Abstract
The detailed analysis of 411 strains of coagulase-negative staphylococci (CoNS) obtained from 40 neutropenic hemato-oncologic patients (61 Hickman catheter episodes) on intensive chemotherapy is described. By random amplification of polymorphic DNA (RAPD) analysis, a total of 88 different genotypes were detected: 51 in air samples and 30 in skin cultures prior to insertion, 12 in blood cultures after insertion, and only 5 involved in catheter-related infections (CRI). Two RAPD genotypes of Staphylococcus epidermidis predominated, and their prevalence increased during patient hospitalization. At insertion, these clones constituted 11 of 86 (13%) CoNS isolated from air samples and 33 of 75 (44%) CoNS isolated from skin cultures. After insertion, their combined prevalence increased to 33 of 62 (53%) in catheters not associated with CRI and 139 of 188 (74%) in catheters associated with CRI (P = 0.0041). These two predominant S. epidermidis clones gave rise to a very high incidence of CRI (6.0 per 1,000 catheter days) and a very high catheter removal rate for CRI, 70%, despite prompt treatment with vancomycin. A likely source of S. epidermidis strains involved in CRI appeared to be the skin flora in 75% of cases. The validity of these observations was confirmed by pulsed-field gel electrophoresis (PFGE) of SmaI DNA macrorestriction fragments of blood culture CoNS isolates. Again, two predominant CoNS genotypes were found (combined prevalence, 60%). RAPD and PFGE yielded concordant results in 75% of cases. Retrospectively, the same two predominant CoNS clones were also found among blood culture CoNS isolates from the same hematology department in the period 1991 to 1993 (combined prevalence, 42%) but not in the period 1978 to 1982. These observations underscore the pathogenic potential of clonal CoNS types that have successfully and persistently colonized patients in this hemato-oncology department.
Coagulase-negative staphylococci (CoNS) have become the most frequently isolated pathogens in intravascular catheter-related infections (CRI), accounting for an estimated 28% of all nosocomial bloodstream infections reported to the National Nosocomial Infections Surveillance System from 1986 through 1989 (3, 16, 30). The emergence of CoNS as the primary pathogen causing CRI has been attributed to the increased use of prosthetic and indwelling devices, the increased use of parenteral nutrition, and the improved survival of the immunocompromised host. In addition, CoNS have been recognized as potentially true nosocomial pathogens rather than harmless culture contaminants not worthy of being reported back from the laboratory to the attending physicians (3, 9, 10). In hemato-oncologic patients, the effective application of antibiotic prophylaxis has facilitated the introduction of more aggressive chemotherapies that have further increased the risk of septicemia by CoNS (35).
Earlier CoNS-genotyping studies have shown persistence of a few CoNS strains in various wards of our hospital. In 1993, more than 30% of CoNS from blood cultures taken from a heart-lung machine during cardiac surgery belonged to a single genotype (33). Furthermore, it was shown that certain CoNS strains permeate the hematology department and colonize its personnel. A number of hemato-oncologic patients were persistently colonized by a single type; from others, however, multiple strains of CoNS were isolated (34). In another controlled study, a high incidence of Hickman CRI due to CoNS in neutropenic patients was demonstrated in the same hematology department (2, 24).
The goal of the present study was to determine prospectively the molecular epidemiology of CoNS involved in CRI within a group of neutropenic hemato-oncologic patients at a large university hospital in The Netherlands. Phenotypic and genotypic typing strategies were used to identify the clonal relatedness of the CoNS strains involved.
(Part of this research was presented at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, La., 15 to 18 September 1996 [23].)
MATERIALS AND METHODS
Patients and materials.
All consecutive hemato-oncological patients receiving intensive chemotherapy between August 1994 and April 1996 were included in the study. Informed consent was obtained from all patients or their parents or guardians, and the study protocol was reviewed and approved by the local medical ethics committee. All patients were fitted with a bilumen Hickman central venous catheter and received antimicrobial prophylaxis with ciprofloxacin and fluconazole. Catheters were inserted under strict aseptic conditions with sonographic and fluoroscopic guidance (17, 27). Hickman CRI were defined as described previously (24).
Culturing.
Cultures from different sites were taken regularly according to the following scheme. (i) Before skin disinfection with 0.5% chlorhexidine in 70% ethanol, cultures were taken from the skin at the insertion site and from air samples. After insertion but prior to closure of the wound, two exit site cultures were taken. (ii) During hospitalization, serial cultures from the exit site, hub interiors, and blood cultures drawn directly from both Hickman catheter channels were taken twice weekly. (iii) In case of fever, extra cultures from the exit site and hub interiors plus at least two blood culture sets each via both Hickman catheter channels and a peripheral vein were taken. As long as fever persisted, these investigations were performed daily. (iv) When indicated, the Hickman catheter was removed by a surgeon and the tip, tunnel, and hub segments were cultured separately.
Isolation and identification of CoNS.
Skin, exit site, hub, and air sample culturing was performed according to standard procedures (14). For blood culturing, the BACTEC 9240 system (Becton Dickinson Diagnostic Instrument Systems, Sparks, Md.) was used. Tip and tunnel segments of removed Hickman catheters were cultured by the semiquantitative roll-plate technique described by Maki et al. (20). Tip, tunnel, and hub segments of removed Hickman catheters were cultured quantitatively after flushing according to Linares et al. (18). All three segments were also cultured in serum broth. Isolates were identified as CoNS based on catalase and tube coagulase tests. CoNS were identified to species level by using the API-Staph 32 test (bioMerieux, Lyon, France) (14). CoNS isolated from clinical materials only, i.e., exit site skin, blood cultures, and removed catheters, were tested for antibiotic susceptibility with the Vitek system (bioMerieux Vitek, Hazelwood, Mo.). MICs were determined by E test (AB Biodisk, Solna, Sweden). Strains were categorized as resistant, intermediately sensitive, or sensitive to the antibiotic used based on National Committee for Clinical Laboratory Standards breakpoints (21). All CoNS isolates were stored at −70°C in glycerol-containing liquid media.
Analysis of genetic relatedness of CoNS.
To obtain bacterial DNA for analysis, CoNS strains were grown overnight at 37°C on brucella blood agar. Between two and five colonies were suspended in a 150-μl solution containing (per liter) 25 mmol of Tris-HCl (pH 8.0), 10 mmol of EDTA, and 50 mmol of glucose. To prepare spheroplasts, 75 μl of a lysostaphin solution (100 μg/ml in water) was added. After incubation at 37°C for 1 h, DNA was isolated according to the protocol described previously (5). The final volume of the DNA solution containing 10 mmol of Tris-HCl (pH 8.0) per liter and 1 mmol of EDTA per liter was 100 μl. The concentration of the purified DNA was determined and adjusted to approximately 10 ng per μl. DNA preparations were stored at −20°C.
According to current recommendations, two techniques were used to type staphylococci at the genotypic level (33, 34). First, all available CoNS isolates from air sample, insertion site skin, exit site, hub, blood, and catheter cultures were genotyped by PCR-based random amplification of polymorphic DNA (RAPD). Analysis of amplification fragment length polymorphisms was performed essentially as described previously (32). Arbitrarily primed PCR was performed with Taq DNA polymerase (0.2 U per assay) (Sphaero Q, Leiden, The Netherlands) in the accompanying buffer system, 0.2 mmol of the respective deoxynucleoside triphosphates per liter and 50 pmol of primer. DNA (50 ng) was added and the mixtures were overlaid with mineral oil. Primers used were the oligonucleotides ERIC 1 and ERIC 2, having the following nucleotide sequences, respectively: 5′-TGTAAGCTCCTGGGGATTCAC-3′ and 5′-AAGTAAGTGACTGGGGTGAGCG-3′. These primers are deduced from enterobacterial repetitive intergenic consensus sequences (36). Cycling (40 times for 1 min at 94°C, 1 min at 25°C, and 2 min at 74°C) was performed in a BioMed model 60 thermocycler (BioMed, Theres, Germany). Analysis of reaction products was by gel electrophoresis in 1 to 2% agarose gels submerged in 0.5× Tris-borate-EDTA (TBE) buffer (28) and subsequent photography with a Polaroid Land camera and high-speed Polaroid sheet film. Data were interpreted by three independent observers, after which consensus was pursued based on additional experimental data or interobserver discussion. Unique DNA banding patterns were indexed with capital letters. When two fingerprints differed with respect to single DNA band-staining intensity only, the same letter was used but a prime was added to the second (e.g., A and A′).
In addition, pulsed-field gel electrophoresis (PFGE) was subsequently performed on all bloodstream CoNS isolates available from patients. For purposes of comparison, available bloodstream CoNS isolates from hemato-oncologic patients admitted to the same hematology department in the periods 1978 to 1982 and 1991 to 1993 were also genotyped by PFGE. PFGE was carried out based on protocols previously described for DNA from Staphylococcus aureus (12, 25). A suspension of bacteria was mixed in a 1:1 ratio with 1% InCert agarose (FMC Bioproducts, Rockland, Maine). Agarose plugs were prepared with Bio-Rad (Veenendaal, The Netherlands) casting forms and incubated with lysostaphin (Sigma Chemical Co., St. Louis, Mo.). Spheroplasts were lysed by incubation of the plugs in buffer containing 1% sodium dodecyl sulfate and 1 mg of proteinase K (Boehringer, Mannheim, Germany) per ml. Plugs were washed six times for 30 min each in a solution containing 10 mmol of Tris-HCl (pH 8.0) per liter and 1 mmol of EDTA per liter and stored at 4°C. DNA from half of a plug was digested by SmaI (Boehringer), and PFGE was carried out in 1% SeaKem GTG agarose gels (FMC Bioproducts). The buffer consisted of 0.5× TBE; the temperature was set at 14°C. Electrophoresis was performed in a Bio-Rad CHEF Mapper. Running time was 22 h, with linear ramping from 2.16 to 44.69 s at an angle of 120° (60°/−60°). The voltage was 6 V/cm, and gel dimensions were 120 by 140 by 5 mm. Gels were stained with ethidium bromide and photographed with instant Polaroid equipment. Differences in banding patterns were documented by at least two independent observers. Genotypes were defined on the basis of identity of the DNA banding patterns. Subtypes differed in the positions of one or two restriction fragments only (34).
RESULTS
In total, 411 CoNS isolates from 61 episodes of Hickman catheter employment in 40 consecutive patients were collected: 389 (95%) belonged to the species Staphylococcus epidermidis; strains of Staphylococcus haemolyticus, Staphylococcus hominis, and Staphylococcus warneri were incidentally isolated. Thirty-six different antibiotic susceptibility patterns were determined: 71% of strains isolated were resistant to methicillin, 89% to penicillin, 55% to gentamicin, 71% to ciprofloxacin, 87% to sulfamethoxazole-trimethoprim, 81% to erythromycin, 56% to clindamycin, 20% to rifampin, and 51% to tetracycline. All were vancomycin susceptible. The majority of strains were resistant to five or more antibiotics.
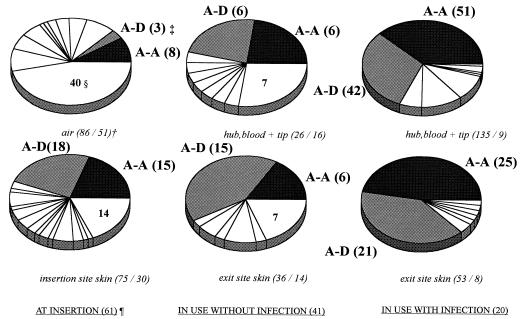
By RAPD, a total of 88 different genotypes of CoNS were found. However, two genotypes of S. epidermidis (types A-A and A-D) predominated. Their combined prevalence increased from 13% at insertion (air sample cultures) to 74% after insertion in catheters associated with CRI (Fig. 1). Their combined prevalence differed significantly between catheters associated with CRI and those not associated with CRI (139 of 188 [74%] and 33 of 62 [53%], respectively [P = 0.0041]). When only blood culture isolates (n = 148) were considered, their combined prevalence rates were 9 of 23 (39%) and 84 of 125 (67%), respectively (P = 0.017 [Fisher’s exact test]). In six of eight catheters (75%) removed because of CRI and yielding positive catheter tip cultures, types A-A and/or A-D were demonstrated (Table 1).
FIG. 1.
Distribution of RAPD genotypes of CoNS during Hickman catheter usage in hemato-oncologic patients. Eighty-eight RAPD genotypes among 411 CoNS isolates from 61 Hickman catheter episodes in 40 patients were detected. Each sector of an individual pie chart represents the contribution of a single RAPD CoNS genotype to the total number of CoNS isolated at the indicated time, site, and infection status. †, number of strains/number of RAPD genotypes isolated; ‡, two-letter codes correspond to codes in Table 1 while numbers are the numbers of strains isolated; §, number of single unique RAPD genotypes isolated at the indicated time, site, and infection status; ¶, number of catheter episodes at the indicated time and infection status.
TABLE 1.
Chronological array of bloodstream CoNS isolates together with genotypically related isolates from air samples, skin, exit sites, and catheter hubsa
| Patient | RAPD type (ERIC 1-ERIC 2) | PFGE type | Chronological isolation of CoNS from different sites after Hickman catheter insertionb |
|---|---|---|---|
| CRI | |||
| 1 | F-E | A | •• ■ |
| I +++++++++ R§ | |||
| 7 | A-A | Icath I, Dcath II | ○□ □• ○□ □••• |
| I ++++++++++++ R§I +++++++++ R§ | |||
| C-C | G | •••• ■ | |
| 8 | A-D | B | ••••■ |
| I ++++++++++++++++++++++++++++++++++++ R§ | |||
| 9cath I | A-D | B | □ ••□••■ |
| I+++++++++ R§ | |||
| A-A | ■ | ||
| 10 | A-A | D | ••■ |
| I +++++++++++++++++++ R§ | |||
| 13 | A-A | Icath I, J + Bcath II | ○□□ □□□•••□ □ ••□•□⊡••• |
| I ++++++++++++++ R§¶I ++++++++++++++++++ R§ | |||
| F-Q | K | • | |
| 15 | A-A | D | ○□ □ □ •• • |
| I +++++++++++++ R§ | |||
| 17 | A-A | D | ○□ □□ •••• |
| I ++++++++++++ R§ | |||
| F-E | L | •• | |
| 31 | A-EE | B + D | ••• |
| I ++++++++++++++++++++ R§ | |||
| JJ-FF | K | • | |
| 5 | A-A | D | ○ □ □ □□□□□•□ |
| I +++++++++++++++++++++++++++++++++++++++++++++++++ NR¶ | |||
| 12 | A-D | B | □ • |
| I +++++++++++++++++++++++++++++++++++++++++++++++++ NR¶ | |||
| 35cath I | A-EE | Q | □ ••• □ |
| I +++++++++++ R¶ | |||
| 2 | A-D | B | □ ⊡ • □ □••• ■ |
| I ++++++++++++ R§¶ | |||
| 4 | A-D | B | • • ■ |
| I +++++++ R§¶ | |||
| A-A | □ □ ■ | ||
| 6 | A-D | B | •• ■ |
| I ++++ R§¶ | |||
| CC or BCCc | |||
| 3 | A-D | B | □• ■ |
| I +++++++++++ NR∥ | |||
| 9cath II | A-D | B | □ • • |
| I +++++++++++++++++++++ NR∥ | |||
| 16 | A-A | D | • |
| I +++++++++++++++++++++++++++++++++++++++++++++++++ NR∥ | |||
| F-N | L | • | |
| 19 | F-U | K | • |
| I +++++++++++++++++++++++++++++++++++++++++++++++++ NR∥ | |||
| 20 | T-I | M | • |
| I ++++++++++++++++++++++++++++ NR∥ | |||
| 22 | A-D | B | □ □ • • |
| I +++++++++++++++++++++++++++++++++++++++++++++++++ NR∥ | |||
| 23 | F-E | L | • |
| I +++++++++++++++++++++++++++++++++++++++++++++++++ NR∥ | |||
| 24 | A-D | B | □ • |
| I +++++++++++++++++++++++++++++++++++++++++++++++++ NR∥ | |||
| 25 | Z-E | N | • |
| I +++++++++++++++++++++++++++++++++++++++++++++++++ NR∥ | |||
| 26 | A-A | D | • |
| I +++++++++++++++++++++++++ NR∥ | |||
| AA-D | O | • | |
| 33 | A-EE | P | □ □•□• • |
| I +++++++++++++++++++++++++++++++++++++++++++++++++ NR∥ | |||
| 35cath II | R-GG | R | •• |
| I ++++++++++++++++++++++++++ NR∥ | |||
| 40 | W-B | E | • • |
| I +++++++++++++++++++++++++++++++++++++++++++++++++ NR∥ |
Isolates from 30 catheter episodes (26 patients) with positive CoNS blood cultures, 20 catheter episodes (18 patients) defined as CRI (17 with positive blood cultures and 3 localized infections without positive blood cultures [not shown]), and 10 catheter episodes defined as catheter colonization or blood culture contamination. One CoNS isolate per day per culture site is shown. Catheter episodes without positive blood cultures are not shown, and genotypically nonrelated CoNS isolates from air samples, skin/exit sites and hubs are not shown.
§, catheter-related bloodstream infection; ¶, catheter-related local infection; ∥, no CRI; I, insertion; R, removed for infection; NR, not removed for infection; +++, 1 week; ○, air sample culture; □, skin/exit site culture; ⊡, hub culture; •, blood culture; ■, tip culture.
Catheter colonization or blood culture contamination.
The diversity in CoNS genotypes decreased inversely from 51 among isolates from air sample cultures to 8 in exit site skin cultures from catheters with CRI (Fig. 1). Twenty-three different S. epidermidis genotypes were isolated after insertion: 17 in catheters not associated with CRI and 11 in catheters associated with CRI. In blood culture isolates, 17 different genotypes of S. epidermidis were detected: 11 in catheters not associated with CRI and 9 in catheters associated with CRI. Only four different genotypes of S. epidermidis were isolated from the surfaces of removed catheters (Table 1). Antibiotic susceptibility patterns varied widely, even within single RAPD genotypes. For example, within RAPD genotype A-A, 20 different antibiotic susceptibility patterns were discerned, while within RAPD genotype A-D, 16 patterns were distinguished. The prevalences of methicillin resistance among RAPD genotypes A-A and A-D were 62.5 and 76%, respectively. Both penicillin and ciprofloxacin resistance was found in 94% of A-A strains and in 90% of A-D strains.
Twenty CRI episodes were encountered in 18 patients: 10 bacteremias (RAPD types A-A [5], A-D [2], A-EE [1], F-E [1], and A-A plus C-C [1]), 6 local infections (A-A [1], A-D [1], A-EE [1], and no positive cultures [3]), and 4 combined infections (A-A [1], A-D [2], and A-A plus A-D [1]). Thus, only five different RAPD genotypes of S. epidermidis (A-A [8], A-D [6], A-EE [2], F-9E [1], and C-C [1]) were involved in these 20 CRI episodes (Table 1; Fig. 2). RAPD genotypes N-D, F-Q, and JJ-FF were considered not relevant, since they were each isolated only once. In 15 of these 20 (75%) episodes the skin was very likely to be the source of these S. epidermidis isolates; there were 10 local infections (with or without bacteremia), and in 5 of 10 “pure” bacteremias the same RAPD genotype was cultured from blood as from exit site skin before the onset of infection (Table 1).
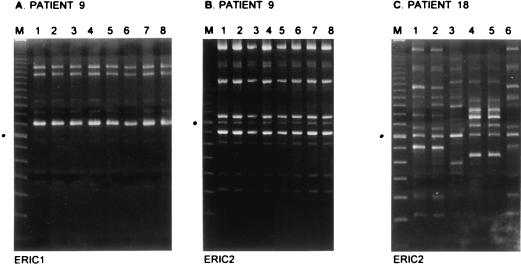
FIG. 2.
RAPD analysis of CoNS isolated from patients fitted with Hickman catheters. For patient 9 (A and B), eight strains derived from blood cultures were analyzed in two separate assays with two different primer species in RAPD (ERIC 1 and ERIC 2), as indicated below the panels. The patient was sampled daily for a full week, and all blood samples were culture positive for CoNS. After catheter removal, catheter tip cultures were also found to be positive. All RAPD fingerprints obtained were identical, demonstrating persistent infection by a single genotype (predominant strain A-D [see also Table 1]). For comparison, panel C displays typing data for strains derived from patient 18. Increased diversity in ERIC 2 fingerprints is shown. Strains were obtained from the catheter exit site, skin, and urine on various occasions spanning a period of more than 3 months. Lanes M display molecular length markers (100-bp ladder; Gibco BRL). The fragment indicated by a black dot on the left of each panel is 800 bp long.
In 13 catheter episodes in 13 patients, positive blood cultures (contamination or colonization) were obtained in the absence of signs or symptoms of CRI. In these episodes, 11 different RAPD genotypes of S. epidermidis (A-A [2], A-D [4], A-EE [1], AA-D [1], F-E [1], F-N [1], F-U [1], R-GG [1], T-I [1], W-B [1], and Z-E [1]) were found. In 5 of the 13 cases, the RAPD genotype found in the blood was demonstrated on skin beforehand (Table 1).
The prospective positive and negative predictive values (PV+ and PV−, respectively) of the serial surveillance cultures for subsequent development of CRIs were low: hub PV+, 33%; hub PV−, 63%; exit site PV+, 33%; exit site PV−, 68%; and for blood cultures via the Hickman catheter, PV+ was 55% and PV− was 88%. Also, colonization with RAPD genotypes A-A and/or A-D did not predict the development of CRI: these clones were isolated from skin or exit sites in 11 of 20 catheters associated with CRI before the onset of infection versus 15 of 41 catheters not associated with CRI (odds ratio, 2.12 [95% confidence interval, 0.715 to 6.28]; P = 0.27).
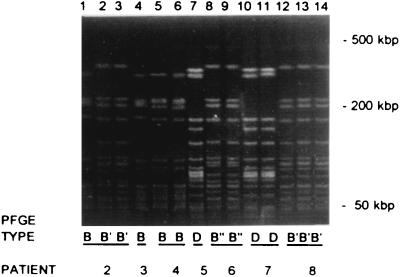
Among the 14 RAPD genotypes of S. epidermidis isolated from 30 catheter episodes with positive blood cultures (17 CRI and 13 non-CRI), 15 different PFGE genotypes were detected. As with RAPD, with PFGE two predominant genotypes of S. epidermidis were found: type B was isolated in 12 of 30 (40%) catheter episodes, and type D was isolated in 8 of 30 (27%) catheter episodes (Table 1; Fig. 3). These predominant genotypes of S. epidermidis persisted throughout the whole study period. Four different PFGE genotypes of S. epidermidis were distinguished among both RAPD genotype A-A (11 genotypes in 10 catheters: B [1], D [7], I [2], and J [1]), and genotype A-EE (4 genotypes in 3 catheters: B [1], D [1], P [1], and Q [1]), while RAPD genotype A-D could not be subtyped further (n = 10 [all PFGE type B]). PFGE genotypes B, D, I, J, P, and Q were confined to RAPD genotypes A-A, A-D, and A-EE. PFGE type K consisted of three different RAPD genotypes (F-Q, F-U, and JJ-FF). RAPD genotype F-E was PFGE typed as A once and as L twice. The seven other RAPD genotypes coincided with one unique PFGE type (Table 1).
FIG. 3.
PFGE of SmaI DNA macrorestriction fragments from CoNS obtained from blood cultures of patients fitted with Hickman catheters. Strains (numbered 1 through 14) were obtained from seven patients, who are indicated by number at the bottom (corresponding with numbering in Table 1). PFGE types are given below the lanes and correspond to PFGE types in Table 1. The positions of some of the reference DNA size markers are indicated on the right.
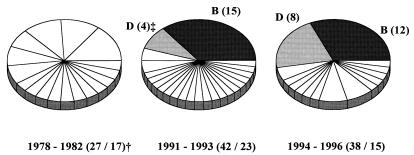
Blood culture CoNS isolates from hemato-oncologic patients admitted to the same hematology department in the periods 1978 to 1982 and 1991 to 1993 were subsequently genotyped by PFGE (one blood culture isolate per genotype per catheter episode). Seventeen PFGE genotypes were found in 27 CoNS isolates from the period 1978 to 1982. No predominant genotypes seemed to be present at that time, and all were genotypically different from the CoNS isolates from the present study. Twenty-three PFGE genotypes were found in 42 CoNS isolates from the period 1991 to 1993. The same two PFGE genotypes (B and D) of S. epidermidis found to be predominant in patient cultures from August 1994 to April 1996 were also found to be present in the period 1991 to 1993. Their combined prevalence has risen from 43% in 1991 to 1993 to 60% in 1994 to 1996 (Fig. 4).
FIG. 4.
PFGE of SmaI DNA macrorestriction fragments from CoNS obtained from blood cultures of patients fitted with Hickman catheters during three different time periods (one isolate per genotype per catheter episode). †, number of strains/number of PFGE genotypes isolated; ‡, one-letter codes correspond to codes in Table 1 while numbers are the numbers of strains isolated.
DISCUSSION
CoNS strains have been implicated frequently in infectious diseases in various groups of patients, although mostly in neonates (13, 15, 22, 33, 34). Persistence of multiresistant strains for prolonged periods has been documented in neonatal intensive care units, suggesting that cross-infections occur regularly (15, 19). Molecular epidemiology of CoNS from blood isolates of neonates with persistent bacteremia has revealed that CRI may be caused persistently by a single strain of CoNS (22, 31). Another study considering CRI by CoNS described colonization and subsequent infection by CoNS strains unique to individual patients (8).
In the present study, it was demonstrated that two clones of S. epidermidis were predominantly involved in colonization and subsequent infection in neutropenic hemato-oncologic patients in a setting with a high incidence of CRI (6.0 per 1,000 catheter days). These two clones were responsible for more than 70% of CRI, and they seem to have persisted for a period of at least 5 years in our hematology department (34). However, colonization itself with one of the two predominant clones could not predict the development of a CRI. Furthermore, these two predominant clones appear to be confined to the hematology department so far, since their PFGE patterns were shown to be totally different from those of CoNS isolated earlier in the cardiopulmonary surgery department and other departments of our hospital (33).
At insertion, RAPD genotypes A-A and A-D of S. epidermidis were found in 13% of air sample cultures and in 44% of skin cultures. During Hickman catheter employment, the prevalence rates of these clones were significantly higher in catheters associated with CRI than in catheters not associated with CRI (74 and 53%, respectively [P = 0.0041]). At removal, these two clones were isolated in 78% of cases. Thus, their prevalence increased during hospitalization, making it very likely that acquisition by cross-contamination in the hematology department occurred frequently. The air of the radiology suite probably became contaminated by CoNS present on the skin of patients under treatment. Furthermore, most Hickman catheters were inserted at the end of the first week following admittance to the hematology department, according to the hemato-oncologic workup protocol. This probably explains why, at the time of Hickman catheter insertion, 44% of patients were already carrying these two clones on their (insertion site) skin. Further genotyping studies of CoNS from the skin of ward-related personnel and air samples from the hematology department, as well as skin cultures from patients upon admittance, are needed to elucidate the transmission routes taken by these strains. However, the skin of the two intervention radiologists inserting all Hickman catheters was free of these two predominant clones of S. epidermidis or any of the other S. epidermidis strains involved in CRI (results not shown).
Besides the fact that these two clones were very predominant, they were possibly also more virulent than other CoNS, since a very high rate of CRI (6.0 per 1,000 catheter days) has been reported from this hematology department, and, importantly, nearly 70% of catheters associated with developed CRI had to be removed prematurely despite prompt and adequate treatment with vancomycin (2, 24). This contrasted sharply with other studies in the literature in which much lower rates of CRI were observed and in which only 30% or fewer catheters had to be removed in the course of a CRI (1, 6, 7, 26, 29, 37).
As 94% of the two predominant strains were ciprofloxacin resistant and all patients received selective antimicrobial prophylaxis with ciprofloxacin, these strains possessed a selective advantage. Perhaps subinhibitory concentrations of ciprofloxacin are able to promote adherence of these two S. epidermidis clones, as has been described recently for S. aureus (4). However, in earlier studies, adherence of a variety of CoNS strains was reduced after incubation with subinhibitory concentrations of ciprofloxacin (38). Furthermore, the pathogenic route in the development of CRI was shown to be different than those found in other studies: in 75% of cases, the most likely source of CRI in this patient group was the exit site skin, while in only two patients could the hub have played a role (18, 37).
In the study of the epidemiology and dynamics of CoNS infections in this neutropenic patient population, genotyping procedures by RAPD and PFGE were shown to be of more value than phenotyping procedures such as species determination by API-Staph 32 and study of antibiotic resistance patterns (8, 33, 34). All but one of bloodstream isolates were identified as S. epidermidis, and antibiotic resistance patterns varied widely, not only between different CoNS genotypes but also within a single genotype. From these results, we conclude that such phenotyping procedures are of little use in determining the pathogenesis and epidemiology of CoNS infection. RAPD and PFGE had about the same discriminating power: PFGE detected 15 different genotypes, and RAPD detected 14 different genotypes. Also, RAPD and PFGE showed concordant results in 75% of cases for all genotypes and in 100% of cases involving RAPD type A-D and PFGE type D.
In our opinion, therefore, RAPD and PFGE are preferred over biotyping, determining antibiotic resistance patterns, bacteriophage typing, sodium dodecyl sulfate-polyacrylamide gel electrophoresis protein profiles, and plasmid profile analysis (11, 22). Although plasmid profile analysis is a simple method that is useful for initial differentiation among isolates, strains readily lose and acquire plasmids, as is also reflected by the diversity in antibiotic resistance patterns, thus yielding misleading results in comparisons of isolates over time (22). Moreover, RAPD and PFGE are easy to perform and, like blotting procedures (22), yield reproducible results over time. Furthermore, as has been shown before, RAPD and PFGE can be used in combination to increase their discriminatory power (33, 34).
In conclusion, two virulent clones of S. epidermidis were shown to be involved in 70% of CRI. These two clones have expanded for at least the last 5 years in our hematology department. Their prevalence may in part be related to the introduction in 1987 of ciprofloxacin as part of the selective antimicrobial prophylaxis of neutropenic hemato-oncologic patients. Apparently, certain strains of CoNS have the capability of firmly establishing themselves among certain groups of critically ill patients in a given geographical setting. Analysis of virulence determinants that provide this selective advantage (adhesins for catheter plastic, capacity to effectively form biofilms, and slime and toxin production, etc.) is the focus of further research.
ACKNOWLEDGMENTS
J. Sluijs was supported by a grant provided by the Erasmus Trust Fund (Erasmus University Rotterdam, Rotterdam, The Netherlands).
We thank Marian Humphrey for critically reviewing the manuscript and Wim Hop for expert statistical advice.
REFERENCES
- 1.Abrahm J L, Mullen J L. A prospective study of prolonged central venous access in leukemia. JAMA. 1982;248:2868–2873. [PubMed] [Google Scholar]
- 2.Bakker J, van Overhagen H, Wielenga J, de Marie S, Nouwen J, de Ridder M A, Lameris J S. Infectious complications of radiologically inserted Hickman catheters in patients with hematologic disorders. Cardiovasc Interventional Radiol. 1998;21:116–121. doi: 10.1007/s002709900226. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee S N, Emori T G, Culver D H, Gaynes R P, Jarvis W R, Horan T, Edwards J R, Tolson J, Henderson T, Martone W J. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. National Nosocomial Infections Surveillance System. Am J Med. 1991;91:86S–89S. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- 4.Bisognano C, Vaudaux P E, Lew D P, Ng E Y W, Hooper D C. Increased expression of fibronectin-binding proteins by fluoroquinolone-resistant Staphylococcus aureus exposed to subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother. 1997;41:906–913. doi: 10.1128/aac.41.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boom R, Sol C J, Salimans M M, Jansen C L, Wertheim-van Dillen P M, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke D E, Raffin T A. Infectious complications of indwelling long-term central venous catheters. Chest. 1990;97:966–972. doi: 10.1378/chest.97.4.966. [DOI] [PubMed] [Google Scholar]
- 7.Darbyshire P J, Weightman N C, Speller D C. Problems associated with indwelling central venous catheters. Arch Dis Child. 1985;60:129–134. doi: 10.1136/adc.60.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominguez M A, Linares J, Pulido A, Perez J L, de Lencastre H. Molecular tracking of coagulase-negative staphylococcal isolates from catheter-related infections. Microb Drug Resist. 1996;2:423–429. doi: 10.1089/mdr.1996.2.423. [DOI] [PubMed] [Google Scholar]
- 9.Dougherty S H. Pathobiology of infection in prosthetic devices. Rev Infect Dis. 1988;10:1102–1117. doi: 10.1093/clinids/10.6.1102. [DOI] [PubMed] [Google Scholar]
- 10.Freeman J, Epstein M F, Smith N E, Platt R, Sidebottom D G, Goldmann D A. Extra hospital stay and antibiotic usage with nosocomial coagulase-negative staphylococcal bacteremia in two neonatal intensive care unit populations. Am J Dis Child. 1990;144:324–329. doi: 10.1001/archpedi.1990.02150270074029. [DOI] [PubMed] [Google Scholar]
- 11.Geary C, Jordens J Z, Richardson J F, Hawcroft D M, Mitchell C J. Epidemiological typing of coagulase-negative staphylococci from nosocomial infections. J Med Microbiol. 1997;46:195–203. doi: 10.1099/00222615-46-3-195. [DOI] [PubMed] [Google Scholar]
- 12.Goering R V, Winters M A. Rapid method for epidemiological evaluation of gram-positive cocci by field inversion gel electrophoresis. J Clin Microbiol. 1992;30:577–580. doi: 10.1128/jcm.30.3.577-580.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huebner J, Pier G B, Maslow J N, Muller E, Shiro H, Parent M, Kropec A, Arbeit R D, Goldmann D A. Endemic nosocomial transmission of Staphylococcus epidermidis bacteremia isolates in a neonatal intensive care unit over 10 years. J Infect Dis. 1994;169:526–531. doi: 10.1093/infdis/169.3.526. [DOI] [PubMed] [Google Scholar]
- 14.Isenberg H D, editor. Clinical microbiology procedures handbook. Washington, D.C: American Society for Microbiology; 1992. [Google Scholar]
- 15.John J F, Jr, Grieshop T J, Atkins L M, Platt C G. Widespread colonization of personnel at a Veterans Affairs medical center by methicillin-resistant, coagulase-negative Staphylococcus. Clin Infect Dis. 1993;17:380–388. doi: 10.1093/clinids/17.3.380. [DOI] [PubMed] [Google Scholar]
- 16.Kloos W E, Bannerman T L. Update on clinical significance of coagulase-negative staphylococci. Clin Microbiol Rev. 1994;7:117–140. doi: 10.1128/cmr.7.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lameris J S, Post P J, Zonderland H M, Gerritsen P G, Kappers-Klunne M C, Schutte H E. Percutaneous placement of Hickman catheters: comparison of sonographically guided and blind techniques. AJR Am J Roentgenol. 1990;155:1097–1099. doi: 10.2214/ajr.155.5.2120941. [DOI] [PubMed] [Google Scholar]
- 18.Linares J, Sitges-Serra A, Garau J, Perez J L, Martin R. Pathogenesis of catheter sepsis: a prospective study with quantitative and semiquantitative cultures of catheter hub and segments. J Clin Microbiol. 1985;21:357–360. doi: 10.1128/jcm.21.3.357-360.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyytikainen O, Saxen H, Ryhanen R, Vaara M, Vuopio-Varkila J. Persistence of a multiresistant clone of Staphylococcus epidermidis in a neonatal intensive-care unit for a four-year period. Clin Infect Dis. 1995;20:24–29. doi: 10.1093/clinids/20.1.24. [DOI] [PubMed] [Google Scholar]
- 20.Maki D G, Weise C E, Sarafin H W. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977;296:1305–1309. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. National Committee for Clinical Laboratory Standards, Villanova, Pa. 1991. Tentative guideline M29-T2. Protection of laboratory workers from infectious disease transmitted by blood, body fluids, and tissue. [Google Scholar]
- 22.Nesin M, Projan S J, Kreiswirth B, Bolt Y, Novick R P. Molecular epidemiology of Staphylococcus epidermidis blood isolates from neonatal intensive care unit patients. J Hosp Infect. 1995;31:111–121. doi: 10.1016/0195-6701(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 23.Nouwen J L, Wielenga J J, van Overhagen H, Kluytmans J A J W, van Belkum A, Sluis J, Verbrugh H A, de Marie S. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. High rate of Hickman catheter (HC) related infections (HCRI) due to coagulase-negative staphylococci (CNS): investigations into the source, abstr. J59; p. 229. [Google Scholar]
- 24.Nouwen, J. L., J. J. Wielenga, H. van Overhagen, J. S. Lameris, J. A. J. W. Kluytmans, W. C. J. Hop, H. A. Verbrugh, and S. de Marie. Hickman catheter-related infections in neutropenic patients: insertion in the operating theater versus the radiology suite. Submitted for publication. [DOI] [PubMed]
- 25.Prevost G, Jaulhac B, Piemont Y. DNA fingerprinting by pulsed-field gel electrophoresis is more effective than ribotyping in distinguishing among methicillin-resistant Staphylococcus aureus isolates. J Clin Microbiol. 1992;30:967–973. doi: 10.1128/jcm.30.4.967-973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raad I, Davis S, Khan A, Tarrand J, Elting L, Bodey G P. Impact of central venous catheter removal on the recurrence of catheter-related coagulase-negative staphylococcal bacteremia. Infect Control Hosp Epidemiol. 1992;13:215–221. doi: 10.1086/646512. [DOI] [PubMed] [Google Scholar]
- 27.Raad I I, Hohn D C, Gilbreath B J, Suleiman N, Hill L A, Bruso P A, Marts K, Mansfield P F, Bodey G P. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15:231–238. [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 29.Simon C, Suttorp M. Results of antibiotic treatment of Hickman-catheter-related infections in oncological patients. Support Care Cancer. 1994;2:66–70. doi: 10.1007/BF00355242. [DOI] [PubMed] [Google Scholar]
- 30.Stillman R I, Wenzel R P, Donowitz L C. Emergence of coagulase negative staphylococci as major nosocomial bloodstream pathogens. Infect Control. 1987;8:108–112. doi: 10.1017/s0195941700067278. [DOI] [PubMed] [Google Scholar]
- 31.Tan T Q, Musser J M, Shulman R J, Mason E O, Jr, Mahoney D H, Jr, Kaplan S L. Molecular epidemiology of coagulase-negative Staphylococcus blood isolates from neonates with persistent bacteremia and children with central venous catheter infections. J Infect Dis. 1994;169:1393–1397. doi: 10.1093/infdis/169.6.1393. [DOI] [PubMed] [Google Scholar]
- 32.van Belkum A, Bax R, Peerbooms P, Goessens W H, van Leeuwen N, Quint W G. Comparison of phage typing and DNA fingerprinting by polymerase chain reaction for discrimination of methicillin-resistant Staphylococcus aureus strains. J Clin Microbiol. 1993;31:798–803. doi: 10.1128/jcm.31.4.798-803.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Belkum A, Kluijtmans J, van Leeuwen W, Goessens W, ter Averst E, Verbrugh H. Investigation into the repeated recovery of coagulase-negative staphylococci from blood taken at the end of cardiopulmonary by-pass. J Hosp Infect. 1995;31:285–293. doi: 10.1016/0195-6701(95)90207-4. [DOI] [PubMed] [Google Scholar]
- 34.van Belkum A, Kluytmans J A J W, van Leeuwen W, Goessens W, ter Averst E, Wielenga J J, Verbrugh H A. Monitoring persistence of coagulase-negative staphylococci in a hematology department using phenotypic and genotypic strategies. Infect Control Hosp Epidemiol. 1996;17:660–667. [PubMed] [Google Scholar]
- 35.van de Leur J J, Dofferhoff A S, van Turnhout J M, Vollaard E J, Clasener H A. Colonisation of oropharynx with staphylococci after penicillin in neutropenic patients. Lancet. 1992;340:861–862. doi: 10.1016/0140-6736(92)92747-4. [DOI] [PubMed] [Google Scholar]
- 36.Versalovic J, Koeuth T, Lupski J R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weightman N C, Simpson E M, Speller D C, Mott M G, Oakhill A. Bacteraemia related to indwelling central venous catheters: prevention, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 1988;7:125–129. doi: 10.1007/BF01963064. [DOI] [PubMed] [Google Scholar]
- 38.Wilcox M H, Finch R G, Smith D G, Williams P, Denyer S P. Effects of carbon dioxide and sub-lethal levels of antibiotics on adherence of coagulase-negative staphylococci to polystyrene and silicone rubber. J Antimicrob Chemother. 1991;27:577–587. doi: 10.1093/jac/27.5.577. [DOI] [PubMed] [Google Scholar]