolved, including optimizing the dosing regimen, identifying accurate efficacy biomarkers, and determining the appropriate timing and frequency of treatment [127].
5.4. Safety and pharmacodynamics of elesclomol in cancer therapy
Based on the published data from clinical trials, no significant adverse effects have been observed thus far when elesclomol was administered either alone or in combination with other chemotherapeutic agents. In a phase I clinical trial involving patients with solid tumors, the maximum tolerated dose of elesclomol was up to 438 mg/m2. The toxicity profile at this dose was similar to paclitaxel alone. With the escalation of elesclomol dose, there was a significant decrease in the total body clearance of paclitaxel [12]. In a phase II trial enrolling patients with stage IV metastatic melanoma, the combination of elesclomol and paclitaxel resulted in a statistically doubling of median PFS and encouraging OS. Significantly, the combinatory therapy exhibited a more tolerable toxicity profile than many other widely used combination regimens for melanoma treatment [15]. Additionally, elesclomol demonstrated a favorable safety profile with minimal toxicity at a dose of 400 mg/m2 in another phase I clinical trial conducted on patients with relapse and refractory acute myeloid leukemia, despite the none-responsiveness of patients to elesclomol [13]. Of note, there have been no reports of patients developing any organic or functional impairment related to elesclomol [123]. Therefore, the high safety profile of elesclomol in cancer therapy is an encouraging characteristic supporting further development of this drug.
The limited efficacy of elesclomol in oncology clinical trials may be partially attributed to its linear pharmacokinetics in vivo, which is characterized by rapid elimination from plasms (biological half-life, 1.06 ± 0.24 h) and a low steady-state apparent volume of distribution (25.1 ± 8.1 L/m(2)) [12]. Consequently, extremely restricted elesclomol in the circulation could enter tumor cells. In addition, the effectiveness of elesclomol-induced cell death in controlling tumor progression might be constrained by the hostile microenvironment within the tumor. Fortunately, the pharmacological properties of anticancer drugs, including their pharmacokinetics and biodistribution have been improved by drug delivery strategies. For instance, nanomedicine for small-molecule therapeutics has focused on the encapsulation and delivery of chemotherapeutic drugs to tumors [128]. To address the issue that elesclomol possesses a short half-life in bloodstream, a ROS-sensitive polymer (PHPM) was designed by researchers and employed to co-encapsulate elesclomol and copper to form nanoparticles (NP@ESCu). These nanoparticles exhibited rapid internalization by tumor cells, leading to a remarkable 4.2-fold increase in intracellular copper levels [118]. NP@ESCu effectively induced cuproptosis in tumor cells by downregulating the expression of LIAS and increasing the PD-L1 expression, enhancing the synergy between cuproptosis and immunotherapy [118]. This study serves as a promising example of leveraging nanomedicines for elesclomol-induced cuproptosis in cancer therapy and may potentially stimulate research efforts in developing nanoscale systems to improve the pharmacological properties of elesclomol. Additionally, to minimize toxicity and broaden its therapeutic window, it is worthwhile to devise antibody-drug conjugates (ADCs) that selectively target tumor antigens for elesclomol delivery.
6. Conclusions and future perspective
Copper is an essential micronutrient whose inherent redox properties make it both beneficial and toxic to the cell. A hereditary or acquired copper unbalance has been associated with a wide range of pathologic conditions, including Menkes disease, Wilson disease, and neurodegenerative disease. Copper also serves as a double-edged sword in carcinogenesis. It exerts tumor-promoting effects by regulating protein kinase cascades, stimulating cell proliferation and migration. It also modulates autophagic pathways, allowing tumors to evade apoptosis and promotes angiogenesis. Moreover, copper can stimulate the expression of immune checkpoint protein PD-L1, suppressing the anti-tumor immune response. While cancer cells typically demand more copper compared to quiescent healthy cells, excessive levels of copper can cause cell death. Copper toxicity has been effectively harnessed by utilization of copper ionophores in antitumor therapy.
The copper ionophore elesclomol is an extensively studied anticancer drug that primarily targets mitochondrial metabolism. Elesclomol–copper complexes induce oxidative stress by disruption of the mitochondrial respiration chain or by indirect non-mitochondrial induction of ROS. Therefore, cancer cells that heavily rely on mitochondrial metabolism, including cancer stem cells, drug-resistance tumor cells and those with lower glycolytic activity, are more vulnerable to elesclomol. The discovery and characterization of cuproptosis, a novel form of programmed cell death that relies on copper ionophores to import copper, has significantly advanced our understanding of the anticancer mechanisms of elesclomol. However, exploring reliable biomarkers for cuproptosis, elucidating copper/copper ionophore-dependent signaling pathways, and dissecting the interplay between ferroptosis and cuproptosis will provide opportunities to get a clear picture of the mechanisms underlying elesclomol-induced cytotoxicity.
Elesclomol failed to yield favorable results in certain oncology clinical trials, but it consistently demonstrated a high safety profile. Importantly, it has been found that serum LDH levels may serve as a potential biomarker for evaluating patients' responsiveness to elesclomol [11]. Selecting cancer types with high mitochondrial metabolism and choosing patients with biomarker-driven approaches may lead to more favorable outcomes for future clinical trials. To enhance the anticancer efficacy of elesclomol, drug delivery strategies such as nanoscale systems should be incorporated into drug development. These technologies can improve the pharmacokinetics, stability, absorption, and exposure of elesclomol within tumors, while also facilitating its administration of synergistic combinations with other therapeutic drugs. Moreover, elucidating the cytotoxic mechanisms of elesclomol is expected to uncover novel pathways that could be therapeutically exploited for the treatment of cancer.
Funding
This review was funded by the National Key Research And Development Plan (2022YFC3401000), the National Natural Science Foundation of China (82022037), the Guangdong Basic and Applied Basic Research Foundation (2021B1515230009), the Key Research and Development Plan of Guangdong Province (2020B0101030006).
Author contributions
J.G.: conceptualization, initial draft preparation; X.W.: initial draft preparation and revision; S.H.: editing; Z.Z.: editing; M.S.: conceptualization, supervision, critical revision, and suggestions. W.H.: conceptualization, supervision, and suggestions. All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to the members of our lab and our partners for attentively reading the text and providing insightful feedback.
Contributor Information
Weiling He, Email: hewling@mail.sysu.edu.cn.
Mei Song, Email: songm7@mail.sysu.edu.cn.
Data availability
Data will be made available on request.
References
- 1.Lutsenko S. Human copper homeostasis: a network of interconnected pathways. Curr. Opin. Chem. Biol. 2010;14:211–217. doi: 10.1016/j.cbpa.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleackley M.R., Macgillivray R.T. Transition metal homeostasis: from yeast to human disease. Biometals. 2011;24:785–809. doi: 10.1007/s10534-011-9451-4. [DOI] [PubMed] [Google Scholar]
- 3.Denoyer D., Masaldan S., La Fontaine S., Cater M.A. Targeting copper in cancer therapy: 'Copper that Cancer'. Metallomics : Integrat. Biometal Sci. 2015;7:1459–1476. doi: 10.1039/c5mt00149h. [DOI] [PubMed] [Google Scholar]
- 4.Margalioth E.J., Schenker J.G., Chevion M. Copper and zinc levels in normal and malignant tissues. Cancer. 1983;52:868–872. doi: 10.1002/1097-0142(19830901)52:5<868::aid-cncr2820520521>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.McAuslan B.R., Reilly W. Endothelial cell phagokinesis in response to specific metal ions. Exp. Cell Res. 1980;130:147–157. doi: 10.1016/0014-4827(80)90051-8. [DOI] [PubMed] [Google Scholar]
- 6.Raju K.S., Alessandri G., Ziche M., Gullino P.M. Ceruloplasmin, copper ions, and angiogenesis. J. Natl. Cancer Inst. 1982;69:1183–1188. [PubMed] [Google Scholar]
- 7.Mandinov L., et al. Copper chelation represses the vascular response to injury. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6700–6705. doi: 10.1073/pnas.1231994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan Q., et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002;62:4854–4859. [PubMed] [Google Scholar]
- 9.Feng W., Ye F., Xue W., Zhou Z., Kang Y.J. Copper regulation of hypoxia-inducible factor-1 activity. Mol. Pharmacol. 2009;75:174–182. doi: 10.1124/mol.108.051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S., et al. Syntheses and antitumor activities of N'1,N'3-dialkyl-N'1,N'3-di-(alkylcarbonothioyl) malonohydrazide: the discovery of elesclomol. Bioorg. Med. Chem. Lett. 2013;23:5070–5076. doi: 10.1016/j.bmcl.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 11.O'Day S.J., et al. Final results of phase III SYMMETRY study: randomized, double-blind trial of elesclomol plus paclitaxel versus paclitaxel alone as treatment for chemotherapy-naive patients with advanced melanoma. J. Clin. Oncol. 2013;31:1211–1218. doi: 10.1200/JCO.2012.44.5585. [DOI] [PubMed] [Google Scholar]
- 12.Berkenblit A., et al. Phase I clinical trial of STA-4783 in combination with paclitaxel in patients with refractory solid tumors. Clin. Cancer Res. : Offic. J. Am. Assoc. Cancer Res. 2007;13:584–590. doi: 10.1158/1078-0432.CCR-06-0964. [DOI] [PubMed] [Google Scholar]
- 13.Hedley D., et al. A phase I study of elesclomol sodium in patients with acute myeloid leukemia. Leuk. Lymphoma. 2016;57:2437–2440. doi: 10.3109/10428194.2016.1138293. [DOI] [PubMed] [Google Scholar]
- 14.Monk B.J., et al. A phase II evaluation of elesclomol sodium and weekly paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube or primary peritoneal cancer: an NRG oncology/gynecologic oncology group study. Gynecol. Oncol. 2018;151:422–427. doi: 10.1016/j.ygyno.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Day S., et al. Phase II, randomized, controlled, double-blinded trial of weekly elesclomol plus paclitaxel versus paclitaxel alone for stage IV metastatic melanoma. J. Clin. Oncol. 2009;27:5452–5458. doi: 10.1200/JCO.2008.17.1579. [DOI] [PubMed] [Google Scholar]
- 16.Chen L., Min J., Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Targeted Ther. 2022;7:378. doi: 10.1038/s41392-022-01229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge E.J., et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat. Rev. Cancer. 2022;22:102–113. doi: 10.1038/s41568-021-00417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandmann O., Weiss K.H., Kaler S.G. Wilson's disease and other neurological copper disorders. Lancet Neurol. 2015;14:103–113. doi: 10.1016/S1474-4422(14)70190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaggelli E., Kozlowski H., Valensin D., Valensin G. Copper homeostasis and neurodegenerative disorders (Alzheimer's, prion, and Parkinson's diseases and amyotrophic lateral sclerosis) Chem. Rev. 2006;106:1995–2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- 20.Mason K.E. A conspectus of research on copper metabolism and requirements of man. J. Nutr. 1979;109:1979–2066. doi: 10.1093/jn/109.11.1979. [DOI] [PubMed] [Google Scholar]
- 21.Harvey L.J., Ashton K., Hooper L., Casgrain A., Fairweather-Tait S.J. Methods of assessment of copper status in humans: a systematic review. Am. J. Clin. Nutr. 2009;89:2009s–2024s. doi: 10.3945/ajcn.2009.27230E. [DOI] [PubMed] [Google Scholar]
- 22.Harvey L.J., et al. Use of mathematical modeling to study copper metabolism in humans. Am. J. Clin. Nutr. 2005;81:807–813. doi: 10.1093/ajcn/81.4.807. [DOI] [PubMed] [Google Scholar]
- 23.Thiele D.J. Integrating trace element metabolism from the cell to the whole organism. J. Nutr. 2003;133:1579S–1580S. doi: 10.1093/jn/133.5.1579S. [DOI] [PubMed] [Google Scholar]
- 24.Ohgami R.S., Campagna D.R., McDonald A., Fleming M.D. The Steap proteins are metalloreductases. Blood. 2006;108:1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J., Petris M.J., Thiele D.J. Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J. Biol. Chem. 2002;277:40253–40259. doi: 10.1074/jbc.M208002200. [DOI] [PubMed] [Google Scholar]
- 26.Liang Z.D., Tsai W.B., Lee M.Y., Savaraj N., Kuo M.T. Specificity protein 1 (sp1) oscillation is involved in copper homeostasis maintenance by regulating human high-affinity copper transporter 1 expression. Mol. Pharmacol. 2012;81:455–464. doi: 10.1124/mol.111.076422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo Y.M., Gybina A.A., Pyatskowit J.W., Gitschier J., Prohaska J.R. Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status. J. Nutr. 2006;136:21–26. doi: 10.1093/jn/136.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts E.A., Sarkar B. Liver as a key organ in the supply, storage, and excretion of copper. Am. J. Clin. Nutr. 2008;88:851s–854s. doi: 10.1093/ajcn/88.3.851S. [DOI] [PubMed] [Google Scholar]
- 29.Öhrvik H., et al. Ctr2 regulates biogenesis of a cleaved form of mammalian Ctr1 metal transporter lacking the copper- and cisplatin-binding ecto-domain. Proc. Natl. Acad. Sci. U.S.A. 2013;110:E4279–E4288. doi: 10.1073/pnas.1311749110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin C., Zhang Z., Wang T., Chen C., James Kang Y. Copper uptake by DMT1: a compensatory mechanism for CTR1 deficiency in human umbilical vein endothelial cells. Metallomics : Integrat. Biometal Sci. 2015;7:1285–1289. doi: 10.1039/c5mt00097a. [DOI] [PubMed] [Google Scholar]
- 31.Okado-Matsumoto A., Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J. Biol. Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 32.Sturtz L.A., Diekert K., Jensen L.T., Lill R., Culotta V.C. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem. 2001;276:38084–38089. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 33.Horng Y.C., Cobine P.A., Maxfield A.B., Carr H.S., Winge D.R. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome C oxidase. J. Biol. Chem. 2004;279:35334–35340. doi: 10.1074/jbc.M404747200. [DOI] [PubMed] [Google Scholar]
- 34.Prohaska J.R. Role of copper transporters in copper homeostasis. Am. J. Clin. Nutr. 2008;88:826S–829S. doi: 10.1093/ajcn/88.3.826S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itoh S., et al. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J. Biol. Chem. 2008;283:9157–9167. doi: 10.1074/jbc.M709463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutsenko S., Barnes N.L., Bartee M.Y., Dmitriev O.Y. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007;87:1011–1046. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- 37.La Fontaine S., Ackland M.L., Mercer J.F. Mammalian copper-transporting P-type ATPases, ATP7A and ATP7B: emerging roles. Int. J. Biochem. Cell Biol. 2010;42:206–209. doi: 10.1016/j.biocel.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamza I., Prohaska J., Gitlin J.D. Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1215–1220. doi: 10.1073/pnas.0336230100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.La Fontaine S., Mercer J.F. Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch. Biochem. Biophys. 2007;463:149–167. doi: 10.1016/j.abb.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 40.Shim H., Harris Z.L. Genetic defects in copper metabolism. J. Nutr. 2003;133:1527S–1531S. doi: 10.1093/jn/133.5.1527S. [DOI] [PubMed] [Google Scholar]
- 41.Moller L.B., Mogensen M., Horn N. Molecular diagnosis of Menkes disease: genotype-phenotype correlation. Biochimie. 2009;91:1273–1277. doi: 10.1016/j.biochi.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Tumer Z., Moller L.B. Menkes disease. Eur. J. Hum. Genet. 2010;18:511–518. doi: 10.1038/ejhg.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaler S.G. ATP7A-related copper transport diseases-emerging concepts and future trends. Nat. Rev. Neurol. 2011;7:15–29. doi: 10.1038/nrneurol.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Czlonkowska A., et al. Wilson disease. Nat. Rev. Dis. Prim. 2018;4:21. doi: 10.1038/s41572-018-0018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huster D. Structural and metabolic changes in Atp7b-/- mouse liver and potential for new interventions in Wilson's disease. Ann. N. Y. Acad. Sci. 2014;1315:37–44. doi: 10.1111/nyas.12337. [DOI] [PubMed] [Google Scholar]
- 46.Maung M.T., et al. The molecular and cellular basis of copper dysregulation and its relationship with human pathologies. Faseb. J. 2021;35 doi: 10.1096/fj.202100273RR. [DOI] [PubMed] [Google Scholar]
- 47.Gupta S.K., Shukla V.K., Vaidya M.P., Roy S.K., Gupta S. Serum and tissue trace elements in colorectal cancer. J. Surg. Oncol. 1993;52:172–175. doi: 10.1002/jso.2930520311. [DOI] [PubMed] [Google Scholar]
- 48.Kuo H.W., Chen S.F., Wu C.C., Chen D.R., Lee J.H. Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol. Trace Elem. Res. 2002;89:1–11. doi: 10.1385/BTER:89:1:1. [DOI] [PubMed] [Google Scholar]
- 49.Diez M., et al. Serum and tissue trace metal levels in lung cancer. Oncology. 1989;46:230–234. doi: 10.1159/000226722. [DOI] [PubMed] [Google Scholar]
- 50.Skrajnowska D., et al. Copper and resveratrol attenuates serum catalase, glutathione peroxidase, and element values in rats with DMBA-induced mammary carcinogenesis. Biol. Trace Elem. Res. 2013;156:271–278. doi: 10.1007/s12011-013-9854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishida S., Andreux P., Poitry-Yamate C., Auwerx J., Hanahan D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc. Natl. Acad. Sci. U.S.A. 2013;110:19507–19512. doi: 10.1073/pnas.1318431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brady D.C., et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature. 2014;509:492–496. doi: 10.1038/nature13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He F., et al. Copper (II) ions activate ligand-independent receptor tyrosine kinase (RTK) signaling pathway. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/4158415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turski M.L., et al. A novel role for copper in Ras/mitogen-activated protein kinase signaling. Mol. Cell Biol. 2012;32:1284–1295. doi: 10.1128/MCB.05722-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baldari S., et al. Effects of copper chelation on BRAF(V600E) positive colon carcinoma cells. Cancers. 2019;11 doi: 10.3390/cancers11050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohno T., et al. Novel role of copper transport protein antioxidant-1 in neointimal formation after vascular injury. Arterioscler. Thromb. Vasc. Biol. 2013;33:805–813. doi: 10.1161/ATVBAHA.112.300862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blockhuys S., Zhang X., Wittung-Stafshede P. Single-cell tracking demonstrates copper chaperone Atox1 to be required for breast cancer cell migration. Proc. Natl. Acad. Sci. U.S.A. 2020;117:2014–2019. doi: 10.1073/pnas.1910722117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagaraju G.P., Dontula R., El-Rayes B.F., Lakka S.S. Molecular mechanisms underlying the divergent roles of SPARC in human carcinogenesis. Carcinogenesis. 2014;35:967–973. doi: 10.1093/carcin/bgu072. [DOI] [PubMed] [Google Scholar]
- 59.Das A., et al. Cysteine oxidation of copper transporter CTR1 drives VEGFR2 signalling and angiogenesis. Nat. Cell Biol. 2022;24:35–50. doi: 10.1038/s41556-021-00822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ash D., et al. The P-type ATPase transporter ATP7A promotes angiogenesis by limiting autophagic degradation of VEGFR2. Nat. Commun. 2021;12:3091. doi: 10.1038/s41467-021-23408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voli F., et al. Intratumoral copper modulates PD-L1 expression and influences tumor immune evasion. Cancer Res. 2020;80:4129–4144. doi: 10.1158/0008-5472.CAN-20-0471. [DOI] [PubMed] [Google Scholar]
- 62.Walshe J.M. Treatment of Wilson's disease with trientine (triethylene tetramine) dihydrochloride. Lancet. 1982;1:643–647. doi: 10.1016/s0140-6736(82)92201-2. [DOI] [PubMed] [Google Scholar]
- 63.Yoshii J., et al. The copper-chelating agent, trientine, suppresses tumor development and angiogenesis in the murine hepatocellular carcinoma cells. Int. J. Cancer. 2001;94:768–773. doi: 10.1002/ijc.1537. [DOI] [PubMed] [Google Scholar]
- 64.Cox C., et al. The role of copper suppression as an antiangiogenic strategy in head and neck squamous cell carcinoma. Laryngoscope. 2001;111:696–701. doi: 10.1097/00005537-200104000-00024. [DOI] [PubMed] [Google Scholar]
- 65.Pan Q., Rosenthal D.T., Bao L., Kleer C.G., Merajver S.D. Antiangiogenic tetrathiomolybdate protects against Her2/neu-induced breast carcinoma by hypoplastic remodeling of the mammary gland. Clin. Cancer Res. : Offic. J. Am. Assoc. Cancer Res. 2009;15:7441–7446. doi: 10.1158/1078-0432.CCR-09-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pass H.I., Brewer G.J., Dick R., Carbone M., Merajver S. A phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: final results. Ann. Thorac. Surg. 2008;86:383–389. doi: 10.1016/j.athoracsur.2008.03.016. discussion 390. [DOI] [PubMed] [Google Scholar]
- 67.Redman B.G., et al. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin. Cancer Res. : Offic. J. Am. Assoc. Cancer Res. 2003;9:1666–1672. [PubMed] [Google Scholar]
- 68.Johansson B. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr. Scand. Suppl. 1992;369:15–26. doi: 10.1111/j.1600-0447.1992.tb03310.x. [DOI] [PubMed] [Google Scholar]
- 69.Sunderman F.W. Efficacy of sodium diethyldithiocarbamate (dithiocarb) in acute nickel carbonyl poisoning. Ann. Clin. Lab. Sci. 1979;9:1–10. [PubMed] [Google Scholar]
- 70.Liu P., et al. Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. Br. J. Cancer. 2012;107:1488–1497. doi: 10.1038/bjc.2012.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu P., et al. Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells. Br. J. Cancer. 2013;109:1876–1885. doi: 10.1038/bjc.2013.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang H., et al. Disulfiram treatment facilitates phosphoinositide 3-kinase inhibition in human breast cancer cells in vitro and in vivo. Cancer Res. 2010;70:3996–4004. doi: 10.1158/0008-5472.CAN-09-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen D., Cui Q.C., Yang H., Dou Q.P. Disulfiram. A clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66:10425–10433. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 74.Lun X., et al. Disulfiram when combined with copper enhances the therapeutic effects of temozolomide for the treatment of glioblastoma. Clin. Cancer Res. : Offic. J. Am. Assoc. Cancer Res. 2016;22:3860–3875. doi: 10.1158/1078-0432.CCR-15-1798. [DOI] [PubMed] [Google Scholar]
- 75.Huang J., et al. A phase I study to repurpose disulfiram in combination with temozolomide to treat newly diagnosed glioblastoma after chemoradiotherapy. J. Neuro Oncol. 2016;128:259–266. doi: 10.1007/s11060-016-2104-2. [DOI] [PubMed] [Google Scholar]
- 76.Huang J., et al. A multicenter phase II study of temozolomide plus disulfiram and copper for recurrent temozolomide-resistant glioblastoma. J. Neuro Oncol. 2019;142:537–544. doi: 10.1007/s11060-019-03125-y. [DOI] [PubMed] [Google Scholar]
- 77.Gehrmann M. Drug evaluation: STA-4783--enhancing taxane efficacy by induction of Hsp70. Curr. Opin. Invest. Drugs. 2006;7:574–580. [PubMed] [Google Scholar]
- 78.Gibellini L., et al. Interfering with ROS metabolism in cancer cells: the potential role of quercetin. Cancers. 2010;2:1288–1311. doi: 10.3390/cancers2021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kirshner J.R., et al. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol. Cancer Therapeut. 2008;7:2319–2327. doi: 10.1158/1535-7163.MCT-08-0298. [DOI] [PubMed] [Google Scholar]
- 80.Nagai M., et al. The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells. Free Radic. Biol. Med. 2012;52:2142–2150. doi: 10.1016/j.freeradbiomed.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 81.Tsvetkov P., et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat. Chem. Biol. 2019;15:681–689. doi: 10.1038/s41589-019-0291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corazao-Rozas P., et al. Mitochondrial oxidative stress is the Achille's heel of melanoma cells resistant to Braf-mutant inhibitor. Oncotarget. 2013;4:1986–1998. doi: 10.18632/oncotarget.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wangpaichitr M., et al. N',N'-Dimethyl-N',N'-bis(phenylcarbonothioyl) propanedihydrazide (elesclomol) selectively kills cisplatin resistant lung cancer cells through reactive oxygen species (ROS) Cancers. 2009;1:23–38. doi: 10.3390/cancers1010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wangpaichitr M., et al. The relationship of thioredoxin-1 and cisplatin resistance: its impact on ROS and oxidative metabolism in lung cancer cells. Mol. Cancer Therapeut. 2012;11:604–615. doi: 10.1158/1535-7163.MCT-11-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ashton T.M., McKenna W.G., Kunz-Schughart L.A., Higgins G.S. Oxidative phosphorylation as an emerging target in cancer therapy. Clin. Cancer Res. : Offic. J. Am. Assoc. Cancer Res. 2018;24:2482–2490. doi: 10.1158/1078-0432.CCR-17-3070. [DOI] [PubMed] [Google Scholar]
- 86.Caro P., et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell. 2012;22:547–560. doi: 10.1016/j.ccr.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ghosh P., Vidal C., Dey S., Zhang L. Mitochondria targeting as an effective strategy for cancer therapy. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21093363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harrington B.S., et al. Drugs targeting tumor-initiating cells prolong survival in a post-surgery, post-chemotherapy ovarian cancer relapse model. Cancers. 2020;12 doi: 10.3390/cancers12061645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sighel D., et al. Inhibition of mitochondrial translation suppresses glioblastoma stem cell growth. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lathia J.D., Mack S.C., Mulkearns-Hubert E.E., Valentim C.L., Rich J.N. Cancer stem cells in glioblastoma. Genes Dev. 2015;29:1203–1217. doi: 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Buccarelli M., et al. Elesclomol-induced increase of mitochondrial reactive oxygen species impairs glioblastoma stem-like cell survival and tumor growth. J. Exp. Clin. Cancer Res. 2021;40:228. doi: 10.1186/s13046-021-02031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marchetto A., et al. Oncogenic hijacking of a developmental transcription factor evokes vulnerability toward oxidative stress in Ewing sarcoma. Nat. Commun. 2020;11:2423. doi: 10.1038/s41467-020-16244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown J.M., Wilson W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 94.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, N.Y.) 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee P., Chandel N.S., Simon M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020;21:268–283. doi: 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kluza J., et al. Inactivation of the HIF-1alpha/PDK3 signaling axis drives melanoma toward mitochondrial oxidative metabolism and potentiates the therapeutic activity of pro-oxidants. Cancer Res. 2012;72:5035–5047. doi: 10.1158/0008-5472.CAN-12-0979. [DOI] [PubMed] [Google Scholar]
- 97.Lu C.W., Lin S.C., Chen K.F., Lai Y.Y., Tsai S.J. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J. Biol. Chem. 2008;283:28106–28114. doi: 10.1074/jbc.M803508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ren Y.J., et al. Silencing of NAC1 expression induces cancer cells oxidative stress in hypoxia and potentiates the therapeutic activity of elesclomol. Front. Pharmacol. 2017;8:804. doi: 10.3389/fphar.2017.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Claps G., et al. The multiple roles of LDH in cancer. Nat. Rev. Clin. Oncol. 2022;19:749–762. doi: 10.1038/s41571-022-00686-2. [DOI] [PubMed] [Google Scholar]
- 100.Koukourakis M.I., et al. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br. J. Cancer. 2003;89:877–885. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gulec S., Collins J.F. Molecular mediators governing iron-copper interactions. Annu. Rev. Nutr. 2014;34:95–116. doi: 10.1146/annurev-nutr-071812-161215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Doguer C., Ha J.H., Collins J.F. Intersection of iron and copper metabolism in the mammalian intestine and liver. Compr. Physiol. 2018;8:1433–1461. doi: 10.1002/cphy.c170045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tang D., Chen X., Kang R., Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xue Q., et al. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy. 2023;19:1982–1996. doi: 10.1080/15548627.2023.2165323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Song W.F., et al. Self-assembled copper-based nanoparticles for glutathione activated and enzymatic cascade-enhanced ferroptosis and immunotherapy in cancer treatment. Small. 2023 doi: 10.1002/smll.202301148. [DOI] [PubMed] [Google Scholar]
- 106.Wu L., Zhou L., Liu D.Q., Vogt F.G., Kord A.S. LC-MS/MS and density functional theory study of copper(II) and nickel(II) chelating complexes of elesclomol (a novel anticancer agent) J. Pharm. Biomed. Anal. 2011;54:331–336. doi: 10.1016/j.jpba.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 107.Garza N.M., Zulkifli M., Gohil V.M. Elesclomol elevates cellular and mitochondrial iron levels by delivering copper to the iron import machinery. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2022.102139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tadini-Buoninsegni F., Smeazzetto S. Mechanisms of charge transfer in human copper ATPases ATP7A and ATP7B. IUBMB Life. 2017;69:218–225. doi: 10.1002/iub.1603. [DOI] [PubMed] [Google Scholar]
- 109.Gao W., et al. Elesclomol induces copper-dependent ferroptosis in colorectal cancer cells via degradation of ATP7A. Mol. Oncol. 2021;15:3527–3544. doi: 10.1002/1878-0261.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cen D., Brayton D., Shahandeh B., Meyskens F.L., Jr., Farmer P.J. Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. J. Med. Chem. 2004;47:6914–6920. doi: 10.1021/jm049568z. [DOI] [PubMed] [Google Scholar]
- 111.Yip N.C., et al. Disulfiram modulated ROS-MAPK and NFkappaB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br. J. Cancer. 2011;104:1564–1574. doi: 10.1038/bjc.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tardito S., et al. Copper binding agents acting as copper ionophores lead to caspase inhibition and paraptotic cell death in human cancer cells. J. Am. Chem. Soc. 2011;133:6235–6242. doi: 10.1021/ja109413c. [DOI] [PubMed] [Google Scholar]
- 113.Liu N., Huang H., Dou Q.P., Liu J. Inhibition of 19S proteasome-associated deubiquitinases by metal-containing compounds. Oncoscience. 2015;2:457–466. doi: 10.18632/oncoscience.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tsvetkov P., et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science (New York, N.Y.) 2022;375:1254–1261. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang X., et al. Copper and cuproptosis-related genes in hepatocellular carcinoma: therapeutic biomarkers targeting tumor immune microenvironment and immune checkpoints. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1123231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou Z., et al. Prognostic significance of cuproptosis-related gene signatures in breast cancer based on transcriptomic data analysis. Cancers. 2022;14 doi: 10.3390/cancers14235771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bao J.H., et al. Identification of a novel cuproptosis-related gene signature and integrative analyses in patients with lower-grade gliomas. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.933973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo B., et al. Cuproptosis induced by ROS responsive nanoparticles with elesclomol and copper combined with alphaPD-L1 for enhanced cancer immunotherapy. Adv. Mater. 2023;35 doi: 10.1002/adma.202212267. [DOI] [PubMed] [Google Scholar]
- 119.Wang W., et al. Ferroptosis inducers enhanced cuproptosis induced by copper ionophores in primary liver cancer. J. Exp. Clin. Cancer Res. 2023;42:142. doi: 10.1186/s13046-023-02720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meng E., et al. CD44+/CD24- ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival. Clin. Exp. Metastasis. 2012;29:939–948. doi: 10.1007/s10585-012-9482-4. [DOI] [PubMed] [Google Scholar]
- 121.Pasto A., et al. Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget. 2014;5:4305–4319. doi: 10.18632/oncotarget.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cierlitza M., et al. Mitochondrial oxidative stress as a novel therapeutic target to overcome intrinsic drug resistance in melanoma cell subpopulations. Exp. Dermatol. 2015;24:155–157. doi: 10.1111/exd.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zheng P., Zhou C., Lu L., Liu B., Ding Y. Elesclomol: a copper ionophore targeting mitochondrial metabolism for cancer therapy. J. Exp. Clin. Cancer Res. 2022;41:271. doi: 10.1186/s13046-022-02485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Soma S., et al. Elesclomol restores mitochondrial function in genetic models of copper deficiency. Proc. Natl. Acad. Sci. U.S.A. 2018;115:8161–8166. doi: 10.1073/pnas.1806296115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guthrie L.M., et al. Elesclomol alleviates Menkes pathology and mortality by escorting Cu to cuproenzymes in mice. Science (New York, N.Y.) 2020;368:620–625. doi: 10.1126/science.aaz8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yuan S., Korolnek T., Kim B.E. Oral elesclomol treatment alleviates copper deficiency in animal models. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.856300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gohil V.M. Repurposing elesclomol, an investigational drug for the treatment of copper metabolism disorders. Expet Opin. Invest. Drugs. 2021;30:1–4. doi: 10.1080/13543784.2021.1840550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Manzari M.T., et al. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021;6:351–370. doi: 10.1038/s41578-020-00269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.