Abstract
Adolescence is often characterized by sleep disturbances that can affect the development of white matter tracts implicated in affective and cognitive regulation, including the cingulate portion of the cingulum bundle (CGC) and the uncinate fasciculus (UF). These effects may be exacerbated in adolescents exposed to early life adversity (ELA). We examined the longitudinal relations between sleep problems and CGC and UF microstructure during adolescence and their relation to depressive symptoms as a function of exposure to ELA. We assessed self-reported sleep disturbances and depressive symptoms and acquired diffusion-weighted MRI scans twice: in early adolescence (9–13 years) and four years later (13–17 years) (N = 72 complete cases). Independent of ELA, higher initial levels and increases in sleep problems were related to increases in depressive symptoms. Further, increases in right CGC fractional anisotropy (FA) mediated the association between sleep problems and depressive symptoms for youth who experienced lower, but not higher, levels of ELA. In youth with higher ELA, higher initial levels of and steeper decreases in sleep problems were associated with greater decreases in right UF FA, but were unrelated to depressive symptoms. Our findings highlight the importance of sleep quality in shaping fronto-cingulate-limbic tract development and depressive symptoms during adolescence.
Keywords: Sleep, Cingulum, Uncinate, Depression, Adolescence, Adversity
Highlights
-
•
Sleep problems in adolescents are associated with increases in depressive symptoms.
-
•
With low early stress, sleep problems increase cingulum cingulate (CGC) integrity.
-
•
Increases in CGC integrity are related to increases in depressive symptoms.
-
•
Sleep problems reduce uncinate fasciculus integrity in youth with more early stress.
-
•
Integrity of uncinate fasciculus is not related to depressive symptoms.
Adolescence is a period during which there are normative changes in sleep behaviors. Pubertal-related shifts in the circadian sleep-wake cycle (Carskadon et al., 1993, Carskadon et al., 1998, Crowley et al., 2018) and the slowing of sleep pressure accumulation throughout the day (Jenni et al., 2005) lead to later sleep times; further, for many adolescents, social and structural changes (e.g., earlier school start times) that require constant or earlier wake-up times result in decreases in sleep duration and greater variability in sleep duration and timing (Crowley et al., 2018). While normative, these sleep disturbances can have significant adverse effects on the developing brain, particularly in white matter tracts that have been implicated in affective and cognitive regulation (Telzer et al., 2013; Guldner et al., 2023) as well as in increased risk for depressive symptoms during adolescence (Gradisar et al., 2022). Moreover, sleep disturbances disproportionately affect youth from adverse backgrounds (April-Sanders et al., 2021; E. J. Park et al., 2021), potentially exacerbating the effects of sleep disturbance on white matter development and depressive symptoms. Indeed, adolescents exposed to adversity are more likely to exhibit more severe and more chronic symptoms of depression than do those who were not exposed to adversity (LeMoult et al., 2020). Similarly, individuals with a history of sleep disturbance are more likely to develop a diagnosis of depression during adolescence than are those without such a history (Gradisar et al., 2022, Scott et al., 2021). Although there is a growing body of research examining the links between sleep disturbances and white matter development during adolescence, few longitudinal studies have delineated the relations between sleep problems and white matter development during adolescence and how they may be altered in those with a history of early adversity. Furthermore, it is not clear whether sleep-related changes in white matter development mediate increases in depressive symptoms in adolescents with a history of early adversity.
Development of several long-range white matter tracts, particularly those connecting frontal regions to parietal and limbic regions, such as the uncinate fasciculus (UF) and the cingulum bundle (particularly the cingulate portion [CGC]), continues through adolescence and into the third decade of life (Asato et al., 2010, Lebel and Deoni, 2018, Peters et al., 2014). The integrity of these fronto-cingulate-limbic tracts during development has been shown to be related to neurocognitive outcomes (Bathelt et al., 2019) and executive functioning (Peters et al., 2014), and its disruption has been associated with symptoms of anxiety and depression (Chahal et al., 2021, Chahal et al., 2022, Olson et al., 2015). The prolonged development of these tracts allows them to be shaped by environmental exposures, such as stressful experiences and poor sleep. Indeed, sleep disturbances during adolescence have been associated with alterations in white matter integrity in numerous tracts, including the UF and cingulum bundle (Cooper et al., 2023, Guldner et al., 2023, Jamieson et al., 2020, Jamieson et al., 2020). For example, Telzer et al. (2015) found that greater variability in sleep duration 1.5 years before (but not concurrent with) the assessment of brain structure was associated with reduced fractional anisoptropy (FA) in frontocortical and frontostriatal tracts, association tracts, projection, and interhemispheric tracts. Studies with more than one assessment of white matter microstructure have found that greater within-person increase in preference for eveningness (i.e., later chronotype) from 12 to 19 years was associated with attenuated changes in FA globally between 17 and 19 years of age (Cooper et al., 2023). Further, greater within-person increases in sleep duration and variability have been associated with greater increases in FA in numerous white matter tracts, including the CGC and UF (Guldner et al., 2023), and greater variability in sleep duration and later chronotype have been associated with poorer physical and mental health outcomes in adolescents (Cooper et al., 2023; H. Park et al., 2016). Together, these studies highlight the long-term effects of sleep problems on white matter development in adolescents.
White matter microstructure of the UF and cingulum bundle have also been found to be altered by experiences of early adversity (Chahal et al., 2021, Dufford et al., 2020, Hanson et al., 2015, Ho et al., 2017, Olson et al., 2015) and to predict responses to subsequent stressful events (Chahal et al., 2022, Hanson et al., 2015). For instance, young adults who experienced more severe childhood maltreatment had lower FA of the UF; further, among those with lower UF FA, subsequent experiences of stressful events were associated with greater increases in internalizing symptoms (Hanson et al., 2015). In addition, using cross-sectional DTI data only from the baseline assessment (i.e., ages 9–13 years) of the current study, we found that during the COVID-19 pandemic, lower fiber density and cross-section of the cingulum bundle at baseline was associated in females with greater increases in depressive symptoms, lower resilience, and higher stress (Chahal et al., 2022).
To date, researchers have documented the adverse effects of early life adversity (ELA) and of sleep disturbances, independently, on fronto-cingulate-limbic white matter integrity. It is not clear, however, whether experiences of ELA interact with and compound the effects of sleep disturbances on white matter development and depressive symptoms during adolescence. Given the overlapping effects of ELA and sleep disturbances on white matter integrity and depressive symptoms during adolescence, it is possible that adolescents who were exposed to ELA are more sensitive to the effects of sleep disturbances (Fuligni et al., 2021). To test this formulation, in the current study we examined the relations among sleep problems, the development of white matter microstructural integrity of tracts that connect fronto-cingulo-limbic regions (i.e., the CGC and the UF), and depressive symptoms during adolescence, and tested whether these effects were moderated by exposure to ELA. Although sleep disturbances have been associated with changes in a number of white matter tracts during adolescence (e.g., Guldner et al., 2023), we focused in this study on the CGC and UF given their overlapping associations with sleep disturbances, ELA, and depressive symptoms. Sleep disturbances, depressive symptoms, and white matter microstructure were assessed at two timepoints – once during early adolescence (9–13 years; Time 1 [T1]) and again approximately four years later (13–17 years; Time 2 [T2]); cumulative exposure to early adversity was assessed at T1. First, we examined whether ELA moderated the association between sleep problems (both initial levels and changes) and changes in depressive symptoms. We hypothesized that greater sleep problems at T1 and greater increases in sleep problems will be associated with greater increases in depressive symptoms and, further, that these associations will be stronger for adolescents exposed to higher levels of ELA. Second, we tested whether ELA moderated associations between sleep problems and white matter microstructural changes in CGC and UF. Consistent with previous studies demonstrating that poor sleep has long-term adverse effects on white matter integrity (Telzer et al., 2015, Guldner et al., 2023), we hypothesized that higher initial levels of, and increases in, sleep problems will be associated with attenuated development of CGC and UF integrity four years later and, further, that these effects will be stronger in adolescents who were exposed to higher levels of ELA. Finally, for associations in which ELA moderated the effect of sleep problems (at T1 and/or changes in sleep problems) on changes in both depressive symptoms and FA, we conducted moderated mediation analyses to test whether changes in FA (CGC and/or UF) from T1 to T2 mediated the association between sleep problems (at T1 and/or changes in sleep problems) and changes in depressive symptoms from T1 to T2, and whether the mediation differed as a function of exposure to ELA. We hypothesized that alterations in CGC and/or UF integrity will mediate the association between greater sleep problems (both initial levels and increases) and greater increases in depressive symptoms, and that these effects will be stronger in youth who experienced greater cumulative severity of ELA.
1. Methods
1.1. Participants
224 children and adolescents (N = 131 females) were recruited starting in September, 2013, from the San Francisco Bay Area to participate in a longitudinal study examining the effects of ELA on psychobiology over puberty. Participants were 9.11–13.98 years of age (M=11.51 years, SD=1.08) at the first timepoint (T1) and 13.07–17.93 years (M=15.61 years, SD=1.12) at the second timepoint (T2), approximately 4 years later. Exposure to early adversity was assessed at T1. Diffusion-weighted MRI scans (T1: N = 142, T2: N = 112, T1 & T2: N = 81) and reports of sleep problems (T1: N = 213, T2: N = 163, T1 & T2 = 157) and depressive symptoms (T1: N = 220, T2: N = 168; T1 & T2 = 166) were obtained from as many participants as possible at both timepoints. All analyses were conducted with the largest number of participants. A total of 155 participants had complete data on ELA, sleep problems, and depressive symptoms for both timepoints. Of those, 72 participants also had good-quality CGC and UF FA data at both timepoints. Participants and their parents or legal guardians gave informed assent and consent, respectively, for study activities and were compensated for their participation. Study procedures were approved by the Stanford University Institutional Review Board and were in accordance with guidelines set forth by the Declaration of Helsinki.
1.2. Measures
1.2.1. Early life adversity
A modified version of the Traumatic Events Screening Inventory for Children (TESI-C; (Ford et al., 2002)) was used to assess more than 30 different types of stressful life experiences (e.g., experienced a traumatic accident, witnessed traumatic accident, experienced illness/injury, etc.). Each type of stress exposure endorsed by participants was followed up with questions to obtain a deep characterization of the experience. For example, participants were asked if they have "ever been in a really bad accident, like a car accident, a fall, or fire.” If this item was endorsed, participants were then asked about when the event happened and whether they or someone else were really hurt. In addition to providing details about stressful events, participants also provided their own subjective rating of the severity of each event. Subsequently, a panel of three trained coders who were blind to the participants’ subjective severity ratings rated the objective severity of each event based on the information obtained at the interview using a modified version of the UCLA Life Stress Interview coding system (Rudolph and Hammen, 1999). Coders rated each event on a 5-point scale with half-point increments, ranging from 0 (non-event or not impactful) to 4 (extremely severe and impactful) (inter-rater intraclass correlation =.99). If there were any discrepancies between coders in ratings for an event, the coders discussed their ratings to arrive at a consensus score for the event that was then used for analyses. Cumulative adversity severity scores were computed by summing the maximum objective severity scores for each type of endorsed stressor (Chahal et al., 2022, King et al., 2020), and ranged from 0 to 22 in this sample.
1.2.2. Sleep disturbances
Participants completed the Youth Self-Report (YSR; Achenbach and Rescorla, 2001), from which we calculated a sleep problems composite generated from five items assessing sleep quality: I sleep less than most kids, I sleep more than most kids during the day and/or night, I have trouble sleeping, I have nightmares, and I feel overtired. Although not a standardized subscale, this sleep composite score has been used in previous research as a measure of overall sleep functioning (e.g., Wang et al., 2016). It has also been shown to correlate strongly with the validated Children’s Sleep Habits Questionnaire (Lionetti et al., 2021, Owens et al., 2000). Each item is rated on a 3-point scale (0 =not true, 1 =somewhat or sometimes, 2 =very true or often true). Response frequencies for each sleep item and the reliabilities (Cronbach’s alpha) for the five-item composite at both T1 and T2 are reported in Table S1. An item-analysis indicated that removing the item assessing hypersomnia (i.e, “I sleep more than most kids during the day and/or night”) increased the relability of the sleep composite. Thus, we removed this item and calculated a sleep problems composite consisting of the sum of the four other items and used this new composite for analyses (range=0–8), with higher scores indicating more sleep problems. Reliabilities for the sleep problems composite at T1 and T2 are 0.66 (95% CI: [0.57, 0.76]) and 0.59 (95% CI: [0.49, 0.70]), respectively.
1.2.3. Depressive symptoms
Participants completed the 10-item short form of the Child Depression Inventory (CDI-S; Kovacs, 2015), a self-report measure of depressive symptoms designed for youth ages 8–17 years. Participants indicated the severity of symptoms of depression they were experiencing over the past two weeks on a three-point scale. The CDI-S measures sadness, pessimism, self-deprecation, self-hate, crying spells, irritability, negative body image, loneliness, lack of friends, and feeling unloved; sleep disturbances were not included as a symptom of depression. A sum score was calculated to represent depressive symptoms, with higher score indicating greater symptoms. Reliabilities for depressive symptoms scale at T1 and T2 are 0.75 (95% CI: [0.70, 0.80]) and 0.85 (95% CI: [0.82, 0.88]), respectively.
1.3. MRI data acquisition
All MRI data were acquired at the Stanford University Center for Cognitive and Neurobiological Imaging (CNI) using a 3 T General Electric (GE) Discovery 750 MRI system (General Electric Healthcare, Milwaukee, WI, USA) with a 32-channel head coil (Nova Medical, Wilmington, MA, USA). A spoiled gradient recall acquisition sequence was used for acquiring T1-weighted images (TR/TE/TI=6.24 s/2.34 s/450 ms; flip angle=12°; 186 sagittal slices; 0.9 mm isotropic voxels) and an echo planar imaging sequence was used for acquiring diffusion-weighted images (TR/TE=8500/93.5 ms; 64 axial slices; 2 mm isotropic voxels; 60 b=2000 diffusion-weighted directions, and 6 b=0 acquisitions at the beginning of the scan; anterior/posterior phase encoding direction).
1.4. Diffusion MRI preprocessing
Diffusion MRI data were processed according to field-consensus procedures using the open source mrVista software distribution developed by the VISTA lab (https://vistalab.stanford.edu/), as outlined in previous work in this sample by our group (Ho et al., 2017, Ho et al., 2020). Specifically, after the T1-weighted images were manually aligned to the anterior commissure-posterior commissure (AC-PC) line, the mean of the non-diffusion-weighted (b=0) images was aligned to the T1 image using a rigid body 3D motion correction with a constrained non-linear warping based on a model of the expected eddy-current distortions. Each registration consisted of estimating the eddy-current/motion correction parameters simultaneously and optimization was performed using a gradient-ascent-type technique within a multi-resolution framework. The eddy-current and motion corrected diffusion-weighted data were then resampled to 2 mm isotropic voxels using a trilinear interpolation algorithm (Ashburner and Friston, 2004). Importantly, the diffusion-weighted directions (bvecs matrix) were adjusted after these alignment and motion correct steps by combining the rotation matrix from the alignment step with the rotation matrix prior to computing the tensors (Leemans and Jones, 2009). We further minimized the effects of motion on the data by excluding directions where relative motion in the translational directions exceeded 5 mm or exceeded 1.5° in the rotational directions and then reperforming the aforementioned eddy-current and motion-corrected steps. All participants included in the present study therefore did not have more than 12 (20%) outlier directions at either timepoint.
1.5. Deterministic tractography of cingulum cingulate and uncinate fasciculus
Whole-brain fiber tracts were mapped onto the ACPC-aligned T1-weighted images with Automated Fiber Quantification (Yeatman et al., 2012; https://yeatmanlab.github.io/pyAFQ/), using a deterministic algorithm with a fourth-order Rungeñ-Kutta path integration method and 1 mm fixed-step size. A continuous tensor field was estimated with trilinear interpolation of the tensor elements. As in previous work (Ho et al., 2017, Ho et al., 2020, Ho et al., 2022), seeds for tractography were selected from a uniform 1 mm 3D grid spanning the whole brain mask (among voxels where FA > 0.3). Path tracing proceeded until FA fell below 0.15 or until the minimum angle between the current and previous path segments was greater than 30°. Streamlines for each of the tracts of interest – left and right CGC, left and right UF – were automatically generated using a two planar waypoint region of interest approach such that tracts were traced along these seeds according to probabilistic fiber groupings based on an established white matter atlas with candidate fibers being assessed based on their similarity to this standard (Mori et al., 2005). Outliers (defined along a Gaussian curve as anything more than 4 standard deviations away from the spatial core) were excluded iteratively. All tracts at each time point were visually assessed and any deviant streamline fibers whose tract profiles differed significantly from known white matter anatomy were manually corrected by one of the co-authors using tools through mrVista. Tracts that were unresolved (e.g., too thin) or errant beyond the scope of this manual correction were excluded. FA was estimated for 100 evenly spaced nodes along each tract of interest and averaged across the entire tract to generate a single metric per tract, as in previous work (Ho et al., 2017, Ho et al., 2020, Ho et al., 2022).
1.6. White matter development
Longitudinal changes in white matter development were assessed by calculating the annualized percentage change (APC) in left and right CGC and UF FA between the two time points. APC is defined as the growth percent (i.e., difference in mean FA between T1 and T2, divided by the mean FA at T1, then multiplied by 100) divided by the time interval in years between T1 and T2 for each participant.
1.7. Statistical analyses
All analyses were conducted in R using the car and lme4 packages for multivariate analysis of covariance (MANCOVA) and linear regression, respectively, and interactions package for visualization. Moderated mediation analyses were conducted using the Hayes’ PROCESS function in R. We used Bonferroni correction to correct for multiple comparisons within each aim/hypothesis. We first tested whether ELA moderated the association between sleep problems at T1 and/or changes in sleep problems from T1 to T2 and changes in depressive symptoms from T1 to T2. The change in levels of depressive symptoms from T1 to T2 (i.e., CDI total scores at T1 subtracted from CDI total scores at T2) was regressed on sleep problems at T1, cumulative severity of ELA, and their interaction, covarying for depressive symptoms at T1, changes in sleep problems from T1 to T2 (sleep problems score at T1 subtracted from sleep problems score at T2), age at T1, and sex. We conducted a separate linear regression with change in depressive symptoms regressed on changes in sleep problems, cumulative severity of ELA, and their interaction, covarying for sleep problems at T1 and the other covariates noted above. We evaluated statistical significance using a corrected alpha = .025 for these analyses.
Next, we conducted two MANCOVAs to test whether ELA moderated associations between sleep problems at T1 and/or changes in sleep problems from T1 to T2 and changes in white matter microstructure. In this analysis, APC of CGC and UF FA were regressed on sleep problems at T1 (or changes in sleep problems), cumulative severity of ELA, and their interaction, covarying for CGC and UF FA at T1, changes in sleep problems (or sleep problems at T1), age at T1, and sex. We conducted separate MANCOVAs for left and right tracts. We evaluated statistical significance at the MANCOVA level, correcting for four comparisons (2 MANCOVAs x 2 hemispheres) for these analyses (corrected alpha =.0125) and followed up significant MANCOVAs with separate linear regressions for APC in CGC and UF FA.
Finally, for associations in which ELA moderated the effect of sleep problems (at T1 and/or changes in sleep problems) and both changes in depressive symptoms and changes in FA, we conducted moderated mediation analyses using Hayes’ PROCESS function in R to test whether changes in FA (CGC and/or UF) from T1 to T2 mediated the association between sleep problems (at T1 and/or changes in sleep problems) and changes in depressive symptoms from T1 to T2, and whether the mediation differed as a function of exposure to ELA. Based on significant findings, we conducted the following moderated mediation models: 1) ELA x sleep problems at T1 on changes in depressive symptoms through APC in right CGC FA; 2) ELA x sleep problems at T1 on changes in depressive symptoms through APC in right UF FA; 3) ELA x changes in sleep problems on changes in depressive symptoms through APC in right CGC FA; and 4) ELA x sleep problems at T1 on changes in depressive symptoms through APC in right UF FA. We used Bonferroni-corrected 98.75% confidence intervals (corrected alpha =.0125 for 4 tests) with 5000 bootstraps to assess the significance of moderated mediation and also reported the uncorrected (i.e., 95%) confidence intervals.
To assess directionality of effects, we also tested the reverse association of whether white matter integrity at T1 (and/or changes in FA) was associated with changes in sleep problems from T1 to T2 and whether early adversity moderated these effects. We conducted separate linear regression models for CGC and UF FA. Changes in sleep problems from T1 to T2 were regressed on CGC (or UF) FA at T1, ELA, and their interactions, covarying for sleep problems at T1, APC in CGC (or UF) FA, age at T1, and sex. Similarly, changes in sleep problems were regressed on APC in CGC (or UF) FA, ELA, and their interactions, covarying for sleep problems at T1, CGC (or UF) FA at T1, age at T1, and sex.
1.8. Sensitivity analyses
Finally, to examine whether the interaction between ELA and sleep problems at T1 (and changes in sleep problems) on development of white matter integrity from T1 to T2 was specific to sleep problems or, alternatively, was due to behavioral/affective problems more broadly, we conducted the same analyses, but using the affective/depressive problems subscale from the YSR instead of sleep problems.
2. Results
Sample characteristics and descriptive statistics are presented in Table 1 and correlations among variables are presented in Table 2. As expected, variables at T1 were correlated with their respective variables at T2. Severity of ELA was positively associated with sleep problems at T1 and T2. CGC and UF FA were positively correlated with each other at both time points.
Table 1.
Sample characteristics and descriptive statistics by timepoint. All descriptive statistics are reported in mean (standard deviation) except for sex and ethnicity, which are reported as number of participants (and percentage) in each category. Items denoted with T1 were measured only at T1. YSR = Youth Self Report. CDI = Child Depression Inventory. CGC = cingulum bundle, cingulate portion. UF = uncinate fasciculus.
| Variable | Time 1 (N = 224) | Time 2 (N = 168) |
|---|---|---|
| Age in years | 11.35 (1.05) | 15.46 (1.11) |
| Sex (M/F) (T1) | 93/131 (41.5%/58.5%) | 72/96 (42.9%/57.1%) |
| Ethnicity (T1) | N (%) | N (%) |
| White | 99 (44.2%) | 68 (42.0%) |
| African American | 19 (8.48%) | 14 (8.64%) |
| Hispanic | 20 (8.93%) | 13 (8.02%) |
| Asian | 24 (10.71%) | 22 (13.58%) |
| Biracial | 48 (21.43%) | 35 (21.60%) |
| Other | 14 (6.25%) | 10 (6.17%) |
| Cumulative Severity of Adversity (T1) | 6.89 (5.47) | 6.55 (5.02) |
| YSR Sleep Problems | 2.35 (1.86) | 2.46 (1.95) |
| CDI Depressive Symptoms | 2.17 (2.38) | 3.58 (3.44) |
| Left CGC FA | 0.48 (0.047) | 0.51 (0.044) |
| Right CGC FA | 0.46 (0.042) | 0.47 (0.045) |
| Left UF FA | 0.46 (0.037) | 0.44 (0.03) |
| Right UF FA | 0.46 (0.035) | 0.43 (0.027) |
Table 2.
Bivariate correlations among variables. L = left, R = right, CGC = cingulum bundle, cingulate portion. UF = uncinate fasciculus, FA = fractional anisotropy. * p < .05, * * p < . 01, * ** p < .001.
| N | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age (T1) | 224 | .85 * ** | -0.11 | -0.12 | 0.18 * | 0.03 | 0.19 * | 0.04 | 0 | 0.22 * | 0 | 0.18 | -0.05 | 0.03 | -0.13 |
| 2. Age (T2) | 157 | -0.17 * | -0.06 | 0.17 | 0.18 | 0.15 | 0.13 | 0.14 | 0.21 * | 0.09 | 0.19 * | -0.02 | -0.09 | -0.05 | |
| 3. Sleep Problems (T1) | 213 | 0.25 * * | -0.1 | 0 | -0.1 | 0 | -0.03 | -0.14 | -0.06 | -0.16 | 0.2 * * | 0.49 * ** | 0.12 | ||
| 4. Sleep Problems (T2) | 163 | -0.04 | 0.01 | -0.01 | 0.04 | 0.05 | 0.05 | -0.04 | 0.04 | 0.29 * ** | 0.16 * | 0.65 * ** | |||
| 5. L CGC FA (T1) | 141 | 0.63 * ** | 0.7 * ** | 0.54 * ** | 0.29 * ** | 0.46 * ** | 0.37 * ** | 0.36 * ** | 0.02 | 0.06 | 0.04 | ||||
| 6. L CGC FA (T2) | 110 | 0.62 * ** | 0.72 * ** | 0.36 * ** | 0.24 * | 0.27 * | 0.22 * | 0.07 | 0.01 | 0.15 | |||||
| 7. R CGC FA (T1) | 138 | 0.78 * ** | 0.24 * * | 0.35 * * | 0.29 * ** | 0.25 * | 0.02 | 0 | 0.05 | ||||||
| 8. R CGC FA (T2) | 111 | 0.29 * * | 0.25 * * | 0.21 | 0.28 * * | 0.17 | 0.07 | 0.2 * | |||||||
| 9. L UF FA (T1) | 142 | 0.8 * ** | 0.7 * ** | 0.51 * ** | -0.02 | 0 | -0.01 | ||||||||
| 10. L UF FA (T2) | 112 | 0.58 * ** | 0.59 * ** | 0.04 | 0.08 | 0.04 | |||||||||
| 11. R UF FA (T1) | 142 | 0.75 * ** | -0.1 | -0.09 | -0.03 | ||||||||||
| 12. R UF FA (T2) | 112 | -0.05 | -0.09 | 0.06 | |||||||||||
| 13. Early life adversity | 224 | 0.28 * ** | 0.2 * * | ||||||||||||
| 14. Depressive Symptoms (T1) | 220 | 0.09 | |||||||||||||
| 15. Depressive Symptoms (T2) | 168 |
2.1. Higher initial levels of and increases in sleep disturbances are related to increases in depressive symptoms
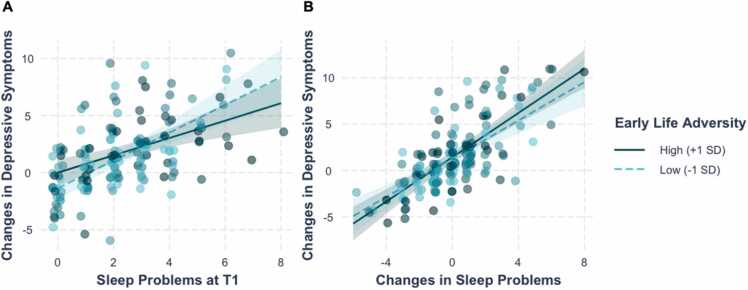
Linear regression analyses indicated that greater sleep problems at T1 were associated with greater increases in depressive symptoms from T1 to T2 (b=0.99, SE=0.16, t(147) = 6.08, p < .001), over and above changes in sleep problems from T1 to T2, sex, age at T1, and depressive symptoms at T1 (Fig. 1 A; Table S2); this effect was marginally (i.e., did not survive correction for multiple comparisons) moderated by severity of ELA (b=−0.047, SE=0.024, t(147) = −2.00, p = .047). Post-hoc simple effects analyses indicated that the effect of sleep problems at T1 on increases in depressive symptoms was stronger for adolescents exposed to lower severity of ELA (b=1.22, SE=0.20, t(147) = 6.05, p < .001) than for adolescents exposed to higher severity of ELA (b=0.76, SE=0.20, t(147) = 3.86, p = .0002). In addition, adolescents who reported increases in sleep problems from T1 to T2 also reported increases in depressive symptoms from T1 to T2 (b=1.11, SE=0.12, t(147) = 9.28), p < .001), over and above sleep problems at T1 (Fig. 1B; Table S3); this effect was not moderated by severity of ELA (b=0.017, SE=0.017, t(147) = 1.00, p = .32).
Fig. 1.
(A) Greater sleep problems at T1 were associated with greater increases in depressive symptoms from T1 to T2, controlling for sex, age at T1, depressive symptoms at T1, and changes in sleep problems from T1 to T2. (B) Increases in sleep problems from T1 to T2 were associated with greater increases in depressive symptoms, controlling for sex, age at T1, depressive symptoms at T1, and sleep problems at T1. Values represent predicted changes in depressive symptoms adjusted for covariates.
2.2. ELA moderates associations between initial levels of sleep disturbances and longitudinal changes in CGC and UF FA
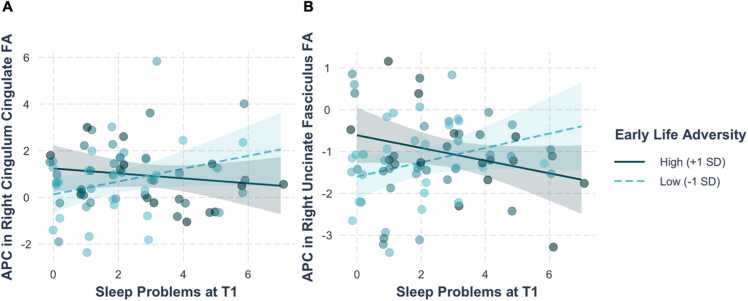
The MANCOVA conducted to test whether ELA moderated associations between sleep problems at T1 and changes in white matter microstructure yielded a significant interaction between ELA and sleep problems at T1 on APC in right CGC and UF FA (F(2, 63)= 5.022, p = .0095; Table S4; Fig. 2). Separate linear regressions indicated that the interaction between ELA and sleep problems at T1 was significant for APC in both right CGC and right UF FA (CGC: b=−0.37, SE=−.016, t(63) = −2.35, p = .022 [Table S5]; UF: b= −0.032, SE= 0.011, t(63) = −2.98, p = .0004 [Table S6]). While the coefficients of the simple slopes indicated that the association between sleep problems at T1 and APC in both right CGC and UF FA were in opposite directions at + /- 1 SD of ELA, the simple slopes were not statistically significant (APC in right CGC: −1 SD ELA: b=0.28, SE=0.15, t(65) = 1.83, p = .07; + 1 SD ELA: b= −0.106, SE= 0.14, t(65) = −0.75, p = .46; APC in right UF: − 1 SD ELA: b= 0.172, SE= 0.10, t(65) = 1.68, p = .10; + 1 SD ELA: b= −0.152, SE= 0.095, t(65) = −1.60, p = .114). Johnson-Neyman analysis indicated that the association between sleep problems at T1 and APC in right UF FA is significant at ELA severity scores greater than 13.35 (+1.18 SD).
Fig. 2.
Early life adversity moderated the association between sleep problems at T1 and annualized percentage change (APC) in (A) right CGC FA and (B) right UF FA, controlling for sex, age at T1, changes in sleep problems, and respective tract FA at T1. Values represent predicted APC in FA adjusted for covariates. CGC = cingulate portion of the cingulum bundle, UF = uncinate fasciculus, FA = fractional anisotropy.
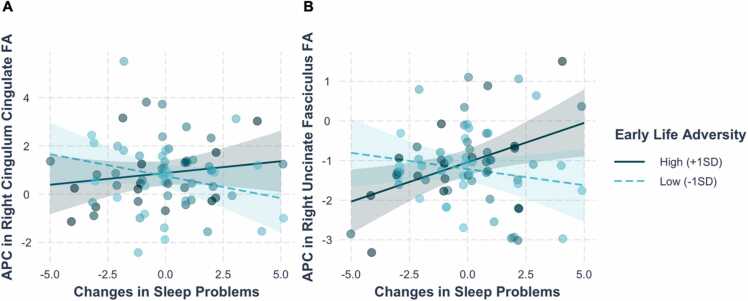
ELA also moderated the association between changes in sleep problems and APC in right CGC and UF FA, over and above sleep problems at T1, F(2,63)= 5.48, p = .0064 (Table S7; Fig. 3). Separate linear regressions indicated that the interaction between ELA and changes in sleep problems was significant for APC in both right CGC and UF FA (CGC: b=0.027, SE=0.013, t(65) = 2.08, p = .042 [Table S8]; UF: b= 0.028, SE= 0.0086, t(67) = 3.21, p = .002 [Table S9]). Simple slope analysis indicated that greater increases in sleep problems were associated with greater increases (smaller decreases) in right UF FA in adolescents who were exposed to higher (+1 SD) levels of ELA (b=0.20, SE=0.08, t(67) = 2.62, p = .01). Changes in sleep problems were not related to changes in right UF FA in adolescents who were exposed to lower (−1 SD) levels of ELA (b=−0.08, SE=0.09, t(67) = −0.99, p = .33). Although simple slope analyses indicated that the coefficients for the effect of changes in sleep disturbances and APC in right CGC were in opposite directions for lower ELA (b=−0.18, SE=0.13, t(65) = −1.41, p = .16) versus higher ELA (b=0.10, SE=0.11, t(65) = 0.85, p = .40), the simple slopes at lower and higher levels of ELA were not statistically significant.
Fig. 3.
Early life adversity (ELA) moderated the association between changes in sleep problems and annualized percentage change (APC) in (A) right CGC FA and (B) right UF FA, controlling for sex, age at T1, sleep problems in T1, and respective tract FA. Simple slope analyses indicated that greater increases in sleep problems were associated with greater APC in right UF FA in adolescents exposed to higher (+1 SD) ELA (p = .01), but not to lower (−1 SD) ELA (p = .33). Values represent predicted APC in FA adjusted for covariates. CGC = cingulate portion of the cingulum bundle, UF = uncincate fasciculus, FA = fractional anisotropy.
The interactions between ELA and sleep problems at T1 (F(2, 63)= 3.37, p = .041; Table S10) and between ELA and changes in sleep problems (F(2, 63)= 2.33, p = .11; Table S13) predicting APC in left CGC (Tables S11 and S14) and UF FA (Tables S12 and S15) were not statistically significant.
Testing the reverse association, the interaction between ELA and CGC FA at T1 (left: p = .80; right: p = .23; Tables S16-S17) and between ELA and UF FA at T1 (left: p = .89; right: p = .30; Tables S18-S19) on changes in sleep problems were not statistically significant, indicating that CGC and UF integrity at baseline were not related to changes in sleep problems. Similarly, the interaction between ELA and APC in CGC FA (left: p = .61; right: p = .23; Tables S20-S21) and between ELA and APC in UF FA (left: p = .09; right: p = .33; Tables S22-S23) on changes in sleep problems were not statistically significant. In addition, across all analyses, sleep problems at T1 were negatively associated with changes in sleep problems, such that greater sleep problems at T1 were associated with a smaller increase (or greater decrease) in sleep problems from T1 to T2 (ps<.001).
2.3. CGC development mediates the association between initial levels of sleep problems and changes in depressive symptoms in adolescents with lower ELA
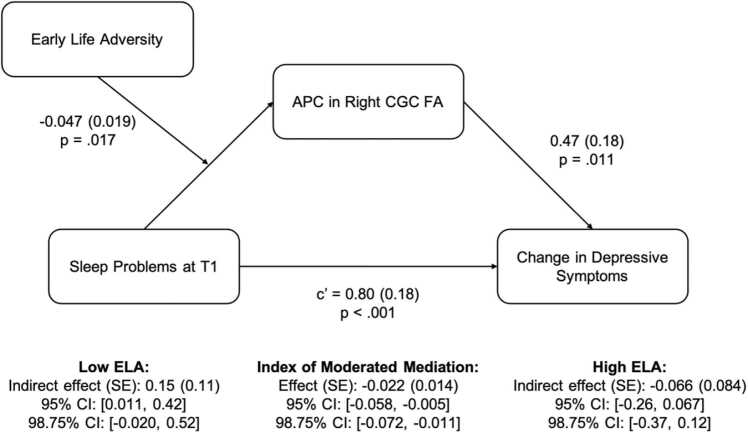
Next, we examined whether APC of right CGC and right UF FA mediated the association between sleep problems at T1 and change in depressive symptoms from T1 to T2, and whether the mediation was moderated by ELA. Covariates included sex, age at T1, change in sleep problems from T1 to T2, respective tract FA at T1, and depressive symptoms at T1. Analyses revealed that the indirect effect of sleep problems at T1 and changes in depressive symptoms through APC in right CGC FA was moderated by ELA (index of moderated mediation = −0.022, SE=0.014, 95% CI: [−0.058, −0.005]; 98.75% CI: [−0.0723, −0.011]), such that the mediation model was marginally significant (i.e., did not survive correction for multiple comparisons) for adolescents with lower levels (−1 SD) of ELA (indirect effect = 0.15, SE=0.11, 95% CI: [0.011, 0.423]; 98.75% CI: [−0.0196, 0.5219]), but not with higher levels (+1 SD) of ELA (indirect effect = −0.066, SE=0.084, 95% CI: [−0.26, 0.67]; 98.75% CI: [−0.369, 0.122]). For adolescents with lower levels of ELA, greater sleep problems at T1 were associated with greater increases in CGC FA (b=0.32, SE=0.15, t(63) = 2.20, p = .0314), which, in turn, were associated with greater increases in depressive symptoms from T1 to T2 (b=0.47, SE=−.18, t(63) = 2.63, p = .011) (Fig. 3). Greater APC in right CGC FA was associated with greater increases in depressive symptoms across all levels of ELA (i.e., the interaction of ELA and APC in right CGC FA was not significant [b=−0.004, SE=0.043, t(62) = −0.10, p = .92]). APC of right UF FA was not associated with changes in depressive symptoms (path b: b=0.48, SE=0.27, t(65) = 1.81, p = .07), and this effect did not differ by ELA (b=0.026, SE=0.037, t(63) = 0.71, p = .48). .
Fig. 4.
Moderated mediation analysis indicated that the indirect effect of sleep problems at T1 and changes in depressive symptoms from T1 to T2 through APC in right CGC FA were moderated by early life adversity (ELA). For adolescents exposed to lower (−1 SD) levels of ELA, greater sleep problems at T1 were associated with greater annualized percentage change (APC) in right CGC FA, which was associated with greater increases in depressive symptoms. APC in right CGC FA did not mediate the association between sleep problems at T1 and changes in depressive symptoms for adoelscents exposed to higher (+1 SD) levels of ELA. Covariates included sex, age at T1, depressive symptoms at T1, changes in sleep problems from T1 to T2, and right CGC FA at T1. CGC = cingulate porition of the cingulum bundle, FA = fractional anisotropy.
The indirect effect of changes in sleep problems on changes in depressive symptoms through APC in right CGC FA was marginally (i.e., did not survive correction for multiple comparisons) moderated by ELA (index of moderated mediation = 0.0186, SE=0.0092, 95% CI: [0.0014, 0.0379], 98.75% CI: [−0.0060, 0.0451]). Further, although the indirect effects were in opposite directions for adolescents with lower ELA (indirect effect = −0.1154, SE=0.0834, 95% CI: [−0.2661, 0.0740], 98.75% CI: [−0.322; 0.148]) than for adolescents with higher ELA (indirect effect = 0.0695, SE=0.0829; 95% CI: [−0.0566, 0.2670], 98.75% CI: [−0.0963, 0.3520]), neither effect was statistically significant. APC in right UF FA was not associated with changes in depressive symptoms (b=0.34, SE=0.27, t(65) = 1.28, p = .21), nor were there any differences in this association across levels of ELA (ELA x APC in right UF FA: b=0.018, SE=0.04, t(63) = 0.49, p = .62), over and above the moderating effect of ELA and changes in sleep problems on APC in right UF FA.
2.4. Sensitivity analyses
To test whether the interaction between ELA and sleep problems at T1 on APC in right CGC and UF from T1 to T2 was specific to sleep problems or, alternatively, was due to affective problems more broadly, we conducted the same MANCOVA, but substituting scores on the affective/depressive problems subscale of the YSR for sleep problems. ELA did not interact significantly with anxious/depressed problems at T1 to predict APC in right CGC and/or UF FA from T1 to T2 (F(2,63)= 1.079, p = .35; Table S24). ELA also did not interact significantly with changes in anxious/depressed problems to predict APC in right CGC and/or UF FA (F(2, 63)= 1.97, p = .148; Table S25).
3. Discussion
The current study examined the interactive effects of early life adversity (ELA) and sleep problems on longitudinal changes in CGC and UF white matter microstructural integrity and depressive symptoms over a four-year period during adolescence. We found that, in adolescents who experienced lower levels of ELA, greater sleep problems early in adolescence were associated with increased FA in right CGC four years later, which was associated with an increase in depressive symptoms across the same time period. We also found that, in adolescents who experienced higher levels of ELA, greater initial levels of sleep problems and greater decreases in sleep problems were associated with a greater decrease in right UF FA four years later; however, changes in UF FA were not associated with changes in depressive symptoms. This study is the first to examine the role of sleep problems in the longitudinal development of CGC and UF integrity and changes in depressive symptoms, and how these associations differ as a function of early adversity.
Consistent with our hypotheses and with previous research (e.g., Guldner et al., 2023; James and Hale, 2017; Talbot et al., 2010), we found that both sleep problems in early adolescence and increases in sleep problems over a four-year interval, independently, were related to increases in depressive symptoms during adolescence. Moreover, these associations were not significantly moderated by severity of ELA. These findings highlight the importance of sleep disturbances in the development and escalation of depressive symptoms during adolescence.
We also found that higher levels of sleep problems at T1 were associated with greater increases in right CGC FA, which were associated with greater increases in depressive symptoms in adolescents who experienced lower levels of ELA. While alterations in the development of the CGC as a function of sleep and ELA were expected, the direction of these effects was counter to our hypotheses. Specifically, we expected that greater sleep problems would be associated with attenuated white matter development (Cooper et al., 2023), and that higher CGC FA would be associated with lower levels of depressive symptoms (Barch et al., 2022, Kliamovich et al., 2021). It is important to note, however, an emerging body of research is documenting higher levels of CGC FA both in adolescents with depression and in adults with chronic sleep disturbances. Interestingly, these findings also appear to be lateralized to the right hemisphere. For example, Cullen et al. (2020) examined the associations between FA across numerous tracts and several dimensions of depression (e.g., lassitude, appetite changes, traumatic intrusion) in adolescents with depression and found that greater right CGC FA was associated with greater lassitude, which is characterized by feelings of exhaustion, low energy, sleepiness, difficulty getting going, and feeling worse in the morning (Cullen et al., 2020). In a study with adults, Lee et al. (2022) found that shift-workers, who regularly experience sleep disturbances due to the desynchronization of their circadian rhythm and homeostatic sleep pressure, exhibited greater right CGC FA than did non-shift-workers. Moreover, in shift-workers, greater right CGC FA was positively correlated with multiple indices of poor sleep quality, including poorer subjective sleep, whereas the opposite pattern (higher CGC FA, better sleep quality) was observed in non-shift-workers. Together, these findings suggest that chronic sleep disturbance and its related depressive symptoms (e.g., low energy, fatigue, loss of interest) are associated with greater right CGC FA. Our findings support this formulation and demonstrate further that, beyond cross-sectional differences in FA, greater sleep problems during adolescence are associated with greater increases of right CGC FA, which are associated with greater increases in depressive symptoms.
In the present study, changes in right CGC FA were found to mediate the association between initial levels of sleep disturbances and increases in depressive symptoms only in adolescents with lower ELA. Although sleep problems at T1 were not associated with changes in right CGC FA for adolescents who had experienced higher ELA, the positive association between changes in right CGC FA and changes in depressive symptoms was also present for these adolescents. The results of our moderated mediation analysis suggests that, while sleep disturbances may be one pathway by which ELA is related to depressive symptoms, this association is perhaps not explained by alterations in fronto-cingulate-limbic white matter development for adolescents with higher levels of ELA.
In contrast to the effects of sleep disturbances on right CGC development, we found that greater sleep disturbances at T1 were associated with greater decreases in right UF FA in adolescents with higher, but not with lower, ELA. In addition, over and above the effects of sleep disturbance at T1, greater decreases in sleep disturbances from T1 to T2 were also associated with greater decreases in right UF FA in adolescents with higher ELA. In our sample, adolescents who had more sleep problems at T1 were more likely to have smaller increases (or greater decreases) in sleep problems from T1 to T2. Considered collectively, these findings suggest that, among adolescents exposed to higher ELA, those who have more sleep problem at T1 also have smaller increases (or greater decreases) in sleep problems from T1 to T2 and, further, that these two patterns of sleep problems are associated with greater decreases in right UF FA. These findings are consistent with previous cross-sectional research that has found lower UF FA in adolescents with more sleep problems and/or with exposure to higher ELA (e.g., (Hanson et al., 2015, Ho et al., 2017, Telzer et al., 2015). Our longitudinal investigation suggests that this lower UF FA is a result of greater decreases in UF FA rather than of attenuated development of UF FA during adolescence. Previous studies that have examined the development of the CGC and UF from childhood through adulthood (8–86 years) found that, whereas UF FA did not change with age, CGC FA increased from childhood through the late-20 s and then remained stable across adulthood (Peters et al., 2014). One longitudinal study that evaluated changes in FA across multiple tracts in individuals 5–32 years of age found that over 70% of individuals showed either no change or decreases in UF FA during adolescent years, and over 80% showed either no change or decreases in UF FA after 19 years (Lebel and Beaulieu, 2011). These findings stand in contrast to the development of the cingulum, with 30–50% of individuals showing increases in FA well into their 20′s (Lebel and Beaulieu, 2011). While speculative, the current findings suggest that, in adolescents who experienced greater ELA, greater sleep disturbances during early adolescence, over and above changes in sleep disturbances across adolescence, are associated with premature degradation of the UF. It will be important to conduct studies examining other indices of white matter integrity, including indices of both macrostructure (e.g., fiber density) and microstructure (e.g., mean, axial, radial diffusivity) integrity, to test this formulation.
We did not find that changes in UF FA were associated with changes in depressive symptoms. The absence of a significant association between UF FA and depressive symptoms is consistent with several studies that also did not find significant differences in UF FA between adolescents with and without depression; importantly, these studies did report significant associations between CGC FA and adolescent depression, suggesting specificity of CGC in adolescent depression (Barch et al., 2022, Cullen et al., 2020, Kliamovich et al., 2021, Uchida et al., 2021). While the function of the UF is not yet clear, researchers have posited that the UF is involved in cognitive control, reward processing, episodic memory, and stress responding (Granger et al., 2021, Kircanski et al., 2019, Olson et al., 2015). Indeed, the UF connects regions in the medial temporal lobe, such as the amygdala and entorhinal cortex, to the orbitofrontal cortex (Olson et al., 2015), regions that have been implicated in these functions. In contrast, the CGC connects frontal regions to subcortical regions (amygdala, striatum, hippocampus) through the subgenual/anterior cingulate (Bubb et al., 2018, Lichenstein et al., 2016, Wakana et al., 2004). The subgenual/anterior cingulate has been implicated in melancholia and depressive symptoms (Mertse et al., 2022), and is part of multiple brain networks involved in emotion processing and regulation, including the default mode, salience, and fronto-parietal networks (Bathelt et al., 2019, Lichenstein et al., 2016), which have also been found to be disrupted in depression and associated with ELA and with sleep disturbances (Akbar et al., 2022, Chahal et al., 2022, Dutil et al., 2018). Taken together, these findings suggest that the CGC specifically underlies associations among ELA, sleep disturbances, and depressive symptoms during adolescence.
We should note three limitations of this study. First, in measuring sleep disturbances we assessed participants’ general experience of multiple sleep problems; thus, this measure is not specific with respect to particular problems/disorders or timeframe (e.g., past week or month). Similarly, we also do not have information about participants’ sleep behaviors (e.g., sleep duration, timing, efficiency); therefore, we cannot distinguish the effects of sleep problems from consequences of sleep behaviors. Nevertheless, it is important to note that our findings converge with results of other studies that examined white matter microstructure as a function of sleep behaviors (Guldner et al., 2023, Telzer et al., 2015) and morning-evening preference (Cooper et al., 2023), suggesting that the effects of sleep disturbances on white matter integrity are robust to differences in the operationalization of sleep disturbances. Future studies might examine possible differences in the neural effects of objectively and subjectively measured sleep behaviors and disturbances. Second, while our examination of longitudinal changes in sleep problems, white matter, and depressive symptoms across two timepoints is a strength of our study, we were not able to investigate potential non-linear effects. Future studies using at least three time points of data will be better positioned to address this question. Finally, we experienced attrition in our DTI data from T1 (N = 142) to T2 (N = 112) (only 72 participants had good quality DTI data and behavioral measures at both timepoints), which reduced statistical power in our analyses. We did, however, obtain significant effects of sleep on brain white matter microstructure; further, our final sample of participants with complete DTI and behavioral data at both timepoints is comparable in size to samples in recently published studies in this area (e.g., Cooper et al., 2023; Guldner et al., 2023).
Despite these limitations, in the current study we demonstrated that greater sleep disturbances during early adolescence and greater longitudinal increases in sleep disturbances are associated with greater increases in depressive symptoms and changes in fronto-cingulate-limbic white matter development across a four-year period. Moreover, these associations differed as a function of exposure to early adversity. These findings highlight the importance of sleep quality in shaping the development of both fronto-cingulate-limbic white matter tracts and depressive symptoms during adolescence.
Funding
This work was supported by the National Institute of Mental Health (R37MH101495 [PI: Gotlib], F32MH135657 [PI: Uy]).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2023.101303.
Appendix A. Supplementary material
Supplementary material
.
Data availability
Data will be made available on request.
References
- Achenbach T.M., Rescorla L.A. Manual for the ASEBA School-Age Forms & Profiles. University of Vermont, Research Center for Children. Youth, Fam. 2001 [Google Scholar]
- Akbar S.A., Mattfeld A.T., Laird A.R., McMakin D.L. Sleep to internalizing pathway in young adolescents (SIPYA): a proposed neurodevelopmental model. Neurosci. Biobehav. Rev. 2022;140 doi: 10.1016/j.neubiorev.2022.104780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- April-Sanders A., Duarte C.S., Wang S., McGlinchey E., Alcántara C., Bird H., Canino G., Suglia S.F. Childhood adversity and sleep disturbances: longitudinal results in Puerto Rican children. Int. J. Behav. Med. 2021;28(1):107–115. doi: 10.1007/s12529-020-09873-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato M.R., Terwilliger R., Woo J., Luna B. White matter development in adolescence: a DTI study. Cereb. Cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. High-dimensional image warping. Hum. Brain Funct. 2004;2:673–694. [Google Scholar]
- Barch D.M., Hua X., Kandala S., Harms M.P., Sanders A., Brady R., Tillman R., Luby J.L. White matter alterations associated with lifetime and current depression in adolescents: Evidence for cingulum disruptions. Depress Anxiety. 2022;39(12):881–890. doi: 10.1002/da.23294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathelt J., Johnson A., Zhang M., Astle D.E. The cingulum as a marker of individual differences in neurocognitive development. Sci. Rep. 2019;9(1):1–16. doi: 10.1038/s41598-019-38894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb E.J., Metzler-Baddeley C., Aggleton J.P. The cingulum bundle: anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 2018;92:104–127. doi: 10.1016/j.neubiorev.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon M.A., Vieira C., Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16(3):258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Carskadon M.A., Wolfson A.R., Acebo C., Tzischinsky O., Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21(8):871–881. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- Chahal R., Kirshenbaum J.S., Ho T.C., Mastrovito D., Gotlib I.H. Greater age-related changes in white matter morphometry following early life stress: Associations with internalizing problems in adolescence. Dev. Cogn. Neurosci. 2021;47 doi: 10.1016/j.dcn.2020.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal R., Miller J.G., Yuan J.P., Buthmann J.L., Gotlib I.H. An exploration of dimensions of early adversity and the development of functional brain network connectivity during adolescence: implications for trajectories of internalizing symptoms. Dev. Psychopathol. 2022;34(2):557–571. doi: 10.1017/S0954579421001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal R., Ho T.C., Miller J.G., Borchers L.R., Gotlib I.H. Sex‐specific vulnerability to depressive symptoms across adolescence and during the COVID‐19 pandemic: The role of the cingulum bundle. JCPP Adv. 2022;2(1):1–13. doi: 10.1002/jcv2.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R., Di Biase M.A., Bei B., Allen N.B., Schwartz O., Whittle S., Cropley V. Development of morning–eveningness in adolescence: implications for brain development and psychopathology. J. Child Psychol. Psychiatry. 2023;64(3):449–460. doi: 10.1111/jcpp.13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley S.J., Wolfson A.R., Tarokh L., Carskadon M.A. An update on adolescent sleep: new evidence informing the perfect storm model. J. Adolesc. 2018;67:55–65. doi: 10.1016/j.adolescence.2018.06.001. (April) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K.R., Brown R., Schreiner M.W., Eberly L.E., Klimes-Dougan B., Reigstad K., Hill D., Lim K.O., Mueller B.A. White matter microstructure relates to lassitude but not diagnosis in adolescents with depression. Brain Imaging Behav. 2020;14(5):1507–1520. doi: 10.1007/s11682-019-00078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufford A.J., Evans G.W., Dmitrieva J., Swain J.E., Liberzon I., Kim P. Prospective associations, longitudinal patterns of childhood socioeconomic status, and white matter organization in adulthood. Hum. Brain Mapp. 2020;41(13):3580–3593. doi: 10.1002/hbm.25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutil C., Walsh J.J., Featherstone R.B., Gunnell K.E., Tremblay M.S., Gruber R., Weiss S.K., Cote K.A., Sampson M., Chaput J. Influence of sleep on developing brain functions and structures in children and adolescents: a systematic review. Sleep. Med. Rev. 2018;42:184–201. doi: 10.1016/j.smrv.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Ford J.D., Racusin R., Rogers K., Ellis C., Schiffman J., Ribbe D., Edwards J. National Center for PTSD and Dartmouth Child Psychiatry Research Group,; 2002. Traumatic events screening inventory for children (TESI-C) Version 8.4. [Google Scholar]
- Fuligni A.J., Chiang J.J., Tottenham N. Sleep disturbance and the long-term impact of early adversity. Neurosci. Biobehav. Rev. 2021;126:304–313. doi: 10.1016/j.neubiorev.2021.03.021. [DOI] [PubMed] [Google Scholar]
- Gradisar M., Kahn M., Micic G., Short M., Reynolds C., Orchard F., Bauducco S., Bartel K., Richardson C. Sleep’s role in the development and resolution of adolescent depression. Nat. Rev. Psychol. 2022;1(9):512–523. doi: 10.1038/s44159-022-00074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger S.J., Leal S.L., Larson M.S., Janecek J.T., McMillan L., Stern H., Yassa M.A. Integrity of the uncinate fasciculus is associated with emotional pattern separation-related fMRI signals in the hippocampal dentate and CA3. Neurobiol. Learn. Mem. 2021;177 doi: 10.1016/j.nlm.2020.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldner S., Sarvasmaa A.S., Lemaître H., Massicotte J., Vulser H., Miranda R., Bezivin –, Frère P., Filippi I., Penttilä J., Banaschewski T., Barker G.J., Bokde A.L., Bromberg U., Büchel C., Conrod P.J., Desrivières S., Flor H., Frouin V., Gallinat J., Martinot J.L. Longitudinal associations between adolescent catch-up sleep, white-matter maturation and internalizing problems. Dev. Cogn. Neurosci. 2023;59 doi: 10.1016/j.dcn.2022.101193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Knodt A.R., Brigidi B.D., Hariri A.R. Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev. Psychopathol. 2015;27:1611–1619. doi: 10.1017/S0954579415000978.Lower. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.C., King L.S., Leong J.K., Colich N.L., Humphreys K.L., Ordaz S.J., Gotlib I.H. Effects of sensitivity to life stress on uncinate fasciculus segments in early adolescence. Soc. Cogn. Affect. Neurosci. 2017;12(9):1460–1469. doi: 10.1093/scan/nsx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.C., Colich N.L., Sisk L.M., Oskirko K., Jo B., Gotlib I.H. Sex differences in the effects of gonadal hormones on white matter microstructure development in adolescence. Dev. Cogn. Neurosci. 2020;42 doi: 10.1016/j.dcn.2020.100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.C., Kulla A., Teresi G.I., Sisk L.M., Rosenberg-Hasson Y., Maecker H.T., Gotlib I.H. Inflammatory cytokines and callosal white matter microstructure in adolescents. Brain, Behav., Immun. 2022;100:321–331. doi: 10.1016/j.bbi.2021.12.003. [DOI] [PubMed] [Google Scholar]
- James S., Hale L. Sleep duration and child well-being: a nonlinear association. J. Clin. Child Adolesc. Psychol. 2017;46(2):258–268. doi: 10.1080/15374416.2016.1204920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D., Broadhouse K.M., McLoughlin L.T., Schwenn P., Parker M.J., Lagopoulos J., Hermens D.F. Investigating the association between sleep quality and diffusion-derived structural integrity of white matter in early adolescence. J. Adolesc. 2020;83:12–21. doi: 10.1016/j.adolescence.2020.06.008. [DOI] [PubMed] [Google Scholar]
- Jamieson D., Broadhouse K.M., Lagopoulos J., Hermens D.F. Investigating the links between adolescent sleep deprivation, fronto-limbic connectivity and the Onset of Mental Disorders: a review of the literature. Sleep. Med. 2020;66:61–67. doi: 10.1016/j.sleep.2019.08.013. [DOI] [PubMed] [Google Scholar]
- Jenni O.G., Achermann P., Carskadon M.A. Homeostatic sleep regulation in adolescents. Sleep. 2005;28(11):1446–1454. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- King L.S., Graber M.G., Colich N.L., Gotlib I.H. Associations of waking cortisol with DHEA and testosterone across the pubertal transition: effects of threat-related early life stress. Psychoneuroendocrinology. 2020;115 doi: 10.1016/j.psyneuen.2020.104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K., Sisk L.M., Ho T.C., Humphreys K.L., King L.S., Colich N.L., Ordaz S.J., Gotlib I.H. Early life stress, cortisol, frontolimbic connectivity, and depressive symptoms during puberty. Dev. Psychopathol. 2019;31(3):1011–1022. doi: 10.1017/S0954579419000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliamovich D., Jones S.A., Chiapuzio A.M., Baker F.C., Clark D.B., Nagel B.J. Sex-specific patterns of white matter microstructure are associated with emerging depression during adolescence. Psychiatry Res. - Neuroimaging. 2021;315 doi: 10.1016/j.pscychresns.2021.111324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Children’s depression inventory (CDI and CDI 2) Encycl. Clin. Psychol. 2015:1–5. doi: 10.1002/9781118625392.wbecp419. [DOI] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Deoni S. The development of brain white matter microstructure. NeuroImage. 2018;182:207–218. doi: 10.1016/j.neuroimage.2017.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Kim M., Kim N., Hwang Y., Lee K.H., Lee J., Lee Y.J., Kim S.J. Evidence of white matter integrity changes in the anterior cingulum among shift workers: a cross-sectional study. Nat. Sci. Sleep. 2022;14:1417–1425. doi: 10.2147/NSS.S369192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans A., Jones D.K. The B‐matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med.: Off. J. Int. Soc. Magn. Reson. Med. 2009;61(6):1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- LeMoult J., Humphreys K.L., Tracy A., Hoffmeister J.A., Ip E., Gotlib I.H. Meta-analysis: exposure to Early Life Stress and Risk for Depression in Childhood and Adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2020;59(7):842–855. doi: 10.1016/j.jaac.2019.10.011. [DOI] [PubMed] [Google Scholar]
- Lichenstein S.D., Verstynen T., Forbes E.E. Adolescent brain development and depression: a case for the importance of connectivity of the anterior cingulate cortex. Neurosci. Biobehav. Rev. 2016;70:271–287. doi: 10.1016/j.neubiorev.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti F., Dellagiulia A., Verderame C., Sperati A., Bodale G., Spinelli M., Fasolo M. The children’s sleep habits questionnaire: identification of sleep dimensions, normative values, and associations with behavioral problems in italian preschoolers. Sleep. Health. 2021;7(3):390–396. doi: 10.1016/j.sleh.2021.03.002. [DOI] [PubMed] [Google Scholar]
- Mertse N., Denier N., Walther S., Breit S., Grosskurth E., Federspiel A., Wiest R., Bracht T. Associations between anterior cingulate thickness, cingulum bundle microstructure, melancholia and depression severity in unipolar depression. J. Affect. Disord. 2022;301:437–444. doi: 10.1016/j.jad.2022.01.035. [DOI] [PubMed] [Google Scholar]
- Mori S., Wakana S., van Zijl P.C.M., Nagae-Poetscher L.M. Elsevier,; 2005. MRI Atlas of Human White Matter. [DOI] [PubMed] [Google Scholar]
- Olson I.R., Heide R.J.V.Der, Alm K.H., Vyas G. Development of the uncinate fasciculus: implications for theory and developmental disorders. Dev. Cogn. Neurosci. 2015;14:50–61. doi: 10.1016/j.dcn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J.A., Spirito A., McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051. doi: 10.1093/sleep/23.8.1d. [DOI] [PubMed] [Google Scholar]
- Park E.J., Kim S.Y., Kim Y., Sung D., Kim B., Hyun Y., Jung K.I., Lee S.Y., Kim H., Park S., Kim B.N., Park M.H. The relationship between adverse childhood experiences and sleep problems among adolescent students: Mediation by depression or anxiety. Int. J. Environ. Res. Public Health. 2021;18(1):1–15. doi: 10.3390/ijerph18010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Tsai K.M., Dahl R.E., Irwin M.R., McCreath H., Seeman T.E., Fuligni A.J. Sleep and inflammation during adolescence. Psychosom. Med. 2016;78(6):677–685. doi: 10.1097/PSY.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B.D., Ikuta T., Derosse P., John M., Burdick K.E., Gruner P., Prendergast D.M., Szeszko P.R., Malhotra A.K. Age-related differences in white matter tract microstructure are associated with cognitive performance from childhood to adulthood. Biol. Psychiatry. 2014;75(3):248–256. doi: 10.1016/j.biopsych.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph K.D., Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective. Child Dev. 1999;70(3):660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Scott J., Kallestad H., Vedaa O., Sivertsen B., Etain B. Sleep disturbances and first onset of major mental disorders in adolescence and early adulthood: a systematic review and meta-analysis. Sleep. Med. Rev. 2021;57 doi: 10.1016/j.smrv.2021.101429. [DOI] [PubMed] [Google Scholar]
- Talbot L.S., McGlinchey E.L., Kaplan K.A., Dahl R.E., Harvey A.G. Vol. 10. American Psychological Association,; 2010. Sleep deprivation in adolescents and adults: changes in affect; pp. 831–841. (In Emotion). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Goldenberg D., Fuligni A.J., Lieberman M.D., Gálvan A. Sleep variability in adolescence is associated with altered brain development. Dev. Cogn. Neurosci. 2015;14:16–22. doi: 10.1016/j.dcn.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida M., Hung Y., Green A., Kelberman C., Capella J., Gaillard S.L., Gabrieli J.D.E., Biederman J. Association between frontal cortico-limbic white-matter microstructure and risk for pediatric depression. Psychiatry Res. - Neuroimaging. 2021;318 doi: 10.1016/j.pscychresns.2021.111396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S., Jiang H., Nagae-Poetscher L.M., van Zijl P.C.M., Mori S. Fiber tract–based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Myall N.J., Wandell B.A., Feldman H.M. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.